Abstract
Purpose of Review
Until recently, cardiac regeneration after myocardial infarction has remained a holy grail in cardiology. Failure of clinical trials using adult stem cells and scepticism about the actual existence of such cells has reinforced the notion that the heart is an irreversibly post-mitotic organ. Recent evidence has drastically challenged this conclusion.
Recent Findings
Cardiac regeneration can successfully be obtained by at least two strategies. First, new cardiomyocytes can be generated from embryonic stem cells or induced pluripotent stem cells and administered to the heart either as cell suspensions or upon ex vivo generation of contractile myocardial tissue. Alternatively, the endogenous capacity of cardiomyocytes to proliferate can be stimulated by the delivery of individual genes or, more successfully, of selected microRNAs.
Summary
Recent experimental success in large animals by both strategies now fuels the notion that cardiac regeneration is indeed possible. Several technical hurdles, however, still need to be addressed and solved before broad and successful clinical application is achieved.
Keywords: Cardiomyocyte, Myocardial infarction, microRNA, Lipid nanoparticle, Regeneration, Stem cells
Introduction
The need to develop novel therapies for heart failure (HF) consequent to myocardial infarction (MI) is impelling. Despite notable progress in the application of devices assisting the failing myocardium [1], HF prognosis remains poor, with mortality estimated at 40% of patients at only 4 years from diagnosis [2]. This is worse than several common cancers. HF is also tremendously expensive, representing 2–3% of national health expenditures in high-income countries, projected to more than doubling in the next 20 years [3, 4].
Most notably, pharmacological treatment of HF uses drugs that have only marginally evolved since the mid-1990s. While high hope is now raised by the unpredicted and somehow surprising cardiovascular effects of SGLT2 inhibitors [5], for which no convincing molecular explanation yet exists, no conceptually novel drugs have been introduced in the management of patients with HF since the angiotensin II receptor blockers [6]. The relatively novel angiotensin receptor-neprilysin inhibitor (ARNI) combination [7] is based on drugs that were both individually developed in the 1990s. In addition, a number of drugs have so far failed in Phase III clinical trials [8]. Even more remarkably, for conditions that are as prevalent as MI and HF, no biological therapy has yet been developed, based on any protein, peptide, antibody, or nucleic acid [9].
The Problems of Cardiomyocyte Loss
It has become progressively clear that a major problem underlying the prevalence of HF is linked to the ageing of the population and the lack of regenerative potential of the heart. Acute myocardial injury can kill as many as 25% of cardiomyocytes (CMs) from the left ventricle, corresponding to up to 1 billion cells [10]. In addition, chronic myocardial disease can kill CMs over prolonged periods of time. This is now clear in a number of pathological conditions, ranging from inherited cardiomyopathies to oncological treatments [11]. CM loss also accompanies physiological ageing [12].
This progressive or sudden loss of contractile cells during life is not paralleled by significant new contractile tissue formation. At least three different types of information are concordant in indicating that the extent of CM renewal in adult life is minimal and certainly clinically negligible. First, 14C-carbon dating of human CM DNA indicated that renewal of these cells in a 70-year-old individual is less than 50% [13], in fact showing that the majority of CMs at any time in adulthood are those generated at birth or immediately afterwards. Second, measurements obtained using mass spectrometry imaging in mice revealed a rate of CM renewal of approximately 1% per year, which raises three times after MI [14]. These values are consistent with those detected by 14C dating. Third, the same information was also obtained by analysing the rate of DNA synthesis in vivo in mice [15].
Lack of CM renewal reflects the incapacity of CMs to replicate. CM replication occurs during embryonic, foetal, and immediate post-natal life, to eventually drop down suddenly in the early neonatal stage [16]. As a consequence, immediately after birth, CM can replicate and drive cardiac regeneration, while this property is irreversibly lost at 7 days in mice. Similar observations also hold true in pigs, in which MI is repaired by complete regeneration in 2-day-old piglets, while invariably leads to scarring in adult animals [17]. An anecdotical report in an infant with acute MI—a very rare condition—reveals that complete cardiac regeneration is also possible immediate after birth in humans [18].
The incapacity of the mammalian heart to regenerate in adulthood contrasts with the evidence in amphibians and fish, in which the regenerative capacity persists throughout life [19, 20]. Of note, regeneration in these animals, similar to neonatal mice and pigs, does not involve the proliferation and differentiation of any cardiac stem cell, but is sustained by the partial de-dedifferentiation of existing CMs that resume proliferation [21, 22]. In adult mice, there is also an attempt at proliferation by CMs bordering the infarcted region [14, 15]; however, this is abortive and the extent of new CM formation is largely below the threshold necessary to provide clinical benefit.
