Abstract
Background
Little is known about the prevalence, predictors, and outcomes of late vasopressor administration which evolves after admission to the ICU.
Research Question
What is the epidemiology of late vasopressor administration in the ICU?
Study Design and Methods
We retrospectively studied a cohort of veterans admitted to the Veterans Administration ICUs for ≥ 4 days from 2014 to 2017. The timing of vasopressor administration was categorized as early (only within the initial 3 days), late (on day 4 or later and none on day 3), and continuous (within the initial 2 days through at least day 4). Regressions were performed to identify patient factors associated with late vasopressor administration and the timing of vasopressor administration with posthospitalization discharge mortality.
Results
Among the 62,206 hospitalizations with at least 4 ICU days, late vasopressor administration occurred in 5.5% (3,429 of 62,206). Patients with more comorbidities (adjusted OR [aOR], 1.02 per van Walraven point; 95% CI, 1.02-1.03) and worse severity of illness on admission (aOR, 1.01 per percentage point risk of death; 95% CI, 1.01-1.02) were more likely to receive late vasopressor therapy. Nearly 50% of patients started a new antibiotic within 24 h of receiving late vasopressor therapy. One-year mortality after survival to discharge was higher for patients with continuous (adjusted hazard ratio [aHR], 1.48; 95% CI, 1.33-1.65) and late vasopressor administration (aHR, 1.26; 95% CI, 1.15-1.38) compared with only early vasopressor administration.
Interpretation
Late vasopressor administration was modestly associated with comorbidities and admission illness severity. One-year mortality was higher among those who received late vasopressor administration compared with only early vasopressor administration. Research to understand optimization of late vasopressor therapy administration may improve long-term mortality.
Key Words: cardiovascular failure, outcomes, persistent critical illness, prolonged ICU stay, sepsis, vasopressors
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted OR; BCMA, Barcode Medication Administration; HR, hazard ratio; IQR, interquartile range; IRR, incidence rate ratio; LOS, length of stay; VA, Veterans Administration; VAPD, Veterans Affairs patient database
FOR EDITORIAL COMMENT, SEE PAGE 440
Early recognition and appropriate treatment of cardiovascular failure improves mortality.1, 2, 3, 4, 5 Most research has focused on initial presentation, particularly in the ED.6 However, presenting diagnoses and pathophysiology on admission to the ICU become less predictive of in-hospital mortality over time as a result of events occurring in the ICU.7, 8, 9 This suggests that caution should be used in generalizing from knowledge about early organ failures to the treatment and prognostic importance of later organ failures.
In several cases, ICU day 4 is used to pragmatically distinguish between aspects of the initial resuscitation and ICU-acquired problems. For example, infections occurring on ICU day 4 and beyond are distinguished from earlier occurring infections, with different recommended management and prognostic implications.10,11 At a single center, it was shown that 78% of patients with long ICU stays develop new late organ failures, most commonly cardiovascular failure, where late was also defined as occurring on day 4 and beyond.12 Although the management of cardiovascular failure remains a core task of the modern ICU, the generalizability of this single-center finding—the frequency and outcome of new late cardiovascular failure—is unknown.
In light of this gap, we sought to evaluate the development of cardiovascular failure in patients in the ICU by evaluating the administration of vasopressor agents in a large health-care system—the US Veterans Administration (VA) system. We specifically hypothesized that late cardiovascular failure would be common and would be driven predominately by the development of sepsis. Therefore, the objectives of the study were the following: (1) to quantify the number of hospitalizations and timing when patients were receiving vasopressor agents, (2) to identify patient factors associated with the development of late vasopressor administration, (3) to understand the extent to which infections are associated with the development of late vasopressor administration, and (4) to measure the 1-year mortality of patients who received late vasopressor administration and survived to discharge and to see if it differs from those who only received early vasopressor administration.
Methods
Study Context
The VA health system is one of the largest integrated health-care delivery systems in the United States with > 9 million beneficiaries and an electronic medical record which can be leveraged to capture granular daily data.13,14
Study Population
Data were abstracted from the Veterans Affairs patient database (VAPD) from 2014 to 2017 and represented > 100 VA hospitals.15 The VAPD contains daily standardized physiological information for all patients hospitalized in the VA. The information is structured at the patient-facility-day and includes pharmacy and laboratory data, and diagnostic codes from the entire VA system.15,16 Analyses from the VA were approved by the institutional review board of the VA Ann Arbor Health System (No. IRB-2016-357).
