Abstract
In this work, commercial anatase TiO2 powders were modified using ultrathin Fe2O3 layer by atomic layer deposition (ALD). The ultrathin Fe2O3 coating having small bandgap of 2.20 eV can increase the visible light absorption of TiO2 supports, at the meantime, Fe2O3/TiO2 heterojunction can effectively improve the lifetime of photogenerated electron–hole pairs. Results of ALD Fe2O3 modified TiO2 catalyst, therefore, showed great visible light driven catalytic degradation of methyl orange compared to pristine TiO2. A 400 cycles of ALD Fe2O3 (~ 2.6 nm) coated TiO2 powders exhibit the highest degradation efficiency of 97.4% in 90 min, much higher than pristine TiO2 powders of only 12.5%. Moreover, an ultrathin ALD Al2O3 (~ 2 nm) was able to improve the stability of Fe2O3-TiO2 catalyst. These results demonstrate that ALD surface modification with ultrathin coating is an extremely powerful route for the applications in constructing efficient and stable photocatalysts.
Subject terms: Photocatalysis, Nanoparticles
Introduction
A rapid industrial development driven by unsustainable technology advances can cause plenty of industrial sewage, spreading chemical hazards into water resources. As a result, water pollution has emerged as one of the most serious environmental issues worldwide1–4. Photocatalytic oxidation technology has shown great prospects in removing the toxic and harmful contaminants in aqueous environment5–7. Semiconductors (e.g. TiO2, ZnO, SnO2) have been widely researched for organic pollutant degradation, however, the large band gap hinders their practical applications8–12. For example, TiO2 with band gap of 3.2 eV can only absorb the ultra-violet light, accounting for only 4–5% of entire solar spectrum13. Therefore, various visible light sensitive photocatalysts has also been widely explored, such as g-C3N4, BiVO4, CdSe, Bi2WO614–19. On the other hand, TiO2 is recognized as one of the excellent materials owning to its good inertness, eco-friendly, low cost, strong oxidizing power, and long-term stability against photo and chemical corrosion9,13,20–22. Thus, plenty of works have been made to extend the absorption spectrum of TiO2 to visible light so to make a full use of solar spectrum. Several different approaches can be employed, including doping23–26 and coupling with small band gap semiconductors or metals27–30.
Small band gap semiconductors not only increase the absorption of visible light but also inhibit photo-generated electrons-holes recombination when constructed as a semiconductor/semiconductor heterojunction structure, thus improving the photocatalytic performance dramatically31. Therefore, various TiO2 based heterojunction photocatalysts have been proposed for visible light photocatalysis, including NiO/TiO232,33, Ag2O/TiO234, CdTe/TiO235, C3N4/TiO236, Bi2O3/TiO237, Cu2O/TiO238, Fe2O3/TiO239, etc. For Fe2O3/TiO2 heterojunction photocatalysts, a variety of composites have been investigated, such as Fe2O3 nanoparticles on TiO2 nanotube40, Fe2O3/TiO2 nanoparticles41, TiO2 coated cubic Fe2O342, Fe2O3 nanosheet/TiO2 hollow sphere39, and Fe2O3 coated TiO243. For instance, Lin et al. demonstrated that Fe2O3 coating can effectively enhance the visible light photocatalytic activity of TiO243. Various fabrication methods were applied, including hydrothermal or solvothermal process and sol–gel, to prepare the heterojunction photocatalysts44–46. Nevertheless, precise control of the interface between Fe2O3 and TiO2 at atomic level by conventional methods remain challenges.
Atomic layer deposition (ALD) is a unique and promising thin film deposition technique based on self limited and saturated surface chemisorption reactions. It can deposit ultrathin, conformal, and uniform layers at sub-nanometer scale, which has attracted great attentions in surface engineering of nanostructures over the years47–49. In catalysts design, ALD enables a conformal layer with precise thickness control and tunable film composition onto another nanostructures with high aspect ratio50. The ALD coating can work as photo-active materials51,52 or surface protection layer53,54. Herein, we modified the commercial anatase TiO2 powders with ultrathin Fe2O3 surface coating by ALD. The photocatalytic performance was investigated by visible light degradation of methyl orange (MO). The ultrathin Fe2O3 coating can enhance the absorption of TiO2 supports for visible light. Fe2O3 modified TiO2 powders show much better visible light photocatalytic degradation of MO than pristine TiO2. A possible mechanism for improved photocatalytic performance is proposed. In addition, an ultrathin ALD Al2O3 (~ 2 nm) was used to promote the long-term durability of TiO2@Fe2O3 catalyst.
Methods
ALD deposition on TiO2 powders
Commercial TiO2 powders with anatase phase (Nanjing Haitai nano materials Co.) were used as supports in this work. Ferrocene (Fe(Cp)2, Suzhou Fornano Corporation Ltd., 99.99%) and ozone were adopted as Fe and oxygen precursors for ALD Fe2O3 deposition. Fe(Cp)2 was vaporized at 85 °C. High purity nitrogen gas (N2, 99.999%) was used as carrier gas at a total flow rate of 750 sccm and a pressure of 6 hPa in our ALD system (Picosun SUNALE™ R-150B). A particular container with porous mesh was used for ALD coating on powders, which has been reported elsewhere24,55, as shown in Fig. 1. Herein, precursors can flow through the TiO2 powders to achieve great conformality. X cycles of ALD Fe2O3 (X = 200, 400, 600, and 800) were coated on TiO2 powder at 300 °C, the samples are marked as TiO2@X-Fe2O3. One cycle of ALD Fe2O3 contains the following four steps, 5 s Fe(Cp)2 injection, 20 s N2 purge, 5 s O3 injection, and 20 s N2 purge. At the same system, 20 cycles of Al2O3 were deposited on TiO2@400-Fe2O3 at 300 °C, where one ALD cycle of Al2O3 is consisted of 5 s trimethylaluminum dose, 20 s N2 purging, 5 s H2O dose, and 20 s N2 purging.
Figure 1.
