Abstract
Background
Respiratory tract infections (RTIs) and interstitial lung disease (ILD) secondary to interleukin (IL) 12/23 or IL-23 antagonists have been reported in autoimmune diseases.
Objective
To assess the risk of RTIs and noninfectious ILD with these drugs.
Methods
We conducted a systematic review and meta-analysis of randomized controlled trials. Risk of RTIs and noninfectious ILD was compared to placebo by Mantel-Haenszel risk difference. We divided RTIs into upper RTIs (URTI), viral URTIs, and lower RTIs (LRTIs) including infectious pneumonia. Noninfectious ILD included ILD, eosinophilic pneumonia, and pneumonitis.
Results
We identified 54 randomized controlled trials including 10,907 patients with 6 IL-12/23 or IL-23 antagonists and 5175 patients with placebo. These drugs significantly increased the risk of RTIs (Mantel-Haenszel risk difference, 0.019; 95% confidence interval, 0.005-0.033; P = .007), which was attributed to URTIs, but not viral URTIs or LRTIs. There was no significant difference in infectious pneumonia and noninfectious ILD between 2 groups.
Limitations
Because of the rarity of infectious pneumonia and ILD, sensitivity analysis was required.
Conclusions
The use of IL-12/23 or IL-23 antagonists for autoimmune diseases increased the risk of URTIs, but not viral URTIs, LRTIs, and noninfectious ILD.
Key words: autoimmune diseases, IL12/23 and IL23 antagonists, meta-analysis, noninfectious interstitial lung disease, respiratory tract infections
Capsule Summary.
-
•
This meta-analysis showed that IL-12/23 or IL-23 antagonists increased the risk of upper respiratory tract infections (URTIs), but not viral URTIs, lower RTIs, and noninfectious interstitial lung disease in autoimmune diseases.
-
•
This result suggests their safe use even during the COVID-19 pandemic, but further observations are required.
The clinical benefit of interleukin (IL) 12 and IL-23 inhibition has been shown in psoriasis and Crohn's disease (CD) by briakinumab1 , 2 or ustekinumab.3 , 4 Furthermore, IL-23–specific antagonists, such as tildrakizumab,5 , 6 risankizumab,7 , 8 guselkumab,9 , 10 and brazikumab,11 have completed phase 2 or 3 trials. Currently, IL-12/23 or IL-23 antagonists are the second most commonly prescribed category of biologics for psoriasis and CD, behind anti–tumor necrosis factor agents.12
However, randomized controlled trials (RCTs) of these drugs reported respiratory tract infections (RTIs) as the most common adverse events.13 Furthermore, the surveillance conducted by the US Food and Drug Administration (FDA) reported the development of noninfectious interstitial lung disease (ILD) after ustekinumab.14 Hence, physicians need evidence to decide whether to continue or hold these drugs, particularly during the current COVID-19 pandemic.15, 16, 17
This systematic review and meta-analysis aimed to determine the risk of RTIs and noninfectious ILD with anti–IL-12/23 or anti–IL-23 agents in autoimmune diseases.
Methods
Search strategy and study selection
This meta-analysis was conducted according to an a priori defined protocol based on Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.18 The protocol of this meta-analysis has been submitted to the International Prospective Register of Systematic Reviews (PROSPERO).19
We searched PubMed/MEDLINE, Google Scholar, Scopus, Embase, ClinicalTrials.gov (https://clinicaltrials.gov/), and the Cochrane database from inception to February 1, 2019, to identify studies assessing the efficacy and safety of anti–IL-12/23 and anti–IL-23 therapies in autoimmune diseases. We also searched abstracts from medical conferences and bibliographies of identified articles for additional references. For Google Scholar, only the first 1000 articles were reviewed because this is the maximum number of results provided by the database. When a study registered in ClinicalTrials.gov or presented as an abstract became later available as an article, data were updated accordingly.20 , 21
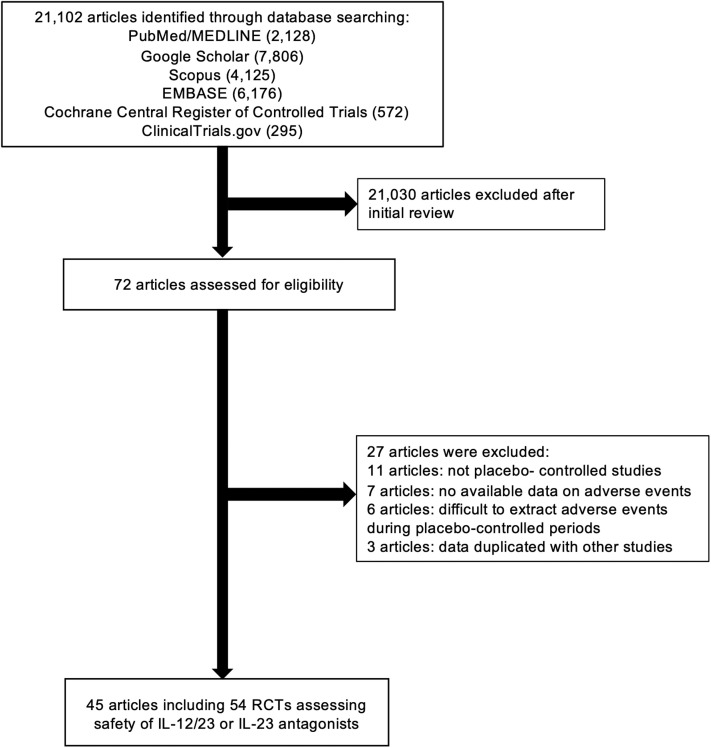
As for inclusion criteria, we considered RCTs reporting the incidence of adverse events, including RTIs and noninfectious ILD, with anti–IL-12/23 and anti–IL-23 therapies. There were no restrictions regarding age, sex, or duration of the study. We imposed no geographic or language restrictions. Two authors (SA and DM) independently screened each of the potential trials, abstract, and/or full article to determine whether they were eligible for inclusion. Area of disagreement or uncertainty were resolved by consensus among the authors. Studies were identified with the terms “briakinumab,” “ustekinumab,” “tildrakizumab,” “guselkumab,” “risankizumab,” “brazikumab,” “mirikizumab,” “LY2525623,” “anti–interleukin-12/23,” “anti–interleukin-23,” “anti–IL-12/23,” “anti–IL-23,” “anti–interleukin-12,” and “anti–IL-12” (both as medical subject headings and free text terms). RCTs without placebo-controlled groups were excluded. The search strategy is described in Fig 1 .
