Abstract
There is a need of experimental studies on biomarkers in patients with anorexia nervosa (PAN), especially in the context of stress, in order to foster understanding in illness maintenance. To this end, the cortisol response to an acute stressor was investigated in n = 26 PAN (BMI: 19.3 ± 3.4 kg/m2), age, and gender matched to n = 26 healthy controls (HC; BMI: 23.08 ± 3.3 kg/m2). For this purpose, salivary cortisol parameters were assessed in two experimental conditions: (1) rest/no intervention and (2) stress intervention (TSST; Trier Social Stress Test). In addition, psychological indicators of stress were assessed (Primary Appraisal Secondary Appraisal, Visual Analogue Scale, and Trier Inventory for the assessment of Chronic Stress), as well as psychological distress, depression, and eating disorder (ED) symptoms. A 2 × 2 × 8 ANOVA demonstrated elevated cortisol levels in PAN in the resting condition. In the stress intervention no significant group effect in terms of cortisol (F (1, 50) = 0.69; p = 0.410; ). A significant condition (F (1, 50) = 20.50; p = 0.000; ) and time effect (F(2.71, 135.44) = 11.27; p = 0.000; ) were revealed, as well as two significant interaction effects. First: Condition × group (F (1, 50) = 4.17, p = 0.046; ) and second: Condition × time (F (2.71, 135.44) = 16.07, p = 0.000, ). In terms of AUCG, no significant differences between both groups were exhibited. Regardless, significant results were evinced in terms of an increase (AUCi: F(1, 50) = 20.66, p = 0.015, ), baseline to peak (+20 min post-TSST: t5 = 16.51 (9.02), p = 0.029) and reactivity (MPAN = 0.73 vs. MHC = 4.25, p = 0.036). In addition, a significant correlation between AUCG and BMI: r (24) = −0.42, p = 0.027 was demonstrated, but not between AUCi and BMI (r (24) = −0.26, p = 0.20). Psychological indices suggested higher levels of chronic and perceived stress in PAN relative to HC. However, stress perception in the stress condition (VAS) was comparable. Additional analyses demonstrated that ED-symptoms are highly correlated with psychological distress and depression, but not with BMI. In addition, it could be demonstrated that reactivity is rather related to ED-symptoms and psychological burden than to BMI. In conclusion, PAN showed elevated basal cortisol levels at rest and exhibited a blunted cortisol reactivity to the TSST as evinced by salivary cortisol parameters. Further, it was shown that weight recovery influences reversibility of hypercortisolemia, i.e., cortisol levels normalize with weight gain. However, HPAA (hypothalamus–pituitary–adrenal axis) irregularities in terms of reactivity persist even at a BMI ≤ 19.3 (±3.4). Our data suggest that pronounced psychological burden in PAN, have a greater impact on the HPAA functionality (secondary to the ED) than BMI itself.
Subject terms: Predictive markers, Human behaviour
Introduction
A growing plethora of studies has increasingly classified eating disorders (EDs) as a relevant cause of mortality, particularly in the case of anorexia nervosa (AN), since it has the highest mortality rate among EDs1. Standardized mortality ratios show at least five times greater rate of death in AN than in the general population1. Only less than half of PAN recovered at follow-up due to severe effects of illness progression on both, mental and physical health2. Maintenance of underweight, fear of weight gain, purging behaviors, constant weight and shape concerns as well as emotional dysregulation are typical characteristic of PAN3–5. Even though, the etiopathogenesis of EDs is still unclear, the role of the HPAA axis6 and stressful life events have been emphasized in the onset and maintenance of EDs7–13. The sympathetic nervous system and the hypothalamus–pituitary–adrenal axis (HPAA) work as mediators of the stress response. Stress activates the HPAA altering cortisol levels as a regulatory function (e.g.,14,15). Cortisol levels gradually increase within a few minutes (~10 min) after stimulation and peaks between 10–30 min. after stress termination16 for details on normal function see14,17. The detection of adequate cortisol release results in a negative feedback loop signaling cessation of cortisol release. This termination is crucial for limiting cortisol exposure in the organism, since chronic exposure is detrimental to health18 and may contribute to the pathophysiology of anxiety and mood disorders19–21. The system can drift from normal, adaptive HPAA functioning in a variety of ways, e.g., by not activating the system when necessary, activating it when dispensable, or not halting it when a stressor is adequately addressed22. An altered HPAA functionality has been mainly observed in individuals, who were exposed to adversity, specially at early life stages14,23. Alterations in PAN manifest in hypercortisolemia (e.g.,10,11) disturbed physiological and behavioral processes24 related to appetite25 and energy expenditure26 and a diminished sensitivity of the hypothalamic and pituitary centers to negative feedback, as evinced in reduced suppression of cortisol after a dexamethasone-suppression test (DST27–29) and a blunted response to acute stress exposure28,30–34. HPAA dysregulation in PAN has been also associated with childhood adversities23,35 and psychological variables, such as pronounced ED-symptomatology, body-image concerns, and psychopathology (e.g., depression, distress36–41). It is thought that these variables induce constant alertness and stress influencing HPAA functioning35,42. In general, cortisol plays an essential role in promoting allostasis, including mediating and suppressing healthy stress responses15. On the other hand, chronic exposure to stress has detrimental effects on health and may contribute to the pathophysiology of affective disorders19–21.
In research related to PAN dominates a great consensus on HPAA hyperactivity (e.g., hypercortisolemia10,28,43–46) at rest, mainly accounted by the well-known effects of starvation and weight loss on HPAA function6,31. Södersten et al.47 even claim that weight related issues, e.g., eating behavior and extreme caloric restriction are major causes for the physiological and psychological changes in PAN. This phenomenon has been additionally supported by research on healthy individuals31,48. An earlier experimental study reported that even healthy individuals, who starve develop similar psychological and physiological symptoms characteristic to AN. Also, in recent years, numerous studies on volunteers free of an ED evinced that extreme caloric restriction (e.g., fasting) resulted in elevated cortisol compared with less drastic diets48–51. Cortisol levels and length of caloric restriction were negative correlated, suggesting an initial increase in cortisol, but a decrease (to baseline) after several hours (e.g.,48,52). This outcome implies, that excessive caloric deprivation results in a temporary HPAA hyperactivity in healthy individuals48. These studies underline the importance of energy deprivation in the development of anorectic symptoms53. In conclusion, elevated baseline cortisol levels are likely observed during extreme caloric restriction in both, HC and PAN. However, hypercortisolemia in HC is temporary. Conversely, reversibility of HPAA dysregulation even in recovered PAN is not always observed. In this regard, some studies reveal that irregularities remain even after weight restorage54–56. In treated/weight recovered PAN, baseline abnormalities in cortisol levels28,54,56, and menstrual cycle57 persisted compared with controls. To counterbalance, literature suggesting otherwise is dominant. Researchers have recurrently demonstrated that HPAA functionality is normalized with weight recovery or treatment. A great body of past and current studies29,58 have namely reported comparable cortisol levels (plasma, salivary) between treated/weight restored patients and controls45,46,59–62 and normalization of other hormones (e.g., leptin, insulin, cortisol63); emphasizing restoration of normal HPAA activity64. Due to the dominance of a variety of studies evincing normalization of HPAA dysregulation, there is a high probability that most of the endocrine functions are restored after therapy/weight recovery. Briefly, predominant literature suggests higher basal cortisol levels (at rest) in PAN compared with HC. Abnormal HPAA activity such as hypercortisolemia is a.o., related to starvation. A great body of literature supports that HPAA irregularities secondary to starvation are temporary in healthy individuals and reversible in PAN after treatment/weight gain. Nevertheless, HPAA activity in terms of the cortisol response to a particular stressor (i.e., reactivity) research often suggests a blunted cortisol response in PAN28,32–34. It is suggested that chronic stress exposure is linked to HPAA hyporeactivity65,66. Even though laboratory induced stress to investigated the HPAA activity has received attention, there are very few experimental studies on the stress response in ED patients using salivary cortisol assessments in the context of psychosocial stress (e.g., TSST). Thus, when looking at the HPAA reactivity outcomes are less abundant and less consistent. In addition, these few studies reveal contradictory results32,67–69. What is more, comparable experimental research on psychosocial stress has focused on bulimia nervosa, binge eating disorder (e.g.,67,70), or mixed groups32,68,71, while outcomes specific to PAN have received little attention. In addition, disorder-specific samples of anorectic adults are still limited in terms of availability as well as in size (e.g.,32,68) and there is no clear consensus emerging from these studies. The current state of experimental research on PAN in terms of the cortisol reactivity to acute psychosocial stress, even by the employment of the same protocol (TSST; Trier Social Stress Test72), and same hormonal parameter (i.e., salivary cortisol) is still inconclusive. For example, comparable studies on PAN report a blunted stress response to the TSST32,34,67 relative to the control group. On the other hand, other studies could not replicate these results, e.g., the study of Monteleone et al.68 described a preserved HPAA activity in PAN in response to the TSST. In a systematic review73 no differences between patient groups (incl. AN) and controls were further observed. Vocks et al.33 also report similarities between ED patients and HC in terms of reactivity, after a mirror exposure task. Given the inconsistencies concerning cortisol reactivity, additional research is needed in order to extend these results and foster understanding on underlying mechanisms. Differences in sample size and population, BMI, treatment status, menstrual cycle, as well as comorbidities, may explain some variation. In terms of sample size, Monteleone et al.68 included only seven patients with AN (vs. eight HC) making findings susceptible to random effects, due to the small sample size. In terms on weight/nutrition and therapy status, some participants were inpatients32, while others outpatients with unknown ED-symptoms, treatment and nutrition status68. In addition, Het et al.32 examined PAN separately, while the results of Monteleone et al.71 refer to patients with an ED as one entity. Furthermore, some patients exhibited greater symptom severity and had comorbid depression and anxiety32, whereas in the study of Monteleone et al.68 these variables were not reported, limiting the comparability of results. The study of Het et al.32 appears to have the highest experimental standards, although total cortisol output in terms of AUCG is not explicitly reported. Still, in this research all confounders were controlled, the stress intervention and cortisol assessment were distinctively standardized. What is more, multiple parameters besides salivary cortisol were considered (i.e., heart rate variability, salivary alpha-amylase) increasing the validity of results. In addition, it provided the largest PAN-sample and information on treatment, nutrition and symptom severity, which allows comprehensive conclusions.
