Abstract
Aim:
The study aimed to find the best reading time for the best accuracy of RUT in optimal time to obtain faster results with lower false rates and consequently save time in commencing treatment of peptic ulcers.
Background:
Rapid urease test is well known to be an accurate test for H.pylori detection in tissue biopsies.
Methods:
Patients with GI problems referring to a university hospital in Tehran who underwent endoscopy and biopsy were entered in the project and three samples of mucosal tissue were captured from the lesser curvature, the antrum and the body of stomach.
Results:
We found 39.6% sensitivity and 95% specificity for the named test in the first 5 minutes as well as PPV = 95.5% and NPV = 37.3% while the accuracy was 54.79%. Except for the specificity which was constantly 95% in all RUT reading times, other diagnostic parameters increased as time went on. The PPV was also higher than 97% after 10 minutes. The highest values of sensitivity, specificity, PPV, NPV and accuracy were achieved after 12 hours including 88.7%, 95%, 97.9%, 76% and 90.41%, respectively.
Conclusion:
To conclude, it seems that there are many different ideas with respect to the rapid urease test in H.pylori detection. However, the current study recommends reading the test optimally after 12 hours but it is suggested more multidisciplinary studies with bigger sample size be carried out to obtain better and more reliable results to be able to generalize in this regard.
Key Words: Helicobacter pylori, Rapid urease test (RUT), Diagnostic value, Pathophysiology
Introduction
H.pylori infection affects more than half the world’s population and more adults in developing countries are infected. H.pylori infection is the main cause of dyspepsia in 10% of people and is also the main correlated factor in duodenal and gastric ulcers in 95% and 70% of cases, respectively (1, 2). Rapid urease test (RUT) is an indirect test for diagnosis of H.pylori according to the presence of urease in the gastric mucosa. RUT is a common rapid cheap and simple diagnostic test and its important advantage over serology tests is in its detection of active infectious agents (3).
Diagnosing H.pylori infection has been made based on biopsies and pathology for a long time and this technique is known as the golden standard test to date. During recent decades, physicians have decided to use techniques like genetic studies as well as urease tests as they are more applicable, faster and probably more accurate and cost-effective.
Rapid urease test (RUT) includes a high urea containing media with an indicator which is sensitive to PH and changes color at different PH rates. A study by Foroutan et al. in 2010 identified RUT 98.57% sensitive, 99.29% specific and accurate in 99.04% of stomach biopsies (4). Regarding the importance of diagnosis time and treatment of peptic ulcers, RUT is a crucial, accurate and fast test. This test needs 24 hours before reading but different reading times have their own different sensitivity and specificity and we may lessen the time to get acceptable results when positive. In 1996 it was disclosed that H.pylori population in the sample tissues strongly affects the speed of obtaining positive results. For instance, a RUT positive result in 20 minutes needs a population of between 3*102 and 3*103 microorganisms (5). Laine et al. raised the importance of multiple tissue biopsies in decreasing the time taken to read RUT results although 40% false negative rate was seen (5).
The current diagnostic study headed to find the best reading time for the best accuracy, sensitivity and specificityof RUT as well as the optimal time of positivity to get faster results with lower false rates and save time in the commencement of treatment of peptic ulcers.
Methods
Through an analytical cross-sectional prospective study, patients with GI problems referring to a university hospital in Tehran who underwent endoscopy and stomach biopsy were enrolled in the current research project. People with active upper GI bleeding, with a history of gastrectomy and hypotension (SBP<90 mmHg) were excluded from this study. Patients who had taken antibioticstwo weeks before the study, including bismuth derivatives and proton pump inhibitors left the study as well.
Endoscopy and biopsy: Heretofore, histopathology was the diagnostic choice to distinguish gastritis and/or peptic ulcers due to Helicobacter pylori (H.pylori). To obtain optimal results, three samples of mucosal tissue from the stomach are routinely captured using large-cap forceps from the lesser curvature, the antrum and the body of stomach. Biopsy samples gatheredfrom the patients were transferred to the pathology department for H&E and Giemsa staining before a microscopic study. H&E stain and microscopy is an excellent test in big populations of H.pylori but the organism is not always abundant enough to achieve perfect microscopic assessment.
Rapid Urease Test (RUT): RUT is a kind of urease test in which hydrolyses of urea products into ammonia and CO2 is performed as follows: The produced ammonia alkalizes samples containing medium to force the PH to change the color of the existing indicator.
