Abstract
The eradication rate of Helicobacter pylori (H. pylori) with proton pump inhibitors, amoxicillin, and clarithromycin has reportedly decreased. Some studies have found probiotics to be useful in eradicating H. pylori, but these effects have not been sufficiently investigated. We aimed to elucidate the role of probiotics in eradicating H. pylori infection. Patients in our hospital with H. pylori infection that received standard treatment from January 2015 to December 2016 were retrospectively evaluated (n = 468). They were divided into three groups based on their treatment regime, being either proton pump inhibitors, amoxicillin, or clarithromycin (PPI group), vonoprazan, amoxicillin, or clarithromycin (VPZ group), and proton pump inhibitors, amoxicillin, or clarithromycin/probiotics (Miya-BM®) (PPI + MBM group). We retrospectively evaluated the H. pylori eradication rate and reported side effects. According to intention-to-treat analyses, the eradication rate of H. pylori was significantly higher in the PPI + MBM group (87.1%) than in the PPI group (70.1%). There was no difference in side effects between any of the three groups. In conclusion, Miya-BM® may have an additive effect when included with eradication therapies for H. pylori.
Keywords: probiotics, eradication therapy of Helicobacter pylori, Miya-BM®
Introduction
Helicobacter pylori (H. pylori) is a known stomach carcinogen,(1,2) and its eradication has been shown to reduce the incidence of stomach cancer.(3) Since 2000, standard H. pylori eradication therapy in Japan has consisted of a combination of proton pump inhibitors (PPI), amoxicillin (AMX), and clarithromycin (CLR). However, the eradication rate for H. pylori with this standard primary eradication therapy has reportedly decreased,(4) with this decrease thought to be correlated with the increase of anti-CLR resistance of the bacteria. Therefore, many researchers have extensively investigated antibiotics that H. pylori are susceptible to.
Vonoprazan (VPZ) is a novel, orally active potassium-competitive acid blocker (P-CAB) discovered and synthesized by Takeda Pharmaceutical Company Ltd., Japan. VPZ provide faster, more potent and sustained gastric acid suppression than standard PPIs. When VPZ was used instead of PPI (with the same antibiotics as mentioned above), there was an increased eradication of H. pylori significantly.(5) However, VPZ has some concerns. We have found that VPZ may increase enteric infection risk more than PPIs by affecting intestinal microbiota. Specifically, VPZ may promote Clostridium difficile (C. difficile) infection by decreasing the genera prevent for the establishment of C. difficile infection such as Blautia and Coprococcus.(6) In addition, VPZ has been reported to dose-dependently increase serum gastrin levels, which have been suspected of causing gastric carcinoids in an animal model.(7) Although the effects of VPZ are promising, the use of PPI in H. pylori eradication might be recommended until the safety of VPZ is confirmed. Therefore, a PPI-based method to improve H. pylori eradication is essential.
In some reports, probiotics have been shown to improve the eradication rate with minimal side effects.(8,9) Miya-BM® (MBM) is a probiotic drug that contains Clostridium butyricum (C. butyricum) MIYAIRI 588. In a preliminary in vitro study, C. butyricum MIYAIRI 588 inhibited the growth of H. pylori.(10) However, there are scarce reports that have examined its effectiveness in eradicating H. pylori. The purpose of this study is to elucidate the efficacy of MBM in the context of H. pylori eradication therapy.
Materials and Methods
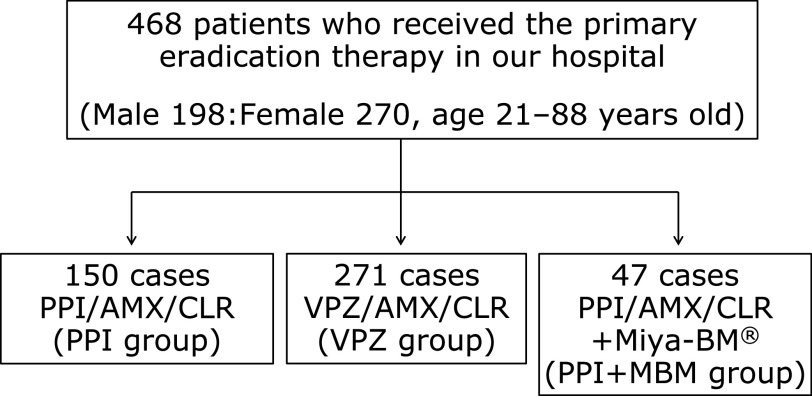
We retrospectively evaluated patients who received the standard primary eradication therapy for H. pylori from January 2015 to December 2016 at Kyoto Prefectural University of Medicine. Patients who dropped out were considered as treatment failures. They were divided into three groups based on their treatment regime, being either PPI/AMX/CLR (PPI group), VPZ/AMX/CLR (VPZ group), and PPI/AMX/CLR/probiotics (Miya-BM®; MBM) (PPI + MBM group). A cohort of 468 patients participated in this study (198 male, 270 female; age 21–88 years) and was divided into three treatment groups: PPI group (n = 150), VPZ group (n = 271), and PPI + MBM group (n = 47) (Fig. 1). Protocols were approved by the Medical Ethics Review Committee of the Kyoto Prefectural University of Medicine (Kyoto, Japan).
