Abstract
Background
Although inhaled therapy reduces exacerbations among patients with COPD, the effectiveness of providing inhaled treatment per risk stratification models remains unclear.
Research Question
Are inhaled regimens that align with the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy associated with clinically important outcomes?
Study Design and Methods
We conducted secondary analyses of Long-term Oxygen Treatment Trial (LOTT) data. The trial enrolled patients with COPD with moderate resting or exertional hypoxemia between 2009 and 2015. Our exposure was the patient-reported inhaled regimen at enrollment, categorized as either aligning with, undertreating, or potentially overtreating per the 2017 GOLD strategy. Our primary composite outcome was time to death or first hospitalization for COPD. Additional outcomes included individual components of the composite outcome and time to first exacerbation. We generated multivariable Cox proportional hazard models across strata of GOLD-predicted exacerbation risk (high vs low) to estimate between-group hazard ratios for time to event outcomes. We adjusted models a priori for potential confounders, clustered by site.
Results
The trial enrolled 738 patients (73.4% men; mean age, 68.8 years). Of the patients, 571 (77.4%) were low risk for future exacerbations. Of the patients, 233 (31.6%) reported regimens aligning with GOLD recommendations; most regimens (54.1%) potentially overtreated. During a 2.3-year median follow-up, 332 patients (44.9%) experienced the composite outcome. We found no difference in time to composite outcome or death among patients reporting regimens aligning with recommendations compared with undertreated patients. Among patients at low risk, potential overtreatment was associated with higher exacerbation risk (hazard ratio, 1.42; 95% CI, 1.09-1.87), whereas inhaled corticosteroid treatment was associated with 64% higher risk of pneumonia (incidence rate ratio, 1.64; 95% CI, 1.01-2.66).
Interpretation
Among patients with COPD with moderate hypoxemia, we found no difference in clinical outcomes between inhaled regimens aligning with the 2017 GOLD strategy compared with those that were undertreated. These findings suggest the need to reevaluate the effectiveness of risk stratification model-based inhaled treatment strategies.
Key Words: COPD, guidelines, inhaled corticosteroids, pharmacotherapy
Abbreviations: 6MWT, 6-min walk test; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; LOTT, Long-term Oxygen Treatment Trial; SGRQ, St. George’s Respiratory Questionnaire
FOR EDITORIAL COMMENT, SEE PAGE 437
Over the past two decades, multiple studies have demonstrated the efficacy of inhaled bronchodilators for reducing respiratory exacerbations while improving symptoms and quality of life for patients with COPD.1, 2, 3, 4, 5 Among patients who have frequent COPD exacerbations, the addition of inhaled corticosteroids (ICSs) reduces the likelihood of acute respiratory illness6, 7, 8; however, physicians should weigh the value of ICS therapy against the risk of pneumonia, with the number needed to harm potentially as low as 16 patients.9, 10, 11, 12, 13 Long-term ICS use has also been associated with the development of diabetes and osteoporosis.14,15 Although inhaled treatments have become standard practice in the management of COPD, previous studies evaluated relatively homogenous patient samples and offer little guidance on the implementation of inhaled therapy at the population level.4, 5, 6,8
Clinical investigations have identified risk factors for mortality and future exacerbations among patients with COPD, including higher symptom burden and history of prior exacerbations.16, 17, 18, 19, 20, 21 Using this evidence, professional societies and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) developed models to both estimate future exacerbation risk and offer inhaled treatment recommendations.22,23 GOLD incorporates new evidence annually,24,25 with the last major update occurring in 2017. Compared with 2011, the 2017 GOLD strategy recommends against using severity of airflow obstruction to predict future exacerbation risk. The GOLD strategies remain the most widely accepted COPD management guidelines in the developed world, published in leading respiratory journals.22,24 However, the benefit of receiving inhaled therapy in accordance with these risk models remains unknown.
Our objective was to understand how the receipt of current guideline-recommended care influences outcomes in clinical practice. Among a cohort of patients with COPD with moderate hypoxemia who participated in the Long-term Oxygen Treatment Trial (LOTT), we determined the association of using an inhaled regimen that aligns with the 2017 GOLD recommendations and clinically important outcomes. We hypothesized that patients who received an aligned regimen, when compared with those who were undertreated, would have lower mortality, fewer moderate and severe COPD exacerbations, increased exercise performance, and improved disease-specific quality of life. Among patients considered low risk for future exacerbations and without an indication for ICSs, we also hypothesized that treatment with ICSs would increase the risk of pneumonia.
Methods
Data and Study Population
We performed secondary analyses of LOTT data.26 LOTT was a randomized trial to determine the efficacy of long-term supplemental oxygen among patients ≥ 40 years of age with stable COPD and moderate resting or exercise-induced hypoxemia. All patients had spirometry-confirmed airflow obstruction (postbronchodilator FEV1/FVC ratio < 0.70), and none had an exacerbation within 30 days of screening.
The trial enrolled 738 patients between 2009 and 2014 at 42 respiratory clinics across the United States, including academic, community-affiliated, and Veterans Affairs medical centers. Patients attended in-person visits at randomization and annually, during which study coordinators performed detailed histories (including medication use), physical examinations, questionnaires, and 6-min walk tests (6MWTs). The trial determined vital status as of August 31, 2015, for all patients who enrolled, and 97% had at least 1 year of hospitalization follow-up data.26,27
COPD Disease Severity Assessment
We used the 2017 GOLD recommendations22 to categorize patients into baseline COPD severity groups. Groups range from A (low symptom burden, low exacerbation risk) through D (high symptom burden, high exacerbation risk) (e-Table 1). We assessed baseline symptoms using the Modified Medical Research Council Scale, with scores ≥ 2 representing high symptom burden.28 Prior to enrollment, LOTT collected 3 months of outpatient exacerbation and 1 year of hospitalization data. We therefore used a modified definition to predict future exacerbation risk, categorizing patients as GOLD group C or D if they reported one or more outpatient exacerbations requiring antibiotics/steroids in the 3 months prior to enrollment or had one or more exacerbations causing hospitalization in the preceding year.
