Abstract
A connection between airway and gut microbiota related to allergen exposure in childhood allergies was not well addressed. We aimed to identify the microbiota alterations in the airway and gut related to mite-specific IgE responses in young children with airway allergies. This study enrolled 60 children, including 38 mite-sensitized children (20 rhinitis and 18 asthma), and 22 non-mite-sensitized healthy controls. Microbiome composition analysis of the throat swab and stool samples was performed using bacterial 16S rRNA sequencing. An integrative analysis of the airway and stool microbial profiling associated with IgE reactions in childhood allergic rhinitis and asthma was examined. The Chao1 and Shannon indices in the airway were significantly lower than those in the stool. Additionally, an inverse association of the airway microbial diversity with house dust mite (HDM) sensitization and allergic airway diseases was noted. Fecal IgE levels were positively correlated with the serum Dermatophagoides pteronyssinus- and Dermatophagoides farinae-specific IgE levels. Airway Leptotrichia spp. related to asthma were strongly correlated with fecal Dorea and Ruminococcus spp., which were inversely associated with fecal IgE levels and risk of allergic rhinitis. Moreover, four airway genera, Campylobacter, Selenomonas, Tannerella, and Atopobium, were negatively correlated with both serum mite-specific and fecal IgE levels. Among them, the airway Selenomonas and Atopobium spp. were positively correlated with stool Blautia and Dorea spp. related to asthma and allergic rhinitis, respectively. In conclusion, airway microbial dysbiosis in response to HDM and its cross-talk with the gut microbial community is related to allergic airway diseases in early childhood.
Subject terms: Asthma, Respiratory tract diseases, Paediatric research, Clinical microbiology, Microbiome
Introduction
Dysbiosis in the gut microbiota is associated with several respiratory infections and lung diseases, including allergy and asthma1,2. There is accumulating evidence that a cross-talk exists between gut and lung. The upper gastrointestinal microbiota affects the development of the airway microbiota either by aspiration or metabolite production, leading to changes in the airway microenvironment3. Conversely, the dysbiosis in the lung microbiota is accompanied by that in the gut microbiota via bloodstream4. In a recent study, airway microbial dysbiosis appears to be strongly associated with sensitization to house dust mites (HDM), potentially contributing to allergic reactions and airway diseases5. However, its contribution to the gut-lung axis associated with allergies remains unclear.
HDM allergy with an elevated immunoglobulin E (IgE) level is strongly implicated in the allergic rhinitis and asthma pathogeneses in children6. Clinically, the comorbidity between these two conditions is high for perennial aeroallergens7,8. Furthermore, IgE is also produced locally in the gut and fecal IgE level could serve as a marker of allergic response to HDM9. The human microbiota mediates the mechanisms of the immune responses, which is essential for regulating mucosal inflammation10. The commensal organisms of the human microbiota that modulate the allergic response to HMD may differ in their molecular immune reactions, leading to different clinical manifestations and phenotypes. However, few studies have addressed the relationships of the microbiota and airway-gut connection in the clinical variants of allergic rhinitis and asthma phenotypes.
Microbial dysbiosis is increasingly being associated with the development and manifestations of allergic diseases11. A comprehensive understanding of the microbiome in response to allergens may provide insights into the pathological conditions affecting different phenotypes and a potential therapy for prevention or treatment of allergies. This study aimed to determine the airway and gut microbial profiles in children with airway allergies using 16S rRNA sequencing. The existence of the association between airway and gut microbiome and its implications in mediating HDM-specific IgE responses for childhood allergic rhinitis and asthma were also examined.
Results
Population characteristics
A total of sixty subjects were enrolled into this study, including 38 children with mite-sensitized allergic rhinitis (n = 20) and asthma (n = 18), and 22 healthy children. Table 1 presents the comparisons of baseline characteristics of children with rhinitis, asthma and healthy controls. Statistically significant differences were noted in the total serum and fecal, and D. pteronyssinus- and D. farinae-specific IgE levels among children with mite-sensitized rhinitis, asthma, and healthy controls (P < 0.05).