We abstracted data from the VAPD for all patients who were admitted to an ICU. All patient exclusion criteria are listed in e-Appendix 1, e-Table 1.
Identification of Vasopressor Administration
Vasopressor administration was defined as any IV receipt of norepinephrine, epinephrine, dopamine, phenylephrine, and vasopressin as recorded in the Barcode Medication Administration (BCMA) files in the Corporate Data Warehouse. The BCMA files include all VA inpatient medication administrations and include drug name and class. The VAPD extracted all vasopressor medications.15 Drug infusion dose was not able to be reliably ascertained; therefore, any administration on a given calendar day was used. Infusions in the operating room were not included.
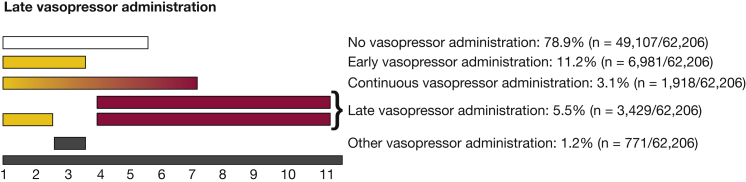
To quantify the timing of vasopressor administration during an ICU admission, we sought to distinguish four time periods. Conceptually, early vasopressor administration reflects the initial resuscitation and stabilization of the patient, whereas late vasopressor administration reflects processes that evolved in the ICU. Unfortunately, there is no reliable way to adjudicate on an individual basis such a distinction at a nationwide scale with the data available. Therefore, following past work distinguishing community- from hospital-acquired pneumonia17 and early from late organ failure, we pragmatically defined the first 3 days in the ICU as early, and day 4 and thereafter as late.12,18 Continuous vasopressor administration was defined as use on ICU day 1 or 2 through at least ICU day 4. Vasopressor administration which was not categorized as early, late, or continuous was defined as other (Fig 1).
Figure 1.
Distribution of vasopressor administration by ICU day in patients with an ICU length of stay of at least 4 d.
A sensitivity analysis was performed to evaluate if admission to the ICU with sepsis was driving the administration of late vasopressor administration.18 Sepsis was defined by the Centers for Disease Control and Prevention definition of sepsis which is an electronic health record-based, diagnostic-code independent definition (e-Appendix 2, e-Fig 1).19
A small number of patients have prolonged ICU stays in the VA, and develop persistent critical illness, which may represent a distinct syndrome of cascading organ failures. We specifically wanted to understand the development of vasopressor administration prior to the onset of persistent critical illness. Using the same methods as in previously published work in Australia and New Zealand8 and Canada,9 we found similar patterns in the onset of persistent illness with 10.7% of the hospitalizations with an ICU length of stay (LOS) > 11 days (e-Appendix 3, e-Tables 2a-c, 3, e-Figs 2, 3). Therefore, we examined daily vasopressor administration from ICU admission through ICU day 11, choosing day 11 for consistency with multiple cross-national sources.
Identification of Late Infection
Among patients who developed late vasopressor administration, antibiotic administration—new or a different class of antibiotic—was reviewed within 24 h of initiation of vasopressors and was used as a surrogate for suspected new infection. The VAPD included extraction from the BCMA files on the administration of antibiotics.15 Culture data were not able to be ascertained.
VA Severity Score
The VA does not use the Acute Physiology and Chronic Health Evaluation IV for severity of illness on admission. For internal risk adjustment, the VA uses an illness severity measure (VA ICU severity score), which predicts 30-day mortality based on several variables (age, admission diagnosis category, 29 comorbid conditions, and 11 laboratory values) (e-Table 3, e-Figs 2, 3). We calculated the VA severity score for each patient admitted to the ICU on the day of admission. This severity score performs similarly to Acute Physiology and Chronic Health Evaluation IV, with a C statistic of 0.874.20
Analysis
We present patient and hospitalization characteristics as counts (percentages), means ± SDs, or medians (interquartile ranges [IQRs]) as appropriate. Elixhauser comorbidities were combined using the method described by van Walraven et al.21 We used hospitalization as the unit of analysis, unless otherwise specified. We used two-sided significance testing and considered a P < .05 to be significant.