Purpose of the study and hypotheses
In summary, experimental studies on the cortisol reactivity specific to PAN are limited and outcomes are ambiguous. Differences in sample size and population compromises generalizability of the results even among equivalent studies using the same protocol (TSST) for stress-induction. Therefore, the main objective of this study is to extend the experimental outcomes in the field of EDs in terms of the cortisol response in PAN after acute stress exposure. For this purpose, we aim to provide a research with a gender and age-matched sample of adults under highly standardized conditions with the following aims. First, investigate the cortisol stress response pattern in a bigger sample (PAN vs. HC) in an experimental setting. Second, examine the relationship between BMI and cortisol response. For this purpose, participants were classified according to ICD-10 in normal-weight (BMI ≤ 25 kg/m2) and anorectic participants (with underweight BMI ≤ 17.5 kg/m2 and weight recovered BMI ≤ 18.5 kg/m2) and reasoned the following hypotheses. First, based on past studies we suggest elevated basal cortisol levels in PAN in the resting condition (H1). This hypothesis relies on predominant literature reporting elevated basal levels (at rest) in PAN (e.g., hypercortisolemia) even after treatment/weight recovery. Second, we expect significant group differences in HPAA reactivity. Thus, we hypothesized that PAN would show a blunted hormonal pattern after stress exposure (TSST). Consequently, AUCi in HC will exhibit a significant increase compared with PAN (H2). This hypothesis is based on past studies related to the HPAA functionality in PAN suggesting abnormal functionality of the HPAA in PAN, as evinced by blunted reactivity to the TSST28,32–34 and also reflected in a diminished sensitivity to negative feedback to the DST27,28 and hypercortisolemia (e.g.,10,11). HPAA dysfunctions in PAN has been often associated with chronic stress8,17–19, pronounced ED-symptoms35,42, and psychopathology (e.g.,36–38). Since PAN in our study exhibit elevated basal cortisol levels, chronic stress, and psychological burden (compared with HC), a decreased sensitivity of the HPAA to negative feedback can be assumed. Hence it is conceivable that PAN’s endocrine system may not perceived acute stress (VAS) (e.g., TSST) as stressful as compared with HC with lower basal cortisol. Therefore, we assume differences in HPAA reactivity between HC and PAN. Furthermore, most of the studies suggest that HPAA functionality is restored after weight recovery. Therefore, we reasoned that our results will support influential literature on the normalization of the HPAA activity in terms of total cortisol output and reactivity with weight recovery (H3; e.g.,46,59). Hence, we expect a positive correlation between BMI and AUCi and a negative correlation between BMI and AUCG in PAN.
Laboratory studies measuring the neuroendocrine responses to a standardized stress task in ED patients and HC is relevant in determining whether AN is characterized by a general dysregulation of the autonomic stress response or not and might help in clarifying inconclusive results. To our knowledge this is the first study that analyses the endocrine response to acute psychosocial stress in a larger group of only PAN under highly standardized conditions.
Material and methods
Study participants
In the present study, n = 26 participants with AN (n = 18 with a BMI > 18.5 Kg/m2 and n = 8 with a BMI < 17.5 Kg/m2) and n = 26 healthy participants (BMI = 23.08 ± 3.3 kg/m2) between 18 and 65 years of age were recruited through online media, newspapers, and bulletin boards at several universities. During the course of the study, PANs were still inpatients at the Polyclinic for Psychotherapy and Psychosomatic in Dresden, Germany, who were diagnosed with AN at the time of admission. During their inpatient treatment some of these patients were asked to participate in our study, but first after reaching a certain level of stability, which was reflected in their BMI (some of the patients were artificially nourished). Please see Table 1 for details. Still, PAN with a higher BMI were also significantly taller (M = 170 ± 5) than PAN with underweight (M = 162.4 ± 5.5). All PAN were re-screened and included in the study after a positive examination of the inclusion criteria. The patients met all diagnostic criteria of AN according to the Structured Clinical Interview (SCID) for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV74,75). However, with the exception that some of the PAN have recovered some weight during the course of inpatient treatment at the clinic and showed a BMI larger than 17.5 Kg/m2 (see Table 1). Exclusion criteria for the clinical group was the presence of other mental disorders (besides depression), health diseases, and intake of any type of medication. HC with a past or a current eating or other mental disorder, a chronic illness, medication treatment, stressful life events in the past 6 months and an abnormal BMI were excluded. HC were age and gender matched to PAN. All participants received an expense allowance of 50 Euro after successful participation on both test days. A description of all N = 52 study participants is provided in Table 1. All participants gave a written informed consent. Ethical approval was obtained from the Ethics Committee of the Medical faculty of the Technical University of Dresden, Germany (No#EK25012013).
Table 1.
Characteristic of the matched participants.
| PAN | HC | t/x2 | p | |||||
|---|---|---|---|---|---|---|---|---|
| Total n (%) | 26 | 26 | ||||||
| Female | 24 (92.3) | 24 (92.3) | ||||||
| Male | 2 (7.7) | 2 (7.7) | ||||||
| Contraceptives | 0 (100) | 6 (25) | 9.474 | 0.003***. | ||||
| Cigarettes/tag | 4.80 (7.07) | 1.20 (3.25) | 23.126 | 0.000***. | ||||
| t | p | |||||||
| M (SD) | PAN BMI | |||||||
| >18.5 | PAN BMI | |||||||
| <17.5 | ||||||||
| BMI | 20.7 (3) | 16.0 (1) | 4.34 | 0.000*** | 23.08 (3.3) | −4.139 | 0.000***. | |
| Age | 26.50 (6.11) | 25.13 4.79 | 0.560 | 0.58 | 25.0 (5.5) | 0.747 | 0.500 | |
| Size | 170.00 (5.00) | 162.38 (5.55) | 3.30 | 0.003* | 168.21(7.05) | 0.452 | 0.653 | |
| Psychological variables | t | p | ||||||
| TICS-9 | 2.00 (.85) | 1.72 (.68) | 0.826 | 0.417 | 1.12 (.51) | 4.254 | 0.000*** | |
| Norm values | ||||||||
| BDI | 52.12 (29.70) | 35.42 (23.29) | 1.32 | 0.20 |
<14 = normal. 14–19 = mild. 20–28 = moderate. 29–63 = severe depression. |
|||
| SCL (GSI) | 15.00 (9.00) | 11.25 (5.36) | 1.07 | 0.29 | Score 11 = Percentile 92%. Score 15 = Percentile 95.2% | |||
| EDI | 154.166 (64.40) | 156.00 (45.91) | 0.072 | 0.93 | Score 155 = Percentile = 85%. | |||
| PASA (SI)-R | −1.04 (1.70) | −1.80 (1.58) | 1.05 | 0.30 | ||||
| PASA (SI)-TSST | 0.74 (1.71) | 0.71 (.97) | 0.211 | 0.83 | ||||
| VAS-R | 37.00 (11.30) | 39.20 (15.40) | −0.43 | 0.66 | ||||
| VAS-TSST | 55.25 (16.0) | 56.40 (6.30) | −0.192 | 0.85 | ||||
| AUCG-R | 503.57 (30.30) | 477.83 (184.84) | 0.22 | 0.82 | ||||
| AUCG-TSST | 550.02 (273.48) | 825.71 (558.559) | −1.71 | 1.00 | ||||
| AUCI-R | −52.05 (246.44) | 32.79 (139.249) | −0.90 | 0.37 | ||||
| AUCI-TSST | 111.65 (202.45) | 250.56 (479.51) | −1.05 | 0.30 |
PAN participants with Anorexia nervosa, HC healthy controls, M mean, SD standard deviation, TICS trier inventory chronic stress, BDI beck depression inventory, EDI eating disorder inventory, SCL Symptom-Checklist-K-9, PASA (SI)-R Primary Appraisal Secondary Appraisal (Stress Index) in Resting Condition, PASA (SI)-TSST primary appraisal secondary appraisal (Stress Index) in stress condition, VAS-R visual analogue scale in resting condition, VAS-TSST visual analogue scale in stress condition.
