Abstract
Background
Admission to high-acuity ICUs has been associated with improved outcomes compared with outcomes in low-acuity ICUs, although the mechanism for these findings is unclear.
Research Question
The goal of this study was to determine if high-acuity ICUs more effectively implement evidence-based processes of care that have been associated with improved clinical outcomes.
Study Design and Methods
This retrospective cohort study was performed in adult ICU patients admitted to 322 ICUs in 199 hospitals in the Philips ICU telemedicine database between 2010 and 2015. The primary exposure was ICU acuity, defined as the mean Acute Physiology and Chronic Health Evaluation IVa score of all admitted patients in a calendar year, stratified into quartiles. Multivariable logistic regression was used to examine relations of ICU acuity with adherence to evidence-based VTE and stress ulcer prophylaxis, and with the avoidance of potentially harmful events. These events included hypoglycemia, sustained hyperglycemia, and liberal transfusion practices (defined as RBC transfusions prescribed for nonbleeding patients with preceding hemoglobin levels ≥ 7 g/dL).
Results
Among 1,058,510 ICU admissions, adherence to VTE and stress ulcer prophylaxis was high across acuity levels. In adjusted analyses, those admitted to low-acuity ICUs compared with the highest acuity ICUs were more likely to experience hypoglycemic events (adjusted OR [aOR], 1.12; 95% CI, 1.04-1.19), sustained hyperglycemia (aOR, 1.07; 95% CI, 1.04-1.10), and liberal transfusion practices (aOR, 1.55; 95% CI, 1.33-1.82).
Interpretation
High-acuity ICUs were associated with better adherence to several evidence-based practices, which may be a marker of high-quality care. Future research should investigate how high-acuity ICUs approach ICU organization to identify targets for improving the quality of critical care across all ICU acuity levels.
Key Words: critical care, evidence-based medicine, guidelines, ICU, patient safety
Abbreviation: APACHE, Acute Physiology and Chronic Health Evaluation
FOR EDITORIAL COMMENT, SEE PAGE 442
Clinical outcomes vary substantially between ICUs in the United States.1 Reasons for such variability include heterogeneous patient case-mix, differences in ICU volume, and variation in staffing models and organizational structures within ICUs.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 ICU acuity, defined as the average patient severity of illness in a given ICU, is another ICU-level factor that has been associated with patient outcomes, independent from ICU volume or individual patient acuity.10 Specifically, compared with ICUs that primarily care for less sick patients (defined as low-acuity ICUs), admission to ICUs with higher average patient severity (ie, high-acuity ICUs) has been associated with shorter ICU and hospital lengths of stay and improved mortality among ICU patients at low risk of dying in a dose-dependent fashion. Identifying mechanisms that may enable high-acuity ICUs to achieve better outcomes than low-acuity ICUs is a crucial step in efforts to improve the quality of care for ICU patients with different risk profiles and across a variety of hospital settings.
One potential mechanism for these findings may be that high-acuity ICUs more effectively implement and standardize evidence-based processes of care that have been associated with improved clinical outcomes. Processes of care are a particularly useful platform for quality improvement because they are typically under greater control by clinicians, reflect specific targets for improvement within defined patient populations, and are generally more actionable than outcome measures or organizational structures.1,11, 12, 13 However, adoption of evidence-based processes of care across ICUs is variable and incomplete.14 Poor adherence to evidence-based processes of care may signal the possibility of low-quality care, which could in turn lead to suboptimal patient outcomes.15
Therefore, the objective of the current study was to determine the relationship between ICU acuity and performance in five evidence-based processes of care among ICU patients admitted to hospitals participating in a large ICU telemedicine program in the United States. We focused on processes of care that are reliably captured by the Philips ICU telemedicine system in an effort to track hospital performance and promote continuous quality improvement among its customers. These include: (1) adherence to VTE prophylaxis; (2) adherence to stress ulcer prophylaxis; (3) avoidance of hypoglycemia; (4) avoidance of sustained hyperglycemia; and (5) avoidance of liberal transfusion practices.16, 17, 18, 19, 20, 21, 22, 23, 24, 25 We hypothesized that high-acuity ICUs would be associated with greater adherence to these evidence-based processes of care than low-acuity ICUs.
