Salmonella causes worldwide foodborne illness, leading to massive disease burden and an estimated 600,000 deaths per year. Salmonella infects orally and invades intestinal epithelial cells using a type 3 secretion system that directly injects effector proteins into host cells. This first step in invasion is tightly regulated by a variety of inputs. In this work, we demonstrate that Salmonella senses the functionality of outer membrane assembly in determining regulation of invasion machinery, and we show that Salmonella uses distinct mechanisms to detect specific perturbations in envelope assembly.
KEYWORDS: Bam, RcsCDB, SPI1, Salmonella, pathogenesis
ABSTRACT
Salmonella enterica serovar Typhimurium uses a type three secretion system (T3SS) encoded on the Salmonella pathogenicity island 1 (SPI1) to invade intestinal epithelial cells and induce inflammatory diarrhea. The SPI1 T3SS is regulated by numerous environmental and physiological signals, integrated to either activate or repress invasion. Transcription of hilA, encoding the transcriptional activator of the SPI1 structural genes, is activated by three AraC-like regulators, HilD, HilC, and RtsA, that act in a complex feed-forward loop. Deletion of bamB, encoding a component of the β-barrel assembly machinery, causes a dramatic repression of SPI1, but the mechanism was unknown. Here, we show that partially defective β-barrel assembly activates the RcsCDB regulon, leading to decreased hilA transcription. This regulation is independent of RpoE activation. Though Rcs has been previously shown to repress SPI1 when disulfide bond formation is impaired, we show that activation of Rcs in a bamB background is dependent on the sensor protein RcsF, whereas disulfide bond status is sensed independently. Rcs decreases transcription of the flagellar regulon, including fliZ, the product of which indirectly activates HilD protein activity. Rcs also represses hilD, hilC, and rtsA promoters by an unknown mechanism. Both dsbA and bamB mutants have motility defects, though this is simply regulatory in a bamB background; motility is restored in the absence of Rcs. Effector secretion assays show that repression of SPI1 in a bamB background is also regulatory; if expressed, the SPI1 T3SS is functional in a bamB background. This emphasizes the sensitivity of SPI1 regulation to overall envelope homeostasis.
IMPORTANCE Salmonella causes worldwide foodborne illness, leading to massive disease burden and an estimated 600,000 deaths per year. Salmonella infects orally and invades intestinal epithelial cells using a type 3 secretion system that directly injects effector proteins into host cells. This first step in invasion is tightly regulated by a variety of inputs. In this work, we demonstrate that Salmonella senses the functionality of outer membrane assembly in determining regulation of invasion machinery, and we show that Salmonella uses distinct mechanisms to detect specific perturbations in envelope assembly.
INTRODUCTION
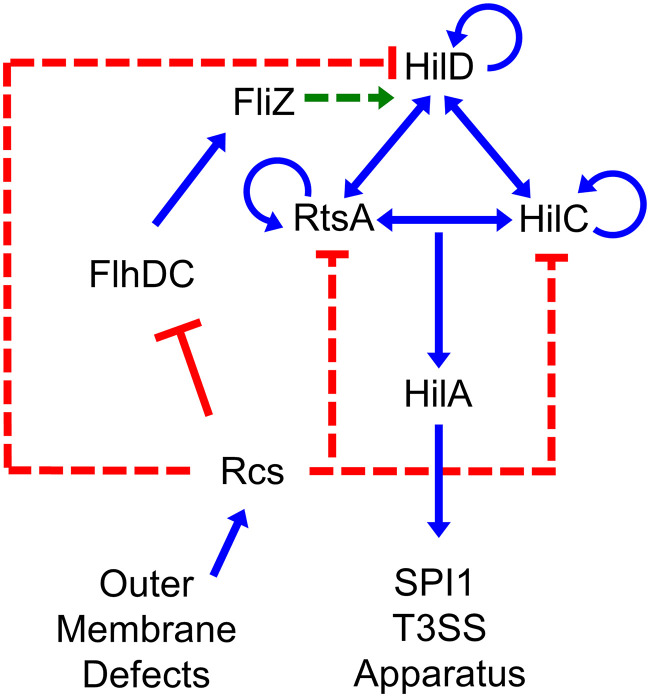
Salmonella serovars are common foodborne pathogens, causing over 1 million cases of disease per year in the United States (1). Salmonella enterica serovar Typhimurium typically causes self-limiting gastroenteritis, but in some immunocompromised individuals infection can result in septicemia and death. Infection is initiated by activating the Salmonella pathogenicity island 1 type 3 secretion system (SPI T3SS) in the distal ileum of the small intestine. This system is tightly controlled by a large number of inputs that regulate the production of the transcriptional activator, HilA. The hilA gene is directly activated by three AraC-like regulators, HilD, HilC, and RtsA, which act in a feed-forward loop to activate expression of themselves, each other, and hilA (Fig. 1) (2). This regulatory circuit responds to a plethora of environmental signals and regulatory systems, leading to precise control of expression and coordination with other cellular systems.
FIG 1.
SPI1 T3SS regulatory circuit. Simplified regulatory model of the SPI1 T3SS and related regulators. Blue lines indicate transcriptional activation, red lines indicate transcriptional repression, and green lines indicate regulation at the protein level. Dotted lines indicate that the exact mechanism of regulation is not known and is likely indirect.
One signal that has been shown to repress hilA expression is a defect in the β-barrel assembly machinery (BAM) complex (3, 4), required for proper assembly of outer membrane β-barrel proteins (OMPs) (5–11). OMPs are synthesized in the cytoplasm, secreted into the periplasm through the Sec translocase (12), and delivered to the multisubunit BAM complex via chaperones (13). The BAM complex consists of the β-barrel OMP BamA, which is highly conserved and essential, and multiple accessory proteins that aid in OMP assembly (6, 8, 14–16). These include BamBCDE, which are all OM lipoproteins whose precise functions are unknown (6, 8, 17). When the system is strained, unfolded OM proteins accumulate in the periplasm, leading to activation of RpoE, which upregulates BAM complex components and outer membrane homeostasis proteins to reduce outer membrane stress (18, 19).
RpoE can negatively regulate genes as well, mainly through small RNAs (sRNAs) that rapidly alter expression, binding mRNAs to block their translation or stimulate degradation (20). The RpoE-regulated sRNAs MicA and RybB reduce OMP synthesis by repressing ompA, lamB, ompC, and ompX translation (21–24). MicC represses translation of ompC and ompD mRNAs (25, 26), while the more recently characterized MicL (RyeF) represses the major OM lipoprotein Lpp (25, 27).
Though RpoE could sense a defective BAM complex and repress hilA, there are other envelope stress responses that could be responsible. PhoPQ and RcsBCD both respond to OM insults (28–30), while EnvZ/OmpR responds to osmotic stress (31) and periplasmic stress (32); all of these systems are known to regulate SPI1 (2, 3, 33–36). The Rcs system responds to β-lactam antibiotics (37–40), lysozyme (41, 42), LPS charge/membrane fluidity (28, 43, 44), and lipoprotein trafficking (45). The Rcs system is activated via a phosphorylation cascade from the sensor kinase RcsC, through the phosphotransfer protein RcsD, to the response regulator RcsB (46). When phosphorylated, RcsB can form homodimers and activate various targets in the cell, including the sRNA RprA, known mainly for increasing translation of the general stationary-phase stress response sigma factor RpoS (47, 48). RcsB can also form heterodimers with several other proteins (49), including RcsA, to activate colonic acid capsule genes and repress flagellar regulatory genes, leading to a biofilm lifestyle (49–51).
Deletion of the nonessential β-barrel assembly protein bamB leads to repression of hilA expression through an unknown mechanism (3, 4). In this work, we show that, although deletion of bamB does activate the RpoE stress response in Salmonella, repression of hilA occurs independently of known RpoE-regulated sRNAs. Rather, a dysfunctional BAM complex stimulates the Rcs response to repress hilA through the flagellar regulatory protein FliZ, which affects HilD protein activity, as well as through transcriptional control of hilD, hilC, and rtsA.
RESULTS
Loss of BamB leads to repression of hilA independently of RpoE.