Fig 1.
Flow chart of the assessment of the studies identified in the meta-analysis. IL, Interleukin; RCT, randomized controlled trial.
Data extraction and quality assessment
All data were independently abstracted in duplicate by 2 authors (SA and DM) by using a data extraction form. Data on the study characteristics, such as author name, year of publication, study design, duration, sample size, age of patients, type of medications, and incidence of events were collected. Several published studies included data from multiple RCTs with different regimens or participant characteristics. For instance, the study published by Gordon et al included a comparison between ustekinumab and placebo and another comparison between risankizumab and placebo.7 These comparisons were considered as separate individual RCTs in our meta-analysis to ensure proper comparison and to avoid selection bias. The Jadad score was used to assess the quality of RCTs.22 We also used Cochrane risk-of-bias assessment instrument to evaluate the quality of the RCTs.23 The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was applied to assess the certainty of evidence obtained from this meta-analysis.24
Outcome assessment
The primary outcome measure of interest was the risk difference (RD) of the development of RTIs and noninfectious ILD among patients on anti–IL-12/IL-23 or anti–IL-23 agents compared with placebo. Subgroup analyses with each monoclonal antibody and underlying disease were performed. Data were analyzed based on the intention-to-treat principle except where indicated. We determined the number of each adverse event from the articles and the ClinicalTrials.gov database. We sorted RTIs into the following 3 categories: (1) upper RTIs (URTIs), (2) viral URTIs, and (3) lower RTIs (LRTIs). Based on Medical Dictionary for Regulatory Activities (MedDRA) Terminology (https://bioportal.bioontology.org/ontologies/MEDDRA), URTIs included the following diagnoses: nasopharyngitis, laryngitis, pharyngitis, rhinitis, sinusitis, tonsillitis, pharyngotonsillitis, tracheobronchitis, and upper respiratory infection. Viral URTIs included influenza and viral URTI. LRTIs were bronchitis, LRTI, and pneumonia. We included the following diseases in noninfectious ILD: ILD, eosinophilic pneumonia, and pneumonitis.
Statistical analysis
We undertook a meta-analysis with a random effects model. Mantel-Haenszel (MH) RD was used as our primary method.25 As for rare events, including infectious pneumonia and noninfectious ILD, we performed the sensitivity analysis as described in the “Results” section because of uncertainty regarding the preferred method for rare events. First, we analyzed data by MH odds ratio (OR), Peto OR,26 and MH risk ratio (RR) by excluding double-zero-event studies. Second, data were pooled among each drug, and then meta-analysis was performed by MH RD, MH OR, Peto OR, and MH RR.27 Finally, data were assessed by MH OR, Peto OR, and MH RR by adding 0.5 continuity correction or treatment arm correction to zero-event studies.28
We evaluated the presence of heterogeneity across trials by using the I 2 statistic. An I 2 value of less than 25% indicated low heterogeneity, 25% to 75% indicated moderate heterogeneity, and greater than 75% indicated considerable heterogeneity.29 Heterogeneity was evaluated by using the Cochran Q statistic with a significance level of P < .10.30 Begg and Egger tests were performed to assess publication bias, and funnel plots were constructed to visualize possible asymmetry when 3 or more studies were available.31 , 32
Statistical analyses were performed using Comprehensive Meta-Analysis Software, version 2.0 (Biostat, Englewood, NJ). All statistical tests except for the Q statistic used a 2-sided P value of .05 for significance.
Results
Study characteristics
We identified 21,102 citations through the literature search, excluded 21,030 titles and abstracts after the initial screening, and assessed 72 studies for eligibility. A final number of 43 full-text articles and 2 studies registered only in ClinicalTrials.gov met all eligibility criteria and included 54 RCTs with a total of 10,907 patients with anti–IL-12/IL-23 or anti–IL-23 antibodies and 5175 with placebo (Fig 1). The 54 RCTs included 1 study of brazikumab (59 patients), 8 of briakinumab (1817 patients), 9 of guselkumab (1321 patients), 5 of risankizumab (830), 5 of tildrakizumab (1596 patients), and 26 of ustekinumab (5284 patients). All of the included studies are randomized, double-blind, placebo-controlled studies and have high scores in the Jadad scoring system. The characteristics and outcomes of the included studies are summarized in Table I .33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60 The mean ages of patients and percentages of male patients were 43.8 years and 66.6% for guselkumab, 42.8 years and 65.4% for risankizumab, 37.6 years and 62.4% for tildrakizumab, 43.3 years and 49.5% for briakinumab, and 43.3 years and 54.0% for ustekinumab. The percentages of studies that permitted use of concomitant drugs (eg, corticosteroids, budesonide, thiopurines, methotrexate, calcineurin inhibitors, or aminosalicylates) during the trials were 40.0% for risankizumab, 38.5% for ustekinumab, 37.5% for briakinumab, 0% for guselkumab, and 0% for tildrakizumab. As for brazikumab, 1 study for CD was included in this analysis and permitted concomitant drugs (Table I).
Table I.