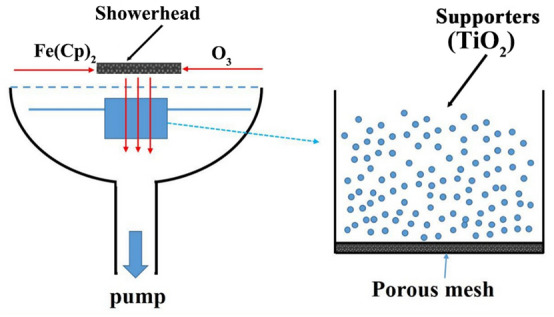
The schematic diagram of coating TiO2 powders by ALD Fe2O3.
Materials characterizations
X-ray diffraction (XRD) using a Rigaku-D/MAX 2000 system was used for crystallinity and phase structure analysis. Scanning electron microscopy (SEM) images were taken using ZEISS Gemini SEM 500 instrument operated at 2 kV. The high-resolution transmission electron microscopy (HRTEM) was performed on a FEI Tecnai F20 S-Twin to observe the microstructures, where TiO2 powders were loaded on the ultra-thin carbon coated copper grids. The surface chemical features and valence band spectra were explored by X-ray photoelectron spectroscopy (XPS) using Thermo Fisher K-Alpha. The adventitious carbon signal (C 1 s = 284.6 eV) was adopted to calibrate the binding energies. UV–visible absorption spectra were conducted on a UV–vis-NIR spectrophotometer (UV-3600, Shimadzu, Japan). Photoluminescence (PL) spectra were collected on a Horiba Jobin Yvon HR800 spectrometer.
Photocatalytic degradation
The photocatalytic performance of Fe2O3 coated TiO2 catalysts was investigated by visible light degradation of MO. 100 ml MO solution (4 mg L−1) with 100 mg photocatalysts were loaded into a glass reactor, which was magnetically stirred at 500 rpm. In order to establish the adsorption/desorption equilibrium between MO and catalysts before irradiation, the MO solutions with catalysts were magnetically stirred for 30 min in darkness. Then, the suspension was irradiated under a 300 W Xe lamp (MircoSolar300, Perfect Light). A 420 nm filter was adopted to cut off UV light. The lamp was placed at 15 cm above the suspension, whose average visible light intensity is around 80 mW cm−2. Water cooling was applied throughout the experiment to maintain the temperature at 25 ± 1 °C. 3 mL solution was collected after each 15 min irradiation. The photocatalysts were removed by centrifugal separation. The residual MO concentration was determined using the absorption at 464 nm by UV–Vis–NIR spectrophotometer. The recycled usage experiment was performed for three times to explore the long-term stability of photocatalysts, e.g. after each photocatalytic experiment, the photocatalysts powders were gathered and rinsed by ethanol and water, then baked for 12 h at 100 °C. At last, a new MO solution was used to evaluate the photocatalytic activity of collected photocatalysts.
Photoelectrochemical measurements
Photoelectrochemical measurements were performed in a three-electrode electrochemical cell at room temperature using 1 M Na2SO4 as the electrolyte. The TiO2 or TiO2@400-Fe2O3 on FTO were used as the working electrode. A Pt wire and Ag/AgCl were used as the counter electrode and the reference electrode, respectively. Photocurrent densities were collected by an electrochemistry workstation (CHI660E, Shanghai) using a potentiostatic method at 0.50 V. Light was chopped on and off cyclically. A solar simulator (300 W Xe lamp, MircoSolar300, Perfect Light) with a 420 nm cut-off filter provides the visible-light irradiation.
Results
Figure 2 depicts the XRD patterns of pristine TiO2 and Fe2O3 coated TiO2 powders. All the samples show the similar characteristic diffraction peaks, in accord with anatase TiO2 (JCPDS No. 21–1,272). This result indicates that ultrathin ALD Fe2O3 would not affect the crystal structure of TiO2, consistent with our previous finding24,55. In addition, signals related to Fe2O3 were absent.
Figure 2.
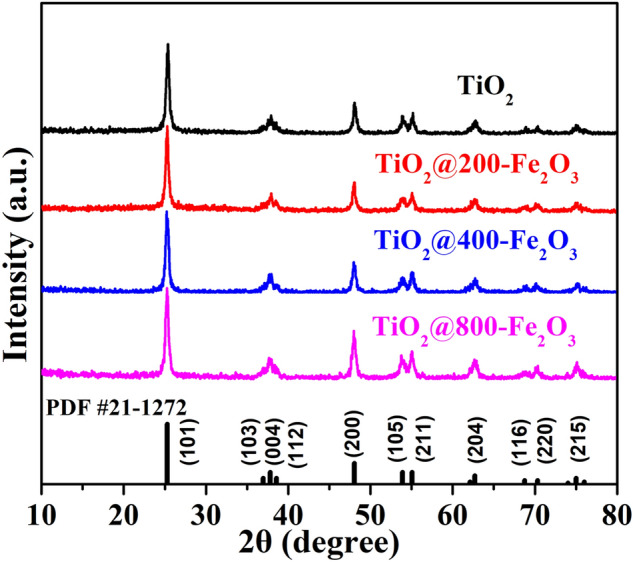
XRD patterns of pristine TiO2 and Fe2O3 coated TiO2.