Over the last years, there has been intense research to unravel the reason why CM proliferation stops irreversibly after birth. A prevalent view is that this is linked to sudden biochemical and mechanical events occurring immediately after birth. Pressure overload [23], sudden increase in oxygen tension and oxidative stress [24], lack of maternal factors [25], changes in hormonal stimulation [26], and switch from glycolytic to oxidative metabolism [27] are all factors that have been associated with the rapid loss of regenerative capacity. Most reasonably, it appears likely that the withdrawal of CMs from the cell cycle and the activation of a hypertrophic gene programme is the consequence of a combination of all these factors.
Factors and Pathways Regulating Cardiomyocyte Proliferation
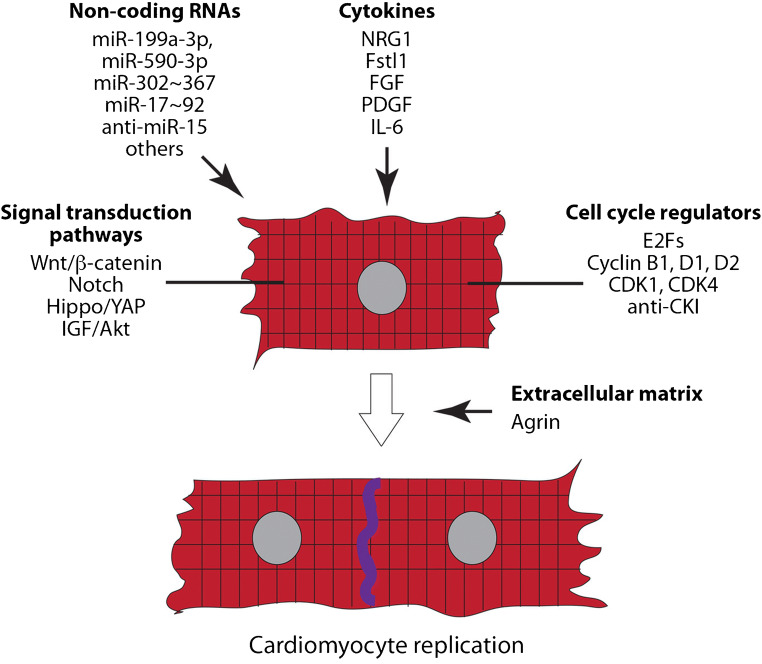
Not unlikely all other cell types, the regulation of CM proliferation occurs through the combined action of a number of factors signalling to CMs from the outside of the plasma membrane or transducing their proliferative signal intracellularly (Fig. 1). These will be briefly reviewed in this section.
FIG. 1.
Intracellular and extracellular proteins, genes, and non-coding RNAs known to regulate cardiomyocyte proliferation and cardiac regeneration. See text for explanation
Growth Factors and Their Receptors
Several growth factors have been identified that can stimulate CM proliferation, especially in conditions when proliferation already occurs spontaneously such as during the embryonic and neonatal stages. These include interleukin-6 (IL-6) [28, 29], platelet-derived growth factor (PDGF) [30], members of the fibroblast growth factor (FGF) family [31, 32], follistatin-like 1 (Fstl1) [33], and neuregulin-1 (NRG1) [34–36]. Paracrine control of CM proliferation was also reported for factors secreted by T-regulatory cells [25] and cardiac resident monocytes [37]. Finally, CM replication was also recently reported to respond to changes in the extracellular matrix, which are mediated agrin [38]. In particular, this factor, which is a component of the neonatal ECM, appears to stimulate CM division through a mechanism that involves the disassembly of the CM membrane-associated dystrophin-glycoprotein complex (DGC) [38].
Signal Transduction Pathways
At least three major signal transduction pathways were shown to participate in the regulation of CM proliferation during the embryonic, foetal, and neonatal life. During embryonic development, CM proliferation is regulated by the Wnt/β-catenin pathway. In the absence of Wnt, the levels of cytoplasmic β-catenin are low since this protein is targeted for degradation by a destruction complex also including glycogen synthase kinase 3β (GSK-3β) [39]. In CMs, inhibition of GSK3-β, through genetic means [40] or by treatment with the inhibitory small molecule BIO [41], results in β-catenin stabilization and translocation to the nucleus, where the protein acts as a transcriptional coactivator of members of the T cell factor (TCF)/Lymphoid enhancer factor (LEF) family of proteins, followed by CM proliferation.
