To the Editor:
Right ventricular (RV) adaptation in pulmonary arterial hypertension (PAH) is poorly understood. Sudden cardiac death (SCD) has been described in PAH,1 but there are no known predictors. Alternative splicing of sodium voltage-gated channel α subunit 5 (SCN5a), a sodium channel integral to myocardial conduction, results in nonfunctional splice variants (SVs) that induce endoplasmic reticulum and mitochondrial stress via the unfolded protein response in left ventricular (LV) failure.2, 3, 4 Expression levels of SVs measured from circulating leukocytes track with fold changes and protein expression in ventricular tissue (including the RV) and arrhythmias in LV failure.2,5 Hypoxia-inducible factor 1α (HIF-1α) induces alternative splicing of SCN5a and is a key regulator of the glycolytic shift in the pulmonary vasculature and RV in PAH.2,6,7 To test the hypothesis that alternative splicing of SCN5a contributes to metabolic reprogramming in PAH, we performed a pilot study to determine whether patients with PAH had elevated levels of circulating SCN5a SVs and whether these levels correlated with markers of RV function and mitochondrial respiration measured from peripheral blood mononuclear cells (PBMCs) (IRB No. 780729-15).
Methods
Patients with Group 1 PAH meeting traditional hemodynamic criteria were eligible.8 Control subjects were recruited from caregivers of patients and from hospital employees. Control subjects were screened by questionnaire to confirm the absence of cardiopulmonary symptoms and cardiac or pulmonary disease, and detailed medical histories were obtained. Expression levels of SVs (E28D), SCN5a, and HIF-1α mRNA were measured.4 SV levels were normalized to absolute values of β-actin and represented as fold changes of E28D/total SCN5a transcripts. PBMCs were concurrently isolated4 and stored immediately in liquid nitrogen. Seahorse XF (Agilent) analysis was performed after batch shipment in seven participants. The first five participants with PAH enrolled underwent same-day fasting 2-deoxy-2-[18F]fluoro-d-glucose (FDG)-PET to detect whether SV expression correlated with FDG uptake in the RV9 as an exploratory end point.
All analyses were conducted with SAS software 9.4 (SAS Institute Inc.). The associations between ln(E28D/total SCN5a) expression levels, mitochondrial respiration, and case/control subject status, respectively, were examined using generalized linear mixed models and assuming a log-normal distribution. Correlations were examined using Spearman rho (α = 0.05).
Results
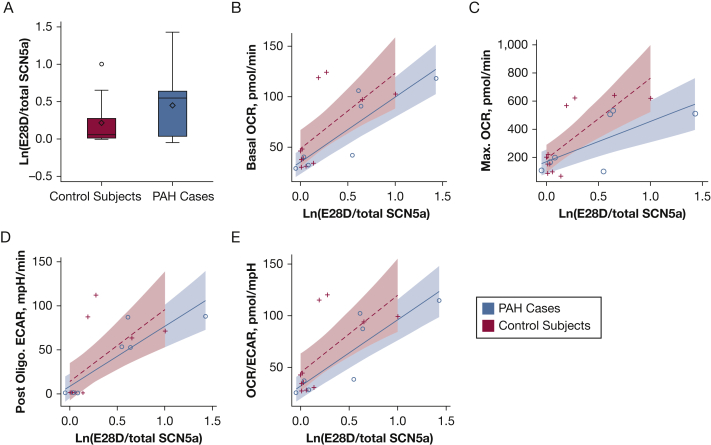
Eleven participants with PAH and 11 age-matched (within 7 years) and sex-matched control subjects were included (Table 1). Circulating SCN5a SV mRNA levels were 23% higher in PAH subjects as compared with matched control subjects despite lower absolute levels of HIF-1α expression (Table 1). In five subjects with PAH with concurrent PET scans, there was a strong correlation between SV expression levels and RV uptake normalized to uptake in the thoracic aorta blood pool (r = 0.89, P = .04) but no relationship to uptake in the pulmonary arteries or LV. There was no correlation between SV expression levels and RV function assessed by echocardiography and brain natriuretic peptide levels in the total sample (n = 11), although these tests were not performed concurrent with blood draw. There were significant inverse correlations between SV expression levels and baseline mean pulmonary artery pressure (r = –0.75, P = .01), and pulmonary vascular resistance (r = –0.70, P = .04), respectively. Subjects with PAH tended to have lower PBMC basal and maximal oxygen consumption rates (OCRs) as well as a lower postoligomycin extracellular acidification rate (ECAR) as compared with control subjects (Table 1). SV expression levels were significantly correlated with basal OCR in subjects with PAH (r = 0.87, P = .01) and control subjects (r = 0.64, P = .03) (Fig 1B). Among subjects with PAH, SV levels correlated with maximum OCR (r = 0.75, P = .05) and postoligomycin ECAR (r = 0.78, P = .04) (Figs 1C and 1D). Basal OCR/basal ECAR correlated with SV levels in PAH subjects (r = 0.87, P = .01) and control subjects (r = 0.64, P = .03) (Table 1, Fig 1E).
