Abstract
Background and introduction
Low and middle-income countries (LMIC) have a considerable burden of neurological disorders. Available profile of neurological disorders in our environment is biased towards neurological admissions. There is a paucity of data on out-patient neurological conditions in sub-Saharan Africa.
Objective
To determine the frequency and demographic data of neurological illnesses being managed at the adult out-patient neurology clinic of the Aga Khan Hospital, Dar es Salaam (AKHD).
Materials and methods
The electronic medical records of all cases with neurological diseases who presented to the adult neurology clinic of the AKHD between January 2018, and December 2019 were retrospectively reviewed and analyzed. Neurological disorders are categorized according to the international classification of diseases version-11(ICD-11).
Results
Of the 1186 patients seen in a period of 2 years, there were 597 (50.4%) females and 588(49.6%) males, with median age (IQR) of 38 (30.0–52.0) and 42 (33.0–54.5) years respectively (p = 0.001). Headache disorders (27.0%); disorders of the nerve root, plexus or peripheral nerves (23.4%); epilepsy (9.3%), cerebrovascular disorders (8.9%); movement disorders (3.6%) and disorders of cognition (3.5%) were the primary neurological conditions encountered. Musculoskeletal disorders (7.5%) and mental/behavioral disorders (5.4%) were other conditions seen in the clinic.
Conclusion
The pattern of neurological disorders in this cohort mirrors that of high-income countries. However, the manpower to tackle these conditions pales in comparison. Increasing the neurology workforce and paying extra attention to non-communicable disorders in SSA is advocated.
Keywords: Adult, Clinic, Disorders, Neurology out-patient, Sub-Saharan Africa, Tanzania
Highlights
-
•
Available profile of neurological disorders in our environment is biased towards neurological admissions.
-
•
We Profile neurological out-patient consultations in Aga Khan Hospital, Dar es Salaam.
-
•
Headache, peripheral nerve disorders, epilepsy and stroke were leading neurological disorders encountered.
-
•
Non-communicable neurological conditions are becoming prevalent in sub-Sahara Africa and they deserve attention.
1. Introduction
Neurological disorders account for more than 6% of the global burden of diseases. More than 1% of all deaths and 28% of all disability-adjusted life years (DALY) is due to neurological disorders [1]. Low and middle-income countries (LMIC) bear the greater proportion of this burden [2],. This trend is on the increase [3] due to several factors such as improvement in maternal and child health, increasing age of populations, and newly recognized disorders of the nervous system [1]. As a result, the global prevalence of neurological disorders is projected to hit 14.7% by 2020 [1]. Ironically, neurological manpower is far from being adequate to manage the present burden, as well as the projected upsurge. With an average of 0.04 neurologists per 100,000 populations in Africa, there is a staggering inadequacy of skilled professionals to effectively tackle the wide spectrum of neurological diseases, and provide timely and efficient preventive, restorative and rehabilitative services [4].
The burden of disease analyses is useful for informing health policy. It helps in identifying not only the fatal but also the non-fatal outcomes for diseases generally and of neurological illnesses in particular. Empirical evidence in our environment shows that the demand for neurological services is increasing. The quest for rapid, quality and evidenced based therapeutic measures in addition to increasing health literacy seems to drive this pursuit [5]. Although in-patient and community pattern of neurological diorders in some regions of Tanzania have been previously descibed [6,7], there is pacuity of data on out-patient spectrum of neurological diorders.Characterizing consultation pattern can reveal the uptake rate of available neurological services. Furthermore, it can enhance the evaluation of individualized therapy; promote appraisal of quality of care, [8] and provide data for strengthening neurology manpower, delivery and expansion of services [9].
This study aims to determine the spectrum of neurological disorders presenting to the adult neurology clinic of AKHD, Tanzania. The study also aims to determine the demographic distribution of the main subcategories of the neurological conditions.
2. Methods
This study was a 2 years retrospective survey of out-patient adult neurological consultations at the AKHD, Tanzania. Dar es Salaam is the economic capital of Tanzania and boasts of a population of 4,364,541, according to the official 2012 census [10]. It lies on latitude 6° 48′ 0″ S, and longitude 39° 17′ 0″ E. Dar es Salaam is one of the fastest-growing cities in the world and attracts a multi-ethnic group of residents. The AKHD, a joint commission international accredited (JCIA) private not for profit institution of health, is one of the oldest private health establishments in the metropolis which provides multidisciplinary secondary and tertiary care to the population. It is a 150 bedded institution that also serves as the teaching facility for the nascent faculty of postgraduate medical education of the Aga Khan University (AKU), Dar es Salaam, Tanzania. The AKHD offers a continuum of care that is coordinated across its primary medical centres (PMCs) in the urban areas of Mbeya, Iringa, Dodoma, Morogoro, and Mwanza and the 18 outreach health centers (OHCs) in Tanzania with 12 0f them being in Dar es Salaam.
The neurology unit of the hospital provides both in-patient and out-patient neurological services to residents of Dar es Salaam and other regions of Tanzania. The neurology unit is supported by a new clinical neurophysiology laboratory established in 2017. The laboratory performs electroencephalography (EEG) - including prolonged and video EEGs, evoked potential studies (EPs), electrodiagnostic (EDx) studies and sleep studies (the sleep laboratory was established in 2019). Radiological support is provided by the radiology department (Headed by AJ) that is equipped with a 1.5Tesla magnetic resonance imaging (MRI) machine, a computerized tomography (CT) scan machine as well as ultrasound machines for vascular and nerve ultrasonography.
The cardiology unit and the pathology departments provide additional supports for diagnostic evaluation of patients. Genetic screening and autoantibodies assay are outsourced. We do not perform nerve biopsies. The neurology clinic is staffed with 1 full time neurologist (PBA) who had fellowship training in clinical neurophysiology. The in-house neurologist is supported by a visiting adult neurologist (EA) and another visiting pediatric neurologist. There is a neurosurgeon (MCM) in the hospital who is also supported by another visiting surgeon. The final neurological diagnoses were arrived at, based on the patient's primary presentation, neurological exam, and subsequent blood, cerebrospinal fluid (CSF), radiological, and/or neurophysiological studies. The scientific committee as well as the health research and ethics committee of AKHD and AKU Tanzania approved the study.
2.1. Data collection
A 2 year medical records (January 1, 2018 to December 31, 2019) of all adult neurology clinic consultations at the AKHD was collected from the health information and statistics (HIS) departments of the hospital. The HIS department operates an electronic medical record therefore; it is easier to trace all new and follow up consultations. Additional records were sought from other departments such as the radiology, cardiac, pathology and neurophysiology units, if clinical consultations were insufficient. The extracted data were patient's age, gender, date of consultation, diagnosis, medications and other adjuvant therapies.