A second signal transduction mechanism relevant to CM proliferation is the Notch pathway. Notch signalling requires cell–cell contact and occurs through engagement of the Notch receptor to one of its ligands, in particular, Jagged1 in the mammalian heart, followed by translocation of the cleaved Notch intracellular domain (ICD) to the nucleus, where it acts as a transcriptional coactivator [42]. Notch regulates proliferation of immature CMs during the foetal and post-natal life [43–45].
A third essential pathway involved in CM proliferation converges in the activation of another transcriptional cofactor, the YAP protein, and its cognate factor TAZ. This is the final positive effector of the inhibitory Hippo pathway. In resting CMs, YAP is maintained inactive by phosphorylation of a kinase cascade, which prevent its nuclear translocation and drives its proteolytic degradation. These kinases include LATS1/2 with its cofactor MOB1 and MST1/2 (Hippo in Drosophila) with its cofactor SAV1. Other inhibitory kinases are TAOK1 and STKL38. Regulation of the Hippo pathway is a main mechanism for CM mechanosensing, which transduces stretch and tension signals from the extracellular environment into the cell nucleus. Genetic deletion of MST1, SAV1, and LATS results in cardiac hyperplasia [46], while overexpression of MST1 [47] or LATS2 [48] leads to post-natal dilated cardiomyopathy. Transgenic overexpression of a constitutively active YAP mutant (YAPS112A) causes CM hyperproliferation [49, 50].
Cell Cycle Regulators
Similar to all cell types, cell cycle regulation in CMs is also governed by a series of positive and negative regulators that converge on cyclin/CDK activation. Work performed over the last several years has shown that overexpression of E2F transcription factor [51–53] or cyclin D1 and D2 [54–56] can lead to CM proliferation. Similar results can be achieved by knocking down the cyclin-dependent kinase inhibitors p21WAF1/CIP1, p27KIP1, and p57KIP2 [57] or Meis1, a homeodomain transcription factor that activates expression of p16INK4a and p21WAF1/CIP1 [58]. The observation that transgenic animals overexpressing cyclin A2 [59, 60], cdk2 [61], cyclin D1 [54], and cyclin D2 [62, 63] have increased CM proliferation is also consistent with these findings.
Stem Cells and Genes for Cardiac Regeneration
Starting from the early 2000s there has been a massive interest in developing innovative strategies for cardiac regeneration. Fuelled by the apparent existence of stem cells in multiple tissues from adult individuals, several clinical attempts were based on the injection, into the infarcted or failing heart, of cells recovered from the bone marrow (unfractionated, immunopurified for c-kit antigen expression or expressing markers of stromal mesenchymal cells [64, 65]) or purified from the heart itself [66, 67]. Despite marginal evidence of clinical benefit in some of these studies due to paracrine effects [68, 69], the current overall consensus is that no proof of actual cardiac regeneration (i.e. formation of new cardiomyocytes and cardiac tissue) was achieved by any of these studies. More in general, no convincing evidence exists that stem cells of any derivation from adult individuals exist that might regenerate the heart [70]. Examination of some of the original studies with c-kit-positive cells has led to the retraction of some of the published findings (http://circ.ahajournals.org/content/129/16/e466.full.pdf+html) and investigation on the integrity of others (10.1016/S0140-6736(14)60608-5).
Embryonic stem cells or iPS cells can be expanded in the laboratory to generate a number of CMs, in the order of 0.5–1 billion, to be directly injected into the heart. This approach has successfully translated into improved cardiac repair in a series of recent studies in infarcted monkeys infused with these cells into the infarcted area [71, 72••]. A more complex approach along the same theme is the ex vivo generation of large patches of myocardial tissue starting form hiPS- or hES-derived CMs based on their integration into 3D contractile tissue [73•, 74]. This is a promising approach to generate contractile tissue, thanks to the spontaneous property of CMs to organize into myocardial-like tissue once subjected to load in the laboratory. The concept of substituting lost cardiac tissue by infusing individual CMs or ex vivo generated cardiac tissue is of intuitive interest, however not devoid of a series of problems. The cells that can be obtained to date from hiPS or hES cells have an embryonic phenotype and generate arrhythmias once injected into the heart; the recipients need to be immunosuppressed to avoid rejection and integration into the cardiac electric and mechanical syncytium is still imperfect. More notably, both CM infusion and cardiac tissue implantation are complex procedures in their nature, which contrasts with the broad demand for cardiac regeneration.