Materials and Methods
This retrospective cohort study was conducted using the Philips eICU Research Institute data repository, which aggregates clinical and administrative data from > 320 participating hospitals across all regions of the United States.26, 27, 28 ICUs participating in this program are geographically dispersed and include critical access, community, and referral hospitals. They also serve communities that are diverse in size, with 14% of ICUs serving communities of >1 million people, 51% serving communities of 100,000 to 999,999, and 34% serving communities of < 100,000. Approximately 20% of participating ICUs serve rural communities, 33% suburban, and 47% urban. Participating ICUs also vary widely with respect to teaching status, academic affiliation, on-site physician staffing models, and hospital size.26 Data from participating ICUs are aggregated centrally in the Philips eICU Research Institute data repository, which contains granular, robust, and standardized admission, discharge, demographic, laboratory, and medication data for all patients admitted to the participating ICUs.10,26,27
Study Population
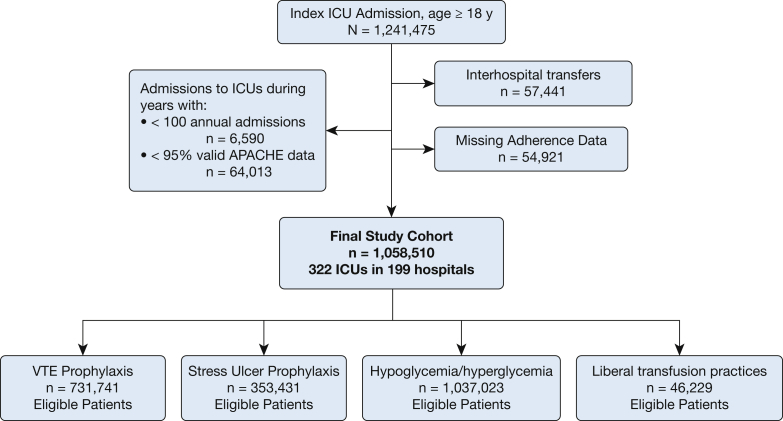
The cohort included patients aged ≥ 18 years admitted to ICUs participating in the Philips ICU telemedicine program between 2010 and 2015. Figure 1 summarizes patient selection. We excluded patients with invalid or incomplete data to calculate an Acute Physiology and Chronic Health Evaluation (APACHE) IVa score. Patients transferred to or from other facilities were excluded, as the entirety of their hospital course and outcomes were unknown. For patients with multiple ICU admissions, all subsequent readmissions were excluded due to non-independence of the outcomes among those ICU admissions. We also excluded admissions to ICUs with < 100 total admissions per year and/or < 95% valid APACHE IVa data in a calendar year for model stability.
Figure 1.
Combined ICU and patient-level exclusion criteria. ACUTE = Acute Physiology and Chronic Health Evaluation.
Exposure and Outcome Variables
The primary exposure was ICU acuity, defined as the mean APACHE IVa score for all patients admitted during a calendar year.10 APACHE IVa is a validated ICU severity-of-illness adjustment system that uses physiologic variables, diagnosis, and comorbidities to predict patient outcomes, including ICU and hospital mortality and length of stay.29 After confirming a near-normal distribution, we categorized ICU acuity into quartiles of low, medium, high, and highest acuity per ICU year, to facilitate comparison of ICUs and interpretability of the results. ICUs could change categories of acuity across individual years of the study period, depending on the relative mean APACHE IVa score in a given year. Teaching hospitals were identified by their membership in the Council of Teaching Hospitals and Health Systems.30
The primary outcomes were appropriate receipt of VTE or stress ulcer prophylaxis among eligible patients and the avoidance of hypoglycemic events, sustained hyperglycemia, and liberal transfusion practices. These patient-level outcomes are reliably tracked by Philips as part of its ICU telemedicine services, which measures the adherence of participating ICUs to evidence-based processes of care.26 Patients admitted to the ICU for > 24 h were considered eligible for VTE prophylaxis unless they were already receiving full-dose anticoagulation or a provider explicitly documented that VTE prophylaxis was not indicated. Adherence to VTE prophylaxis occurred if there was documentation of administration of either pharmacologic (eg, unfractionated heparin, low-molecular-weight heparin, warfarin, or other anticoagulant) or mechanical (ie, intermittent compression device) prophylaxis within 48 h of ICU admission among eligible patients.
Patients were considered eligible for stress ulcer prophylaxis if their active medical problems included head injury, serious burns, or coagulopathy, or if they were mechanically ventilated for > 24 h. Adherence to stress ulcer prophylaxis occurred if there was documentation of administration of pharmacologic (ie, antacid, histamine2-receptor antagonist, proton pump inhibitor, sucralfate) prophylaxis within 24 h of identification of a risk factor for stress ulcers.