***p ≤ 0.001; *p ≤ 0.05.
Procedures
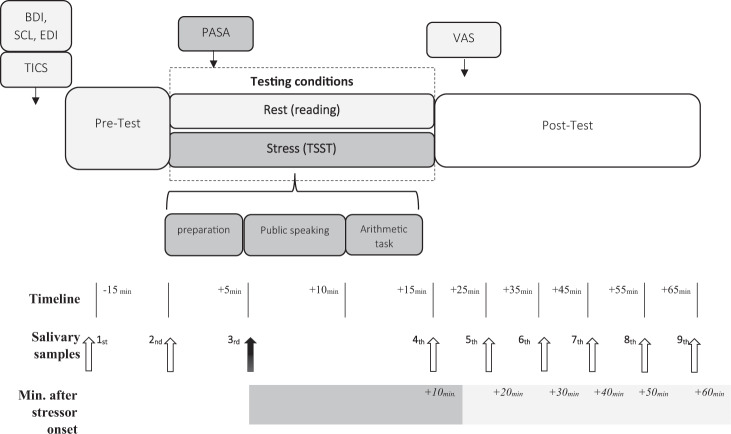
PAN (n = 26) and HC (n = 26) were scheduled on consecutive days for two different experimental conditions (stress and rest) on a given weekday. The procedures took place between 2:00 and 4.00 p.m., in order to standardized cortisol measurements affecting its circadian rhythm. In addition, all healthy females and a small part of PANs (n = 5) were submitted to the TSST only during the luteal phase, since some of the patients were amenorrhoeic (n = 8) and some didn’t submit any information about their cycle (n = 13). Throughout the entire experiment a total of nine salivary samples were collected. Upon arrival, participants signed an informed consent form and completed psychological measures. The acclimatization phase of the participants lasted around 15 min. Acclimatization of the participants to the laboratory settings facilitate pre-study cortisol levels to return to baseline76, thereby allowing a strong TSST responses and low inter-individual variability77. The testing sequence was randomized: 26 participants initially completed the resting condition, while the other 26 started with the stress condition. On the next day, all participants returned to complete the remaining condition. The stress condition consisted on the exposure to an acute stressor (TSST), while during the resting condition participants read in quiet and in a neutral environment. The entire experimental procedure took around 90 min and was divided in three phases: (1) pre-test, (2) intervention phase (rest or stress) and (3) post-test. The experimental conditions were set as follows. (1) During the pre-test phase (~30 min.), all participants completed psychological questionnaires. Salivary samples were collected 15 min. prior intervention (stress or rest) and immediately at the start. prior intervention. (2) In the following 15 min, participants were either exposed to the stress (TSST) or resting condition (quiet reading/room). At the beginning of the assigned condition, all participants filled out the Primary Appraisal Secondary Appraisal (PASA) for the purpose of evaluating stress perception elicited by the corresponding condition. Participants, who were submitted to the stress condition (TSST) underwent three test phases (5 min each = 1. preparation, 2. interview, 3. math task). The resting condition took place in a secluded and quiet laboratory room, where fordable literature was suggested to counteract possible superfluous arousal effects. At this stage, three salivary samples were collected every 10 min. (3) Immediately after the assigned condition (either stress or rest), the participants completed the Visual Analog Scale (VAS) for subjective stress perception assessment with reference to the respective condition. Along the post-test, five salivary samples were collected every 10 min. Please see Fig. 1 for an overview of the procedure.
Fig. 1. Timeline and design of experimental conditions.
Trier Inventory for the assessment of Chronic Stress; PASA Primary Appraisal Secondary Appraisal questionnaire, VAS Visual Analogue Scale, BDI Beck Depression Inventory, SCL Symptom Check List-K-9, EDI Eating Disorder Inventory, TSST Trier Social Stress Test.
Cortisol samples and analyses
Cortisol concentrations were measured by means of salivary samples collected by a Salivette® (Sarstedt, Nümbrecht, Germany). This procedure consists in salivary moistening of a cotton roll for 1 min and placing it into a salivette-device immediately afterwards. Salivettes were refrigerated at 2–8 °C. Salivary cortisol analyses were run after centrifugation using the luminescence immunoassay test method. The latter procedure provides an intra- and inter-assay coefficient of variation below 9.0%, which is considered as robust and valid78.
Stress intervention
Trier Social Stress Test (TSST79). The TSST is known for its highly standardized protocol procedure and its effectiveness in eliciting a stress reaction. This protocol was applied in the stress condition for laboratory stress induction purposes. The TSST is also an internationally established test for inducing an acute psychosocial stress response and its effectiveness has been demonstrated in countless studies worldwide76,80. It conveys uncontrollability, social evaluation, and arithmetic tasks. Subjective perception and cognitive evaluation of stress was assessed on account of the scales PASA and VAS, which were submitted during and after the 15 min stress test.
Psychological assessments
Trier Inventory for Chronic Stress (TICS-981,82). This scale is based on a larger version conveying 57 items and was applied to measure perceived chronic stress during the last 3 months. The short version consists of nine items representing the main dimensions of the long version (i.e., work and social overload, pressure to perform, work discontent, excessive demands at work, lack of social recognition, social tensions, isolation and chronic worrying). Item ratings range from 0 to 5 (0 = never, 1 = rarely, 2 = sometimes, 3 = often, 4 = very often). Higher values indicate greater stress. Satisfactory psychometrics have been demonstrated in many studies and exhibits good reliability between Cronbach’s Alpha α = 0.88–0.9182–84.
Primary Appraisal Secondary Appraisal (PASA85). This measure evaluates four cognitive appraisal processes threat, challenge, self-concept of own abilities, and control expectancy based on a six-point rating scale. Relying on the four primary scales two secondary scales and a stress index are calculated. Primary appraisal encompasses threat and challenge, while secondary appraisal includes self-concept of own abilities and control expectancy. The stress index is calculated by subtracting the primary appraisal from the secondary appraisal and offers a global assessment. This scale was applied to evaluate stress perception (acute stress intervention). The scale has shown satisfactory psychometric values86.
Visual Analog Scale (VAS87). This tool serves as a visual aid scale and it is widely applied to quantify self-report instruments. In our study it was employed immediately after both experimental conditions (stress vs. rest) to rate stress perception from 0 (no stress) to 100 (maximum stress). Satisfactory psychometric properties and usefulness and economy have been evinced in a variety of studies87–89.
Symptom-Check-List-9 (SCL-K-990). This scale is a short version of the Symptom-Checklist-90-Revided91, which is one of the most applied self-rating scales for assessing general psychopathology. It comprises 90 items to be rated on a 5-point Likert scale (from 0 = never to 4 = very often), which indicate symptom severity in nine main psychopathological dimensions: somatic symptoms, interpersonal sensitivity, obsessive–compulsive behaviors, anxiety and depressive symptoms, hostility, phobic symptoms, paranoid tendencies, and psychoticism, and are also comprised in the short version. The scale also provides a global severity index (GSI) as an indicator of overall psychological distress, with higher scores reflecting higher levels of psychopathological distress as well as a greater symptom severity. For the purpose of the present study, we only reported GSI-values. The sum score is calculated by addition of the scores of all scales. The psychometric properties are satisfactory and its reliability values range from α = 0.83 to 0.879292–94. Normative percentile values specific to age and gender are provided94.
Beck Depression Inventory (BDI95,96). This scale was applied to measure depressive symptoms in PAN. The BDI is a self-report questionnaire consisting in 21 items, which describe symptoms and attitudes to be rated in terms of intensity from 0 to 3 (total score range: 0–63). The total score is calculated by addition of the items. Higher scores indicate greater severity of symptoms. Cut-off values are established as follow: <14 = normal, 14–19 = mild depression, 20–28 = moderate depression, 29–63 = severe depression. The psychometric properties of the BDI are satisfactory. Cronbach’s Alpha ranges from α = 0.89 to 0.9497,98.
Eating Disorder Inventory (EDI99,100). The EDI was applied to measure ED related symptoms and attitudes relevant to pathological eating behavior. The scale is composed of 64 items comprised in 8 dimensions: Drive for Thinness, Bulimia (B), Body Dissatisfaction (BD), Ineffectiveness (I), Perfectionism (P), Interpersonal Distrust (ID)I Interoceptive Awareness (IA), and Maturity Fears (MF). The items are to be rated on a six-point rating scale (0 = never to 5 = always). Reported Cronbach’s Alpha range from α = 0.72 to 0.92100,101. The values of the PAN in the present study are based on the percentile values provided102.
Statistical analysis
The optimum statistical sample size was calculated with the G*power program (version: 3.1.9.2.). Based on a medium effect size of Cohen’s f = 0.25, two groups (HC vs. PAN), n = 8 repetitions, a significant level of p = 0.05, power of 80% (1–β = 0.80), and after Bonferroni-correction, a total sample size of n = 26 for within-subjects factor and n = 52 for between-subjects factor was needed. For the analyses of the cortisol output of anorectic adults and healthy controls after a stress condition. A 2 (condition: rest vs. TSST) × 8 (time) × 2 (PAN vs. HC)-factorial ANOVA for repeated measures with time and condition as within-factor and group as between-factor was calculated with SPSS (version 26). The assumption of sphericity was controlled by Mauchly’s test, and if required, the Greenhouse-Geisser correction was applied. Prior to data analyses, participants were first matched for age and gender. In the next step, sociodemographic and life-style variables (e.g., smoking and use of contraceptives) between both groups were examined by means of the independent t-test and chi-square test. Second, the cortisol pathway response after stress induction in both groups (over eight measurement points: −5, +0, +10, +20, +30, +40, +50, +60) were analyzed by a two-factorial ANOVA for repeated measures with the between-factor group (N = 52) and the within-factor time (n = 26). The pre-test measure of −15 min was not included in the analyses, since elevated pre-test cortisol levels could possibly mask laboratory induced stress, resulting in falsely altered responses77. Use of oral contraceptive and smoking status were tested to control influencing factors on cortisol. Since cortisol data was not normally distributed, we worked with values subjected to log transformations. Moreover, the following cortisol parameters were calculated: area under the curve (AUC) with respect to ground (AUCG) and increase (AUCI), as well as reactivity and peak-base values were calculated. Reactivity is defined as the change in salivary cortisol during the experimental phase and is calculated as the difference between the first and the last sample, while peak-base is the time from baseline to peak-value103. An ANOVA was further computed to evaluate differences in the derived cortisol parameters, in basal levels and stress appraisal between the research groups. To specify the cortisol response to the TSST, the participants were categorized into non-responders and responders based on an increase of at least 1.5 nmol/L104.