A possibly simpler and translatable possibility is to re-awaken the endogenous potential of CMs to proliferate and thus mimic what spontaneously occurs in the neonatal mammalian heart or in amphibians and fish throughout life. This is quite efficient in transgenic mouse models. For example, mice transgenic for activated YAP or knock out for the inhibitor Mst1 kinase or for the Mst1 co-factor Salvador can regenerate the heart after MI [50, 75, 76••]. For some of the activators, regeneration was also reported when their genes were administered using viral vectors. Among others, this was reported for YAP [77] or the Notch intracellular domain [43, 45]. However, exogenous delivery is not fraught with problems. Permanent overexpression YAP needs to be taken with caution, given the tumour suppressor role that the Hippo pathway exerts in several cancers [78]. In the case of Notch, this pathway is instead inactivated in adult hearts by suppressive epigenetic modifications at Notch-responsive promoters [79]. Finally, CM replication and cardiac regeneration was shown to be achievable by the simultaneous delivery of multiple cell cycle regulators [80]; this approach, however, appears quite problematic for therapeutic applications.
Stimulation of Endogenous Cardiac Regeneration by microRNAs
An appealing strategy to achieve cardiac regeneration is to manipulate the CM proliferative potential by microRNAs (miRNAs). These short, double-stranded RNA molecules control virtually any aspect of cell biology, including proliferation. This also holds true for CMs (reviewed in ref. [81]). The miRNAs stimulating endogenous CM proliferation can be classified into one of three categories. First, several pro-regenerative miRNAs include molecules that are highly expressed in ES cells and are required to maintain pluripotency. These include members of the miR-302~367 and miR-miR-290 families, which share the same seed sequence [82, 83]. Activation of the miR-302-367 cluster after MI in mice induces cardiac regeneration, as does the transient delivery of some of its members as synthetic molecules [84]. A second group of miRNAs that can induce CM regeneration includes a series of miRNAs involved in tumorigenesis. These include the miR-17~92 cluster (also named OncomiR1) [85, 86], and its paralogue clusters miR-106b~25 and miR-106a~363 [87, 88]. Also, in this case, both transgenic expression of the miR-17~92 cluster cluster [89] or the administration of the cluster member miR-19a/19b [90] led to cardiac regeneration. A third group of miRNAs was identified through two large screenings of human miRNAs [91, 92]. The most prominent and studied of these miRNAs is miR-199a-3p, which was shown to stimulate cardiac repair both when expressed from an AAV9 vector [91] or a synthetic RNA molecule [93] in mice and from an AAV6 vector in pigs [94••]. Several other miRNAs are also capable, to a various extent, to induce cardiomyocyte proliferation (extensively reviewed in ref. [81]), indicating that the withdrawal of CMs from the cell cycle at birth is not an irreversible process but a regulated one, with the possibility of reverting it through the manipulation of gene expression.
The identification of miRNAs with the potential to stimulate cardiac regeneration appears particularly appealing for translational purposes, as these molecules can be developed as treatments fitting virtually all patients and do not require extensive ex vivo manipulation, as instead is required for stem cell applications. MicroRNAs can be delivered either as expressed from viral vectors or as synthetic molecules. In the former case, AAV vectors now appear as the vectors of choice for cardiac gene delivery [95]. Using AAVs to deliver genes promoting cardiac regeneration, however, is not devoid of problems. These vectors persist virtually indefinitely in CMs, while, to date, no promoter has been described in humans or non-human primates from which expression can be turned off. This creates obvious safety issues and efficacy concerns as far as CMs are concerned, as the stimulation of proliferation in these cells requires the transient disassembly of the sarcomeric apparatus and cell dedifferentiation [22, 96, 97]. There appears to be a need, therefore, to apply the pro-proliferative stimulus transitorily. In addition, endogenous transcription of a miRNA gene is followed by processing of the pri-miRNA by the cellular RNAi machinery. This leads to the generation of both miRNA strands, which can be not necessarily desirable. These problems surfaced in the only large animal study so far performed to test the efficacy of one miRNA, miR-199a, to drive cardiac regeneration in pigs [94••]. In this study, cardiac regeneration and improvement of cardiac function was very significant at 1 month after treatment of infarcted pigs; however, several animals developed fatal arrhythmias at longer times. The hearts of the treated animals showed the presence of proliferating and undifferentiated cells, along with the expression of both the pro-regenerative miR-199a-3p strand but also of its complementary miR-199a-5p strand, which is known to exert undesirable effects in the heart [94••].
One of the possible strategies to overcome this limitation of AAV-mediated gene delivery is to resort to the possibility of administering the miRNAs as synthetic RNA molecules. Already available evidence shows that a single intramyocardial injection miR-199a-3p or miR-590-3p mimics using a lipofectamine-based formulation can stimulate a regenerative response in mice [93]. Similar results were also obtained by a single intramyocardial injection of miR-19a/19b [90] or miR-302b/c mimics [98] or the daily intravenous administration of miR302b/c [84], miR-19a/19b [90], or miR-708 [99].