The Philips ICU telemedicine program defines hypoglycemia as at least one documented glucose value < 50 mg/dL during a patient’s ICU stay. Sustained hyperglycemia is defined as the time-weighted average daily glucose value ≥ 180 mg/dL during a patient’s ICU stay. Liberal transfusion is defined as any transfusion of RBCs during a patient’s ICU stay with a preceding documented hemoglobin level ≥ 7 g/dL. Patients with admitting diagnoses of GI bleeding, hemorrhage (except CNS), an APACHE admitting diagnosis, or active diagnosis of acute coronary syndrome, trauma, or sepsis were excluded from the latter analysis, as were patients with a ≥ 3 g/dL drop in hemoglobin level in the 12 h preceding the transfusion.
Analysis
We restricted each analysis to patients eligible for each intervention as defined previously. For the blood transfusion outcome, only those patients who had received any transfusion of RBCs during their ICU stay were evaluated. All variables were summarized by using standard descriptive statistics. Unadjusted differences between ICU acuity levels were estimated by using χ2 tests and Wilcoxon rank sum tests, as appropriate. Table 1 summarizes the results of the descriptive statistics performed.
Table 1.
Characteristics and Unadjusted Outcomes of Patients Stratified According to ICU Acuity
| Variable | Low-Acuity ICUs (n = 224,294) | Medium-Acuity ICUS (n = 237,128) | High-Acuity ICUs (n = 260,372) | Highest Acuity ICUs (n = 336,716) |
|---|---|---|---|---|
| Age, y | 62.8 ± 17.1 | 63.8 ± 16.7 | 63.4 ± 17.0 | 63.5 ± 16.9 |
| Male sex | 120,002 (53.5) | 128,884 (54.4) | 139,807 (53.7) | 180,612 (53.6) |
| Race | ||||
| White | 170,591 (76.1) | 179,034 (75.5) | 202,091 (77.6) | 251,840 (74.8) |
| Black | 24,442 (10.9) | 28,747 (12.1) | 25,948 (10.0) | 40,643 (12.1) |
| Other | 29,261 (13.0) | 29,347 (12.4) | 32,333 (12.4) | 44,233 (13.1) |
| APACHE IVa score | 47.8 ± 21.8 | 52.4 ± 22.7 | 56.1 ± 24.7 | 62.8 ± 27.8 |
| Admission source | ||||
| ED | 121,960 (54.4) | 127,231 (53.7) | 142,233 (54.6) | 183,399 (54.5) |
| Operating room | 34,083 (15.2) | 44,405 (18.7) | 40,496 (15.6) | 43,639 (13.0) |
| Ward transfer | 28,105 (12.5) | 33,127 (14.0) | 40,475 (15.5) | 57,411 (17.1) |
| Direct admission | 21,524 (9.6) | 16,110 (6.8) | 18,818 (7.2) | 26,122 (7.8) |
| Other | 18,622 (8.3) | 16,255 (6.9) | 18,350 (7.0) | 26,145 (7.8) |
| Admitting diagnosis | ||||
| Cardiac | 55,795 (24.9) | 74,383 (31.4) | 68,566 (26.3) | 74,456 (22.1) |
| Diabetic ketoacidosis | 4,126 (1.8) | 4,992 (2.1) | 5,689 (2.2) | 7,969 (2.4) |
| GI bleeding | 9,876 (4.4) | 12,646 (5.3) | 15,349 (5.9) | 23,062 (6.8) |
| Neurologic | 42,292 (18.9) | 24,772 (10.4) | 27,658 (10.6) | 30,777 (9.1) |
| Overdose | 6,025 (2.7) | 6,742 (2.8) | 8,004 (3.1) | 10,280 (3.1) |
| Respiratory | 5,246 (2.3) | 7,282 (3.1) | 7,473 (2.9) | 10,167 (3.0) |
| Sepsis | 34,884 (15.6) | 44,781 (18.9) | 56,180 (21.6) | 95,483 (28.4) |
| Trauma | 16,115 (7.2) | 9,174 (3.9) | 11,833 (4.5) | 12,124 (3.6) |
| Other | 49,935 (22.3) | 52,356 (22.1) | 59,620 (22.9) | 72,398 (21.5) |
| ICU LOS, d | 2.8 ± 3.5 | 2.9 ± 3.7 | 2.9 ± 3.7 | 3.2 ± 4.1 |
| ICU mortality | 8,493 (3.8) | 10,682 (4.5) | 14,287 (5.5) | 25,367 (7.5) |
| Hospital LOS, d | 6.8 ± 6.5 | 7.4 ± 7.0 | 7.7 ± 7.2 | 8.7 ± 8.2 |
| Hospital mortality | 15,640 (7.0) | 18,648 (7.9) | 22,914 (8.8) | 39,397 (11.7) |
Data are presented as mean ± SD or No. (%). APACHE = Acute Physiology and Chronic Health Evaluation; LOS = length of stay.
