Abstract
Currently there are no commercially available hydrocortisone formulations for the treatment of children with congenital adrenal hyperplasia (CAH) that allow for smaller doses (0.1-1.25 mg) and incremental adjustments needed to control excess androgen production and avoid the negative effects of overtreatment. This lack of availability has led physicians to recommend dividing hydrocortisone 5-mg tablets into 4 to 6 pieces, compounding capsules or hydrocortisone suspension, or crushing 5- or 10-mg tablets in 5 or 10 mL of water. We report a case of iatrogenic Cushing syndrome in a 6-year 11-month-old girl with salt-wasting CAH treated with hydrocortisone tablets that were administered after crushing and dispersing into water to obtain the prescribed dose. She presented with poor growth, increasing body mass index (BMI), excess downy hair, round facies, and gastric ulcers. Her hydrocortisone dose was 8.1 mg/m2/day. Results for all adrenal steroid concentrations were undetectable at 8 am, 12 hours after her last dose. The year prior to presentation her parents began dissolving 10 mg of hydrocortisone in 10 mL of water and using this preparation over the course of 24 hours, which coincided with rapid increase of BMI. We switched her to a pharmacy-compounded alcohol-free hydrocortisone suspension with total daily doses ranging from 6.5 to 8.2 mg/m2/day, which resulted in resolution of her cushingoid features, a decrease in BMI, and catch-up growth. Our case highlights that manipulation of hydrocortisone tablets by parents can result in great variability in dosing and the need for commercially available pediatric formulations allowing for smaller dosing required in young children.
Keywords: congenital adrenal hyperplasia, hydrocortisone, Cushing syndrome, pharmacokinetics
Patients with congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency require lifelong glucocorticoid therapy. Hydrocortisone is used in growing children because of the negative effects on growth of long-acting glucocorticoids [1]. Hydrocortisone has a short half-life especially in children with CAH [2] and is usually given 3 times a day with a recommended daily dose of 10 to 15 mg/m2/day [1]. In infancy and early childhood, smaller doses and incremental adjustments are required to reach a dose that is enough to prevent increased androgen production and exposure, but not excessive to avoid causing hypercortisolism. However, this balance is very hard to achieve in practice because the smallest US Food and Drug Administration (FDA)-approved commercial formulation currently available in the United States is the 5-mg hydrocortisone tablet with a single score.
This lack of an appropriate pediatric formulation has led to various manipulations of the 5-mg hydrocortisone tablet to deliver prescribed doses under 2.5 mg. Some parents are instructed to split tablets into 4 or more pieces, or to crush tablets to create a solution, both of which could lead to great dose variability with resultant hypocortisolemia or hypercortisolemia [3-5]. We report a case of iatrogenic Cushing syndrome in a patient treated with hydrocortisone tablets that were administered after crushing and dispersing into water by parents to obtain the prescribed dose.
Case Presentation
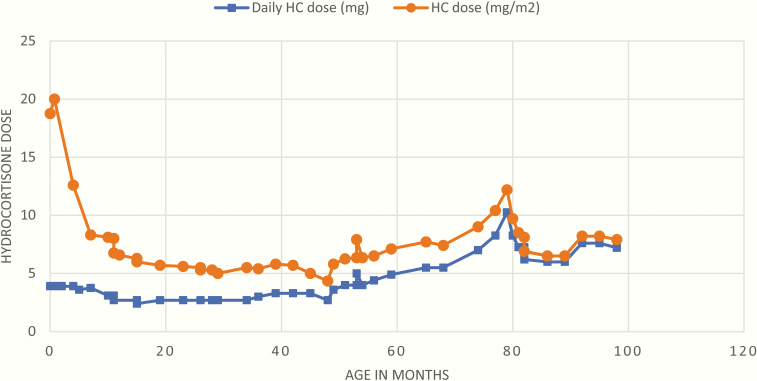
A 6-year 11-month old female presented to our CAH clinic for a consultation regarding her endocrine management. She was diagnosed with salt-wasting (SW) CAH shortly after birth, when presenting with atypical genitalia (Prader grade 4), hyperkalemia (8.3 mmol/L), and hyponatremia (130 mmol/L). Family history was significant for simple-virilizing CAH in a paternal second cousin and nonclassic CAH in a paternal aunt. Initial evaluation revealed a 46,XX karyotype and elevated baseline adrenal steroids (17-hydroxyprogesterone; 17-OHP = 33 800 ng/dL, androstenedione; D4A = 404 ng/dL, testosterone = 353 ng/dL), and plasma renin activity (3677 ng/mL/h). Pelvic ultrasound showed normal female anatomy. Treatment was started on day of life 6, with hydrocortisone 1.3 mg 3 times daily (18.7 mg/m2/day), fludrocortisone 0.1 mg daily, and sodium chloride 10.2 mEq/kg/day. Hydrocortisone was initially administered using a compounded 2-mg/mL suspension. Her dose was decreased at age 4 months to 1.2 mg 3 times a day (12.6 mg/m2/d) because of undetectable androgen levels. At age 5 months, the patient underwent genital reconstructive surgery, during which she was noted to be markedly hypertensive. Workup was negative and included plasma metanephrines and echocardiogram. Dimercaptosuccinic acid scan was performed because of history of vesicoureteral reflux and showed no scarring. She was discharged on captopril 15 mg and amlodipine 2.5 mg daily. Her salt was reduced, her fludrocortisone was decreased to 0.05 mg daily, and her hydrocortisone formulation was changed to tablets that were initially quartered and given 3 times daily, assuming the suspension might have produced inaccurate dosing. As her hydrocortisone doses were further reduced in the following months, parents were instructed to crush the tablets and make a solution in water, then draw the prescribed amount (Fig. 1 depicts daily hydrocortisone dosing since birth).