Conclusions
The notion that regeneration of the cardiac muscle can be achieved by either the implantation of ex vivo generated CMs or through the stimulation of the proliferative capacity of endogenous cells to proliferate remains exciting. It challenges a long-standing dogma that damage in post-mitotic tissue is intrinsically irreversible and offers hope for treatment to the vast number of patients with post-ischemic HF. Both the stem-cell and the endogenous regeneration approaches, however, still require significant improvement before extensive clinical application. In the case of stem cells, ES- or iPS-derived CMs are still immature, their electrical and mechanical coupling to endogenous CM is limited and the process of deriving the large number of cells required for each patient is demanding for scaling up. Maturation appears improved when CMs are used to form ex vivo, 3D cardiac tissue; however, the problem of integrating large patches of engineered myocardium into the preexisting one still remains.
In the case of endogenous cardiac regeneration, this approach appears more amenable to application in a large number of patients and less demanding in terms of development. However, a main problem remains the efficacy of delivery of genes or miRNAs to the infarcted myocardium. The experiments with lipid-mediated delivery in mice appear promising, but translation of these findings into large animals will require extensive experimentation. In this respect, it is however exciting to note that the field of small RNA delivery has made substantial progress over the last decade, with the generation of lipid nanoparticles (LNPs) with neutral surface charge, and of methods to load small nucleic acids into these particles [100]. The first LNP delivering an RNAi therapeutic molecule to reach the market was patisiran in 2018—this drug lowers the hepatic levels of transthyretin for the treatment of hereditary transthyretin-induced amyloidosis [101]. Several other LNP formulations of siRNAs and miRNAs for other applications are in the pipeline of clinical experimentation. Whether an LNP delivering one of the pro-proliferative miRNAs for CMs might be effective in inducing cardiac regeneration in large animals and is thus amenable to clinical translation awaits the results of ongoing experimentation.
Funding Information
This work was supported by the European Research Council (ERC) Advanced Grant 787971 “CuRE,” by the British Heart Foundation (BHF) Programme Grant RG/19/11/34633 and the King’s College London BHF Centre of Research Excellence grant RE/18/2/34213 and by grants 825670 “CardioReGenix” and 874764 “REANIMA” from the European Commission Horizon 2020 programme, along with the continuous support of Fondazione CRTrieste, Trieste, Italy.
Compliance with Ethical Standards
Conflict of Interest
The author declares that no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Myocardial Disease
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Birks EJ. Molecular changes after left ventricular assist device support for heart failure. Circ Res. 2013;113(6):777–791. doi: 10.1161/CIRCRESAHA.113.301413. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG, American Heart Association Advocacy Coordinating Committee. Council on Arteriosclerosis, Thrombosis and Vascular Biology. Council on Cardiovascular Radiology and Intervention. Council on Clinical Cardiology. Council on Epidemiology and Prevention. Stroke Council Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L, Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, el Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, el Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and National Burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb SS, Dickstein K, Fleck E, Kostis J, Levine TB, LeJemtel T, DeKock M. Hemodynamic and neurohormonal effects of the angiotensin II antagonist losartan in patients with congestive heart failure. Circulation. 1993;88(4 Pt 1):1602–1609. doi: 10.1161/01.cir.88.4.1602. [DOI] [PubMed] [Google Scholar]
- 7.Califf RM. LCZ696: too good to be true? Eur Heart J. 2015;36(7):410–412. doi: 10.1093/eurheartj/ehu501. [DOI] [PubMed] [Google Scholar]
- 8.Kaye DM, Krum H. Drug discovery for heart failure: a new era or the end of the pipeline? Nat Rev Drug Discov. 2007;6(2):127–139. doi: 10.1038/nrd2219. [DOI] [PubMed] [Google Scholar]
- 9.Packer M. The future treatment of heart failure? Eur Heart J. 2018;39(1):5–7. doi: 10.1093/eurheartj/ehx745. [DOI] [PubMed] [Google Scholar]
- 10.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47(9):1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 12.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26(4):1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 13.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am J Phys. 1997;272(1 Pt 2):H220–H226. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 16.Sedmera D, Reckova M, DeAlmeida A, Coppen SR, Kubalak SW, Gourdie RG, et al. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat Rec A Discov Mol Cell Evol Biol. 2003;274(1):773–777. doi: 10.1002/ar.a.10085. [DOI] [PubMed] [Google Scholar]
