1. Introduction
Death from the novel coronavirus disease (COVID-19) predominantly occurs by refractory hypoxemia [1]. Some Authors have suggested that this type of respiratory failure differs from typical Acute Respiratory Distress Syndrome (ARDS) [2]. Patients with COVID-19 can present with (i) higher respiratory system compliance, and (ii) lower amount of non-ventilated lung tissue than expected given the severity of hypoxemia. These two elements may suggest a different primary cause of gas exchange impairment in COVID-19 compared to ARDS of another origin: excessive perfusion of small non-normally ventilated lung regions rather than a large anatomical shunt [3]. In line with this model, a recent series has revealed the presence of diffuse pulmonary endothelial injury and microthrombosis in COVID-19 lung pathology [4]. Given the novelty of COVID-19, its treatment is currently grounded on our knowledge of ARDS. Modern supportive therapy of ARDS generally consists of endotracheal intubation and mechanical ventilation with supplemental oxygen and Positive End Expiratory Pressure (PEEP), along with muscle paralysis, prone positioning, and ExtraCorporeal Membrane Oxygenation (ECMO) for the most severe cases [5,6]. Inhaled nitric oxide (iNO) can be considered as rescue therapy for hypoxemia due to its potent vasodilator effect on the pulmonary circulation [7]. International guidelines [5], and experts in the field [6,[8], [9], [10]], all suggest considering iNO even for refractory hypoxemia due to COVID-19. However, there are no strong clinical data to support this indication.
Herein we describe the response to iNO in a small group of mechanically ventilated patients with severe COVID admitted to our Intensive Care Unit.
2. Methods
As part of our routine clinical practice for ARDS, we administered a 30-min test dose of 20 ppm to ten adults with COVID-19 treated with invasive mechanical ventilation and with a partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen ratio (FiO2) around or below 100 mmHg. All patients had been previously ventilated in prone position for 12 to 16 h (one session) with only a minor, if any, improvement in arterial oxygenation. By the time iNO was tested, they were already sedated and ventilated in a controlled mode in the supine position. A tank with 450 ppm NO in nitrogen was connected to the inspiratory limb of the ventilatory circuit; NO was delivered during the inspiratory phase using a digital system (Optikonox, Air Liquide, Milan, Italy). Blood gases were recorded immediately before and at 30 min of NO breathing with the same ventilatory settings. Based on the individual response to the test, we then considered to prolong or discontinue the use of iNO. Data collection and presentation were approved by our IRB (protocol number 233/20); informed consent was obtained according to local policy for critically ill patients.
3. Results
Ten patients with a mean age of 55 ± 9 years received iNO after 3.6 ± 2.1 days of mechanical ventilation (range 1–7). By that time, the median FiO2 was 80% (70–90 IQR); mean (±SD) PEEP was 15 ± 3 cmH2O. Mean static respiratory system compliance was 44 ± 12 ml/cmH2O, and >50 ml/cmH2O in four patients. With iNO, mean PaO2 went from 62 ± 9 to 64 ± 14 mmHg (p = 0.427, paired t-test); mean PaO2/FiO2 from 81 ± 19 to 84 ± 22 mmHg (p = 0.325) (Fig. 1 ). Arterial pH, partial pressure of arterial carbon dioxide, heart rate, and mean arterial pressure did not significantly change throughout the test.
Fig. 1.
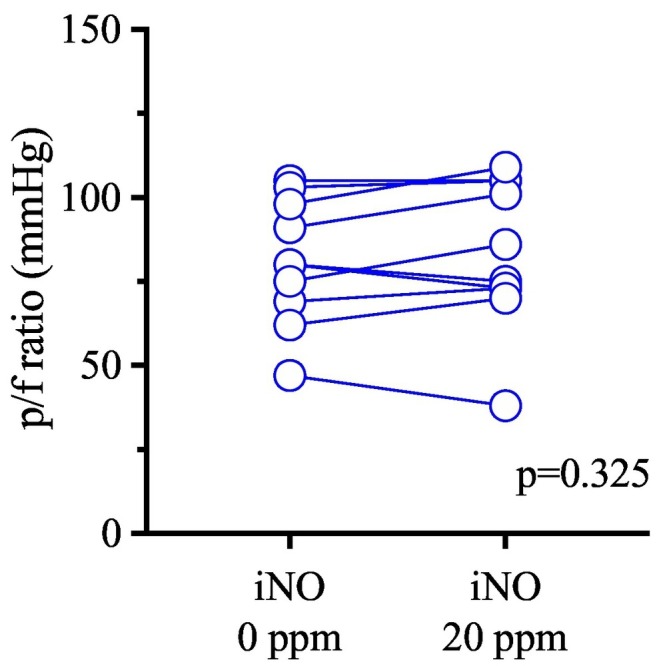
Effect of inhaled nitric oxide on arterial oxygenation in mechanically ventilated patients with COVID-19.
Partial pressure of oxygen to fraction of inspired oxygen ratio (p/f ratio) of patients before and at 30 min of 20 ppm inhaled nitric oxide (iNO) treatment. P-value refers to the paired t-test.
Considering the negligible effect on oxygenation and the potential side effects of prolonged iNO [7], we discontinued its use in all cases. All 10 patients underwent other sessions of ventilation in prone position, and none received ECMO (at the discretion of the attending physicians). Mean duration of mechanical ventilation was 34 ± 21 days (range 9–62). Eight out of ten patients (80%) were discharged alive from the hospital.
4. Discussion
iNO usually relieves hypoxemia [7] by dilating the blood vessels of lung regions open to ventilation (and iNO), thus diverting blood flow away from areas of alveolar consolidation and reducing the functional shunt. However, in this small series of patients with severe hypoxemia due to COVID-19, it did not significantly improve arterial oxygenation.
We do not have a precise explanation for our findings. We suspect that diffuse loss of pulmonary vascular tone, with increased perfusion around alveolar consolidations [3], may have blunted the vasodilatory and “blood stealing” response to iNO in other ventilated lung regions. Further investigation is required to identify those patients who may benefit the most from iNO and the most appropriate dose to be used.
Financial disclosures
None.
Declaration of Competing Interest
Authors declare no conflict of interest for this manuscript.
References
- 1.Phua J., Weng L., Ling L., Egi M., Lim C.-M., Divatia J.V. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir. Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang M., Som A., Mendoza D.P., Flores E.J., Reid N., Carey D. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthay M.A., Aldrich J.M., Gotts J.E. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir. Med. 2020;8:433–434. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karam O., Gebistorf F., Wetterslev J., Afshari A. The effect of inhaled nitric oxide in acute respiratory distress syndrome in children and adults: a Cochrane Systematic Review with trial sequential analysis. Anaesthesia. 2017;72:106–117. doi: 10.1111/anae.13628. [DOI] [PubMed] [Google Scholar]
- 8.Lei C., Su B., Dong H., Bellavia A., Di Fenza R., Safaee Fakhr B. Protocol of a randomized controlled trial testing inhaled Nitric Oxide in mechanically ventilated patients with severe acute respiratory syndrome in COVID-19 (SARS-CoV-2) medRxiv. 2020:1–14. doi: 10.1101/2020.03.09.20033530. [DOI] [Google Scholar]
- 9.Kobayashi J., Murata I. Nitric oxide inhalation as an interventional rescue therapy for COVID-19-induced acute respiratory distress syndrome. Ann. Intensive Care. 2020;10:61. doi: 10.1186/s13613-020-00681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rello J., Storti E., Belliato M., Serrano R. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.01028-2020. (Published 2020 May 21) [DOI] [PMC free article] [PubMed] [Google Scholar]