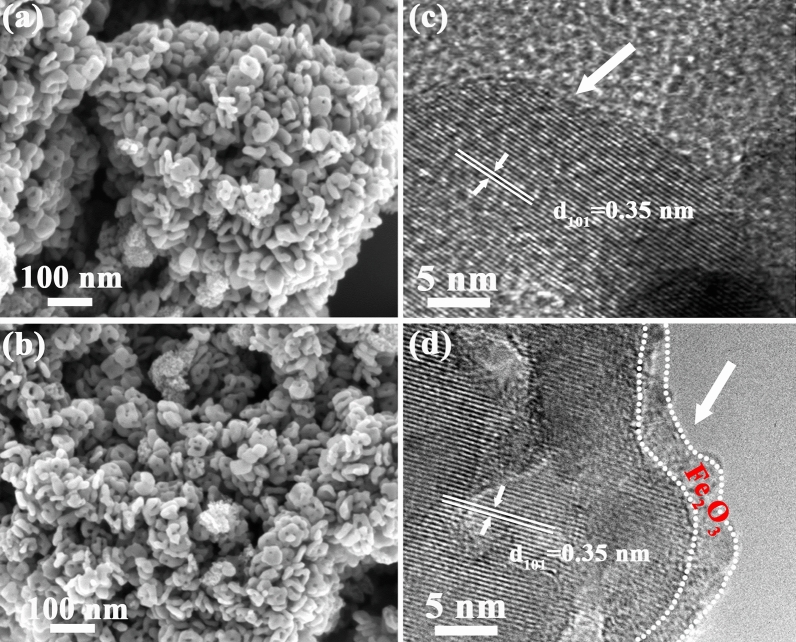
SEM images of TiO2 powders without and with 400 cycles ALD Fe2O3 deposition are shown in Fig. 3a,b. It can be seen that the pristine TiO2 powder exhibits a disk-like morphology with a diameter of approximately 40 nm and a thickness of approximately 10 nm, with severe aggregation. After ALD Fe2O3 deposition, it was observed that Fe2O3 coated TiO2 exhibited almost identical morphology, indicating that ultra-thin Fe2O3 coating did not have significant influence on particle size and morphology of TiO2. HRTEM was further conducted to observe the microstructure of TiO2@400-Fe2O3 and pristine TiO2 powders as shown in Fig. 3c,d. While pristine TiO2 shows a sharp well-ordered surface with good crystallinity (Fig. 3c), an amorphous layer of ~ 2.6 nm formed on the TiO2 surface was observed which was contributed to ultra-thin Fe2O3 layer (400 cycles) formed by ALD deposition. Moreover, both samples exhibit the lattice spacing of 0.35 nm, corresponding to (101) planes of anatase TiO2. Based on above XRD and TEM data, it was speculated that an ultra-thin amorphous Fe2O3 is coated on TiO2 nanoparticles surface without significantly modifying the morphology of the catalyst supports.
Figure 3.
SEM images of (a) pristine TiO2 and (b) TiO2@400-Fe2O3. TEM images of (c) pristine TiO2 and (d) TiO2@400-Fe2O3.
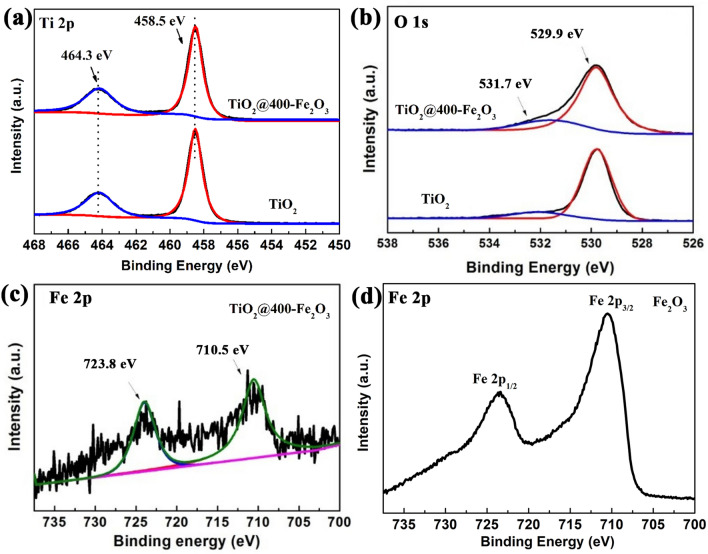
To further determine the successful deposition of ALD Fe2O3, XPS was performed to characterize the surface chemical features of ALD Fe2O3 coated TiO2 powders. Figure 4a shows the Ti 2p spectra of TiO2@400-Fe2O3 and pristine TiO2 powders. The spectra can be fitted into two peaks at 464.3 eV and 458.5 eV, which can be assigned to Ti 2p1/2 and Ti 2p3/2 peaks28. In O 1 s spectra (Fig. 4b), both samples present the main peak at 529.9 eV related to Ti–O bonding from TiO2. In addition, there is a peak at 531.7 eV can be ascribed to the surface -OH56. Figure 4c exhibits the Fe 2p spectrum of TiO2@400-Fe2O3, Fe 2p1/2 and Fe 2p3/2 peaks locate at 723.8 eV and 710.5 eV, in accord with Fe–O bonding value in Fe2O357. Based on XPS data, the Fe atom ratio (Fe/Fe + Ti) is determined to be 1.1% in TiO2@400-Fe2O3. It is anticipated that an ultra-thin amorphous Fe2O3 is coated on TiO2 nanoparticles surface successfully. Due to the low content of Fe2O3, Fe 2p spectrum shows a bad signal to noise ratio. Therefore, the Fe 2p spectrum of ALD Fe2O3 film is also presented for reference, as shown in Fig. 4d which can exhibit much better signal to noise ratio.
Figure 4.
XPS spectra of (a) Ti 2p and (b) O 1s for TiO2 and TiO2@400-Fe2O3, Fe 2p XPS spectra for (c) TiO2@400-Fe2O3 and (d) Fe2O3 film.
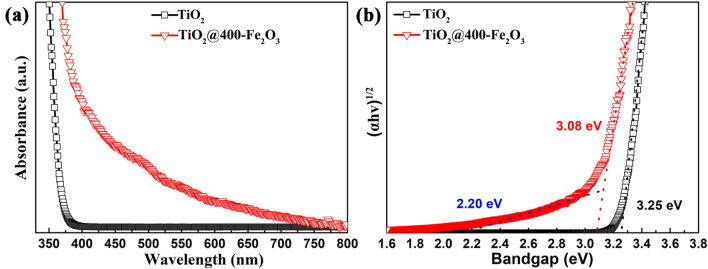
The influence of ultra-thin Fe2O3 coating on absorption of TiO2 powders in visible light was explored using UV–Vis diffuse reflectance spectra, as illustrated in Fig. 5a. The spectrum of pristine TiO2 powders is also plotted for comparison. Pristine TiO2 powders exhibit the absorption edge of around 371 nm without noticeable visible light absorption. However, noticeable absorption in the visible light region from 390 to 750 nm can be observed after ultra-thin Fe2O3 surface modification. The relationship of the absorption edge with the photon energy (hν) for the indirect bandgap semiconductor is shown in the following formula: (αhν)1/2 = A(hν-Eg), where α and A are the absorption coefficient and absorption constant, respectively. Since the absorption coefficient α is determined by the scattering and reflectance spectra based on Kubelka–Munk theory, therefore, the bandgap values can be determined by the intercept of the tangent lines. As depicted from Fig. 5b, only one tangent line can be extrapolated for pristine TiO2 powders showing a bandgap of 3.25 eV, while two bandgap values can be obtained from the plots for TiO2@400-Fe2O3 powders, attributing to Fe2O3 coating with a bandgap value of ~ 2.20 eV and TiO2 supports with a bandgap value of ~ 3.08 eV. It can be concluded that ultra-thin Fe2O3 coating results in a smaller bandgap which can increase the absorption of TiO2 powder support in visible light.