Figure 1.
Patient’s total daily hydrocortisone (HC) dose in milligrams and milligrams divided by meters squared (mg/m2) since birth. Starting at age 65 months, her total daily prescribed dose increased to greater than 5 mg, which necessitated her parents to dissolve 10 mg of hydrocortisone in 10 mL of water rather than 5 mg of hydrocortisone in 5 mL of water. Orange circles represent total daily hydrocortisone dose in milligrams and blue squares represent total daily hydrocortisone dose in milligrams divided by meters squared.
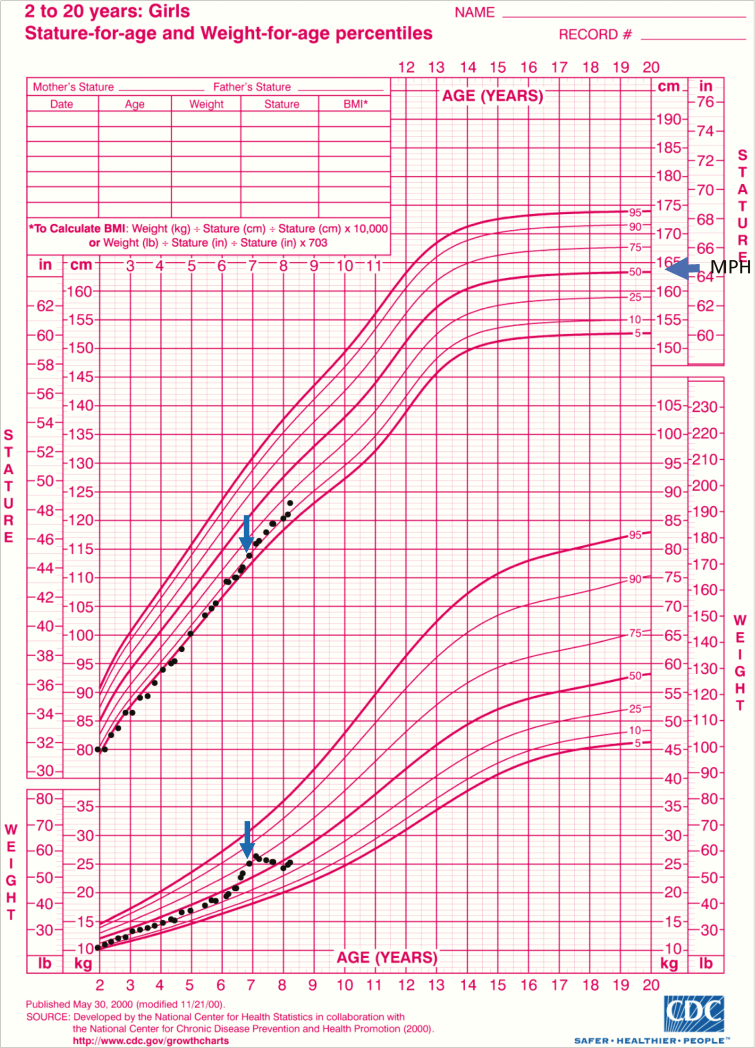
At age 12 months, the parents sought a second opinion because of concerns about poor linear growth (15th percentile) compared to her parental target height (50th percentile) (Fig. 2) and persistently undetectable adrenal androgens. At that time her hydrocortisone dose was 6.6 mg/m2/day. Advice was given to target 17-OHP levels between 400 and 1200 ng/dL, and her doses were titrated accordingly. Until age 4 years, her daily hydrocortisone doses ranged between 4.35 and5.8 mg/m2/day divided 3 times daily. At age 3.5 years, she was noted to have “mild global increased lanugo, and slightly rounded cheeks.” Cushingoid features continued to be described in subsequent visits, with increased downy hair growth on face, upper arms and back. At age 4 years, her linear growth dropped to the fourth percentile. At that time, she was taken off fludrocortisone completely and without tapering for a trial period because her treating endocrinologist questioned her diagnosis of SW-CAH based on her low daily hydrocortisone dose and unexplained hypertension. Two days later she developed fatigue, vomiting, hypotension, polyuria, and natriuresis (urine Na 125 mmol/L). Fludrocortisone was restarted with subsequent resolution of her symptoms. Her hydrocortisone was split into 4 doses a day every 6 hours starting at 6 am (5.8 mg/m2/d). Angiotensin-converting enzyme inhibitor was stopped to allow for better monitoring of her fludrocortisone dose. At age 4.4 years, the family sought a third opinion. CYP21A2 molecular testing confirmed the diagnosis and revealed compound