2.2. Categorization
The identified cases were classified according to the international classifications of diseases version 11 (ICD11) and cases confirmed as neurological were further analyzed. We have chosen to use ICD11 classification since the WHO website has officially approved six ICD-11 linearization [11] of which neurology is one. According to the ICD11 classification, neurological cases are classified as follows [1] movement disorders, [2] disorders with neurocognitive impairment as a major feature, [3] multiple sclerosis or other white matter disorders, [4] epilepsy or seizures, [5] headache disorders, [6] cerebrovascular diseases, (7)spinal cord disorders excluding trauma, [8] motor neuron diseases or related disorders, [9] disorders of nerve root, plexus and peripheral nerves, [10] diseases of neuromuscular junction or muscles [11], cerebral palsy, [12] nutritional or toxic disorders of the nervous system, [13] diseases of cerebrospinal fluid pressure or flow, [14] disorders of autonomic nervous system, [15] human prion diseases, [16] disorders of consciousness, [17] other disorders of the nervous system and, [18] post procedural disorders of the nervous system.
2.3. Statistical analysis
Descriptive statistics was performed to determine the frequency of neurological disorders as well as gender and age distribution. Continuous variables were summarized as means (standard deviation) when data was normally distributed. Skewed data were summarized as median (interquartile range, IQR). Pearson chi square test was used to determine between group differences of categorical data. Means and medians were compared using either the student's t-test or Mann–Whitney's U test for paired comparisons. All statistical analyses were performed using SPSS version 22.0 (SPSS, Chicago, Illinois). A P-value <0.05 was considered to be statistically significant.
3. Results
3.1. Demography and frequency of classes of neurological disorders
One thousand, one hundred and eighty-six (1186) patients were seen during the period, with an overall median age (IQR) of 40 (32.0–53.0) years. There were 597 (50.4%) females and 588 (49.6%) males with median age (IQR) of 38 (30.0–52.0) and 42 (33.0–54.5) years respectively (p = 0.001). Table 1 shows the age distribution of the patients according to gender. Females within the age of 21–30 years were significantly more than males (p = 0.004), while males between the ages of 71–80 years were significantly more than females (p = 0.003). Table 2 shows the categories of neurological disorders according to ICD 11 Classification. During the period under review, we did not see disorders of [1] autonomic nervous system, [2] Human Prion diseases, and disorders of consciousness; hence, these disorders did not reflect on Table 2. Other conditions not categorized under section 8 of ICD 11 classifications were included as disorders classified elsewhere.
Table 1.
Age and gender distribution of the patients
| Age distribution | Total |
Males |
Females |
X2 | OR | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| 1,186 (100%) | 588 (100%) | 597 (100%) | |||||
| < 20 years | 96 (8.1) | 42 (7.1) | 54 (9.0) | 1.46 | 1.29 | 0.85–1.97 | 0.227 |
| 21–30 years | 157 (13.2) | 61 (10.4) | 96 (16.1) | 8.46 | 1.66 | 1.18–2.34 | 0.004* |
| 31–40 years | 352 (29.7) | 168 (28.6) | 184 (30.8) | 0.75 | 1.12 | 0.87–1.43 | 0.386 |
| 41–50 years | 235 (19.8) | 132 (22.4) | 103 (17.3) | 4.96 | 0.72 | 0.54–0.96 | 0.026* |
| 51–60 years | 171 (14.4) | 85 (14.5) | 86 (14.4) | 0.00 | 0.99 | 0.72–1.38 | 0.990 |
| 61–70 years | 84 (7.1) | 42 (7.1) | 42 (7.0) | 0.00 | 0.99 | 0.63–1.54 | 0.949 |
| 71–80 years | 67 (5.7) | 45 (7.7) | 22 (3.7) | 8.70 | 0.46 | 0.27–0.78 | 0.003* |
| >81 years | 23 (1.9) | 13 (2.2) | 10 (1.7) | 0.74 | 0.70 | 0.31–1.59 | 0.391 |
X2 = Pearson chi square; OR = odd ratio; * = significant value.
Table 2.
Neurological Disorders according to ICD 11 Classification.
| ICD 11 classifications | Frequency |
Neurological disorders |
Male |
Female |
P value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Movement disorders | 43(3.6) | Parkinson’s disease | 5 (19.2) | 2 (11.8) | 0.259 |
| Parkinson’s plus syndrome | 3 (11.5) | 1 (5.9) | |||
| Drug induced Parkinsonism | 3 (11.5) | 0 (0.0) | |||
| Essential tremor | 1 (3.8) | 4 (23.5) | |||
| Other tremors | 1 (3.8) | 3 (17.6) | |||
| Dystonia | 8 (30.1) | 4 (23.5) | |||
| Others | 5 (19.2) | 3 (17.6) | |||
| Total | 26 (100) | 17 (100) | |||
| Disorders with neurocognitive impairment as a major feature | 41 (3.5) | Alzheimer’s disease | 3 (15.0) | 10 (47.6) | 0.018* |
| Vascular dementia | 12 (60.0) | 6 (28.6) | |||
| Dementia with lewy bodies | 3 (15) | 2 (9.5) | |||
| Normal pressure hydrocephalus | 2 (10.0) | – | |||
| Mild cognitive impairment, no dementia | 0 (0.0) | 3(14.3) | |||
| Total | 20 (100) | 21(100) | |||