(NH2)2 CO + 2 H2O → CO2 + H2O + 2NH3
This study used RUT agar kits by Shimanzim ®, Iran with phenol red as PH indicator. Positive results for H.pylori were considered when the color of the kits changed from light orange into pink/red in 24 hours. Color changes were recorded in 5, 10, 20 and 60 minutes and then after 2, 6, 12 and 24 hours.
Statistics: Data entered in the SPSS and EPI info16 and frequency and central tendency indices as well as the assessments for accuracy, sensitivity and specificity of RUT were compared with the golden standard pathology. The power of the study was headed to be 0.8 beside type one error (α = 0.05) and the confidence interval (CI = 95%).
Ethics: The current study recruited candidate patients for diagnostic endoscopy regarding the medical indications and physician decision by census. There was no obligation to do the tests with no indication. The costs of the diagnostic procedures were covered by the project and the participants with positive results for H.pylori infection were prescribed for treatment. All the participants’ information was secured by the principle investigator.
Results
In the present study 264 patients with endoscopy and biopsy results were enrolled and among them 118 had used antibiotics in recent weeks and were excluded from the study. Finally, 146 patients including 86 (58.9%) females and 60 (41.1%) males participated in this project. The mean age and standard deviation were reported 45.36 ± 17.41 years with 25% of patients who were over 59.25 years.
Nausea and vomiting were reported by 30 subjects (20.5%) while 64 (43.8%) had abdominal pain when fasting but 60 (41.1%) complained of stomachache after eating. Flatulence (42.5%), heartburn (26%), haematochesia (2.7%), melena (8.2%), anemia (5.5%) and occult blood (2.7%) were the other findings in the studied population. A history of upper GI bleeding was raised in 14 (9.6%) and among them, 2 (1.4%) had had it in the recent month. Six patients (4.1%) in recent 1-6 months, 2 (1.4%) in previous 6-12 months and 2 (1.4%) in the last year had Upper GI bleeding.
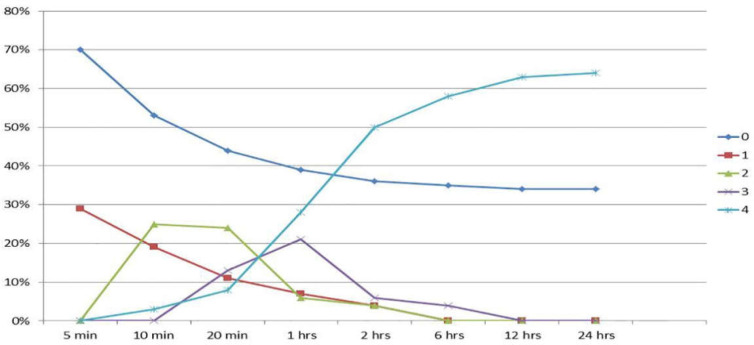
Concerning RUT, 102 (69.9%) showed negative results after 5 minutes compared to 44 (30.1%) positive casesof H.pylori. The RUT results were followed for 24 hours in the participants. Table 1 and figure 1 illustrate the findings in this regard in more detail as well as the trend of each stage of the test throughout the study.
Table1.
Frequency of RUT and pathology results
| Pathology |
Total(%) | |||
|---|---|---|---|---|
| Yes (%) | No(%) | |||
| RUT5 | Yes | 42(95.5) | 2(4.5) | 44(100) |
| No | 64(62.7) | 38(37.3) | 102(100) | |
| RUT10 | Yes | 66(97.1) | 2(2.9) | 68(100) |
| No | 40(51.3) | 38(48.7) | 78(100) | |
| RUT20 | Yes | 78(97.5) | 2(2.5) | 80(100) |
| No | 26(40.6) | 38(59.4) | 64(100) | |
| RUT1hrs | Yes | 86(97.7) | 2(2.3) | 88(100) |
| No | 18(32.1) | 38(67.9) | 56(100) | |
| RUT2hrs | Yes | 90(97.8) | 2(2.2) | 92(100) |
| No | 14(26.9) | 38(73.1) | 52(100) | |
| RUT6hrs | Yes | 92(97.9) | 2(2.1) | 94(100) |
| No | 12(24) | 38(76) | 50(100) | |
| RUT12hrs | Yes | 94(97.9) | 2(2.1) | 96(100) |
| No | 12(24) | 38(76) | 50(100) | |
| RUT24hrs | Yes | 94(97.9) | 2(2.1) | 96(100) |
| No | 12(24) | 38(76) | 50(100) | |
Figure 1.