We performed logistic regression analysis to identify patient characteristics (age, sex, race, and comorbidities), which were associated with late vasopressor administration (yes/no) while adjusting for severity of illness, type of ICU, hospital LOS prior to ICU admission, admission diagnosis, and receipt of major surgery. To account for the number of days a patient could have cardiovascular organ failure, we performed a Poisson regression adjusting for the same covariates to evaluate which patient characteristics were associated with the outcome, the number of days of late vasopressor administration.
Kaplan-Meier curves were used to evaluate 90-day in-hospital and 1-year postdischarge mortality. Among survivors, log-rank tests were performed to compare the unadjusted 1-year mortality of those who received any vasopressors and those who never received any vasopressors. The 90-day in-hospital and 1-year postdischarge mortality were evaluated using a Cox regression adjusting for patient characteristics, ICU type, and severity of illness on admission. The 90-day in-hospital and 1-year postdischarge mortality were pragmatically chosen to understand if there was a difference in inpatient mortality and postdischarge mortality. The 1-year postdischarge mortality was chosen given the last cohort was admitted in 2017 and death records were only available until 2018. The data were right-side censored at the end of 2018. Only the first hospitalization was used for patients with multiple admissions during the study period.
We conducted all analyses with Stata software 15.1 (StataCorp) and SAS 9.4 (SAS Institute). All code is available at GitHub (https://github.com/CCMRcodes/Late-Vasopressor).
Results
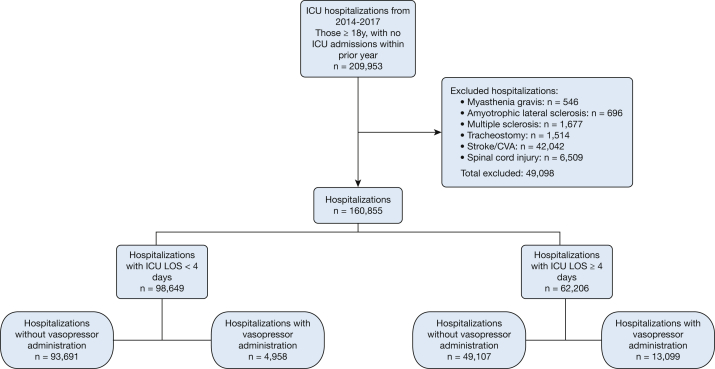
Of the 160,855 patients admitted to VA ICUs from 2014 to 2017, 62,206 had an ICU LOS ≥ 4 days (Fig 2). Such patients had a median age of 68 years (IQR, 62-73), were predominately men, and were predominately white (Table 1).
Figure 2.
Flow diagram of hospitalizations from 2014 to 2017. CVA = cerebral vascular accident.
Table 1.
Demographic and Clinical Features of Patients Who Remained in the ICU ≥ 4 Days (n = 62,206)
| Demographic/Clinical Feature | Value |
|---|---|
| Male | 96.4 (59,970) |
| Age, Y | 68 (62-73) |
| Race | |
| White | 72.3 (45,003) |
| Black | 20.3 (12,597) |
| Other | 7.4 (4,606) |
| Type of ICU | |
| Medical | 55.2 (34,329) |
| Surgical | 38.0 (23,651) |
| Other | 6.8 (4,226) |
| Elixhauser Comorbidity | 4 (2-5) |
| ICU LOS | 5 (4-8) |
| Hospital LOS | 9 (6-15) |
| In-hospital death | 7.9 (4,931) |
| Discharge location | |
| Home | 89.2 (55,480) |
| Transfer to another acute care facility | 3.1 (1,926) |
| Other/death | 7.7 (4,800) |
Values are % (No.) or median (interquartile range). LOS = length of stay.