Table 1.
Comparison of the clinical and epidemiologic characteristics between mite-sensitized rhinitis and asthma, and non-mite-sensitized healthy controls.
| Characteristics | Mite-sensitized | P-value | Mite-sensitized | Non-mite-sensitized | P-value | |
|---|---|---|---|---|---|---|
| Rhinitis (n = 20) | Asthma (n = 18) | Total (n = 38) | Controls (n = 22) | |||
| Age (yr) | 4.35 ± 0.40 | 4.39 ± 0.53 | 0.848 | 4.37 ± 0.45 | 4.59 ± 0.36 | 0.178 |
| Sex, male | 14 (70.0%) | 12 (66.7%) | 0.460 | 26 (68.4%) | 10 (45.5%) | 0.266 |
| Probiotic supplements | 6 (30.0%) | 10 (55.6%) | 0.370 | 16 (42.1%) | 8 (36.4%) | 1.000 |
| Cesarean delivery | 6 (30.0%) | 6 (33.3%) | 1.000 | 12 (31.6%) | 6 (27.3%) | 1.000 |
| Maternal atopy | 6 (30.0%) | 10 (55.6%) | 0.524 | 16 (42.1%) | 10 (45.5%) | 0.858 |
| Passive smoking | 8 (40.0%) | 10 (55.6%) | 0.740 | 18 (47.4%) | 12 (54.5%) | 0.705 |
| Household income | ||||||
| Low, 500,000 NTD | 10 (50.0%) | 9 (50.0%) | 0.276 | 19 (50.0%) | 10 (45.5%) | 0.152 |
| Medium, 500,000–1,000,000 NTD | 4 (20.0%) | 7 (38.9%) | 11 (28.9%) | 12 (54.5%) | ||
| High, > 1,000,000 NTD | 6 (30.0%) | 2 (11.1%) | 8 (21.1%) | 0 (0.0%) | ||
| Allergen-specific IgE, kU/L | ||||||
| D. pteronyssinus | 54.58 ± 42.63 | 20.8 ± 34.07 | 0.065 | 38.58 ± 41.53 | 3.27 ± 8.69 | 0.021 |
| D. farinae | 38.63 ± 38.21 | 12.49 ± 18.64 | 0.095 | 26.25 ± 32.62 | 1.41 ± 3.33 | 0.011 |
| Total serum IgE, kU/L | 623.20 ± 632.22 | 164.52 ± 192.84 | 0.043 | 405.93 ± 521.29 | 44.92 ± 36.31 | 0.008 |
| Total fecal IgE, kU/L | 25.20 ± 32.45 | 4.63 ± 3.94 | 0.182 | 15.46 ± 25.39 | 1.92 ± 1.26 | 0.003 |
All P-values < 0.05, which is in bold, are significant.
Data shown are mean ± SD or number (%) of patients as appropriate.
yr year, NTD New Taiwan Dollar, IgE immunoglobulin E.