Figure 5.
(a) UV–Vis diffuse reflectance spectra and (b) Tauc plot of TiO2 with and without 400 cycles Fe2O3 coating.
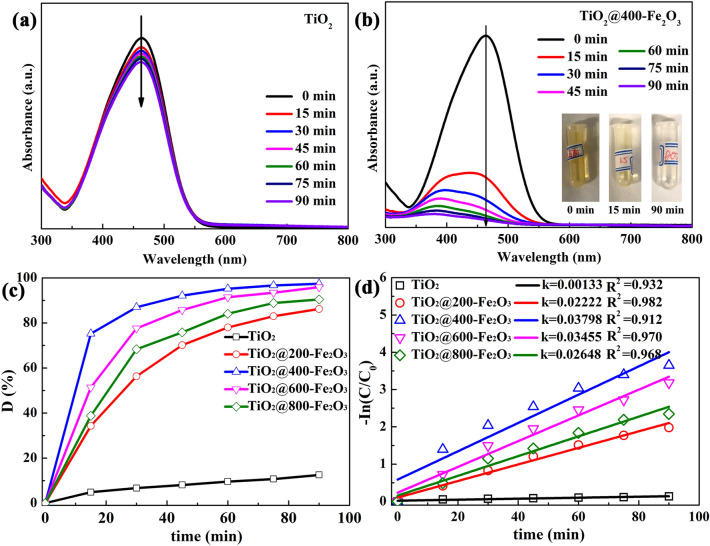
The visible light photocatalytic activity of TiO2 and Fe2O3 coated TiO2 catalysts was compared by degrading MO. All samples exhibit low adsorption capacity of MO molecules. As reported in our previous work, MO is selected here for its stability under visible light irradiation in the absence of catalysts24. Figure 6a shows the evolution of UV–vis absorption spectra of MO solution in the presence of pristine TiO2 under visible light irradiation. It can been seen that the absorption peaks at 464 nm decreases slightly after 90 min, exhibiting very poor photocatalytic activity due to its large bandgap. In contrast, the peak intensity at 464 nm fades rapidly for TiO2@400-Fe2O3 with the irradiation time extending, as shown in Fig. 6b. In addition, the orange MO solution turns into colorless after 90 min, as presented in the insert of Fig. 6b, indicating the degradation of MO. Figure 6c compares visible light photocatalytic activity of Fe2O3 coated TiO2 catalysts. It can be found that a much-improved photocatalytic degradation efficiency of ~ 86.2% can be achieved with only 200 cycles of ALD Fe2O3 modification. And the TiO2@400-Fe2O3 photocatalysts display the highest photocatalytic degradation efficiency of 97.4%. In comparison with reported Fe2O3-TiO2 heterojunction catalysts for photodegradation of organic pollutants and antibiotics, ALD Fe2O3 coated TiO2 (TiO2@400-Fe2O3) in this work exhibit excellent removal efficiency for MO degradation, as summarized in Table 1. The photocatalytic degradation efficiency decreases to 95.8% and 90.4% for TiO2@600-Fe2O3 and TiO2@800-Fe2O3 samples, respectively, along with further increasing the thickness of ALD Fe2O3 coating. The reduced efficiency can be ascribed to the fact that more Fe2O3 coating would introduce more recombination sites for photoinduced electron–hole pairs42, diminishing the photocatalytic efficiency.
Figure 6.
UV–vis absorption spectra of MO exposed to different irradiation time in the presence of (a) pristine TiO2 and (b) TiO2@400-Fe2O3 catalysts under visible light irradiation. The inserts are the photos of MO solution before and after irradiation. (c) Visible light photocatalytic degradation curves of MO and (d) −In(C/C0) vs. time curves by using TiO2 with and without Fe2O3 coating as catalysts.
Table 1.
Comparison of photocatalytic activity for degradation of organic pollutants using TiO2–Fe2O3 based catalysts.
| Catalysts | Method | Power of Xe lamp (W) | Organic pollutants | Time (min) | D (%) | Ref | |
|---|---|---|---|---|---|---|---|
| type | C (mg L-1) | ||||||
| Fe2O3 decorated TiO2 | calcination | 350 | MBa | 3.2 | 80 | 64.5 | 58 |
| Fe2O3-Doped TiO2 | Sol–gel | 500 | MB | 10 | 120 | 100 | 44 |
| Fe2O3/TiO2 nanofibers | Electrospinning + calcination | 800 | RhBb | 5 | 180 | 53.6 | 59 |
| Fe2O3 decorated TiO2 | hydrothermal | 500 | RhB | 4.8 | 270 | 77.8 | 45 |
| Fe2O3@SiO2@TiO2 | vapor-thermal | 300 | RhB | 4.8 | 60 | 100 | 46 |
| Fe2O3 coated TiO2 | solvothermal | 300 | TCc | 50 | 90 | 100 | 39 |
| Core–shell Fe2O3@TiO2 | Precipitation | 350 | RhB | 10 | 360 | 71.0 | 60 |
| Core–shell TiO2@Fe2O3 | hydrothermal | 300 | MO | 10 | 16 | 96.6 | 43 |
| Core–shell C@TiO2@Fe2O3 | Impregnation | 500 | MB | 20 | 240 | 80.8 | 61 |
| Fe2O3/TiO2 composites | Impregnation | 500 | Orange II | 20 | 240 | 53.4 | 62 |
| Fe2O3 coated TiO2 | ALD | 300 | MO | 4 | 90 | 97.4 | This work |
a MB is Methylene Blue, b RhB is Rhodamine B, c TC is Tetracycline.
