Abstract
The unique symbiosis between a vertebrate salamander, Ambystoma maculatum, and unicellular green alga, Oophila amblystomatis, involves multiple modes of interaction. These include an ectosymbiotic interaction where the alga colonizes the egg capsule, and an intracellular interaction where the alga enters tissues and cells of the salamander. One common interaction in mutualist photosymbioses is the transfer of photosynthate from the algal symbiont to the host animal. In the A. maculatum–O. amblystomatis interaction, there is conflicting evidence regarding whether the algae in the egg capsule transfer chemical energy captured during photosynthesis to the developing salamander embryo. In experiments where we took care to separate the carbon fixation contributions of the salamander embryo and algal symbionts, we show that inorganic carbon fixed by A. maculatum embryos reaches 2% of the inorganic carbon fixed by O. amblystomatis algae within an egg capsule after 2 h in the light. After 2 h in the dark, inorganic carbon fixed by A. maculatum embryos is 800% of the carbon fixed by O. amblystomatis algae within an egg capsule. Using photosynthesis inhibitors, we show that A. maculatum embryos and O. amblystomatis algae compete for available inorganic carbon within the egg capsule environment. Our results confirm earlier studies suggesting a role of heterotrophic carbon fixation during vertebrate embryonic development. Our results also show that the considerable capacity of developing A. maculatum embryos for inorganic carbon fixation precludes our ability to distinguish any minor role of photosynthetically transferred carbon from algal symbionts to host salamanders using bicarbonate introduced to the egg system as a marker.
Keywords: symbiosis, carbon fixation, photosymbiosis, mutualism, heterotroph, alga, salamander, embryo
Introduction
During embryonic development, egg capsules of multiple amphibian species found in the Northern Hemisphere are colonized by a green alga, Oophila amblystomatis. Particular attention has been given to the conspicuous association between the common spotted salamander of North America (Ambystoma maculatum) and its O. amblystomatis symbiont (Orr, 1888; Gilbert, 1942). While recent studies have focused on the unique facultative endosymbiotic association of algal cells inside the embryo host (Kerney et al., 2011, 2019; Burns et al., 2017), there is a long history of research into the ecto-symbiotic association between free-living Oophila inside the embryonic egg capsule (Kerney, 2011). This intracapsular Oophila has a role in oxygenating the egg capsule microenvironment (Gilbert, 1942, 1944; Bachmann et al., 1986; Pinder and Friet, 1994; Mills and Barnhart, 1999; Bianchini et al., 2012) and potentially in removal of nitrogenous waste from the host (Goff and Stein, 1978; Bianchini et al., 2012; Small et al., 2014). Other modes of interaction between alga and embryo during the ecto-symbiotic stage of this association are relatively unexplored. One intriguing possibility is that the intracapsular Oophila fixes carbon from the atmosphere, uses energy from the sun to build fixed carbon into energy storage molecules like carbohydrates, and transfers that chemical energy to the salamander by exporting metabolically active compounds (Hammen and Hutchison, 1962; Goff and Stein, 1978; Graham et al., 2013, 2014). Such a mechanism is at play in other animal-alga photosymbioses, such as the coral-dinoflagellate mutualism, where the photosymbiont captures and transfers energy to the animal in the form of sugars and sugar alcohols like glucose and glycerol in nutrient-poor waters (Venn et al., 2008; Tremblay et al., 2012; Raven, 2017). However, none of these parallel naturally occurring animal-algal photosymbioses include a vertebrate host.
There is an under-appreciated controversy in the published literature on metabolite transfer within the Oophila – A. maculatum symbiosis. Recent studies have found evidence of 14C-labeled photosynthate transfer from Oophila to their embryonic spotted-salamander hosts (Graham et al., 2013, 2014). However, an earlier study (Hammen and Hutchison, 1962), which also used a 14C label to detect intracapsular Oophila photosynthate transfer, came to the opposite conclusion: “The results of these experiments indicate that the facultative mutualism of Ambystoma embryos and the alga Oophila is not simply one of photosynthetic carbon dioxide fixation by the alga with subsequent transport of labeled carbohydrate to the embryo” (Hammen and Hutchison, 1962). Instead, this earlier report found that the embryos themselves were fixing a considerable amount of inorganic carbon, in the form of either carbon dioxide or bicarbonate ions. The possibility of heterotrophic carbon fixation was not explored by either recent paper and may be a confounding variable in their analysis and subsequent conclusions.
In aquatic systems, carbon dioxide (CO2) and bicarbonate () are critical for energy storage and central metabolism (Brinson et al., 1981; Boston et al., 1989). Heterotrophs primarily get the carbon they need from organic molecules like sugars and lipids while plants and algae collect carbon from the air or water and build it into the requisite organic compounds (Allen et al., 2005). There are, however, several metabolic processes common to both plants and animals that require inorganic carbon in the form of carbon dioxide or bicarbonate as substrates. Those include the urea cycle where the initial step of ammonia removal involves combining ammonium ions with bicarbonate and ATP by the enzyme carbamoyl phosphate synthetase to eventually remove the nitrogen in urea (Holden et al., 1999); the citric acid cycle where the enzyme pyruvate carboxylase combines carbon dioxide with pyruvate to produce oxaloacetate to fill in TCA intermediates (Jitrapakdee et al., 2006); and fatty acid biosynthesis where the first committed step involves adding bicarbonate to acetyl-CoA to produce malonyl-CoA, the fatty acid building block precursor (Blanchard and Waldrop, 1998).