heterozygosity with 3 CYP21A2 pathogenic variants (Intron 2G/Intron 2G-P453S), which is consistent with SW-CAH in 85% of cases [6]. The consultant endocrinologist was concerned about the accuracy of the hydrocortisone formulation because parents dissolved 5-mg tablets in 5 mL of water, either after crushing or by letting the tablet dissolve over time in warm water at room temperature. The parents would then use that solution for the rest of the day. The endocrinologist advised parents to instead cut the tablets into halves and quarters, because it was assumed to produce more accuracy, and her dose was changed accordingly (7.9 mg/m2/d). However, a month later the parents were advised by their home endocrinologist to go back to dispersing tablets in water at a dose of 6.5 mg/m2/day.
Figure 2.
Poor linear growth illustrated by patient’s height tracking along the fifth percentile despite midparental height (MPH) being at the 50th percentile. Catch-up growth is evident after the patient was switched from dispersed hydrocortisone tablets to the pharmacy-compounded alcohol-free suspension. A peak of weight gain was also observed prior to presentation. Arrows represent time of switching to pharmacy-compounded suspension.
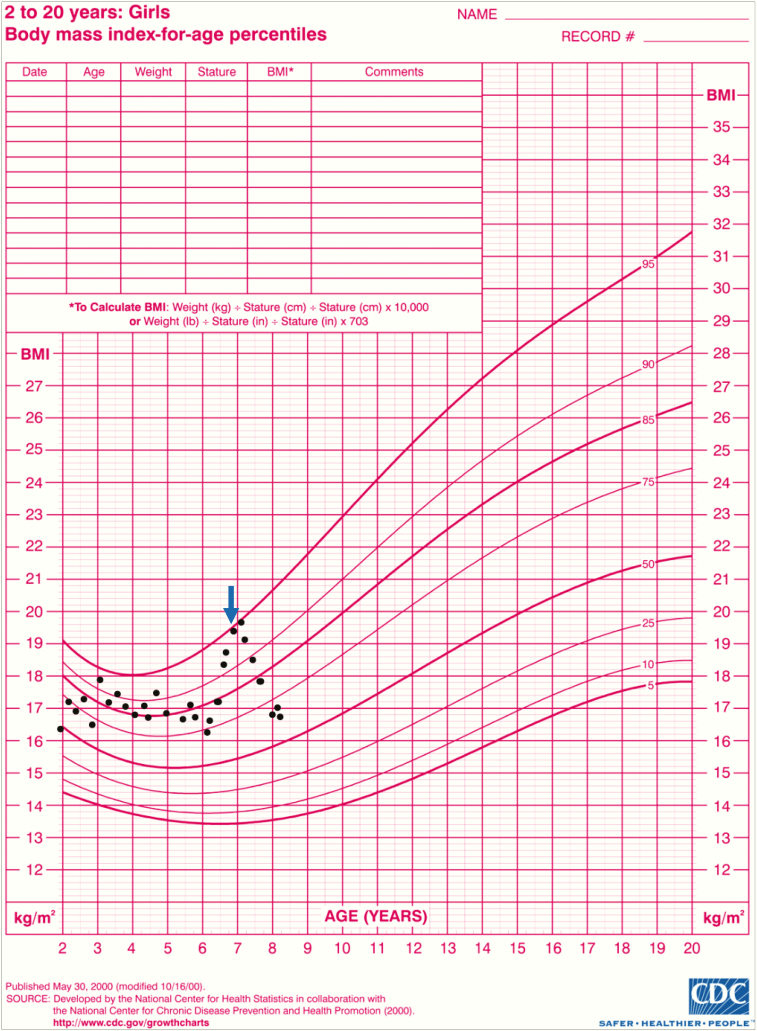
From age 5 to 6.5 years, her hydrocortisone dose gradually increased from 7.1 mg/m2/day to 12.2 mg/m2/day. Because the total daily dose exceeded 5 mg, her parents needed to make a 10-mg/10-mL solution using 2 5-mg tablets and used that solution over 24 hours. During this period, the patient continued to grow along the fifth percentile and to show increased downy hair growth, poor energy, and emotional lability. She also required higher doses of amlodipine (5 mg to 7.5 mg) to control her blood pressure. Her weight increased from 19.1 kg at 6 years (30th percentile, –0.51 SD) to 24.9 kg at 6.8 years (74th percentile, 0.7 SD), with a sharp increase in her body mass index (BMI) from 16.5 kg/m2 (77th percentile, 0.74 SD) to 19.38 kg/m2 (94th percentile, 1.61 SD) in the same time frame (Fig. 3; parental consent was obtained for all the photos presented in the figures).