Subsequently, a correlation was performed between BMI and AUCG, and BMI and AUCI values in order to investigate the relationship between the cortisol and BMI in PAN. Thereafter, a further t-test for independent samples was computed to assess group differences (PAN vs. HC) in chronic stress (TICS-9) and further stress appraisal scales (PASA and VAS). Moreover, psychological measures (EDI, BDI, SCL-K-9) in PAN were evaluated and compared with available norm values. For exploratory purposes we computed correlations (Pearson-Product-Moment-Correlations) between BMI values and psychological measures. Lastly, we calculated t-test to assess the data of only PAN. We compared the data of underweight anorectic patients (BMI < 17.5 Kg/m2) to weight recovered patients (BMI > 18.5 Kg/m2).
Results
Psychological measures
A summary of the sociodemographic variables of all study participants is displayed in Table 1. In terms of randomization of the procedure, there was no sequence effect. PAN and HC were successfully matched for age and gender. Significant differences between the two groups in terms of BMI use of contraceptives, smoking, and chronic stress were exhibited. As observed, none of the members of the clinical group used contraceptives, but smoked significantly more than the healthy controls. In addition, PAN reported higher values of chronic and perceived stress, as well as depression in comparison to HC and norm values respectively. In addition, PAN showed pronounced values in terms of general psychological distress (GSI) and ED symptomatology (EDI) according to percentile values (see Table 1). Since some of the PAN had recovered some weight (BMI > 17.5 Kg/m2), while others were still underweight (BMI < 17.5 Kg/m2) we re-analyzed data comparing the above-mentioned group categories in PAN. Further analyses (e.g., t-tests) showed neither significant differences between the two groups in terms of psychological measures nor in cortisol (see Table 1). As shown in Table 2, VAS after stress induction was reflected in values higher than 0 without a significant difference between both groups (t(1, 50) = 0.195; p = 0.71), indicating a successful stress induction. In terms of appraisal (PASA), significant differences between the groups were exhibited only in particular subscales: Threat (t(1, 50) = 2.101, p = 0.041), Self-Concept t(1,50) = −4.802, p = 0.000), and Secondary Appraisal (t(1, 50) = −3.332; p = 0.002) as well as in the Stress-index (t(1, 50) = 2.750; p = 0.008), whereby PAN showed a higher stress index. PASA-values were correlated with different cortisol parameters, however no significant correlations were shown (e.g., AUCG and PASA (SI), r (25) = −0.108, p = 0.608; AUCG and PASA (SI), r (25) = −0.199, p = 0.339). In general, PAN evaluated the experimental procedures as more threatening and experience themselves as less influential over the ongoing circumstances. Further, they felt less capable of coping with the given situation than healthy adults (see Table 3). Additional analyses revealed significant correlations between BMI and BDI (r (25) = 0.402, p = 0.046), but not between BMI and EDI (r (25) = 0.332, p = 0.098) or BMI and GSI (r (25) = 0.323, p = 0.107). This means that BMI is rather associated with depression rather than with ED-symptoms or distress. Significant and high correlations were also observed between EDI and GSI, r (26) = 0.733, p = 0.000, EDI and BDI r (25) = 0.664, p = 0.000, but not between EDI and BMI r (26) = 0.332, p = 0.098. These results suggest that EDI is rather associated with distress and depression rather than with BMI. Reactivity correlated high with EDI r (26) = −0.505, p = 0.008 and moderately with GSI r (26) = −0.392, p = 0.048, but not with BMI r (26) = −0.114, p = 0.56. Therefore, the higher the ED-symptoms and distress, the weaker the reactivity. In conclusion these outcomes propose that reactivity is rather related to ED-symptoms and psychological burden than to BMI.
Table 2.
Cortisol parameters and Subjective Appraisal in PAN and HC - Conditions and Groups.
| PAN | HC | ANOVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | Condition | Condition | Condition | Group | Interaction | ||||||||
| AUC | Rest | Stress | Rest | Stress | F (df) | p | η2 | F | p | η² | F(df) | p | η2 |
| AUCG | 495.65 (266.49) | 634.85 (393.76) | 372.41 (171.98) | 693.12 (330.65) | 39.19 (1, 50) | .000*** | .439 | .086(1, 50) | .770 | .002 | 7.19 (1, 50) | 0.010* | 0.126 |
| AUCI | −25.94 (219.83) | 154.393 (310.68) | 8.94 (114.98) | 312.37 (342.07) | 20.91 (1, 50) | .000*** | .295 | 20.66(1, 50) | .015* | .113 | 2.96 (1,50) | 0.091 | 0.056 |
|
Baseline (t-15 min.) |
11.53 (6.80) | 11.00 (6.00) | 7.49 (3.11) | 8.68 (4.44) | 177 (1, 50) | .676 | .004 | 6.54 (1, 50) | .014* | .116 | 1.22 (1, 50) | 0.274 | 0.024 |
| (t-1 min.) | 10.22 (6.41) | 9.42 (5.21) | 7.12 (3.71) | 7.46 (4.00) | .076 (1, 50) | .784 | .002 | 5.48 (1, 50) | .023* | .099 | .454 (1, 50) | 0.503 | 0.009 |
| Subjective Appraisal | |||||||||||||
| PASA - SI | −1.38 (1.62) | .70 (1.49) | −2.40 (.98) | −.23 (.87) | 120.508 (1, 49) | .000*** | .711 | 10.59 (1, 49) | .002** | .178 | .045 (1, 49) | 0.833 | 0.001 |
| VAS | 37.39 (12.62) | 55.62 (13.49) | 30.49 (10.72) | 54.89 (13.22) | 114.205 (1, 49) | .000*** | .700 | 1.737 (1 49) | .197 | .034 | 2.39 (1, 49) | 0.128 | 0.047 |
PAN all participants with Anorexia nervosa, HC healthy controls, M Mean, SD standard deviation, AUCG area under the curve with respect to the ground, AUCI area under the curve in respect to the increase, PASA primary appraisal secondary appraisal, VAS visual analogue scale.
*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Table 3.
Influence of the stress condition on subjective appraisal and hormonal response in PAN and HC.
| PAN | HC | t | p | |
|---|---|---|---|---|
| PASA | M (SD) | |||
| Subjective appraisal | ||||
| Threat | 4.30 (1.15) | 3.72 (0.788) | 2.10 | 0.041* |
| Challenge | 4.59 (0.97) | 4.58 (0.533) | 0.01 | 0.987 |
| Self-concept | 2.93 (1.11) | 4.20 (0.74) | −4.80 | 0.000*** |
| Control expectancy | 4.55 (0.68) | 4.57 (0.868) | −0.10 | 0.915 |
| Primary appraisal | 4.44 (0.98) | 4.15 (0.57) | 1.28 | 0.206 |
| Secondary appraisal | 3.74 (0.70) | 4.40 (0.68) | −3.33 | 0.002*** |
| VAS | 55.62 (13.49) | 54.89 (12.22) | 0.19 | 0.84 |
| Hormonal response | M (SD) | t | p | |
| Derived parameters | ||||
| Peak-base | 7.5 (8.53) | 10.7 (9.04) | −2.25 | 0.029* |
| Reactivity | 0.73 (5.10) | 4.25 (6.30) | −2.17 | 0.036* |
PAN all participants with Anorexia nervosa, HC healthy controls, M mean, SD standard deviation, PASA primary appraisal secondary appraisal, VAS visual analogue scale.
*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Cortisol response and total output
Due to a skewed distribution of the cortisol data, statistical analyses were computed on log-transformed values. The pre-test measure of −15 min was not included in the analyses, since pre-test cortisol levels were elevated in spite of the acclimatization phase (as recommended by 78 and 79) and including it may result in falsely altered responses77. Cortisol values and further derived parameters are displayed in Tables 2–4. There were no significant differences between responders and non-responders (z = −0.363, p = 0.717).
Table 4.
Cortisol values of PAN and HC in nmol/l at nine measurement timepoints.
| Salivary samples | aMin. | Rest | Stress | Condition | Rest | Stress |
|---|---|---|---|---|---|---|
| PAN | HC | |||||
| M (SD) | M (SD) | |||||
| 1 | −20 | 11.53 (6.80) | 11.00 (6.00) | 7.49 (3.11) | 8.68 (4.44) | |
| 2 | −5 | 10.22 (6.41) | 9.42 (5.21) | 7.12 (3.71) | 7.47 (4.00) | |
| 3 | 0 | 10.23 (6.33) | 9.51 (4.77) | 7.46 (3.77) | 8.25 (3.88) | |
| 4 | 10 | 9.91 (6.28) | 11.84 (8.37) | 7.34 (3.49) | 10.98 (5.74) | |
| 5 | 20 | 8.88 (5.42) | 13.88 (9.99) | 6.66 (3.45) | 16.51 (9.02) | |
| 6 | 30 | 9.73 (6.18) | 14.24 (10.52) | 7.32 (4.00) | 14.31 (8.24) | |
| 7 | 40 | 10.28 (6.07) | 12.28 (8.68) | 7.42 (3.63) | 13.77 (6.43) | |
| 8 | 50 | 10.03 (5.18) | 11.36 (5.56) | 7.68 (3.73) | 13.01 (6.29) | |
| 9 | 60 | 9.17 (5.17) | 10.15 (5.63) | 7.38 (3.69) | 11.71 (5.73) | |
PAN all participants with Anorexia nervosa, HC healthy controls, M mean, SD standard deviation.
aMin. relative to stressor onset.
As predicted (H1), baseline cortisol levels at rest (resting condition) were higher in PAN in contrast to those of healthy individuals, while no sig. baseline differences were found in the stress condition (see Table 2).