Identification of the airway and stool bacterial community composition and abundance
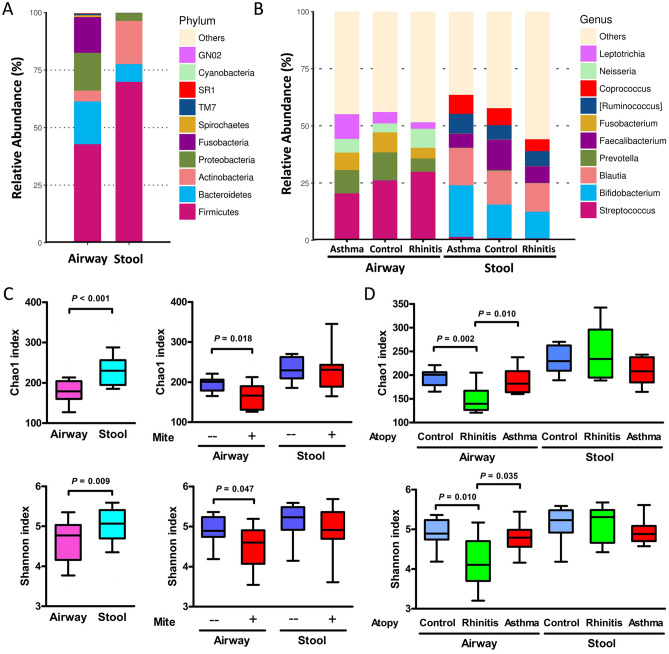
The taxonomic classification of the airway microbiota showed a high prevalence of phylum Firmicutes (42.8% of the total number of sequences obtained) followed by those of the phyla Bacteroidetes (18.6%), Proteobacteria (16.4%), and Fusobacteria (15.5%), whereas the phyla Firmicutes (69.9%), Actinobacteria (18.8%), Bacteroidetes (7.7%), and Proteobacteria (3.4%) were predominantly found in the stool microbiota (Fig. 1a). The most common genera in the airway microbiota were Streptococcus (24.5%), Prevotella (8.9%), and Fusobacterium (7.7%), whereas those in the stool microbiota were Bifidobacterium (16.0%), Blautia (14.3%), and Faecalibacterium (9.2%). The genera of airway and stool microbiota categorized by atopic diseases are shown in Fig. 1b.
Figure 1.
Airway and stool microbial composition and abundance at the phylum (A) and the genus level (B). Differences and comparisons of bacterial richness and diversity between airway and stool microbiota related to mite sensitization (C) and atopic diseases (D). Each bar represents the top ten enriched class categories ranked by the relative abundance in each group. Bacterial richness is calculated as the Chao1 index, and diversity is calculated as the Shannon index. The box-plot shows the median and the 10th, 25th, 75th and 90th percentile.
Bacterial richness and diversity categorized by atopic indices and diseases
Figure 1c shows the bacterial richness and diversity categorized by the sample types and atopic indices. Significantly lower Chao1 and Shannon indices were found in the airway microbiota than in the stool microbiota. In the airway microbiota, these indices were significantly reduced in children with mite sensitization, and were significantly lower in children with mite-sensitized rhinitis but not asthma than those in the healthy children without mite sensitization (Fig. 1d). However, in the stool microbiota, no difference was noted in the bacterial richness and diversity regarding the mite sensitization and its relevance to rhinitis and asthma. Furthermore, bacterial richness and diversity were not significantly different with regard to factors contributing to atopic diseases including sex, probiotics use, cesarean delivery, maternal atopy, passive smoking, and household income characteristics of the patients (Supplementary Figure S1).
Abundance of bacterial taxa in the airway and stool for rhinitis and asthma
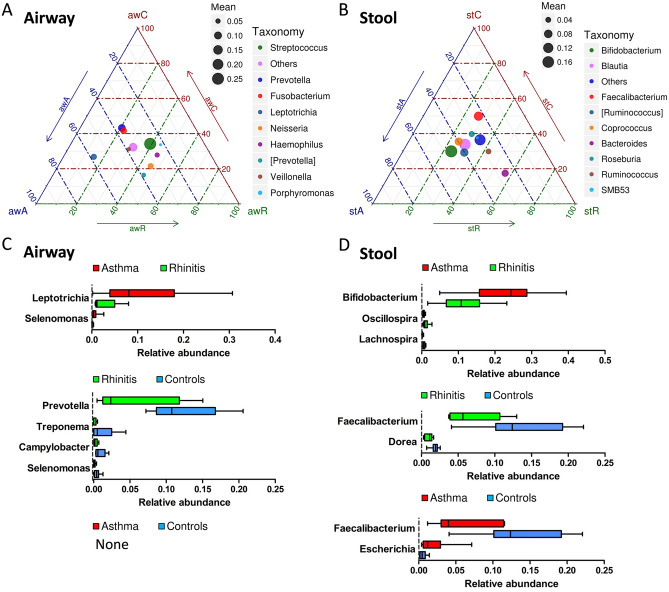
The ternary plots of OTU distribution were generated to display the bacterial community composition and abundance contributing to rhinitis and asthma (Fig. 2a,b). The genera Neisseria and Haemophilus in the airway and Bacteroides in the stool microbiota were orientated toward mite-sensitized rhinitis. Contrarily, Leptotrichia in the airway and Bifidobacterium, Ruminococcus, and Blautia in the stool microbiota were orientated toward mite-sensitized asthma. Figure 2c,d show the differences in the abundance of the members of different genera among children with rhinitis and asthma and healthy controls using the MetaStat method. The genera Prevotella, Treponema, Campylobacter, and Selenomonas in the airway, and Faecalibacterium and Dorea in the stool microbiota were significantly reduced in children with mite-sensitized rhinitis compared with those in healthy controls. However, compared with children with mite-sensitized rhinitis, those with mite-sensitized asthma had significantly increased Leptotrichia and Selenomonas in the airway and Bifidobacterium in the stool microbiota.