Heterotrophic carbon fixation was first described by Harlan Wood in his studies of propionibacteria (Wood et al., 1941) and later in animals through his work on pigeon liver physiology (Wood et al., 1945; Kresge et al., 2005). The discovery of heterotrophic carbon fixation was at first met with skepticism, but later revealed in multiple bacterial and animal systems (Kresge et al., 2005). While there are six canonical autotrophic pathways to carbon fixation (Berg, 2011), many additional carboxylases have been characterized in heterotrophic cellular physiology (Erb, 2011). The scale of these “dark carbon fixation” pathways are often overlooked and may have considerable bearing on models of global carbon cycling (Baltar and Herndl, 2019). Coincidentally, many of the animal systems that revealed metazoan carbon fixation utilized amphibian embryos (Biggers and Bellve, 1974). Several definitive studies on carbon fixation by frog embryos were performed by Nobel laureate Stanley Cohen (1954, 1963), who later went on to discover nerve and epidermal growth factors (Shampo, 1999). Interestingly, the study of heterotrophic carbon fixation in amphibian embryos has received little research attention since the 1970’s despite decades of biochemical and molecular research into the mechanisms of development (Elinson and del Pino, 2011) and parallel research attention into the mechanisms of carbon fixation (Gong et al., 2016).
In amphibians, carbon fixation was noted during early development of embryos of several frog species in the genus Rana (Cohen, 1954, 1963; Flickinger, 1954) and in the European newt Triton (Tiedemann and Tiedemann, 1954). Those early studies generally concluded that in early development, carbon fixation proceeded via the action of carbamoyl phosphate synthetase, feeding into both de novo pyrimidine biosynthesis and nascent mRNA production, as well as directly into the urea cycle (Biggers and Bellve, 1974). Carbon fixation has been noted in other vertebrates as well, including fish (Mounib and Eisan, 1969) and mammals (Wales et al., 1969). In whole animals and embryos, the bulk of carbon fixation was observed to be tissue-specific reflecting the metabolic demands of different cell types (Biggers and Bellve, 1974).
Here, we revisit carbon fixation and translocation experiments taking care to separate algal and salamander contributions. We found that heterotrophic carbon fixation by salamander embryos is considerable and precludes our ability to distinguish metabolite transfer of algal-fixed carbon from heterotrophic carbon fixation by the salamander using bulk measurements of whole embryos or egg capsules. Our results are incompatible with the notion that a measurable quantity of photosynthetically fixed carbon is transferred from alga to salamander in the egg capsule and are in agreement with the results from Hammen and Hutchison, 1962. These results further indicate that more precise imaging of labeled carbon is required to definitively reveal whether algal metabolites are transferred to the host during the extracellular and intracellular portions of this symbiosis.
Materials and Methods
Egg Mass Collection and Maintenance
Ambystoma maculatum egg clutches were collected from Michaux State Forest in central Pennsylvania (PA Fish and Boat Commission permit PA-727 type A) and Castamine Maine in March/April, 2019 (ME Department of Inland Fisheries and Wildlife permit 2020-590). A. maculatum egg clutches were maintained in modified Holtfreter’s solution (15 mM NaCl, 0.6 mM NaHCO3, 0.2 mM KCl, 0.2 mM CaCl2, 0.2 mM MgSO4⋅7H20) at 4°C to control the rate of development of the salamander embryos and with constant light to maintain intracapsular algae. Prior to carbon fixation experiments, egg clutches were transferred to 18°C with a 12 h/12 h light/dark cycle for several days to facilitate development (Hammen and Hutchison, 1962; Graham et al., 2013, 2014). Development was monitored by visual inspection using a binocular microscope. When embryos reached Harrison stage 32–34 (Harrison, 1969; Figure 1), individual eggs were removed from the jelly mass. Algal presence was confirmed by the green color of individual eggs.
FIGURE 1.
Typical stage 33 A. maculatum embryo from these experiments. (A) Single egg capsule containing stage 33 embryo. Green hue is from intracapsular algae. (B) Decapsulated stage 33 embryo. Scale bar: 1 mm.
14C Bicarbonate Incubation
Basic experimental design and methods for 14C bicarbonate incubation experiments are visualized in Supplementary Figure S1.