Characteristics of randomized controlled trials of IL-12/23 or IL-23 antagonists∗
| Drugs | Target | Disease | References | Age, y | Sex, % male | Study duration, wk | Concomitant therapies during trials | Regimen, mg (unless noted otherwise) | Jadad score | Patients, N |
RTIs, n |
Infectious pneumonia, n |
Noninfectious ILD, n |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-12/23 | Placebo | IL-12/23 | Placebo | IL-12/23 | Placebo | IL-12/23 | Placebo | ||||||||||
| Brazikumab | IL-23 | CD | Sands et al (2017)11 | 37 | 38 | 12 | Yes | 700 IV at wk 0, 4 | 5 | 59 | 60 | 9 | 11 | 0 | 0 | 0 | 0 |
| Guselkumab | IL-23 | Psoriasis | Ohtsuki et al (2018)33 | 50 | 68 | 16 | No | 50, 100 SC at wk 0, 4, 12 | 5 | 128 | 64 | 22 | 7 | 0 | 0 | 0 | 0 |
| †NCT0290533120 | 46 | 68 | 16 | No | 100 SC at wk 0, 4, 12 | 4 | 62 | 16 | 13 | 1 | 0 | 0 | 0 | 0 | |||
| Nemoto et al (2018)34 | NA | 60 | 24 | No | 10, 30, 100, 300 SC (s) | 5 | 20 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | |||
| Blauvelt et al (2017)35 | 44 | 73 | 16 | No | 100 SC at wk 0, 4, 12 | 5 | 329 | 174 | 55 | 26 | 0 | 0 | 0 | 0 | |||
| Reich et al (2017)10 | 44 | 70 | 16 | No | 100 SC at wk 0, 4, 12 | 5 | 494 | 248 | 51 | 26 | 0 | 0 | 0 | 0 | |||
| Gordon et al (2015)9 | 44 | 72 | 16 | No | 5, 50, 200 SC at wk 0, 4, q12 wk; 15, 100 SC q8 wk | 5 | 207 | 42 | 25 | 3 | 0 | 0 | 0 | 0 | |||
| PPP | Sofen et al (2014)36 | 43 | 63 | 24 | No | 10, 30, 100, 300 SC (s) | 4 | 20 | 4 | 5 | 0 | 0 | 0 | 0 | 0 | ||
| Healthy volunteer | Terui et al (2018)37 | 52 | 29 | 24 | No | 200 SC at wk 0, 4 | 5 | 25 | 24 | 8 | 9 | 0 | 0 | 0 | 0 | ||
| Zhuang et al (2016)38 | 27 | 96 | 16 | No | 0.03, 0.1, 0.3, 1, 3, 10 mg/kg IV; 3 mg/kg SC (s) | 5 | 36 | 11 | 5 | 1 | 0 | 0 | 0 | 0 | |||
| Risankizumab | IL-23 | Psoriasis | Gordon et al (2018)7 | 48 | 71 | 16 | No | 150 SC at wk 0, 4 | 5 | 304 | 102 | 37 | 8 | 0 | 0 | 0 | 0 |
| Gordon et al (2018)7 | 47 | 68 | 16 | No | 150 SC at wk 0, 4 | 5 | 294 | 98 | 21 | 4 | 0 | 0 | 0 | 0 | |||
| Krueger et al (2015)39 | 42 | 81 | 24 | No | 0.01, 0.05, 0.25, 1, 3, 5 mg/kg IV; 0.25, 1 mg/kg SC (s) | 5 | 31 | 8 | 11 | 2 | 0 | 0 | 0 | 0 | |||
| Ankylosing spondylitis | Baeten et al (2018)64 | 38 | 70 | 16 | Yes | 18 SC (s); 90, 180 SC at wk 0, 8, 16 | 5 | 119 | 40 | 31 | 5 | 0 | 0 | 0 | 0 | ||
| CD | Feagan et al (2017)8 | 39 | 37 | 12 | Yes | 200, 600 IV at wk 0, 4, 8 | 5 | 82 | 39 | 10 | 5 | 1 | 1 | 0 | 0 | ||
| Tildrakizumab | IL-23 | Psoriasis | Reich et al (2017)5 | 46 | 67 | 12 | No | 100, 200 SC at wk 0, 4 | 5 | 617 | 154 | 69 | 17 | 0 | 0 | 0 | 0 |
| Reich et al (2017)5 | 46 | 71 | 12 | No | 100, 200 SC at wk 0, 4 | 5 | 621 | 156 | 76 | 12 | 0 | 0 | 0 | 0 | |||
| Papp et al (2015)6 | 43 | 74 | 16 | No | 5, 25, 100, 200 SC at wk 0, 4, 16 | 5 | 308 | 45 | 63 | 11 | 0 | 0 | 0 | 0 | |||
| Healthy volunteer | Khalilieh et al (2018)40 | 26 | 38 | 28 | No | 0.1, 0.5, 3, 10 mg/kg IV (s) | 5 | 22 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Khalilieh et al (2018)40 | 27 | 62 | 20 | No | 50, 200 SC (s) | 5 | 28 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Briakinumab | IL-12 IL-23 |
Psoriasis | Gordon et al (2012)1 | 46 | 69 | 12 | No | 200 SC wk 0, 4; 100 SC wk 8 | 5 | 981 | 484 | 114 | 40 | 2 | 0 | 0 | 0 |
| Gottlieb et al (2011)41 | 43 | 67 | 12 | No | 200 SC wk 0, 4; 100 SC wk 8 | 5 | 138 | 68 | 19 | 8 | 0 | 0 | 0 | 0 | |||
| Strober et al (2011)42 | 45 | 64 | 12 | No | 200 SC wk 0, 4; 100 SC wk 8 | 5 | 139 | 72 | 20 | 6 | 0 | 0 | 0 | 0 | |||
| Kimball et al (2008)43 | 46 | 75 | 12 | No | 200 SC (s); 100 SC q2wks for 12 wk; 200 SC q1wk for 4 wk; 200 SC q2wks or q1wk for 12 wk | 5 | 150 | 30 | 38 | 3 | 0 | 0 | 0 | 0 | |||
| CD | Panaccione et al (2015)2 | 36 | 51 | 12 | Yes | 200, 400, 700 IV at wk 0, 4, 8 | 5 | 200 | 46 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Mannon et al (2004)44 | 43 | 25 | 28 | Yes | 1, 3 mg/kg SC at wk 0; q1wk from wk 4 to 10 | 5 | 32 | 8 | 2 | 0 | 0 | 0 | 0 | 0 | |||
| Mannon et al (2004)44 | 40 | 20 | 25 | Yes | 1, 3 mg/kg SC q1wk for 7 wk | 5 | 31 | 8 | 2 | 0 | 0 | 0 | 0 | 0 | |||
| MS | Vollmer et al (2011)45 | 47 | 25 | 24 | No | 200 SC q2wk; 200 SC q1wk | 5 | 146 | 69 | 51 | 29 | 0 | 0 | 0 | 0 | ||
| Ustekinumab | IL-12 IL-23 |