Figure 2.
OTU distribution of ternary plots and significant differential expression of bacteria at the genus level across rhinitis, asthma, and healthy controls in airway (A, C) and stool (B, D). In ternary plots, the colors represent the genera to which corresponding OTUs were assigned. The size of plotted dots corresponds to the abundance of the OTUs with respect to each disease. The position of each circle is determined by the contribution of the indicated diseases to the total abundance. OTU, operational taxonomic unit.
Association of airway and stool microbiota related to fecal and serum IgE levels
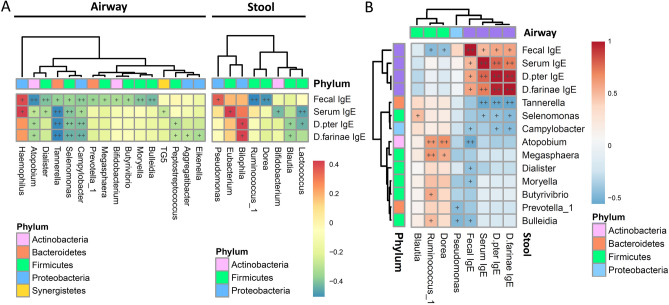
Spearman’s rank correlation coefficients between the airway and stool microbiota grouped by bacterial phylum are shown in Supplementary Figure S2. The genera Atopobium, Megasphaera, and Leptotrichia in the airway microbiota were significantly negatively correlated with Akkermansia, but positively correlated with Dorea and Ruminococcus in the stool microbiota. Figure 3a presents the correlations of the airway and stool microbiota with the fecal and serum IgE levels. Airway microbiota appeared to be mostly correlated with the total fecal IgE levels. Atopobium, Bulleidia, Moryella, and Dialister in the airway and Dorea and Ruminococcus in the stool microbiota were found to be negatively correlated with the total fecal IgE levels (P < 0.01). Conversely, Haemophilus in the airway and Bilophila, Eubacterium, and Pseudomonas in the stool microbiota were positively correlated with the IgE levels. Furthermore, Tannerella, Selenomonas, and Campylobacter in the airway microbiota were significantly negatively correlated with the total fecal, serum and mite-specific IgE levels.
Figure 3.
Heatmap of Spearman’s rank correlation coefficients between airway and stool microbiota associated with total fecal IgE and serum mite-specific IgE levels. Airway and stool genera of bacteria significantly correlated with fecal and serum IgE levels (A). FDR-adjusted correlations between IgE-related airway and stool genera of bacteria, and fecal and serum IgE levels (B). Color intensity represents the magnitude of correlation. Red color represents positive correlations; blue color represents negative correlations. + symbol means a P-value < 0.05; ++ symbol means a P-value < 0.01.
Figure 3b depicts the FDR-adjusted correlations between the IgE-related airway and stool genera of bacteria. Their detailed scatter plots of these correlations are shown in Supplementary Figure S3. The total fecal IgE levels were significantly positively correlated with those of specific IgE levels to D. pteronyssinus and D. farinae (P < 0.01). Additionally, Selenomonas in the airway was positively correlated with Blautia in the stool microbiota. Furthermore, Atopobium and Megasphaera in the airway were significantly positively correlated with both Dorea and Ruminococcus.