Figure 3.
Rapid body mass index (BMI) increase between ages 6 and 7 years, corresponding with using 10 mg of hydrocortisone in 10 mL of water. Significant improvement noted after switching to the pharmacy-compounded suspension (arrow).
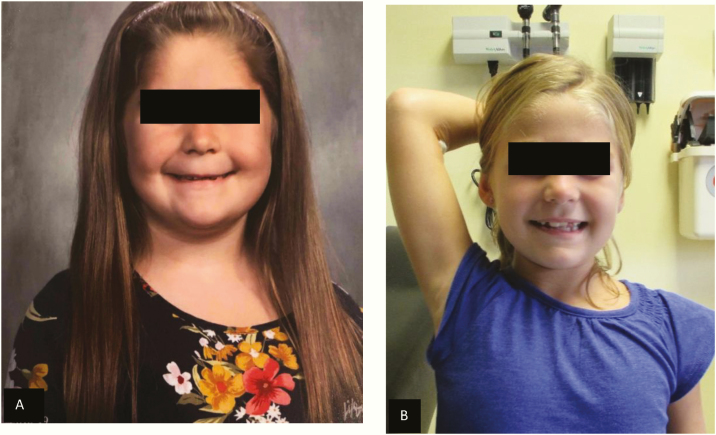
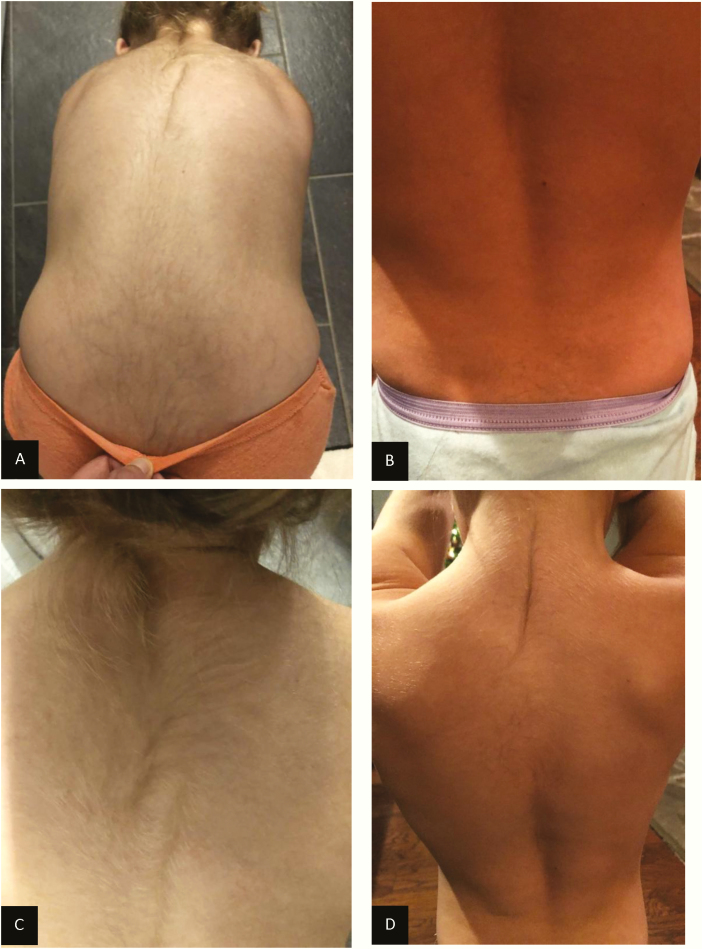
The patient presented at our CAH clinic at age 6.9 years with a weight of 25.1 kg (74th percentile and 0.7 SD), height of 113.8 (ninth percentile and –1.3 SD), and a BMI of 19.38 (94th percentile and 1.61 SD) (Figs. 2 and 3). Her physical exam was significant for round facies, a buffalo hump, and downy body hair over the upper back with a large whorl, as well as on her arms, legs, forehead, and sideburns (Figs. 4 and 5). She had no acanthosis nigricans or hyperglycemia. Her blood pressure was at the 92nd percentile systolic and 96th percentile diastolic based on the August 2017 American Academy of Pediatrics clinical practice guideline, despite treatment with amlodipine. Her CAH regimen consisted of 2 mg hydrocortisone at 6 am, 1.75 mg at noon, 1.5 mg at 6 pm, and 2 mg at midnight (8.1 mg/m2/d), and fludrocortisone 0.1 mg daily.