| Multiple sclerosis and other white matter disorders | 4 (0.3) | Neurosarcoidosis | 1 (100) | – | - |
| Multiple sclerosis | – | 2 (66.6) | |||
| Clinically isolated syndrome | – | 1 (33.3) | |||
| Total | 1 (100) | 3 (100) | |||
| Epilepsy or seizures | 111(9.3) | Epilepsy due to structural or metabolic condition | 26 (48.1) | 24 (42.1) | 0.545 |
| Genetic or presumed genetic condition | 25 (46.3) | 27 (47.4) | |||
| Epileptic Encephalopathy | 2 (3.7) | 2 (3.5) | |||
| Seizure due to acute causes | 0 | 3 (5.3) | |||
| Single unprovoked seizures | 1 (1.9) | 1 (1.8) | |||
| Total | 54 (100) | 57 (100) | |||
| Headache disorders | 320 (27.0) | Migraine | 54 (42.9) | 120 (61.9) | <0.001* |
| Tension type headache | 18 (14.3) | 40(20.6) | |||
| Chronic daily headache | 7 (5.6) | 6(3.1) | |||
| Trigeminal autonomic celphalagias | 6 (4.8) | 4(2.1) | |||
| Cervicogenic headaches | 20 (15.9) | 19(9.8) | |||
| Others | 21 (16.7) | 5(2.6) | |||
| Total | 126 (100) | 194(100) | |||
| Cerebrovascular disease | 106 (8.9) | Ischaemic stroke | 63 (87.5) | 34(100) | 0.210 |
| Haemorrhagic stroke | 6 (8.3) | - | |||
| Subarachnoid haemorrhage | 1 (1.4) | - | |||
| Transient ischaemic attack | 2 (2.8) | - | |||
| Total | 72 (100) | 34 (100) | |||
| Spinal cord disorders excluding trauma | 4 (0.3) | Potts disease | 1 (50) | 0.306 | |
| HTLV associated myelopathy | 1(50) | – | |||
| Compressive myelopathy from prolapsed disc | 1(50) | – | |||
| Transverse myelitis | 1 (50) | ||||
| Total | 2(100) | 2 (100) | |||
| Motor neuron diseases or related disorders | 4 (0.3) | Amyotrophic lateral sclerosis | 2(100) | 1 (50) | 0.248 |
| HIV associated motor neuron disease | – | 1 (50) | |||
| Total | 2 (100) | 2 (100) | |||
| Disorders of nerve root, plexus or peripheral nerves | 278 (23.4) | Cervical radiculopathy | 41 (27.0) | 29 (23.0) | 0.401 |
| Lumbar radiculopathy | 40 (26.3) | 38 (30.2) | |||
| Cervical & lumbar polyradiculopathy | 2 (1.3) | 4 (3.2) | |||
| Brachial plexopathy | 2 (1.3) | - | |||
| Mononeuropathy | 40 (26.3) | 39 (31.0) | |||
| Polyneuropathy | 27 (17.8) | 16 (12.7) | |||
| Total | 152 (100) | 126 (100) | |||
| Disease of neuromuscular junction or muscles | 9(0.75) | Myasthenia gravis | 3 (75.0) | 5 (100) | 0.236 |
| Duchene muscular dystrophy | 1 (25.0) | – | |||
| Total | 3 (100) | 5 (100) | |||
| Cerebral palsy | 1 (0.08) | – | 1 (100) | – | |
| Nutritional or toxic disorders of the nervous system | 3 (0.25) | Alcohol withdraw syndrome | 1 (50.0) | – | 0.223 |
| Korsakoff syndrome | 1 (50.0) | – | |||
| Vitamin B 12 deficiency | – | 1(100) | |||
| Total | 3 (100) | 1(100) | |||
| Disorders of cerebrospinal fluid pressure or flow | 2(0.16) | Idiopathic intracranial hypertension | – | 1(100) | |
| Arachnoid cyst | 1(100) | – | |||
| Total | 1 (100) | 1 (100) | |||
| Post procedural disorders of the nervous system | 2 (0.16) | Post dura puncture headache | – | 2 (100) | – |
| Other conditions classified elsewhere | 258 (21.7) | 0.265 | |||
| Mental, behavioural or neurodevelopmental disorders | 58 (4.9) | Anxiety and fear related disorders | 17 (13.8) | 16 (11.8) | |
| Functional neurological disorders | 10 (8.1) | 10 (7.4) | |||
| Neurodevelopmental disorders | 3 (2.4) | 2 (1.5) | |||
| Musculoskeletal disorders | 90 (7.5) | Cervical and lumbar sprain and/or spasm | 40 (32.5) | 50 (37.0) | |
| Central nervous system (CNS) tumor | 6 (0.05) | Meningioma, schwannoma, pituitary adenoma | 4 (3.2) | 2 (1.5) | |
| Central nervous system (CNS) infections | 9 (0.7) | TB meningitis and Cryptococci meningitis | 1 (0.8) | 8 (5.9) | |
| Disorders of vestibular function | 24 (2.0) | Benign positional vertigo and Meniere’s disease | 14 (11.3) | 10 (7.4) | |
| Sleep and wake disorders | 9(0.7) | Insomnia, sleep apnea & restless leg syndrome | 7 (5.7) | 2 (1.5) | |
| Fibromyalgia | 15(1.3) | – | 6 (4.9) | 9 (6.6) | |
| Other painful conditions unspecified | 25 (2.1) | – | 11 (8.9) | 14 (10.3) | |
| General medical conditions | 22(1.8) | – | 10 (8.1) | 12 (8.9) | |
| Total | 123 (100) | 135 (100) | |||
| Total | 1186 (100) | ||||
3.2. Headache disorders
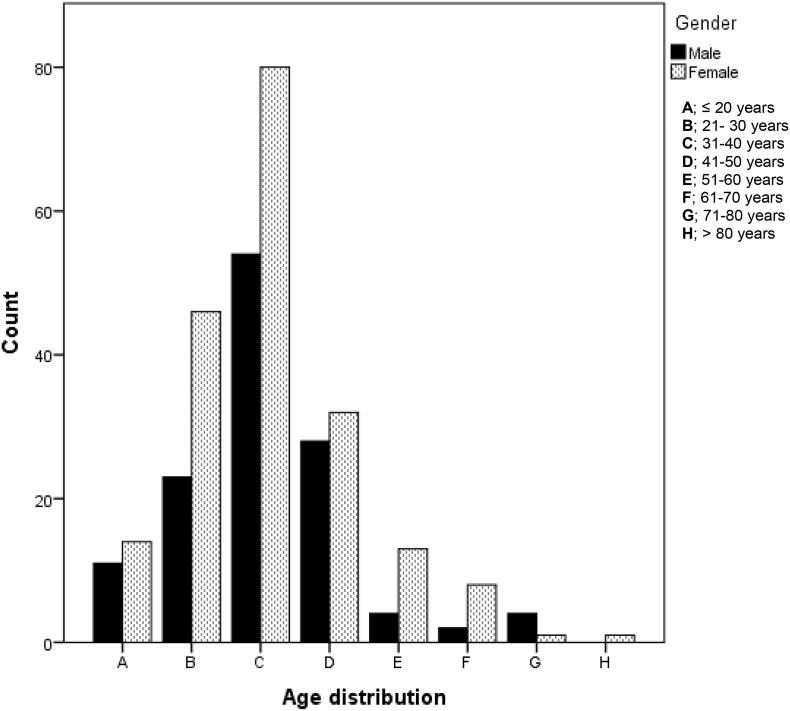
Three hundred and twenty patients (27.0%) consulted the clinic for headache disorders. The mean age ± SD was 36.27 ± 12.27. There was no difference in age between the genders (p = 0.958). Migraine (54.2%), followed by tension type headache (18.1%), were the predominant primary headaches. Thirteen (4.0%) patients had chronic daily headache while 10 patients (3.1%) had trigeminal autonomic cephalagias (cluster headache, 6 (0.5%); paroxysmal hemicranias, 3(0.25%); cluster tic syndrome = 1 (0.008%). Table 3 shows the other types of headaches. Females were the predominant sufferer of headache disorders (p ≤0.001). Fig. 1 shows age and gender distribution of headache disorders in this study. The modal age distribution for both genders was 31–40 years.