We chose the 2017 GOLD recommendations for our primary analyses because this strategy represents the most recent paradigm shift in the management of COPD. Although LOTT occurred prior to the publication of the 2017 recommendations, we think this approach is reasonable because (1) the diagnostic criteria and classes of inhaled medications available to treat COPD have not changed and (2) our goal was not to assess physician adherence to guidelines but instead to identify the association of guideline-concordant treatment with outcomes.
Exposure
Our primary exposure was the inhaled regimen reported at enrollment, classified as aligning with, undertreating, or potentially overtreating based on the 2017 GOLD recommendations (e-Table 2). In general, undertreating regimens frequently lacked recommended long-acting bronchodilators, whereas overtreating regimens contained potentially unnecessary ICSs.
Outcomes
Our primary composite outcome was time from enrollment in LOTT to death or first COPD-related hospitalization (severe exacerbation). Secondary outcomes included individual components of the composite outcome, time to first moderate or severe COPD exacerbation, follow-up 6MWT distance, and follow-up COPD-specific quality of life measured by the English language St. George’s Respiratory Questionnaire (SGRQ).
Statistical Analyses
For the primary exposure, we generated multivariable Cox proportional hazards models clustered by clinical site to estimate between-group hazard ratios for time to event outcomes (primary composite, mortality, and exacerbation outcomes). We evaluated proportional hazards assumptions using Schoenfeld residuals and stratified by covariates with violations (GOLD stage and BODE index).29 We used multivariable linear regression to construct generalized estimating equations that estimated between-group differences in 6MWT distance and SGRQ total score at follow-up visits. We excluded patients who did not complete these tests during follow-up. We estimated separate models across risk strata (high vs low) for all analyses. We chose a priori covariates that might influence the receipt of inhaled regimens and outcomes.
Secondary Analyses
We investigated the association of ICS use with pneumonia among a restricted cohort of patients at low risk for future exacerbation and without an identifiable indication for ICSs (GOLD groups A and B). We defined pneumonia as any inpatient or outpatient encounter for which the primary/secondary diagnosis was pneumonia and/or pneumonia-specific treatment was administered. To minimize confounding by indication, we propensity matched patients on the likelihood of receiving ICSs. We used negative binomial regression to estimate between-group incidence rate ratios for pneumonia.
Updating the 2011 GOLD strategy, the 2017 recommendations removed severity of airflow obstruction from the disease severity assessment. Seeking to understand how this update may have influenced the association of receiving guideline-recommended care with outcomes, we repeated analyses after categorizing COPD severity and inhaled regimens per the 2011 GOLD strategy.
We prespecified sensitivity analyses that (1) updated inhaled regimens and primary exposure categories every 12 months and (2) adjusted for randomization to long-term supplemental oxygen. We conducted a post hoc analysis in which we excluded patients reporting daily systemic glucocorticoid use. We performed analyses using Stata versions 15.1 and 14.1 (StataCorp). The institutional review board of the VA Puget Sound Healthcare System approved this study (MIRB 01382). Additional details are available in e-Methods, e-Appendix 1.
Results
Patient Characteristics
We included all 738 randomized LOTT participants in our primary time to event analysis (Table 1). Mean patient age was 68.8 years; 73.4% were men. We classified 163 (22.1%), 408 (55.3%), 37 (5.0%), and 130 (17.6%) patients as 2017 GOLD groups A through D, respectively. Most patients (n = 468, 63.4%) had an FEV1 < 50% predicted. Patients classified as high risk for future exacerbations (groups C and D) reported prior pneumonia and participation in pulmonary rehabilitation more frequently than low-risk patients (groups A and B). Overall, comorbidity and estimated risk of death (BODE index) was similar between risk groups. Group A and B patients had no exacerbations in the 3 months prior to enrollment, whereas group C and D patients reported a mean of 1.1 exacerbations during this period.
Table 1.