Discussion
Gut microbiota dysbiosis involved in lung diseases in the gut-lung axis has been widely observed, but the contribution of the allergen exposure on the airway microbiota to this axis remains unclear. This study has demonstrated a modulation of HDM exposure on the microbiota composition alteration predominantly in the airway. An association of the particular subsets of airway microbial dysbiosis and gut microbial community related to allergies supports the existence of cross-talk between the gut and lung contributing to allergic airway diseases.
The human microbiota is extremely diverse and differs significantly at several body sites12. The gut is the most densely colonized surface, whereas airway is one of the least-populated surfaces of the human body. In this study, similar to the gut, the most predominant phylum detected in the airways was Firmicutes. However, the diversity of the airway microbiota was significantly lower than that of the gut microbiota. In addition, an inverse association between the HDM sensitization and microbial diversity of the airway but not of the gut was noted, indicating the alteration of the airway microbiota composition may specifically represent the impact of aeroallergen exposure on allergies.
Allergic reactions to HDM play a significant role in various childhood atopic diseases13. In this study, the fecal IgE levels were strongly correlated with the serum total and mite-specific IgE levels, indicating the gut-lung connection and the possibility of a relation of allergic diseases between gut and lung1. Dysbiosis in gut microbiota has been implicated in several lung diseases, including allergy and asthma14. However, mite-sensitized airway diseases appeared to be only associated with microbial dysbiosis in the airway than in the gut5,9. Despite the fact that these findings support the existence of a cross-talk between the gut and lung, the changes in the airway microbial community related to HDM may take part in the composition of the gut microbiota in childhood rhinitis and asthma.
A modulation between microbial dysbiosis and responses to allergens potentially contributes to the susceptibility to allergic airway diseases5,9. Leptotrichia spp. in the airway was found to be significantly in the interaction of mite sensitization with asthma but not with rhinitis5, which is consistent with the findings of this study. Nevertheless, in the gut, a reduction of Dorea and Ruminococcus spp. inversely associated with the fecal IgE levels was strongly related to children with mite-sensitized rhinitis but not asthma in this study. However, airway Leptotrichia spp. was strongly correlated with gut Dorea and Ruminococcus spp., indicating that the microbial dysbiosis linked to HDM between gut and lung may influence the phenotypes observed in allergic airway diseases.
The microbial dysbiosis and interactions with the gut-lung axis and its implications have a complex effect on health and airway diseases3. In this study, airway Campylobacter, Selenomonas, Tannerella, and Atopobium were strongly correlated with both serum mite-specific and fecal IgE levels. Among them, Campylobacter and Selenomonas were significantly reduced in children with rhinitis. Most importantly, airway Selenomonas and Atopobium spp. were positively related to stool Blautia and Dorea spp., respectively. In the intestinal tract, Blautia spp. primarily provides beneficial anti-inflammatory effects15, whereas Dorea spp. has shown to be associated with childhood rhinitis9. These results suggest that the particular subsets of airway microbial dysbiosis associated HDM-specific IgE responses can interact with corresponding gut microbiome, contributing to the susceptibility to allergic airway diseases.
Gut microbiota coordinates to shape mucosal immunity and contribute to modulating inflammation and allergic reactions16. In this study, reduced Faecalibacterium spp. accompanied with increased Escherichia spp. in the gut microbiota were significantly associated with a higher risk of asthma. The genus Faecalibacterium, a butyrate-producing bacterium, plays an essential role in maintaining the intestinal epithelium integrity for anti-inflammatory activities17,18. Furthermore, butyrate can acidify the local gastrointestinal microenvironment, making it less suitable for the overgrowth of pathogenic species such as Escherichia19. However, both the genera Faecalibacterium and Escherichia were not correlated with IgE levels in this study. These observations elucidate a potential role of certain gut bacteria in a reaction involving other components of the immune system apart from IgE antibodies for childhood asthma.
