Abstract
The family of p21-activated kinases (PAKs) are oncogenic proteins that regulate critical cellular functions. PAKs play central signaling roles in the integrin/CDC42/Rho, ERK/MAPK, PI3K/AKT, NF-κB, and Wnt/β-catenin pathways, functioning both as kinases and scaffolds to regulate cell motility, mitosis and proliferation, cytoskeletal rearrangement, and other cellular activities. PAKs have been implicated in both the development and progression of a wide range of cancers, including breast cancer, pancreatic melanoma, thyroid cancer, and others. Here we will discuss the current knowledge on the structure and biological functions of both group I and group II PAKs, as well as the roles that PAKs play in oncogenesis and progression, with a focus on thyroid cancer and emerging data regarding BRAF/PAK signaling.
Keywords: cancer, thyroid cancer, PAK, p21-activated kinase, BRAF
Thyroid cancer is the most common malignancy of classical endocrine tissues and its incidence has risen since the 1970s, with a more dramatic rise over the past 3 decades. It is estimated that there will be 52 890 new cases and 2180 deaths in 2020 in the United States (1). The more rapid rise in incidence can be attributed mostly to an increase in use of neck ultrasound and other radiographic methods in clinical practice. Notably, while the overall incidence of thyroid cancer has stabilized in the past several years with changes in clinical guidelines, the rate at which patients succumb to thyroid cancer has continued to increase (2-4). These data suggest that current therapies for more aggressive forms of thyroid cancer have not improved survival rates for the disease. Indeed, beyond surgery, thyrotropin (TSH) suppression and iodine-131 therapy (treatments that have been used for decades), the current Food and Drug Administration-approved and experimental treatments improve progression-free survival but have not caused durable complete remissions from the disease. This emphasizes the need for improved therapeutic targeting, defining mechanisms of resistance to these compounds, and for better stratification of thyroid cancer to select patients for potential earlier targeted intervention (5, 6).
Approximately 95% of thyroid cancers derive from follicular thyroid cells and, of those, ~90% are classified as well differentiated, which are further subclassified as follicular thyroid cancer, papillary thyroid cancer (PTC), and Hürthle cell thyroid cancer (HCC). PTC is the most common histological subtype of thyroid cancer and overall has an excellent prognosis due to its typical early stage at diagnosis and slow growth pattern. However, due to its high frequency, PTC also accounts for the largest number of patients with more aggressive thyroid cancer. PTC development is dependent primarily upon activation of the mitogen activated protein kinase (MAPK) pathway via activating mutation in BRAF (most commonly resulting in BRAFV600E), RAS mutations (mostly NRAS), and gene rearrangements such as RET/PTC that are found mostly in pediatric and/or radiation-induced thyroid cancers (7). By contrast, follicular thyroid cancers are most often characterized by activation of PI3K signaling (8), and Hürthle cell thyroid cancers are characterized by a unique pattern of chromosomal loss and activation of mTOR signaling (9). In all cases, as the cancers become more aggressive and more poorly differentiated, they typically gain mutations in the hTERT promoter, TP53, and display genomic instability and reduced expression of thyroid differentiation proteins such as the sodium–iodide symporter and the TSH receptor; in rare cases these can evolve into anaplastic cancer. Effective treatment options for these tumors become progressively more limited as they dedifferentiate.
p21-Activated kinases (PAKs) are a family of serine/threonine kinases that were first identified in mouse brain tissue in 1994 (PAK1) (10). Functional roles for these proteins have been reported in the regulation of the cytoskeleton and cell motility, proliferation, apoptosis, and gene transcription, as well as oncogenic transformation (11). It has been demonstrated that PAKs are overexpressed and/or overactivated in thyroid, pancreatic, colorectal, breast, and ovarian cancers (12). There are currently 6 known isoforms of PAK that are divided into 2 groups based on their structural homology and their ability to be activated by Rho family GTPases (13, 14). Group I consists of PAKs 1-3 and group II consists of PAKs 4-6. Current findings that have been made in the biological functions of the 2 groups of PAK will be summarized here, with particular focus on the molecular pathways involved in transformation and progression in thyroid cancer.
Group I PAK Structure and Function
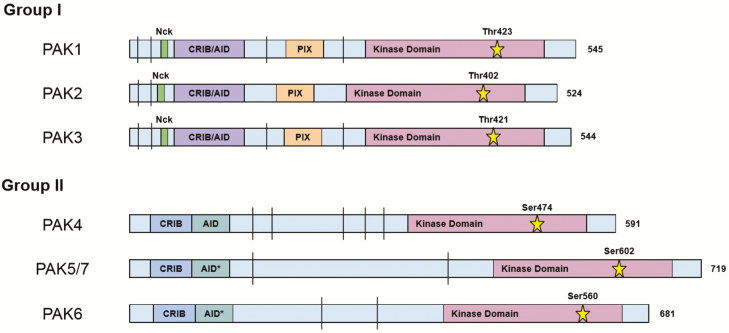
Group I, and to a lesser extent group II, PAKs are homologous to the STE20 family of proteins found in yeast, where they have overlapping roles in extracellular signal transduction and cytoskeletal rearrangement, indicating an ancient origin and conserved function (15). All PAKs, regardless of group assignment, possess a N-terminal p21-binding domain (PBD), alternatively known as a CDC42 and RAC1 binding (CRIB) domain, a C-terminal kinase domain, and an isoform-dependent number of conserved proline-rich regions (Fig. 1). PAKs 1, 2, and 3 also possess an Auto-Inhibitory Domain (AID) that overlaps with the PBD. Group I PAKs share greater than 88% sequence similarity of the PBD/AID region and over 93% in the kinase domain (13). Another specific feature of group I PAKs is the presence of 3 separate SH3 (Src homology 3) binding domains, 2 of which bind to well-known adaptor proteins Nck (16) and Grb (17) and the other binds to β-PIX (PAK interacting exchange factor, which is also referred to as Rho guanine nucleotide exchange factor 7 [ARHGEF7]) (15). These adaptor proteins recruit signal transduction intermediaries to receptor tyrosine kinases, thereby coupling extracellular messages to signal transduction cascades within the cell. Thus, it is very likely that these are the primary recruiters of group I PAKs to the plasma membrane for activation (18, 19). Supporting this concept, disruption of the Nck and PAK1 interaction inactivates the kinase (20), while constitutive recruitment of PAK1 to the plasma membrane by myristoylation of the SH3 domain results in activation of PAK1 (21). PAK1 also contains 3 nuclear localization sequences in the N-terminal domain, allowing these PAKs to enter the nucleus and interact with target chromatin (22) and histone H3 (22, 23).
Figure 1.