In terms of the cortisol response, a 2 (condition: rest vs. TSST) × 8 (time: −5, +0, +10, +20, +30, +40, +50, +60) × 2 factorial ANOVA for repeated measures with time and condition as within-factor and group effect (PAN vs. HC) as between-factor revealed the following results. A group effect was not observed F (1, 50) = 0.69; p = 0.410; ). This means that both groups exhibited comparable cortisol mean values. Further analyses showed a highly significant condition effect (F (1, 50) = 20.50; p = 0.000; ) accounting for almost 30% of the variance in participants’ cortisol values. Moreover, a highly significant effect of time over the eight measurement points was evident (F (2.71, 135.44) = 11.27; p = 0.000; ). Further, two significant interaction effects were observed (1. Condition × group and 2. Condition × time). First (marginally significant), condition × group: (F (1, 50) = 4.17, p = 0.046; ), indicating that regardless of time, significant variation in cortisol was observed as a function of both, condition and group. Second (highly significant), condition × time: F (2.71, 135.44) = 16.07, p = 0.000, . This outcome indicates significantly different cortisol response patterns. All participants demonstrated higher levels of salivary cortisol after the stress intervention, than during the resting condition. When controlling for oral contraceptive use, the effect of condition and group on cortisol was attenuated but remained significant. In spite of that, the effect of factor time on cortisol was marginally affected by cigarette smoking (p = 0.045; ), but not by oral contraceptive use. As aforementioned, PAN smoked significantly more than HC.
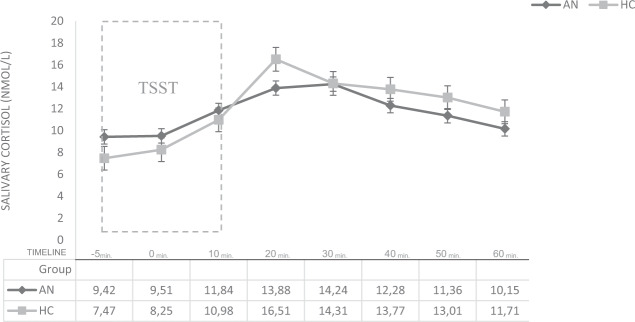
In terms of reactivity, both groups (HC and PAN) showed a TSST-induced cortisol response. However, as predicted (H2), HC demonstrated a higher increase in salivary cortisol (to 221.313% of the baseline) than PAN (151.167%)—see Fig. 2. Also, in terms of an increase (AUCI), significant differences were revealed between both groups (HC vs. PAN)—see Table 2. This increase was also clearly visible. As illustrated in Fig. 2, healthy participants reacted with a greater cortisol increase (from baseline to peak, +20 min. post-stressor onset: t5 = 16.51 (9.02), p = 0.029) and reactivity (MPAN = 0.73 vs. MHC = 4.25, p = 0.036) to the stress intervention, when compared with the patient group. As assumed (H2), these results indicate altogether a blunted cortisol reactivity in PAN. Further, our data notably shows (see Table 4) a delayed cortisol response in PAN (after 30 min.) from stressor onset (i.e., public speaking), when compared with the control group (after 20 min.)—as illustrated in Fig. 2. Concerning our last hypothesis related to PAN (H3), we expected a significant inverse correlation between total cortisol output (AUCG) in the stress condition and BMI. The results confirm this hypothesis = r (24) = −0.42, p = 0.027. This outcome indicates that PAN with a higher BMI released less total cortisol in response to the stress condition (AUCG). We also hypothesized a positive correlation between a cortisol increase (AUCi) and BMI. However, this could not be demonstrated = (r (24) = −0.26, p = 0.20). Please insert Fig. 2.
Fig. 2. Cortisol increase in terms of AUCi.
Significant cortisol increase in PAN vs. HC in the stress condition p > 0.001. PAN individuals with Anorexia Nervosa, HC healthy controls.
Discussion
The present study investigated the salivary cortisol response to a psychosocial stressor under highly standardized laboratory conditions in inpatients with AN and a matched sample of healthy participants. Our data could replicate the results of many studies showing elevated basal levels in PAN (H1) relative to HC. In terms of reactivity, both groups displayed an increase in cortisol after stress induction. However, HC showed a common cortisol response often seen in healthy adults (i.e., relatively rapid cortisol onset followed by decreased values with time progression), while PAN exhibited a delayed and blunted cortisol response pattern (H2). Overall, no significant group (HC vs. PAN) differences were shown in total cortisol output. Furthermore, our data indeed suggests reversibility of hypercortisolemia with a higher BMI, but with persistent irregularities in cortisol reactivity (H3). Altogether, underweight anorectic (BMI < 17.5 Kg/m2) and weight recovered patients (BMI > 18.5 Kg/m2) show comparable results in terms of cortisol response, reactivity, and psychological measures. Concerning psychological variables, PAN generally reported greater chronic and perceived stress relative to HC. PAN also felt more threatened and less resourceful when confronted with the stress intervention, in contrast to HC. What is more, they reported pronounced symptoms of depression (BDI), psychological distress (GSI), and ED-symptomatology according to norm values. Additional analyses revealed significant correlations between BMI and depression, but not between BMI and EDI or GSI. Also, EDI and GSI as well as EDI and BDI correlated high. Reactivity correlated high with EDI and moderately with GSI but not with BMI. In conclusion these outcomes propose that ED-symptoms and reactivity are related to psychological burden than to BMI.
In summary, the outcomes in the present study reflect previous findings in the field of research concerning PAN and reveal comparable outcomes related to the cortisol response in the context of studies using the TSST. In line with the majority of studies on cortisol in PAN (as previously described), our data could also demonstrate elevated basal cortisol levels in AN at rest. Therefore, our first hypothesis could be confirmed. Further, our study was able to replicate the results of Het et al.32 and Zonnevylle-Bender et al.34 showing a blunted HPAA reactivity to acute stress (TSST). The latter was also evinced by Vocks et al.33, however stress exposure was implemented by responding to a mirror task. Notwithstanding, Monteleone et al.68 reported a preserved cortisol response to the TSST in PAN, however this occurred at significantly higher hormone levels (pre-and-post stressor). Possible sources of discrepancies between this outcome and our findings might arise as a result of methodological dissimilarities, including sample size, patient populations, and cortisol assessments (e.g., confounding variables), as revised in the meta-analyses of Monteleone et al.73. Notable difference in sample sizes and mixed clinical groups, are for example noticed in Monteleone’s68 study. In such, only seven patients with were compared with eight HC, making results susceptible to random fluctuations. A further explanation may lie in the characteristics of the PAN. As mentioned, our participants were inpatients still receiving intensive treatment and the majority (n = 18) had a larger BMI (≥17.5–19 kg/m2) than normally observed in affected subjects (BMI < 17.5 Kg/m2). On the other hand, the participants in the study of Monteleone et al.68 were outpatients with a quite low BMI (M = 16.3, SD = 1.2) and possibly malnourished (starvation-mode). The effects of starvation are well known for triggering HPA-axis hyperactivity (e.g.,25,105), resulting in higher cortisol levels. Such were indeed evident in PAN throughout their entire experiment, when compared with HC. The researchers assumed a hyperactivity of the HPAA, but with a “preserved” reactivity68. Considered by itself, this pattern could be interpreted more as an irregularity, than an average response to stress. A common response to stress as presented in healthy individuals, is characterized by a rapid onset followed by a quick recovery76,79. This pattern common to HC was also displayed by the control group described in the current study and is line with responses reported in a recent metanalysis77. What is more, cortisol assessment could have been probably affected by the participants’ menstrual cycle. The female participants in our study were tested in their luteal phase, however many of the PANs did not provide data on their cycle and some were amenorrhoeic, while participants in Monteleone’s68 study were tested during their follicular phase. It is assumed that stress responsivity varies across the menstrual cycle75–77. Beyond that, PAN in our study smoked significantly more than HC, which have may affected cortisol levels, since smoking affects the endocrine system106.
After all, further considerations may be plausible in terms of the blunted cortisol response pattern seen in PAN in our study. As reported by past studies, besides caloric restriction/starvation, other variables affect HPAA functionality e.g., chronic stress, of childhood adversities, psychological burden, and pronounced eating pathology. As aforementioned, the PAN in the present study were still in therapy and demonstrated relatively high levels of chronic and perceived stress, as well as psychological burden and ED-symptoms. Thus, it is conceivable that this increased level of chronic stress together with the pronounced threat perception (PASA) and psychopathology (BDI, GSI) accounted for HPAA dysregulation in PAN to a certain extent. It is known that stress appraisal is a relevant determinant of the cortisol response85 and chronic stress results in long-lasting HPAA activity dysregulation107, e.g., attenuated reactivity resulting in a blunted hormonal response51,65. Correspondingly, Lelli et al.35 reported that patients with a history of childhood adversities (CHA) exhibited an attenuated cortisol response before and after therapy. Analogous results were presented in the research of Monteleone et al.71: PAN with CHA demonstrated a blunted cortisol response to the TSST as compared with controls (HC and PAN without CHA), but with a comparable amount of overall cortisol production, as in the case of the present experiment. Hence, we speculate that the PAN in our study, have also had been affected by adverse experiences in the past. This assumption is based on the blunted response, pronounced ED-symptoms and psychological burden (e.g., GSI, BDI) even while being treated. However, psychological burden alone is also related with a blunted cortisol response to acute stress36,38–41 as presented in PANs in our current study. Therefore, it is conceivable that the observed symptomology (i.e., pronounced chronic and perceived stress, ED-symptoms, depression and psychological distress) might have accounted for the blunted response to the TSST.