The degradation data were also fitted by the pseudo-first-order kinetics. The rate constant k can be determined by In(Ct/C0) = −kt at low initial pollutant concentration. Herein, C0 is the initial MO concentration, while the Ct is the MO concentration after irradiation time of t. k is the first-order rate constant (min−1). The −In(Ct/C0) vs. t curves are plotted in Fig. 6d. It can be seen that −In(Ct/C0) has a linear relationship with t, indicating the photocatalytic degradation of MO by Fe2O3 modified TiO2 catalysts obeys the first-order kinetics. The first-order rate constant (k) is determined to be 3.8 × 10−2 min−1 for TiO2@400-Fe2O3, which is much larger than pristine TiO2 of 1.3 × 10–3 min−1. The result indicates that ALD Fe2O3 modification can effectively enhance the visible light photocatalytic performance of TiO2 supports.
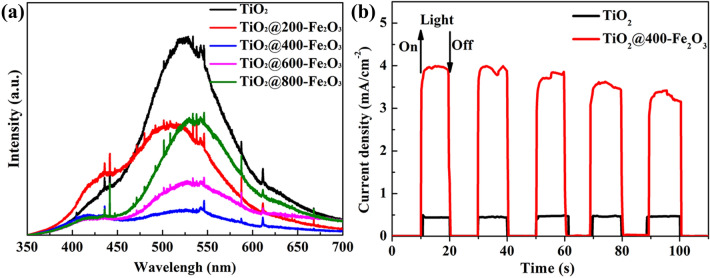
PL and photocurrent response measurements were conducted to explore the recombination rate of photo-generated electron–hole pairs. The lower the PL intensity, the higher separation efficiency of electron–hole pairs in the catalysts. Figure 7a shows the PL spectra of pristine TiO2 and various cycles of ALD Fe2O3 coated TiO2. It can be seen that all the Fe2O3 coated TiO2 exhibit lower intensity than pristine TiO2, indicating that the coupling of TiO2 and Fe2O3 can effectively inhibit the recombination of the photo-generated electron–hole pairs. Moreover, it can be seen that the 400 cycles Fe2O3 coating results in the lowest intensity, thicker Fe2O3 coating would increase the recombination of the photo-generated electron–hole pairs. The PL data are consistent well with the results of photocatalytic degradation of MO. Figure 7b presents the photocurrent response curves of TiO2 and TiO2@400-Fe2O3, it can be seen that TiO2@400-Fe2O3 exhibits a much higher photocurrent density than pristine TiO2, indicating a more efficient separation of the photoexcited electron–hole pairs.
Figure 7.
(a) PL spectra and (b) photocurrent response curves of TiO2 and Fe2O3 coated TiO2.
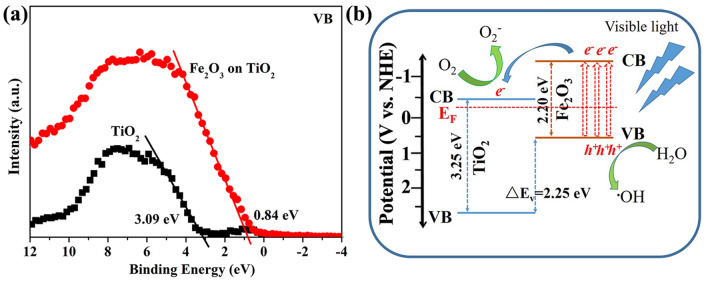
The band alignment for Fe2O3 coated TiO2 was determined by measuring the valence band offset ∆Ev (VBO) using XPS. Figure 8a shows VB spectra of TiO2 and Fe2O3–TiO2 determined by linear extrapolation method, respectively. The VB of TiO2 and Fe2O3–TiO2 are found to be 3.09 eV and 0.84 eV, respectively. The VB of Fe2O3 is higher than that of TiO2, and ∆Ev at the interface of Fe2O3-TiO2 is estimated to be 2.25 eV. The optical bandgaps of TiO2 and Fe2O3 have been determined to be 3.25 and 2.20 eV, respectively, in Fig. 5. Therefore, the conduction band offset ∆Ec (CBO) at the interface of Fe2O3-TiO2 is estimated to be 1.20 eV. Considering the band structure of TiO2 vs. standard hydrogen electrode (NHE)62–64, the energy band structure of Fe2O3 coated TiO2 can be depicted in Fig. 8b. Under visible light, TiO2 shows no photo-electronic response due to its large band gap. Only Fe2O3 can be excited, yielding photo-generated electron from its VB to CB. Due to the aligned equilibrium of Fermi level at the interface of TiO2 and Fe2O3, as shown in Fig. 8b, the photogenerated electrons can transfer from CB of Fe2O3 to that of TiO2 driven by the built-in electric field and the concentration gradient of electrons, while remaining the holes in VB of Fe2O360,62–64. Therefore, the separation efficiency of photoinduced electron–hole pairs can be improved, which has been demonstrated by PL and photocurrent response results in Fig. 7. There are a large number of literatures focusing on the photocatalytic activity of Fe2O3-TiO2 composites catalyst60,62–64. It is widely accepted that OH. radicals can be formed via the reaction of water and photogenerated holes in VB of Fe2O3. And electrons in CB of TiO2 can react with oxygen to form O2-. These radicals with high activities can degrade organic molecules into harmless substances.
Figure 8.
(a) Valence band spectra of TiO2 and Fe2O3–TiO2. (b) Schematic illustration of energy band structure of Fe2O3 coated TiO2 and proposed charge transfer mechanism during visible light irradiation.