- 17.Ye L, D'Agostino G, Loo SJ, Wang CX, Su LP, Tan SH, et al. Early regenerative capacity in the porcine heart. Circulation. 2018;138(24):2798–2808. doi: 10.1161/CIRCULATIONAHA.117.031542. [DOI] [PubMed] [Google Scholar]
- 18.Haubner BJ, Schneider J, Schweigmann U, Schuetz T, Dichtl W, Velik-Salchner C, Stein JI, Penninger JM. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ Res. 2016;118(2):216–221. doi: 10.1161/CIRCRESAHA.115.307017. [DOI] [PubMed] [Google Scholar]
- 19.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 20.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187(2):249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, MacRae CA, Stainier DYR, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canseco DC, Kimura W, Garg S, Mukherjee S, Bhattacharya S, Abdisalaam S, Das S, Asaithamby A, Mammen PPA, Sadek HA. Human ventricular unloading induces cardiomyocyte proliferation. J Am Coll Cardiol. 2015;65(9):892–900. doi: 10.1016/j.jacc.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet SW, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Asaithamby A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157(3):565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zacchigna S, Martinelli V, Moimas S, Colliva A, Anzini M, Nordio A, Costa A, Rehman M, Vodret S, Pierro C, Colussi G, Zentilin L, Gutierrez MI, Dirkx E, Long C, Sinagra G, Klatzmann D, Giacca M. Paracrine effect of regulatory T cells promotes cardiomyocyte proliferation during pregnancy and after myocardial infarction. Nat Commun. 2018;9(1):2432. doi: 10.1038/s41467-018-04908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, Lunn D, Bigley RB, Yu H, Wang J, Smith M, Gillett E, Muroy SE, Schmid T, Wilson E, Field KA, Reeder DM, Maden M, Yartsev MM, Wolfgang MJ, Grützner F, Scanlan TS, Szweda LI, Buffenstein R, Hu G, Flamant F, Olgin JE, Huang GN. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science. 2019;364(6436):184–188. doi: 10.1126/science.aar2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56(2):130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 28.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, Gise A, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, Pu WT. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121(5):1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Przybyt E, Krenning G, Brinker MG, Harmsen MC. Adipose stromal cells primed with hypoxia and inflammation enhance cardiomyocyte proliferation rate in vitro through STAT3 and Erk1/2. J Transl Med. 2013;11:39. doi: 10.1186/1479-5876-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinrichsen R, Haunso S, Busk PK. Different regulation of p27 and Akt during cardiomyocyte proliferation and hypertrophy. Growth Factors. 2007;25(2):132–140. doi: 10.1080/08977190701549835. [DOI] [PubMed] [Google Scholar]
- 31.Kardami E, Banerji S, Doble BW, Dang X, Fandrich RR, Jin Y, Cattini PA. PKC-dependent phosphorylation may regulate the ability of connexin43 to inhibit DNA synthesis. Cell Commun Adhes. 2003;10(4–6):293–297. doi: 10.1080/cac.10.4-6.293.297. [DOI] [PubMed] [Google Scholar]
- 32.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103(42):15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525(7570):479–485. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273(17):10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 35.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 36.D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17(5):627–638. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- 37.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111(45):16029–16034. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, Tzahor E. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547(7662):179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, MacDonald BT, Zhang X, Abreu JG, Peng L, He X. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340(6134):867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh AP, Umbarkar P, Guo Y, Force T, Gupte M, Lal H. Inhibition of GSK-3 to induce cardiomyocyte proliferation: a recipe for in situ cardiac regeneration. Cardiovasc Res. 2019;115(1):20–30. doi: 10.1093/cvr/cvy255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006;13(9):957–963. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 42.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of notch intracellular domain. Nature. 1999;398(6727):518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 43.Collesi C, Zentilin L, Sinagra G, Giacca M. Notch1 signaling stimulates proliferation of immature cardiomyocytes. J Cell Biol. 2008;183(1):117–128. doi: 10.1083/jcb.200806091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Croquelois A, Domenighetti AA, Nemir M, Lepore M, Rosenblatt-Velin N, Radtke F, Pedrazzini T. Control of the adaptive response of the heart to stress via the Notch1 receptor pathway. J Exp Med. 2008;205(13):3173–3185. doi: 10.1084/jem.20081427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campa VM, Gutierrez-Lanza R, Cerignoli F, Diaz-Trelles R, Nelson B, Tsuji T, et al. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J Cell Biol. 2008;183(1):129–141. doi: 10.1083/jcb.200806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto S, Yang G, Zablocki D, Liu J, Hong C, Kim SJ, Soler S, Odashima M, Thaisz J, Yehia G, Molina CA, Yatani A, Vatner DE, Vatner SF, Sadoshima J. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111(10):1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsui YY, Nakano NN, Shao DD, Gao SS, Luo WW, Hong CC, Zhai P, Holle E, Yu X, Yabuta N, Tao W, Wagner T, Nojima H, Sadoshima J. Lats2 is a negative regulator of myocyte size in the heart. Circ Res. 2008;103(11):1309–1318. doi: 10.1161/CIRCRESAHA.108.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4(196):ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110(34):13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirshenbaum LA, Abdellatif M, Chakraborty S, Schneider MD. Human E2F-1 reactivates cell cycle progression in ventricular myocytes and represses cardiac gene transcription. Dev Biol. 1996;179(2):402–411. doi: 10.1006/dbio.1996.0270. [DOI] [PubMed] [Google Scholar]
- 52.Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, Schneider MD. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest. 1997;100(11):2722–2728. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Amerongen MJ, Diehl F, Novoyatleva T, Patra C, Engel FB. E2F4 is required for cardiomyocyte proliferation. Cardiovasc Res. 2010;86(1):92–102. doi: 10.1093/cvr/cvp383. [DOI] [PubMed] [Google Scholar]
- 54.Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, Kim KK, Field LJ. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997;99(11):2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamamori-Adachi M, Ito H, Sumrejkanchanakij P, Adachi S, Hiroe M, Shimizu M, Kawauchi J, Sunamori M, Marumo F, Kitajima S, Ikeda MA. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res. 2003;92(1):e12–e19. doi: 10.1161/01.res.0000049105.15329.1c. [DOI] [PubMed] [Google Scholar]
- 56.Busk PK, Hinrichsen R, Bartkova J, Hansen AH, Christoffersen TE, Bartek J, et al. Cyclin D2 induces proliferation of cardiac myocytes and represses hypertrophy. Exp Cell Res. 2005;304(1):149–161. doi: 10.1016/j.yexcr.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 57.Di Stefano V, Giacca M, Capogrossi MC, Crescenzi M, Martelli F. Knockdown of cyclin-dependent kinase inhibitors induces cardiomyocyte re-entry in the cell cycle. J Biol Chem. 2011;286(10):8644–8654. doi: 10.1074/jbc.M110.184549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497(7448):249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, Wolgemuth DJ. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem. 2004;279(34):35858–35866. doi: 10.1074/jbc.M404975200. [DOI] [PubMed] [Google Scholar]
- 60.Woo YJ, Panlilio CM, Cheng RK, Liao GP, Atluri P, Hsu VM, Cohen JE, Chaudhry HW. Therapeutic delivery of cyclin A2 induces myocardial regeneration and enhances cardiac function in ischemic heart failure. Circulation. 2006;114(1 Suppl):I206–I213. doi: 10.1161/CIRCULATIONAHA.105.000455. [DOI] [PubMed] [Google Scholar]
- 61.Liao HS, Kang PM, Nagashima H, Yamasaki N, Usheva A, Ding B, Lorell BH, Izumo S. Cardiac-specific overexpression of cyclin-dependent kinase 2 increases smaller mononuclear cardiomyocytes. Circ Res. 2001;88(4):443–450. doi: 10.1161/01.res.88.4.443. [DOI] [PubMed] [Google Scholar]
- 62.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res. 2005;96(1):110–118. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 63.Hassink RJ, Pasumarthi KB, Nakajima H, Rubart M, Soonpaa MH, de la Riviere AB, Doevendans PA, Field LJ. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res. 2008;78(1):18–25. doi: 10.1093/cvr/cvm101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 65.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 67.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Zimmet H, Porapakkham P, Porapakkham P, Sata Y, Haas SJ, Itescu S, Forbes A, Krum H. Short- and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Eur J Heart Fail. 2012;14(1):91–105. doi: 10.1093/eurjhf/hfr148. [DOI] [PubMed] [Google Scholar]
- 69.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116(8):1413–1430. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisen J, et al. Cardiomyocyte regeneration: a consensus statement. Circulation. 2017;136(7):680–686. doi: 10.1161/CIRCULATIONAHA.117.029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018;36(7):597–605. doi: 10.1038/nbt.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao ML, et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation. 2017;135(19):1832–1847. doi: 10.1161/CIRCULATIONAHA.116.024145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med. 2016;8(363):363ra148. doi: 10.1126/scitranslmed.aaf8781. [DOI] [PubMed] [Google Scholar]