Table 3.
More detailed diagnoses not reflected on Table 2
| ICD 11 (n = frequency) | Diagnosis (n) |
|---|---|
| Movement disorders | |
| Parkinson’s Plus syndrome (n = 4) | Vascular parkinsonism (1), progressive supranuclear palsy (2),multiple system atrophy (1) |
| Dystonia (n = 12) | Hemifacial spasm (5),Meige syndrome (1),cervical dystonia (2),tardive dyskinesia (2), writer’s cramp (1),generalized dystonia (1) |
| Others (n = 8) | Hereditary ataxia (1),myoclonus(3),Tremor-dystonia (1),suspected SCA (3) |
| Disorders with neurocognitive impairment as a major feature | |
| Others (n = 3) | Mild cognitive impairment, no dementia. |
| Headache | |
| Trigeminal autonomic celphalagias | Cluster headache (6), paroxysmal hemicranias(3), cluster –tic syndrome (1) |
| Other headaches | Medication overuse headache(1),nummular headache (1),sinus headache (7), post traumatic headache (12),primary stabbing headache (1),hypnic headache (1),TMJ subluxation (1), unclassified (2) |
| Nerve root, plexus and nerves | |
| Mononeuropathy (n = 84)a | Bell’s palsy(16), trigeminal neuralgia(18), numb cheek syndrome(1), optic neuropathy (1), peripheral Horner’s syndrome (2) Intercostal neuralgia (4), carpal tunnel syndrome(15), ulnar neuropathy(16), radial neuropathy (2), femoral neuropathy (1), peroneal neuropathy(3),piriformis syndrome (1), sciatic neuropathy (1). |
| Polyneuropathy (n = 43) | Guillain Barré syndrome (4), CIDP (1), sensory polyneuropathy (30), sensory-motor polyneuropathy(3), small fiber neuropathy(5) |
5 patients had concurrent ulnar neuropathy and carpal tunnel syndrome hence the higher frequency of mononeuropathy; GBS, Guillain–Barré syndrome; CIDP, Chronic inflammatory demyelinating polyneuropathy; SCA, spinocerebellar degeneration; TMJ, temporomandibular joint subluxation.
Fig. 1.
Age and Gender distribution for Headache disorders.
3.3. Disorders of nerve root, plexus, and peripheral nerves
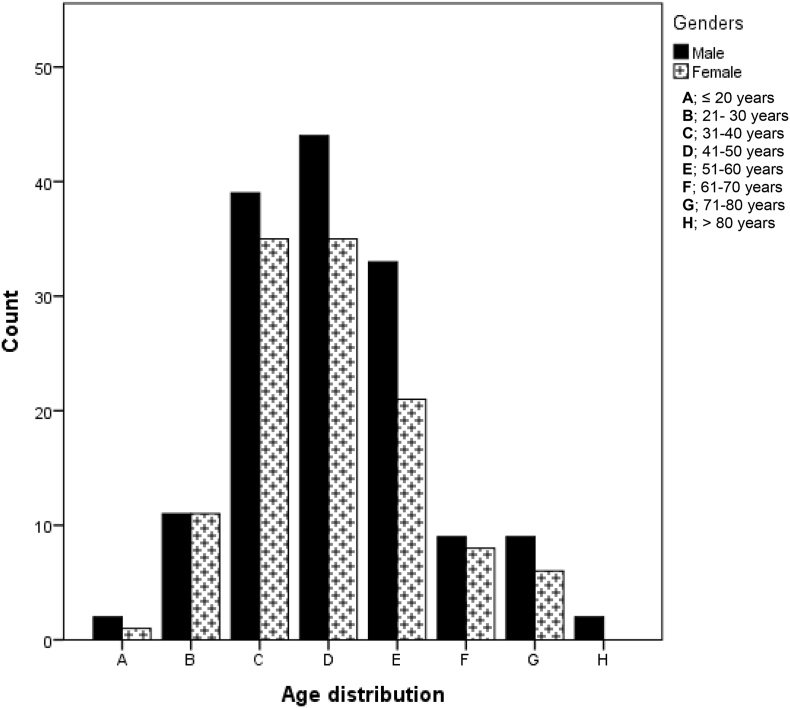
Of the 278 patients with mean ± SD of 46.15 ± 13.26 years belonging to this category, 154 (55.4%) had cervical, lumbar spondylotic radiculopathy or polyradiculopathy. Seventy-nine patients had mononeuropathy, while 43 patients had polyneuropathy, constituting 28.4% and 15.5%, respectively. There was no significant gender difference in this category (p = 0.401). Fig. 4. shows the age and gender distribution of the patients in this group. The females in this category had two modal age range; 31-40 years and 41–40 years. The modal age range for males was 41–50 years. Detailed diagnosis of the mononeuropathy and polyneuropathy subgroup is presented in Table 3.
Fig. 4.
Age and gender distribution for disorders of nerve roots, plexus and peripheral nerves.