PAK isoform structure. Linear representation of groups I and II PAK proteins, with the numbers to right indicating the total number of amino acid residues. Overlapping CRIB (CDC42/RAC interactive binding) domain, also known as the PBD, and AID (Auto-Inhibitory Domain) for group I PAKs is shown in purple. Separate CRIB domain and AID for group II PAKs are shown in blue and aquamarine, respectively. Nck and PIX binding regions are shown in green and orange, respectively. The kinase domain is shown in plum and the catalytic residue is represented by the star, with the corresponding residue number above. The vertical bar (|) indicates proline-rich segments that are potential binding regions for proteins with a SH3 domain. The asterisk indicates that the AID for these proteins, PAK5 and 6, may not actually serve as an inhibitory domain and needs further characterization. Drawing is not to scale.
As described in detail below, group I PAKs are thought to exist in 2 states: (1) as an inactive dimer in which kinase activity is autoinhibited by binding of the AID of a second PAK molecule, and (2) as an active monomer via phosphorylation of the kinase domain. Genetic, structural, and biochemical studies provide evidence for asymmetric homodimerization of group I PAKs leading to inactivation. In this model, the kinase domain of 1 PAK binds to the AID of the other monomer and vice versa, limiting the ability of each kinase to autophosphorylate, a required step for activation 24-26). Other findings also indicate that the 2 inactive monomers exist in different conformations, similar to an enzyme–product complex, and that this is important in the transition from inactive to active states via trans autophosphorylation (27). However, this traditional model of dimerization as the primary means of PAK inactivation has recently been challenged by solution-phase structural analysis, which unexpectedly showed that over 95% of the unphosphorylated PAK1 is in a monomeric state, with concordant results across multiple analytical methods (Sedimentation velocity analytical ultracentrifugation, Liquid chromatography -mass spectrometry, and High performance liquid chromatography-small angle X-ray scattering) (28). High performance liquid chromatography-small angle X-ray scattering data indicated that the unphosphorylated monomers were more compact than their phosphorylated counterparts (28). These findings suggest that group I PAK autoinhibition may also be intramolecular due to folding of PAK monomers.
The transition to an active monomer is primarily initiated by the binding of GTP-bound CDC42 or RAC1 to the PBD, destabilizing the interaction between the AID and kinase domain (29). Multiple autophosphorylation events are necessary for full kinase activity. There are serine and threonine residues between the PBD/AID region and the kinase region that when autophosphorylated are thought to act as a multistep activation switch and are necessary for autoinhibition suppression and maintenance of full activity (25, 26). This transition also results in the phosphorylation of the catalytic threonine residue in the kinase domain (Thr423 in PAK1, Thr402 in PAK2, and Thr421 in PAK3), often serving as a marker of group I PAK activation (29, 30). Alternate modes of activation that may not require GTPase binding have been described, but their physiological relevance is less understood, including autoinhibitory structure disruption via binding of sphingosine (31), direct phosphorylation by AKT (32), PDK1 (33), and JAK2 (34), caspase-3 binding during apoptosis for PAK2 (35), and via binding of PIX with subsequent recruitment to GIT1 (36). Inactivation of group I PAKs occurs through dephosphorylation by phosphatases (37, 38), direct binding and stabilization of the kinase domains (39), and via phosphorylation by PKA (40). Specifically, multiple members of the POPX family of phosphatases, PP1 and PP2, dephosphorylate PAKs both at autoinhibitory serines and at the catalytic threonine in the kinase domain, depending on the PAK isoform (37, 38). In addition, PAK activation can be blocked by the binding of proteins that interfere with access either of GTPases to the PBD/AID region, as is the case with Merlin (41), hPIP1 (42), and CRIPak (43), or that interfere with the kinase domain, such as PITSLRE (44) and Nischarin (45).
Group I PAKs have distinct tissue-specific expression patterns. PAK1 is expressed is most tissues, but to a greater extent in thyroid, brain, and muscle (13). PAK2 is ubiquitously expressed and PAK3 is the most restricted of group I, expressed primarily in brain, cartilage, liver, and spleen (13). Bone marrow, pancreas, peripheral nervous system, and thymus tissues show little to no expression of either group I or group II PAKs (13). Group I PAKs have been shown to be especially important in both neural and cardiac development and function (11).
PAKs are central nodes in a number of signaling cascades and have more than 30 identified direct substrates (13, 46). Cytoskeletal rearrangement and regulation of cell membrane extensions that are important for cell motility were among the first functions discovered for PAK1. Acting as an effector of external stimuli, PAK1 directly phosphorylates Myosin Light Chain Kinase (MLCK) (47), Tubulin cofactor B (TCoB) (48), OP18/stathmin (49), and vimentin (50) (among others), leading to dynamic cytoskeletal morphologies such as membrane ruffling and filopodia formation (51, 52). Activated PAK1 has also been shown to relocalize from the cytosol to focal adhesions and dynamic actin structures near the plasma membrane in response to external stimuli, further illustrating a role in cytoskeletal reorganization and cell motility (53, 54). Group I PAKs also play a role in proliferation promoted by cell adhesion signals through integrin-mediated CDC42 and RAC1 activation (55).
PAKs play an important activation role for MAPK signaling. PAK1 directly phosphorylates MEK1 and RAF1, both integral components of the MAPK pathway, thereby mediating growth factor–driven cell cycle progression (56, 57). RAF1 (CRAF) Ser338 is a direct phosphorylation target of PAKs 1 and 2, an event that enhances CRAF activity and activation of the MAPK pathway (57). Additionally, a kinase-independent role in MAPK signaling has also been observed for PAK1, with PAK1 serving as a scaffold for MEK1/2 and CRAF at the cell membrane, allowing CRAF to phosphorylate its primary target, MEK1/2 (58). A more detailed review of BRAF–PAK interactions and signaling effects are described in later sections of this review in the context of thyroid cancer.
PAK1 also affects cell survival and proliferation by regulating several other signaling cascades. PAK1 activates NF-κB signaling by directly phosphorylating NF-κB-inducing kinase (59) and by coordinately increasing expression of the cell cycle regulator Cyclin D1 (60). In addition, PAK1 has been shown to be necessary for PI3K-mediated activation of CRAF and cell cycle progression in some systems (61). Finally, RAC and CDC42 GEFs (ie, PIX, SOS, VAV), which are necessary for these GTPases to be activated, are stimulated by activated PI3K as a result of fibronectin or growth factors, and this in turn activates PAK1 (62, 63). Thus, group I PAK activation is important for a number of key proliferative cellular pathways.