Trend of RUT results during 24 hours: 0=no changes in the color of the specimen; 1=+1: Shows a color change in only a part of the tissue; 2=+2: Color change in all the tissue with 1 mm margin around it; 3=+3: Between +2 and +4; 4=+4: Complete color change in the whole solution
The golden standard test in this work was histopathology of stomach biopsies as discussed in the methodology section. Overall, 94 patients (64.4%) presented with H.pylori infection in the body of their stomach based on the above test but the rest were negative. The antrum seemed to be more infected than the body as reported by positive pathologic results for 98 (67.1%). So, the positivity of pathology for infection was 106 (72.6%) regardless of the location of it.
A strong linear correlation was found between RUT results and pathology results in biopsies from body part of the stomach at different times of the test from 5 minutes to 24 hours (P value < 0.001). A similar correlation was shown in a comparison between antrum pathologic studies and RUT (P value < 0.001) with rather higher coefficients.
Interestingly, body infection was obviously correlated with antrum infection in pathology (P value < 0.001; r = 0.639). People with positive pathology results for H.pylori infection were overall around 10 years older than the people with negative results (48.47 ± 16.46 vs.37.1 ± 17.33 years) (P value < 0.001). City of settlement, sub nationality and education were not matters of significant difference in pathology.
In order to find the sensitivity, specificity and predictive values for RUT, we compared the findings with the golden diagnostic test and found 39.6% sensitivity and 95% specificity for the named test in the first 5 minutes as well as PPV = 95.5% and NPV = 37.3% while the accuracy was 54.79% (table 2). Except for the specificity which was constantly 95% in all RUT reading times, other diagnostic parameters were
Table2.
Sensitivity, specificity, PPV, NPV and accuracy of RUT in different reading times
| RUT | Sensitivity (%) CI 95% |
Specificity (%) CI 95% |
PPV (%) CI 95% |
NPV (%) CI 95% |
Accuracy CI 95% |
|---|---|---|---|---|---|
| 5 min | 39.6 26.8-54 |
95 73.1-99.7 |
95.5 75.1-99.8 |
37.3 24.5-51.9 |
54.79 |
| 10 min | 62.3 47.9-74.9 |
95 73.1-99.7 |
97.1 82.9-99.8 |
48.7 32.7-65 |
71.23 |
| 20 min | 75 60.08-85.5 |
95 73.1-99.7 |
97.5 85.3-99.9 |
59.4 40.8-75.8 |
80.55 |
| 1 hour | 82.7 69.2-91.3 |
95 73.1-99.7 |
97.7 86.5-99.9 |
67.9 47.6-83.4 |
86.11 |
| 2 hours | 86.5 73.6-94 |
95 73.1-99.7 |
97.8 87-99.9 |
73.1 51.9-87.6 |
88.88 |
| 6 hours | 88.5 75.9-95.2 |
95 73.1-99.7 |
97.9 87.3-99.9 |
76 54.5-89.8 |
90.27 |
| 12 hours | 88.7 76.3-95.3 |
95 73.1-99.7 |
97.9 87.5-99.9 |
76 54.5-89.8 |
90.41 |
| 24 hours | 88.7 76.3-95.3 |
95 73.1-99.7 |
97.9 87.5-99.9 |
76 54.5-89.8 |
90.41 |
growing as time went on. The PPV was also higher than 97% after 10 minutes and had the least changes as can be seen in table 4. Finally, the highest values of sensitivity, specificity, PPV, NPV and accuracy were achieved after 12 hours including 88.7%, 95%, 97.9%, 76% and 90.41%, respectively and the future results continued to be exactly the same.
In terms of more details in diagnostic value of RUT, we calculated positive and negative likelihood ratios for the 12th hour of the test using the following equations:
The above likelihood ratio for RUT results means that its positivity can unremarkably increase the probability of gastritis and/or ulcers while negative results show a more reliable role of the test to rule out H.pylori active infection.