3.4. Epilepsy and seizures
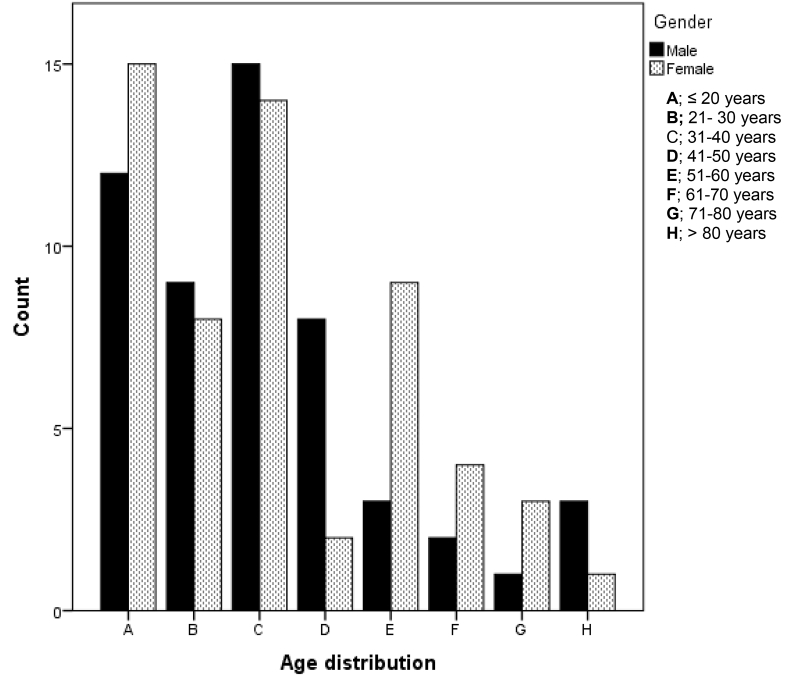
Of the 111 patients in this group, 50 (45%) had epilepsy due to structural or metabolic conditions while 52(46.8%) had genetic/ presumed genetic epilepsy. Four patients (3.6%) had epileptic encephalopathy while 3 (2.7%) patients had seizures due to acute causes. Two cases (1.8%) of unprovoked seizures were seen. The median age (IQR) of patients in this group was 33.0 (20.0–49.0). There was no gender difference between males and females (p = 0.626). Fig. 2 shows the age and gender distribution of epilepsy and seizure disorders. The modal age range of females was <20 years, while males had a modal age range of 31–40 years.
Fig. 2.
Age and gender distribution for Epilepsy and Seizures.
3.5. Cerebrovascular disease
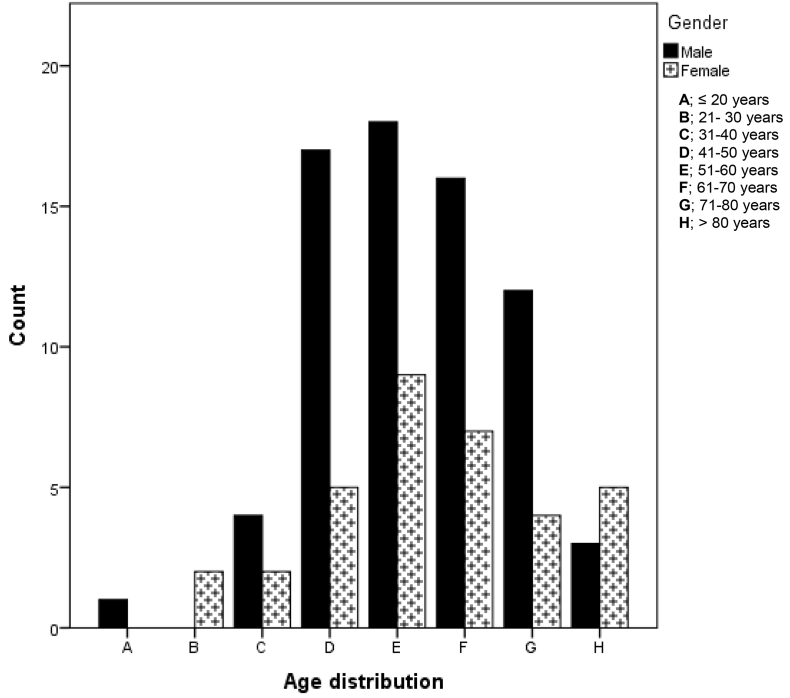
One hundred and six (8.9%) patients with median age (IQR) of 57.0 (49.5–69.0), had cerebrovascular diseases. There was no gender no age difference between the genders (p = 0.782). All the patients had brain imaging (CT and/or MRI) as well as vascular imaging (CT angiography or MR angiography) hence, adjudication of stroke types, arterial territory, and aetiology was possible for all cases. Ischaemic stroke predominated this group at 91.5%. Intracranial haemorrhage (intracerebral and subarachnoid haemorrhage) was the diagnosis in 6.6% patient. There were 2(1.9%) cases of transient ischaemic attacks (TIA). Although a preponderance of male ischaemic stroke survivors was noted, there was no significant difference between the genders (p = 0.21). Fig. 3 shows the age and gender distribution of patients with cerebrovascular disorders. The modal age range for both sexes was 51–60 years.
Fig. 3.
Age and gender distribution for cerebrovascular diseases.
3.6. Movement disorders
Forty-three cases (3.6%), with median age (IQR) of 52. 50 (34.0–65.75) had movement disorders. There was no difference in age between the genders (p = 1.0). Dystonia and Parkinson's disease were the most common conditions in this group at 27.9% and 16.3%, respectively. The median (IQR) of patients with Parkinsonian disorder was 62 (49–73), while that of the dystonia group was 40 (29–54.5). Focal and segmental dystonia made up more than 90% of the cohort with dystonia. There was no gender difference in this group (p = 0.259). Table 3 shows more detailed diagnoses of the other subcategories in this group.
3.7. Disorders with neurocognitive impairment as a major feature
Forty-one patients (3.5%) had neurocognitive disorders and vascular dementia was the most common condition in this category (43.9%). Alzheimer's disease (AD) was the next most common (31.7). The mean age (SD) of this cohort is 66.4(15.3), with an age range of 30–82. There was a significant gender difference in this category (p = 0.018); more females had Alzheimer's disease while more males had vascular dementia. There were 5 (12.2%) patients who had Dementia with lewy bodies (DLB) and 2 patients (4.9%) with normal pressure hydrocephalus. Mild cognitive impairment (MCI) was present in 3 (7.3%) patients.
3.8. Disease of neuromuscular junction or muscles
Nine patients (0.75%) belonged to this category. With 8 patients (0.67%) diagnosed with myasthenia gravis and 1(0.08%) case of Duchene muscular dystrophy. The median (IQR) of the patients with myasthenia gravis was 41.0(34.7–45.0) with a female: male ratio of 1:1. Of the myasthenia gravis cases, 2 were ocular myasthenia and 6 were generalized. The patient thought to be having Duchene muscular dystrophy declined genetic testing. However, his phenotypic features are no doubt in keeping with the disorder.
3.9. Multiple sclerosis (MS) and other white matter disorders
Four patients with mean age ± SD of 26.0 ± 8.41 belonged to this category. The male to female ratio in this category was 3:1. Two (0.17%) had MS (0.34%) and one (0.08) had a clinically isolated syndrome. These were all females. The male patient had neurosarcoidosis. This case was diagnosed from an outside facility. The patient was being followed up in our facility.