Baseline Patient Characteristics, Stratified by Exacerbation Risk
| Variable | Overall Cohort (N = 738) | Low-Risk Groups A and B (n = 571) |
High-Risk Groups C and D (n = 167) |
|---|---|---|---|
| Demographics | |||
| Age, y | 68.8 ± 7.5 | 69.0 ± 7.5 | 68.1 ± 7.4 |
| Male | 542 (73.4) | 422 (73.9) | 120 (71.9) |
| BMI, kg/m2 | 28.6 ± 6.5 | 28.4 ± 6.6 | 29.2 ± 5.7 |
| Current smoker | 202 (27.4) | 165 (28.9) | 37 (22.2) |
| Pack-year history | 61.4 ± 32.9 | 61.5 ± 32.7 | 61.0 ± 33.9 |
| Randomized to oxygen | 368 (49.9) | 287 (50.2) | 81 (48.5) |
| 24-h oxygen | 220 (29.8) | 170 (29.8) | 50 (29.9) |
| Nocturnal/ambulatory oxygen only | 148 (20.0) | 117 (20.4) | 31 (18.6) |
| Indication for supplemental oxygen | |||
| Resting Spo2 88%-94% | 133 (18.0) | 105 (18.4) | 28 (16.8) |
| Ambulatory Spo2 80%-88% | 319 (43.2) | 239 (41.9) | 80 (47.9) |
| Both | 286 (38.8) | 227 (39.8) | 59 (35.3) |
| Charlson comorbidity index score | 5.0 ± 1.9 | 5.0 ± 1.9 | 4.9 ± 1.9 |
| Prior pneumonia | 394 (53.4) | 283 (49.6) | 111 (66.5) |
| Pulmonary rehabilitation | 220 (29.8) | 157 (27.5) | 63 (37.7) |
| COPD severity | |||
| BODE indexa | |||
| 0-2 | 165 (22.7) | 136 (24.2) | 29 (17.5) |
| 3-4 | 282 (38.7) | 217 (38.6) | 65 (39.2) |
| 5-6 | 210 (28.8) | 155 (27.6) | 55 (33.1) |
| 7-10 | 71 (9.8) | 54 (9.6) | 17 (10.2) |
| GOLD stage | |||
| I | 26 (3.5) | 24 (4.2) | 2 (1.2) |
| II | 244 (33.1) | 185 (32.4) | 59 (35.3) |
| III | 357 (48.4) | 279 (48.9) | 78 (46.7) |
| IV | 111 (15.0) | 83 (14.5) | 28 (16.8) |
| Exacerbations in past 3 mo | 0.3 ± 0.6 | 0.0 ± 0.0 | 1.1 ± 0.9 |
| Baseline SGRQ total score | 50.0 ± 17.9 | 48.9 ± 17.9 | 53.8 ± 17.7 |
| Baseline 6MWT distance, m | 318 ± 99 | 322 ± 101 | 307 ± 91 |
Values are mean ± SD or No. (%). 6MWT = 6-min walk test; GOLD = Global Initiative for Chronic Obstructive Lung Disease; SGRQ = St. George’s Respiratory Questionnaire; Spo2 = pulse oximetry oxygen saturation.
n = 728; 10 patients could not complete baseline 6MWT.
Regimen Alignment With GOLD Recommendations
Patients most often reported using combination long-acting beta agonist (LABA), long-acting muscarinic antagonist (LAMA), and ICS (42.6%) (triple therapy) (Table 2). Combination LABA/ICS was also commonly used (20.7%). Patients infrequently reported LABA or LAMA monotherapy. Compared with group A and B patients, a higher percentage of group C and D patients reported use of daily systemic glucocorticoids. Overall, 233 patients (31.6%) reported regimens that aligned with the 2017 GOLD recommendations. Most baseline regimens potentially overtreated (54.1%).
Table 2.
Baseline COPD Treatment Regimens, Stratified by COPD Exacerbation Risk
| Treatment Regimen | Overall Cohort (N = 738) | Groups A and B (n = 571) | Groups C and D (n = 167) |
|---|---|---|---|
| Inhalers | |||
| Any short-acting | 637 (86.3) | 475 (83.2) | 162 (97.0) |
| LABA only | 17 (2.3) | 15 (2.6) | 2 (1.2) |
| LAMA only | 72 (9.8) | 60 (10.5) | 12 (7.2) |
| ICS only | 35 (4.7) | 26 (4.6) | 9 (5.4) |
| LABA + LAMA | 23 (3.2) | 19 (3.3) | 4 (2.4) |
| LAMA + ICS | 28 (3.8) | 31 (3.7) | 7 (4.2) |
| LABA + ICS | 153 (20.7) | 127 (22.2) | 26 (15.6) |
| LABA + LAMA + ICS | 314 (42.6) | 219 (38.4) | 95 (56.9) |
| Systemic medications | |||
| Glucocorticoids | 61 (8.3) | 32 (5.6) | 29 (17.4) |
| Theophylline | 50 (6.8) | 37 (6.5) | 13 (7.8) |
| 2017 GOLD alignment | |||
| Not aligned | 505 (68.4) | 461 (80.7) | 44 (26.3) |
| Undertreated | 106 (14.4) | 83 (14.5) | 23 (13.8) |
| Potentially overtreated | 399 (54.1) | 378 (66.2) | 21 (12.6) |
| Aligned | 233 (31.6) | 110 (19.3) | 123 (73.7) |
Values are No. (%). ICS = inhaled corticosteroid; LABA = long-acting beta agonist; LAMA = long-acting muscarinic antagonist. See Table 1 legend for expansion of other abbreviation.