Discussion
The current study showed the most effective values of the assessed diagnostic factors at the 12th hour of RUT test and recommendsthat the best time to read RUT results is after 12 hours instead of 24 hours. We used three biopsy samples for each patient because we could not find the effect of size and number of biopsies on the rate and time of positive results in RUT similar to what happened in Laine’s study published in 1996. They found that two biopsy samples could make results positive 30 minutes earlier in 56% of cases compared with a single biopsy sample (5). They also found better sensitivity using two samples although 40% false negative cases occurred in earlier readings. Most of the false negative results in RUT may be due to taking antibiotics, PPIs and presence of intestinal metaplasia as Uotani clarified before (6). RUT, using urease, has superiorityover serologic tests because of detecting the active phase of infection and not latent infections (6). In any case, regarding the minimum number of organisms to make the results positive (105), the quality and location of the samples are the most important parts of the test. For instance, atrophic lesions in the stomach cannot be good sampling sites because of few organisms and by pushing the results to false negativity more than even a single sample from duodenal non-atrophic lesions which perfectly provide enough of a H.pylori population to make RUT sensitivity and specificity more optimal and consequently more reliable (6). However, this minimum number of organisms is not significantly important because the organisms usually exceed 105 and this would provide high sensitivity in easier steps of the test as the current study showed (7-11).
A study by Levin et al. in 2005 showed 100% specificity of the test disregarding the time of reading but around 31% of early negative results changed into positive in RUT24 and 9.8% of the whole cases were positive in results in histopathology but reported negative by both RUT0 and RUT24 (12). They believed that these false negative cases were due to imperfect sampling, in that PPIs or other medications could not alter the sensitivity and specificity of RUT at all. On the contrary, a study in 2000 in Greece distinguished that RUT, with its own diagnostic values, could not discover all the positive cases of H.pylori infection reported by histopathology, especially during the first 24 hours (13). Similarly, Talebi et al. in a letter to the Saudi journal of gastroenterology in 2011 asked physicians not to rely on RUT as a sensitive test of detection for H.pylori but on the contrary Foroutan et al. who declared the test as being a reliable test in 2010 because of finding 30% false negative results by the test (4, 14).
Peptic ulcers are causative factors of dyspepsia in 10% of cases and 95% of duodenal and 70% of gastric ulcers are mainly due to H.pylori (2). So, controlling the infection is a crucial treatment for curing patients. In this regard, accurate fast detection plays the most predominant role and RUT is usually faster than histopathologic studies, especially if the results are reliable enough after 12 hours as our study has shown. Unlike histopathology, RUT does not need any specialty or subspecialty nor any complex process to make it cheaper. The other advantage is theability of carrying out RUT using portable kits with no necessary equipment , possible in the most remotest of areas.
The prevalence of the disease is important when likelihood ratios for positive and negative RUT results are concerned. There are several studies on the worldwide prevalence of H.pylori infection; some of which have been conducted in Iran showing 90% infection rate among adults and involvement of children in more than 50% before the age of 15 (15, 16, 17).
Among hospital referrals of dyspepsia, a range of 60-80% is usually reported for the prevalence of H.pylori infection by histopathology (18-20). Looking at the mentioned rates, likelihood ratios would be applied as follows (Probability Pre):
For positive RUT results:
Odds Post = Odds Pre × LR + = 2.33×17.74 = 41.33
The odds ratio here shows the actual effect of LR on the probability of H.pylori infection among patients with dyspepsia. Regarding the values of LR+, when a patient with dyspepsia refers to us and the RUT is positive in 12 hours, it means our patient is suffering from H.pylori active infection with a 98% chance. In other words we can increase the chance of infection diagnosis from 70% at the beginning to 98% by a simple 12-hour test in a hospital setting. For negative results the probability Post was obtained at 22% showing that the first 70% chance of H.pylori changes into 22% if RUT is negative in 12 hours. So, RUT positive results can draw up the diagnosis to 28% whilst 48% in negative results. With these diagnostic values, is it not feasible that we can rely on RUT and reduce the use of pathologic studies in this regard, especially in areas where we have a lack of equipment, personnel, and finance?