3.10. Other neurological conditions
As shown in Table 2, other less frequent disorders included spinal cord disorders excluding trauma (0.3%), motor neuron diseases or related disorders (0.3%) and nutritional or toxic disorders of the nervous system (0.25%), disorders of cerebrospinal fluid pressure or flow (0.16) and post procedural disorders of the nervous system (0.16%).There was a case of spastic cerebral palsy (0.08%).
3.11. Other conditions classified elsewhere
Aside from the core neurological disorders detailed above, other illnesses that were commonly encountered in the clinic are as summarized in Table 2. Musculoskeletal diseases were quite prevalent in this group, as it accounted for 7.6% of cases. These cases mostly consist of cervical and lumbar sprain and/or spasm. The next most common group of conditions is disorders of mental, behavioral and neurodevelopmental disorders. Among this cohort, anxiety and fear related disorders made up 2.9%, followed by dissociative neurological symptom disorders (functional neurological disorders) at 1.7%. Other painful conditions made up 2.1%, while disorders of vestibular function constituted 2.0%. Sleep and wake disorders (0.7%), CNS tumor (0.5%), and CNS infections (0.7%) were other conditions encountered in this group.
4. Discussion
This study has profiled the spectrum of out-patient neurological disorders encountered in an adult neurology clinic of a private academic hospital in Tanzania. In this cohort of 1186 patients, we noted that headache disorders; disorders of the nerve root, plexus and peripheral nerves, epilepsy and cerebrovascular diseases were the predominant illnesses for which neurological consultations were sought. These four groups of disorders accounted for close to 70% of all the cases. This pattern is not in contrast to what is obtainable in out-patient neurology clinics in high-income countries (HIC). In Europe, headache, epilepsy, functional or psychological disorders, neuromuscular and peripheral nerve disorders as well as MS and related diseases are the predominant out-patient neurological cases [12,13,14].
In Africa, Siddiqi et al. in Zambia, found that the pattern of out-patient neurological conditions was similar to what might be expected in HIC [15] and Tegueu et al. in Cameroun [16] noted the same. Extant literatures show that CNS infectious diseases, cerebrovascular disorders and epilepsy including febrile convulsions were dominant neurological diagnoses in sub-Saharan African; including previous studies from Tanzania [17,18,19,6,20]. It should be noted, however, that these studies were conducted majorly among in-patient admissions hence;, our comparison is somewhat cautious because acute neurological cases like stroke and CNS infections are more likely to be admission cases [15].. The above being said, our study almost mirrors the pattern in the community when compared to the prevalence study by Dewhurst et al. [7] among older people in northern Tanzania. Aside for the dominance of movement disorders in that survey; headache, neuropathies and stroke constituted the bulk of other neurological diseases. In contrast to our study, Sarfo et [2] in Ghana found stroke to be the principal out-patient neurological diagnosis, followed by epilepsy and movement disorders. Variation in the service organization, referral bias, and the type of health facilities (government vs. private) could be responsible for the dissimilarities, although this assumption could be termed a priori. Ours, being a private health institution, patients can request for neurological consultations without a referral from a primary care physician or other medical specialties.
Headache is the most frequent neurological diagnosis in this cohort. This finding is similar to patterns in other studies in HIC [12,13,14] and SSA [16].. The high proportion of headache disorders in our group may not be unrelated to the high prevalence of the major primary headache disorders in Tanzania [21,22,23,24]. Migraine, Tension-type headache (TTH) and medication overuse headache (MOH) are the 3 headache disorders of public health importance but migraine is the most disabling. Migraine is the second cause of disability globally [25]. It is the first cause of disability in people below 50 years [26]. Migraine affects one in ten people worldwide indicating a recent rise [27]. and higher prevalence is found among females, students, and urban residents just like our study suggests [28]. Although efforts at lifting the burden of headache are being instituted and recognized on the global level [ 29], similar efforts are not visible in LMIC, especially Africa. Advocacy work to mitigate the burden of headache disorders is hugely needed and it is recommended that these endeavors should be championed by patients and practitioners [29].
The global burden of stroke has been well described [30]. Within the combined burden of neurological disorders, cerebrovascular diseases as measured in the GBD 2010, contribute an enormous proportions of DALY [3] Among in-patients in sub-Saharan Africa, stroke is the leading cause of neurological admission [6,31,32]. However, in this out-patient cohort, it is the fourth most common condition; in agreement with other studies [15,16] This pattern would not be said to reflect the prevalence of stroke in the community as Dar es Salaam has been known to have a high incidence of stroke [33]. The rising burden of stroke in Tanzania is well documented [34], our institution is stroke ready and has an approved stroke fast pathway for triaging acute stroke cases in the emergency room. Patients who qualified for intravenous thrombolysis are offered the service. Mechanical thrombectomy program is yet to begin. Cases of cerebral haemorrhage requiring surgical intervention are managed, discharged and followed up by the neurosurgical team. This could account for the low frequency of haemorrhagic stroke in our cohort. Although Sarfo et al. reported a higher proportion stroke in their out-patient facility, the modal age range of cerebrovascular disease in our study mirrors that in Kumasi [2]. The demographic, nutritional and epidemiological transition that is happening across LMIC is undoubtedly driving the incidence of cardiovascular risk such as metabolic syndrome, hypertension and diabetes. Staunching this trend with proven preventive measures is indeed obligatory.
Over 85% of people living with epilepsy are found in low- and middle-income countries, including sub-Saharan Africa [35]. Epilepsy accounted for 9.3% of all the cases seen during this period. This proportion is similar to the studies [2,15,16]. The aetiology of epilepsy in Africa has been well described [36]. In this cohort of patients with epilepsy secondary to structural or metabolic brain disorders, we found that stroke, CNS trauma, mesial temporal sclerosis, and atrophy/neurodegenerative disorders were responsible for most cases in the category. Aside infections, non –communicable diseases play a corresponding and significant role in the onset of epilepsy in SSA. This underscores the need to increase public awareness of preventive measures against conditions such as traumatic brain injury, stroke and dementia. Stoke advocacy and public education would need to be escalated as well. Stroke survivors are significantly at a high risk of seizures [37] hence prevention is key. In the same manner, perinatal issues need to be well communicated and emphasized to the public.