Whole Eggs
For experiments with whole eggs, individual eggs were removed from the jelly mass by gentle manipulation with gloved hands, and transferred to individual scintillation vials in 2.5 ml modified Holtfreter’s solution without sodium bicarbonate per egg. To each egg, 1.5 μl of 14C sodium bicarbonate [Perkin Elmer, 37 MBq/mL] was added. Adapting the protocol from Graham et al. (2014), five individual eggs per experiment were incubated in the light or dark for 2 h. Following the initial incubation with 14C bicarbonate, eggs were removed from the solution and rinsed with ultra-pure water to remove unincorporated 14C bicarbonate from outside the egg. Following the rinse, eggs were transferred to 2.5 ml modified Holtfreter’s solution (not radioactive) per egg and incubated in the dark for 2 additional hours. After the dark incubation, eggs were pierced with jeweler’s forceps, and the embryo was removed. Intracapsular fluid, including all algae, and the egg membrane were retained in one scintillation vial. Embryos were washed three times with Holtfreter’s solution without sodium bicarbonate to remove carry-over algae and were placed into a separate scintillation vial. Embryos were homogenized in 500 μl of 2M HCl and incubated for 24 h in an open vial in a chemical fume hood to remove unincorporated sodium bicarbonate as CO2. 500 μl of 2M HCl was added to the algal fraction, which was also incubated for 24 h in an open vial in a chemical fume hood to remove unincorporated sodium bicarbonate. After 24 h incubation, 10 mL of scintillation cocktail (Perkin Elmer, Ultima Gold LLT) was added to all scintillation vials before vortexing, and radioactivity was assayed using a Perkin Elmer Tri-Carb 3110TR scintillation counter. Results are reported as disintegrations per minute (DPM) as a measure of 14C incorporation.
Decapsulated Embryo Experiments
For experiments where embryos were removed from eggs prior to incubation with 14C bicarbonate, individual eggs were removed from the jelly mass by gentle manipulation with gloved hands. Eggs were pierced with jeweler’s forceps and embryos were transferred to clean Holtfreter’s solution without bicarbonate; algae were discarded. Embryos were transferred through clean Holtfreter’s solution without bicarbonate three times to remove exogenous algae. Individual decapsulated and washed embryos were transferred to scintillation vials containing 2.5 ml modified Holtfreter’s solution without bicarbonate. Controls consisting of killed embryos were completed by transferring decapsulated, washed embryos to a solution of 0.6× PBS containing 2.5% (w/v) glutaraldehyde and incubating the embryos in that solution for 15 min at room temperature. Following incubation in glutaraldehyde, embryos were washed three times in Holtfreter’s solution without bicarbonate.
Cultured algae were labeled by adding 300 μL of 14C bicarbonate (at 37 MBq/mL) to 100 mL of Oophila algae in exponential growth in AF6 medium (at around 200,000 cells per mL) and incubating algae with 14C bicarbonate for 4 h at 16°C under 260 μmol photons/m2/s. Following incubation, algae were transferred to 50 mL falcon tubes and centrifuged at 300 × g for 8 min. The supernatant was discarded and algae were resuspended in 20 mL complete Holtfreter’s solution. Algae were pelleted a second time at 300 × g for 8 min, and then resuspended in 1 mL complete Holtfreter’s solution. In parallel, unlabeled algae were subjected to the same centrifugation and wash procedure. Unlabeled algae from the resuspended pellet were counted on a hemocytometer.
For experiments with pre-labeled algae, a volume of 14C labeled algae approximately equivalent to 150,000 algal cells was added to live and glutaraldehyde killed decapsulated and washed embryos. Live and dead embryos were incubated with 14C bicarbonate (1.5 μL at 37 MBq/mL per embryo in 2.5 mL Holtfreter’s without bicarbonate) or 14C labeled algae for 2 h in the dark at 16°C. Following incubation with 14C sodium bicarbonate or 14C labeled algae, embryos were washed three times with unlabeled Holtfreter’s solution, transferred to individual scintillation vials, crushed in 500 μL of 2M HCl, incubated for 24+ hours in open vials in a chemical fume hood to remove unincorporated bicarbonate, mixed with 10 mL scintillation cocktail, and subjected to scintillation counting as described above.
Photosynthesis Inhibitor Experiments
Individual eggs were removed from the jelly mass by gentle manipulation with gloved hands. Six eggs per experiment were placed together in a 50 mL falcon tube in 15 mL modified Holtfreter’s solution without sodium bicarbonate. Eggs were pre-incubated in the light at 16°C with two photosynthesis inhibitors, DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) and DBMIB (2,5-dibromo-3-methyl-6-isopropylbenzoquinone) at 20 μM final concentration for 1 h. Following pre-incubation, 9 μl of 14C sodium bicarbonate, was added to each 50 mL Falcon tube containing six eggs. Eggs were incubated in the light or dark for 2 h. Following incubation, eggs were removed from the Falcon tubes, washed with ultra-pure water, opened with jeweler’s forceps, and separated into individual scintillation vials. Intracapsular fluid with algae and the egg membrane from one egg were collected in one vial, each embryo was washed three times with Holtfreter’s solution and placed in a separate scintillation vial. Algae and embryos were processed as described above for scintillation counting.
Statistical Analyses
Statistical analyses were performed in the R programming language (R Core Team, 2017). Plots were generated using the ggplot2 package (Wickham, 2016). One-way ANOVA followed by the non-parametric Games-Howell post hoc test (used due to violation of homogeneity of variance in the data) was performed using the “userfriendlyscience” package (Peters, 2018).