Risk of Exacerbations and Mortality
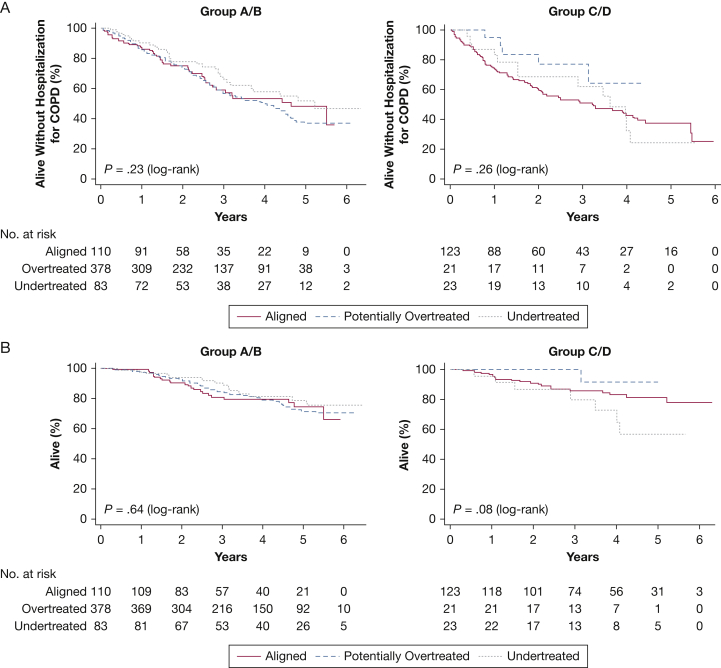
For the primary composite outcome, median follow-up was 2.3 years, during which 332 patients (44.9%) died or experienced hospitalization for COPD; 111 group A and B patients (19.4%) and 28 group C and D patients (20.4%) died. Figure 1 shows Kaplan-Meier curves for the primary composite outcome (Fig 1A) and death (Fig 1B), stratified by predicted future exacerbation risk. We found no significant difference in time to death and/or first hospitalization for COPD among patients reporting baseline inhaled regimens that undertreated or potentially overtreated compared with those reporting regimens that aligned with recommendations in the 2017 GOLD strategy (Table 3). Among patients at low risk for future exacerbations (groups A and B), those reporting regimens that potentially overtreated were 42% more likely to experience an initial exacerbation during follow-up compared with patients reporting regimens that aligned with strategy recommendations (hazard ratio, 1.42; 95% CI, 1.09-1.87) (e-Fig 1, Table 3). Among group C and D patients, we found no significant difference in time to first exacerbation by category of baseline inhaled regimen.
Figure 1.
A-B, Kaplan-Meier analyses for the primary composite outcome and death by category of primary exposure. A, Time to event analysis for death or first COPD-related hospitalization, stratified by exacerbation risk (N = 738); median follow-up 2.29 y. Data for patients who neither died nor had a first hospitalization for COPD were censored at the time of their last interview. A total of 248 patients (43.4%) in groups A and B and 84 patients (50.3%) in groups C and D experienced the composite outcome. B, Time to event analysis for death, stratified by exacerbation risk (N = 738); median follow-up 3.46 y. Data for patients who were alive as of August 31, 2015, were censored at the time of their last interview. A total of 111 patients (19.4%) in groups A and B and 28 patients (16.8%) in groups C and D died. P values were generated from log-rank tests.
Table 3.
Relative Risk of Experiencing Time to Event Outcomes by COPD Treatment Classification
| Outcome by Exposure | 2017 Groups A and B |
2017 Groups C and D |
||
|---|---|---|---|---|
| Crude Hazard Ratio (95% CI) | Adjusted Hazard Ratioa (95% CI) | Crude Hazard Ratio (95% CI) | Adjusted Hazard Ratioa (95% CI) | |
| Primary outcome | ||||
| Death/first hospitalization for COPDb | ||||
| Aligned | Referent | Referent | Referent | Referent |
| Undertreated | 0.78 (0.46-1.34) | 0.92 (0.61-1.40) | 0.96 (0.66-1.40) | 0.93 (0.57-1.52) |
| Overtreated | 1.09 (0.71-1.67) | 1.02 (0.67-1.54) | 0.47 (0.20-1.09) | 0.55 (0.24-1.28) |
| Secondary outcomes | ||||
| Deathb | ||||
| Aligned | Referent | Referent | Referent | Referent |
| Undertreated | 0.73 (0.31-1.76) | 0.86 (0.39-1.88) | 2.09 (0.94-4.64) | 1.76 (0.74-4.19) |
| Overtreated | 0.93 (0.54-1.60) | 0.96 (0.63-1.47) | 0.32 (0.12-0.90) | 0.52 (0.08-3.46) |
| First hospitalization for COPDc | ||||
| Aligned | Referent | Referent | Referent | Referent |
| Undertreated | 0.83 (0.43-1.64) | 0.94 (0.57-1.54) | 0.72 (0.45-1.18) | 0.83 (0.41-1.71) |
| Overtreated | 1.22 (0.76-1.97) | 1.08 (0.68-1.71) | 0.42 (0.14-1.27) | 0.46 (0.17-1.24) |
| First COPD exacerbationd | ||||
| Aligned | Referent | Referent | Referent | Referent |
| Undertreated | 0.91 (0.53-2.15) | 0.96 (0.61-1.49) | 0.93 (0.65-1.34) | 1.00 (0.55-1.81) |
| Overtreated | 1.60 (1.19-2.15) | 1.42 (1.09-1.87) | 1.24 (0.80-1.93) | 1.05 (0.53-2.04) |
Cox proportional hazard models adjusted for baseline age, sex, Charlson comorbidity index score, smoking status, and number of all-cause hospitalizations in year before randomization, stratified by baseline Global Initiative for Chronic Obstructive Lung Disease stage and BODE index.
Includes all 738 patients enrolled in the Long-term Oxygen Treatment Trial.
Excludes patients who died prior to experiencing an initial COPD-related hospitalization (n = 43).
Excludes patients who died prior to experiencing an initial COPD exacerbation (n = 39).
Functional Status and Quality of Life
Overall, 572 patients completed at least one follow-up 6MWT, whereas 645 patients had at least one follow-up SGRQ score. Among low-risk patients (groups A and B), those reporting baseline inhaled regimens that undertreated walked a mean of 12 m further (95% CI, 2-22 m) than patients reporting regimens that aligned with the 2017 strategy. We observed a similar relationship among patients at high risk for future exacerbation (mean 6MWT distance 6 m further for those undertreated vs aligned; 95% CI, −3 to 15 m). We did not detect a significant difference in follow-up mean SGRQ score by category of baseline inhaled regimen, regardless of future exacerbation risk (Table 4).