Overall, 18,057 hospitalizations (11.2% of 160,855) required vasopressor administration. Most were long stay hospitalizations (Fig 2), with 13,099 (21.1% of 62,206) with ICU LOS ≥ 4 days vs 4,958 (5.0% of 98,649) with ICU LOS < 4 days. Among all patients in the ICU, vasopressors were given only on ICU days 1 to 3 in 11,939 hospitalizations (4,958 with ICU LOS < 4 days and 6,981 with ICU LOS ≥ 4 days), whereas 5,347 received them on day 4 or after.
Among patients admitted to the ICU for at least 4 days, late vasopressor administration occurred in 5.5% (3,429 of 62,206). Late vasopressor administration occurred in 9.4% (1,690 of 9,048) and 4.8% (2,574 of 53,158) among patients with and without sepsis on admission (see Figure 1 for late definitions and e-Appendix 2 and e-Figure 1 for additional information on analysis stratified by sepsis present on admission). The median ICU day for the start of late vasopressor administration was ICU day 6 (IQR, 5-7 days), with a median duration of 1 day (IQR, 1-2 days).
Of the patients with late vasopressor administration, nearly one-half (1,639 of 3,429) transitioned to a new antibiotic or a new class of antibiotic within 24 h of the receipt of new vasopressors. The median duration of the new antibiotic was 3 days (IQR, 2-5 days).
Among patients with at least 4 ICU days, patients with more comorbidities (adjusted OR [aOR], 1.02 per van Walraven point; 95% CI, 1.02-1.03; incidence rate ratio [IRR], 1.02; 95% CI, 1.02-1.03) and higher severity of illness (aOR, 1.01 per percent; 95% CI, 1.01-1.02; IRR, 1.01; 95% CI, 1.01-1.02) at hospital admission had higher odds and higher rates of late vasopressor administration. Neither age (aOR, 0.98 per year; 95% CI, 0.94-1.01; IRR, 0.98; 95% CI, 0.95-1.01) nor sex (aOR, 1.03 for women vs men; 95% CI, 0.84-1.26; IRR, 1.03; 95% CI, 0.84-1.26) were associated with higher odds or rates of late vasopressor administration (Table 2).
Table 2.
Predictors of Late Vasopressor Administration
| Variable | Logistic Regression |
Poisson Regression |
||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR | P Value | Adjusted OR | 95% CI | P Value | IRR | 95% CI | P Value | |
| Age (per decade) | 1.09 | < .01 | 0.98 | 0.94-1.01 | .19 | 0.98 | 0.95-1.01 | .17 |
| Female (vs male) | 0.85 | .10 | 1.03 | 0.84-1.26 | .80 | 1.03 | 0.84-1.26 | .80 |
| Race (vs white) | ||||||||
| Black | 0.84 | < .01 | 0.87 | 0.79-0.95 | < .01 | 0.87 | 0.79-0.95 | < .01 |
| Other | 1.27 | < .01 | 1.18 | 1.04-1.33 | .01 | 1.18 | 1.04-1.33 | .01 |
| ICU type (vs medical) | ||||||||
| Surgical | 0.91 | < .01 | 1.14 | 1.03-1.25 | .01 | 1.13 | 1.02-1.26 | .02 |
| Others | 0.77 | < .01 | 0.74 | 0.63-0.87 | < .01 | 0.74 | 0.63-0.87 | < .01 |
| Elixhauser (per van Walraven point) | 1.03 | < .01 | 1.02 | 1.02-1.03 | < .01 | 1.02 | 1.02-1.03 | < .01 |
| Va risk score (per percent) | 1.02 | < .01 | 1.01 | 1.01-1.02 | < .01 | 1.01 | 1.01-1.02 | < .01 |
| Operations | 2.54 | < .01 | 2.36 | 2.19-2.54 | < .01 | 2.36 | 2.18-2.55 | < .01 |
| Hospital LOS prior to ICU admission (per day) | 1.03 | < .01 | 1.03 | 1.02-1.04 | < .01 | 1.03 | 1.01-1.04 | < .01 |
Association of patient-level characteristics comparing patients who received late vasopressors with those who did not receive late vasopressors. IRR = incidence rate ratio; VA = Veterans Administration. See Table 1 legend for expansion of other abbreviation.