Results
A. maculatum Embryos Fix Carbon
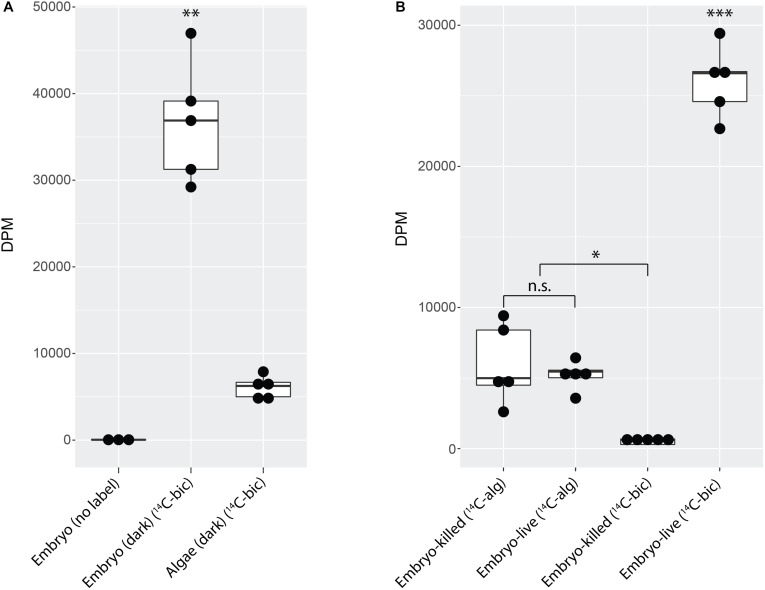
Decapsulated A. maculatum embryos (Figure 1B) incubated in the dark incorporated inorganic carbon into their tissues as acid stable forms (Figure 2A) while control embryos had no such signal (p < 0.01). Decapsulated Embryos incubated in the dark exhibited significantly more 14C-bicarbonate incorporation than total algae from whole eggs (as in Figure 1A) incubated in the dark (p < 0.01) indicating that the elevated radioactive signal in the embryos could not have come from residual algae on or near the dark incubated embryos (Figure 2A).
FIGURE 2.
A. maculatum embryos fix carbon. Box and whisker plots showing raw data (dark circles) range (thin lines) averages (thick horizontal lines) and upper and lower quartiles (box). In each plot, the y-axis represents radioactivity assayed per sample (DPM, disintegrations per minute); the x-axis represents the treatments: (A) embryo or algae incubated in the dark without (no label) or with 14C-bicarbonate (14C-bic). (B) Glutaraldehyde-killed or live embryo (embryo-killed and embryo-live, respectively) incubated in the light with 14C-labeled alga (14C-alg) or with 14C-bicarbonate. n.s. = not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Significance levels were determined by ANOVA followed by the Games-Howell post-hoc test.
To explicitly test whether residual algae that persisted through wash steps could account for the fixed carbon signal from embryos, 14C-bicarbonate or pre-labeled O. amblystomatis cultures were added to live or glutaraldehyde killed A. maculatum embryos. When incubated with 14C-bicarbonate, live embryos exhibited an elevated fixed carbon signal compared to glutaraldehyde killed embryos (p < 0.05). There was no significant difference between live or dead embryos when incubated with pre-labeled algae (p = 0.9) (Figure 2B). The result suggests that some algae persist through the washes but living A. maculatum embryos do not actively accrue additional measurable photosynthate from lab-cultured algae in these conditions. We note that although cultured algae are in a different transcriptional state from intracapsular algae (Kerney et al., 2019), lab-cultured algae are able to interact with and invade A. maculatum embryos ex situ, similar to algae in the intracapsular environment (Kerney et al., 2019). In this study, decapsulated embryos incubated in the dark accumulated significantly more fixed carbon (Figure 2B) compared to embryos incubated with pre-labeled algae (p < 0.001) or embryos killed with glutaraldehyde prior to incubation with bicarbonate (p < 0.001).
A. maculatum Embryos Compete With O. amblystomatis Algae for Bicarbonate
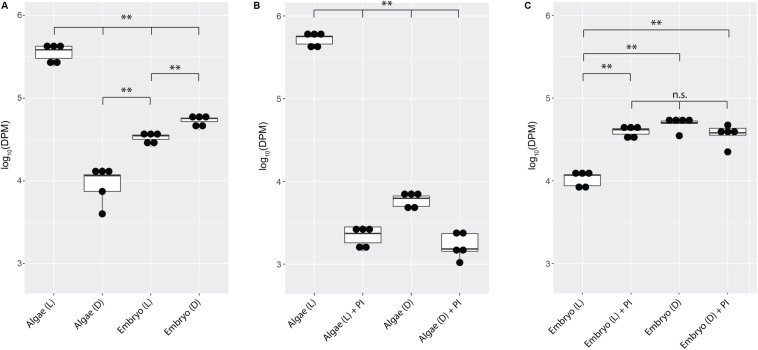
Embryos from whole eggs incubated in the dark fixed significantly more carbon than embryos from whole eggs incubated in the light (Figure 3A, p < 0.01). To test whether this was due to greater inorganic carbon availability when algae are not actively photosynthesizing (i.e., in the dark), photosynthesis inhibitors were added to the eggs in the light and the dark. Chemical inhibition of algal photosynthesis in the light decreased carbon fixation in the algae (Figure 3B, p < 0.01), and resulted in elevated fixed carbon levels in salamander embryos, reproducing the effect of placing the egg in the dark (Figure 3C).