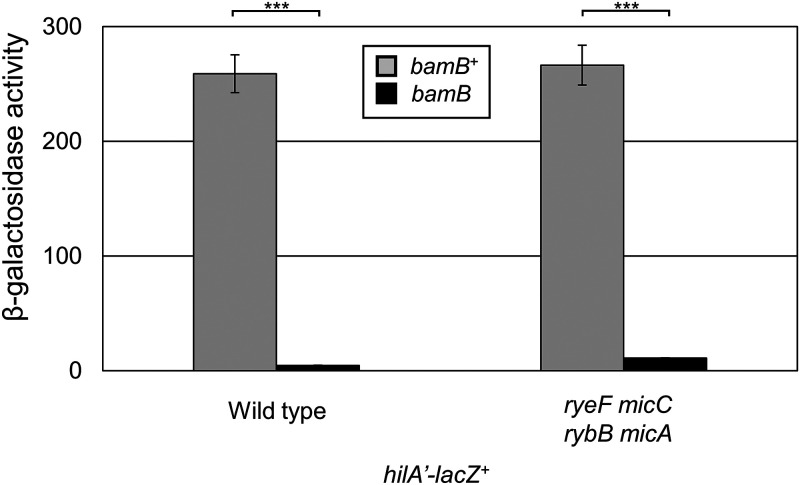
Deletion of bamB (yfgL), encoding a component of the β-barrel assembly machinery, has been shown to repress expression of the SPI1 T3SS (3, 4, 52). We first wanted to confirm that deletion of bamB leads to repression of the main SPI1 activator hilA using a hilA’-lacZ+ transcriptional fusion. In the bamB mutant background, hilA expression decreased dramatically, demonstrating that loss of BamB leads to loss of SPI1 expression (Fig. 2).
FIG 2.
Loss of BamB represses SPI1 expression independent of RpoE-activated sRNAs. β-Galactosidase activity was measured from cells containing a hilA’-lacZ+ transcriptional fusion in the indicated backgrounds. Cells were grown overnight, subcultured 1:100, and then grown with mild aeration for 6 h at 37˚C in HSLB. β-Galactosidase activity units are defined as (micromoles of ONP formed per minute) × 106/(OD600 × milliliters of cell suspension) and are reported as mean ± standard deviation where n = 3. Strains used: JS749, JS1040, JS2367, and JS2368.
The BAM complex is required to properly fold and assemble outer membrane β-barrel proteins (5–11). Misfolded outer membrane proteins induce the RpoE regulon, which includes periplasmic chaperones, periplasmic proteases, and small RNAs that block translation of certain outer membrane protein mRNAs, relieving stress on the BAM system (19). Mutations affecting the BAM complex are known to stimulate the RpoE response in E. coli (53). We first hypothesized that the repression of SPI1 seen in the bamB mutant was mediated through RpoE. We confirmed that that deletion of bamB activates RpoE in Salmonella by monitoring expression from an rpoE’-lacZ+ transcriptional fusion (see Fig. S1 in the supplemental material).
Previous data had suggested that deletion of bamB leads to repression of hilA through repression of hilD translation (3), and because negative regulatory effects from RpoE tend to be sRNA mediated (12), we hypothesized that hilA is repressed through an RpoE-activated sRNA. We deleted the known RpoE-regulated sRNA genes micA, micC, micL (ryeF), and rybB and examined hilA expression in the presence and absence of BamB (Fig. 2). Deletion of RpoE-activated sRNAs did not alleviate repression of hilA expression, demonstrating that deletion of bamB integrates into SPI1 regulation via some other mechanism.
To test if RpoE could repress SPI1 expression independently of known regulated sRNAs, we monitored expression of the hilA’-lacZ+ fusion in a strain overexpressing OmpX, known to induce the RpoE regulon (54). Expression of OmpX led to activation of RpoE, as demonstrated by increased expression from an rpoE’-lacZ+ fusion (Fig. S1), but this had no effect on SPI1 expression, suggesting that loss of bamB must repress SPI1 independently of RpoE.
Rcs mediates repression of SPI1 in the absence of bamB.
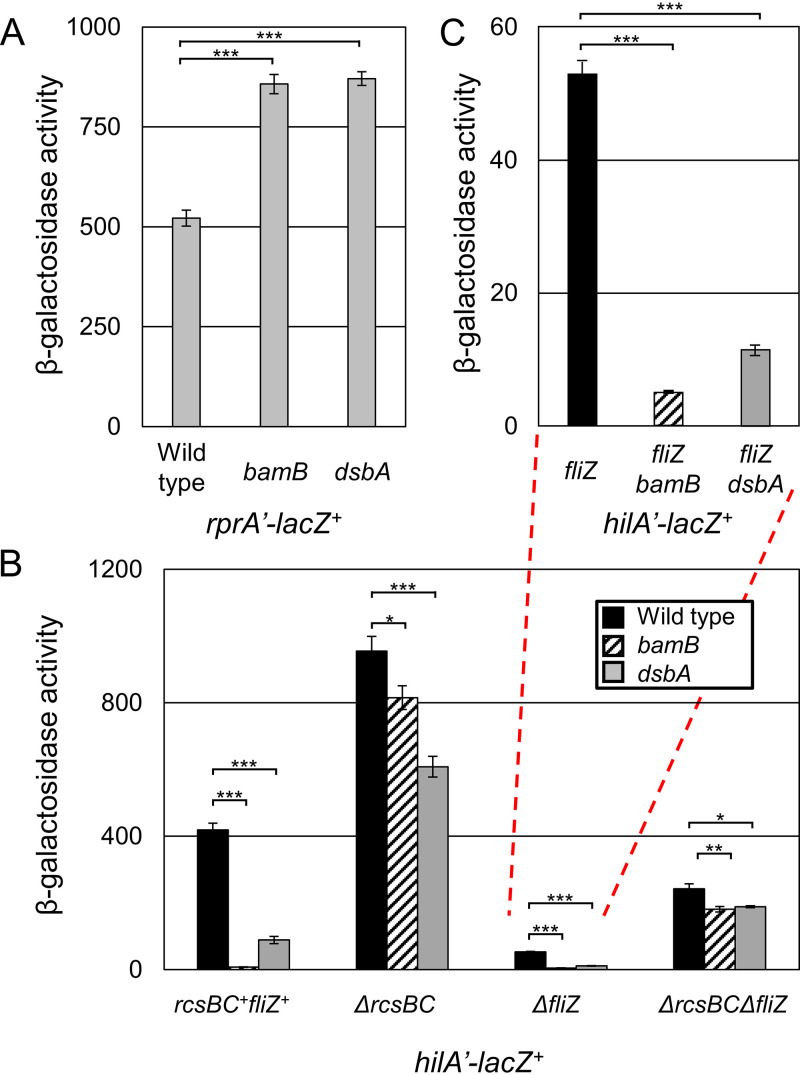
To identify the mediator of hilA repression in the absence of bamB, we performed a transposon mutagenesis in a hilA’-lacZ+ bamB background, screening for mutants with increased expression of hilA on LB agar containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Subsequent β-galactosidase assays identified four MudCm insertions (55) that conferred greater than a 5-fold increase in hilA expression in the bamB background (Fig. S2). DNA sequence analyses (56) indicated that two of the insertions were in topA, encoding topoisomerase I, one was in yjiY, encoding an inner membrane transporter (57), and one was in rcsD (yojN), encoding part of the RcsBCD signal transduction system (58). To confirm these phenotypes, we transduced the MudCm insertions into strains containing the hilA’-lacZ+ with or without bamB. The yjiY-associated insertion had the most modest effect and functions through an unknown mechanism. MudCm insertions associated with topA and rcsD alleviated repression most dramatically. The mutations in topA are more likely causing indirect increases in hilA expression and we did not follow up with this mutation (59). Given the role of the Rcs system in sensing envelope stress (46) and the fact that this system had been previously shown to affect SPI1 expression, it seemed likely that the bamB effect could be mediated primarily through Rcs.
To confirm that the Rcs regulon was induced in the absence of bamB, we fused the promoter of the Rcs-regulated sRNA gene rprA to lacZ and integrated that fusion into the lambda attachment site. In the absence of bamB, rprA expression roughly doubled, confirming activation of the Rcs regulon (Fig. 3A). We previously demonstrated that Salmonella uses Rcs to sense disulfide bond status in the periplasm and repress hilA expression when disulfide bond formation is defective (35). We confirmed that Rcs is activated in the absence of dsbA; deletion of dsbA also led to doubling of rprA expression, similar to effects seen in the bamB deletion background.
FIG 3.
BAM dysfunction stimulates Rcs, which represses SPI1. β-Galactosidase activity was measured from cells containing the indicated transcriptional fusion. Cells were grown overnight, subcultured 1:100, and then grown with mild aeration for 6 h at 37˚C in HSLB medium. Panel C represents a replotting of the indicated data in panel B. β-Galactosidase activity units are defined as (micromoles of ONP formed per minute) × 106/(OD600 × milliliters of cell suspension) and are reported as mean ± standard deviation where n = 3. P values are indicated as follows from unpaired t tests: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Strains used were JS2376, JS2377, and JS2378 (A) and JS749, JS1040, JS754, JS775, JS776, JS2379, JS778, JS2380, JS780, JS782, JS2381, and JS783 (B).
Disulfide bond status and β-barrel assembly affect SPI1 expression through Rcs.
The dsbA mutant was shown to repress SPI1 both through activation of Rcs, which represses flhDC, encoding the main transcriptional regulator of the flagellar regulon, and by preventing proper assembly of the flagellar apparatus, thereby blocking export of the anti-sigma factor FlgM (35). Both of these effects decreased expression of fliZ, encoding a protein that indirectly controls HilD activity (60, 61).