Furthermore, this blunted cortisol response in PAN was evident in spite of a higher BMI, since PAN are mostly underweight (BMI < 17.5 Kg/m2). In relation to this parameter, we predicted that irregularities in hormonal response to an acute stressor (TSST) are likely to normalize in individuals with a higher weight. Therefore, we expected an inverse correlation between BMI and AUCG and a positive correlation between BMI and AUCI and (H3). A significant inverse linear correlation between total cortisol output (AUCG) and BMI was indeed observable, suggesting reversibility of hypercortisolemia with weight recovery. Still, HPAA irregularities in terms of reactivity persisted. Thus, this hypothesis was only partially confirmed. In the present study, a lack of hypersecretion of total salivary cortisol output was also manifested in similar AUCG-values between PAN and controls, since total cortisol output was comparable (also confirmed by the 2 × 2 × 8 ANOVA). Similarities in total cortisol output were also reported in comparable studies32,34,71. The former finding is also in accordance with abundant studies reporting a normalization of hypercortisolemia (e.g.,60,64,108,109) in weight recovered PANs. Likewise, earlier studies support our outcome58, e.g., Walsh et al.29 examined the adrenocortical activity in underweight and weight recovered anorexia patients during a 24 h period. The results revealed that as anorectic patients recovered weight, the rate of cortisol production decreased. Other researchers have also replicated this outcome. These finding proposes that dysregulated activity of the HPAA of symptomatic PAN is related to extreme emaciation or underweight. Consequently, it is concluded that altered activity of the HPAA in PAN in terms of total cortisol release is a state-dependent phenomenon. On the other hand, our results did not confirm that reactivity normalizes with weight gain (non-sig. AUCI). The results therefore suggest that the HPAA response to acute stress is still affected in PAN. Therefore, we assume that hypersecretion of cortisol does attenuate with a higher BMI, but irregularities in terms of reactivity persist (even at a BMI = 19Kg/m2). Nevertheless, PAN had still notable ED-symptoms and were still receiving inpatient treatment. In addition, full realimentation (in the sense of weight restorage) and recovery was not concluded. Consequently, it is not clear whether this pattern may change after full remission. In conclusion, this outcome implies that HPAA functionality is not fully restored after weight recovery and that functionality is sensitive to different types of stressors: e.g., bodily stressors (e.g., starvation) acutely affect cortisol production, while chronic stressors affect reactivity long-term. This suggest, that even though hormonal alterations are reversed by refeeding, weight recovery does not restore the full range of HPAA functionality110. Karin et al. recently proposed a detailed explanation of this phenomenon. The researchers elucidated that prolonged HPA activation enlarges the functional masses of the pituitary corticotropes as well as adrenal cortex and that their recovery from stress takes weeks even after stress cessation. In short, this mechanism clarifies why ACTH responses remain blunted for weeks even after cortisol production is normalized/regulated. This explains the normal cortisol output (AUCG) with a higher BMI, despite blunted reactivity in our sample of PAN.
Overall, these findings raise the question whether other variables besides BMI and chronic stress promoted maintenance of the blunted cortisol response in anorexia patients. As previously stated, massage therapy resulted in comparable hormone levels between PAN and controls, without significant weight changes. Qualitative studies also claim that interventions aiming at weight changes are not beneficial for recovery in patients with an ED111. In this light, it seems reasonable to consider other psychological variables as influential factors, such as depression, distress, stress appraisal as well as coping skills. Indeed, our data showed that ED-symptoms are highly correlated with psychological distress and depression, but not with BMI. In addition, it could be demonstrated that reactivity is rather related to ED-symptoms and psychological burden than to BMI. As a matter of fact, recovered patients (weight remission), still experience poor well-being112,113 and low quality of life113,114 despite significant treatment response112 even after 2 years of therapy, compared with controls115,116. Particularly, fully recovered ED patients highlight factors such as social support and the development of new coping skills as essential to the process of (mental and physical) recovery117–119.
In summary, the main results of our study replicated past research showing elevated basal cortisol levels (at rest) and a blunted cortisol response in PAN in response to acute stress exposure. In addition, our data emphasized that with weight gain, hypercortisolemia might be attenuated, but HPA-axis irregularities in terms of cortisol reactivity may persist. Additional analyses of our data attests past outcomes on the detrimental effects of chronic and perceived stress, as well as psychological burden on the HPAA activity. As such, this outcome is a major strength of the present study since it fosters understanding in the psychology of affected individuals and provides input for further potential designs aiming a differentiated analysis on the particular functionality of the HPAA in terms of cortisol production and reactivity. Importantly, it suggests that psychological burden has a higher impact on ED maintenance than weight itself. A further strength of our research were the highly standardized procedures and laboratory settings: Both investigated groups were clearly separated in normal-weight controls and anorectic individuals, who were perfectly age and gender match, excluding confounding factors by means of strict inclusion criteria. In addition, we employed a highly standardized psychosocial stress test (TSST) and methodological procedures for accurate cortisol assessment purposes, by taking confounders into account (e.g., menstrual cycle, smoking, contraceptive) and considering strict exclusion criteria (e.g., acute or chronic illness, mental disorders, medication, or substance intake). Notwithstanding, limiting factors are the lack of male participants (limited generalizability) and the lack of data related to measurement of illness duration, number of days at the clinic and childhood adversities. Future research might benefit from including this additional data in future studies. Thereupon, it remains to clarify whether HPAA reactivity can be completely restored, not only after weight recovery, but specially after full remission of psychological burden (e.g., depression and distress) and perhaps after regaining healthy levels of well-being.
The aim of the present study was to study the cortisol response of PAN elicited by an acute stressor. A further purpose was to investigate the relationship between BMI and the cortisol response. In conclusion, compared with healthy controls, inpatients with AN evinced a blunted hormonal response to stress, which indicated alterations in the HPAA. On the other hand, our data supported partial reversibility of HPAA dysfunction: while cortisol output was regulated with weight recovery, irregularities in HPAA reactivity persisted. Based on our data we suppose that in addition to weight recovery, other psychological variables might affect HPAA reactivity (e.g., stress appraisal, depression, and psychological distress). In addition, our study makes clear that weight recovery is not crucial for ED recovery. Even after weight recovery PAN still deal with ED-symptoms, depression, and psychological distress. Consequently, it is advisable to address psychological burden in therapy sessions.
Acknowledgements
Open access funding provided by Projekt DEAL.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/8/2021
A Correction to this paper has been published: 10.1038/s41398-021-01326-6
References
- 1.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch. Gen. Psychiatry. 2011;68:724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 2.Harbottle EJ, Birmingham CL, Sayani F. Anorexia nervosa: a survival analysis. Eat. Weight Disord. 2008;13:e32–4. [PubMed] [Google Scholar]
- 3.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol. Behav. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DSM-5 American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. (American Psychiatric Publishing, Arlington, 2013).
- 5.Wildes JE, Ringham RM, Marcus MD. Emotion avoidance in patients with anorexia nervosa: initial test of a functional model. Int. J. Eat. Disord. 2010;43:398–404. doi: 10.1002/eat.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culbert KM, Racine SE, Klump KL. Hormonal factors and disturbances in eating disorders. Curr. Psychiatry Rep. 2016;18:65. doi: 10.1007/s11920-016-0701-6. [DOI] [PubMed] [Google Scholar]
- 7.Corstorphine E, Mountford V, Tomlinson S, Waller G, Meyer C. Distress tolerance in the eating disorders. Eat. Behav. 2007;8:91–97. doi: 10.1016/j.eatbeh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychological Bull. 2004;130:19. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- 9.Monteleone AM, et al. Early traumatic experiences impair the functioning of both components of the endogenous stress response system in adult people with eating disorders. Psychoneuroendocrinology. 2020;115:104–644. doi: 10.1016/j.psyneuen.2020.104644. [DOI] [PubMed] [Google Scholar]
- 10.Lo Sauro CL, Ravaldi C, Cabras PL, Faravelli C, Ricca V. Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology. 2008;57:95–115. doi: 10.1159/000138912. [DOI] [PubMed] [Google Scholar]
- 11.Miller KK. Endocrine effects of anorexia nervosa. Endocrinol. Metab. Clin. 2013;42:515–528. doi: 10.1016/j.ecl.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pike KM, et al. Toward an understanding of risk factors for anorexia nervosa: a case-control study. Psychological Med. 2008;38:1443–1453. doi: 10.1017/S0033291707002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojo L, Conesa L, Bermudez O, Livianos L. Influence of stress in the onset of eating disorders: data from a two-stage epidemiologic controlled study. Psychosom. Med. 2006;68:628–635. doi: 10.1097/01.psy.0000227749.58726.41. [DOI] [PubMed] [Google Scholar]
- 14.Lovallo, W. R. Stress and health: biological and psychological interactions. Sage publications, (Thousand Oaks, London, 2015).