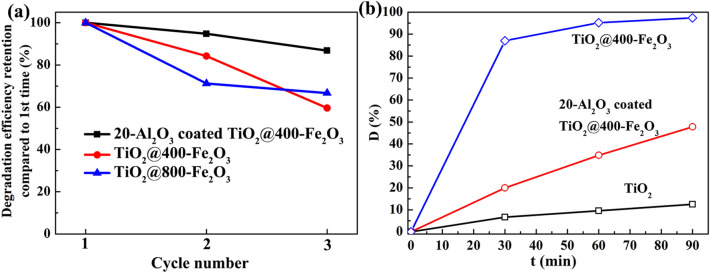
The stability of photocatalysts is one of the significant factors for practical applications, therefore, TiO2@400-Fe2O3 and TiO2@800-Fe2O3 catalysts were tested in recycling experiments of MO photodegradation. As shown in Fig. 9a, compared to the first usage, both TiO2@400-Fe2O3 and TiO2@800-Fe2O3 catalysts exhibit a declining degradation efficiency. The degradation efficiency retention compared to the first usage is only 59.6% and 66.7% for TiO2@400-Fe2O3 and TiO2@800-Fe2O3, respectively. It may be ascribed to the fact that stability of Fe2O3 during photocatalytic reactions is reduced due to photo-corrosion42,65. ALD coatings have been widely used as surface protection layer to protect active materials from photo-corrosion53,54. Therefore, an ultrathin Al2O3 (~ 2 nm) protective layer was deposited on catalysts surface by ALD in the same system to improve the stability of Fe2O3 coated TiO2 catalysts. Figure 9b shows the photocatalytic degradation of MO using both TiO2@400-Fe2O3 with Al2O3 passivation. The photocatalytic degradation efficiency decreases to 47.8% in 90 min, which is ascribed to the fact that Al2O3 coating with large band gap would also hinder the photogenerated carriers form migrating to the surface of electrode62,66. But it is still much better than that of pristine TiO2. More importantly, the stability of catalysts is persistent, as there is limited decline of degradation efficiency after three usage as shown in Fig. 8a, retaining 86.8% compared to the first usage. The results indicate that thin Al2O3 can act as a physical shell to protect Fe2O3 from photo-corrosion. This can be ascribed to that Al2O3 can prevent the direct contact between solution and Fe2O3.
Figure 9.
(a) Three cycles of MO degradation for TiO2@400-Fe2O3, TiO2@800-Fe2O3 and 20 cycles Al2O3 coated TiO2@400-Fe2O3 in 90 min. (b) Comparison of MO degradation curves for pristine TiO2, TiO2@400-Fe2O3, and 20 cycles Al2O3 coated TiO2@400-Fe2O3.
Conclusions
In summary, commercial anatase TiO2 powders were modified using ultrathin ALD Fe2O3 surface coating. The ultrathin Fe2O3 layer with small bandgap of ~ 2.20 V can increase the absorption of TiO2 supports in visible light. In addition, Fe2O3/TiO2 heterojunction can suppress the photoinduced electron–hole pairs recombination. The above results indicate excellent visible light photocatalytic activity for the Fe2O3 modified TiO2 powders. 400 cycles Fe2O3 (~ 2.6 nm) coated TiO2 photocatalysts show excellent degradation efficiency of 97.4% in 90 min, far above the performance of pristine TiO2 powders with only 12.5%. Moreover, an ultrathin Al2O3 (~ 2 nm) can improve the recycling usage performance of Fe2O3 coated TiO2 catalyst effectively. As conclusions, ALD surface modification with ultrathin film is a promising route for improving the visible light activity and long-term stability of photocatalysts.
Acknowledgements
This work is supported by Natural Science Foundation of China (51802150, 51721001, and 51571111) and Jiangsu Province (BK20170645, and BK20201087).
Author contributions
#Y.Q.C. and T.Q.Z. contributed equally in this work. X.R.Z., T.Q.Z. and Y.Q.C. prepared samples and tested the photocatalytical performance. C.L. conducted the SEM test. C.L and Q.R. performed XPS characterization. J.B.F performed TEM test. Y.Q.C. drafted the manuscript. A.D.L. supervised the whole work. W.M.L. and A.D.L. revised the manuscript. All authors critically read and commented on the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yan-Qiang Cao and Tao-Qing Zi.
References
- 1.Schultz DM, Yoon TP. Solar synthesis: prospects in visible light photocatalysis. Science. 2014;343:1239176. doi: 10.1126/science.1239176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu C, Li G, Kumar S, Yang K, Jin R. Phase transformation synthesis of novel Ag2O/Ag2CO3 heterostructures with high visible light efficiency in photocatalytic degradation of pollutants. Adv. Mater. 2014;26:892–898. doi: 10.1002/adma.201304173. [DOI] [PubMed] [Google Scholar]
- 3.Dong W, et al. Preparation of secondary mesopores in mesoporous anatase-silica nanocomposites with unprecedented-high photocatalytic degradation performances. Adv. Funct. Mater. 2016;26:964–976. [Google Scholar]
- 4.Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science. 2001;293:269–271. doi: 10.1126/science.1061051. [DOI] [PubMed] [Google Scholar]
- 5.Mills A, Davies RH, Worsley D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993;22:417–425. [Google Scholar]
- 6.Malato S, Fernández-Ibáñez P, Maldonado MI, Blanco J, Gernjak W. Decontamination and disinfection of water by solar photocatalysis: recent overview and trends. Catal. Today. 2009;147:1–59. [Google Scholar]
- 7.Byrne C, Subramanian G, Pillai SC. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018;6:3531–3555. [Google Scholar]
- 8.Cao YQ, et al. Photocatalytic activity and photocorrosion of atomic layer deposited ZnO ultrathin films for the degradation of methylene blue. Nanotechnology. 2015;26:024002. doi: 10.1088/0957-4484/26/2/024002. [DOI] [PubMed] [Google Scholar]
- 9.Xing Z, et al. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B. 2018;225:452–467. [Google Scholar]
- 10.Parale VG, Kim T, Phadtare VD, Yadav HM, Park H-H. Enhanced photocatalytic activity of a mesoporous TiO2 aerogel decorated onto three-dimensional carbon foam. J. Mol. Liq. 2019;277:424–433. [Google Scholar]
- 11.Kim T, et al. Facile synthesis of SnO2 aerogel/reduced graphene oxide nanocomposites via in situ annealing for the photocatalytic degradation of methyl orange. Nanomaterials. 2019;9:358. doi: 10.3390/nano9030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parale VG, et al. SnO2 aerogel deposited onto polymer-derived carbon foam for environmental remediation. J. Mol. Liq. 2019;287:110990. [Google Scholar]