Table 1.
Participant Characteristics and Association of mRNA Expression Levels and Markers of Mitochondrial Respiration by PAH Status
| Variable | PAH | Control | P Value |
|---|---|---|---|
| No. | 11 | 11 | … |
| Age, y | 58 (52-65) | 60 (48-65) | … |
| Female, No. (%) | 9 (82) | 10 (91) | … |
| White, No. (%) | 9 (82) | 9 (82) | … |
| BMI, kg/m2 | 31 (26-38) | 29 (24-33) | … |
| WBC count, × 10–9/L | 6.0 (4.7-8.5) | 6.8 (6.1-7.7) | … |
| Diabetes mellitus, No. (%) | 0 (0) | 2 (18) | … |
| Arrhythmia, No. (%) | |||
| Atrial fibrillation | 3 (27) | 0 (0) | … |
| Premature ventricular contractions | 1 (9) | 0 (0) | … |
| Idiopathic PAH | 6 (54) | … | … |
| Markers of disease severitya | |||
| 6-min walk distance, m | 382 (201-472) | … | … |
| Functional class III/IV, No. (%) | 3 (27) | … | … |
| BNP, pg/mL | 76.8 (14.4-114.8) | … | … |
| Echocardiographic parameters | |||
| RVSP, mm Hg | 39 (33-66) | … | … |
| TAPSE, cm | 2.1 (1.8-2.3) | … | … |
| Hemodynamics | |||
| Right atrial pressure, mm Hg | 7 (5-13) | … | … |
| Mean PAP, mm Hg | 40 (32-49) | … | … |
| PCWP, mm Hg | 11 (10-15) | … | … |
| Cardiac output, L/min | 6.9 (6.5-7.5) | … | … |
| Cardiac index, L/min/m2 | 3.6 (3.3-3.9) | … | … |
| PVR, Wood units | 3.9 (3.0-6.4) | … | … |
| PAH therapies, No. (%) | |||
| Calcium channel blockers | 2 (18) | … | … |
| Phosphodiesterase-5 inhibitors | 8 (72) | … | … |
| Endothelin receptor antagonists | 7 (64) | … | … |
| Prostacyclin analogs | 6 (55) | … | … |
| Combination | 9 (82) | … | … |
| mRNA expression levels | |||
| ln(E28D/total SCN5a) | 1.6 (1.2-2.1) | 1.2 (1.0-1.5) | .047 |
| ln(HIF-1α/β-actin) | 0.6 (0.3-1.1) | 0.9 (0.8-1.1) | .10 |
| Mitochondrial respiration | |||
| Basal OCR, pmol/min | 48.7 (32.6-72.8) | 55.4 (37.2-82.5) | .28 |
| Maximal OCR, pmol/min | 214.1 (118.3-387.3) | 227.8 (124.5-416.8) | .82 |
| Basal ECAR, mpH/min | 30.9 (25.4-37.5) | 28.8 (23.8-34.9) | .53 |
| Postoligomycin ECAR, mpH/min | 5.0 (1.1-22.3) | 6.3 (1.5-26.4) | .06 |
| Basal OCR/basal ECAR, pmol/mpH | 1.6 (1.0-2.6) | 1.9 (1.4-2.6) | .39 |
BNP = brain natriuretic peptide; ECAR = extracellular acidification rate; HIF = hypoxia inducible factor; OCR = oxygen consumption rate; PAH = pulmonary arterial hypertension; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RVSP = right ventricular systolic pressure; TAPSE = tricuspid annular plane systolic excursion.
Clinical data collected at time point closest to blood draw.
Figure 1.
A, Expression levels of SCN5a splice variants [ln(E28D/total SCN5a)] in case subjects with PAH vs control subjects. Box is mean (diamond), median (center line) and interquartile range; whiskers are minimum and maximum observations. B-E, Correlations between basal (B) and maximum (C) OCR, postoligomycin ECAR (D), and the basal OCR/ECAR ratio (E) in subjects with PAH (blue) and control subjects (red). ECAR = extracellular acidification rate; OCR = oxygen consumption rate; PAH = pulmonary arterial hypertension.