When stratifying patients by timing of vasopressor administration, in an unadjusted model, in-hospital 90-day mortality was higher among patients with late and continuous vasopressor administration compared with patients with only early vasopressor administration. In an adjusted Cox regression model controlling for patient characteristics on admission, ICU type, and severity of illness on admission, the adjusted hazard ratio (aHR) for in-hospital mortality was higher for patients who received continuous (aHR, 2.53; 95% CI, 2.26-2.84) or late vasopressor administration (aHR, 2.09; 95% CI, 1.88-2.33) compared with patients who only received early vasopressor administration.
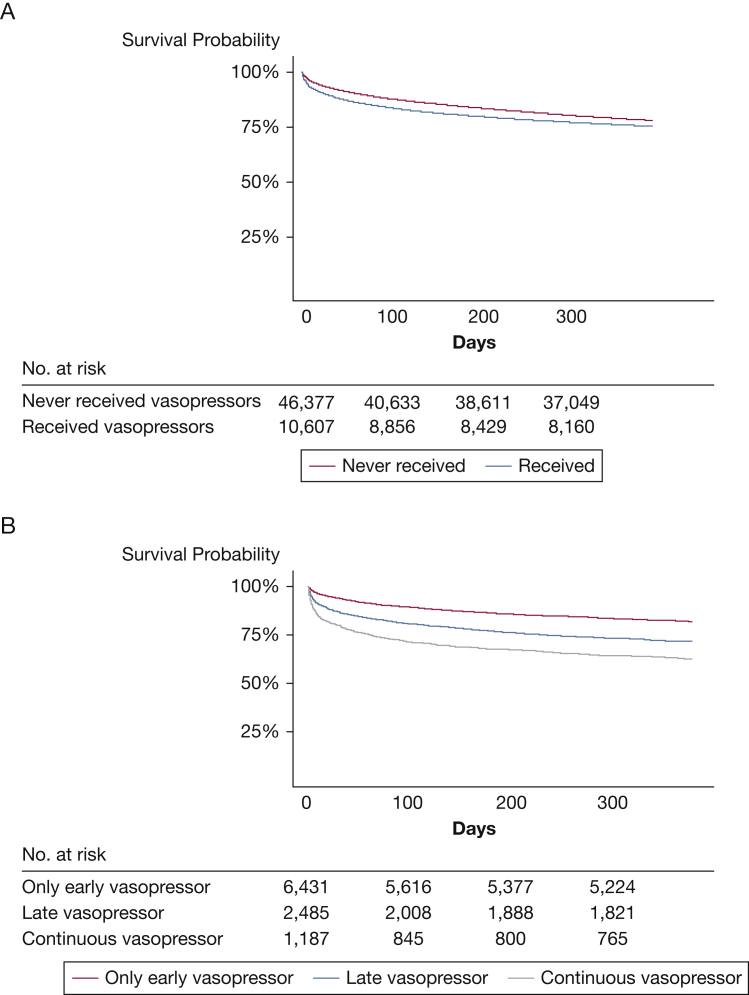
Among those who survived to hospital discharge, patients who received any vasopressor administration had a worse 1-year mortality compared with those with no vasopressor requirements (unadjusted log-rank P < .01) (Fig 3A). In an unadjusted model, when stratifying patients by timing of vasopressor administration, patients with late and continuous vasopressor administration had higher 1-year postdischarge mortality compared with those with only early vasopressor administration (Fig 3B). Among those who survived to hospital discharge, in an adjusted Cox regression model controlling for patient characteristics, ICU type, and severity of illness on admission, the aHR for mortality in the 1 year was higher for patients who received continuous (aHR, 1.48; 95% CI, 1.33-1.65) or late vasopressor administration (aHR, 1.26; 95% CI, 1.15-1.38) compared with patients who only received early vasopressor administration.
Figure 3.
A-B, Kaplan-Meier survival curves 1 year after hospitalization discharge among those with an ICU LOS ≥ 4 d. A, Entire cohort by vasopressor administration; B, Vasopressor-receiving cohort by timing of vasopressor administration.