Fig. 1.
Study design. Patients who received the standard primary eradication therapy for H. pylori were divided into three groups based on their treatment regime, being either PPI/AMX/CLR (PPI group), VPZ/AMX/CLR (VPZ group), and PPI/AMX/CLR/Miya-BM® (PPI + MBM group). PPI, proton pump inhibitors; AMX, amoxicillin; CLR, clarithromycin.
Statistical analyses
We retrospectively evaluated the H. pylori eradication rate and the treatments’ side effects. Intention-to-treat (ITT) and per-protocol (PP) analyses excluded patients lost to follow-up or prematurely withdrew before completion of the study. Significant differences between the two groups were analyzed using Student’s t test. Differences with p<0.05 were considered to be statistically significant.
Results
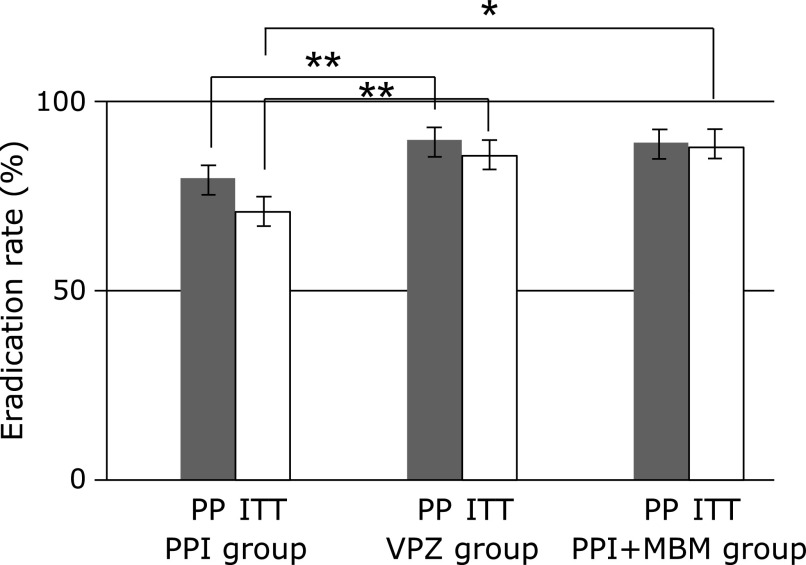
In an ITT analysis, the eradication rates were 70.1% in the PPI group, 84.9% in the VPZ group, and 87.1% in the PPI + MBM group. In a PP analysis, the eradication rate was 81.7% in the PPI group, 89.8% in the VPZ group, and 89.1% in the PPI + MBM group (Fig. 2). There was a significant difference in the eradication rate between the PPI and VPZ groups both in ITT and PP analyses (p = 0.0008 in ITT analysis; p = 0.0073 in PP analysis). Additionally, ITT analysis of the eradication rate in the PPI + MBM group was significantly higher than that of the PPI group (p = 0.0195). The main side effect was diarrhea, eruption, and stomatitis. Fortunately, there were no side effects severe enough to require hospitalization. Side effects were reported in 14.7% of the PPI group, 10.0% of the VPZ group, and 19.1% of the PPI + MBM group. There was no significant difference between the three groups (Table 1).
Fig. 2.
The eradication rate of three groups. In an ITT analysis, the eradication rate was 70.1% in PPI group, 84.9% in VPZ group, and 87.1% in PPI + MBM group. In a PP analysis, the eradication rate was 81.7% in PPI group, 89.8% in VPZ group, and 89.1% in PPI + MBM group. (n = 150 in PPI group, n = 271 in VPZ group, n = 47 in PPI + MBM group). ITT, intention-to-treat; PP, per-protocol. *p<0.05, **p<0.01.
Table 1.