Fecal microbiota composition is dynamic and individualized depending on the influence of diet, exposition to ingested probiotic bacteria, and intestinal environmental conditions. Bifidobacteria in the probiotics are particularly effective at modulating the immune response and protecting against allergic diseases20. However, the probiotic bacteria could be mostly retrieved in the subjects’ stools without affecting microbial diversity21. In this study, the genus Bifidobacterium spp. was conversely enriched in asthma children, which could be particularly explained by the high usage rate of yogurt and probiotic supplements for allergy as in this study.
One major limitation of this study is it`s relatively small sample size that may not be representative of the entire population. The cross-sectional design of our study also limits the ability to make causal inferences. However, the analysis of outcomes for an age-matched comparison design eliminates the dissimilarities in the microbial compositions across a wide range of age groups. Any contamination will also not have systematically influenced our results because of using the same protocols and procedures for cases and control subjects. Most importantly, the oropharynx is constantly exposed to microbes from both the upper and lower respiratory tract, where would be representative of the microbes related to airway diseases. Moreover, an integrated airway and gut microbiome analysis in this study provides a comprehensive overview of the regulatory network of microbiome in allergic airway diseases.
In conclusion, an inverse association of the airway microbial diversity with HDM sensitization and allergic airway diseases suggests that the modulation between airway microbiota composition and HDM responses may potentially play a role in the susceptibility to airway allergies. A significant association between the particular subsets of HDM-associated airway microbial dysbiosis and gut microbial community corroborates the cross-talk between the gut and lung contributing to allergic airway diseases. However, further research is required for functional studies to elucidate the molecular mechanisms of these associations.
Methods
Study population
A cross-sectional case–control study nested within PATCH birth cohort recruited since 2007 was conducted to investigate the gut and airway microbial profiles in children aged 4–5 years diagnosed with mite-sensitized asthma alone or rhinitis alone for the first time, and healthy controls. Atopic diseases were evaluated using the ISAAC questionnaire and were physician-diagnosed by the same pediatric pulmonologist at the outpatient clinics5,22. Healthy children without a history of atopic conditions or infections were enrolled as controls. Children suffered an upper airway infection or chronic viral infection mimicking atopic diseases were excluded. Information regarding demographic data and factors related to atopic diseases were collected. This study was approved by the Ethics Committee of Chang Gung Memory Hospital (No. 104-3757B). All experiments and methods in this study were performed in accordance with relevant guidelines and regulations, and written informed consent was obtained from the parents or guardians of all study subjects.
Collections of throat swab and stool samples
Samples of throat swab and stool were collected in subjects without receiving antibiotics therapy for at least 4 weeks23. Sterile cotton swabs were rubbed by physician around the oropharynx with swab rotation at least three times to collect throat swab samples5. Throat swab were frozen and stored at − 80 °C immediately after sampling. Clean specimen bottles were used to collect fresh stools by parents from each child according to the instructions on proper method of collection9. The parents were telephone-informed to collect stool samples 3 days before an outpatient appointment. The stool samples were stored in the freezer at home directly after collection and carefully transported to our laboratory in cooled condition using ice cubes, and were then stored at − 80 °C until use.
Measurement of total and allergen-specific IgE levels
Total serum and allergen-specific serum IgE levels were examined as described in our previous study24. Total serum IgE levels were measured by ImmunoCAP (Phadia, Uppsala, Sweden), and specific IgE levels to aeroallergens (Dermatophagoides pteronyssinus and Dermatophagoides farinae) were determined using a commercial assay for IgE (ImmunoCAP Phadiatop Infant; Phadia). Mite sensitization was defined as D. pteronyssinus- or D. farinae-specific IgE levels 17.5 kU/L (class 4–6) to confirm the presence of sensitization25. Total fecal levels of IgE were measured using Immunoglobulin E ELISA Kit (Immundiagnostik AG, Bensheim, Germany) according to the manufacturer’s instructions.