Psoriasis | Gordon et al (2018)7 | 48 | 71 | 16 | No | 45 SC (wt ≤ 100 kg); 90 SC (wt > 100 kg) at wk 0, 4 | 5 | 100 | 102 | 12 | 8 | 0 | 0 | 0 | 0 |
| Gordon et al (2018)7 | 47 | 68 | 16 | No | 45 SC (wt ≤ 100 kg), 90 SC (wt > 100 kg) at wk 0, 4 | 5 | 99 | 98 | 9 | 4 | 0 | 0 | 0 | 0 | |||
| Landells et al (2015)46 | 15 | 49 | 12 | No | 0.75 mg/kg SC (wt ≤ 60 kg); 45 SC (60 < wt ≤ 100 kg); 90 SC (wt > 100 kg); 0.375 mg/kg SC (wt ≤ 60 kg); 22.5 SC (60 < wt ≤ 100 kg); 45 SC (>100 kg) at wk 0, 4 | 5 | 73 | 37 | 16 | 13 | 0 | 0 | 0 | 0 | |||
| Lebwohl et al (2015)47 | 45 | 69 | 12 | No | 45 SC (wt ≤ 100 kg); 90 SC (wt > 100 kg) at wk 0, 4 | 5 | 300 | 309 | 38 | 37 | 0 | 0 | 0 | 0 | |||
| Lebwohl et al (2015)47 | 45 | 68 | 12 | No | 45 SC (wt ≤ 100 kg), 90 SC (wt > 100 kg) at wk 0, 4 | 5 | 313 | 313 | 32 | 39 | 0 | 0 | 0 | 0 | |||
| Zhu et al (2013)48 | 40 | 77 | 12 | No | 45 SC at wk 0, 4 | 5 | 160 | 161 | 28 | 21 | 0 | 0 | 0 | 0 | |||
| Igarashi et al (2012)61 | 46 | 80 | 12 | No | 45, 90 SC at wk 0, 4 | 5 | 126 | 32 | 21 | 4 | 0 | 0 | 1 | 0 | |||
| Tsai et al (2011)49 | 40 | 85 | 12 | No | 45 SC at wk 0, 4 | 5 | 61 | 60 | 12 | 10 | 0 | 0 | 0 | 0 | |||
| Papp et al (2008)3 | 45 | 69 | 12 | No | 45, 90 SC at wk 0, 4 | 5 | 820 | 410 | 119 | 59 | 1 | 1 | 0 | 0 | |||
| Leonardi et al (2008)50 | 45 | 69 | 12 | No | 45, 90 SC at wk 0, 4 | 5 | 510 | 255 | 109 | 52 | 0 | 1 | 0 | 0 | |||
| Krueger et al (2007)51 | 46 | 59 | 20 | No | 45, 90 SC at wk 0, q4wk | 5 | 252 | 67 | 95 | 24 | 1 | 0 | 0 | 0 | |||
| PsA | Ritchlin et al (2014)62 | 49 | 47 | 16 | Yes | 45, 90 SC at wk 0, 4, 16 | 5 | 207 | 104 | 31 | 15 | 0 | 0 | 0 | 1 | ||
| Mclnnes et al (2013)52 | 48 | 52 | 16 | Yes | 45, 90 SC at wk 0, 4, 16 | 5 | 409 | 205 | 33 | 18 | 0 | 0 | 0 | 0 | |||
| Gottlieb et al (2009)53 | 50 | 59 | 12 | Yes | 90 SC q1wk for 4 wk | 5 | 76 | 70 | 20 | 12 | 0 | 0 | 0 | 0 | |||
| CD | Feagan et al (2016)54 | 37 | 40 | 8 | Yes | 130 mg, 6 mg/kg IV (s) | 5 | 495 | 245 | 23 | 13 | 0 | 0 | 0 | 0 | ||
| Feagan et al (2016)54 | 39 | 50 | 8 | Yes | 130 mg, 6 mg/kg IV (s) | 5 | 419 | 208 | 24 | 10 | 0 | 0 | 0 | 0 | |||
| Sandborn et al (2012)4 | 39 | 41 | 8 | Yes | 1, 3, 6 mg/kg IV (s) | 5 | 394 | 132 | 41 | 8 | 0 | 0 | 0 | 0 | |||
| Sandborn et al (2008)55 | 40 | 55 | 8 | Yes | 90 SC at wk 0, 1, 2, 3; 4.5 mg/kg IV at wk 0 | 5 | 52 | 52 | 6 | 3 | 0 | 0 | 0 | 0 | |||
| Atopic dermatitis | Khattri et al (2017)56 | 37 | 63 | 16 | No | 45 SC (wt ≤ 100 kg), 90 SC (wt > 100 kg) at wk 0, 4, 16 | 5 | 16 | 16 | 2 | 1 | 0 | 0 | 0 | 0 | ||
| Saeki et al (2017)51 | 39 | 71 | 24 | No | 45, 90 SC at wk 0, 4 | 5 | 52 | 27 | 15 | 7 | 0 | 0 | 0 | 0 | |||
| GVHD | †NCT0171340021 | 53 | 63 | 52 | Yes | 45 SC (wt ≤ 100 kg), 90 SC (wt > 100 kg) at day -1 and day 20 after transplantation | 5 | 15 | 15 | 1 | 3 | 0 | 0 | 0 | 0 | ||
| SLE | van Vollenhoven et al (2018)58 | 40 | 3 | 24 | Yes | 260 (wt 35-55 kg), 390 (55 < wt ≤ 85), 520 (wt > 85 kg) IV wk 0; 90 SC q8wk | 5 | 60 | 42 | 21 | 12 | 1 | 0 | 0 | 0 | ||
| Sarcoidosis | Judson et al (2014)63 | 50 | 51 | 44 | Yes | 180 SC wk 0; 90 SC wk 8, 16, 24 | 5 | 60 | 58 | 40 | 35 | 3 | 0 | 1 | 0 | ||
| MS | Segal et al (2008)59 | 37 | 36 | 37 | No | 27, 90, 180 SC wk 0, 1, 2, 3, 7, 11, 15, 1990 SC q8wks | 5 | 200 | 49 | 73 | 17 | 0 | 0 | 0 | 0 | ||
| PPPP | Bissonnette et al (2014)60 | 55 | 10 | 16 | No | 45 SC (wt < 100 kg), 90 SC (wt ≥ 100 kg) at wk 0, 4, 16 | 5 | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| PPP | Bissonnette et al (2014)60 | 50 | 0 | 16 | No | 45 SC (wt < 100 kg); 90 SC (wt ≥ 100 kg) at wk 0, 4, 16 | 5 | 5 | 8 | 0 | 1 | 0 | 1 | 0 | 0 | ||
CD, Crohn's disease; GVHD, graft-versus-host disease; IL, interleukin; ILD, interstitial lung disease; IV, intravenous; MS, multiple sclerosis; NA, not available; PPP, palmoplantar pustulosis; PPPP, palmoplantar pustular psoriasis; PsA, psoriatic arthritis; q, every; RTI, respiratory tract infection; (s), single dose; SC, subcutaneous; SLE, systemic lupus erythematosus; wt, weight.
Regarding age and sex, data from overall patients in each trials or patients treated IL-12/23 or IL-23 antagonists were used.
This study was registered in ClinicalTrails.gov but later became available as an article.