Table 4.
Mean Follow-Up 6MWT Distance and Total SGRQ Score by COPD Treatment Classification
| Outcome by Exposure | 2017 Groups A and B |
2017 Groups C and D |
||
|---|---|---|---|---|
| Crude β1 Coefficient (95% CI) | Adjusted β1 Coefficienta (95% CI) | Crude β1 Coefficient (95% CI) | Adjusted β1 Coefficientb (95% CI) | |
| Follow-up 6MWT (m) | ||||
| Aligned | Referent | Referent | Referent | Referent |
| Undertreated | 9 (−3 to 15) | 12 (2 to 22) | 7 (−14 to 29) | 7 (−23 to 37) |
| Overtreated | 6 (0 to 19) | 6 (−3 to 15) | −8 (−31 to 16) | −23 (−53 to 8) |
| Follow-up SGRQ score | ||||
| Aligned | Referent | Referent | Referent | Referent |
| Undertreated | −1.1 (−2.9 to 0.6) | −1.1 (−3.1 to 0.9) | −0.5 (−3.2 to 2.3) | −0.3 (−3.2 to 2.7) |
| Overtreated | 0.2 (−1.0 to 1.5) | 0.2 (−1.1 to 1.5) | 0.1 (−3.6 to 3.9) | 0.3 (−3.7 to 4.3) |
See Table 1 legend for expansion of abbreviations.
Multivariable linear regression model with an exchangeable correlation matrix linked on patient identity, adjusted for baseline 6MWT distance, age, sex, Charlson comorbidity index score, smoking status, BODE index, Global Initiative for Chronic Obstructive Lung Disease stage, and site. A total of 572 patients had at least one outcome measure.
Multivariable linear regression model with an exchangeable correlation matrix linked on patient identity, adjusted for baseline SGRQ score, age, sex, Charlson comorbidity index score, smoking status, BODE index, Global Initiative for Chronic Obstructive Lung Disease stage, and site. A total of 645 patients had at least one outcome measure.
Secondary Analyses
Risk of Pneumonia
After restricting the cohort to patients at low risk for future exacerbations (groups A and B), we propensity matched patients on the likelihood of reporting baseline ICSs use. The matched cohort contained 332 patients (166 per group), with small differences in baseline characteristics and similar likelihood of receiving ICSs between groups (e-Fig 2, e-Table 3). The incidence of pneumonia was higher among patients who reported ICS use vs not (mean episodes 0.43 vs 0.25, respectively) (e-Fig 3), with those reporting ICS use 64% more likely to develop pneumonia during follow-up (incidence rate ratio, 1.64; 95% CI, 1.01-2.66). We obtained similar results using an adjusted negative binomial regression model instead of propensity matching (incidence rate ratio, 1.73; 95% CI, 1.00-3.00).
GOLD 2011 Analyses
When we assessed baseline disease severity according to the 2011 GOLD strategy,23 we classified 209 patients (28.3%) as low risk (groups A and B) and 529 patients (71.7%) as high risk (groups C and D) for future exacerbations (e-Table 4). Overall, 490 patients (66.4%) reported baseline inhaled regimens that aligned with the 2011 GOLD strategy (e-Table 5). In general, the results of the 2011 analyses were similar to those for 2017. However, we now observed a higher exacerbation risk among potentially overtreated patients across strata of predicted future risk (e-Figs 4, 5; e-Table 6).
Sensitivity Analyses
We obtained similar results when we updated patient-reported inhaled regimens at 12-month intervals (e-Table 7), excluded patients reporting daily systemic glucocorticoid use, or adjusted for randomization to supplemental oxygen (data not shown).
Discussion
The efficacy of inhaled therapy in the management of COPD is well established, with studies focusing on the comparative effectiveness of individual regimens.6,30 To our knowledge, our study is the first to evaluate the relationship between receiving inhaled treatment in accordance with the 2017 GOLD recommendations and developing clinically important outcomes. Among a cohort of patients with moderate hypoxemia, we found no significant difference in time to death and/or first COPD-related hospitalization among patients whose inhaled regimens aligned with the 2017 GOLD recommendations compared with those who were undertreated. Quality of life was also similar between groups. Among low-risk patients, potential overtreatment was associated with higher risk of experiencing a COPD exacerbation, whereas potentially unnecessary ICS use was associated with higher risk of pneumonia.
Our findings suggest that choosing inhaled regimens based on the 2017 GOLD strategy provides little added benefit when compared with approaches that undertreat. One possible explanation is that the inhaled treatment recommendations are overly broad, allowing for multiple acceptable regimens within each disease severity group. Clinical trials demonstrating the efficacy of inhaled therapies typically compare two or three regimens.2,4, 5, 6,8 In contrast, our study evaluated the relative effectiveness of treatment categories containing a wide range of inhaled therapies.18 This heterogeneity may dilute the effectiveness of specific regimens and explain our findings. Narrowing treatment recommendations within disease severity groups (eg, at least dual bronchodilator therapy among patients with frequent exacerbations)4,5,8 might improve outcomes.