Figure 4.
A, Patient showing cushingoid facies at time of presentation. B, resolution of cushingoid features a year after switching to alcohol-free hydrocortisone suspension.
Figure 5.
A and C, Increased growth of dark hair on patient’s back while on the homemade hydrocortisone liquid. B and D, Diminished hair growth and pigmentation on the upper back after switching to pharmacy-compounded alcohol-free hydrocortisone suspension.
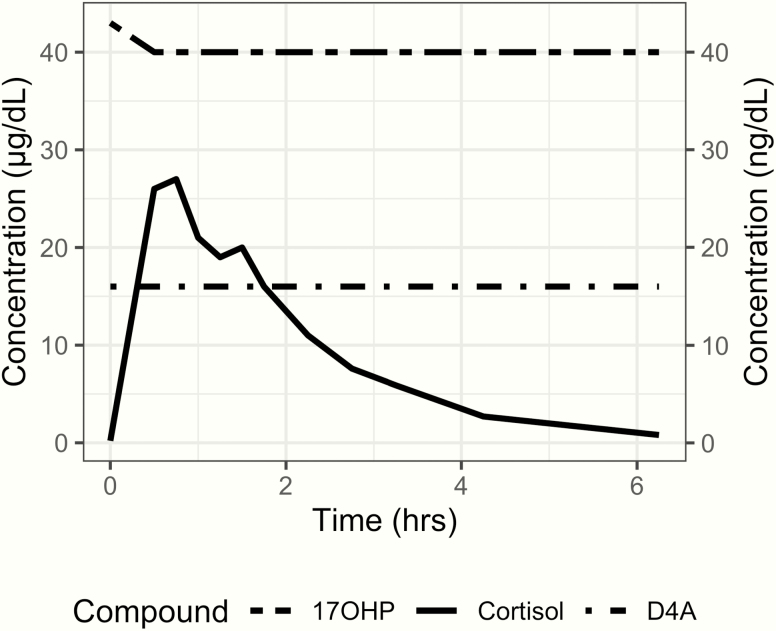
To determine her cortisol pharmacokinetic and pharmacodynamic response, the patient underwent 6-hour timed serial measurements of her cortisol, 17-OHP, and D4A after receiving her prescribed hydrocortisone dose at 8 am. Her baseline 17-OHP concentration was 43 ng/dL and her adrenocorticotropic hormone, cortisol, and D4A concentrations were undetectable, even though her last dose of hydrocortisone was given the previous evening at 8 pm (12 hours prior to her 8 am dose), suggesting chronic oversuppression of her hypothalamic-pituitary-adrenal axis. Throughout the test her 17-OHP and D4A levels remained undetectable. Her cortisol pharmacokinetic response was significant for low clearance (Fig. 6 and Table 1). Her growth factors and thyroid function tests were in the normal range.
Figure 6.
Patient’s cortisol and adrenal steroids timed measurements during a 6-hour period following her morning hydrocortisone dose. 17-hydroxyprogesterone (17 OHP) and androstenedione (D4A) were suppressed at baseline and remained undetectable throughout the test.
Table 1.
Patient’s cortisol pharmacokinetics
| Parameter | Patient value | Lower range | Patient value normalized | Upper range |
|---|---|---|---|---|
| CL | 52.3 dL/h | 46-86 dL/h/m2 | 58.8 dL/h/m2 | 136-210 dL/h/m2 |
| Vd | 78.7 dL | 77-126 dL/m2 | 88.4 dL/m2 | 187-237 dL/m2 |
| Half-life, h | 1.0 | 0.7-0.9 | 1.2-1.8 |
Abbreviations: CL, clearance; Vd, volume of distribution.
Based on our findings we changed her formulation to an extemporaneously compounded, alcohol-free hydrocortisone suspension based on a published method [7] made by our compounding pharmacy that has been found to have comparable bioavailability to tablets [8]. Her regimen was changed to 2 mg at 6 am, 1.2 mg at 8 am, 1.8 mg at 2 pm, and 1.2 mg at 9 pm (6.9 mg/m2/d). Over the next year, her daily hydrocortisone dose ranged between 6.5 and 8.2 mg/m2/day, divided 4 times a day at the same intervals, using the compounded suspension.