Parkinsonian disorders and dystonia are the leading movement disorders in this cohort. As already established, the Parkinsonian disorders are common in the elderly. We saw two patients with early-onset Parkinson's disease, but further exploration of genetic underpinning could not be performed. The dystonia group was younger, though focal dystonia; Hemifacial spasm in particular was the dominant type in this cohort. In larger neurology practice, cervical dystonia is the most frequent focal dystonia [38]. The effort by the MDS to improve the diagnosis and management, including training and education of healthcare workers, is yielding fruits in Africa [39]. The frequency of disorders of cognition was comparable to that of movement disorders in our study and Alzheimer's disease trailed vascular dementia in rate. The dominance of the female gender with AD in this cohort reflects the already established pattern. About two-thirds of patients with AD are women [40]. Sex and gender differences in risk factors, symptom presentation, mortality and treatment are significant contributors to this pattern. A focus on these differences will lead to improved care and treatment [41].
Disorders of the peripheral nerves form a substantial proportion of patients in our study, and it is similar to the survey by stone et al. [12] We have gone ahead to detail the spectrum of these disorders (Table 3) and narrow down the diagnosis with electrophysiological data when available. The lead author's subspecialty interest is in the area of peripheral nerve disorders, and this bias could have influenced referrals. Vestibular disorders and mental health disorders, in particular, functional neurological disorders, are encountered frequently by neurologists. Our data supports this. Recent insights from brain imaging and neurophysiology studies are helping to differentiate functional and feigned symptoms. Although these patients would benefit from both psychiatrist and neurologist review, the pendulum is again swinging in the direction of neurologists as the physician to take responsibility for functional neurological disorders [42].
4.1. Study limitations
Its retrospective observational single center design limits our study. Undoubtedly, the findings would not represent the pattern of neurological disorders in the general population. Furthermore, this study population is highly selective, consisting of middle and high-income earners who have good insurance coverage or can pay cash for their healthcare costs. The strength of our study lies however, in the ability to thoroughly investigate our patients aside from other clinical evaluations as neurology demands. The electronic record system also ensured complete data capturing. Certainly, diagnostic confidence and accuracy is high in this cohort.
5. Conclusion
In this cohort of urban dwellers who accessed care in a private academic hospital, we noted that non-communicable diseases of headache, peripheral nervous disorders, epilepsy and stroke were the leading cause of neurological consultation. Efforts at mitigating the burden of these illnesses at the population level might stem the raging tide of non-communicable neurological disorders in our environment. Future expansion of neurology services should explore establishment of specialty clinics devout to specific conditions such as headache, neuromuscular, epilepsy and stroke.
Funding
None.
Authors' contributions
PBA conceived the idea of the study and its design. OMA, REM, MCM (1), SMA, and NM collected and curated the data. PBA drafted the manuscript. AJ, MCM (2), and EA reviewed edited the manuscript for intellectual content. All authors approved the final manuscript.
Declaration of Competing Interest
The authors declare no conflicts of interest regarding this work.
Acknowledgments
None.
Contributor Information
Philip B. Adebayo, Email: philip.adebayo@akhst.org.
Mandela C. Makakala, Email: mandela.makakala@akhst.org.
Ahmed M. Jusabani, Email: ahmed.jusabani@akhst.org.
References
- 1.Menken M., Munsat T.L., Toole J.F. The global burden of disease study: implications for neurology. Arch. Neurol. 2000;57(3):418–420. doi: 10.1001/archneur.57.3.418. http://www.ncbi.nlm.nih.gov/pubmed/10714674 Accessed October 23, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Sarfo F.S., Akassi J., Badu E., Okorozo A., Ovbiagele B., Akpalu A. Profile of neurological disorders in an adult neurology clinic in Kumasi. Ghana eNeurological. Sci. 2016;3:69–74. doi: 10.1016/j.ensci.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy K.S. Global Burden of Disease Study 2015 provides GPS for global health 2030. Lancet (London, England) 2016;388(10053):1448–1449. doi: 10.1016/S0140-6736(16)31743-3. [DOI] [PubMed] [Google Scholar]
- 4.Akinyemi R.O., Owolabi M.O., Adebayo P.B. Task-shifting training improves stroke knowledge among Nigerian non-neurologist health workers. J. Neurol. Sci. 2015;359(1–2):112–116. doi: 10.1016/j.jns.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philip A.B., Akolade I., Ahmed W., Abayomi O.S. Pattern and influence of in-patient neurological consultations in a Nigerian tertiary health institution: a brief survey. ISSN. 2012;1(7):2277–2879. http://www.onlineresearchjournals.org/JMMSR Accessed November 5, 2016. [Google Scholar]
- 6.Laizer S., Kilonzo K., Urasa S., Maro V., Walker R., Howlett W. Neurological disorders in a consultant hospital in northern Tanzania. A cohort study. eNeurologicalSci. 2019;14:101–105. doi: 10.1016/j.ensci.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewhurst F., Dewhurst M.J., Gray W.K. The prevalence of neurological disorders in older people in Tanzania. Acta Neurol. Scand. 2013;127(3):198–207. doi: 10.1111/j.1600-0404.2012.01709.x. [DOI] [PubMed] [Google Scholar]
- 8.Akachi Y., Kruk M.E. Quality of care: measuring a neglected driver of improved health. Bull. World Health Organ. 2017 doi: 10.2471/BLT.16.180190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renggli S., Mayumana I., Mboya D. Towards improved health service quality in Tanzania: appropriateness of an electronic tool to assess quality of primary healthcare 11 medical and health sciences 1117 public health and health services. BMC Health Serv. Res. 2019;19(1):55. doi: 10.1186/s12913-019-3908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United Nations Human Settlements Programme. UNHS, United Nations . UN-HABITAT. 2008. Economic Commission for Africa. The state of African cities 2008 : a framework for addressing urban challenges in Africa; p. 207. Accessed August 30, 2019. [Google Scholar]
- 11.Downloads. 2020. https://icd.who.int/dev11/downloads/ Accessed May 15, 2020. [Google Scholar]