Alternatively, the disease severity assessment in the 2017 GOLD strategy may lack the precision necessary to adequately risk-stratify patients and guide treatment. Studies evaluating the predictive accuracy of the 2007, 2011, and 2017 GOLD strategies for mortality and exacerbations reported low area under the receiver operator curves (between 0.62 and 0.68).25,26 Although prior exacerbations strongly predict future events,19 additional independent risk factors for death and future exacerbations exist.20,21,31, 32, 33 Moreover, COPD is a heterogenous disease with multiple phenotypes. For example, patients with COPD with elevated eosinophil levels experience more frequent exacerbations and may benefit from eosinophil-targeted treatment.34, 35, 36, 37, 38 Although the 2019 strategy incorporates this latter evidence,24 the 2017 risk prediction models and treatment recommendations follow a one size fits all approach. The observed outcomes among patients in this cohort may reflect this misclassification by the 2017 strategy.
It is also possible, however, that inhaled therapy is less effective when applied to the broader population using risk stratification models. Trials establishing the efficacy of inhaled regimens were almost exclusively performed among patients with high symptom burden, moderate to severe obstruction, and at least one exacerbation in the year prior to enrollment.4,5,8 In contrast, comparative effectiveness studies have evaluated inhaled treatments without accounting for a patient’s GOLD group.6,30 Our findings suggest that additional studies are necessary to truly establish the effectiveness of providing inhaled treatment based on GOLD risk models.
The higher exacerbation risk we observed among potentially overtreated, low-risk patients is intriguing. Because LOTT collected only 3 months of outpatient exacerbation data, we may have misclassified patients who are truly high risk for exacerbation. We performed multiple comparisons and type 1 error could also explain this result. However, if either explanation was true, we would not have expected the 2011 analyses (in which we reclassified > 300 patients from low to high risk) to yield similar results. Alternatively, it is possible that both the 2011 and 2017 strategies misclassified the exacerbation risk of patients in this cohort. Consistent with prior studies,39,40 regimens in the present cohort that potentially overtreated contained ICSs. Clinically, COPD exacerbations are often diagnosed concurrently with pneumonia, which may explain the observed difference in exacerbation risk. Our findings support the 2017 strategy’s recommendations to limit ICS use only to patients with a history of recurrent exacerbations.
Our findings are consistent with the only prior study evaluating outcomes in association with adherence to inhaled treatment recommendations based on risk stratification models.41 Among Swiss adults with physician-diagnosed COPD, the authors found no difference in symptoms or exacerbation frequency among patients whose regimens aligned with the 2011 GOLD strategy compared with those whose regimens did not. Nearly 50% of patients initially enrolled in that study were lost to follow-up and therefore did not contribute outcome data. Moreover, the investigators were unable to ensure the validity of spirometry data and consequently could not guarantee the accuracy of COPD diagnoses. In contrast, outcomes were ascertained, and the validity of spirometry was confirmed, for all patients in our study.
Our study has several strengths. We had robust ascertainment of outcomes during a median follow-up of 2.3 years. Few studies have as comprehensive data regarding spirometry, medication use, symptoms, and exercise performance. Although LOTT was a pragmatic randomized trial performed throughout the United States, certain aspects of this study limit the generalizability of our findings. LOTT recruited participants from respiratory clinics only. Moreover, all patients who enrolled in the LOTT cohort had moderate hypoxemia and airflow obstruction confirmed by spirometry. None had a respiratory exacerbation within 30 days of screening. Future studies should address these limitations.
Multiple aspects of our study limit our conclusions. As with all observational pharmacoepidemiology studies, we cannot exclude confounding by indication. Physicians in LOTT may have recognized other independent risk factors for death and future exacerbations not captured by the GOLD strategies and escalated inhaled treatment accordingly. The higher exacerbation risk among potentially overtreated, low-risk patients could have resulted from this bias. Although we adjusted for multiple confounders, additional unmeasured factors may have influenced our results. LOTT relied on patient report to ascertain inhaled medication use and patients may not have been taking the regimens they reported. This might explain why only 86% of patients reported use of short-acting bronchodilators. We classified relatively few regimens as under- or potentially overtreating, which likely produced the imprecise variance estimates observed among group C and D patients. Moreover, we performed multiple comparisons, which increases the risk of type 1 error. Finally, we used a modified definition of increased exacerbation risk and may have inadvertently misclassified low-risk patients whom the 2017 strategy would have considered high risk for future exacerbations. However, the distribution of disease severity groups and categories of inhaled regimens in our study was similar to those observed in prior investigations based on the 2017 strategy.39,40,42,43 It is therefore unlikely that our modified definition of exacerbation risk significantly affected our results.
Interpretation
In this post hoc secondary analysis of LOTT data, we did not find a significant difference in time to death or first hospitalization for COPD, exacerbations, or quality of life between patients reporting regimens that aligned with recommendations in the 2017 GOLD strategy compared with those who were undertreated. Potential overtreatment among low-risk patients was associated with a higher risk of COPD exacerbation. Our findings highlight the need for additional studies to evaluate the effectiveness of risk stratification model-based inhaled treatment strategies in real-world practice.