To conclude, it seems that there are many different ideas with respect to rapid urease test in H.pylori detection. However, the current study recommends reading the test optimally after 12 hours and we also advise more multidisciplinary studies be carried out with a bigger sample size to obtain better and more reliable results to be able to generalize in this regard.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Ford AC, Gurusamy KS, Delaney B, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive people. Cochrane Database Syst Rev. 2016;4:CD003840. doi: 10.1002/14651858.CD003840.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz C, Schütte K, Malfertheiner P. Helicobacter pylori and Other Gastric Microbiota in Gastroduodenal Pathologies. Dig Dis. 2016;34:210–6. doi: 10.1159/000443353. [DOI] [PubMed] [Google Scholar]
- 3.Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 2015;3:9. doi: 10.3978/j.issn.2305-5839.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foroutan M, Loloei B, Irvani S, Azargashb E. Accuracy of rapid urease test in diagnosing Helicobacter pylori infection in patients using NSAIDs. Saudi J Gastroenterol. 2010;16:110–2. doi: 10.4103/1319-3767.61238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laihe L. The influence of size or number of biopsies on rapid urease test results. Gastrointest Endosc. 1996;43:49–52. doi: 10.1016/s0016-5107(96)70260-2. [DOI] [PubMed] [Google Scholar]
- 6.Uotani T, David Y. Graham. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 2015;3:9–15. doi: 10.3978/j.issn.2305-5839.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mégraud F, Bessède E, Lehours P. Current methods used for the diagnosis of Helicobacter pylori infection. In: Buzás GM, editor. Helicobacter pylori - A Worldwide Perspective 2014. Oak Park: Bentham Science; 2014. pp. 234–58. [Google Scholar]
- 8.Calvet X, Sánchez-Delgado J, Montserrat A, Lario S, Ramírez-Lázaro MJ, Quesada M, et al. Accuracy of diagnostic tests for Helicobacter pylori: a reappraisal. Clin Infect Dis. 2009;48:1385–91. doi: 10.1086/598198. [DOI] [PubMed] [Google Scholar]
- 9.Al-Humayed SM, Ahmed ME, Bello CS, Tayyar MA. Comparison of 4 laboratory methods for detection of Helicobacter pylori. Saudi Med J. 2008;29:530–2. [PubMed] [Google Scholar]
- 10.Redéen S, Petersson F, Törnkrantz E, Levander H, Mårdh E, Borch K. Reliability of Diagnostic Tests for Helicobacter pylori Infection. Gastroenterol Res Pract. 2011;2011:940650. doi: 10.1155/2011/940650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaira D, Perna F. How useful is the rapid urease test for evaluating the success of Helicobacter pylori eradication therapy? Nat Clin Pract Gastroenterol Hepatol. 2007;4:600–1. doi: 10.1038/ncpgasthep0966. [DOI] [PubMed] [Google Scholar]
- 12.Levin DA, Watermeyer G, Mohamed N, Epstein DP, Hlatshwayo SJ, Metz DC. Evaluation of a locally produced rapid urease test for the diagnosis of Helicobacter pylori infection. S Afr Med J. 2007;97:1281–4. [PubMed] [Google Scholar]
- 13.Archimandritis A, Tzivras M, Sougioultzis S, Papaparaskevas I, Apostolopoulos P, Avlami A, et al. Rapid urease test is less sensitive than histology in diagnosing Helicobacter pylori infection in patients with non-variceal upper gastrointestinal bleeding. J Gastroenterol Hepatol. 2000;15:369–73. doi: 10.1046/j.1440-1746.2000.02171.x. [DOI] [PubMed] [Google Scholar]
- 14.Bezmin Abadi AT, Taghvaei T, Wolfram L. Inefficiency of rapid urease test for confirmation of Helicobacter pylori. Saudi J Gastroenterol. 2011;17:84–5. doi: 10.4103/1319-3767.74441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseini E, Poursina F, Van de Wiele T, Ghasemian Safaei H, Adibi P. Helicobacter pylori in Iran: A systematic review on the association of genotypes and gastroduodenal diseases. J Res Med Sci. 2012;17:280–92. [PMC free article] [PubMed] [Google Scholar]
- 16.Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S, et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57:37–42. doi: 10.1136/jcp.57.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magalhães Queiroz DM, Luzza F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2006;11:1–5. doi: 10.1111/j.1478-405X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 18.Yasir Sh, Moin F, Syed Akhtar M. Frequency of Helicobacter Pylori Infection on Histopathology in Patients with Dyspepsia. Am J Clin Med Res. 2014;2:53–56. [Google Scholar]
- 19.Mustapha SK, Bolori MT, Ajayi N, Nggada H, Pindiga U, Gashau W, et al. Endoscopic Findings and The Frequency Of Helicobacter Pylori Among Dyspeptic Patients In North-Eastern Nigeria. Internet J Gastroenterol. 2006;6:12. [Google Scholar]
- 20.Tariq Siddiqui Sh, Naz E, Mirza T, Mirza T, Aziz S, Ali A. Frequency of Helicobacter pylori in biopsy proven gastritis and its association with lymphoid follicle formation. J Pak Med Assoc. 2011;61:138–41. [PubMed] [Google Scholar]