Discussion
Key Findings
In a national cohort of veterans admitted to the ICU, we found that one in nine received any vasopressors and this increased to one in five among those with an ICU LOS of at least 4 days. Patients with a higher initial comorbidity burden and severity of illness, but not greater age, were somewhat more likely to be administered late vasopressors. Nearly 50% of patients with late vasopressor administration had a new antibiotic or a different class of antibiotic initiated within 24 h, suggesting that vasopressor administration was frequently associated with clinical concerns for recurrent or new sepsis. Late vasopressor therapy was associated with an increased in-hospital mortality and, among survivors, an increased 1-year postdischarge mortality compared with those who used vasopressors only in the first 3 days of an ICU stay.
Relationship to Previous Studies
Previous work on cardiovascular failure focused on the early presentation of cardiovascular failure (eg, early goal-directed therapy for sepsis and early revascularization for cardiogenic shock).1, 2, 3, 4, 5 Mortality rates from early cardiovascular failure have improved with the advancement of early detection strategies and the initiation of the appropriate treatment.22,23 Consequently, more patients have survived their initial pathologies but have continued to remain in the ICU. Previous work has focused on identifying ways to limit the duration of IV vasopressors as a way of shortening ICU stays. For example, corticosteroids have reduced the duration of vasopressors but with potential adverse consequences.24,25 Midodrine has been shown in several small studies to be a beneficial adjunct in stopping IV vasopressors and is currently being evaluated in a clinical trial.26, 27, 28, 29 These adjuncts (corticosteroids and midodrine) have been studied early in the ICU course.24, 25, 26
There has been little past work systematically studying the epidemiology of late cardiovascular failure. Using data from the 1990s, Rosenberg et al7 showed that organ failures present later in an ICU stay (eg, after interhospital transfer) had a different association with in-hospital mortality than those present on initial ICU presentation. In a single-center cohort study, 50 patients with prolonged ICU stays were found to frequently develop new late organ failures on and after ICU day 4. The most common organ failure was cardiovascular failure.12 Our results validate and expand this concept by evaluating a large, national cohort of patients admitted to the ICU for at least 4 days. This national scope offers generalizability, while still maintaining a high level of clinical granularity with linked 1-year mortality outcomes.
Study Implications
Cardiovascular failure which occurs later in the ICU course may have been assumed to have implications similar to cardiovascular failure which occurred on presentation. Our work questions this assumption. These data demonstrate that when a patient develops the need for vasopressors in their ICU stay has important mortality implications—even if the patient survives the hospitalization. Similar elevated postdischarge mortality has been found in sepsis, and to a lesser degree, acute hypoxic respiratory failure.30, 31, 32
New late vasopressor administration in this large national health system is not rare—and may benefit from targeted research with a more nuanced understanding of the physiology driving the administration of late vasopressor utilization in the ICU, rather than being treated by analogy to hypotension newly presenting to the ED. Our data raise an urgent question about the extent to which the in-ICU and postdischarge mortality, that may be attributable to late cardiovascular failure, are modifiable by differences in practice.
Our findings also imply that certain patient characteristics on admission (eg, severity of illness, comorbidities, race, hospital LOS prior to admission to ICU, ICU type) are associated with late vasopressor administration. However, the individual effect sizes are very small. Whether these can be meaningfully aggregated into a useful context-specific risk stratification tool should be a subject of future work.33,34
Our findings demonstrate a higher mortality during and after hospitalizations with late vasopressor administration compared with those hospitalizations with early vasopressor administration. This implies that the timing of cardiovascular failure during the ICU stay matters and has different survival implications. The mechanisms driving this mortality difference need to be discerned while in the ICU and in the posthospitalization period (eg, post-ICU clinics).
Additionally, our findings of associated changes in antibiotic therapy imply that sepsis—or clinical concern for sepsis—may be partially driving the development of late vasopressor administration because nearly one-half of the patients receive a new antibiotic class within 24 h of developing a requirement for vasopressors. However, this interpretation must be tempered by the high propensity of US hospitals to administer antibiotics, and emphasizes the need for more accurate point-of-care sepsis diagnostics.35 Future work would benefit from targeted prospective research with a more nuanced understanding of how infections are being worked up in the ICU, rather than being identified by analogy to the administration of antibiotics.
Strengths and Limitations
Our study has several strengths. We examined a national cohort with detailed daily physiological data collected over a 3-year period encompassing 62,346 hospitalizations with linked mortality data. These granular data allowed us to relate the timing of the need for vasopressors use with long-term mortality and explore the development of late vasopressor administration with the development of new infections. We have also shown that few patient characteristics are associated with late vasopressor therapy administration.