To explore how Rcs senses β-barrel protein assembly compared to disulfide bond status, we deleted bamB or dsbA in the presence and absence of rcsBC and tested expression of a hilA’-lacZ+ fusion. Deletion of rcsBC led to increased hilA expression, demonstrating that in SPI1-inducing conditions, Rcs partially represses hilA (Fig. 3B). In the absence of rcsBC, deletion of dsbA repressed hilA, but much less than in an rcsBC+ background, because the dsbA effect functions through Rcs and FlgM (35). Most importantly, in the absence of rcsBC, deletion of bamB had only a minor effect on hilA expression. This demonstrates that the Rcs system does mediate repression of hilA when the BAM complex is not fully functional.
The Rcs system represses the flagellar operon by downregulating the master flagellar regulator FlhDC (51). Flagellar regulation has been shown to integrate into SPI1 regulation, mainly through the FlhDC-activated flagellar regulator FliZ (60, 61). To test if the bamB effect is integrated through FliZ similarly to dsbA, we tested the effects of dsbA and bamB mutants on hilA expression in the absence of rcsBC and fliZ. In the absence of fliZ, deletion of either dsbA or bamB significantly decreased hilA expression (Fig. 3C). This shows that bamB and dsbA repressive effects integrate into SPI1 at least in part independently of FliZ. When both rcsBC and fliZ are deleted, we see almost no further repression of SPI1 in the bamB and dsbA mutant backgrounds, proving that these systems are the main integration point for SPI1 regulation. Together, these data show that, although FliZ is a key link between Rcs and SPI1 regulation, Rcs regulates SPI1 through some additional mechanism.
Rcs senses disulfide bond status and β-barrel assembly through distinct mechanisms.
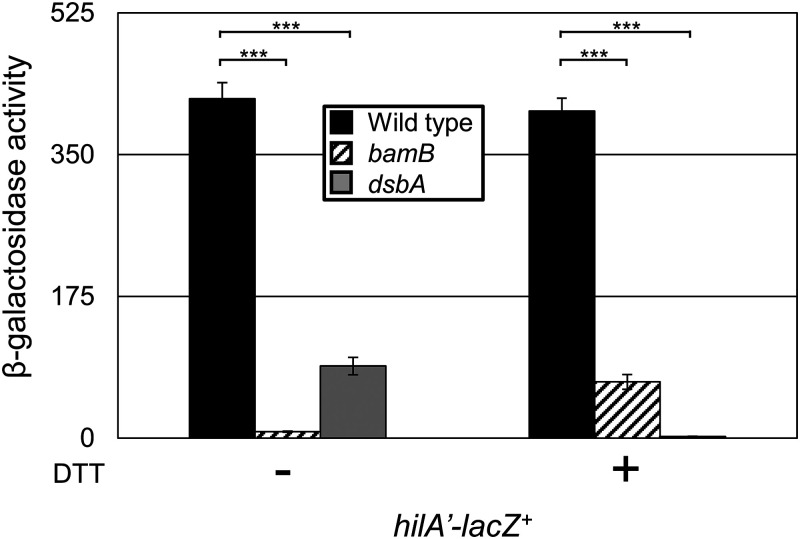
Lin et al. showed that the dsbA effect is amplified in the presence of a reducing agent that further disrupts disulfide bond formation in Salmonella (35). If the mechanism of sensing disulfide status and BAM function are the same, we would expect dithiothreitol (DTT) to amplify the effects of the bamB mutant as well. The addition of the reducing agent DTT further decreased hilA expression in the dsbA deletion background, as expected (Fig. 4). In contrast, DTT partially suppressed the repression of hilA expression conferred by loss of BamB. These data strongly suggest that the mechanism of SPI1 regulation, and perhaps the mechanism of Rcs activation, differs in the bamB and dsbA backgrounds.
FIG 4.
Rcs senses disulfide bond status and BAM functionality through distinct mechanisms. β-Galactosidase activity was measured from cells containing a hilA’-lacZ+ transcriptional fusion in the designated backgrounds. Cells were grown overnight, subcultured 1:100, and then grown with mild aeration for 6 h at 37˚C in HSLB medium in the presence or absence of 2 mM DTT. β-Galactosidase activity units are defined as (micromoles of ONP formed per minute) × 106/(OD600 × milliliters of cell suspension) and are reported as mean ± standard deviation where n = 3. P values are indicated as follows from unpaired t tests: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Strains used: JS749, JS1040, and JS754.
The bamB effect depends on the signaling protein RcsF, whereas the dsbA effect is RcsF independent.
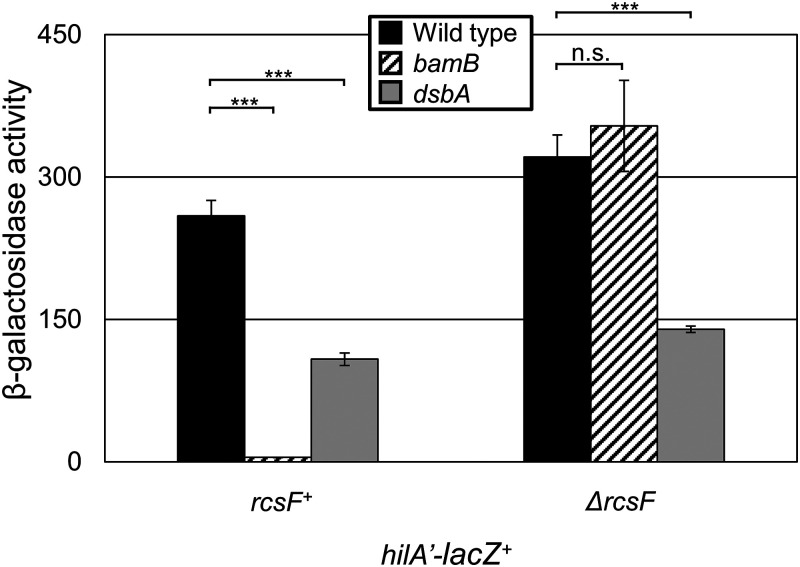
We originally proposed that deletion of dsbA affected disulfide bond formation in the sensor protein RcsF, which contains two disulfide bonds (62) and senses a subset of signals that activate RcsBCD. To sense outer membrane stress, RcsF is threaded through abundant β-barrel proteins, which require BAM for proper folding (63, 64). To test if the dsbA effect is functioning at the level of RcsF, we deleted dsbA in the absence of rcsF and monitored expression of hilA. Deletion of dsbA led to repression of hilA regardless of rcsF (Fig. 5), showing that dsbA affects expression independently of the disulfide bonds in RcsF. In contrast, deletion of rcsF completely suppressed the decrease in hilA expression conferred by loss of BamB. Thus, the outer membrane defect caused by deletion of bamB is sensed by RcsF, whereas disulfide bond status appears to activate Rcs through some other mechanism.
FIG 5.
RcsF senses β-barrel assembly but not disulfide bond status. β-Galactosidase activity was measured from cells containing a hilA’-lacZ+ transcriptional fusion in the designated backgrounds. Cells were grown overnight, subcultured 1:100, and then grown with mild aeration for 6 h at 37˚C in HSLB. β-Galactosidase activity units are defined as (micromoles of ONP formed per minute) × 106/(OD600 × milliliters of cell suspension) and are reported as mean ± standard deviation where n = 3. P values are indicated as follows from unpaired t tests: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Strains used: JS749, JS1040, JS754, and JS2383-JS2385.
Flagellar gene expression responds to β-barrel assembly.