In addition to their roles in regulating cell motility and proliferation, group I PAKs also play a role in inhibition of apoptosis. For example, PAK1 is reported to phosphorylate and inactivate the proapoptotic protein BCL2 associated agonist of cell death (BAD) by subsequently reducing the affinity of binding between BAD and antiapoptotic proteins (64). PAK1 also phosphorylates and inactivates the proapoptotic protein BimL in a similar manner (65). Finally, PAK1 phosphorylates forkhead transcription factor (FKHR), which results in cytoplasmic mislocation of the protein and inhibits its transcriptional activity, ultimately preventing the transcription of downstream targets including several proapoptotic genes (66). PAK2, on the other hand, appears to have a direct role in the later stages of caspase-3-mediated apoptosis. PAK2 is cleaved by caspase-3 and this fragment is then myristoylated and relocalized to the cell membrane. Membrane-bound PAK2 subsequently mediates apoptosis through JNK activation. Abrogation of this myristoylation greatly reduced the apoptotic effects of the caspase-3–generated PAK2 fragment (67).
Group I PAKs also play a role in the progression of mitosis and the cytoskeletal dynamics required for cell division. PAK1 interacts with histone H3 during chromosome condensation and phosphorylates it at Ser10, a requirement for the progression of mitosis during prophase (23). PAK1 is also phosphorylated by Cyclin B1/CDC2 (also called CDK1) at Thr212 during mitosis, with high levels found from metaphase to telophase (68). Additionally, PAK1 Thr212 is enriched in microtubule-organizing centers during mitosis (23, 68). PAK activation is also implicated in centrosome separation. Tiam1, a GEF for RAC1, localizes at the centrosome and is phosphorylated by CDK1, a modification that leads to subsequent activation of colocalized PAK1 and 2 (69). Cells depleted of Tiam1 or PAK1 and 2 both had defects in chromosomal alignment on the metaphase plate (69). PAKs, in complex with β-PIX and GIT1, have also been shown to phosphorylate and activate the centrosome-associated kinase Aurora-A, a critical regulator of prometaphase chromosome alignment, anaphase sister chromatid separation, and cytokinesis (70). PLK1 (Polo-like kinase 1), which is necessary for the G2/M transition as well as proper bipolar spindle formation and spindle body tension, is also a direct phosphorylation target of PAK1 (71). All this information together suggests that group I PAKs play important roles in cell cycle progression and maintaining the proper cytoskeletal mechanisms necessary for division.
Group II PAK Structure and Function
The less studied PAKs 4, 5, and 6 make up group II, and share N-terminal PBD and C-terminal kinase domains with group I, albeit with considerably less sequence similarity (Fig. 1). Group II PAKs do not appear to use the AID for autoinhibition, but rather for subcellular localization, and instead autoinhibit in cis through the Pseudosubstrate (PS) domain near the PBD (72, 73). This structure only allows for activation in the presence of a binding partner with a classical SH3 (Src homology 3) domain. Similar to group I PAKs, PAK4 (73) and PAK5 (74) have also been shown to contain at least 1 nuclear localization sequence signal in the N-terminal domain. Group II PAKs were originally thought to be constitutively active due to basal phosphorylation of the catalytic threonine in the kinase domain, but evidence of the PS/AID domain and the increased activity of mutant PAK4 lacking the PBD/AID/PS domains suggests regulation of activity (75). Another distinguishing feature of group II is the lack of a PIX binding domain and lack of binding to the Nck adaptor protein (75). CDC42, and to a lesser extent RAC1, can still interact with the PBD of these kinases; however, they are not involved in activation of the kinase domain, but rather for subcellular localization as seen with PAK4 (76).
Group II PAKs also have isoform-specific tissue distribution patterns. PAK4 is expressed mainly in testis, prostate, cervix, and colon tissue, PAK5 is found primarily in brain and eye tissue, while PAK6 is highly expressed in brain, prostate, and placenta (13). PAK4 is particularly important for neural development and cardiovascular regeneration (13).
Similar to group I PAKs, functions that have been described to group II PAKs are regulation of cytoskeletal dynamics, cell proliferation, and apoptosis. For example, PAK6 is directly stimulated by MKK6, part of the p38–MAPK pathway, possibly as a stress response (77). Apoptosis is inhibited by PAK5 via the phosphorylation of BAD (78), as described above for PAK1. Most of the knowledge about group II PAKs comes from studying PAK4. PAK4, in a similar fashion to PAK1, is involved in growth factor–stimulated signaling (79). Hepatocyte growth factor recruits PAK4 to its receptor when colocalized near lamellipodia, along with the Gab1 adaptor protein (79). The interaction between PAK4 and Gab1 is PAK kinase independent and enhances cell migration and invasion in vitro (79). PAK4 also participates in Wnt/β-catenin signaling, a key pathway in embryonic development and cell proliferation, via direct phosphorylation of β-catenin on Ser675; this helps translocate it into the nucleus, as well as by preventing β-catenin degradation overall (80). Cytoskeletal rearrangement is also regulated by PAK4, which has been shown to phosphorylate GEF-H (81, 82) and Paxillin (83). These are important in the dissolution of stress fibers and focal adhesion complex organization. PAK4 also directly phosphorylates LIMK1 which promotes actin filament stability (84).
PAKs in Cancer
The above data demonstrate that PAKs regulate a diverse set of cellular functions that suggest a potential role in promoting cancer development and progression. While PAK point mutations or indels are rarely observed in human cancers, its activation is critical to potentiate the activity of upstream oncogenic regulators, such as RAC1 and RAS in skin tumors (7). PAKs 1 and 4 are particularly overexpressed isoforms in cancer. PAK1 amplification/overexpression has been found in thyroid (85), ovarian (86), colon (87), breast (60), pancreatic (88), and brain cancers (89), where it is generally associated with a poor prognosis in association with increased invasiveness. PAKs promote malignant transformation and cancer progression in a variety of manners, including enhanced invasiveness, motility, and proliferation, promoting epithelial to mesenchymal transition (EMT), inhibiting apoptosis, and promoting angiogenic induction (90).
Augmented cell proliferation is one of the classical hallmarks of cancer (91) and PAKs stand at the intersection of multiple cell signaling pathways involved in regulating this process. For example, PAK1 and PAK4 share many of the same substrates and regulate oncogenic MAPK signaling at several levels in the cascade, as described (92). Integrin and CDC42/RAC1 signaling enable anchorage independent growth in part via PAK (11). PAK1 also influences the activation of the NF-κB pathway (93) via NF-κB-inducing kinase (59) and RAS/RAF1-mediated activation 93-95), which contributes to oncogenesis by promoting cell survival and has been shown to be upregulated in many different types of cancer (96). PAKs also promote cancer cell survival through the inhibition of proapoptotic proteins as described earlier (11).
EMT is widely considered to be a feature to enable both local invasion and metastases of solid tumors. PAKs contribute to EMT by activating the Wnt/β-catenin pathway and through direct phosphorylation of SNAIL1, resulting in loss of cell adherence via the downregulation of E-cadherin (80, 97). Additionally, PAK1 phosphorylates SHARP, which, in turn, activates Notch signaling, another embryonic development pathway implicated in EMT and cancer (98, 99). The dynamic rearrangement of the cytoskeleton and cellular polarity is critical for local tissue invasion and often is coupled with EMT. PAKs phosphorylate cytoskeletal regulators such as Merlin (100), DLC1 (65), TCoB (101), LIMK (102), OP18/stathmin (103, 104), filamin (105), and vimentin (106), which are particularly active in invasive cancers.