RTIs
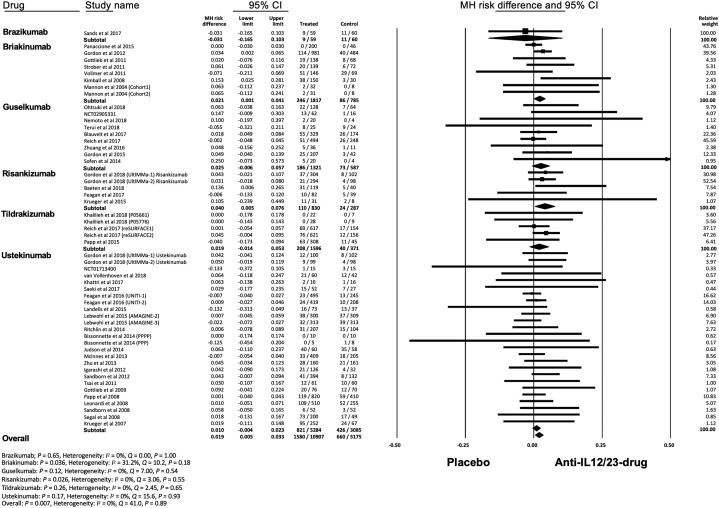
Meta-analysis with a random effects model showed that the overall risk of RTIs with anti–IL-12/IL-23 or anti–IL-23 agents was significantly higher than that of placebo (MH RD, 0.019; 95% confidence interval [CI], 0.005-0.033; P = .007) (Fig 2 ). The number needed to harm of RTIs was 58.8. Subgroup analysis showed a significantly increased risk of RTIs with briakinumab (MH RD, 0.021; 95% CI, 0.001-0.041; P = .036) and risankizumab (MH RD, 0.040; 95% CI, 0.005-0.076; P = .026). Heterogeneity was absent (I 2 = 0%) in overall and subgroup analyses except for briakinumab (I 2 = 31%). Funnel plot showed no asymmetry, therefore suggesting there were no small-study effects or publication biases, which was supported by Begg and Egger tests (Supplemental Fig 1; available via Mendeley at https://doi.org/10.17632/j7dfkr2s8v.1). We also assessed the differential risk of RTIs by underlying disease and showed a significantly increased risk of RTIs in psoriasis (MH RD, 0.023; 95% CI, 0.010-0.036; P < .001) and ankylosing spondylitis (MH RD, 0.136; 95% CI, 0.006-0.265; P = .040) (Supplemental Fig 2; available via Mendeley at https://doi.org/10.17632/j7dfkr2s8v.1).
Fig 2.
Meta-analysis of the Mantel-Haenszel (MH) risk difference of respiratory tract infections with IL-12/23 and IL-23 antagonists. CI, Confidence interval; IL, interleukin.
We divided RTIs into URTIs, viral URTIs, and LRTIs and investigated each risk with IL-12/23 or IL-23 inhibitors. The overall risk of URTIs was significantly higher in the treatment group compared to placebo (MH RD, 0.017; 95% CI, 0.005-0.029; P = .006) (Supplemental Fig 3, A; available via Mendeley at https://doi.org/10.17632/j7dfkr2s8v.1). Subgroup analysis showed an elevated risk of URTIs with risankizumab (MH RD, 0.028; 95% CI, 0.004-0.053; P = .024). No publication bias was detected by Begg and Egger tests (Supplemental Fig 3, B).
Anti–IL-12/IL-23 or anti–IL-23 agents did not increase the overall risks of viral URTIs (MH RD, 0.001; 95% CI, -0.002 to 0.003; P = .60) and LRTIs (MH RD, 0; 95% CI, -0.002 to 0.002; P = .71) (Supplemental Figs 4, A and 5, A; available via Mendeley at https://doi.org/10.17632/j7dfkr2s8v.1). Heterogeneity was absent (I 2 = 0%) in these analyses. Publication bias was indicated in the analysis of viral URTIs (Begg: P < .001; Egger: P = .019) (Supplemental Fig 4, B) and LRTIs (Begg: P < .001; Egger: P = .55) (Supplemental Fig 5, B), but the funnel plots did not appear asymmetric on visual inspection.
Infectious pneumonia and noninfectious ILD
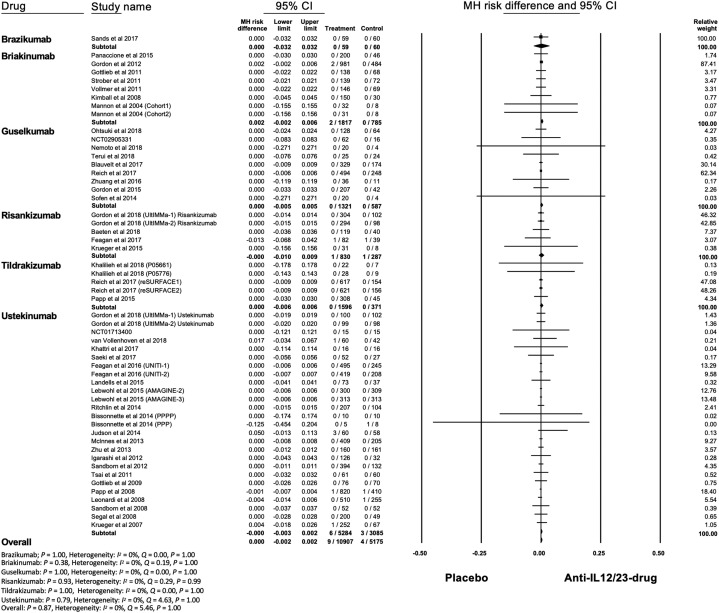
The total numbers of infectious pneumonia were 9 and 4 cases in the treatment group and placebo, respectively. Mycobacterium tuberculosis and viral pneumonia were not reported. The overall risk of infectious pneumonia was not significantly increased in the treatment group compared to placebo (MH RD, 0; 95% CI, -0.002 to 0.002; P = .87) (Fig 3 ). Heterogeneity was absent (I 2 = 0%). The funnel plot was not asymmetric, indicating no publication bias, which was supported by Egger test (P = .93) but not Begg test (P < .001) (Supplemental Fig 6; available via Mendeley at https://doi.org/10.17632/j7dfkr2s8v.1).
Fig 3.
Meta-analysis of Mantel-Haenszel (MH) risk difference of infectious pneumonia with IL-12/23 and IL-23 antagonists. CI, Confidence interval; IL, interleukin.