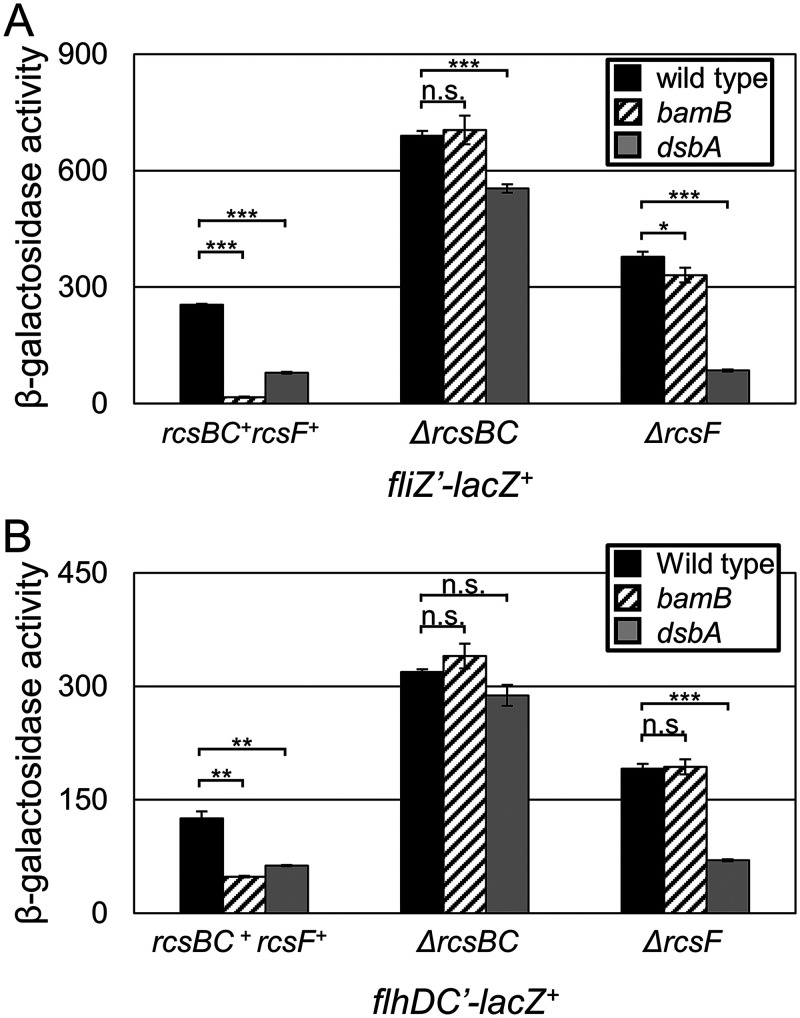
To confirm that the bamB effect functions partially through flagellar regulation, we monitored expression of both flhDC and fliZ. Expression from both the fliZ’-lacZ+ and flhDC’-lacZ+ fusions was significantly reduced in the absence of bamB (Fig. 6). This repression was completely alleviated in the absence of rcsBC, demonstrating that all regulatory effects are dependent on RcsBC. In contrast, deletion of dsbA had an additional slight repressive effect on fliZ expression independent of RcsBC, whereas flhDC expression was not affected. These data suggest that bamB represses SPI1 by activating Rcs to repress flhDC and, therefore, fliZ. The dsbA mutation also affects fliZ transcription independently of Rcs, presumably due to the inability to export FlgM, leading to decreased expression from the FliA-dependent promoter (35).
FIG 6.
Rcs repression of flagellar regulators. β-Galactosidase activity was measured from cells containing the indicated lacZ transcriptional fusions. Cells were grown overnight, subcultured 1:100, and then grown with mild aeration for 6 h at 37˚C in HSLB medium. β-Galactosidase activity units are defined as (micromoles of ONP formed per minute) × 106/(OD600 × milliliters of cell suspension) and are reported as mean ± standard deviation where n = 3. P values are indicated as follows from unpaired t tests: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Strains used were JS696, JS785, JS794, JS788, and JS2392-JS2396 (A) and JS2347 and JS2405-JS2412 (B).
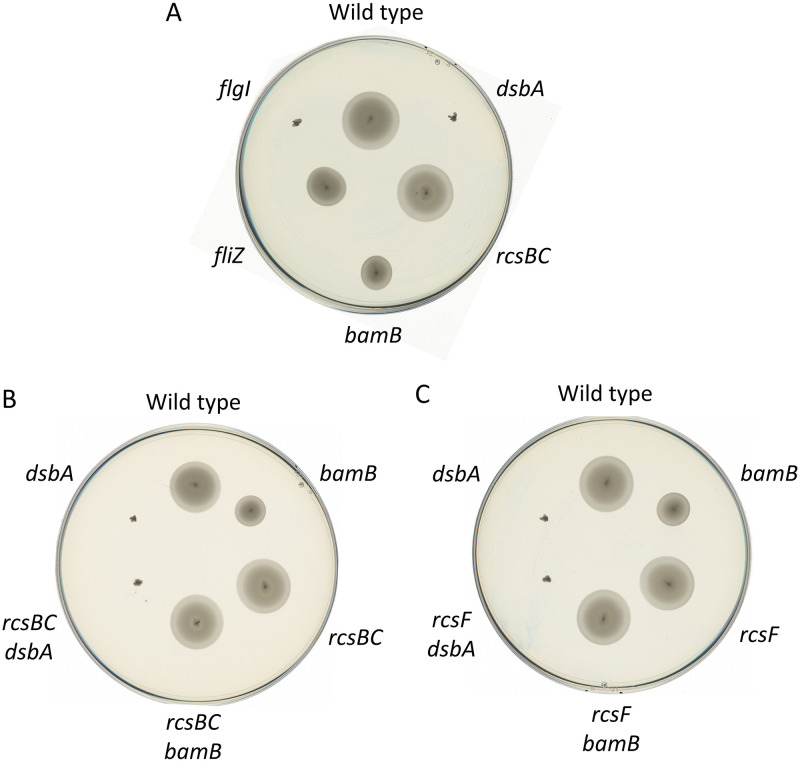
To test how BamB and DsbA affect flagellar motility, we inoculated various Salmonella strains on motility agar (Fig. 7). Loss of BamB conferred a modest motility defect. In contrast, loss of DsbA conferred a complete loss of motility, apparently equivalent to deletion of flgI, encoding a flagellar structural protein previously shown to require DsbA to fold appropriately (65). Further deletion of either rcsBC or rcsF completely suppressed the bamB motility defect but had no effect in the dsbA background (Fig. 7B and C). This suggests that the effect on motility caused by loss of BamB is due to activation of the Rcs system via RcsF, and thus the Bam complex, or at least BamB, is not required to build a functional flagellum. Loss of DsbA, on the other hand, activates Rcs, leading to repression of flagellar expression (Fig. 6), but also leads to an inability to assemble a functional apparatus.
FIG 7.
Rcs represses motility. Colonies of the indicated strains were stabbed into motility agar and incubated at 37C for 11 h. Strains used were as follows: wild type, JS1039, JS326, JS745, JS746, and JS747 (A); wild type, JS1039, JS745, JS2413, JS2414, and JS326 (B); and wild type, JS1039, JS2382, JS2415, JS326, and JS2416 (C).
β-Barrel assembly affects hilD, hilC, and rtsA transcription.
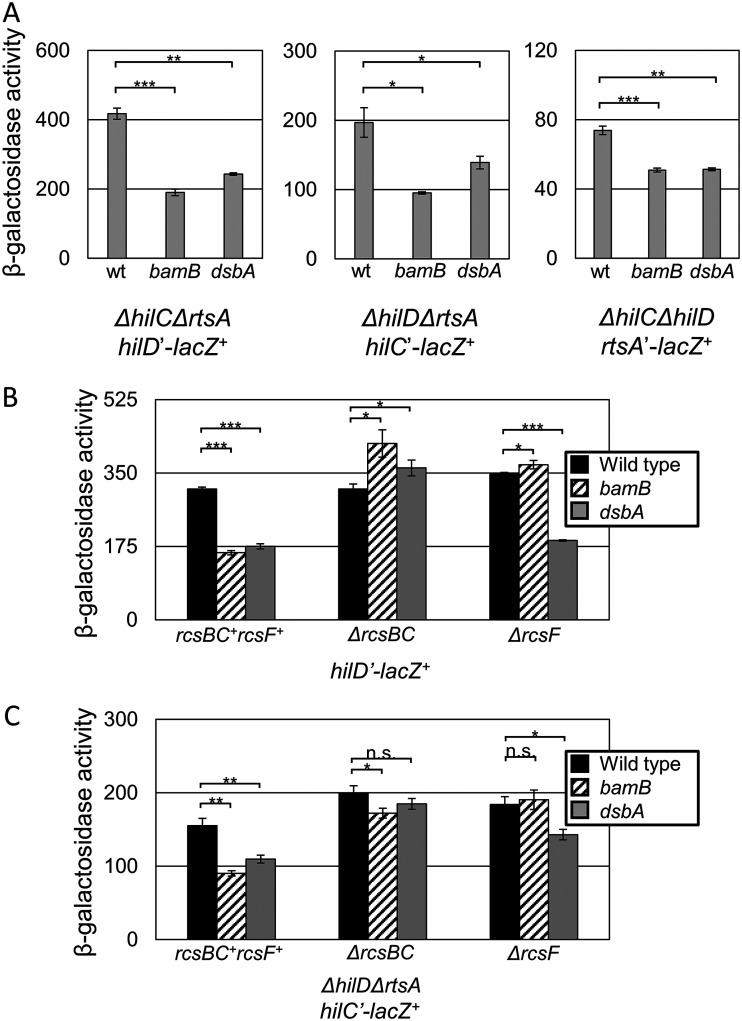
Because repression of SPI1 expression caused by loss of bamB is partially independent of FliZ (Fig. 3B) and because Lin et al. suggested that part of the dsbA effect is HilD dependent, we tested if the bamB effect also functions through hilD, hilC, or rtsA transcription. Because HilD, HilC, and RtsA activate expression of themselves and each other, we monitored expression of the promoters in strains deleted for all three regulators. Surprisingly, expression from all three promoters was significantly decreased in both the bamB and dsbA backgrounds (Fig. 8A). This is the first case of significant signal integration at the level of hilC transcription that we have observed. To test if this effect was dependent on Rcs, we monitored expression from a hilD’-lacZ+ fusion and examined both bamB and dsbA effects in the presence and absence of rcsBC or rcsF. Because this fusion is a deletion of hilD, we expect no expression from hilC or rtsA, so any effect on hilD transcription should be specific to the hilD promoter (33). Expression of hilD was repressed about 2-fold in an RcsBC-dependent manner (Fig. 8B). As above, loss of RcsF suppressed the decreased expression in the bamB background but had no effect in the dsbA mutant background. We observed a similar pattern on a hilC’-lacZ+ transcriptional fusion (Fig. 8C). Together, these data demonstrate that Rcs integrates into SPI1 regulation through hilD, hilC, and rtsA transcription, a combinatorial effect repressing SPI1 expression that could explain the remaining repressive phenotype observed in the absence of fliZ (Fig. 3).