FIGURE 3.
A. maculatum embryos compete with O. amblystomatis algae for bicarbonate. In each image, the y-axis represents radioactivity assayed per sample (DPM, disintegrations per minute); the x-axis represents the treatments. (A) Algae from whole eggs incubated in the light (L) or dark (D). (B) Cultured algae incubated in the light or dark with and without photosynthesis inhibitors (PI). (C) Carbon fixation by embryos in the whole egg environment, in the light or dark with and without photosynthesis inhibitors. n.s. = not significant, ∗∗p < 0.01. Significance levels were determined by ANOVA followed by the Games-Howell post-hoc test.
Discussion
In plants and algae, carbon dioxide is seen as a building block, a molecule captured from the air or surrounding fluid and fixed into organic matter as part of photosynthesis. In animals, carbon dioxide is largely a by-product of respiration that is also co-opted as an important biological buffer in body fluids. In addition to these roles, carbon dioxide and its hydration product, bicarbonate, participate in several biosynthetic reactions outside of photosynthesis. All animals can presumably re-capture respiratory carbon for use in biosynthetic pathways (Windmueller and Spaeth, 1980; Marini, 2016). Aquatic animals, and their eggs and embryos in particular, however, have access to environmental carbon dioxide dissolved in water and can import that exogenous carbon for use in biosynthetic processes (Flickinger, 1954; Mounib and Eisan, 1973).
In addition to biosynthetic roles, fixation of intracapsular carbon dioxide by amphibian embryos may be important for localized pH regulation. Amphibian embryos vary in their pH tolerances, and A. maculatum are particularly sensitive (Pierce, 1985). Their mortality can increase from <1% to >60% by lowering the pH from 7.0 to 6.0 and approaches 100% by pH 4.0 (Pough, 1976). Previous research in the A. maculatum-Oophila symbiosis found a pH of 4.5 decreases the partial pressure of oxygen in the egg capsule and increases intracapsular ammonia as well as embryonic ammonia and lactate (Bianchini et al., 2012). These changes are inferred to have a direct effect on algal cellular physiology, which interferes with the net benefit Oophila confers to the host embryos. The balance between algal and embryo manipulation of local pH through the action of excreted carboxylases (Shiraiwa et al., 1993) and photosynthetic and heterotrophic carbon fixation may additionally influence the rate and quantity of carbon fixation at different embryonic stages (Cohen, 1954).
During the salamander-alga symbiosis, the carbon fixation processes observed in other animals are likely active in A. maculatum embryos. The results presented here are consistent with heterotrophic fixation of exogenous bicarbonate by A. maculatum embryos (Table 1). As demonstrated in other systems, such carbon fixation is necessary for proper development, and here we provide evidence that the algae and salamander compete for exogenously supplied bicarbonate (Table 1). Our results are consistent with the findings of Hammen and Hutchison, 1962, suggesting that there is no measurable exchange of photosynthate from ecto-symbiotic algae to salamander embryos. The studies suggesting such an exchange (Graham et al., 2013, 2014) were likely only measuring variability in heterotrophic carbon fixation between individual embryos and did not control for the possibility that the salamander embryos themselves were fixing significant quantities of exogenously supplied bicarbonate.
TABLE 1.
Results summary.
| Experiment | Embryo | Algae |
| Decapsulated embryo–light | +++ | na |
| Decapsulated embryo–dark | +++ | na |
| Decapsulated embryo–killed | − | na |
| Decapsulated embryo+labeled algae | − | na |
| Whole egg–light | ++ | ++++ |
| Whole egg–dark | +++ | + |
| Whole egg–light–PI | +++ | + |
| Whole egg–dark–PI | +++ | + |
The number of pluses (+) indicates the relative amount of inorganic carbon assimilated under the indicated conditions: (+) low, (++) moderate, (+++) high, (++++) max observed. A dash (−) indicates that no carbon assimilation was observed above background. An na indicates “not applicable” in experiments where algae were not present or were pre-labeled. “PI” indicates the addition of photosynthesis inhibitors in those experiments.
Further studies on endosymbiotic algae are needed to reveal whether intracellular algal metabolites which are produced by the subset of endosymbiotic Oophila inside A. maculatum host cells are assimilated by their embryonic hosts. Our previous transcriptomic analysis has revealed the metabolic shift of intracellular algae from oxidative phosphorylation to fermentation (Burns et al., 2017), which may coincide with the formation of glycerol, formate, or acetate (Catalanotti et al., 2013), which are used in other photosynthetic endosymbioses (glycerol; Léon and Galván, 1995) or ectosymbiotic bacterial associations (formate and acetate; Den Besten et al., 2013; Karasov and Douglas, 2013). While there is no evidence that endosymbiotic Oophila enable A. maculatum embryos to utilize photosynthesis as a direct energy source, the chemical dialogue between this intracellular mutualist and its vertebrate host is a fascinating research topic for subsequent studies.
