Abstract
Background
Liver diseases contribute a prominent global burden of mortality and morbidity. The current therapies of liver diseases have numerous limitations including severe adverse effects. This denotes that new more effective, safer, and cheaper drugs are required and medicinal plants used in traditional medicines often offer ideal opportunities. Accordingly, the present study aimed to evaluate the in vivo hepatoprotective and in vitro radical scavenging activities of dried rhizome extracts of Rumex abyssinicus (R. abyssinicus), which is traditionally claimed to provide hepatoprotection.
Materials and Methods
Hepatoprotective activity of extracts was evaluated using carbon tetrachloride (CCl4)-induced liver injury in mice. Pre- and post-treatment models were employed to test the effect of the extracts and silymarin (standard drug). Serum biochemical markers and liver histopathology were used as parameters to evaluate hepatoprotective activities whereas in vitro radical scavenging activity was tested by 2, 2-diphenyl-2-picrylhydrazyl hydrate (DPPH) assay.
Results and Conclusion
Oral administration of CCl4 (1 ml/kg) significantly (P<0.001) raised the serum levels of liver enzyme markers compared to the normal control group. Pre-treatment with 125, 250, and 500 mg/kg of R. abyssinicus extract reduced the serum level of CCl4-induced rise in liver enzyme markers with the highest reduction observed at a dose of 500 mg/kg. Likewise, in the post-treatment model, the crude extract and butanol fraction at dose 500 mg/kg reduced levels of liver enzymes. Histopathological examinations revealed lesser liver damage of extract-treated mice compared to the toxic (CCl4-treated) controls. The in vitro radical scavenging activity of the different extracts showed concentration-dependent radical scavenging activity. Thus, the results of this study may justify the traditional use of the plant as a hepatoprotective agent.
Conclusion
Results of serum biochemical markers and histopathological examinations of CCl4-induced mice models, in the present study, show the hepatoprotective potential of extracts from the rhizome of R. abyssinicus.
Keywords: carbon tetrachloride, hepatoprotective, liver disease, Rumex abyssinicus
Introduction
Liver disease is a cluster term for an array of problems that influences the tissues, structures, and cells of the human liver.1,2 Generally, liver diseases contribute a prominent global burden of mortality and morbidity.3 For instance, nearly 2000 cases of acute liver failure occur yearly in the United States.4 Hospital-based analyses denoted that acute viral hepatitis, chronic hepatitis, hepatocellular carcinoma, and cirrhosis were amenable for at least 12% of medical admissions and over 20% of hospital mortality in numerous parts of Africa.5 Likewise, a clinical study in Ethiopia shows that liver disease accounted for 12% of hospital admissions and 31% of hospital mortality.6
One important factor responsible for the development of liver disorder is oxidative stress, often resulting from exposure of the liver to free radicals derived from some xenobiotics and drugs. The most common oxidative liver damage occurs via lipid peroxidation.7,8 Similarly, CCl4 causes liver toxicity via free radical-associated damage to liver cells and simulates most human hepatopathologies which makes it a suitable model to assess the efficacy of liver-protective agents.9 CCl4 is metabolized in the liver resulting in the formation of free radicals like trichloromethyl and trichloromethyl peroxyl radicals, which react with macromolecules such as proteins, lipids, and DNA.10 Although hepatocytes are protected from free radical-mediated attack by an intrinsic enzymatic and non-enzymatic antioxidant defense system, this antioxidant defense is compromised in certain settings, resulting in oxidative stress.11–13 In this regard, understanding the impact of oxidative stress and capacity of antioxidants to minimize liver damage may serve an important role in developing more effective hepatoprotective natural products, especially considering that nearly 50% of drugs used in liver diseases are either natural products or their derivatives.14
In the present situation, there is an enhanced tendency to discover antioxidants from natural sources as most of the currently available drugs for the treatment of hepatic illnesses are inadequate and have been associated with severe adverse reactions.14–19 Despite that there have been lots of plant-based medicines used to treat liver ailments,19 such medicines were not scientifically characterized even in pre-clinical studies using animal models. Rumex abyssinicus Jacq. (Polygonaceae), commonly known as “Spinach Rhubarb”, is a medicinal and food additive plant that is widely distributed throughout North Africa, Ethiopia, and in the highlands of tropical Africa.20,21 The tender shoots and leaves are edible and rhizomes are used to refine butter that give it a rich yellow color, while its roots are utilized for therapy.20 As a traditional medicinal plant, R. abyssinicus roots are claimed to treat various diseases including hypertension, itching skin, vitiligo,22 toothaches, cancer,23 malaria, constipation, neuralgia, rheumatism, migraine, ear problems, rabies, scabies, wound, stomachache, typhus, diabetes, and hepatitis.24,25 Besides, previous studies show that this plant demonstrated different pharmacological activities including anthelmintic,26 anticancer,27 wound healing,28 antibacterial,29 diuretic, and analgesia.20 Taking the above viewpoints into account, the current study was intended to investigate antioxidant and hepatoprotective potential of R. abyssinicus root extracts.