FIG 8.
Rcs represses hilD, hilC, and rtsA transcription. β-Galactosidase activity was measured from cells containing the indicated lacZ transcriptional fusions in the designated backgrounds. Cells were grown overnight, subcultured 1:100, and then grown with mild aeration for 6 h at 37˚C in HSLB medium. β-Galactosidase activity units are defined as (micromoles of ONP formed per minute) × 106/(OD600 × milliliters of cell suspension) and are reported as mean ± standard deviation where n = 3. P values are indicated as follows from unpaired t tests: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Strains used were JS2201, JS2386-JS2391, JS2198, and JS2191 (A); JS488 and JS2397-2404 (B); and JS2198, JS2388-JS2389, and JS2417-JS2422 (C).
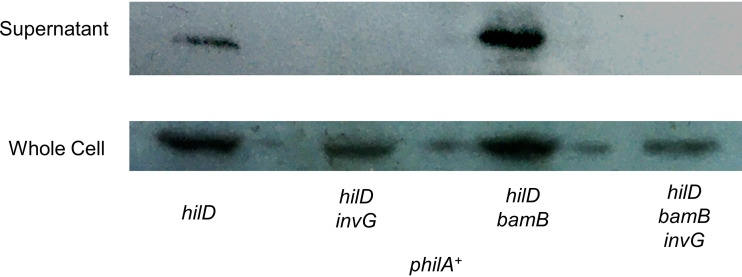
SPI1 can assemble in the absence of bamB.
Effects on β-barrel assembly could lead to outer membrane stress that affects proper assembly of the SPI1 T3SS. Alternatively, repression of SPI1 may just be regulatory and if bypassed, a functional T3SS could assemble and secrete effectors. To test this, we deleted hilD and expressed hilA from a plasmid to bypass regulatory repression in a bamB background. As expected, we observed SopB secretion in the hilD pHilA background (Fig. 9). To confirm the functionality of this assay, we deleted invG, a structural protein of the SPI1 T3SS that is required for a functional apparatus (66–68). As expected, SopB was produced but not secreted in the absence of InvG. Deletion of bamB had no effect on SopB production or secretion in the pHilA background. When we deleted invG in the bamB background, secretion was abolished, demonstrating that this is SPI1-dependent export. Together, these data suggest that defects in β-barrel assembly lead to repression of the SPI1 T3SS but do not destroy its functionality (4).
FIG 9.
The SPI1 T3SS can function in the absence of bamB. Cells were grown overnight, subcultured 1:100 into 10 ml HSLB-Amp and grown standing for 18 h at 37˚C. Cells were pelleted and supernatant was TCA precipitated. Western blotting was performed using anti-FLAG to identify SopB levels within the cell and the cell culture supernatant as indicated. The strain used was JS2425-JS2428 containing plasmid pHilA+.
DISCUSSION
Expression of the SPI1 T3SS is tightly controlled in response to various regulatory inputs. This allows Salmonella to activate or repress the invasion apparatus depending on environmental conditions that can indicate location within the host, as well as the physiological status within the bacterium (33–35, 60, 69–71). Here, we show that Salmonella specifically senses outer membrane β-barrel protein assembly through RcsF, the outer membrane lipoprotein sensor. RcsF stimulates the RcsCDB system, leading to repressed expression of flhDC, encoding the master flagellar regulator. This in turn represses SPI1 by decreasing expression of the flagellar regulator FliZ that indirectly activates HilD protein activity (35, 60). We also show that Rcs represses transcription of hilD, hilC, and rtsA by an unknown mechanism. Although the SPI1 T3SS can function if expressed in a bamB null strain, it is presumably deleterious to assemble the machine and invade while dealing with significant outer membrane stress, or, perhaps, when some particular protein or aspect of outer membrane integrity is deficient. Salmonella has clearly evolved complex mechanisms for appropriate activation of the invasion apparatus and senses critical bacterial functions via several different mechanisms.
Though RcsCDB has previously been shown to sense hindered disulfide bond status and repress SPI1 through flagellar regulatory pathways, bamB and dsbA deletions activate Rcs using clearly different mechanisms. We originally proposed that loss of dsbA activates through RcsF, which contains multiple disulfide bonds (62). It was subsequently shown that mutating the cysteine residues in RcsF leads to decreased stimulation of the Rcs response (72). Here, we show that deletion of dsbA stimulates Rcs through an unknown RcsF-independent mechanism. This is in stark contrast to a bamB mutant, in which activation of Rcs is strictly RcsF dependent. Consistent with independent mechanisms of activation, the addition of the reducing agent DTT partially suppresses the bamB phenotype. DTT presumably disrupts the key disulfide bonds in RcsF, reducing its signal transduction role and alleviating stimulation of Rcs in the absence of bamB. In contrast, the addition of DTT exacerbates the dsbA phenotype to stimulate Rcs, suggesting that disulfide bond status in some other protein(s) may be affected to activate signaling. One enticing hypothesis is that the repressor of Rcs, IgaA, which contains four conserved periplasmic cysteines (73), becomes dysfunctional in the dsbA background. This is difficult to test because igaA is an essential gene, but disruption of its disulfide bonds inhibits its ability to repress Rcs (73, 74). Further studies will be required to demonstrate this mechanism conclusively.
Another key difference between dsbA and bamB mutants is motility. Both stimulate Rcs, which represses flagellar regulatory pathways, leading to clear motility defects in both backgrounds. In the case of defective β-barrel protein assembly, motility is restored in the absence of RcsBC, meaning that the motility defects are solely regulatory. In contrast, in the absence of dsbA, we see Rcs-independent motility defects. This is likely due to key disulfide bonds in FlgI that are required for proper assembly of the flagellar apparatus (75). Without proper assembly, flagellar regulatory systems are repressed by the anti-sigma factor FlgM, which remains intracellular and bound to the sigma factor FliA, leading to further repression of SPI1 via decreased expression of fliZ from the FliA-dependent promoter (35, 76, 77).
In addition, we expand upon how Rcs controls SPI1 regulation. Most signals that integrate into SPI1 regulation have previously been shown to function at the level of hilD translation or through HilD protein, as in the case of FliZ (60). However, Rcs activation also represses hilD, hilC, and rtsA transcription. This is a rare example of a regulatory input acting through hilC. Decreased expression of these critical activators certainly contributes to downregulation of SPI1. The mechanism of this regulation is unknown, although we do not believe that it is direct. Rcs could, for example, be functioning through H-NS, which contributes to silencing of hilD, hilC, rtsA, and hilA (33, 78–80).
The role of the Rcs system in pathogenesis is complex. The negative regulatory protein IgaA was originally identified by partial loss of function mutations that allowed Salmonella to proliferate in fibroblasts, normally nonpermissive for replication (81). However, in mice, activation of the system by point mutations in igaA (82) or rcsC (30) attenuates Salmonella virulence. Knocking out capsule production partially, but not completely, suppressed this loss in virulence (30). These studies included intraperitoneal infections, so the virulence defect is not simply due to decreased expression of flagella or the SPI1 T3SS, which are neither expressed nor required systemically (2, 83). Deleting downstream Rcs components also suppressed the virulence defect caused by the respective activating mutations (30, 82). These results would suggest that the Rcs system is normally “off” during Salmonella infection. However, Detweiler et al. (84) showed that rscC loss of function mutants were attenuated after several weeks of infection in NRAMP+/+ mice. Thus, while inappropriate activation of RcsCDB is clearly detrimental, proper regulation is important during infection.
MATERIALS AND METHODS
Media, reagents, and enzymatic assays.