Author’s Note
This manuscript has been released as a pre-print at BioRxiv (Burns et al., 2020).
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the animal study because research on pre-hatchling archosaur and ectotherm embryos does not require Institutional Animal Care and Use Committee protocol approval according to Public Health Service policy (NIH 2015; National Research Council 2011). Embryos were treated with the same ethical standards as free-living larvae and care was taken to minimize stress and the number of embryos used in this study. National Research Council (2011) Guide for the Care and Use of Laboratory Animals, Eighth Edition. That National Academies Press. Washington D.C. https://doi.org/10.17226/12910. NIH Office of Laboratory Animal Welfare (2015) Public Health Service Policy on the Humane Care and Use of Laboratory Animals. United States Department of Health and Human Services. https://olaw.nih.gov/policies-laws/phs-policy.htm.
Author Contributions
JB and SD conceived, planned, and conducted the experiments. JB performed the data analysis. JB, SD, and RK discussed the results and implications and co-wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Challenge Yourself to Change (CYTC) community group in East Stroudsburg, PA, especially Rocky Sayles, Marquise Long, Dominic Kaps, Julius Patterson, and others who put on waders when collecting spotted salamander egg masses that contributed to this research. The authors also thank Darryl Speicher, Roger Spotts, and Kettle Creek Environmental Education Center for aid in egg mass collection and Hui Yang for thoughtful comments on drafts of the manuscript.
Footnotes
Funding. This work was supported by the Gordon and Betty Moore Foundation Grant #GBMF5604 (https://doi.org/10.37807/GBMF5604). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01815/full#supplementary-material
References
- Allen A. P., Gillooly J. F., Brown J. H. (2005). Linking the global carbon cycle to individual metabolism. Funct. Ecol. 19 202–213. 10.1111/j.1365-2435.2005.00952.x [DOI] [Google Scholar]
- Bachmann M. D., Carlton R. G., Burkholder J. M., Wetzel R. G. (1986). Symbiosis between salamander eggs and green algae: microelectrode measurements inside eggs demonstrate effect of photosynthesis on oxygen concentration. Can. J. Zool. 64 1586–1588. 10.1139/z86-239 [DOI] [Google Scholar]
- Baltar F., Herndl G. J. (2019). Ideas and perspectives: is dark carbon fixation relevant for oceanic primary production estimates? Biogeosciences 16 3793–3799. 10.5194/bg-16-3793-2019 [DOI] [Google Scholar]
- Berg I. A. (2011). Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl. Environ. Microbiol. 77 1925–1936. 10.1128/AEM.02473-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini K., Tattersall G. J., Sashaw J., Porteus C. S., Wright P. A. (2012). Acid water interferes with salamander-green algae symbiosis during early embryonic development. Physiol. Biochem. Zool. 85 470–480. 10.1086/667407 [DOI] [PubMed] [Google Scholar]
- Biggers J., Bellve A. (1974). “Carbon dioxide in developmental systems,” in Proceedings of the Carbon Dioxide and Metabolic Regulations Satellite SYmposium of the XXV Internaitonal Congress of Physiology, Monaco. [Google Scholar]
- Blanchard C. Z., Waldrop G. L. (1998). Overexpression and kinetic characterization of the carboxyltransferase component of acetyl-CoA carboxylase. J. Biol. Chem. 273 19140–19145. 10.1074/jbc.273.30.19140 [DOI] [PubMed] [Google Scholar]
- Boston H. L., Adams M. S., Madsen J. D. (1989). Photosynthetic strategies and productivity in aquatic systems. Aquat. Bot. 34 27–57. 10.1016/0304-3770(89)90049-1 [DOI] [Google Scholar]
- Brinson M. M., Lugo A. E., Brown S. (1981). Primary productivity, decomposition and consumer activity in freshwater wetlands. Annu. Rev. Ecol. Evol. Sci. 12 123–161. 10.1146/annurev.es.12.110181.001011 [DOI] [Google Scholar]
- Burns J. A., Kerney R., Duhamel S. (2020). Heterotrophic carbon fixation in a salamander-alga symbiosis. bioRxiv [Preprint]. 10.1101/2020.02.14.948299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. A., Zhang H., Hill E., Kim E., Kerney R. (2017). Transcriptome analysis illuminates the nature of the intracellular interaction in a vertebrate-algal symbiosis. eLife 6:e22054 10.7554/eLife.22054.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalanotti C., Yang W., Posewitz M. C., Grossman A. R. (2013). Fermentation metabolism and its evolution in algae. Front. Plant Sci. 4:150. 10.3389/fpls.2013.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. (1954). The metabolism of 14CO2 during amphibian development. J. Biol. Chem. 211, 337–54. [PubMed] [Google Scholar]