Next, we performed patient-level multivariable analyses using mixed effects logistic regression with ICU as the random effect to account for the non-independence within sites. Hospital discharge year was included as a fixed effect to prevent confounding by practice changes among or within hospitals over time.31,32 We adjusted for potential confounders selected a priori, including severity of illness determined by using the APACHE IVa score, year of hospital discharge, ICU type, ICU volume, number of hospital beds, and hospital teaching status. Because the APACHE IVa score is in part derived from patient-level variables such as age, diagnosis, and chronic comorbidities, we did not include these variables as additional covariates in the models. ICU-level covariates were aggregated over patients within an ICU, and all analyses were done at the patient level.
We also performed several planned secondary analyses. First, ICU acuity was defined as a continuous, rather than categorical, variable. Next, the study cohort was restricted to patients at low risk of dying given prior observational evidence of improved outcomes associated with admission of this population to high-acuity ICUs compared with admission to low-acuity ICUs.10 Patients at low risk of dying were defined as those with APACHE IVa-predicted hospital mortality ≤ 3%.10,33 Finally, a post hoc exploratory analysis was performed to test for interaction between ICU acuity and teaching status to gain insight into additional organizational factors that may be associated with adherence to evidence-based practices.
P values < .05 were considered to be significant. All analyses were completed by using Stata version 14 (StataCorp, LLC). All data were de-identified, and the study was considered exempt from human subjects review by the Veteran Affairs Portland Healthcare System Institutional Review Board.
Results
The final study population included 1,058,510 patients admitted to 322 ICUs in 199 hospitals. Table 1 summarizes characteristics and unadjusted outcomes of patients based on admission to ICUs of varying acuity levels. Cardiac diagnoses were the most common admitting diagnosis, and the ED was the most common admission source across all quartiles of ICU acuity. Approximately 60% of the ICUs were mixed medical-surgical units. Hospitals varied widely in their number of hospital beds and annual average patient volume, and the majority of ICUs were nonteaching (Table 2).30
Table 2.
Characteristics of ICUs Stratified According to ICU Acuity
| Characteristic | Low-Acuity ICUs (n = 131) | Medium-Acuity ICUs (n = 151) | High-Acuity ICUs (n = 146) | Highest Acuity ICUs (n = 120) |
|---|---|---|---|---|
| ICU type | ||||
| Medical-surgical | 78 (59.5) | 87 (57.6) | 86 (58.9) | 70 (58.3) |
| Specialty/other | 53 (40.5) | 64 (42.4) | 60 (41.1) | 50 (41.7) |
| Hospital bed size | ||||
| < 100 | 21 (16.0) | 20 (13.2) | 14 (9.6) | 6 (5.0) |
| 100-249 | 40 (30.5) | 42 (27.8) | 37 (25.3) | 18 (15.0) |
| 250-499 | 19 (14.5) | 25 (16.6) | 33 (22.6) | 36 (30.0) |
| ≥ 500 | 25 (19.1) | 41 (27.2) | 46 (31.5) | 46 (38.3) |
| Missing | 26 (19.8) | 23 (15.2) | 16 (11.0) | 14 (11.7) |
| Teaching hospitals | 20 (15.3) | 24 (15.9) | 30 (20.5) | 33 (27.5) |
Data are presented as No. (%). Counts represent the number of unique ICUs at each acuity level. Because of the way ICU acuity was defined, ICUs could belong to more than one quartile over the study period.
For VTE and stress ulcer prophylaxis, overall adherence was high with minimal variability in performance (96.6% of 731,741 eligible patients and 90.5% of 353,431 eligible patients, respectively) (e-Fig 1). Adjusted analyses of these two care processes showed similarly high rates of adherence with no clinically meaningful differences across levels of ICU acuity (e-Table 1, e-Appendix 1). In contrast, patients experienced the potentially harmful events of sustained hyperglycemia and liberal transfusion practices more frequently, with increased variability in practice across ICUs (e-Fig 2). In adjusted analyses for the outcomes of hypoglycemia, sustained hyperglycemia, and liberal transfusion practices, patients admitted to low-, medium-, and high-acuity ICUs were more likely to experience hypoglycemia and sustained hyperglycemia compared with those admitted to the highest acuity ICUs. For the transfusion outcome, increasing ICU acuity was associated with lower odds of liberal transfusion practices in a dose-dependent fashion (Table 3).
Table 3.