PAKs also have been implicated in promoting angiogenesis in vitro. Vascular endothelial growth factor secretion, a proangiogenic feature of many cancer cells, is increased in response to PAK1 activation in breast cancer epithelial cells (107). PAK2 is reported to regulate vascular development and maintenance, but as yet has not been shown to play a defined role in cancer (108).
PAKs play a role in allowing transformed cells to progress through the cell cycle, as evidenced by PAK1-mediated increase in Cyclin D1 levels and PAK4-mediated decrease in cyclin-dependent kinase inhibitor 1C levels in breast cancer cells (60, 109). Experimentally, breast (60, 66, 109), colorectal 110-112), and skin cancer (113, 114) progression and the development of schwannomas (115) are inhibited by molecular knockdown or pharmacologic inhibition of PAKs 1 or 4, in vitro and in vivo, with evidence of reduced downstream signaling, cell cycle progression, and increased apoptosis. Finally, one of the major challenges in treating aggressive cancer is the development of drug resistance. Interestingly, activation of PAKs 1, 4, 5, and 6 has been shown to be important in resistance to cytotoxic chemotherapy by reactivation of cell cycle and survival signals, and targeting PAKs has resulted in renewed sensitivity to standard chemotherapies (88, 116-122). These data together have created interest in pursuing PAKs as potential therapeutic targets for several cancer types in different contexts (12, 90).
PAKs in Thyroid Cancer
A role for PAKs in papillary thyroid cancer (PTC) was first reported when PAKs were predicted to be one of the key signaling nodes identified in the analysis of global mRNA expression in the invasive fronts versus central regions of aggressive PTCs (123). The invasive fronts of these tumors also showed high expression of genes in the transforming growth factor-β, PI3K, and integrin pathways and low expression of cell adhesion genes. These, together, implicated an EMT regardless of the initiating oncogene, although most of the cancers had MAPK-activating mutations (113). Subsequent studies confirmed not only EMT but also that PAK1 was overexpressed and functionally critical for PTC invasion and EMT pathway activation (85).
In vitro studies demonstrated that group I PAKs were necessary for thyroid cancer cell migration, invasion, and proliferation (85, 124, 125). Further siRNA studies analyzing all 6 isoforms demonstrated these functions were primarily dependent on PAK1, with modest contributions from PAK4, suggesting a broad role for PAK1 in thyroid cancer cells (85). A role for PAK1 in thyroid cancer proliferation and cell motility is further supported by studies defining that PAK1 is a functional target of microRNA 7 (miR-7), an inhibitor of thyroid cancer growth (125). PAK4 has been shown to block the proliferative response of thyroid cells to TSH in vitro (126). Thus, it appears that PAK1 is the most important isoform in cancer cells with activated MAPK, while PAK4 may be relevant to PKA signaling, which is regulated by TSH, in thyroid cells.
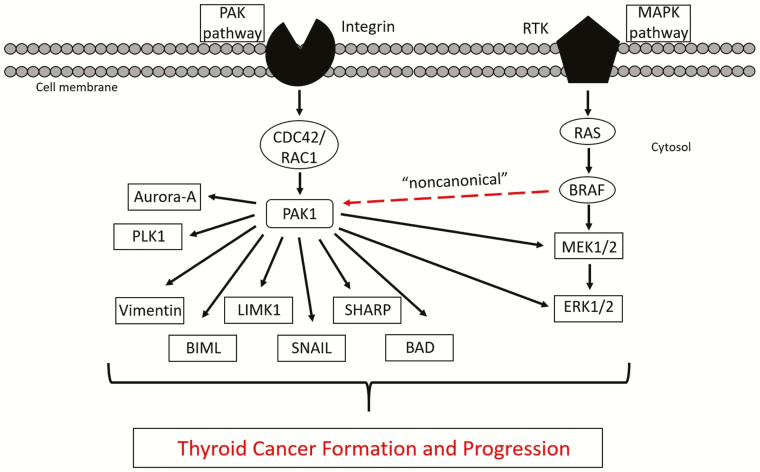
PTC initiation is driven primarily by MAPK activation and the BRAFV600E mutation is particularly common (127). It is associated with more aggressive tumors and is overrepresented in clinical trial populations. These associations are particularly strong when it occurs in common with mutations in the hTERT promoter, TP53, or in PI3K regulatory genes, and similar relationships have been shown for other MAPK activating mutations, such as those in NRAS, that are common in follicular forms of thyroid cancer (7, 128, 129). Due to the known ability of PAKs to augment MAPK signaling by direct phosphorylation of CRAF (61, 130), ARAF (131), and MEK1 (132), there has been interest in determining the role of PAK in MAPK-mediated tumorigenesis. For example, in skin tumor models, PAK activity has been shown to be required for RAS-induced transformation and growth (110, 113, 133, 134). In thyroid cancer cell lines, it has been shown that inhibition of group I PAK activity, and PAK1 specifically, inhibits cellular proliferation in cancer cells with BRAFV600E or HRAS-activating mutations (124). It, therefore, was hypothesized that in thyroid cancer cells, PAK-mediated phosphorylation of MEK1 or BRAF, at the cognate site for CRAF, would further activate MAPK signaling. Unexpectedly, inhibition of PAK activity did not alter BRAF-mediated MAPK signaling. However, PAK activation was shown to be dependent on BRAF, a feature not described previously and termed “noncanonical” (Fig. 2). Due to the known scaffold functions of PAK (58), further studies demonstrated that BRAF colocalized and coimmunoprecipitated with PAK, particularly during mitosis. In addition, the regulation of PAK by BRAF was not altered either by MEK inhibitors or MEK depletion, suggesting that BRAF regulation of PAK is independent of MEK (124). Finally, BRAF siRNA-mediated inhibition of invasion was rescued by both constitutively active MEK and PAK, further suggesting that both pathways are important in BRAF-mediated effects in thyroid cancer cells (124). These data suggested that BRAF might regulate thyroid cancer development and/or progression not only through MEK activation, but also by activation of PAKs.
Figure 2.
PAK/MAPK signaling and the downstream effectors involved in oncogenesis and progression. A restricted view of the PAK/MAPK signaling cascades, and a limited list of PAK substrates that illustrates the diverse mechanisms by which PAK dysregulation can lead to oncogenesis and progression. Overactivation of PAK, whether upstream through mutations in BRAF or RAC1, or PAK overexpression/amplification, lead to transformation exemplary of the hallmarks of cancer. The “noncanonical” activation of PAK by BRAF is noted with a red line.