- 13.Linsebigler AL, Lu G, Yates JT., Jr Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 1995;95:735–758. [Google Scholar]
- 14.Paul DR, et al. Synthesis, characterization and application of silver doped graphitic carbon nitride as photocatalyst towards visible light photocatalytic hydrogen evolution. Int. J. Hydrogen Energy. 2019 doi: 10.1016/j.ijhydene.2019.06.061. [DOI] [Google Scholar]
- 15.Paul DR, Sharma R, Nehra SP, Sharma A. Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 2019;9:15381–15391. doi: 10.1039/c9ra02201e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devina, et al. Silver doped graphitic carbon nitride for the enhanced photocatalytic activity towards organic dyes. J. Nanosci. Nanotechnol. 2019;19:5241–5248. doi: 10.1166/jnn.2019.16838. [DOI] [PubMed] [Google Scholar]
- 17.Patidar D, Yadav A, Paul DR, Sharma A, Nehra SP. Nanohybrids cadmium selenide-reduced graphene oxide for improving photo-degradation of methylene blue. Physica E. 2019;114:113560. [Google Scholar]
- 18.Fu H, Pan C, Yao W, Zhu Y. Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B. 2005;109:22432–22439. doi: 10.1021/jp052995j. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, et al. Graphitic carbon nitride quantum dots and nitrogen-doped carbon quantum dots co-decorated with BiVO4 microspheres: a ternary heterostructure photocatalyst for water purification. Sep. Purif. Technol. 2019;226:117–127. [Google Scholar]
- 20.Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J. Photochem. Photobiol., C. 2000;1:1–21. [Google Scholar]
- 21.Nakata K, Fujishima A. TiO2 photocatalysis: design and applications. J. Photochem. Photobiol., C. 2012;13:169–189. [Google Scholar]
- 22.Qian R, et al. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: an overview. Catal. Today. 2019;335:78–90. [Google Scholar]
- 23.Zhou F, Yan C, Sun Q, Komarneni S. TiO2/Sepiolite nanocomposites doped with rare earth ions: Preparation, characterization and visible light photocatalytic activity. Microporous Mesoporous Mater. 2019;274:25–32. [Google Scholar]
- 24.Cao Y-Q, et al. TiOxNy modified TiO2 powders prepared by plasma enhanced atomic layer deposition for highly visible light photocatalysis. Sci. Reports. 2018;8:12131. doi: 10.1038/s41598-018-30726-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J, et al. Photocatalytic enhancement of floating photocatalyst: layer-by-layer hybrid carbonized chitosan and Fe–N–codoped TiO2 on fly ash cenospheres. Appl. Surf. Sci. 2017;391:236–250. [Google Scholar]
- 26.Lee H, Kim B-J, Park Y-K, Kim J-S, Jung S-C. Assessment of photocatalytic performance of Fe/N-TiO2 photocatalysts prepared by liquid phase plasma process. Catal. Today. 2019 doi: 10.1016/j.cattod.2019.07.008. [DOI] [Google Scholar]
- 27.Choi T, Kim J-S, Kim JH. Transparent nitrogen doped TiO2/WO3 composite films for self-cleaning glass applications with improved photodegradation activity. Adv. Powder Technol. 2016;27:347–353. [Google Scholar]
- 28.Guo X, et al. Porous TiB2-TiC/TiO2 heterostructures: Synthesis and enhanced photocatalytic properties from nanosheets to sweetened rolls. Appl. Catal. B. 2017;217:12–20. [Google Scholar]
- 29.Zhao H, et al. Synthesis of hierarchically meso-macroporous TiO2/CdS heterojunction photocatalysts with excellent visible-light photocatalytic activity. J. Colloid Interface Sci. 2018;512:47–54. doi: 10.1016/j.jcis.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, et al. Mesoporous black TiO2-x/Ag nanospheres coupled with g-C3N4 nanosheets as 3D/2D ternary heterojunctions visible light photocatalysts. J. Hazard. Mater. 2018;343:181–190. doi: 10.1016/j.jhazmat.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Hou J, Cheng H, Yang C, Takeda O, Zhu H. Hierarchical carbon quantum dots/hydrogenated-γ-TaON heterojunctions for broad spectrum photocatalytic performance. Nano Energy. 2015;18:143–153. [Google Scholar]
- 32.Wang M, et al. TiO2/NiO hybrid shells: p–n junction photocatalysts with enhanced activity under visible light. J. Mater. Chem. A. 2015;3:20727–20735. [Google Scholar]
- 33.Liu J, et al. Black NiO–TiO2 nanorods for solar photocatalysis: recognition of electronic structure and reaction mechanism. Appl. Catal. B. 2018;224:705–714. [Google Scholar]
- 34.Zhou W, et al. Ag2O/TiO2 nanobelts heterostructure with enhanced ultraviolet and visible photocatalytic activity. ACS Appl. Mater. Interfaces. 2010;2:2385–2392. doi: 10.1021/am100394x. [DOI] [PubMed] [Google Scholar]
- 35.Gong Y, et al. All-solid-state Z-scheme CdTe/TiO2 heterostructure photocatalysts with enhanced visible-light photocatalytic degradation of antibiotic waste water. Chem. Eng. J. 2018;350:257–267. [Google Scholar]
- 36.Hao R, Wang G, Jiang C, Tang H, Xu Q. In situ hydrothermal synthesis of g-C3N4/TiO2 heterojunction photocatalysts with high specific surface area for Rhodamine B degradation. Appl. Surf. Sci. 2017;411:400–410. [Google Scholar]
- 37.Sood S, Mehta SK, Sinha ASK, Kansal SK. Bi2O3/TiO2 heterostructures: synthesis, characterization and their application in solar light mediated photocatalyzed degradation of an antibiotic, ofloxacin. Chem. Eng. J. 2016;290:45–52. [Google Scholar]
- 38.Yang L, et al. High efficient photocatalytic degradation of p-nitrophenol on a unique Cu2O/TiO2 p–n heterojunction network catalyst. Environ. Sci. Technol. 2010;44:7641–7646. doi: 10.1021/es101711k. [DOI] [PubMed] [Google Scholar]