ICU Acuity and Odds of Experiencing Hypoglycemia, Sustained Hyperglycemia, or Liberal Transfusion Practices
| ICU Acuity | Hypoglycemia (n = 1,037,023a) | Hyperglycemia (n = 1,037,023a) | Transfusion (n = 46,229a) |
|---|---|---|---|
| Highest | Reference | Reference | Reference |
| High | 1.10 (1.05-1.15) | 1.05 (1.03-1.07) | 1.08 (0.98-1.18) |
| Medium | 1.11 (1.05-1.18) | 1.04 (1.02-1.06) | 1.41 (1.25-1.59) |
| Low | 1.12 (1.04-1.19) | 1.07 (1.04-1.10) | 1.55 (1.33-1.82) |
Data are presented as OR (95% CI).
Total number of eligible patients for each outcome.
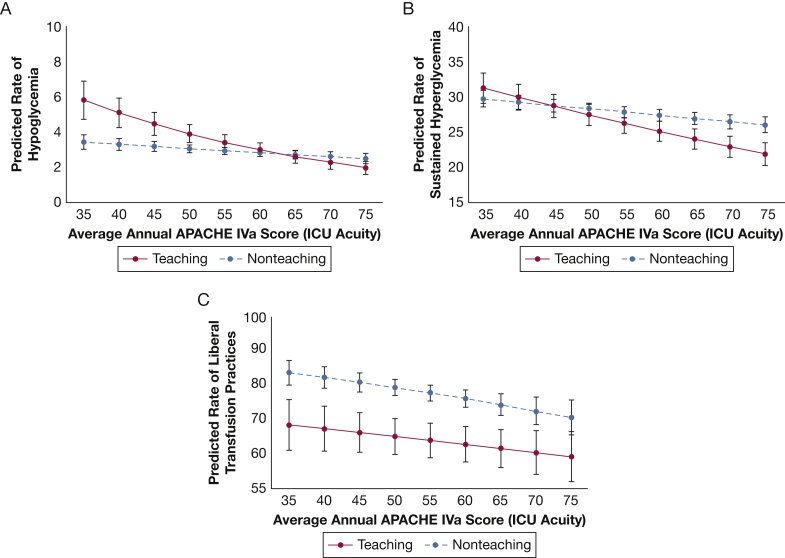
In post hoc exploratory analyses with ICU acuity as a continuous variable and including an interaction term between ICU acuity and teaching status, we found that low-acuity teaching hospitals had higher predicted probability of experiencing hypoglycemia and sustained hyperglycemia compared with low-acuity nonteaching hospitals. As ICU acuity increased, the predicted probability of experiencing hypoglycemia and sustained hyperglycemia decreased, and this effect was more pronounced among teaching hospitals (Figs 2A and 2B). In contrast, teaching hospitals had lower predicted probability of liberal transfusion practices compared with nonteaching hospitals, regardless of ICU acuity level (Fig 2C). In analyses restricted to only low-risk patients, adherence to VTE and stress ulcer prophylaxis remained high (195,508 of 204,293 [95.7%] and 41,191 of 50,529 [83.5%] eligible patients, respectively), with no significant differences across acuity levels. There were also no differences across ICU acuity levels for hypoglycemia or sustained hyperglycemia outcomes. For the transfusion outcome, we found a similar pattern as in the primary analysis, with increasing ICU acuity associated with lower odds of liberal transfusion practices in a dose-dependent fashion.
Figure 2.
Expected rate of (A) hypoglycemic events, (B) sustained hyperglycemia, and (C) liberal transfusion practices based on ICU acuity as defined by the average APACHE IVa score per ICU year. Each line represents results for teaching hospitals compared with nonteaching hospitals. APACHE IVa scores < 10 or > 70 were collapsed into two groups given the small number of patients in the study cohort with scores beyond these thresholds. See Figure 1 legend for expansion of abbreviation.