At her follow-up visit at age 8 years, her linear growth started catching up and was tracking along the 10th percentile (see Fig. 1). Her hypertension improved and her local nephrologist weaned the amlodipine down to 3 mg, from 7.5 mg at initial presentation to our clinic. Her cushingoid features significantly improved compared to her initial presentation (see Fig. 4). Her body hair had decreased in density and pigmentation (see Fig. 5). Her BMI decreased to 16.79 kg/m2 (68th percentile, 0.48 SD), after reaching a peak of 20.46 (96th percentile, 1.81 SD) at age 7 (see Fig. 3).
Discussion
Our patient’s clinical and biochemical presentation was consistent with iatrogenic Cushing syndrome although her total daily hydrocortisone dose (8.1 mg/m2/d) was less than the recommended range of 10 to 15 mg/m2/day [1]. Her case highlights the health risk of parents manipulating hydrocortisone tablets (splitting, crushing, and dissolving) to give prescribed doses in increments less than 2.5 mg and the need for a commercially available pediatric formulation that allows small incremental doses.
Hydrocortisone tablets have poor aqueous solubility (0.28 mg/mL at 25°C) that makes forming a stable homogeneous mixture in water very difficult. This leads to sedimentation and unequal distribution of the drug particles in different zones of the container, resulting in increased variability of the intended dose. In our case, the patient was receiving higher than intended doses that led to overtreatment. Her sharp increase in weight and BMI corresponded to her total daily dose exceeding 5 mg, thus requiring the parents to use 2 tablets (5 mg) to make a 10-mg/10-mL hydrocortisone solution that resulted in unequal distribution and higher ingested doses than intended. Dispersion of tablets into liquid followed by withdrawal of the required volume is associated with variability in dosing [9]. Watson et al found that among parents who administer 10-mg hydrocortisone tablets dispersed in water, the dose the children received was outside the ± 20% range of the target dose of 2.5 mg in more than half the cases, with some individual doses reaching beyond 250% of the desired target dose [5].
Other methods of manipulation besides dispersing tablets in water, such as splitting scored tablets, can also lead to wide variability of dosing. Verrue and colleagues found that tablet-splitting devices were superior to knives or scissors yet there were still large dose deviations [10]. Madathilethu et al reported that splitting 10-mg hydrocortisone tablets, which are quarter scored, allowing them to be divided into halves and quarters, were found to produce unacceptable dose variations [3]. A survey that targeted physicians from 16 countries in Europe [11] revealed that 60% of them used divided adult hydrocortisone tablets and 55% used unlicensed individualized capsules, with the prescribed doses reported to be as small as 0.5 mg. Another survey in the United Kingdom showed that parents are usually advised to either disperse the 10 mg tablets in water to obtain the required dose or to quarter them to obtain 2.5 mg [5].
A commercially available hydrocortisone cypionate suspension was removed from the market in 2001 following a study that showed that patients required higher daily hydrocortisone doses, had higher D4A levels, and increased weight gain and hypertension compared those taking the tablets [12]. However, the authors indicated their study results were specific to the bioavailability of hydrocortisone cypionate, but not to other forms of hydrocortisone suspension such as alcohol-free hydrocortisone suspension prepared from tablets or powder by a compounding pharmacy.
Multiple compounded liquid formulations of hydrocortisone have been studied and have been shown to be stable [13-17]. These formulations were made by adding inactive ingredients that increase the solubility of hydrocortisone such as polysorbate 80 and β-cyclodextrin [14, 18], or included a 1:1 mixture of suspending vehicles with high colloidal activity, such as ORA-plus, and ORA-Sweet, a flavoring vehicle to improve palatability [17, 19]. Alcohol-free compounded solutions of either 2 mg/mL [17] and 1 mg/mL [19] have been shown to be stable in amber plastic bottles and syringes stored at 4°C or 25°C for 90 days with excellent dose repeatability. Sarafoglou and colleagues compared the bioavailability of extemporaneously compounded alcohol-free hydrocortisone suspension to tablets and found that based on a milligram divided by meters squared dose–normalized area-under-the-curve analysis, the absorption of the alcohol-free hydrocortisone suspension was not different compared to commercial hydrocortisone tablets in children with CAH [8]. The study found that Cmax values, and Tmax values were also similar to those among patients taking tablets, strongly supporting the notion of comparable bioavailability [8]. In addition, adrenal steroid concentrations, weight gain, and growth were comparable between children on tablets and suspension. Despite all this cumulating evidence on the suitability of hydrocortisone suspensions for treatment of children with CAH, there has been no effort to commercially market an alcohol-free suspension. Currently, such formulation is available only through compounding pharmacies.