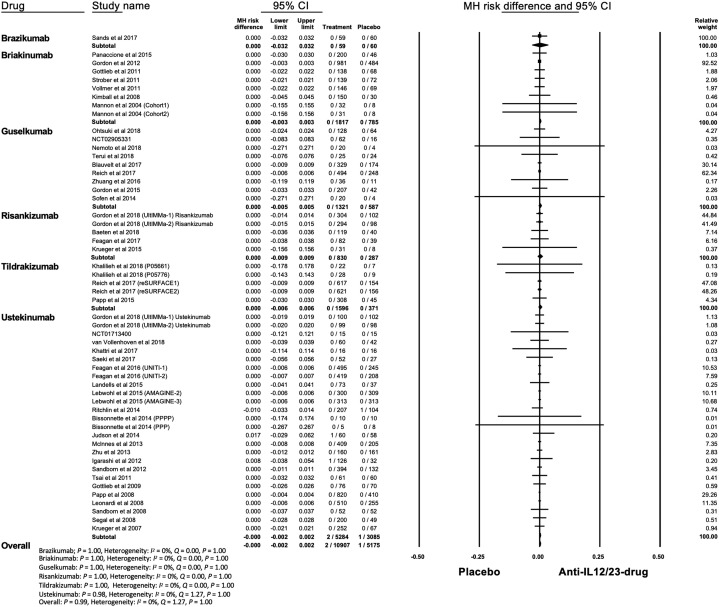
In terms of noninfectious ILD, 2 and 1 cases were identified in the treatment and placebo groups, respectively. All 3 cases were reported in trials of ustekinumab and occurred within 16 weeks after initiation of the trial.61, 62, 63 The overall risk of ILD was not significantly increased in the treatment group (MH RD, 0; 95% CI, -0.002 to 0.002; P = .99) (Fig 4 ). Heterogeneity was absent (I 2 = 0%). Begg (P < .001) test was suggestive of publication bias, however the funnel plot was not asymmetric (Supplemental Fig 7; available via Mendeley at https://doi.org/10.17632/j7dfkr2s8v.1).
Fig 4.
Meta-analysis of the Mantel-Haenszel (MH) risk difference of noninfectious interstitial lung disease with IL-12/23 and IL-23 antagonists. CI, Confidence interval; IL, interleukin.
The sensitivity analysis showed consistent results (Supplemental Tables 1-6; available via Mendeley at https://doi.org/10.17632/rdxgpw9yxk.2), except the analysis with 0.5 constant correction of zero-event studies showed a lower risk of infectious pneumonia (Supplemental Table 7; available via Mendeley at https://doi.org/10.17632/rdxgpw9yxk.2) and ILD in the treatment group (Supplemental Table 8; available via Mendeley at https://doi.org/10.17632/rdxgpw9yxk.2).
Grading the quality of evidence
Based on the GRADE criteria, the overall quality of evidence for this analysis was moderate because infectious pneumonia and ILD were rare events (Supplemental Tables 9 and 10; available via Mendeley at https://doi.org/10.17632/rdxgpw9yxk.2).
Discussion
Our meta-analysis showed that IL-12/23 or IL-23 inhibitors increased the risk of RTIs, especially URTIs, but not viral URTIs and LRTIs, and noninfectious ILD in autoimmune diseases.
We found that risankizumab and briakinumab particularly enhanced the risk of RTIs and hypothesized that concomitant therapies during the trials might differentiate the risk of RTIs. In terms of anti–IL-23 agents, risankizumab showed a higher rate of RCTs that permitted concomitant therapies (40.0%) compared with guselkumab (0%) and tildrakizumab (0%). Among RCTs of risankizumab, the only study reporting an increased risk of RTIs was performed in patients with ankylosing spondylitis, who were permitted to use conventional disease-modifying antirheumatic drugs or low-dose systemic steroids.64 This suggests that combination therapy of anti–IL-23 agents with immunosuppressants might work synergistically to surface the risk of RTIs. As for anti–IL-12/IL-23 agents, each of briakinumab and ustekinumab has a similar percentage of RCTs that permitted concomitant drugs (37.5% for briakinumab and 38.5% for ustekinumab). Other potential risk factors such as age and sex were not different among the drugs. Given that briakinumab has been withdrawn from the application with the FDA because of severe adverse events,1 the difference in risk of RTIs among the 2 drugs would be explained by different properties of these drugs.
Our study might support that anti–IL-12/23 and anti–IL-23 therapies can be safely used for autoimmune diseases even during the current COVID-19 pandemic. However, given that influenza vaccination is generally recommended for patients with autoimmune diseases receiving immunosuppressive therapies,65 viral URTIs caused by influenza virus could be prevented in both the treatment and placebo groups, and our finding regarding the risk of viral URTIs might be affected. In addition, studies included in this analysis were conducted before the pandemic and the risk of severe acute respiratory syndrome coronavirus 2 in patients with anti–IL-12/23 or anti–IL-23 therapies could not be assessed. Further studies, particularly during the current pandemic, are necessary to provide enough evidence to ensure the safety of these drugs. Additional investigations are also needed to understand the differential risk of RTIs in psoriasis and ankylosing spondylitis and the mechanism of ILD by IL-12/23 inhibition because patients who are indicated for these drugs, namely psoriasis, rheumatoid arthritis, and inflammatory bowel disease, all carry an increased risk of lung disease.66, 67, 68
IL-12 and IL-23 have fundamental functions in host defense. IL-12 promotes the differentiation of naive T cells into interferon gamma–producing T helper type (Th) 1 cells, which contribute to viral clearance69 and prevent infections of Mycobacterium and Salmonella species.70 Meanwhile, IL-23 maintains IL-17–producing Th17 cells, and the deficiency of IL-17 immunity results in infections of Candida species.71 , 72 Our results showed that IL-12/23 or IL-23 antagonists did not increase the risk of LRTIs and infectious pneumonia, including Mycobacteria tuberculosis or any virus. As for viral RTIs, previous studies showed that IL-17–knockout mice had lower levels of lung inflammation by influenza virus compared with the wild type.73 A study of the Middle East respiratory syndrome coronavirus showed that a patient with a poor outcome had an increased level of IL-17 expression in the lung.69 These data suggest that IL-12/23 or IL-23 inhibitors might theoretically be preventive for severe acute respiratory syndrome coronavirus 2–induced pneumonia rather than detrimental in autoimmune diseases during the COVID-19 pandemic.