Side effects of eradication therapy of H. pylori
| PPI group n = 150 (cases) | VPZ group n = 271 (cases) | PPI + MBM group n = 47 (cases) | |
|---|---|---|---|
| Diarrhea | 6 | 10 | 4 |
| Eruption | 7 | 6 | 2 |
| Stomatitis | 4 | 3 | 1 |
| Heartburn, abdominal pain | 2 | 4 | 1 |
| Others | 4 | 3 | 0 |
| The incident of side effects | 15.30% | 9.60% | 17.00% |
Discussion
In this study, we reported the additive effects of MBM, a probiotic, on H. pylori eradication when combined with conventional treatment regimes. So far, the most frequently studied probiotics are butyric acid bacteria, particularly Lactobacillus sp.(11,12) During its viable bacterial preparations, butyric acid bacteria form spores that are not easily affected by antibacterial drugs even if they are not imparting artificial resistance.(13) Further, it is reported that butyric acid bacteria are useful for the prevention and treatment of diarrhea.(14) Therefore, we chose MBM as the probiotic for this study.
We suspect three factors that contribute to the effectiveness of MBM, the first one being its antibacterial properties. It is known that butyric acid has an antibacterial property that inhibits the growth of H. pylori.(10) As mentioned above, butyric acid bacteria are less susceptible to antibacterial agents and can survive even if exposed to antibacterial agents by forming spores. It is also known that butyric acid bacteria continue to germinate and proliferate after the concentration of antibacterial agents in the digestive tract decreases, thereby resuming their role as a probiotic.(14) Further, the butyric acid damages the integrity of the H. pylori cell envelope, which is necessary for many vital cell functions such as transport, peptidoglycan biosynthesis and cross-linking, as well as the synthesis of lipids. Disturbance of the cell envelope causes a destabilization of the cell membrane and, ultimately, cell death.(15)
The second factor that we suspect to contribute to the effectiveness of MBM is its capacity to inhibit H. pylori from adhering to gastric epithelial cells. Gastritis associated with H. pylori infection is triggered by such adhesion. The effects of C. butyricum on adhesion of H. pylori to human gastric cancer cells (MKN45) was reported by Takahashi et al.(10) Since C. butyricum inhibits H. pylori from adhering to human gastric cancer cells, it may inhibit H. pylori from adhering to gastric epithelial cells in the same way.
The third factor that we suspect to contribute to the effectiveness of MBM in our study is its capacity to improve the gut microbiota. It is known that probiotic supplementation can make the gut microbiota more resilient to the effects of antibiotics. The benefits of “good” antibiotic-resistant bacteria in the gut—such as C. butyricum—may increase the eradication rate of H. pylori.(16)
It has been reported that probiotic supplementation, together with typical eradication therapies for H. pylori, reduces unpleasant side effects.(8,17) However, in the present study, we found no significant differences in the prevalence of side effects between the three groups. Zheng et al.(11) revealed that H. pylori eradication rates had remarkably improved with combination therapy of a lactobacillus-containing probiotic, but there was no significant decrease in adverse side effects. To our knowledge, no publications exist to date that describes in detail the mechanism by which probiotics reduce or maintain the prevalence of adverse side effects. The effect of probiotics on negative side effects is still ambiguous and requires further study.
There are some limitations to this study—first, we were limited by the relatively small sample size from one center, as well as the capacity to only investigate retrospectively. Second, the prevalence of side effects could not be consistently determined, since doctors could not have known of our intentions while they were treating their patients. Therefore, an accurate analysis may not have been performed.
Third, factors affecting the eradication rate and the ratio of drug sensitivity were not investigated in this study. For example, it is known that CYP2C19 metabolizes PPI, and the serum concentration of PPI is affected by a patient’s CYP2C19 gene polymorphism, which has been reported to affect its therapeutic effect. As a rapid metabolizer, CYP2C19 may deem our H. pylori eradication therapy as ineffective.(18) Furthermore, an antibiotic susceptibility test by bacterial culture from each patient’s gastric biopsy is recommended to improve the eradication rate ultimately.(19) It is reported that the eradication rates are dependent on the susceptibility of the strain to metronidazole and CLR,(20) and the eradication rate can be improved by performing a susceptibility test and selecting an appropriate antimicrobial agent. However, in clinical practice, this procedure is not feasible and infrequently performed in Japan.
Despite these limitations, our study reported that the eradication rate of H. pylori was significantly increased by adding MBM with conventional PPI-based therapy when compared to that without MBM, being comparable to VPZ-based eradication therapy.
In conclusion, the supplemental use of Miya-BM® in H. pylori eradication therapy with PPI is recommended until the safeness of VPZ will be confirmed.
Author Contributions
YN supervised this study, and RM and OH were responsible for its conception, design, and critical revision. AM and YS contributed to data analysis and interpretation.