Acknowledgments
Author contributions: T. K. had full access to the data and assumes responsibility for the integrity and accuracy of data analysis. T. K., L. J. S., L. M. D., S. S. C., M. G., A. D. B., D. H. A., and L. C. F. contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript. E. U., R. C., J. A. C., G. J. C., P. T. D., A. L. F., S. E. G., R. E. K., F. J. M., R. J. P., D. S., A. S., T. S., J. K. S., J. T., R. W., and R. D. Y. provided the source data and contributed substantially to data interpretation and writing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. C. reports receiving institutional grant funding from GlaxoSmithKline, Boehringer-Ingelheim, Astellas, and AstraZeneca; reports receiving consulting fees from Astellas, Regeneron, and Genentech; reports serving on advisory boards for Boehringer-Ingelheim, AstraZeneca, GlaxoSmithKline, and Medimmune; reports serving in the speakers bureaus for GlaxoSmithKline, AstraZeneca, and Boehringer-Ingelheim; and owns stock in Inogen. A. L. F. reports being a coinvestigator on a PCORI clinical trial, reports travel support from AstraZeneca, reports consulting fees from Novartis, and chairs a randomized trial adjudication committee for Icon Medical Imaging. F. J. M. reports serving on the steering committees for Bayer, Boehringer Ingelheim, Centocor, Gilead Sciences, Takeda Pharmaceuticals, Afferent Pharmaceuticals, Forest Laboratories, Janssen, GlaxoSmithKline, AstraZeneca, and Pearl Therapeutics; serving on advisory boards for Boehringer Ingelheim, Genentech Ikaria, Kadmon, Takeda Pharmaceuticals, Pfizer, Veracyte, Forest Laboratories, Janssen, GlaxoSmithKline, AstraZeneca, Bellerophon Therapeutics, Novartis, Pearl Therapeutics, Roche, Sunovion Pharmaceuticals, Theravance Biopharma, and Concert Pharmaceuticals; serving on data and safety monitoring boards for Biogen and GlaxoSmithKline; receiving fees for participating in continuing medical education activities from AcademicCME, MedEd Consulting, Continuing Education, Potomac Center for Medical Education, CME incite, Annenberg Center for Health Sciences at Eisenhower, Integritas Communications, inThought Research, Miller Medical Communications, Prime Healthcare, WebMD, and PeerView Academic Network; receiving consulting fees from Axon Communications, Johnson & Johnson, Clarion Communications, Adept Field Solutions, Amgen, Proterixbio, Unity Biotechnology, and Lucid Communique Medical Education; and receiving lecture fees from AstraZeneca. J. K. S. reports being a consultant for Grifols, CSL Behring, Takeda, 23andMe, Vertex, Arrowhead Pharmaceuticals, and InhibRx; and serves on the Board of Directors for the Alpha-1 Foundation and the American Respiratory Care Foundation. R. W. reports receiving consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, ContraFect, GlaxoSmithKline, Janssen, Mylan, Novartis, Pfizer, Pulmonx, Roche, Spiration, Sunovion Pharmaceuticals, Teva Pharmaceutical Industries, Theravance, Verona Pharma, and Vertex Pharmaceuticals; and grant support from Boehringer Ingelheim, GlaxoSmithKline, Teva Pharmaceutical Industries, and Pearl Therapeutics. D. H. A. reports serving on the data monitoring committee for Novartis. None declared (T. K., L. J. S., L. M. D., E. U., S. S. C., M. G., A. D. B., J. A. C., G. J. C., P. T. D., S. E. G., R. E. K., R. J. P., D. S., A. S., T. S., J. T., R. D. Y., L. C. F.).
Role of sponsors: The funding sources had no role in the study design, data analysis, interpretation, or writing of the manuscript.
Additional information:The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The Long-term Oxygen Treatment Trial was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH) and Department of Health and Human Services [Contracts HHSN268200736183C, HHSN268200736184C, HHSN268200736185C, HHSN268200736186C, HHSN268200736187C, HHSN268200736188C, HHSN268200736189C, HHSN268200736190C, HHSN268200736191C, HHSN268200736192C, HHSN268200736193C, HHSN268200736194C, HHSN268200736195C, HHSN268200736196C, HHSN268200736197C, Y1-HR-7019-01, Y1-HR-8076-01], in cooperation with the Centers for Medicare and Medicaid Services, Department of Health and Human Services. Additional funding support was provided by Dr Laura Feemster’s NIH NHLBI K23 award [Grant HL 111116] and an NIH institutional training grant [Grant T32 HL 007287].
Supplementary Data
References
- 1.Kew K.M., Dias S., Cates C.J. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;3:CD010844. doi: 10.1002/14651858.CD010844.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelmeier C., Hederer B., Glaab T. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 3.Karner C., Chong J., Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;7:CD009285. doi: 10.1002/14651858.CD009285.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedzicha J.A., Banerji D., Vogelmeier C.F. Indacaterol-glycopyrronium for COPD. N Engl J Med. 2016;375(9):899–900. doi: 10.1056/NEJMc1609305. [DOI] [PubMed] [Google Scholar]
- 5.Calverley P.M.A., Anzueto A.R., Carter K. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337–344. doi: 10.1016/S2213-2600(18)30102-4. [DOI] [PubMed] [Google Scholar]
- 6.Vestbo J., the Salford Lung Study Investigators Effectiveness of fluticasone furoate-vilanterol in COPD. N Engl J Med. 2016;375(26):2607. doi: 10.1056/NEJMc1613713. [DOI] [PubMed] [Google Scholar]
- 7.Yang I.A., Clarke M.S., Sim E.H.A., Fong K.M. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipson D.A., Criner G.J., Lomas D.A. Single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;379(6):592–593. doi: 10.1056/NEJMc1807380. [DOI] [PubMed] [Google Scholar]
- 9.Calverley P.M., Anderson J.A., Celli B. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 10.Ernst P., Gonzalez A.V., Brassard P., Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176(2):162–166. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
- 11.Suissa S., Coulombe J., Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177–1183. doi: 10.1378/chest.15-0627. [DOI] [PubMed] [Google Scholar]
- 12.Suissa S., Patenaude V., Lapi F., Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029–1036. doi: 10.1136/thoraxjnl-2012-202872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crim C., Dransfield M.T., Bourbeau J. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27–34. doi: 10.1513/AnnalsATS.201409-413OC. [DOI] [PubMed] [Google Scholar]