Limitations
First, this study did not assess the long-term effect of IL-12/23 or IL-23 antagonists on RTIs and ILD. However, 92.6% (50/54) of the included studies reported RTIs during placebo-controlled phases. The FDA reported whether the onset of ILD was acute or subacute,14 so our data would most likely include the incidence of these events. Second, regarding infectious pneumonia and ILD, many studies had zero events in both arms (87% [47/54] and 94% [51/54], respectively). Thus, we undertook comprehensive analyses that either included or excluded double-zero-event studies. The analysis with 0.5 constant correction showed a lower risk of these events in the treatment group. We also used treatment arm correction because this method performed better than 0.5 constant correction to examine rare events.74 Third, our study may not reflect the risk in patients at high risk for RTIs because of the possible exclusion of patients with recent RTIs or chronic lung disease in clinical trials. Fourth, we categorized RTIs into URTIs, viral URTIs, and LRTIs based on MedDRA, which is widely used in clinical trials, but not so much in clinical research. Furthermore, the included studies were conducted before the pandemic. Hence, it does not provide evidence of whether there is an increase in RTIs or ILD during the pandemic in patients receiving IL-12/23 or IL-23 antagonists, nor whether these agents can be continued after a diagnosis of COVID-19. A meta-analysis of real-world studies of COVID-19 in patients with autoimmune diseases is needed.
Conclusion
This meta-analysis showed that IL-12/23 or IL-23 antagonists had an increased risk of URTIs, but not viral URTIs, LRTIs including infectious pneumonia, and noninfectious ILD.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: Not applicable.
Reprints not available from the authors.
References
- 1.Gordon K.B., Langley R.G., Gottlieb A.B., et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:304–314. doi: 10.1038/jid.2011.304. [DOI] [PubMed] [Google Scholar]
- 2.Panaccione R., Sandborn W.J., Gordon G.L., et al. Briakinumab for treatment of Crohn's disease: results of a randomized trial. Inflamm Bowel Dis. 2015;21:1329–1340. doi: 10.1097/MIB.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papp K.A., Langley R.G., Lebwohl M., et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 4.Sandborn W.J., Gasink C., Gao L.L., et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519–1528. doi: 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- 5.Reich K., Papp K.A., Blauvelt A., et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276–288. doi: 10.1016/S0140-6736(17)31279-5. [DOI] [PubMed] [Google Scholar]
- 6.Papp K., Thaci D., Reich K., et al. Tildrakizumab (MK-3222), an anti–interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930–939. doi: 10.1111/bjd.13932. [DOI] [PubMed] [Google Scholar]
- 7.Gordon K.B., Strober B., Lebwohl M., et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650–661. doi: 10.1016/S0140-6736(18)31713-6. [DOI] [PubMed] [Google Scholar]
- 8.Feagan B.G., Sandborn W.J., D'Haens G., et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn's disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699–1709. doi: 10.1016/S0140-6736(17)30570-6. [DOI] [PubMed] [Google Scholar]
- 9.Gordon K.B., Duffin K.C., Bissonnette R., et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136–144. doi: 10.1056/NEJMoa1501646. [DOI] [PubMed] [Google Scholar]
- 10.Reich K., Armstrong A.W., Foley P., et al. Efficacy and safety of guselkumab, an anti–interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Sands B.E., Chen J., Feagan B.G., et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn's disease: a phase 2a study. Gastroenterology. 2017;153:77–86. doi: 10.1053/j.gastro.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 12.Frontanilla D., Vasiliou C., Sawalhi-Leckenby N., Kurniawan A. Crohn's disease: disease coverage. Datamonitor Healthcare. 2018. https://pharmastore.informa.com/wp-content/uploads/2018/08/crohnsdisease_5415.pdf Available at: Accessed April 2020.
- 13.Lopez-Ferrer A., Laiz A., Puig L. The safety of ustekinumab for the treatment of psoriatic arthritis. Expert Opin Drug Saf. 2017;16:733–742. doi: 10.1080/14740338.2017.1323864. [DOI] [PubMed] [Google Scholar]
- 14.Brinker A., Cheng C., Chan V. Association of noninfectious pneumonia with ustekinumab use. JAMA Dermatol. 2019;155:221–224. doi: 10.1001/jamadermatol.2018.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zumla A., Niederman M.S. Editorial: the explosive epidemic outbreak of novel coronavirus disease 2019 (COVID-19) and the persistent threat of respiratory tract infectious diseases to global health security. Curr Opin Pulm Med. 2020;26:193–196. doi: 10.1097/MCP.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebwohl M., Rivera-Oyola R., Murrell D.F. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217–1218. doi: 10.1016/j.jaad.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monteleone G., Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J Crohns Colitis. 2020;14:1334–1336. doi: 10.1093/ecco-jcc/jjaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Booth A. PROSPERO's progress and activities 2012/13. Syst Rev. 2013;2:111. doi: 10.1186/2046-4053-2-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris L.K., Ott E., Jiang J., et al. Efficacy and safety of guselkumab, administered with a novel patient-controlled injector (One-Press), for moderate-to-severe psoriasis: results from the phase 3 ORION study. J Dermatolog Treat. 2020;31:152–159. doi: 10.1080/09546634.2019.1587145. [DOI] [PubMed] [Google Scholar]
- 21.Pidala J., Beato F., Kim J., et al. In vivo IL-12/IL-23p40 neutralization blocks Th1/Th17 response after allogeneic hematopoietic cell transplantation. Haematologica. 2018;103:531–539. doi: 10.3324/haematol.2017.171199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadad A.R., Moore R.A., Carroll D., et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Altman D.G., Gotzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt G.H., Oxman A.D., Vist G., et al. GRADE guidelines: 4. rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich J.O., Adhikari N.K., Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5. doi: 10.1186/1471-2288-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brockhaus A.C., Bender R., Skipka G. The Peto odds ratio viewed as a new effect measure. Stat Med. 2014;33:4861–4874. doi: 10.1002/sim.6301. [DOI] [PubMed] [Google Scholar]
- 27.Friedenreich C.M. Methods for pooled analyses of epidemiologic studies. Epidemiology. 1993;4:295–302. doi: 10.1097/00001648-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Bongartz T., Sutton A.J., Sweeting M.J., et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J., Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. [Google Scholar]
- 31.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 32.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohtsuki M., Kubo H., Morishima H., et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053–1062. doi: 10.1111/1346-8138.14504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemoto O., Hirose K., Shibata S., Li K., Kubo H. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689–696. doi: 10.1111/bjd.16236. [DOI] [PubMed] [Google Scholar]