Acknowledgments
This work was partly supported by Grant of Industry-Academia-Government Collaboration of “Field for Knowledge Integration and Innovation” (FKII) to YN (No. 16824414) from the Ministry of Agriculture, Forestry and Fisheries of Japan, and the contract research expenses (J192001018 survey of H. pylori infection in high school students).
Conflict of Interest
The authors disclose a conflict of interest with the following companies. OH received lecture fees from AstraZeneca KK (Osaka, Japan) and Daiichi Sankyo Company (Tokyo, Japan). YN received a scholarship fund from EA Pharma. Co. Ltd. and collaboration research fund from FUJIFILM Medical Co., Ltd. and Taiyo Kagaku Co., Ltd., and has been paid lecture fees by Mylan EPD Co., Takeda Pharma. Co. Ltd., Mochida Pharma. Co. Ltd., EA Pharma. Co. Ltd., Otsuka Pharma. Co. Ltd., Nippon Kayaku Co. Ltd., and Miyarisan Pharma. Co. Ltd. These funds partly funded the present research. Neither the funding agency nor any outside organization has participated in study design or have any competing of interest. These companies had final approval of the manuscript.
References
- 1.Handa O, Naito Y, Yoshikawa T. Redox biology and gastric carcinogenesis: the role of Helicobacter pylori. Redox Rep 2011; 16: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Handa O, Naito Y, Yoshikawa T. Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm Res 2010; 59: 997–1003. [DOI] [PubMed] [Google Scholar]
- 3.Sugano K. Effect of Helicobacter pylori eradication on the incidence of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2019; 22: 435–445. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Takahashi S, Suzuki H, et al. Changes in the first line Helicobacter pylori eradication rates using the triple therapy—a multicenter study in the Tokyo metropolitan area (Tokyo Helicobacter pylori study group). J Gastroenterol Hepatol 2014; 29 Suppl 4: 29–32. [DOI] [PubMed] [Google Scholar]
- 5.Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016; 65: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otsuka T, Sugimoto M, Inoue R, et al. Influence of potassium-competitive acid blocker on the gut microbiome of Helicobacter pylori-negative healthy individuals. Gut 2017; 66: 1723–1725. [DOI] [PubMed] [Google Scholar]
- 7.Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther 2015; 42: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armuzzi A, Cremonini F, Bartolozzi F, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2001; 15: 163–169. [DOI] [PubMed] [Google Scholar]
- 9.Canducci F, Armuzzi A, Cremonini F, et al. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther 2000; 14: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Kamiya S. Studies of the effect of Clostridium butyricum on Helicobacter pylori in several test models including gnotobiotic mice. J Med Microbiol 2000; 49: 635–642. [DOI] [PubMed] [Google Scholar]
- 11.Zheng X, Lyu L, Mei Z. Lactobacillus-containing probiotic supplementation increases Helicobacter pylori eradication rate: evidence from a meta-analysis. Rev Esp Enferm Dig 2013; 105: 445–453. [DOI] [PubMed] [Google Scholar]
- 12.Shimbo I, Yamaguchi T, Odaka T, et al. Effect of Clostridium butyricum on fecal flora in Helicobacter pylori eradication therapy. World J Gastroenterol 2005; 11: 7520–7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko N, Nakayama T, Ichikawa N. Susceptibility of spore-forming butyric acid bacteria to antimicrobial agents. Yakugaku Zasshi 2012; 132: 849–853. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 14.Egashira K, Kitahara T, Kashiwagi K, et al. Investigation for proper use of probiotics in Nagasaki University Hospital of Medicine and Dentistry. Yakugaku Zasshi 2006; 126: 1155–1161. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 15.Yonezawa H, Osaki T, Hanawa T, et al. Destructive effects of butyrate on the cell envelope of Helicobacter pylori. J Med Microbiol 2012; 61 (Pt 4): 582–589. [DOI] [PubMed] [Google Scholar]
- 16.Oh B, Kim BS, Kim JW, et al. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter 2016; 21: 165–174. [DOI] [PubMed] [Google Scholar]
- 17.Cremonini F, Di Caro S, Covino M, et al. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol 2002; 97: 2744–2749. [DOI] [PubMed] [Google Scholar]
- 18.Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther 2000; 14: 963–978. [DOI] [PubMed] [Google Scholar]
- 19.Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection—recent developments in diagnosis. World J Gastroenterol 2014; 20: 9299–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alba C, Blanco A, Alarcón T. Antibiotic resistance in Helicobacter pylori. Curr Opin Infect Dis 2017; 30: 489–497. [DOI] [PubMed] [Google Scholar]