- Cohen S. (1963). 14CO2 fixation and the accumulation of malonic acid in amphibian hybrids (R. pipiens - female x R. sylvatica - male). Exp. Cell Res. 29 207–211. 10.1016/0014-4827(63)90376-8 [DOI] [PubMed] [Google Scholar]
- Den Besten G., van Eunen K., Groen A. K., Venema K., Reijngoud D. J., Bakker B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54 2325–2340. 10.1194/jlr.r036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinson R. P., del Pino E. M. (2011). Developmental diversity of amphibians. Wiley Interdiscip. Rev. Dev. Biol. 1 345–369. 10.1002/wdev.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb T. J. (2011). Carboxylases in natural and synthetic microbial pathways. Appl. Environ. Microbiol. 77 8466–8477. 10.1128/AEM.05702-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger R. A. (1954). Utilization of 14CO2 by developing amphibian embryos, with special reference to regional incorporation into individual embryos. Exp. Cell Res. 6 172–180. 10.1016/0014-4827(54)90159-7 [DOI] [PubMed] [Google Scholar]
- Gilbert P. W. (1942). Observations on the eggs of Ambystoma maculatum with especial reference to the green algae found within the egg envelopes. Ecology 23 215–227. 10.2307/1931088 [DOI] [Google Scholar]
- Gilbert P. W. (1944). The alga-egg relationship in Ambystoma maculatum, a case of symbiosis. Ecology 25 366–369. 10.2307/1931284 [DOI] [Google Scholar]
- Goff L. J., Stein J. R. (1978). Ammonia: basis for algal symbiosis in salamander egg masses. Life Sci. 22 1463–1468. 10.1016/0024-3205(78)90641-0 [DOI] [PubMed] [Google Scholar]
- Gong F., Cai Z., Li Y. (2016). Synthetic biology for CO2 fixation. Sci. China Life Sci. 59 1106–1114. 10.1007/s11427-016-0304-2 [DOI] [PubMed] [Google Scholar]
- Graham E. R., Fay S. A., Davey A., Sanders R. W. (2013). Intracapsular algae provide fixed carbon to developing embryos of the salamander Ambystoma maculatum. J. Exp. Biol. 216 452–459. 10.1242/jeb.076711 [DOI] [PubMed] [Google Scholar]
- Graham E. R., McKie-Krisberg Z. M., Sanders R. W. (2014). Photosynthetic carbon from algal symbionts peaks during the latter stages of embryonic development in the salamander Ambystoma maculatum. BMC Res. Notes 7:764. 10.1186/1756-0500-7-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. S., Hutchison V. H. (1962). Carbon dioxide assimilation in the symbiosis of the salamander Ambystoma maculatum and the alga Oophila amblystomatis. Life Sci. 1 527–532. 10.1016/0024-3205(62)90113-3 [DOI] [Google Scholar]
- Harrison R. G. (1969). “Harrison stages and description of the normal development of the spotted salamander, Amblystoma punctatum,” in Organization and Development of the Embryo, ed. Wilens S. (London: Yale University Press; ), 44–66. [Google Scholar]
- Holden H. M., Thoden J. B., Raushel F. M. (1999). Carbamoyl phosphate synthetase: an amazing biochemical odyssey from substrate to product. Cell. Mol. Life. Sci. 56 507–522. 10.1007/s000180050448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitrapakdee S., Vidal-Puig A., Wallace J. C. (2006). Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell. Mol. Life. Sci. 63 843–854. 10.1007/s00018-005-5410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov W. H., Douglas A. E. (2013). Comparative digestive physiology. Compr. Physiol. 3 741–783. 10.1002/cphy.c110054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerney R. (2011). Symbioses between salamander embryos and green algae. Symbiosis 54 107–119. 10.1007/s13199-011-0134-2 [DOI] [Google Scholar]
- Kerney R., Kim E., Hangarter R. P., Heiss A. A., Bishop C. D., Hall B. K. (2011). Intracellular invasion of green algae in a salamander host. Proc. Natl. Acad. Sci. U.S.A. 108 6497–6502. 10.1073/pnas.1018259108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerney R., Leavitt J., Hill E., Zhang H., Kim E., Burns J. (2019). Co-cultures of Oophila amblystomatis between Ambystoma maculatum and Ambystoma gracile hosts show host-symbiont fidelity. Symbiosis 78 73–85. 10.1007/s13199-018-00591-2 [DOI] [Google Scholar]
- Kresge N., Simoni R., Hill R. (2005). The discovery of heterotrophic carbon dioxide fixation by Harland G. Wood. J. Biol. Chem. 280:e15. [Google Scholar]