- 75.Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140(23):4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leach JP, Heallen T, Zhang M, Rahmani M, Morikawa Y, Hill MC, et al. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature. 2017;550(7675):260–264. doi: 10.1038/nature24045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PRR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115(3):354–363. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kakiuchi-Kiyota S, Schutten MM, Zhong Y, Crawford JJ, Dey A. Safety considerations in the development of hippo pathway inhibitors in cancers. Front Cell Dev Biol. 2019;7:156. doi: 10.3389/fcell.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Felician G, Collesi C, Lusic M, Martinelli V, Ferro MD, Zentilin L, Zacchigna S, Giacca M. Epigenetic modification at Notch responsive promoters blunts efficacy of inducing Notch pathway reactivation after myocardial infarction. Circ Res. 2014;115(7):636–649. doi: 10.1161/CIRCRESAHA.115.304517. [DOI] [PubMed] [Google Scholar]
- 80.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell. 2018;173(1):104–116. doi: 10.1016/j.cell.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Braga L, Ali H, Secco I, Giacca M. Non-coding RNA therapeutics for cardiac regeneration. Cardiovasc Res. 2020. 10.1093/cvr/cvaa071. [DOI] [PMC free article] [PubMed]
- 82.Barroso-del Jesus A, Lucena-Aguilar G, Menendez P. The miR-302-367 cluster as a potential stemness regulator in ESCs. Cell Cycle. 2009;8(3):394–398. doi: 10.4161/cc.8.3.7554. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40(12):1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med. 2015;7(279):279ra38. doi: 10.1126/scitranslmed.3010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gruszka R, Zakrzewska M. The oncogenic relevance of miR-17-92 cluster and its paralogous miR-106b-25 and miR-106a-363 clusters in brain tumors. Int J Mol Sci. 2018;19(3):879. doi: 10.3390/ijms19030879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mehlich D, Garbicz F, Wlodarski PK. The emerging roles of the polycistronic miR-106b approximately 25 cluster in cancer—a comprehensive review. Biomed Pharmacother. 2018;107:1183–1195. doi: 10.1016/j.biopha.2018.08.097. [DOI] [PubMed] [Google Scholar]
- 89.Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, Pu WT, Liao R, Wang DZ. Mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013;112(12):1557–1566. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao F, Kataoka M, Liu N, Liang T, Huang ZP, Gu F, Ding J, Liu J, Zhang F, Ma Q, Wang Y, Zhang M, Hu X, Kyselovic J, Hu X, Pu WT, Wang J’, Chen J, Wang DZ. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat Commun. 2019;10(1):1802. doi: 10.1038/s41467-019-09530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492(7429):376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 92.Diez-Cunado M, Wei K, Bushway PJ, Maurya MR, Perera R, Subramaniam S, et al. miRNAs that induce human cardiomyocyte proliferation converge on the hippo pathway. Cell Rep. 2018;23(7):2168–2174. doi: 10.1016/j.celrep.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lesizza P, Prosdocimo G, Martinelli V, Sinagra G, Zacchigna S, Giacca M. Single-dose intracardiac injection of pro-regenerative microRNAs improves cardiac function after myocardial infarction. Circ Res. 2017;120(8):1298–1304. doi: 10.1161/CIRCRESAHA.116.309589. [DOI] [PubMed] [Google Scholar]
- 94.Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature. 2019;569(7756):418–422. doi: 10.1038/s41586-019-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zacchigna S, Zentilin L, Giacca M. Adeno-associated virus vectors as therapeutic and investigational tools in the cardiovascular system. Circ Res. 2014;114(11):1827–1846. doi: 10.1161/CIRCRESAHA.114.302331. [DOI] [PubMed] [Google Scholar]
- 96.Aguirre A, Montserrat N, Zacchigna S, Nivet E, Hishida T, Krause MN, Kurian L, Ocampo A, Vazquez-Ferrer E, Rodriguez-Esteban C, Kumar S, Moresco JJ, Yates JR, III, Campistol JM, Sancho-Martinez I, Giacca M, Izpisua Belmonte JC. In vivo activation of a conserved microRNA program induces mammalian heart regeneration. Cell Stem Cell. 2014;15(5):589–604. doi: 10.1016/j.stem.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, Cavanaugh CA, Zhou S, Kanade R, Atluri P, Morrisey EE, Burdick JA. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng. 2017;1:983–992. doi: 10.1038/s41551-017-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deng S, Zhao Q, Zhen L, Zhang C, Liu C, Wang G, Zhang L, Bao L, Lu Y, Meng L, Lü J, Yu P, Lin X, Zhang Y, Chen YH, Fan H, Cho WC, Liu Z, Yu Z. Neonatal heart-enriched miR-708 promotes proliferation and stress resistance of cardiomyocytes in rodents. Theranostics. 2017;7(7):1953–1965. doi: 10.7150/thno.16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kulkarni JA, Cullis PR, van der Meel R. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 2018;28(3):146–157. doi: 10.1089/nat.2018.0721. [DOI] [PubMed] [Google Scholar]
- 101.Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary Transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]