16S rRNA gene amplification and sequencing
Throat swab bacterial DNA was extracted using a FastDNA Spin Kit for Soil (MP Biomedical, Solon, OH, USA) and fecal bacterial DNA was extracted from the same amount of feces (0.5 g) using a FastDNA Spin Kit for Feces (MP Biomedical, Solon, OH, USA) following the manufacturer’s instructions. The variable region V3-V4 encoded for 16S rRNA gene was amplified by using bacteria/archaeal primer 341F/805R for throat swab samples and 341F/806R for stool samples respectively with the barcodes5,26. Sequencing libraries were generated using the NEBNext Ultra II DNA Library Prep Kit for Illumina (New England BioLabs, USA) and subjected to sequencing on an Illumina HiSeq 2,500 platform (Illumina, Inc., San Diego, CA, USA) according to the manufacturer’s instructions. DNA extraction kits without clinical sample as negative controls and reagent controls for potential kit contamination were performed, but did not detect any amplicons in both controls for subsequent 16S rNRA sequencing. The sequence raw data and mapping file for all the samples included in this study have been originally deposited in Figshare (https://figshare.com/s/e337e0b26af010885553).
Microbiome data analysis
Analysis of microbiome data was performed using the software ‘‘Quantitative Insights into Microbial Ecology’’ (QIIME 1.9.1)27. Assembled sequences were clustered into operational taxonomic units (OTUs) using Uparse software at 97% sequence identity, and taxonomy classification was assigned based on the Greengenes 16S rRNA Database version 13.828. To normalize variation in sequence depth across samples, OTUs abundance information was rarefied to the minimum sequence depth using the QIIME script (single_rarefaction.py). As described in our previous study5, richness of each sample was calculated with the Chao1 index and diversity accounting for both relative abundance and evenness was evaluated with Shannon index. The ternary plot of OTU relative abundance was generated to visualize the distribution of bacterial communities among rhinitis, asthma, and healthy controls with the ggtern extension package to R software (Lucent Technologies, NJ, USA, version 3.5.1)29. This plot showed the proportion of the abundance of the genera per group as positions in the triangle using barycentric coordinates. The Metastat method was used to test the differences in the microbial composition of microbiome between groups with adjusted P-values for multiple comparisons30. In the species with significant differences, OTUs presented in at least 10% of samples with a mean proportional abundance of > 0.01% were further selected31.
Statistical analysis
Comparisons of baseline characteristics between children with rhinitis and asthma, and between with mite-sensitized allergic airway diseases and healthy controls were done with non-parametric tests such as Mann–Whitney U test, chi-square test, and Fisher’s exact test. The correlation coefficients between the gut and airway bacterial compositions, and fecal and serum specific IgE levels were calculated using Spearman’s correlation test with false discovery rate (FDR) adjustment for multiple measurements in R software (Lucent Technologies, NJ, USA, version 3.3.1). Statistical analysis was performed by using the Statistical Package for the Social Sciences (SPSS Statistics for Windows Version 20.0; Armonk, NY). All statistical hypothesis tests were 2-tailed and a P-value < 0.05 was considered significant.
Supplementary information
Acknowledgements
This study was financially supported by research Grants of CMRPG2G0651-3 and CMRPG2E0301 from the Chang Gung Memorial Hospital, Taiwan, and MOST 108-2314-B-182A-087 of the Ministry of Science and Technology in Taiwan. We are extremely grateful for all the members of the Clinical Informatics and Medical Statistics Research Centre, Chang Gung University for the helpful comments and technical assistance.
Author contributions
C.-Y.C. and S.-C.S. designed and supervised the study. Y.-L. C. and M.-H.T. performed experimental work and interpretation. C.-J.W and M.-H.C. performed statistical analyses and presented the data. C.-C.C. was responsible for clinical evaluation of the children and data collection. All authors discussed the results and reviewed the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available duo to the personal privacy of subjects but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chih-Yung Chiu, Email: pedchestic@gmail.com.
Shih-Chi Su, Email: zenith5862@hotmail.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-70528-7.