There are several limitations to our study. First, we used a cohort of veterans who are disproportionately white men and may not be representative of other cohorts. However, the cohort also included 3.6% women (n = 2,236) and 27.7% nonwhite patients (n = 17,203), numbers that would be substantial by themselves in many contexts. Second, we used vasopressor administration as a surrogate for cardiovascular failure. Third, it is unknown if changes in patient’s code status or limitations of care contributed to the differences in mortality and if those changes were related to the ICU admission or decisions to administer vasopressors. Finally, we do not know if the patients had a documented infection when antibiotics were initiated.
Conclusions
In patients admitted to the ICU for at least 4 days, late vasopressor therapy administration was not uncommon and 1-year mortality was higher for patients who received late vasopressor therapy and survived to hospital discharge compared with those who only received vasopressors early. Research aimed at understanding what is driving late vasopressor therapy administration may be a target for improving long-term mortality.
Acknowledgments
Author contributions: E. M. V. is accountable for all aspects of the work. E. M. V. designed the study, performed the statistical analyses, interpreted the results, and compiled the manuscript. S. M. B., R. B., and J. M. provided critical revisions of the manuscript. D. J. M., X. Q. W., and S. S. performed statistical analyses and provided critical revisions for the manuscript. T. J. I. designed the study, refined the analyses, assisted in interpreting the findings, and provided critical revisions of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. This work does not represent the official views of the U.S. Government or Department of Veterans Affairs.
Additional information: The e-Appendixes, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [Grants T32 HL7749-25 to Dr Viglianti andK12 HL138039-02 to Drs Viglianti and Iwashyna], the Department of Veterans Affairs Health Services Research & Development service [Grants IIR 13-079, 17-219 to Drs Iwashyna], and a Canada Research Chair in Critical Care Nephrology [to Dr Bagshaw].
Supplementary Data
References
- 1.Liu V.X., Fielding-Singh V., Greene J.D. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howell M.D., Davis A.M. Management of sepsis and septic shock. JAMA. 2017;317(8):847–848. doi: 10.1001/jama.2017.0131. [DOI] [PubMed] [Google Scholar]
- 3.Hayden G.E., Tuuri R.E., Scott R. Triage sepsis alert and sepsis protocol lower times to fluids and antibiotics in the ED. Am J Emerg Med. 2016;34(1):1–9. doi: 10.1016/j.ajem.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochman J.S., Sleeper L.A., Webb J.G. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295(21):2511–2515. doi: 10.1001/jama.295.21.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Diepen S., Katz J.N., Albert N.M. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 6.Seymour C.W., Gesten F., Prescott H.C. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg A.L., Hofer T.P., Strachan C., Watts C.M., Hayward R.A. Accepting critically ill transfer patients: adverse effect on a referral center's outcome and benchmark measures. Ann Intern Med. 2003;138(11):882–890. doi: 10.7326/0003-4819-138-11-200306030-00009. [DOI] [PubMed] [Google Scholar]
- 8.Iwashyna T.J., Hodgson C.L., Pilcher D. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4(7):566–573. doi: 10.1016/S2213-2600(16)30098-4. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw S.M., Stelfox H.T., Iwashyna T.J., Bellomo R., Zuege D., Wang X. Timing of onset of persistent critical illness: a multi-centre retrospective cohort study. Intensive Care Med. 2018;44(12):2134–2144. doi: 10.1007/s00134-018-5440-1. [DOI] [PubMed] [Google Scholar]
- 10.Langer M., Cigada M., Mandelli M., Mosconi P., Tognoni G. Early onset pneumonia: a multicenter study in intensive care units. Intensive Care Med. 1987;13(5):342–346. doi: 10.1007/BF00255791. [DOI] [PubMed] [Google Scholar]
- 11.Kalil A.C., Metersky M.L., Klompas M. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viglianti E.M., Kramer R., Admon A.J. Late organ failures in patients with prolonged intensive care unit stays. J Crit Care. 2018;46:55–57. doi: 10.1016/j.jcrc.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kizer K.W., Fonseca M.L., Long L.M. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42(3):283–298. [PubMed] [Google Scholar]