Discussion
In this retrospective cohort study of ICUs participating in an ICU telemedicine program across the United States, we found very high rates of VTE and stress ulcer prophylaxis with minimal variability across ICUs. Furthermore, we found that increasing ICU acuity was associated with decreased odds of potentially harmful events, including hypoglycemia, sustained hyperglycemia, and liberal transfusion practices. Superior adherence to evidence-based practices may be a marker of high-quality care and correlate with better adherence to other best practices, thereby offering a mechanism for the previous finding that high-acuity ICUs are associated with improved risk-adjusted patient outcomes, including length of stay and mortality.10
We selected five evidence-based processes of care based on literature review and available data from the Philips eICU Research Institute.17, 18, 19, 20, 21, 22,24,27 Although prior literature has linked these process measures to improved patient outcomes, adherence to them may also represent a signal of high-quality care that is otherwise unmeasured. For example, a 2008 study by Werner et al13 found that the unmeasured elements of care associated with aspirin administration are nearly 10 times greater than aspirin’s direct pharmacologic contribution to improved mortality among patients with acute myocardial infarction. Similarly, it stands to reason that ICUs with better adherence to evidence-based practices may also perform better in unmeasured care processes that affect patient outcomes, such as care coordination and local infrastructure for quality improvement. Taken together, these results support using multiple performance measures to assess ICU quality, including structure, process, and outcomes, and suggest further opportunity to improve patient outcomes through the study of ICUs with exceptional performance. Using mixed methods approaches to study high-performing ICUs may be particularly helpful in efforts to enable the identification of currently unmeasured factors that contribute to outstanding outcomes in high-performing hospitals, with the goal of disseminating these findings across all hospitals.13
Our results also expand on previous studies reporting a volume-outcome relationship in critical care. A large systematic review and meta-analysis found that patients at the highest risk of death were most likely to benefit from admission to a high-volume center.7 However, ICU and/or hospital-level organizational factors were found to be major determinants of the observed volume-outcome relationship. A 2017 study evaluating the volume-outcome relation in sepsis found that select evidence-based processes of care were more likely to be implemented at high-volume hospitals.34 Our study adds to the literature by showing an association between ICU acuity and adherence to evidence-based processes of care, independent of ICU volume. These results inform debates regarding regionalization of critical care by offering additional insight into factors that enable certain ICUs to perform better than others.
We also found that ICUs within a large ICU telemedicine program have very high adherence to both VTE and stress ulcer prophylaxis with minimal variability across hospitals. This finding is consistent with results from a large multicenter observational study evaluating the relation between individual processes of care that varied among ICU telemedicine interventions and patient outcomes. Specifically, authors found that several individual components of the ICU telemedicine interventions were associated with better outcomes, including prompt remote intensivist review, improved adherence to evidence-based practices, reduced response time to alarms, and the real-time use of performance measures.35 Better adherence to evidence-based practices such as VTE and stress ulcer prophylaxis may therefore represent plausible mechanisms for the potential effectiveness of the ICU telemedicine intervention.
In post hoc exploratory analyses, we found that teaching status influenced the relationship between ICU acuity and the odds of experiencing hypoglycemia, sustained hyperglycemia, and liberal transfusion practices but in different patterns. At lower levels of ICU acuity, teaching hospitals had higher predicted probabilities of hypoglycemia and sustained hyperglycemia, but this pattern was reversed at higher levels of ICU acuity. In contrast, teaching hospitals had lower predicted probability of liberal transfusion practices regardless of ICU acuity levels. The mechanisms behind these findings are unclear and warrant further exploration. For example, earlier literature has shown that the use of protocols for glucose management in the ICU is associated with better glucose control and improved outcomes.36 Teaching and nonteaching hospitals may differ in their use of protocols and the role of nurses, pharmacists, and trainees in glucose management across ICUs, potentially contributing to the variability in outcomes we observed. Similarly, teaching hospitals are more often located in academic medical centers,14 which more commonly have dedicated intensivists with specific training in evidence-based transfusion practices and other common diagnoses normally treated in the ICU. Taken together, our findings suggest that high-acuity ICUs may have organizational environments that are more conducive to systems-level quality improvement interventions (including more timely data collection and reporting) than low-acuity ICUs. More research is needed to understand how high-acuity ICUs approach ICU organization to identify targets to improve the quality of critical care across all levels of ICU acuity.
Our study has several limitations. First, although the study cohort included a diverse mix of ICUs that vary in location, size, type, and teaching status, they are all part of an ICU telemedicine program, which is in itself an ICU-level intervention that has been associated with increased adherence to some evidence-based practices.35,37,38 Second, we did not have access to data on other hospital characteristics or other organizational factors (eg, the use of checklists, default order sets, staffing patterns such as the presence of a clinical pharmacist on rounds or patient-to-nurse ratios) and therefore could not account for such characteristics in analyses. Third, we restricted each analysis to only patients who were eligible for each intervention, and the methods to determine both patient eligibility and to define the process variables under study rely on chart documentation and may carry a risk of misclassification bias. However, our study focuses on comparisons between the low-acuity and highest acuity ICUs, thereby maximizing the differences between exposure variables. Fourth, for patients in the dataset who had only one glucose measurement per day, the average daily glucose value consisted of that single glucose result.
Conclusions
Compared with patients admitted to low-acuity ICUs, we found that admission to higher-acuity ICUs was associated with better adherence to evidence-based practices. These findings suggest that high-acuity ICUs may more effectively implement and standardize these evidence-based processes of care that have been associated with improved patient outcomes. Future research should investigate how high-acuity ICUs approach ICU organization to identify targets to improve the quality of critical care across all ICU acuity levels.