- 39.Ren L, et al. Defects-engineering of magnetic γ-Fe2O3 ultrathin nanosheets/mesoporous black TiO2 hollow sphere heterojunctions for efficient charge separation and the solar-driven photocatalytic mechanism of tetracycline degradation. Appl. Catal. B. 2019;240:319–328. [Google Scholar]
- 40.Xu Z, et al. Sulfate functionalized Fe2O3 nanoparticles on TiO2 nanotube as efficient visible light-active photo-fenton catalyst. Ind. Eng. Chem. Res. 2015;54:4593–4602. [Google Scholar]
- 41.Hassan ME, Chen Y, Liu G, Zhu D, Cai J. Heterogeneous photo-Fenton degradation of methyl orange by Fe2O3/TiO2 nanoparticles under visible light. J. Water Process Eng. 2016;12:52–57. [Google Scholar]
- 42.Zheng X, Fu W, Kang F, Peng H, Wen J. Enhanced photo-Fenton degradation of tetracycline using TiO2-coated α-Fe2O3 core–shell heterojunction. J. Ind. Eng. Chem. 2018;68:14–23. [Google Scholar]
- 43.Lin Z, Liu P, Yan J, Yang G. Matching energy levels between TiO2 and α-Fe2O3 in a core–shell nanoparticle for visible-light photocatalysis. J. Mater. Chem. A. 2015;3:14853–14863. [Google Scholar]
- 44.Cao X, Luo S, Liu C, Chen J. Synthesis of bentonite-supported Fe2O3-doped TiO2 superstructures for highly promoted photocatalytic activity and recyclability. Adv. Powder Technol. 2017;28:993–999. [Google Scholar]
- 45.Cheng G, et al. A novel protocol to design TiO2–Fe2O3 hybrids with effective charge separation efficiency for improved photocatalysis. Adv. Powder Technol. 2017;28:665–670. [Google Scholar]
- 46.Yu X, Liu S, Yu J. Superparamagnetic γ-Fe2O3@SiO2@TiO2 composite microspheres with superior photocatalytic properties. Appl. Catal. B. 2011;104:12–20. [Google Scholar]
- 47.Liu M, Li X, Karuturi SK, Tok AIY, Fan HJ. Atomic layer deposition for nanofabrication and interface engineering. Nanoscale. 2012;4:1522–1528. doi: 10.1039/c2nr11875k. [DOI] [PubMed] [Google Scholar]
- 48.Knez M, Nielsch K, Niinistö L. Synthesis and surface engineering of complex nanostructures by atomic layer deposition. Adv. Mater. 2007;19:3425–3438. [Google Scholar]
- 49.Meng X, et al. Atomic layer deposition for nanomaterials synthesis and functionalization in energy technology. Mater. Horizons. 2017;4:133–154. [Google Scholar]
- 50.O’Neill BJ, et al. Catalyst design with atomic layer deposition. ACS Catal. 2015;5:1804–1825. [Google Scholar]
- 51.Liu J, et al. Enhanced photochemical catalysis of TiO2 inverse opals by modification with ZnO or Fe2O3 using ALD and the hydrothermal method. Mater. Res. Express. 2018;5:025509. [Google Scholar]
- 52.Hiltunen A, et al. Design aspects of all atomic layer deposited TiO2–Fe2O3 scaffold-absorber photoanodes for water splitting. Sustain. Energy Fuels. 2018;2:2124–2130. [Google Scholar]
- 53.Long J, et al. High-quality ZnO inverse opals and related heterostructures as photocatalysts produced by atomic layer deposition. Appl. Surf. Sci. 2018;454:112–120. [Google Scholar]
- 54.Cheng Q, et al. Al2O3 and SiO2 atomic layer deposition layers on ZnO photoanodes and degradation mechanisms. ACS Appl. Mater. Interfaces. 2017;9:16138–16147. doi: 10.1021/acsami.7b01274. [DOI] [PubMed] [Google Scholar]
- 55.Zhao X-R, et al. Photocatalytic properties of Co3O4-coated TiO2 powders prepared by plasma-enhanced atomic layer deposition. Nanoscale Res. Lett. 2017;12:497. doi: 10.1186/s11671-017-2269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan X, et al. The interplay of sulfur doping and surface hydroxyl in band gap engineering: mesoporous sulfur-doped TiO2 coupled with magnetite as a recyclable, efficient, visible light active photocatalyst for water purification. Appl. Catal. B. 2017;218:20–31. [Google Scholar]
- 57.Nefedov VI, Salyn YV, Leonhardt G, Scheibe R. A comparison of different spectrometers and charge corrections used in X-ray photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 1977;10:121–124. [Google Scholar]
- 58.Cheng L, Qiu S, Chen J, Shao J, Cao S. A practical pathway for the preparation of Fe2O3 decorated TiO2 photocatalyst with enhanced visible-light photoactivity. Mater. Chem. Phys. 2017;190:53–61. [Google Scholar]
- 59.Liu H, et al. Highly flexible Fe2O3/TiO2 composite nanofibers for photocatalysis and utraviolet detection. J. Phys. Chem. Solids. 2018;121:236–246. [Google Scholar]
- 60.Zhang X, et al. One-dimensional mesoporous Fe2O3@TiO2 core–shell nanocomposites: rational design, synthesis and application as high-performance photocatalyst in visible and UV light region. Appl. Surf. Sci. 2014;317:43–48. [Google Scholar]
- 61.Li J, Liu Z, Wang D, Zhu Z. Visible-light responsive carbon–anatase–hematite core–shell microspheres for methylene blue photodegradation. Mater. Sci. Semicond. Process. 2014;27:950–957. [Google Scholar]
- 62.Peng L, Xie T, Lu Y, Fan H, Wang D. Synthesis, photoelectric properties and photocatalytic activity of the Fe2O3/TiO2 heterogeneous photocatalysts. Phys. Chem. Chem. Phys. 2010;12:8033. doi: 10.1039/c002460k. [DOI] [PubMed] [Google Scholar]
- 63.Sun B, et al. Magnetic Fe2O3/mesoporous black TiO2 hollow sphere heterojunctions with wide-spectrum response and magnetic separation. Appl. Catal. B. 2018;221:235–242. [Google Scholar]
- 64.Liu J, et al. 3D flower-like α-Fe2O3@TiO2 core–shell nanostructures: general synthesis and enhanced photocatalytic performances. ACS Sustain. Chem. Eng. . 2015;3:2975–2984. [Google Scholar]
- 65.Krýsa J, et al. α-Fe2O3/TiO2 stratified photoanodes. J. Photochem. Photobiol., A. 2018;366:12–17. [Google Scholar]
- 66.Jiao W, et al. Hollow hematite single crystals deposited with ultra-thin Al2O3 by atom layer deposition for improved photoelectrochemical performance. Dalton Trans. 2017;46:10635–10640. doi: 10.1039/c7dt00504k. [DOI] [PubMed] [Google Scholar]