Compounding is an important part of the health care system because it can allow for flexibility in dosing and individualized therapy, which can be of great benefit on a small scale. However, compounded drugs are less strictly regulated than licensed medications by the FDA and are exempt from good practice manufacturing regulations, which can be concerning for inconsistent quality among different compounding pharmacies [20]. It is important that the prescribing physician be familiar with the reliability of their local compounding pharmacy. There have been reports of compounded hydrocortisone capsules associated with iatrogenic Cushing [21, 22]. In Neumann et al, examination of 61 batches of hydrocortisone capsules showed that 21.4% failed the uniformity analysis, with 3.6% not containing hydrocortisone at all [21], underscoring the need for the development of licensed pediatric hydrocortisone formulations that provide a safe and accurate method for hydrocortisone administration to children with CAH. Preventing cortisol underexposure or overexposure during early childhood is critical to ensure optimal growth and weight gain and to prevent adverse outcomes on cardiovascular and bone health and glucose metabolism due to insulin resistance associated with overtreatment [23-30]. A pediatric formulation in development, but not yet available in the United States, is hydrocortisone granules (0.5 mg each) (Alkindi), which has been reported to have good absorption and bioavailability [11, 31].
Acknowledgments
Financial Support: This work was supported in part by the Office of Orphan Products Development of the Food and Drug Administration (award number R01FDR0006100). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the FDA or FDA’s Office of Orphan Products Development.
Glossary
Abbreviations
- 17-OHP
17-hydroxyprogesterone
- BMI
body mass index
- CAH
congenital adrenal hyperplasia
- D4A
androstenedione
- FDA
US Food and Drug Administration
- SW
salt-wasting
Additional Information
Disclosure Summary: Dr Sarafoglou receives research support from Spruce Biosciences, Alexion, Inc, and Neurocrine Biosciences. The other authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References and Notes
- 1. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(11):4043-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarafoglou K, Zimmerman CL, Gonzalez-Bolanos MT, Willis BA, Brundage R. Interrelationships among cortisol, 17-hydroxyprogesterone, and androstenendione exposures in the management of children with congenital adrenal hyperplasia. J Investig Med. 2015;63(1):35-41. [DOI] [PubMed] [Google Scholar]
- 3. Madathilethu J, Roberts M, Peak M, Blair J, Prescott R, Ford JL. Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. BMJ Paediatr Open. 2018;2(1):e000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daniel E, Whitaker MJ, Keevil B, Wales J, Ross RJ. Accuracy of hydrocortisone dose administration via nasogastric tube. Clin Endocrinol (Oxf). 2019;90(1):66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Watson C, Webb EA, Kerr S, Davies JH, Stirling H, Batchelor H. How close is the dose? Manipulation of 10mg hydrocortisone tablets to provide appropriate doses to children. Int J Pharm. 2018;545(1-2):57-63. [DOI] [PubMed] [Google Scholar]
- 6. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21(3):245-291. [DOI] [PubMed] [Google Scholar]
- 7. Allen LV. Formulations: hydrocortisone 2 mg/mL oral liquid. Int J Pharm Compd. 2004;8(1):56. [Google Scholar]
- 8. Sarafoglou K, Gonzalez-Bolanos MT, Zimmerman CL, Boonstra T, Yaw Addo O, Brundage R. Comparison of cortisol exposures and pharmacodynamic adrenal steroid responses to hydrocortisone suspension vs. commercial tablets. J Clin Pharmacol. 2015;55(4):452-457. [DOI] [PubMed] [Google Scholar]
- 9. Abu-Geras D, Hadziomerovic D, Leau A, et al. Accuracy of tablet splitting and liquid measurements: an examination of who, what and how. J Pharm Pharmacol. 2017;69(5):603-612. [DOI] [PubMed] [Google Scholar]
- 10. Verrue C, Mehuys E, Boussery K, Remon JP, Petrovic M. Tablet-splitting: a common yet not so innocent practice. J Adv Nurs. 2011;67(1):26-32. [DOI] [PubMed] [Google Scholar]
- 11. Whitaker MJ, Spielmann S, Digweed D, et al. Development and testing in healthy adults of oral hydrocortisone granules with taste masking for the treatment of neonates and infants with adrenal insufficiency. J Clin Endocrinol Metab. 2015;100(4):1681-1688. [DOI] [PubMed] [Google Scholar]