- 15.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 16.Foley P, Kirschbaum C. Human hypothalamus–pituitary–adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci. Biobehav. Rev. 2010;35:91–96. doi: 10.1016/j.neubiorev.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch. Intern. Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 18.Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol. Metab. Clin. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- 19.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen BS, Gianaros PJ. Stress-and allostasis-induced brain plasticity. Annu. Rev. Med. 2010;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handwerger K. Differential patterns of HPA activity and reactivity in adult posttraumatic stress disorder and major depressive disorder. Harv. Rev. Psychiatry. 2009;17:184–205. doi: 10.1080/10673220902996775. [DOI] [PubMed] [Google Scholar]
- 23.Monteleone AM, et al. Childhood trauma and cortisol awakening response in symptomatic patients with anorexia nervosa and bulimia nervosa. Int. J. Eat. Disord. 2015;48:615–621. doi: 10.1002/eat.22375. [DOI] [PubMed] [Google Scholar]
- 24.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Lawson EA, et al. Increased hypothalamic-pituitary-adrenal drive is associated with decreased appetite and hypoactivation of food motivation neurocircuitry in anorexia nervosa. Eur. J. Endocrinol./Eur. Federation Endocr. Soc. 2013;169:639. doi: 10.1530/EJE-13-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellini G, et al. Association between resting energy expenditure, psychopathology and HPA-axis in eating disorders. World J. Clin. Cases. 2014;2:257. doi: 10.12998/wjcc.v2.i7.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brambilla F, et al. Psychoimmunoendocrine investigation in anorexia nervosa. Neuropsychobiology. 1993;27:9–16. doi: 10.1159/000118947. [DOI] [PubMed] [Google Scholar]
- 28.Putignano P, et al. Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur. J. Endocrinol. 2001;145:165–171. doi: 10.1530/eje.0.1450165. [DOI] [PubMed] [Google Scholar]
- 29.Walsh BT, et al. The production rate of cortisol declines during recovery from anorexia nervosa. J. Clin. Endocrinol. Metab. 1981;53:203–205. doi: 10.1210/jcem-53-1-203. [DOI] [PubMed] [Google Scholar]
- 30.Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. Jama. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 31.Fichter MM, Pirke KM. Effect of experimental and pathological weight loss upon the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 1986;11:295–305. doi: 10.1016/0306-4530(86)90015-6. [DOI] [PubMed] [Google Scholar]
- 32.Het S, et al. Blunted neuroendocrine stress reactivity in young women with eating disorders. J. Psychosom. Res. 2015;78:260–267. doi: 10.1016/j.jpsychores.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Vocks S, Legenbauer T, Wächter A, Wucherer M, Kosfelder J. What happens in the course of body exposure?: Emotional, cognitive, and physiological reactions to mirror confrontation in eating disorders. J. Psychosom. Res. 2007;62:231–239. doi: 10.1016/j.jpsychores.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Zonnevylle-Bender MJ, et al. Adolescent anorexia nervosa patients have a discrepancy between neurophysiological responses and self-reported emotional arousal to psychosocial stress. Psychiatry Res. 2005;135:45–52. doi: 10.1016/j.psychres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Lelli L, Castellini G, Cassioli E, Monteleone AM, Ricca V. Cortisol levels before and after cognitive behavioural therapy in patients with eating disorders reporting childhood abuse: a follow-up study. Psychiatry Res. 2019;275:269–275. doi: 10.1016/j.psychres.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Carroll D, Ginty AT, Whittaker AC, Lovallo WR, de Rooij SR. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neurosci. Biobehav. Rev. 2017;77:74–86. doi: 10.1016/j.neubiorev.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiksdal A, et al. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology. 2019;102:44–52. doi: 10.1016/j.psyneuen.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hori H, et al. Psychological coping in depressed outpatients: association with cortisol response to the combined dexamethasone/CRH test. J. Affect. Disord. 2014;152:441–447. doi: 10.1016/j.jad.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Peeters F, Nicholson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosom. Med. 2003;65:836–841. doi: 10.1097/01.psy.0000088594.17747.2e. [DOI] [PubMed] [Google Scholar]
- 41.Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23:411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 42.Scantamburlo G, Ansseau M, Legros JJ. Role of the neurohypophysis in psychological stress. L’encephale. 2001;27:245. [PubMed] [Google Scholar]
- 43.Paszynska E, Dmitrzak-Weglarz M, Tyszkiewicz-Nwafor M, Slopien A. Salivary alpha-amylase, secretory IgA and free cortisol as neurobiological components of the stress response in the acute phase of anorexia nervosa. World J. Biol. Psychiatry. 2016;17:266–273. doi: 10.3109/15622975.2016.1163419. [DOI] [PubMed] [Google Scholar]
- 44.Paszynska E, et al. Is there a link between stress and immune biomarkers and salivary opiorphin in patients with a restrictive-type of anorexia nervosa? World J. Biol. Psychiatry. 2020;21:1–10. doi: 10.1080/15622975.2019.1593502. [DOI] [PubMed] [Google Scholar]
- 45.Shibuya I, et al. Changes in salivary cortisol levels as a prognostic predictor in children with anorexia nervosa. Int. J. Psychophysiol. 2011;82:196–201. doi: 10.1016/j.ijpsycho.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Da Luz Neto LM, et al. Differences in cortisol concentrations in adolescents with eating disorders: a systematic review. J. de. Pediatr. (Vers.ão em Port.ês.) 2019;95:18–26. doi: 10.1016/j.jped.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Södersten P, Bergh C, Leon M, Zandian M. Dopamine and anorexia nervosa. Neurosci. Biobehav. Rev. 2016;60:26–30. doi: 10.1016/j.neubiorev.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura Y, Walker BR, Ikuta T. Systematic review and meta-analysis reveals acutely elevated plasma cortisol following fasting but not less severe calorie restriction. Stress. 2016;19:151–157. doi: 10.3109/10253890.2015.1121984. [DOI] [PubMed] [Google Scholar]
- 49.Bergendahl M, et al. Short-term fasting suppresses leptin and (Conversely) activates disorderly growth hormone secretion in midluteal phase women—a clinical research center study. J. Clin. Endocrinol. Metab. 1999;84:883–894. doi: 10.1210/jcem.84.3.5536. [DOI] [PubMed] [Google Scholar]
- 50.Pasiakos SM, Caruso CM, Kellogg MD, Kramer FM, Lieberman HR. Appetite and endocrine regulators of energy balance after 2 days of energy restriction: Insulin, Leptin, Ghrelin, and DHEA-S. Obesity. 2011;19:1124–1130. doi: 10.1038/oby.2010.316. [DOI] [PubMed] [Google Scholar]
- 51.Tomiyama AJ, et al. Low calorie dieting increases cortisol. Psychosom. Med. 2010;72:357–364. doi: 10.1097/PSY.0b013e3181d9523c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazurak N, et al. Effects of a 48-h fast on heart rate variability and cortisol levels in healthy female subjects. Eur. J. Clin. Nutr. 2013;67:401–406. doi: 10.1038/ejcn.2013.32. [DOI] [PubMed] [Google Scholar]
- 53.Keys, A., Brožek, J., Henschel, A., Mickelsen, O., & Taylor, H. L. The biology of human starvation, Vols. 2. (Univ. of Minnesota Press, Minnesota, 1950).
- 54.Grinspoon S, et al. Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. Am. J. Clin. Nutr. 2001;73:865–869. doi: 10.1093/ajcn/73.5.865. [DOI] [PubMed] [Google Scholar]
- 55.Lawson EA, et al. Increased hypothalamic-pituitary-adrenal drive is associated with decreased appetite and hypoactivation of food-motivation neurocircuitry in anorexia nervosa. Eur. J. Endocrinol. 2013;169:639–47. doi: 10.1530/EJE-13-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayer L, et al. Body fat redistribution after weight gain in women with anorexia nervosa. Am. J. Clin. Nutr. 2005;81:1286–1291. doi: 10.1093/ajcn/81.6.1286. [DOI] [PubMed] [Google Scholar]
- 57.Brambilla F, et al. Persistent amenorrhoea in weight-recovered anorexics: psychological and biological aspects. Psychiatry Res. 2003;118:249–257. doi: 10.1016/s0165-1781(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 58.Doerr P, Fichter M, Pirke KM, Lund R. Relationship between weight gain and hypothalamic pituitary adrenal function in patients with anorexia nervosa. J. Steroid Biochem. 1980;13:529–537. doi: 10.1016/0022-4731(80)90209-5. [DOI] [PubMed] [Google Scholar]
- 59.Hart S, et al. Anorexia nervosa symptoms are reduced by massage therapy. Eat. Disord. 2001;9:289–299. doi: 10.1080/106402601753454868. [DOI] [PubMed] [Google Scholar]
- 60.Monteleone AM, et al. Underweight subjects with anorexia nervosa have an enhanced salivary cortisol response not seen in weight restored subjects with anorexia nervosa. Psychoneuroendocrinology. 2016;70:118–121. doi: 10.1016/j.psyneuen.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Oskis, A., Loveday, C., Hucklebridge, F., Wood, D., & Clow, A. Recovery from adolescent anorexia nervosa and associations with diurnal patterns of salivary stress hormones: a case report. Case reports in psychiatry2012, 798512. 10.1155/2012/798512 (2012). [DOI] [PMC free article] [PubMed]
- 62.Schüle C, Sighart C, Hennig J, Laakmann G. Mirtazapine inhibits salivary cortisol concentrations in anorexia nervosa. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2006;30:1015–1019. doi: 10.1016/j.pnpbp.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 63.Herpertz S, et al. Longitudinal changes of circadian leptin, insulin and cortisol plasma levels and their correlation during refeeding in patients with anorexia nervosa. Eur. J. Endocrinol. 2000;142:373–379. doi: 10.1530/eje.0.1420373. [DOI] [PubMed] [Google Scholar]
- 64.Bailer UF, Kaye WH. A review of neuropeptide and neuroendocrine dysregulation in anorexia and bulimia nervosa. Curr. Drug Targets-CNS Neurological Disord. 2003;2:53–59. doi: 10.2174/1568007033338689. [DOI] [PubMed] [Google Scholar]
- 65.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Hellhammer J, Schlotz W, Stone AA, Pirke KM, Hellhammer D. Allostatic load, perceived stress, and health: a prospective study in two age groups. Ann. N. Y. Acad. Sci. 2004;1032:8–13. doi: 10.1196/annals.1314.002. [DOI] [PubMed] [Google Scholar]
- 67.Ginty AT, Phillips AC, Higgs S, Heaney JL, Carroll D. Disordered eating behaviour is associated with blunted cortisol and cardiovascular reactions to acute psychological stress. Psychoneuroendocrinology. 2012;37:715–724. doi: 10.1016/j.psyneuen.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Monteleone P, et al. Abnormal diurnal patterns of salivary α-amylase and cortisol secretion in acute patients with anorexia nervosa. World J. Biol. Psychiatry. 2011;12:455–461. doi: 10.3109/15622975.2011.590226. [DOI] [PubMed] [Google Scholar]
- 69.Monteleone AM, et al. The vulnerability to interpersonal stress in eating disorders: The role of insecure attachment in the emotional and cortisol responses to the trier social stress test. Psychoneuroendocrinology. 2019;101:278–285. doi: 10.1016/j.psyneuen.2018.12.232. [DOI] [PubMed] [Google Scholar]
- 70.Klatzkin RR, Baldassaro A, Hayden E. The impact of chronic stress on the predictors of acute stress-induced eating in women. Appetite. 2018;123:343–351.. doi: 10.1016/j.appet.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Monteleone AM, et al. Deranged emotional and cortisol responses to a psychosocial stressor in anorexia nervosa women with childhood trauma exposure: evidence for a “maltreated ecophenotype”? J. Psychiatr. Res. 2018;104:39–45. doi: 10.1016/j.jpsychires.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 72.Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Monteleone AM, Treasure J, Kan C, Cardi V. Reactivity to interpersonal stress in patients with eating disorders: a systematic review and meta-analysis of studies using an experimental paradigm. Neurosci. Biobehav. Rev. 2018;87:133–150. doi: 10.1016/j.neubiorev.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 74.First, M. B., Gibbon, M., Spitzer, R. L., Benjamin, L. S. & Williams, J. B. Structured Clinical Interview for DSM-IV® Axis II Personality Disorders SCID-II. American Psychiatr. Pub. 1–80 (1997).