- 35.Blauvelt A., Papp K.A., Griffiths C.E., et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 36.Sofen H., Smith S., Matheson R.T., et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032–1040. doi: 10.1016/j.jaci.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Terui T., Kobayashi S., Okubo Y., et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309–316. doi: 10.1001/jamadermatol.2017.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhuang Y., Calderon C., Marciniak S.J., Jr., et al. First-in-human study to assess guselkumab (anti-IL-23 mAb) pharmacokinetics/safety in healthy subjects and patients with moderate-to-severe psoriasis. Eur J Clin Pharmacol. 2016;72:1303–1310. doi: 10.1007/s00228-016-2110-5. [DOI] [PubMed] [Google Scholar]
- 39.Krueger J.G., Ferris L.K., Menter A., et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116–124. doi: 10.1016/j.jaci.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Khalilieh S., Hodsman P., Xu C., et al. Pharmacokinetics of tildrakizumab (MK-3222), an anti-IL-23 monoclonal antibody, after intravenous or subcutaneous administration in healthy subjects. Basic Clin Pharmacol Toxicol. 2018;123:294–300. doi: 10.1111/bcpt.13001. [DOI] [PubMed] [Google Scholar]
- 41.Gottlieb A.B., Leonardi C., Kerdel F., et al. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:652–660. doi: 10.1111/j.1365-2133.2011.10418.x. [DOI] [PubMed] [Google Scholar]
- 42.Strober B.E., Crowley J.J., Yamauchi P.S., Olds M., Williams D.A. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661–668. doi: 10.1111/j.1365-2133.2011.10419.x. [DOI] [PubMed] [Google Scholar]
- 43.Kimball A.B., Gordon K.B., Langley R.G., et al. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Arch Dermatol. 2008;144:200–207. doi: 10.1001/archdermatol.2007.63. [DOI] [PubMed] [Google Scholar]
- 44.Mannon P.J., Fuss I.J., Mayer L., et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 45.Vollmer T.L., Wynn D.R., Alam M.S., Valdes J. A phase 2, 24-week, randomized, placebo-controlled, double-blind study examining the efficacy and safety of an anti-interleukin-12 and -23 monoclonal antibody in patients with relapsing-remitting or secondary progressive multiple sclerosis. Mult Scler. 2011;17:181–191. doi: 10.1177/1352458510384496. [DOI] [PubMed] [Google Scholar]
- 46.Landells I., Marano C., Hsu M.C., et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594–603. doi: 10.1016/j.jaad.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Lebwohl M., Strober B., Menter A., et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X., Zheng M., Song M., et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS) J Drugs Dermatol. 2013;12:166–174. [PubMed] [Google Scholar]
- 49.Tsai T.F., Ho J.C., Song M., et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL) J Dermatol Sci. 2011;63:154–163. doi: 10.1016/j.jdermsci.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Leonardi C.L., Kimball A.B., Papp K.A., et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 51.Krueger G.G., Langley R.G., Leonardi C., et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 52.McInnes I.B., Kavanaugh A., Gottlieb A.B., et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 53.Gottlieb A., Menter A., Mendelsohn A., et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633–640. doi: 10.1016/S0140-6736(09)60140-9. [DOI] [PubMed] [Google Scholar]
- 54.Feagan B.G., Sandborn W.J., Gasink C., et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 55.Sandborn W.J., Feagan B.G., Fedorak R.N., et al. A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 56.Khattri S., Brunner P.M., Garcet S., et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol. 2017;26:28–35. doi: 10.1111/exd.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saeki H., Kabashima K., Tokura Y., et al. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomized, double-blind, placebo-controlled, phase II study. Br J Dermatol. 2017;177:419–427. doi: 10.1111/bjd.15493. [DOI] [PubMed] [Google Scholar]
- 58.van Vollenhoven R.F., Hahn B.H., Tsokos G.C., et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. 2018;392:1330–1339. doi: 10.1016/S0140-6736(18)32167-6. [DOI] [PubMed] [Google Scholar]
- 59.Segal B.M., Constantinescu C.S., Raychaudhuri A., et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 60.Bissonnette R., Nigen S., Langley R.G., et al. Increased expression of IL-17A and limited involvement of IL-23 in patients with palmo-plantar (PP) pustular psoriasis or PP pustulosis; results from a randomised controlled trial. J Eur Acad Dermatol Venereol. 2014;28:1298–1305. doi: 10.1111/jdv.12272. [DOI] [PubMed] [Google Scholar]
- 61.Igarashi A., Kato T., Kato M., et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242–252. doi: 10.1111/j.1346-8138.2011.01347.x. [DOI] [PubMed] [Google Scholar]
- 62.Ritchlin C., Rahman P., Kavanaugh A., et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73:990–999. doi: 10.1136/annrheumdis-2013-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Judson M.A., Baughman R.P., Costabel U., et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014;44:1296–1307. doi: 10.1183/09031936.00000914. [DOI] [PubMed] [Google Scholar]
- 64.Baeten D., Ostergaard M., Wei J.C., et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018;77:1295–1302. doi: 10.1136/annrheumdis-2018-213328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopez A., Mariette X., Bachelez H., et al. Vaccination recommendations for the adult immunosuppressed patient: a systematic review and comprehensive field synopsis. J Autoimmun. 2017;80:10–27. doi: 10.1016/j.jaut.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Kawamoto H., Hara H., Minagawa S., et al. Interstitial pneumonia in psoriasis. Mayo Clin Proc Innov Qual Outcomes. 2018;2:370–377. doi: 10.1016/j.mayocpiqo.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathai S.C., Danoff S.K. Management of interstitial lung disease associated with connective tissue disease. BMJ. 2016;352:h6819. doi: 10.1136/bmj.h6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Black H., Mendoza M., Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131:524–532. doi: 10.1378/chest.06-1074. [DOI] [PubMed] [Google Scholar]
- 69.Faure E., Poissy J., Goffard A., et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9:e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casanova J.L., Abel L., Quintana-Murci L. Immunology taught by human genetics. Cold Spring Harb Symp Quant Biol. 2013;78:157–172. doi: 10.1101/sqb.2013.78.019968. [DOI] [PubMed] [Google Scholar]
- 71.Yen D., Cheung J., Scheerens H., et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teng M.W., Bowman E.P., McElwee J.J., et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 73.Crowe C.R., Chen K., Pociask D.A., et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sweeting M.J., Sutton A.J., Lambert P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]