Strains were routinely grown at 37°C in Luria-Bertani (LB) medium containing 1% salt, termed HSLB. SOC medium was used to recover all transformants (85). Strains containing temperature-sensitive plasmids pCP20 and pKD46 were grown at 30°C. Motility agar contained 0.3% Bacto agar, 1% tryptone, and 0.5% NaCl. When necessary, antibiotics were used as follows: ampicillin 50 μg/ml, chloramphenicol 20 μg/ml, and kanamycin 50 μg/ml. Primers were purchased from IDT; plasmids were constructed using NEBuilder HiFi DNA Assembly and Invitrogen restriction enzymes. Strains for β-galactosidase assays were grown overnight in LB containing 0% salt (NSLB) at 37°C, and subcultured 1:100 in HSLB with or without 2 mM dithiothreitol (DTT) and then grown for 6 h, at which point the β-galactosidase was performed as previously described (86). β-Galactosidase activity units are defined as (micromoles of o-nitrophenol formed per minute) × 106/(optical density at 600 nm [OD600] × millilters of cell suspension). The data are expressed as means ± standard deviations with three biological replicates (n = 3).
Strain and plasmid construction.
All strains and plasmids used in this study are described in Table S1 in the supplemental material. All strains are derivatives of Salmonella enterica serovar Typhimurium ATCC 14028 (American Type Culture Collection). Deletions and simultaneous insertion of antibiotic resistance cassettes were constructed using λ-red-mediated recombination as previously described (87, 88), with the indicated endpoints. All deletions were checked by PCR analysis and transduced into clean wild-type backgrounds using P22 HT105/1 int-201 transduction (85). When necessary, antibiotic cassettes were removed using the FLP recombinase, encoded on the temperature-sensitive pCP20 plasmid (89).
To build the rprA-lacZ fusion, the promoter of rprA was cloned into pDX1 (35) using primers rprA promoter forward (5′-TCT AGA GGA TCC CCG GGT ACT TAT CAA TTC AAC GCA CAC-3′) and rprA promoter reverse (5′-TTG TCG GAT CCC CGG GAA TTT GTG CTA ATA GTA GGC ATG-3′). PDX1 was digested with EcoRI and KpnI. The fusion was then integrated into the lambda attachment site using λInt produced from the CRIM helper plasmid pINT-ts (90). Integration of a single copy was confirmed by PCR.
The SopB-3×FLAG fusion was constructed using primers pSUB11 sopB fw (5′-TTG GCA GTC AGT AAA AGG CAT TTC TTC ATT AAT CAC ATC TGA CTA CAA AGA CCA TGA CGG-3′) and pSUB11 sopB rev (5′-TAA ACG ATT TAA TAG ACT TTC CAT ATA GTT ACC TCA AGA CCA TAT GAA TAT CCT CCT TAG-3′) as previously described (91).
MudCm insertions.
An estimated 40,000 MudCm insertions were created in a hilA’-lacZ+ bamB mutant background and plated on LB agar containing 50 μg/ml X-Gal and 20 μg/ml chloramphenicol (55). Dark blue colonies were isolated and β-galactosidase assays were performed as described above. MudCm insertions were located by semirandom PCR, first amplifying using primers INSEQB (5′-GGC CAC GCG TCG ACT AGT CAN NNN NNN NNN ACG CC-3′) and MudL1 (5′-CCC ATC AGA TCC CGA ATA ATC C-3′) (56). A 2.5-μl aliquot of that product was used as a template for primers INSEQD (5′-GGC CAC GCG TCG ACT AGT AC-3′) and MudL2 (5′-GCA AGC CCC ACC AAA TCT AAT CC-3′). The final PCR product was sequenced using primer MudL3 (5′-CGA CTA AAA TTT GCA CTA CAG GC-3′).
Western blotting of secreted proteins.
SopB was detected by Western blotting as previously described (35). Strains were grown for 10 h with mild aeration at 37°C, then subcultured 1:100 in 10 ml of HSLB containing ampicillin at a concentration of 50 μg/ml, and grown standing for 18 h at 37°C. The culture supernatant was then filter sterilized using a 0.22-μm filter and concentrated to 1 ml using an Amicon-15 filter with a nominal molecular weight limit of 30,000. Proteins were precipitated with a final concentration of 10% cold trichloroacetic and by incubating them on ice for 30 min. Precipitated proteins were collected by centrifugation at 20,000 × g for 30 min at 4°C. The supernatant was then removed, and the trichloroacetic acid precipitate was washed with 1 ml of ice-cold 95% isopropanol. The samples were then centrifuged for 20 min at 20,000 × g and the supernatant was removed. The pellet was washed a final time in 95% isopropanol and allowed to air dry. The pellet was then suspended in 15 μl of 50 mM Tris (pH 8), and 15 μl of 2 × SDS loading buffer was added. The primary antibody used was monoclonal anti-FLAG BioM2 antibody produced in mouse (Sigma-Aldrich, St. Louis, Missouri), and the secondary was Pierce high-sensitivity streptavidin-horseradish peroxidase (Thermo Fisher Scientific, Waltham, MA).
ACKNOWLEDGMENTS
We thank Tom Silhavy and members of both the Slauch lab and the Vanderpool lab for helpful discussions, Dongxia Lin and Kyungsub Kim for constructing strains, and Paola Parraga for construction of the pHilA plasmid.
This work was supported by the National Institutes of Health grant R01 GM120182.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellermeier CD, Ellermeier JR, Slauch JM. 2005. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol 57:691–705. doi: 10.1111/j.1365-2958.2005.04737.x. [DOI] [PubMed] [Google Scholar]
- 3.Golubeva YA, Sadik AY, Ellermeier JR, Slauch JM. 2012. Integrating global regulatory input into the Salmonella pathogenicity island 1 type III secretion system. Genetics 190:79–U497. doi: 10.1534/genetics.111.132779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fardini Y, Chettab K, Grepinet O, Rochereau S, Trotereau J, Harvey P, Amy M, Bottreau E, Bumstead N, Barrow PA, Virlogeux-Payant I. 2007. The YfgL lipoprotein is essential for type III secretion system expression and virulence of Salmonella enterica serovar Enteritidis. Infect Immun 75:358–370. doi: 10.1128/IAI.00716-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. 2003. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 6.Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz N, Falcone B, Kahne D, Silhavy TJ. 2005. Chemical conditionality: a genetic strategy to probe organelle assembly. Cell 121:307–317. doi: 10.1016/j.cell.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Sklar JG, Wu T, Gronenberg LS, Malinverni JC, Kahne D, Silhavy TJ. 2007. Lipoprotein SmpA is a component of the YaeT complex that assembles outer membrane proteins in Escherichia coli. Proc Natl Acad Sci U S A 104:6400–6405. doi: 10.1073/pnas.0701579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ES, Werner JN, Misra R. 2006. Differential effects of yfgL mutation on Escherichia coli outer membrane proteins and lipopolysaccharide. J Bacteriol 188:7186–7194. doi: 10.1128/JB.00571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuong P, Bennion D, Mantei J, Frost D, Misra R. 2008. Analysis of YfgL and YaeT interactions through bioinformatics, mutagenesis, and biochemistry. J Bacteriol 190:1507–1517. doi: 10.1128/JB.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun M, Silhavy TJ. 2002. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol Microbiol 45:1289–1302. doi: 10.1046/j.1365-2958.2002.03091.x. [DOI] [PubMed] [Google Scholar]
- 12.Ricci DP, Silhavy TJ. 2012. The Bam machine: a molecular cooper. Biochim Biophys Acta 1818:1067–1084. doi: 10.1016/j.bbamem.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sklar JG, Wu T, Kahne D, Silhavy TJ. 2007. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev 21:2473–2484. doi: 10.1101/gad.1581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatzeva-Topalova PZ, Walton TA, Sousa MC. 2008. Crystal structure of YaeT: conformational flexibility and substrate recognition. Structure 16:1873–1881. doi: 10.1016/j.str.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowles TJ, Jeeves M, Bobat S, Dancea F, McClelland D, Palmer T, Overduin M, Henderson IR. 2008. Fold and function of polypeptide transport-associated domains responsible for delivering unfolded proteins to membranes. Mol Microbiol 68:1216–1227. doi: 10.1111/j.1365-2958.2008.06225.x. [DOI] [PubMed] [Google Scholar]
- 16.Anwari K, Poggio S, Perry A, Gatsos X, Ramarathinam SH, Williamson NA, Noinaj N, Buchanan S, Gabriel K, Purcell AW, Jacobs-Wagner C, Lithgow T. 2010. A modular BAM complex in the outer membrane of the alpha-proteobacterium Caulobacter crescentus. PLoS One 5:e8619. doi: 10.1371/journal.pone.0008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Malinverni JC, Sliz P, Silhavy TJ, Harrison SC, Kahne D. 2007. Structure and function of an essential component of the outer membrane protein assembly machine. Science 317:961–964. doi: 10.1126/science.1143993. [DOI] [PubMed] [Google Scholar]