- 14.Suissa S., Kezouh A., Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123(11):1001–1006. doi: 10.1016/j.amjmed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Loke Y.K., Cavallazzi R., Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66(8):699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- 16.Sundh J., Janson C., Lisspers K., Montgomery S., Stallberg B. Clinical COPD questionnaire score (CCQ) and mortality. Int J Chron Obstruct Pulmon Dis. 2012;7:833–842. doi: 10.2147/COPD.S38119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundh J., Janson C., Lisspers K., Stallberg B., Montgomery S. The dyspnoea, obstruction, smoking, exacerbation (DOSE) index is predictive of mortality in COPD. Prim Care Respir J. 2012;21(3):295–301. doi: 10.4104/pcrj.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura K., Izumi T., Tsukino M., Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 19.Hurst J.R., Vestbo J., Anzueto A. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 20.Mullerova H., Maselli D.J., Locantore N. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 21.Soler-Cataluna J.J., Martinez-Garcia M.A., Roman Sanchez P., Salcedo E., Navarro M., Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogelmeier C.F., Criner G.J., Martinez F.J. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(6) doi: 10.1183/13993003.00214-2017. [DOI] [PubMed] [Google Scholar]
- 23.Qaseem A., Wilt T.J., Weinberger S.E. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 24.Singh D., Agusti A., Anzueto A. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5) doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 25.Soriano J.B., Lamprecht B., Ramirez A.S. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient data. Lancet Respir Med. 2015;3(6):443–450. doi: 10.1016/S2213-2600(15)00157-5. [DOI] [PubMed] [Google Scholar]
- 26.Long-Term Oxygen Treatment Trial Research Group. Albert R.K., Au D.H. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617–1627. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusen R.D., Criner G.J., Sternberg A.L. The long-term oxygen treatment trial for chronic obstructive pulmonary disease: rationale, design, and lessons learned. Ann Am Thorac Soc. 2018;15(1):89–101. doi: 10.1513/AnnalsATS.201705-374SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajiro T., Nishimura K., Tsukino M., Ikeda A., Koyama H., Izumi T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(4):1185–1189. doi: 10.1164/ajrccm.158.4.9802091. [DOI] [PubMed] [Google Scholar]
- 29.Hosmer D.W., Lemeshow S., May S. 2nd ed. John Wiley & Sons; Hoboken, NJ: 2011. Applied Survival Analysis: Regression Modeling of Time to Event Data. [Google Scholar]
- 30.Suissa S., Dell’Aniello S., Ernst P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest. 2019;155(6):1158–1165. doi: 10.1016/j.chest.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Santibanez M., Garrastazu R., Ruiz-Nunez M. Predictors of hospitalized exacerbations and mortality in chronic obstructive pulmonary disease. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0158727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yohannes A.M., Mülerová H., Lavoie K. The association of depressive symptoms with rates of acute exacerbations in patients with COPD: Results from a 3-year longitudinal follow-up of the ECLIPSE cohort. J Am Med Dir Assoc. 2017;18(11):955–959.e6. doi: 10.1016/j.jamda.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Iyer A.S., Bhatt S.P., Garner J.J. Depression is associated with readmission for acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):197–203. doi: 10.1513/AnnalsATS.201507-439OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim V.L., Coombs N.A., Staples K.J. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J. 2017;50(4) doi: 10.1183/13993003.00853-2017. [DOI] [PubMed] [Google Scholar]
- 35.Couillard S., Larivee P., Courteau J., Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151(2):366–373. doi: 10.1016/j.chest.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Pascoe S., Locantore N., Dransfield M.T., Barnes N.C., Pavord I.D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 37.Hastie A.T., Martinez F.J., Curtis J.L. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi: 10.1016/S2213-2600(17)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavord I.D., Chanez P., Criner G.J. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh S., Anderson W.H., Putcha N. Alignment of inhaled chronic obstructive pulmonary disease therapies with published strategies. Analysis of the global initiative for chronic obstructive lung disease recommendations in SPIROMICS. Ann Am Thorac Soc. 2019;16(2):200–208. doi: 10.1513/AnnalsATS.201804-283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh M.J., Huang S.Y., Yang T.M. The impact of 2011 and 2017 Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) guidelines on allocation and pharmacological management of patients with COPD in Taiwan: Taiwan Obstructive Lung Disease (TOLD) study. Int J Chron Obstruct Pulmon Dis. 2018;13:2949–2959. doi: 10.2147/COPD.S176065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jochmann A., Scherr A., Jochmann D.C. Impact of adherence to the GOLD guidelines on symptom prevalence, lung function decline and exacerbation rate in the swiss COPD cohort. Swiss Med Wkly. 2012;142:w13567. doi: 10.4414/smw.2012.13567. [DOI] [PubMed] [Google Scholar]
- 42.Gayle A., Dickinson S., Morris K., Poole C., Mathioudakis A.G., Vestbo J. What is the impact of GOLD 2017 recommendations in primary care? - a descriptive study of patient classifications, treatment burden and costs. Int J Chron Obstruct Pulmon Dis. 2018;13:3485–3492. doi: 10.2147/COPD.S173664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Criner R.N., Labaki W.W., Regan E.A. Mortality and exacerbations by global initiative for chronic obstructive lung disease groups ABCD: 2011 versus 2017 in the COPDGene® cohort. Chronic Obstr Pulm Dis. 2019;6(1):64–73. doi: 10.15326/jcopdf.6.1.2018.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.