In vivo studies further support a fundamental role of PAK signaling in PTC progression. Induction of BRAFV600E in the thyroid in mice leads to PTC development associated with increased PAK isoform expression and activity. Treatment of mice harboring doxycycline-inducible thyroid-specific BRAFV600E overexpression develop PTC with activation of both PAK and ERK. Pretreatment of these mice with the group I PAK inhibitor G-5555 inhibited BRAFV600E-induced PTC development with reduced PAK and maintained ERK activation, consistent with an important role for PAK in BRAFV600E-induced PTC development (135). Moreover, in thyroid cancer cells with a BRAFV600E mutation, combination of the BRAFV600E-specific kinase inhibitor, vemurafenib, with G-5555 was synergistic with inhibition of both PAK and MEK signaling, suggesting that inhibition of both signals may be therapeutically beneficial (135). Taken together, these data suggest that there are both overlapping and independent PAK and MEK signaling cascades and that there may be therapeutic value in inhibiting the MEK-independent cascade. Importantly, because BRAF-mediated PAK activity occurs via a nonkinase mechanism, it is not inhibited by available BRAF kinase inhibitors. Similar data have been reported in melanoma models in which PAK activation is mechanistically necessary for resistance to BRAF and/or MEK inhibitors in BRAFV600E or mutated RAS-driven cancer models (133, 136, 137). Finally, while these data have focused on group I PAKs, PAK4 has been implicated in therapeutic resistance to MEK inhibition in RAS-mediated colorectal cancer (92) and pancreatic adenocarcinoma (138), and anaplastic thyroid cancer resistance (139) to the VEGFR inhibitor, Lenvatinib, although the mechanisms are less well defined. Group I PAKs have also been shown to be required for tumors induced by activated RAC1P29S (113, 114, 140) and NF2 (41, 141, 142) mutants, 2 uncommon but reported genomic events in thyroid cancer. Taken together, PAKs represent a rational therapeutic target for aggressive thyroid cancer, particularly those that harbor RAS or BRAF gene mutations as drivers.
Summary and Future Directions
PAKs are a family of serine/threonine kinases that have diverse cellular functions regulating cytoskeletal dynamics, mitosis, cell survival/growth, cell cycle progression, and gene transcription. The role of PAK as a necessary signaling molecule for oncogenic transformation driven by a number key pathways implicates its potential both as a biomarker for signaling activity and as a therapeutic target. New data in thyroid cancer and melanoma models suggest a potential specific role in MAPK-driven tumors, both for combinatorial primary treatment approaches and as a key mechanistic player in therapeutic resistance to BRAF and MEK-targeted therapies that are in clinical practice and/or clinical trials. Overall, the PAKs are promising therapeutic targets warranting further translational study.
Acknowledgments
Financial Support: This work was supported by funding from National Institutes of Health to MDR (Grant # R01CA102572, and R01CA227847).
Glossary
Abbreviations
- AID
Auto-Inhibitory Domain
- CRIB
CDC42 and RAC1 binding
- EMT
epithelial to mesenchymal transition
- MAPK
mitogen activated protein kinase
- PAK
p21-activated kinase
- PBD
p21-binding domain
- PS
Peseudosubstrate
- PTC
papillary thyroid cancer
- TSH
thyrotropin
Additional Information
Disclosure Summary: The authors report no conflicts of interest
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Howlader NAMN, Krapcho M, Miller D, et al. SEER Cancer Statistics Review. 1975–2016. SEER Website; 2020. https://seer.cancer.gov/statfacts/html/thyro.html. Accessed March 15, 2020. [Google Scholar]
- 2. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99(2):E276-E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan KL, Li S, Tseng CH, et al. Rising incidence and incidence-based mortality of thyroid cancer in California, 2000–2017. J Clin Endocrinol Metab. 2020;105(6):1770-1777. [DOI] [PubMed] [Google Scholar]
- 5. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621-630. [DOI] [PubMed] [Google Scholar]
- 7. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375(23):2307. [DOI] [PubMed] [Google Scholar]
- 8. Brzezianska E, Pastuszak-Lewandoska D. A minireview: the role of MAPK/ERK and PI3K/Akt pathways in thyroid follicular cell-derived neoplasm. Front Biosci (Landmark Ed). 2011;16(2):422-439. [DOI] [PubMed] [Google Scholar]
- 9. Ganly I, Ricarte Filho J, Eng S, et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J Clin Endocrinol Metab. 2013;98(5):E962-E972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367(6458):40-46. [DOI] [PubMed] [Google Scholar]
- 11. Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene. 2009;28(28):2545-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6(6):459-471. [DOI] [PubMed] [Google Scholar]
- 13. Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100(2):97-108. [DOI] [PubMed] [Google Scholar]
- 14. Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol. 2002;34(7):713-717. [DOI] [PubMed] [Google Scholar]
- 15. Zhao ZS, Manser E. PAK and other Rho-associated kinases–effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386(Pt 2):201-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of the Nck adapter protein with p21-activated kinase (PAK1). J Biol Chem. 1996;271(42):25746-25749. [DOI] [PubMed] [Google Scholar]
- 17. Puto LA, Pestonjamasp K, King CC, Bokoch GM. p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J Biol Chem. 2003;278(11):9388-9393. [DOI] [PubMed] [Google Scholar]
- 18. Pawson T. Protein modules and signalling networks. Nature. 1995;373(6515):573-580. [DOI] [PubMed] [Google Scholar]
- 19. Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1(5):253-259. [DOI] [PubMed] [Google Scholar]
- 20. Kiosses WB, Hood J, Yang S, et al. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ Res. 2002;90(6):697-702. [DOI] [PubMed] [Google Scholar]
- 21. Lu W, Katz S, Gupta R, Mayer BJ. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr Biol. 1997;7(2):85-94. [DOI] [PubMed] [Google Scholar]
- 22. Singh RR, Song C, Yang Z, Kumar R. Nuclear localization and chromatin targets of p21-activated kinase 1. J Biol Chem. 2005;280(18):18130-18137. [DOI] [PubMed] [Google Scholar]
- 23. Li F, Adam L, Vadlamudi RK, et al. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 2002;3(8):767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lei M, Lu W, Meng W, et al. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102(3):387-397. [DOI] [PubMed] [Google Scholar]
- 25. Tu H, Wigler M. Genetic evidence for Pak1 autoinhibition and its release by Cdc42. Mol Cell Biol. 1999;19(1):602-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chong C, Tan L, Lim L, Manser E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem. 2001;276(20):17347-17353. [DOI] [PubMed] [Google Scholar]