- Léon R., Galván F. (1995). Glycerol photoproduction by free and Ca-alginate entrapped cells of Chlamydomonas reinhardtii. J. Biotechnol. 42 61–67. 10.1016/0168-1656(95)00069-3 [DOI] [Google Scholar]
- Marini J. C. (2016). Interrelationships between glutamine and citrulline metabolism. Curr. Opin. Clin. Nutr. 19 62–66. 10.1097/mco.0000000000000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills N. E., Barnhart M. C. (1999). Effects of hypoxia on embryonic development in two Ambystoma and two Rana species. Physiol. Biochem. Zool. 72 179–188. 10.1086/316657 [DOI] [PubMed] [Google Scholar]
- Mounib M. S., Eisan J. S. (1969). Metabolism of pyruvate and glyoxylate by eggs of salmon (Salmo salar). Comp. Biochem. Physiol. 29 259–264. 10.1016/0010-406x(69)91742-3 [DOI] [PubMed] [Google Scholar]
- Mounib M. S., Eisan J. S. (1973). Fixation of carbon dioxide and some of the enzymes involved in cod eggs. Int. J. Biochem. 4 207–212. 10.1016/0020-711x(73)90014-1 [DOI] [Google Scholar]
- Orr H. (1888). Memoirs: note on the development of amphibians, chiefly concerning the central nervous system; with additional observations on the hypophysis, mouth, and the appendages and skeleton of the head. J. Cell Sci. 2 295–324. [Google Scholar]
- Peters G. (2018). Userfriendlyscience: Quantitative Analysis Made Accessible. R package version 0.7.2. Available online at: https://userfriendlyscience.com (accessed July 22, 2020). [Google Scholar]
- Pierce B. A. (1985). Acid tolerance in amphibians. BioScience. 35 239–243. 10.2307/1310132 [DOI] [Google Scholar]
- Pinder A., Friet S. (1994). Oxygen transport in egg masses of the amphibians Rana sylvatica and Ambystoma maculatum: convection, diffusion and oxygen production by algae. J. Exp. Biol. 197 17–30. [DOI] [PubMed] [Google Scholar]
- Pough F. H. (1976). Acid precipitation and embryonic mortality of spotted salamanders, Ambystoma maculatum. Science 192 68–70. 10.1126/science.3852 [DOI] [PubMed] [Google Scholar]
- R Core Team (2017). R: A Language And Environment For Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Raven J. A. (2017). “Symbiosis involving photosynthetic organisms,” in Algal and Cyanobacteria Symbioses, eds Grube M., Seckbach J., Muggia A. (Singapore: World Scientific; ), 3–41. 10.1142/9781786340580_0001 [DOI] [Google Scholar]
- Shampo M. A. (1999). Stamp vignette on medical science: Stanley Cohen–Nobel Laureate for growth factor. Mayo Clin. Proc. Rochester 74:600 10.4065/74.6.600 [DOI] [PubMed] [Google Scholar]
- Shiraiwa Y., Goyal A., Tolbert N. E. (1993). Alkalization of the medium by unicellular green algae during uptake of dissolved inorganic carbon. Plant Cell Physiol. 34 649–657. 10.1093/oxfordjournals.pcp.a078467 [DOI] [Google Scholar]
- Small D. P., Bennett R. S., Bishop C. D. (2014). The roles of oxygen and ammonia in the symbiotic relationship between the spotted salamander Ambystoma maculatum and the green alga Oophila amblystomatis during embryonic development. Symbiosis 64 1–10. 10.1007/s13199-014-0297-8 [DOI] [Google Scholar]
- Tiedemann H., Tiedemann H. (1954). Einbau von14CO2 in gefurchte und ungefurchte Eihälften und in verschiedene entwicklungsstadien von Triton. Naturwissenschaften 41 535–535. 10.1007/BF00623059 [DOI] [Google Scholar]
- Tremblay P., Grover R., Maguer J. F., Legendre L., Ferrier-Pagès C. (2012). Autotrophic carbon budget in coral tissue: a new 13C-based model of photosynthate translocation. J. Exp. Biol. 215 1384–1393. 10.1242/jeb.065201 [DOI] [PubMed] [Google Scholar]
- Venn A. A., Loram J. E., Douglas A. E. (2008). Photosynthetic symbioses in animals. J. Exp. Bot. 59 1069–1080. 10.1093/jxb/erm328 [DOI] [PubMed] [Google Scholar]
- Wales R. G., Quinn P., Murdoch R. N. (1969). The fixation of carbon dioxide by the eight-cell mouse embryo. J. Reprod. Fertil. 20 541–543. 10.1530/jrf.0.0200541 [DOI] [PubMed] [Google Scholar]
- Wickham H. (2016). Ggplot2: Elegant Graphics For Data Analysis. Berlin: Springer. [Google Scholar]
- Windmueller H. G., Spaeth A. E. (1980). Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J. Biol. Chem. 255 107–112. [PubMed] [Google Scholar]
- Wood H. G., Vennesland B., Evans E. A. (1945). The mechanism of carbon dioxide fixation by cell-free extracts of pigeon liver: distribution of labeled carbon dioxide in the products. J. Biol. Chem. 159 153–158. [Google Scholar]
- Wood H. G., Werkman C. H., Hemingway A., Nier A. O. (1941). Heavy carbon as a tracer in heterotrophic carbon dioxide assimilation. J. Biol. Chem. 139 365–376. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.