- 14.US Department of Veteran Affairs Veteran Health Administration. https://www.va.gov/health/aboutvha.asp
- 15.Wang X.Q., Vincent B.M., Wiitala W.L. Veterans Affairs patient database (VAPD 2014-2017): building nationwide granular data for clinical discovery. BMC Med Res Methodol. 2019;19(1):94. doi: 10.1186/s12874-019-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fihn S.D., Francis J., Clancy C. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood) 2014;33(7):1203–1211. doi: 10.1377/hlthaff.2014.0054. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 18.van Vught L.A., Klein Klouwenberg P.M., Spitoni C. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315(14):1469–1479. doi: 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 19.Rhee C., Dantes R., Epstein L. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Render M.L., Deddens J., Freyberg R. Veterans Affairs intensive care unit risk adjustment model: validation, updating, recalibration. Crit Care Med. 2008;36(4):1031–1042. doi: 10.1097/CCM.0b013e318169f290. [DOI] [PubMed] [Google Scholar]
- 21.van Walraven C., Austin P.C., Jennings A., Quan H., Forster A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 22.Iwashyna T.J., Cooke C.R., Wunsch H., Kahn J.M. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaukonen K.M., Bailey M., Suzuki S., Pilcher D., Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesh B., Finfer S., Cohen J. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378(9):797–808. doi: 10.1056/NEJMoa1705835. [DOI] [PubMed] [Google Scholar]
- 25.Annane D., Renault A., Brun-Buisson C. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378(9):809–818. doi: 10.1056/NEJMoa1705716. [DOI] [PubMed] [Google Scholar]
- 26.Levine A.R., Meyer M.J., Bittner E.A. Oral midodrine treatment accelerates the liberation of intensive care unit patients from intravenous vasopressor infusions. J Crit Care. 2013;28(5):756–762. doi: 10.1016/j.jcrc.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Whitson M.R., Mo E., Nabi T. Feasibility, utility, and safety of midodrine during recovery phase from septic shock. Chest. 2016;149(6):1380–1383. doi: 10.1016/j.chest.2016.02.657. [DOI] [PubMed] [Google Scholar]
- 28.Poveromo L.B., Michalets E.L., Sutherland S.E. Midodrine for the weaning of vasopressor infusions. J Clin Pharm Ther. 2016;41(3):260–265. doi: 10.1111/jcpt.12375. [DOI] [PubMed] [Google Scholar]
- 29.Anstey M.H., Wibrow B., Thevathasan T. Midodrine as adjunctive support for treatment of refractory hypotension in the intensive care unit: a multicenter, randomized, placebo controlled trial (the MIDAS trial) BMC Anesthesiol. 2017;17(1):47. doi: 10.1186/s12871-017-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prescott H.C., Sjoding M.W., Langa K.M., Iwashyna T.J., McAuley D.F. Late mortality after acute hypoxic respiratory failure. Thorax. 2018;73(7) doi: 10.1136/thoraxjnl-2017-210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott H.C., Osterholzer J.J., Langa K.M., Angus D.C., Iwashyna T.J. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankar-Hari M., Harrison D.A., Ferrando-Vivas P., Rubenfeld G.D., Rowan K. Risk factors at index hospitalization associated with longer-term mortality in adult sepsis survivors. JAMA Netw Open. 2019;2(5) doi: 10.1001/jamanetworkopen.2019.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwashyna T.J., Viglianti E.M. Patient and population-level approaches to persistent critical illness and prolonged intensive care unit stays. Crit Care Clin. 2018;34(4):493–500. doi: 10.1016/j.ccc.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwashyna T.J., Hodgson C.L., Pilcher D. Towards defining persistent critical illness and other varieties of chronic critical illness. Crit Care Resusc. 2015;17(3):215–218. [PubMed] [Google Scholar]
- 35.Baggs J., Fridkin S.K., Pollack L.A., Srinivasan A., Jernigan J.A. Estimating national trends in inpatient antibiotic use Among US hospitals from 2006 to 2012. JAMA Intern Med. 2016;176(11):1639–1648. doi: 10.1001/jamainternmed.2016.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.