- 27. Wang J, Wu JW, Wang ZX. Structural insights into the autoactivation mechanism of p21-activated protein kinase. Structure. 2011;19(12):1752-1761. [DOI] [PubMed] [Google Scholar]
- 28. Sorrell FJ, Kilian LM, Elkins JM. Solution structures and biophysical analysis of full-length group A PAKs reveal they are monomeric and auto-inhibited in cis. Biochem J. 2019;476(7):1037-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gatti A, Huang Z, Tuazon PT, Traugh JA. Multisite autophosphorylation of p21-activated protein kinase gamma-PAK as a function of activation. J Biol Chem. 1999;274(12):8022-8028. [DOI] [PubMed] [Google Scholar]
- 30. Zenke FT, King CC, Bohl BP, Bokoch GM. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J Biol Chem. 1999;274(46):32565-32573. [DOI] [PubMed] [Google Scholar]
- 31. Bokoch GM, Reilly AM, Daniels RH, et al. A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J Biol Chem. 1998;273(14):8137-8144. [DOI] [PubMed] [Google Scholar]
- 32. Zhou GL, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol Cell Biol. 2003;23(22):8058-8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. King CC, Gardiner EM, Zenke FT, et al. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J Biol Chem. 2000;275(52):41201-41209. [DOI] [PubMed] [Google Scholar]
- 34. Rider L, Shatrova A, Feener EP, Webb L, Diakonova M. JAK2 tyrosine kinase phosphorylates PAK1 and regulates PAK1 activity and functions. J Biol Chem. 2007;282(42):30985-30996. [DOI] [PubMed] [Google Scholar]
- 35. Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276(5318):1571-1574. [DOI] [PubMed] [Google Scholar]
- 36. Loo TH, Ng YW, Lim L, Manser E. GIT1 activates p21-activated kinase through a mechanism independent of p21 binding. Mol Cell Biol. 2004;24(9):3849-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koh CG, Tan EJ, Manser E, Lim L. The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr Biol. 2002;12(4):317-321. [DOI] [PubMed] [Google Scholar]
- 38. Zhan Q, Ge Q, Ohira T, Van Dyke T, Badwey JA. p21-activated kinase 2 in neutrophils can be regulated by phosphorylation at multiple sites and by a variety of protein phosphatases. J Immunol. 2003;171(7):3785-3793. [DOI] [PubMed] [Google Scholar]
- 39. Zhao ZS, Manser E, Chen XQ, Chong C, Leung T, Lim L. A conserved negative regulatory region in alphaPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18(4):2153-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howe AK, Juliano RL. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol. 2000;2(9):593-600. [DOI] [PubMed] [Google Scholar]
- 41. Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12(4):841-849. [DOI] [PubMed] [Google Scholar]
- 42. Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M. Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1. Proc Natl Acad Sci U S A. 2001;98(11):6174-6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Talukder AH, Meng Q, Kumar R. CRIPak, a novel endogenous Pak1 inhibitor. Oncogene. 2006;25(9):1311-1319. [DOI] [PubMed] [Google Scholar]
- 44. Chen S, Yin X, Zhu X, et al. The C-terminal kinase domain of the p34cdc2-related PITSLRE protein kinase (p110C) associates with p21-activated kinase 1 and inhibits its activity during anoikis. J Biol Chem. 2003;278(22):20029-20036. [DOI] [PubMed] [Google Scholar]
- 45. Alahari SK. Nischarin inhibits Rac induced migration and invasion of epithelial cells by affecting signaling cascades involving PAK. Exp Cell Res. 2003;288(2):415-424. [DOI] [PubMed] [Google Scholar]
- 46. Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72(1):743-781. [DOI] [PubMed] [Google Scholar]
- 47. Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283(5410):2083-2085. [DOI] [PubMed] [Google Scholar]
- 48. Vadlamudi RK, Barnes CJ, Rayala S, et al. p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B. Mol Cell Biol. 2005;25(9):3726-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of microtubule destabilizing activity of Op18/stathmin downstream of Rac1. J Biol Chem. 2004;279(7):6196-6203. [DOI] [PubMed] [Google Scholar]
- 50. Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem. 2006;281(45):34716-34724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manser E, Huang HY, Loo TH, et al. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17(3):1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7(3):202-210. [DOI] [PubMed] [Google Scholar]
- 53. Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138(6):1265-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145(4):837-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Slack-Davis JK, Eblen ST, Zecevic M, et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol. 2003;162(2):281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem. 2005;280(44):36609-36615. [DOI] [PubMed] [Google Scholar]
- 57. Tran NH, Frost JA. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J Biol Chem. 2003;278(13):11221-11226. [DOI] [PubMed] [Google Scholar]
- 58. Wang Z, Fu M, Wang L, et al. p21-activated kinase 1 (PAK1) can promote ERK activation in a kinase-independent manner. J Biol Chem. 2013;288(27):20093-20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Foryst-Ludwig A, Naumann M. p21-activated kinase 1 activates the nuclear factor kappa B (NF-kappa B)-inducing kinase-Ikappa B kinases NF-kappa B pathway and proinflammatory cytokines in Helicobacter pylori infection. J Biol Chem. 2000;275(50):39779-39785. [DOI] [PubMed] [Google Scholar]
- 60. Balasenthil S, Sahin AA, Barnes CJ, et al. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004;279(2):1422-1428. [DOI] [PubMed] [Google Scholar]
- 61. Chaudhary A, King WG, Mattaliano MD, et al. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr Biol. 2000;10(9):551-554. [DOI] [PubMed] [Google Scholar]
- 62. Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142(2):573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17(8):4406-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem. 2005;280(26):24698-24705. [DOI] [PubMed] [Google Scholar]
- 65. Vadlamudi RK, Bagheri-Yarmand R, Yang Z, et al. Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes. Cancer Cell. 2004;5(6):575-585. [DOI] [PubMed] [Google Scholar]
- 66. Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 2003;535(1-3):6-10. [DOI] [PubMed] [Google Scholar]
- 67. Vilas GL, Corvi MM, Plummer GJ, Seime AM, Lambkin GR, Berthiaume LG. Posttranslational myristoylation of caspase-activated p21-activated protein kinase 2 (PAK2) potentiates late apoptotic events. Proc Natl Acad Sci U S A. 2006;103(17):6542-6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Banerjee M, Worth D, Prowse DM, Nikolic M. Pak1 phosphorylation on t212 affects microtubules in cells undergoing mitosis. Curr Biol. 2002;12(14):1233-1239. [DOI] [PubMed] [Google Scholar]
- 69. Whalley HJ, Porter AP, Diamantopoulou Z, White GR, Castañeda-Saucedo E, Malliri A. Cdk1 phosphorylates the Rac activator Tiam1 to activate centrosomal Pak and promote mitotic spindle formation. Nat Commun. 2015;6(1):7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20(2):237-249. [DOI] [PubMed] [Google Scholar]