Discussion
In this small study, we have shown that prevalent PAH patients have increased circulating SCN5a SV levels as compared with matched control subjects and that these levels track with increased oxygen consumption and markers of glycolysis in PBMCs, and in a small subset, RV FDG uptake. Sudden death and altered cellular energetics have been described in PAH,9,10 but posttranscriptional modification of SCN5a—a possible link between these phenomena—has not been reported.
We were likely underpowered to detect differences in HIF-1α expression and mitochondrial respiration between subjects with PAH and control subjects. This may be explained by the inclusion of prevalent PAH patients receiving therapy, discordance between transcript and phenotype, and/or transcriptional and metabolic differences in peripheral PBMCs vs the pulmonary circulation and RV. It is also possible that SCN5a splicing is long-lasting and delayed from upstream triggers and downstream effects (eg, the unfolded protein response, which triggers an interorganelle stress response).3
The association between SV levels and basal OCR/basal ECAR and RV metabolic activity supports a relationship between increased SV accumulation and increased aerobic glycolysis, consistent with known impairments in glucose oxidation in PAH.10 This may be due to upregulated but inefficient mitochondrial activity to meet adenosine triphosphate demand coupled with increased glycolysis. An increase in mitochondrial mass, fatty acids, and reactive oxygen species could lead to simultaneous increases in both OCR and ECAR. Surprisingly, higher SV levels were inversely correlated with measurements of pulmonary afterload. Clinical data were collected as close as possible to blood draw, but only PET scans were performed on the same day as phlebotomy. We included only well-controlled patients without clinical evidence of RV failure. The RV in PAH may be unique and characterized by different SVs than those measured here; it is also possible that mitochondrial respiration varies by cellular compartment. Limitations of our study include the small sample size, lack of tissue-level mRNA, and missing data.
Our knowledge of metabolic markers of disease in humans living with PAH is limited. The results from this study provide data to support a novel and, to our knowledge, not previously reported relationship between alternative splicing and cellular metabolism in PAH. We believe this may be one of the first studies to assess mitochondrial function among living patients with PAH from a peripheral blood draw. This may be the first step toward point-of-care testing for RV metabolic changes (and possibly SCD) in this highly lethal condition.
Acknowledgments
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank all of the participants who volunteered to take part in this study.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES: The authors have reported to CHEST the following: C. E. V. has received grants to her institution from United Therapeutics, and from Eiger for the conduct of a clinical trial; and has served as a past consultant for Acceleron Pharma. S. D. has a patent pending on diagnostics on sudden cardiac death in pulmonary arterial hypertension (No. 20180094317). J. R. K. receives grants to his institution from United Therapeutics and Actelion and serves as a nonreimbursed consultant for Bayer. None declared (D. B., T. N. G., A. P., M. W., I. K., A. Z.).
FUNDING/SUPPORT: This work was completed with support from the 2015 CHEST Foundation Research Grant in Pulmonary Arterial Hypertension, an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences [P20 GM103652], and the National Institutes of Health [R01-HL141268].
References
- 1.Tonelli A.R., Arelli V., Minai O.A. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188(3):365–369. doi: 10.1164/rccm.201209-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao G., Xie A., Huang S.C. Role of RBM25/LUC7L3 in abnormal cardiac sodium channel splicing regulation in human heart failure. Circulation. 2011;124(10):1124–1131. doi: 10.1161/CIRCULATIONAHA.111.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao G., Xie A., Zhang J. Unfolded protein response regulates cardiac sodium current in systolic human heart failure. Circ Arrhythm Electrophysiol. 2013;6(5):1018–1024. doi: 10.1161/CIRCEP.113.000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang L.L., Pfahnl A.E., Sanyal S. Human heart failure is associated with abnormal C-terminal splicing variants in the cardiac sodium channel. Circ Res. 2007;101(11):1146–1154. doi: 10.1161/CIRCRESAHA.107.152918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao G., Brahmanandam V., Raicu M. Enhanced risk profiling of implanted defibrillator shocks with circulating SCN5A mRNA splicing variants: a pilot trial. J Am Coll Cardiol. 2014;63(21):2261–2269. doi: 10.1016/j.jacc.2014.02.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R., Wu Y., Zhao M. Role of HIF-1α in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297(4):L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadi A., Ohira H., Mielniczuk L.M. FDG PET imaging for identifying pulmonary hypertension and right heart failure. Curr Cardiol Rep. 2015;17(1):555. doi: 10.1007/s11886-014-0555-7. [DOI] [PubMed] [Google Scholar]
- 8.Simonneau G., Gatzoulis M.A., Adatia I. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Lundgrin E.L., Park M.M., Sharp J. Fasting 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Ann Am Thorac Soc. 2013;10(1):1–9. doi: 10.1513/AnnalsATS.201206-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W., Koeck T., Lara A.R. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A. 2007;104(4):1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]