- 18.Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. 2003. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell 113:61–71. doi: 10.1016/s0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 19.Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. 2006. Conserved and variable functions of the sigma E stress response in related genomes. PLoS Biol 4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat Rev Microbiol 9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bossi L, Figueroa-Bossi N. 2007. A small RNA downregulates LamB maltoporin in Salmonella. Mol Microbiol 65:799–810. doi: 10.1111/j.1365-2958.2007.05829.x. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen AA, Eriksen M, Gilany K, Udesen C, Franch T, Petersen C, Valentin-Hansen P. 2005. Regulation of ompA mRNA stability: the role of a small regulatory RNA in growth phase-dependent control. Mol Microbiol 58:1421–1429. doi: 10.1111/j.1365-2958.2005.04911.x. [DOI] [PubMed] [Google Scholar]
- 23.Coornaert A, Lu A, Mandin P, Springer M, Gottesman S, Guillier M. 2010. MicA sRNA links the PhoP regulon to cell envelope stress. Mol Microbiol 76:467–479. doi: 10.1111/j.1365-2958.2010.07115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansen J, Rasmussen AA, Overgaard M, Valentin-Hansen P. 2006. Conserved small non-coding RNAs that belong to the sigma E regulon: role in down-regulation of outer membrane proteins. J Mol Biol 364:1–8. doi: 10.1016/j.jmb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Zhang A, Blyn LB, Storz G. 2004. MicC, a second small-RNA regulator of Omp protein expression in Escherichia coli. J Bacteriol 186:6689–6697. doi: 10.1128/JB.186.20.6689-6697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeiffer V, Papenfort K, Lucchini S, Hinton JC, Vogel J. 2009. Coding sequence targeting by MicC RNA reveals bacterial mRNA silencing downstream of translational initiation. Nat Struct Mol Biol 16:840–846. doi: 10.1038/nsmb.1631. [DOI] [PubMed] [Google Scholar]
- 27.Guo MS, Updegrove TB, Gogol EB, Shabalina SA, Gross CA, Storz G. 2014. MicL, a new sigmaE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev 28:1620–1634. doi: 10.1101/gad.243485.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konovalova A, Mitchell AM, Silhavy TJ. 2016. A lipoprotein/beta-barrel complex monitors lipopolysaccharide integrity transducing information across the outer membrane. Elife 5:e15276. doi: 10.7554/eLife.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalebroux ZD, Miller SI. 2014. Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity. Curr Opin Microbiol 17:106–113. doi: 10.1016/j.mib.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouslim C, Delgado M, Groisman EA. 2004. Activation of the RcsC/YojN/RcsB phosphorelay system attenuates Salmonella virulence. Mol Microbiol 54:386–395. doi: 10.1111/j.1365-2958.2004.04293.x. [DOI] [PubMed] [Google Scholar]
- 31.Slauch JM, Garrett S, Jackson DE, Silhavy TJ. 1988. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol 170:439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerken H, Misra R. 2010. MzrA-EnvZ interactions in the periplasm influence the EnvZ/OmpR two-component regulon. J Bacteriol 192:6271–6278. doi: 10.1128/JB.00855-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer AD, Kim K, Slauch JM. 2019. PhoP-mediated repression of the SPI1 type 3 secretion system in Salmonella enterica serovar Typhimurium. J Bacteriol 201:e00264-19. doi: 10.1128/JB.00264-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K, Golubeva YA, Vanderpool CK, Slauch JM. 2019. Oxygen-dependent regulation of SPI1 type three secretion system by small RNAs in Salmonella enterica serovar Typhimurium. Mol Microbiol 111:570–587. doi: 10.1111/mmi.14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin D, Rao CV, Slauch JM. 2008. The Salmonella SPI1 type three secretion system responds to periplasmic disulfide bond status via the flagellar apparatus and the RcsCDB system. J Bacteriol 190:87–97. doi: 10.1128/JB.01323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas RL, Lee CA. 2001. Roles of HilC and HilD in regulation of hilA expression in Salmonella enterica serovar Typhimurium. J Bacteriol 183:2733–2745. doi: 10.1128/JB.183.9.2733-2745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laubacher ME, Ades SE. 2008. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J Bacteriol 190:2065–2074. doi: 10.1128/JB.01740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sledjeski DD, Gottesman S. 1996. Osmotic shock induction of capsule synthesis in Escherichia coli K-12. J Bacteriol 178:1204–1206. doi: 10.1128/jb.178.4.1204-1206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrieres L, Clarke DJ. 2003. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K-12 and controls the expression of a regulon in response to growth on a solid surface. Mol Microbiol 50:1665–1682. doi: 10.1046/j.1365-2958.2003.03815.x. [DOI] [PubMed] [Google Scholar]
- 40.Sailer FC, Meberg BM, Young KD. 2003. beta-Lactam induction of colanic acid gene expression in Escherichia coli. FEMS Microbiol Lett 226:245–249. doi: 10.1016/S0378-1097(03)00616-5. [DOI] [PubMed] [Google Scholar]
- 41.Miajlovic H, Cooke NM, Moran GP, Rogers TR, Smith SG. 2014. Response of extraintestinal pathogenic Escherichia coli to human serum reveals a protective role for Rcs-regulated exopolysaccharide colanic acid. Infect Immun 82:298–305. doi: 10.1128/IAI.00800-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callewaert L, Vanoirbeek KG, Lurquin I, Michiels CW, Aertsen A. 2009. The Rcs two-component system regulates expression of lysozyme inhibitors and is induced by exposure to lysozyme. J Bacteriol 191:1979–1981. doi: 10.1128/JB.01549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farris C, Sanowar S, Bader MW, Pfuetzner R, Miller SI. 2010. Antimicrobial peptides activate the Rcs regulon through the outer membrane lipoprotein RcsF. J Bacteriol 192:4894–4903. doi: 10.1128/JB.00505-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girgis HS, Liu Y, Ryu WS, Tavazoie S. 2007. A comprehensive genetic characterization of bacterial motility. PLoS Genet 3:1644–1660. doi: 10.1371/journal.pgen.0030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiba Y, Miyagawa H, Nagahama H, Matsumoto K, Kondo D, Matsuoka S, Matsumoto K, Hara H. 2012. Exploring the relationship between lipoprotein mislocalization and activation of the Rcs signal transduction system in Escherichia coli. Microbiology 158:1238–1248. doi: 10.1099/mic.0.056945-0. [DOI] [PubMed] [Google Scholar]
- 46.Wall E, Majdalani N, Gottesman S. 2018. The complex Rcs regulatory cascade. Annu Rev Microbiol 72:111–139. doi: 10.1146/annurev-micro-090817-062640. [DOI] [PubMed] [Google Scholar]
- 47.Navarro Llorens JM, Tormo A, Martínez-García E. 2010. Stationary phase in Gram-negative bacteria. FEMS Microbiol Rev 34:476–495. doi: 10.1111/j.1574-6976.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- 48.Papenfort K, Espinosa E, Casadesus J, Vogel J. 2015. Small RNA-based feedforward loop with AND-gate logic regulates extrachromosomal DNA transfer in Salmonella. Proc Natl Acad Sci U S A 112:E4772–81. doi: 10.1073/pnas.1507825112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wehland M, Bernhard F. 2000. The RcsAB box. Characterization of a new operator essential for the regulation of exopolysaccharide biosynthesis in enteric bacteria. J Biol Chem 275:7013–7020. doi: 10.1074/jbc.275.10.7013. [DOI] [PubMed] [Google Scholar]
- 50.Ferrieres L, Aslam SN, Cooper RM, Clarke DJ. 2007. The yjbEFGH locus in Escherichia coli K-12 is an operon encoding proteins involved in exopolysaccharide production. Microbiology 153:1070–1080. doi: 10.1099/mic.0.2006/002907-0. [DOI] [PubMed] [Google Scholar]
- 51.Francez-Charlot A, Laugel B, Van Gemert A, Dubarry N, Wiorowski F, Castanié-Cornet M-P, Gutierrez C, Cam K. 2003. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol Microbiol 49:823–832. doi: 10.1046/j.1365-2958.2003.03601.x. [DOI] [PubMed] [Google Scholar]
- 52.Amy M, Velge P, Senocq D, Bottreau E, Mompart F, Virlogeux-Payant I. 2004. Identification of a new Salmonella enterica serovar Enteritidis locus involved in cell invasion and in the colonisation of chicks. Res Microbiol 155:543–552. doi: 10.1016/j.resmic.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Mahoney TF, Ricci DP, Silhavy TJ. 2016. Classifying beta-barrel assembly substrates by manipulating essential Bam complex members. J Bacteriol 198:1984–1992. doi: 10.1128/JB.00263-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mecsas J, Welch R, Erickson JW, Gross CA. 1995. Identification and characterization of an outer membrane protein, OmpX, in Escherichia coli that is homologous to a family of outer membrane proteins including Ail of Yersinia enterocolitica. J Bacteriol 177:799–804. doi: 10.1128/jb.177.3.799-804.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elliott T. 1993. Transport of 5-aminolevulinic acid by the dipeptide permease in Salmonella typhimurium. J Bacteriol 175:325–331. doi: 10.1128/jb.175.2.325-331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chun KT, Edenberg HJ, Kelley MR, Goebl MG. 1997. Rapid amplification of uncharacterized transposon-tagged DNA sequences from genomic DNA. Yeast 13:233–240. doi:. [DOI] [PubMed] [Google Scholar]