- 71. Maroto B, Ye MB, von Lohneysen K, Schnelzer A, Knaus UG. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene. 2008;27(36):4900-4908. [DOI] [PubMed] [Google Scholar]
- 72. Ha BH, Davis MJ, Chen C, et al. Type II p21-activated kinases (PAKs) are regulated by an autoinhibitory pseudosubstrate. Proc Natl Acad Sci U S A. 2012;109(40):16107-16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Baskaran Y, Ng YW, Selamat W, Ling FT, Manser E. Group I and II mammalian PAKs have different modes of activation by Cdc42. EMBO Rep. 2012;13(7):653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wen YY, Wang XX, Pei DS, Zheng JN. p21-Activated kinase 5: a pleiotropic kinase. Bioorg Med Chem Lett. 2013;23(24):6636-6639. [DOI] [PubMed] [Google Scholar]
- 75. Rane CK, Minden A. P21 activated kinases: structure, regulation, and functions. Small GTPases. 2014;5:e28003-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Abo A, Qu J, Cammarano MS, et al. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 1998;17(22):6527-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kaur R, Liu X, Gjoerup O, et al. Activation of p21-activated kinase 6 by MAP kinase kinase 6 and p38 MAP kinase. J Biol Chem. 2005;280(5):3323-3330. [DOI] [PubMed] [Google Scholar]
- 78. Cotteret S, Jaffer ZM, Beeser A, Chernoff J. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol. 2003;23(16): 5526-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Paliouras GN, Naujokas MA, Park M. Pak4, a novel Gab1 binding partner, modulates cell migration and invasion by the Met receptor. Mol Cell Biol. 2009;29(11):3018-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li Y, Shao Y, Tong Y, et al. Nucleo-cytoplasmic shuttling of PAK4 modulates β-catenin intracellular translocation and signaling. Biochim Biophys Acta. 2012;1823(2):465-475. [DOI] [PubMed] [Google Scholar]
- 81. Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12(5):699-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci. 2005;118(Pt 9):1861-1872. [DOI] [PubMed] [Google Scholar]
- 83. Dong JM, Lau LS, Ng YW, Lim L, Manser E. Paxillin nuclear-cytoplasmic localization is regulated by phosphorylation of the LD4 motif: evidence that nuclear paxillin promotes cell proliferation. Biochem J. 2009;418(1):173-184. [DOI] [PubMed] [Google Scholar]
- 84. Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem. 2001;276(34): 32115-32121. [DOI] [PubMed] [Google Scholar]
- 85. McCarty SK, Saji M, Zhang X, et al. Group I p21-activated kinases regulate thyroid cancer cell migration and are overexpressed and activated in thyroid cancer invasion. Endocr Relat Cancer. 2010;17(4):989-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Brown LA, Kalloger SE, Miller MA, et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer. 2008;47(6):481-489. [DOI] [PubMed] [Google Scholar]
- 87. Carter JH, Douglass LE, Deddens JA, et al. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res. 2004;10(10):3448-3456. [DOI] [PubMed] [Google Scholar]
- 88. Zhou W, Jubb AM, Lyle K, et al. PAK1 mediates pancreatic cancer cell migration and resistance to MET inhibition. J Pathol. 2014;234(4):502-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Aoki H, Yokoyama T, Fujiwara K, et al. Phosphorylated Pak1 level in the cytoplasm correlates with shorter survival time in patients with glioblastoma. Clin Cancer Res. 2007;13(22 Pt 1):6603-6609. [DOI] [PubMed] [Google Scholar]
- 90. Rane CK, Minden A. P21 activated kinase signaling in cancer. Semin Cancer Biol. 2019;54(1):40-49. [DOI] [PubMed] [Google Scholar]
- 91. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. [DOI] [PubMed] [Google Scholar]
- 92. Tabusa H, Brooks T, Massey AJ. Knockdown of PAK4 or PAK1 inhibits the proliferation of mutant KRAS colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT signaling. Mol Cancer Res. 2013;11(2):109-121. [DOI] [PubMed] [Google Scholar]
- 93. Frost JA, Swantek JL, Stippec S, Yin MJ, Gaynor R, Cobb MH. Stimulation of NFkappa B activity by multiple signaling pathways requires PAK1. J Biol Chem. 2000;275(26):19693-19699. [DOI] [PubMed] [Google Scholar]
- 94. Li S, Sedivy JM. Raf-1 protein kinase activates the NF-kappa B transcription factor by dissociating the cytoplasmic NF-kappa B-I kappa B complex. Proc Natl Acad Sci U S A. 1993;90(20):9247-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Norris JL, Baldwin AS Jr. Oncogenic Ras enhances NF-kappaB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways. J Biol Chem. 1999;274(20):13841-13846. [DOI] [PubMed] [Google Scholar]
- 96. Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2(9):823-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 2005;65(8):3179-3184. [DOI] [PubMed] [Google Scholar]
- 98. Vadlamudi RK, Manavathi B, Singh RR, Nguyen D, Li F, Kumar R. An essential role of Pak1 phosphorylation of SHARP in Notch signaling. Oncogene. 2005;24(28):4591-4596. [DOI] [PubMed] [Google Scholar]
- 99. Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;11(6):745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277(12):10394-10399. [DOI] [PubMed] [Google Scholar]
- 101. Hendrix MJ, Seftor EA, Chu YW, Trevor KT, Seftor RE. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev. 1996;15(4):507-525. [DOI] [PubMed] [Google Scholar]
- 102. Davila M, Frost AR, Grizzle WE, Chakrabarti R. LIM kinase 1 is essential for the invasive growth of prostate epithelial cells: implications in prostate cancer. J Biol Chem. 2003;278(38):36868-36875. [DOI] [PubMed] [Google Scholar]
- 103. Brattsand G. Correlation of oncoprotein 18/stathmin expression in human breast cancer with established prognostic factors. Br J Cancer. 2000;83(3):311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Roos G, Brattsand G, Landberg G, Marklund U, Gullberg M. Expression of oncoprotein 18 in human leukemias and lymphomas. Leukemia. 1993;7(10):1538-1546. [PubMed] [Google Scholar]
- 105. Flanagan LA, Chou J, Falet H, Neujahr R, Hartwig JH, Stossel TP. Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J Cell Biol. 2001;155(4):511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Singh S, Sadacharan S, Su S, Belldegrun A, Persad S, Singh G. Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 2003;63(9):2306-2311. [PubMed] [Google Scholar]
- 107. Bagheri-Yarmand R, Vadlamudi RK, Wang R-A, Mendelsohn J, Kumar R. Vascular endothelial growth factor up-regulation via p21-activated Kinase-1 signaling regulates heregulin-β1-mediated angiogenesis. J Biol Chem. 2000;275(50):39451-39457. [DOI] [PubMed] [Google Scholar]
- 108. Radu M, Lyle K, Hoeflich KP, Villamar-Cruz O, Koeppen H, Chernoff J. p21-Activated kinase 2 regulates endothelial development and function through the Bmk1/Erk5 pathway. Mol Cell Biol. 2015;35(23):3990-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li Y, Wang D, Zhang H, et al. P21-activated kinase 4 regulates the cyclin-dependent kinase inhibitor p57(kip2) in human breast cancer. Anat Rec (Hoboken). 2013;296(10):1561-1567. [DOI] [PubMed] [Google Scholar]