Acknowledgments
Author contributions: K. C. V., J. Y. S., and O. B. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. K. C. V., O. B., M. O. H., C. G. S., and M. P. K. contributed to the conception and design of this study. O. B. contributed to data acquisition. K. C. V. and J. Y. S. contributed to the analysis of data. K. C. V., J. Y. S., O. B., M. O. H., C. G. S., D. R. S., and M. P. K. contributed to interpretation of data. All authors have made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; have contributed to drafting the article for important intellectual content; and have provided final approval of the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: O. B. is an employee of Philips Healthcare. None declared (K. C. V., J. Y. S., M. O. H., C. G. S., D. R. S., M. P. K.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: This article was reviewed and approved by Craig Lilly, MD; Louis Gidel, MD, PhD; Richard Riker, MD; Leo Celi, MD, MS, MPH; Teresa Rincon, RN, BSN, eCCRN; Theresa Davis, PhD, RN, NE-BC, CHTP; and Michael Waite, MD of the Philips eICU Research Institute Publications Committee. They were not compensated for this review.
Additional information: The e-Appendix, e-Figures and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Department of Veterans Affairs did not have a role in the conduct of the study; in the collection, management, analysis, or interpretation of data; or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the US Government.
FUNDING/SUPPORT: Dr Vranas is supported by grant 5K12HL133115. Dr Harhay is supported by grants K99HL141678 and R00HL141678. Dr Slatore is supported by resources from the VA Portland Health Care System. Dr Sullivan is supported by grant K07CA190706.
Supplementary Data
References
- 1.Checkley W., Martin G.S., Brown S.M. Structure, process, and annual ICU mortality across 69 centers: United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study. Crit Care Med. 2014;42(2):344–356. doi: 10.1097/CCM.0b013e3182a275d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halm E.A., Lee C., Chassin M.R. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–520. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 3.Durairaj L., Torner J.C., Chrischilles E.A., Vaughan Sarrazin M.S., Yankey J., Rosenthal G.E. Hospital volume-outcome relationships among medical admissions to ICUs. Chest. 2005;128(3):1682–1689. doi: 10.1378/chest.128.3.1682. [DOI] [PubMed] [Google Scholar]
- 4.Kahn J.M., Goss C.H., Heagerty P.J., Kramer A.A., O'Brien C.R., Rubenfeld G.D. Hospital volume and the outcomes of mechanical ventilation. New Engl J Med. 2006;355(1):41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 5.Multz A.S., Chalfin D.B., Samson I.M. A "closed" medical intensive care unit (MICU) improves resource utilization when compared with an "open" MICU. Am J Respir Crit Care Med. 1998;157(5 pt 1):1468–1473. doi: 10.1164/ajrccm.157.5.9708039. [DOI] [PubMed] [Google Scholar]
- 6.Bellani G., Laffey J.G., Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen Y.L., Wallace D.J., Yordanov Y. The volume-outcome relationship in critical care: a systematic review and meta-analysis. Chest. 2015;148(1):79–92. doi: 10.1378/chest.14-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin A.J., Simpson K.N., Ford D.W. Volume-mortality relationships during hospitalization with severe sepsis exist only at low case volumes. Ann Am Thorac Soc. 2015;12(8):1177–1184. doi: 10.1513/AnnalsATS.201406-287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaieski D.F., Edwards J.M., Kallan M.J., Mikkelsen M.E., Goyal M., Carr B.G. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190(6):665–674. doi: 10.1164/rccm.201402-0289OC. [DOI] [PubMed] [Google Scholar]
- 10.Vranas K.C., Jopling J.K., Scott J.Y. The association of ICU acuity with outcomes of patients at low risk of dying. Crit Care Med. 2018;46(3):347–353. doi: 10.1097/CCM.0000000000002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn J.M., Gould M.K., Krishnan J.A. An official American Thoracic Society workshop report: developing performance measures from clinical practice guidelines. Ann Am Thorac Soc. 2014;11(4):S186–S195. doi: 10.1513/AnnalsATS.201403-106ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown S.E., Ratcliffe S.J., Halpern S.D. An empirical comparison of key statistical attributes among potential ICU quality indicators. Crit Care Med. 2014;42(8):1821–1831. doi: 10.1097/CCM.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner R.M., Bradlow E.T., Asch D.A. Does hospital performance on process measures directly measure high quality care or is it a marker of unmeasured care? Health Serv Res. 2008;43(5 pt 1):1464–1484. doi: 10.1111/j.1475-6773.2007.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohn R., Madden V., Kahn J.M. Diffusion of evidence-based intensive care unit organizational practices: a state-wide analysis. Ann Am Thorac Soc. 2017;14(2):254–261. doi: 10.1513/AnnalsATS.201607-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauman K.A., Hyzy R.C. ICU 2020: five interventions to revolutionize quality of care in the ICU. J Intensive Care Med. 2014;29(1):13–21. doi: 10.1177/0885066611434399. [DOI] [PubMed] [Google Scholar]