- 57.Kristoficova I, Vilhena C, Behr S, Jung K. 2017. BtsT, a novel and specific pyruvate/H(+) symporter in Escherichia coli. J Bacteriol 200:e00599-17. doi: 10.1128/JB.00599-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeda S, Fujisawa Y, Matsubara M, Aiba H, Mizuno T. 2001. A novel feature of the multistep phosphorelay in Escherichia coli: a revised model of the RcsC → YojN → RcsB signalling pathway implicated in capsular synthesis and swarming behaviour. Mol Microbiol 40:440–450. doi: 10.1046/j.1365-2958.2001.02393.x. [DOI] [PubMed] [Google Scholar]
- 59.Cameron AD, Dorman CJ. 2012. A fundamental regulatory mechanism operating through OmpR and DNA topology controls expression of Salmonella pathogenicity islands SPI-1 and SPI-2. PLoS Genet 8:e1002615. doi: 10.1371/journal.pgen.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chubiz JE, Golubeva YA, Lin D, Miller LD, Slauch JM. 2010. FliZ regulates expression of the Salmonella pathogenicity island 1 invasion locus by controlling HilD protein activity in Salmonella enterica serovar typhimurium. J Bacteriol 192:6261–6270. doi: 10.1128/JB.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chilcott GS, Hughes KT. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol Mol Biol Rev 64:694–708. doi: 10.1128/mmbr.64.4.694-708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadokura H, Tian H, Zander T, Bardwell JC, Beckwith J. 2004. Snapshots of DsbA in action: detection of proteins in the process of oxidative folding. Science 303:534–537. doi: 10.1126/science.1091724. [DOI] [PubMed] [Google Scholar]
- 63.Konovalova A, Perlman DH, Cowles CE, Silhavy TJ. 2014. Transmembrane domain of surface-exposed outer membrane lipoprotein RcsF is threaded through the lumen of beta-barrel proteins. Proc Natl Acad Sci U S A 111:E4350–8. doi: 10.1073/pnas.1417138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho SH, Szewczyk J, Pesavento C, Zietek M, Banzhaf M, Roszczenko P, Asmar A, Laloux G, Hov AK, Leverrier P, Van der Henst C, Vertommen D, Typas A, Collet JF. 2014. Detecting envelope stress by monitoring beta-barrel assembly. Cell 159:1652–1664. doi: 10.1016/j.cell.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 65.Dailey FE, Berg HC. 1993. Mutants in disulfide bond formation that disrupt flagellar assembly in Escherichia coli. Proc Natl Acad Sci U S A 90:1043–1047. doi: 10.1073/pnas.90.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collazo CM, Zierler MK, Galan JE. 1995. Functional analysis of the Salmonella typhimurium invasion genes invl and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol 15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 67.Kaniga K, Trollinger D, Galan JE. 1995. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol 177:7078–7085. doi: 10.1128/jb.177.24.7078-7085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaniga K, Tucker S, Trollinger D, Galan JE. 1995. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol 177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iyoda S, Kamidoi T, Hirose K, Kutsukake K, Watanabe H. 2001. A flagellar gene fliZ regulates the expression of invasion genes and virulence phenotype in Salmonella enterica serovar Typhimurium. Microb Pathog 30:81–90. doi: 10.1006/mpat.2000.0409. [DOI] [PubMed] [Google Scholar]
- 70.Ellermeier JR, Slauch JM. 2008. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol 190:476–486. doi: 10.1128/JB.00926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim K, Palmer AD, Vanderpool CK, Slauch JM. 2019. The small RNA PinT contributes to PhoP-mediated regulation of the Salmonella pathogenicity island 1 type III secretion system in Salmonella enterica serovar Typhimurium. J Bacteriol 201:e00312-19. doi: 10.1128/JB.00312-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rogov VV, Rogova NY, Bernhard F, Lohr F, Dotsch V. 2011. A disulfide bridge network within the soluble periplasmic domain determines structure and function of the outer membrane protein RcsF. J Biol Chem 286:18775–18783. doi: 10.1074/jbc.M111.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pucciarelli MG, Rodriguez L, Garcia-Del Portillo F. 2017. A disulfide bond in the membrane protein IgaA is essential for repression of the RcsCDB system. Front Microbiol 8:2605. doi: 10.3389/fmicb.2017.02605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cano DA, Domínguez-Bernal G, Tierrez A, Garcia-Del Portillo F, Casadesús J. 2002. Regulation of capsule synthesis and cell motility in Salmonella enterica by the essential gene igaA. Genetics 162:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hizukuri Y, Yakushi T, Kawagishi I, Homma M. 2006. Role of the intramolecular disulfide bond in FlgI, the flagellar P-ring component of Escherichia coli. J Bacteriol 188:4190–4197. doi: 10.1128/JB.01896-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karlinsey JE, Tanaka S, Bettenworth V, Yamaguchi S, Boos W, Aizawa SI, Hughes KT. 2000. Completion of the hook-basal body complex of the Salmonella typhimurium flagellum is coupled to FlgM secretion and fliC transcription. Mol Microbiol 37:1220–1231. doi: 10.1046/j.1365-2958.2000.02081.x. [DOI] [PubMed] [Google Scholar]
- 77.Kutsukake K, Iino T. 1994. Role of the FliA-FlgM regulatory system on the transcriptional control of the flagellar regulon and flagellar formation in Salmonella typhimurium. J Bacteriol 176:3598–3605. doi: 10.1128/jb.176.12.3598-3605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olekhnovich IN, Kadner RJ. 2007. Role of nucleoid-associated proteins Hha and H-NS in expression of Salmonella enterica activators HilD, HilC, and RtsA required for cell invasion. J Bacteriol 189:6882–6890. doi: 10.1128/JB.00905-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schechter LM, Jain S, Akbar S, Lee CA. 2003. The small nucleoid-binding proteins H-NS, HU, and Fis affect hilA expression in Salmonella enterica serovar Typhimurium. Infect Immun 71:5432–5435. doi: 10.1128/iai.71.9.5432-5435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olekhnovich IN, Kadner RJ. 2006. Crucial roles of both flanking sequences in silencing of the hilA promoter in Salmonella enterica. J Mol Biol 357:373–386. doi: 10.1016/j.jmb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Cano DA, Martínez-Moya M, Pucciarelli MG, Groisman EA, Casadesús J, García-Del Portillo F. 2001. Salmonella enterica serovar Typhimurium response involved in attenuation of pathogen intracellular proliferation. Infect Immun 69:6463–6474. doi: 10.1128/IAI.69.10.6463-6474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Domínguez-Bernal G, Pucciarelli MG, Ramos-Morales F, García-Quintanilla M, Cano DA, Casadesús J, García-del Portillo F. 2004. Repression of the RcsC-YojN-RcsB phosphorelay by the IgaA protein is a requisite for Salmonella virulence. Mol Microbiol 53:1437–1449. doi: 10.1111/j.1365-2958.2004.04213.x. [DOI] [PubMed] [Google Scholar]
- 83.Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. 2010. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci U S A 107:17733–17738. doi: 10.1073/pnas.1006098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Detweiler CS, Monack DM, Brodsky IE, Mathew H, Falkow S. 2003. VirK, SomA andRcsC are important for systemic Salmonella enterica serovar Typhimurium infection and cationic peptide resistance. Mol Microbiol 48:385–400. doi: 10.1046/j.1365-2958.2003.03455.x. [DOI] [PubMed] [Google Scholar]
- 85.Maloy SR, Stewart VJ, Taylor RK. 1996. Genetic analysis of pathogenic bacteria: a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- 86.Slauch JM, Silhavy TJ. 1991. Genetic fusions as experimental tools. Methods Enzymol 204:213–248. doi: 10.1016/0076-6879(91)04011-c. [DOI] [PubMed] [Google Scholar]
- 87.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ellermeier CD, Janakiraman A, Slauch JM. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290:153–161. doi: 10.1016/S0378-1119(02)00551-6. [DOI] [PubMed] [Google Scholar]
- 89.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 90.Haldimann A, Wanner BL. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol 183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uzzau S, Figueroa-Bossi N, Rubino S, Bossi L. 2001. Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci U S A 98:15264–15269. doi: 10.1073/pnas.261348198. [DOI] [PMC free article] [PubMed] [Google Scholar]