References
- 1.Anand S, Mande SS. Diet, Microbiota and Gut-Lung connection. Front. Microbiol. 2018;9:2147. doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frati, F. et al. The Role of the Microbiome in Asthma: The Gut(-)Lung Axis. Int. J. Mol. Sci.20 (2018). [DOI] [PMC free article] [PubMed]
- 3.Marsland BJ, Trompette A, Gollwitzer ES. The Gut-Lung axis in respiratory disease. Ann. Am. Thorac. Soc. 2015;12(Suppl 2):S150–156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 4.Sze MA, et al. Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS ONE. 2014;9:e111228. doi: 10.1371/journal.pone.0111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu CY, et al. Airway microbial diversity is inversely associated with mite-sensitized rhinitis and asthma in early childhood. Sci. Rep. 2017;7:1820. doi: 10.1038/s41598-017-02067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderon MA, et al. Respiratory allergy caused by house dust mites: What do we really know? J. Allergy Clin. Immunol. 2015;136:38–48. doi: 10.1016/j.jaci.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Bugiani M, et al. Allergic rhinitis and asthma comorbidity in a survey of young adults in Italy. Allergy. 2005;60:165–170. doi: 10.1111/j.1398-9995.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 8.Nelson, V., Couroux, P., Sadoway, T., Patel, P. & Salapatek, A. M. Comorbidity of allergic rhinitis and asthma to birch allergen in an environmental exposure chamber. Eur. Respir. J.46 (2015).
- 9.Chiu CY, et al. Gut microbial dysbiosis is associated with allergen-specific IgE responses in young children with airway allergies. World Allergy Organ. J. 2019;12:100021. doi: 10.1016/j.waojou.2019.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 11.Huang YJ, et al. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017;139:1099–1110. doi: 10.1016/j.jaci.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ursell LK, et al. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J. Allergy Clin. Immunol. 2012;129:1204–1208. doi: 10.1016/j.jaci.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu CY, et al. Sensitization to food and inhalant allergens in relation to atopic diseases in early childhood: a birth cohort study. PLoS ONE. 2014;9:e102809. doi: 10.1371/journal.pone.0102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hufnagl, K., Pali-Schц╤ll, I., Roth-Walter, F. & Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Seminars in immunopathology42, 75–93 (2020). [DOI] [PMC free article] [PubMed]
- 15.Jenq RR, et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Transpl. 2015;21:1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Zhu L, Qin S. Gut microbiota modulation on intestinal mucosal adaptive immunity. J. Immunol. Res. 2019;2019:4735040–4735040. doi: 10.1155/2019/4735040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demirci, M. et al. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol (Madr) (2019). [DOI] [PubMed]
- 19.Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17:592–602. doi: 10.1016/j.chom.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akay HK, et al. The relationship between bifidobacteria and allergic asthma and/or allergic dermatitis: a prospective study of 0–3 years-old children in Turkey. Anaerobe. 2014;28:98–103. doi: 10.1016/j.anaerobe.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Laursen MF, et al. Administration of two probiotic strains during early childhood does not affect the endogenous gut microbiota composition despite probiotic proliferation. BMC Microbiol. 2017;17:175. doi: 10.1186/s12866-017-1090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cave AJ, Atkinson LL. Asthma in preschool children: a review of the diagnostic challenges. J. Am. Board Fam. Med. 2014;27:538–548. doi: 10.3122/jabfm.2014.04.130276. [DOI] [PubMed] [Google Scholar]
- 23.Costa M, Weese JS. Methods and basic concepts for microbiota assessment. Vet. J. 2019;249:10–15. doi: 10.1016/j.tvjl.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Chiu, C. Y. et al. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatr. Allergy Immunol. (2015). [DOI] [PubMed]
- 25.Zhang C, et al. House dust mite and storage mite IgE reactivity in allergic patients from Guangzhou, China. Asian Pac. J. Allergy Immunol. 2012;30:294–300. [PubMed] [Google Scholar]
- 26.Caporaso JG, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald D, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton, N. E. & Ferry, M. ggtern: Ternary Diagrams Using ggplot2. J. Stat. Softw.87 (2018).
- 30.White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 2009;5:e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crusell MKW, et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome. 2018;6:89. doi: 10.1186/s40168-018-0472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available duo to the personal privacy of subjects but are available from the corresponding author on reasonable request.