- 12. Merke DP, Cho D, Calis KA, Keil MF, Chrousos GP. Hydrocortisone suspension and hydrocortisone tablets are not bioequivalent in the treatment of children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(1):441-445. [DOI] [PubMed] [Google Scholar]
- 13. Santovena A, Llabré M, Farina JB. Quality control and physical and chemical stability of hydrocortisone oral suspension: an interlaboratory study. Int J Pharm Compd. 2010;14(5):430-435. [PubMed] [Google Scholar]
- 14. Fawcett JP, Boulton DW, Jiang R, Woods DJ. Stability of hydrocortisone oral suspensions prepared from tablets and powder. Ann Pharmacother. 1995;29(10):987-990. [DOI] [PubMed] [Google Scholar]
- 15. Gupta VD. Chemical stability of hydrocortisone in Humco simple syrup and Ora-Sweet vehicles. Int J Pharm Compd. 2010;14(1):76-77. [PubMed] [Google Scholar]
- 16. Gupta VD. Chemical stabilities of hydrocortisone in an oral liquid dosage form without suspending agents. Int J Pharm Compd. 2007;11(3):259-261. [PubMed] [Google Scholar]
- 17. Manchanda A, Laracy M, Savji T, Bogner RH. Stability of an alcohol-free, dye-free hydrocortisone (2 mg/mL) compounded oral suspension. Int J Pharm Compd. 2018;22(1):66-75. [PubMed] [Google Scholar]
- 18. Orlu-Gul M, Fisco G, Parmar D, Gill H, Tuleu C. A new reconstitutable oral paediatric hydrocortisone solution containing hydroxypropyl-β-cyclodextrin. Drug Dev Ind Pharm. 2013;39(7):1028-1036. [DOI] [PubMed] [Google Scholar]
- 19. Chong G, Décarie D, Ensom MH.. Stability of hydrocortisone in extemporaneously compounded suspension. J Inform Pharmacother. 2003;13:100-110. [Google Scholar]
- 20. Gudeman J, Jozwiakowski M, Chollet J, Randell M. Potential risks of pharmacy compounding. Drugs R D. 2013;13(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neumann U, Burau D, Spielmann S, et al. Quality of compounded hydrocortisone capsules used in the treatment of children. Eur J Endocrinol. 2017;177(2):239-242. [DOI] [PubMed] [Google Scholar]
- 22. Barillas JE, Eichner D, Van Wagoner R, Speiser PW. Iatrogenic Cushing syndrome in a child with congenital adrenal hyperplasia: erroneous compounding of hydrocortisone. J Clin Endocrinol Metab. 2018;103(1):7-11. [DOI] [PubMed] [Google Scholar]
- 23. Bomberg EM, Addo OY, Kyllo J, et al. The relation of peripubertal and pubertal growth to final adult height in children with classic congenital adrenal hyperplasia. J Pediatr. 2015;166(3):743-750. [DOI] [PubMed] [Google Scholar]
- 24. Halper A, Hooke MC, Gonzalez-Bolanos MT, et al. Health-related quality of life in children with congenital adrenal hyperplasia. Health Qual Life Outcomes. 2017;15(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halper A, Sanchez B, Hodges JS, Dengel DR, Petryk A, Sarafoglou K. Use of an aromatase inhibitor in children with congenital adrenal hyperplasia: impact of anastrozole on bone mineral density and visceral adipose tissue. Clin Endocrinol (Oxf). 2019;91(1):124-130. [DOI] [PubMed] [Google Scholar]
- 26. Halper A, Sanchez B, Hodges JS, et al. Bone mineral density and body composition in children with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2018;88(6):813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maccabee-Ryaboy N, Thomas W, Kyllo J, et al. Hypertension in children with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2016;85(4):528-534. [DOI] [PubMed] [Google Scholar]
- 28. Sarafoglou K, Addo OY, Turcotte L, et al. Impact of hydrocortisone on adult height in congenital adrenal hyperplasia—the Minnesota Cohort. J Pediatr. 2014;164(5):1141-1146.e1. [DOI] [PubMed] [Google Scholar]
- 29. Sarafoglou K, Forlenza GP, Yaw Addo O, et al. Obesity in children with congenital adrenal hyperplasia in the Minnesota Cohort: importance of adjusting body mass index for height-age. Clin Endocrinol (Oxf). 2017;86(5):708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mooij CF, Kroese JM, Claahsen-van der Grinten HL, Tack CJ, Hermus AR. Unfavourable trends in cardiovascular and metabolic risk in paediatric and adult patients with congenital adrenal hyperplasia? Clin Endocrinol (Oxf). 2010;73(2):137-146. [DOI] [PubMed] [Google Scholar]
- 31. Neumann U, Whitaker MJ, Wiegand S, et al. Absorption and tolerability of taste-masked hydrocortisone granules in neonates, infants and children under 6 years of age with adrenal insufficiency. Clin Endocrinol (Oxf). 2018;88(1):21-29. [DOI] [PubMed] [Google Scholar]