- 75.Wittchen, H. U., Wunderlich, U., Gruschwitz, S. & Zaudig, M. SKID-I: Strukturiertes klinisches interview für DSM-IV, Achse I. psychische störungen 68–70. 10.1026//0084-5345.28.1.68 (1997).
- 76.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bull. 2004;130:355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 77.Goodman WK, Janson J, Wolf JM. Meta-analytical assessment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test. Psychoneuroendocrinology. 2017;80:26–35. doi: 10.1016/j.psyneuen.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 78.Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. Mol. Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- 79.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 80.Kudielka BM, Hellhammer DH, Kirschbaum C, Harmon-Jones E, Winkielman P. Ten years of research with the Trier Social Stress Test—revisited. Soc. Neurosci.: Integrating Biol. Psychol. Explan. Soc. Behav. 2007;56:83. [Google Scholar]
- 81.Schulz, P., Schlotz, W., & Becker, P. Trierer Inventar zum Chronischen Stress (TICS)[Trier Inventory for Chronic Stress (TICS)]. 8–19. 10.1026//0012-1924.45.1.8 (2004).
- 82.Petrowski, K., Kliem, S., Albani, C., Hinz, A., & Brähler, E. Norm values and psychometric properties of the short version of the Trier Inventory for Chronic Stress (TICS) in a representative German sample. PLoS ONE, 14, e0222277. 10.1371/journal.pone.0222277 (2019). [DOI] [PMC free article] [PubMed]
- 83.Petrowski K, et al. Factor structure and psychometric properties of the english version of the trier inventory for chronic stress (TICS-E) BMC Med. Res. Methodol. 2018;18:18. doi: 10.1186/s12874-018-0471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petrowski K, Paul S, Albani C, Brähler E. Factor structure and psychometric properties of the Trier Inventory for Chronic Stress (TICS) in a representative German sample. BMC Med. Res. Methodol. 2012;12:42. doi: 10.1186/1471-2288-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaab J, Rohleder N, Nater UM, Ehlert U. Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30:599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Carpenter R. A review of instruments on cognitive appraisal of stress. Arch. Psychiatr. Nurs. 2016;30:271–279. doi: 10.1016/j.apnu.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Lesage FX, Berjot S, Deschamps F. Clinical stress assessment using a visual analogue scale. Occup. Med. 2012;62:600–605. doi: 10.1093/occmed/kqs140. [DOI] [PubMed] [Google Scholar]
- 88.Herhaus B, Päßler S, Petrowski K. Stress-related laboratory eating behavior in adults with obesity and healthy weight. Physiol. Behav. 2018;196:150–157. doi: 10.1016/j.physbeh.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 89.Domes G, Stächele T, von Dawans B, Heinrichs M. Effects of internet-based stress management on acute cortisol stress reactivity: Preliminary evidence using the Trier Social Stress Test for Groups (TSST-G) Psychoneuroendocrinology. 2019;105:117–122. doi: 10.1016/j.psyneuen.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Klaghofer, R. & Brähler, E. Konstruktion und Teststatistische Prüfung einer Kurzform der SCL-90–R. Z. Klin. Psychol. Psychiatr. Psychother. 19–38 (2001).
- 91.Franke, G. H., & Derogatis, L. R. SCL-90-R: symptom-Checkliste von LR Derogatis: deutsche version: manual. (Beltz Test, Göttingen, 2002).
- 92.Prinz U, et al. Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry. 2013;13:104. doi: 10.1186/1471-244X-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sereda Y, Dembitskyi S. Validity assessment of the symptom checklist SCL-90-R and shortened versions for the general population in Ukraine. BMC Psychiatry. 2016;16:1. doi: 10.1186/s12888-016-1014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petrowski K, Schmalbach B, Kliem S, Hinz A, Brähler E. Symptom-Checklist-K-9: Norm values and factorial structure in a representative German sample. PloS ONE. 2019;14:e0213490. doi: 10.1371/journal.pone.0213490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beck AT, Ward C, Mendelson M, Mock J, Erbaugh J. Beck depression inventory (BDI) Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 96.Hautzinger, M., Bailer, M., Worall, H., & Keller, F. Beck-depressions-inventar (BDI). (Huber, Bern, 1994).
- 97.Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San. Antonio, TX: Psychological Corporation. 1996;1:82. [Google Scholar]
- 98.Kühner C, Bürger C, Keller F, Hautzinger M. Reliabilität und validität des revidierten beck-depressionsinventars (BDI-II) Der Nervenarzt. 2007;78:651–656. doi: 10.1007/s00115-006-2098-7. [DOI] [PubMed] [Google Scholar]
- 99.Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int. J. Eat. Disord. 1983;2:15–34. [Google Scholar]
- 100.Thiel, A. & Paul, T. Entwicklung einer deutschsprachigen Version des Eating-Disorder-Inventory (EDI). Zh. Differe. Diagn. Psychol. 267–278 (1988).
- 101.Dinkel A, Berth H, Exner C, Rief W, Balck F. Deutsche adaptation der restraint scale zur erfassung gezügelten essverhaltens. Diagnostica. 2005;51:67–74. [Google Scholar]
- 102.Kappel, V., Thiel, A., Holzhausen, M., Jaite, C., Schneider, N., Pfeiffer, E. & Salbach-Andrae, H. Eating disorder inventory-2 (EDI-2). Diagnostica. 27–144. 10.1026/0012-1924/a000069 (2012).
- 103.Fekedulegn DB, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom. Med. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- 104.Miller R, Plessow F, Kirschbaum C, Stalder T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosom. Med. 2013;75:832–840. doi: 10.1097/PSY.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 105.Schorr M, Lawson EA, Dichtel LE, Klibanski A, Miller KK. Cortisol measures across the weight spectrum. J. Clin. Endocrinol. Metab. 2015;100:3313–3321. doi: 10.1210/JC.2015-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. Metab. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- 107.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 108.Buehren K, et al. Association between neuroendocrinological parameters and learning and memory functions in adolescent anorexia nervosa before and after weight recovery. J. Neural Transm. 2011;118:963–968. doi: 10.1007/s00702-010-0567-4. [DOI] [PubMed] [Google Scholar]
- 109.Pellegrino F, et al. Investigation of salivary cortisol response to awakening in underweight and weight-restored patients with anorexia nervosa. Eur. Psychiatry. 2017;41:S283. [Google Scholar]
- 110.Karin, O. et al. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. bioRxiv 892596. 10.1101/2020.01.01.892596 (2020). [DOI] [PMC free article] [PubMed]
- 111.Doll HA, Petersen SE, Stewart-Brown SL. Eating disorders and emotional and physical well-being: Associations between student self-reports of eating disorders and quality of life as measured by the SF-36. Qual. Life Res. 2005;14:705–717. doi: 10.1007/s11136-004-0792-0. [DOI] [PubMed] [Google Scholar]
- 112.Jenkins PE, Hoste RR, Meyer C, Blissett JM. Eating disorders and quality of life: a review of the literature. Clin. Psychol. Rev. 2011;31:113–121. doi: 10.1016/j.cpr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 113.Tomba E, Tecuta L, Schumann R, Ballardini D. Does psychological well-being change following treatment? An exploratory study on outpatients with eating disorders. Compr. Psychiatry. 2017;74:61–69. doi: 10.1016/j.comppsych.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 114.Padierna A, Quintana JM, Arostegui I, Gonzalez N, Horcajo MJ. Changes in health related quality of life among patients treated for eating disorders. Qual. Life Res. 2002;11:545–552. doi: 10.1023/a:1016324527729. [DOI] [PubMed] [Google Scholar]
- 115.De la Rie SM, Noordenbos G, Van Furth EF. Quality of life and eating disorders. Qual. Life Res. 2005;14:1511–1521. doi: 10.1007/s11136-005-0585-0. [DOI] [PubMed] [Google Scholar]
- 116.Cockell SJ, Zaitsoff SL, Geller J. Maintaining change following eating disorder treatment. Professional Psychol.: Res. Pract. 2004;3:527. [Google Scholar]
- 117.Pettersen G, Rosenvinge JH. Improvement and recovery from eating disorders: a patient perspective. Eat. Disord. 2002;10:61–71. doi: 10.1002/erv.425. [DOI] [PubMed] [Google Scholar]
- 118.Bardone-Cone AM, Hunt RA, Watson HJ. An overview of conceptualizations of eating disorder recovery, recent findings, and future directions. Curr. Psychiatry Rep. 2018;20:79. doi: 10.1007/s11920-018-0932-9. [DOI] [PubMed] [Google Scholar]
- 119.Las Hayas C, López de Arroyabe E, Calvete E. Resilience in family caregivers of persons with acquired brain injury. Rehabilitation Psychol. 2015;60:295. doi: 10.1037/rep0000040. [DOI] [PubMed] [Google Scholar]