- 16.Weissman G.E., Gabler N.B., Brown S.E., Halpern S.D. Intensive care unit capacity strain and adherence to prophylaxis guidelines. J Crit Care. 2015;30(6):1303–1309. doi: 10.1016/j.jcrc.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearon C., Akl E.A., Comerota A.J. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shekelle P.G., Pronovost P.J., Wachter R.M. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158(5 pt 2):365–368. doi: 10.7326/0003-4819-158-5-201303051-00001. [DOI] [PubMed] [Google Scholar]
- 19.Cook D.J., Fuller H.D., Guyatt G.H. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994;330(6):377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 20.Finfer S., Chittock D., Li Y. Intensive versus conventional glucose control in critically ill patients with traumatic brain injury: long-term follow-up of a subgroup of patients from the NICE-SUGAR study. Intensive Care Med. 2015;41(6):1037–1047. doi: 10.1007/s00134-015-3757-6. [DOI] [PubMed] [Google Scholar]
- 21.Capes S.E., Hunt D., Malmberg K., Gerstein H.C. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 22.Capes S.E., Hunt D., Malmberg K., Pathak P., Gerstein H.C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 23.Gale S.C., Sicoutris C., Reilly P.M., Schwab C.W., Gracias V.H. Poor glycemic control is associated with increased mortality in critically ill trauma patients. Am Surg. 2007;73(5):454–460. doi: 10.1177/000313480707300507. [DOI] [PubMed] [Google Scholar]
- 24.Hebert P.C., Wells G., Blajchman M.A. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 25.Kahn S.R., Lim W., Dunn A.S. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lilly C.M., Zuckerman I.H., Badawi O., Riker R.R. Benchmark data from more than 240,000 adults that reflect the current practice of critical care in the United States. Chest. 2011;140(5):1232–1242. doi: 10.1378/chest.11-0718. [DOI] [PubMed] [Google Scholar]
- 27.McShea M., Holl R., Badawi O., Riker R.R., Silfen E. The eICU research institute—a collaboration between industry, health-care providers, and academia. IEEE Eng Med Biol Mag. 2010;29(2):18–25. doi: 10.1109/MEMB.2009.935720. [DOI] [PubMed] [Google Scholar]
- 28.eICU Collaborative Research Database. https://eicu-crd.mit.edu/
- 29.Zimmerman J.E., Kramer A.A., McNair D.S., Malila F.M. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34(5):1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 30.Council of Teaching Hospitals and Health Systems. https://www.aamc.org/members/coth/
- 31.Localio A.R., Berlin J.A., Ten Have T.R., Kimmel S.E. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001;135(2):112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 32.Wunsch H., Linde-Zwirble W.T., Angus D.C. Methods to adjust for bias and confounding in critical care health services research involving observational data. J Crit Care. 2006;21(1):1–7. doi: 10.1016/j.jcrc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman J.E., Kramer A.A. A model for identifying patients who may not need intensive care unit admission. J Crit Care. 2010;25(2):205–213. doi: 10.1016/j.jcrc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Fawzy A., Walkey A.J. Association between hospital case volume of sepsis, adherence to evidence-based processes of care and patient outcomes. Crit Care Med. 2017;45(6):980–988. doi: 10.1097/CCM.0000000000002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lilly C.M., McLaughlin J.M., Zhao H., Baker S.P., Cody S., Irwin R.S. A multicenter study of ICU telemedicine reengineering of adult critical care. Chest. 2014;145(3):500–507. doi: 10.1378/chest.13-1973. [DOI] [PubMed] [Google Scholar]
- 36.Bull S.V., Douglas I.S., Foster M., Albert R.K. Mandatory protocol for treating adult patients with diabetic ketoacidosis decreases intensive care unit and hospital lengths of stay: results of a nonrandomized trial. Crit Care Med. 2007;35(1):41–46. doi: 10.1097/01.CCM.0000249825.18677.D2. [DOI] [PubMed] [Google Scholar]
- 37.Lilly C.M., Cody S., Zhao H. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175–2183. doi: 10.1001/jama.2011.697. [DOI] [PubMed] [Google Scholar]
- 38.Vranas K.C., Slatore C.G., Kerlin M.P. Telemedicine coverage of intensive care units: a narrative review. Ann Am Thorac Soc. 2018;15(11):1256–1264. doi: 10.1513/AnnalsATS.201804-225CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.