- 12.Stone J., Carson A., Duncan R. Who is referred to neurology clinics? - the diagnoses made in 3781 new patients. Clin. Neurol. Neurosurg. 2010;112(9):747–751. doi: 10.1016/j.clineuro.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Matías-Guiu J.A., García-Azorín D., García-Ramos R., Basoco E., Elvira C., Matías-Guiu J. Estudio de la asistencia neurológica ambulatoria en la Comunidad de Madrid: Impacto del modelo de libre elección de hospital. Neurologia. 2015;30(8):479–487. doi: 10.1016/j.nrl.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins A., Menken M., DeFriese G. A record of patient encounters in neurological practice in the United Kingdom. J. Neurol. Neurosurg. Psychiatry. 1989;52(4):436–438. doi: 10.1136/jnnp.52.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqi O.K., Atadzhanov M., Birbeck G.L., Koralnik I.J. The spectrum of neurological disorders in a Zambian tertiary care hospital. J. Neurol. Sci. 2010;290(1–2):1–5. doi: 10.1016/j.jns.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tegueu C.K., Nguefack S., Doumbe J., Fogang Y.F., Mbonda P.C., Mbonda E. The spectrum of neurological disorders presenting at a neurology clinic in Yaoundé, Cameroon. Pan. Afr. Med. J. 2013;14 doi: 10.11604/pamj.2013.14.148.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osuntokun B.O. The pattern of neurological illness in tropical Africa. Experience at Ibadan, Nigeria. J. Neurol. Sci. 1971;12(4):417–442. doi: 10.1016/0022-510X(71)90110-9. [DOI] [PubMed] [Google Scholar]
- 18.Kwasa TO The pattern of neurological disease at Kenyatta National Hospital. East Afr. Med. J. 1992;69(5):236–239. [PubMed] [Google Scholar]
- 19.Winkler A., Stelzhammer B., Kerschbaumsteiner K. The prevalence of headache with emphasis on tension-type headache in rural Tanzania: a community-based study. Cephalalgia. 2009;29(12):1317–1325. doi: 10.1111/j.1468-2982.2009.01885.x. [DOI] [PubMed] [Google Scholar]
- 20.Birbeck G.L., Meyer A.-C., Ogunniyi A. Nervous system disorders across the life course in resource-limited settings. Nature. 2015;527(7578):S167–S171. doi: 10.1038/nature16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dent W., Spiss H., Helbok R., Matuja W., Scheunemann S., Schmutzhard E. Prevalence of migraine in a rural area in South Tanzania: a door-to-door survey. Cephalalgia. 2004;24(11):960–966. doi: 10.1111/j.1468-2982.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 22.Matuja W.B. Headache: pattern and features as experienced in a neurology clinic in Tanzania. East Afr. Med. J. 1991;68(12):935–943. http://www.ncbi.nlm.nih.gov/pubmed/1800090 Accessed May 7, 2017. [PubMed] [Google Scholar]
- 23.Matuja W.B., Mteza I.B., Rwiza H.T. Headache in a nonclinical population in Dar es Salaam, Tanzania. A community-based study. Headache. 1995;35(5):273–276. doi: 10.1111/j.1526-4610.1995.hed3505273.x. http://www.ncbi.nlm.nih.gov/pubmed/7775191 Accessed May 6, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Winkler A.S., Dent W., Stelzhammer B. Prevalence of migraine headache in a rural area of northern Tanzania: a community-based door-to-door survey. Cephalalgia. 2010;30(5):582–592. doi: 10.1111/j.1468-2982.2009.01994.x. [DOI] [PubMed] [Google Scholar]
- 25.Vos T., Allen C., Arora M. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steiner T.J., Stovner L.J., Vos T., Jensen R., Katsarava Z. Migraine is first cause of disability in under 50s: will health politicians now take notice? J. Headache Pain. 2018;19(1) doi: 10.1186/s10194-018-0846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woldeamanuel Y.W., Cowan R.P. Migraine affects 1 in 10 people worldwide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J. Neurol. Sci. 2017;372:307–315. doi: 10.1016/j.jns.2016.11.071. [DOI] [PubMed] [Google Scholar]
- 28.Woldeamanuel Y.W., Andreou A.P., Cowan R.P. Prevalence of migraine headache and its weight on neurological burden in Africa: a 43-year systematic review and meta-analysis of community-based studies. J. Neurol. Sci. 2014;342(1–2):1–15. doi: 10.1016/j.jns.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Steiner T.J. Lifting the burden: the global campaign to reduce the burden of headache worldwide. J. Headache Pain. 2005;6(5):373–377. doi: 10.1007/s10194-005-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thrift A.G., Thayabaranathan T., Howard G. Global stroke statistics. Int. J. Stroke. October 2016 doi: 10.1177/1747493016676285. [DOI] [PubMed] [Google Scholar]
- 31.Owolabi L.F., Shehu M.Y., Shehu M.N., Fadare J. Pattern of neurological admissions in the tropics: experience at Kano, Northwestern Nigeria. Ann. Indian Acad. Neurol. 2010;13(3):167–170. doi: 10.4103/0972-2327.70875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lester F.T. Neurological diseases in Addis Ababa, Ethiopia. Afr. J. Med. Med. Sci. 1979;8(1–2):7–11. [PubMed] [Google Scholar]
- 33.Walker R., Whiting D., Unwin N. Stroke incidence in rural and urban Tanzania: a prospective, community-based study. Lancet Neurol. 2010;9(8):786–792. doi: 10.1016/S1474-4422(10)70144-7. [DOI] [PubMed] [Google Scholar]
- 34.Walker R.W., Viney R., Green L. Trends in stroke admissions to a Tanzanian hospital over four decades: a retrospective audit. Tropical Med. Int. Health. 2015;20(10):1290–1296. doi: 10.1111/tmi.12547. [DOI] [PubMed] [Google Scholar]
- 35.Ngugi A.K., Bottomley C., Kleinschmidt I., Sander J.W., Newton C.R. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51(5):883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ba-Diop A., Marin B., Druet-Cabanac M., Ngoungou E.B., Newton C.R., Preux P.M. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014;13(10):1029–1044. doi: 10.1016/S1474-4422(14)70114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myint P.K., Staufenberg E.F.A., Sabanathan K. Post-stroke seizure and post-stroke epilepsy. Postgrad. Med. J. 2006;82(971):568–572. doi: 10.1136/pgmj.2005.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stacy M. Epidemiology, clinical presentation, and diagnosis of cervical dystonia. Neurol. Clin. 2008;26(SUPPL.1):23–42. doi: 10.1016/S0733-8619(08)80003-5. [DOI] [PubMed] [Google Scholar]
- 39.African Section. 2020. https://www.movementdisorders.org/MDS-Africa Accessed May 19, 2020. [Google Scholar]
- 40.2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 2017;13(4):325–373. doi: 10.1016/j.jalz.2017.02.001. [DOI] [Google Scholar]
- 41.Mielke M.M. Sex and gender differences in Alzheimer’s disease dementia. Psychiatr. Times. 2018;35(11):14. [PMC free article] [PubMed] [Google Scholar]
- 42.Stone J., Hallett M., Carson A., Bergen D., Shakir R. Functional disorders in the neurology section of ICD-11: a landmark opportunity. Neurology. 2014;83(24):2299–2301. doi: 10.1212/WNL.0000000000001063. [DOI] [PMC free article] [PubMed] [Google Scholar]