- 110. Chow HY, Dong B, Valencia CA, et al. Group I Paks are essential for epithelial- mesenchymal transition in an Apc-driven model of colorectal cancer. Nat Commun. 2018;9(1):3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. He H, Huynh N, Liu KH, et al. P-21 activated kinase 1 knockdown inhibits β-catenin signalling and blocks colorectal cancer growth. Cancer Lett. 2012;317(1):65-71. [DOI] [PubMed] [Google Scholar]
- 112. Huynh N, Liu KH, Baldwin GS, He H. P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via ERK- and AKT-dependent pathways. Biochim Biophys Acta. 2010;1803(9):1106-1113. [DOI] [PubMed] [Google Scholar]
- 113. Araiza-Olivera D, Feng Y, Semenova G, Prudnikova TY, Rhodes J, Chernoff J. Suppression of RAC1-driven malignant melanoma by group A PAK inhibitors. Oncogene. 2018;37(7):944-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Uribe-Alvarez C, Guerrero-Rodriguez SL, Rhodes J, Cannon A, Chernoff J, Araiza-Olivera D. Targeting effector pathways in RAC1(P29S)-driven malignant melanoma [Published online ahead of print February 17, 2020]. Small GTPases. 2020:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chow HY, Stepanova D, Koch J, Chernoff J. p21-Activated kinases are required for transformation in a cell-based model of neurofibromatosis type 2. PLoS One. 2010;5(11):e13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Qing H, Gong W, Che Y, et al. PAK1-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Tumour Biol. 2012;33(4):985-994. [DOI] [PubMed] [Google Scholar]
- 117. Park MH, Lee HS, Lee CS, et al. p21-Activated kinase 4 promotes prostate cancer progression through CREB. Oncogene. 2013;32(19):2475-2482. [DOI] [PubMed] [Google Scholar]
- 118. Shu XR, Wu J, Sun H, Chi LQ, Wang JH. PAK4 confers the malignance of cervical cancers and contributes to the cisplatin-resistance in cervical cancer cells via PI3K/AKT pathway. Diagn Pathol. 2015;10(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Moon SU, Kim JW, Sung JH, et al. p21-Activated kinase 4 (PAK4) as a predictive marker of gemcitabine sensitivity in pancreatic cancer cell lines. Cancer Res Treat. 2015;47(3):501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. He S, Feng M, Liu M, et al. P21-activated kinase 7 mediates cisplatin-resistance of esophageal squamous carcinoma cells with Aurora-A overexpression. PLoS One. 2014;9(12):e113989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang DG, Zhang J, Mao LL, et al. p21-Activated kinase 5 affects cisplatin-induced apoptosis and proliferation in hepatocellular carcinoma cells. Tumour Biol. 2015;36(5):3685-3691. [DOI] [PubMed] [Google Scholar]
- 122. Chen J, Lu H, Yan D, et al. PAK6 increase chemoresistance and is a prognostic marker for stage II and III colon cancer patients undergoing 5-FU based chemotherapy. Oncotarget. 2015;6(1):355-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vasko V, Espinosa AV, Scouten W, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci U S A. 2007;104(8):2803-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. McCarty SK, Saji M, Zhang X, et al. BRAF activates and physically interacts with PAK to regulate cell motility. Endocr Relat Cancer. 2014;21(6):865-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yue K, Wang X, Wu Y, Zhou X, He Q, Duan Y. microRNA-7 regulates cell growth, migration and invasion via direct targeting of PAK1 in thyroid cancer. Mol Med Rep. 2016;14(3):2127-2134. [DOI] [PubMed] [Google Scholar]
- 126. Xie X, Shi X, Guan H, et al. P21-activated kinase 4 involves TSH induced papillary thyroid cancer cell proliferation. Oncotarget. 2017;8(15):24882-24891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fagin JA. Genetics of papillary thyroid cancer initiation: implications for therapy. Trans Am Clin Climatol Assoc. 2005;116(1):259-269; discussion 269. [PMC free article] [PubMed] [Google Scholar]
- 128. Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bae JS, Choi SK, Jeon S, et al. Impact of NRAS mutations on the diagnosis of follicular neoplasm of the thyroid. Int J Endocrinol. 2014;2014:289834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. King AJ, Sun H, Diaz B, et al. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396(6707):180-183. [DOI] [PubMed] [Google Scholar]
- 131. Tran NH, Wu X, Frost JA. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J Biol Chem. 2005;280(16):16244-16253. [DOI] [PubMed] [Google Scholar]
- 132. Frost JA, Steen H, Shapiro P, et al. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. Embo J. 1997;16(21):6426-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lu H, Liu S, Zhang G, et al. PAK signalling drives acquired drug resistance to MAPK inhibitors in BRAF-mutant melanomas. Nature. 2017;550(7674):133-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Baker NM, Yee Chow H, Chernoff J, Der CJ. Molecular pathways: targeting RAC-p21-activated serine-threonine kinase signaling in RAS-driven cancers. Clin Cancer Res. 2014;20(18):4740-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Knippler CM, Saji M, Rajan N, Porter K, La Perle KMD, Ringel MD. MAPK- and AKT-activated thyroid cancers are sensitive to group I PAK inhibition. Endocr Relat Cancer. 2019;26(8):699-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Feddersen CR, Schillo JL, Varzavand A, et al. Src-Dependent DBL family members drive resistance to vemurafenib in human melanoma. Cancer Res. 2019;79(19):5074-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Babagana M, Johnson S, Slabodkin H, Bshara W, Morrison C, Kandel ES. P21-activated kinase 1 regulates resistance to BRAF inhibition in human cancer cells. Mol Carcinog. 2017;56(5):1515-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Thillai K, Lam H, Sarker D, Wells CM. Deciphering the link between PI3K and PAK: An opportunity to target key pathways in pancreatic cancer? Oncotarget. 2017;8(8):14173-14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Khan HY, Ge J, Nagasaka M, et al. Targeting XPO1 and PAK4 in 8505C anaplastic thyroid cancer cells: putative implications for overcoming lenvatinib therapy resistance. Int J Mol Sci. 2019;21(1):237-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lionarons DA, Hancock DC, Rana S, et al. RAC1(P29S) induces a mesenchymal phenotypic switch via serum response factor to promote melanoma development and therapy resistance. Cancer Cell. 2019;36(1):68-83.e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cooper J, Giancotti FG. Molecular insights into NF2/Merlin tumor suppressor function. FEBS Lett. 2014;588(16):2743-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. López-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009;29(15):4235-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]