Materials and Methods
Chemicals
2, 2-Diphenyl-2-picrylhydrazyl hydrate (DPPH), formalin, xylene, Carbon tetrachloride (CCl4) (Sigma-Aldrich, Germany), ascorbic acid (S.D. Fine Chemical Limited, India), silymarin (Zhejiang Chemicals Hangzhou, China), methanol (Amaira Petro Chem Pvt. Ltd, France), chloroform (ACS, ISO, Merck), ethyl acetate (Loba Chemie Pvt. Ltd., India), n-hexane (Qualikems Fine Chem Pvt. Ltd, India), and n-butanol (Faiz Chemical Pvt. Ltd, France). All the chemicals and reagents used were of analytical grade.
Plant Materials
Rhizomes of Rumex abyssinicus were collected from a locality near Gondar city (12.6030° N, 37.4521° E), northern Ethiopia. The plant was authenticated by Mr. Abiyu Enyew (a botanist) at the Herbarium, Biology Department, Faculty of Natural and Computational Science, University of Gondar where a voucher specimen (No. BA001) was deposited.
Experimental Animals
Healthy female Swiss albino mice (weighing 20–30 g and ages 8 to 12 weeks) were obtained from the animal house of Department of Pharmacology, School of Pharmacy, Mekelle University. Animals were housed in polypropylene cage (6–10 animals per cage), sustained under standard condition (12 h light and 12 h dark cycle; 24°C ± 1°C) and permitted to freely access of standard pellet diet and water ad libitum. After randomize grouping and before initiation of the experiment, animals were acclimatized for 5 days to the laboratory conditions. All procedures used during the study complied with the Guide for the Care and Use of Laboratory Animals30 and ethical approval to conduct the study was obtained from the Health Research Ethics Review Committee of College of Health Sciences, Mekelle University with ERC number 1024/2017.
Preparation of Crude Extract and Fractions
Dried powder of R. abyssinicus rhizome (600 g) was macerated in 80% methanol for 3 days with manual agitation and occasional shaking using a rotary shaker (BIBBY Stuart Rotary Shaker S01 UK). At the end of 3 days maceration, the filtrate was separated from the marc (residue), and the marc was re-macerated twice with the same volume of 80% methanol, again each time for 3 days. Extract filtration was made using Whatman No.1 filter paper, and the solvent was evaporated in an oven at 40°C. To prepare solvent fractions, 20 g of the dried crude extract was dissolved in 200 ml distilled water and partitioned into different solvent of increasing polarity as per the procedure described by Otsuka (2006):31 n-hexane (200 ml x 3), chloroform (200 ml x 3), ethyl acetate (200 ml x 3), n-butanol (200 ml x 3) fractions were prepared by successive solvent-solvent extraction in separating funnel. The filtrates obtained were distilled and concentrated under reduced pressure at a temperature between 40°C and 45°C in a rotary evaporator and dried in an oven (Genlab). All the crude extract and fractions were stored in a refrigerator until used in further studies.
Acute Oral Toxicity Study
Acute oral toxicity was determined according to the Organization of Economic Co-operation Development (OECD)-425 guidelines.32 Adult nulliparous and non-pregnant female albino mice were starved for 4 h but with free access to water ad libitum and were acclimatized for 5 days before the study. A total of five animals were employed for this test and received 2000 mg/kg dose of crude extract dissolved in distilled water orally using oral gavages. One animal was dosed in the first day and observed continuously for determination of any sign of toxicity during the first 24 h. Then, the other four animals were dosed similarly since no sign of toxicity was manifested by the first mice. The mice were observed individually for gross behavioral changes at least once during the first 30 min after dosing, periodically with special attention given during the first 4 h of the 24 h, and daily thereafter, for a total of 14 days.
DPPH Radial Scavenging Activity Assay
The free radical scavenging potential of R. abyssinicus crude extract, solvent fractions, and ascorbic acid used as a positive standard control were determined using 2, 2-diphenyl-2-picrylhydrazyl hydrate (DPPH) assay following the procedure described by Braca et al (2001):33 Briefly, 3 ml of 0.004% methanolic solution of DPPH was mixed with 30 µL of various concentrations (1000, 500, 250, 125, and 62.5 µg/ml) of each test sample (methanol solutions) in different test tubes. After 30-min incubation in the dark at room temperature, absorbance values were measured at 517 nm using a spectrophotometer (JENWEY6405). Each sample was measured in triplicate and the readings averaged.
Hepatoprotective Activity Models
Pre-Treatment Model
This study was done as per the procedure described by Bhat et al (2014)34 with some modifications in the technique of blood collection and experimental animals used. Female Swiss albino mice were used for this study and randomly allocated into six experimental groups, each group consisting of six mice. Group I served as a normal control group and received distilled water (10ml/kg, p.o.); group II served as toxic control (CCl4) and received distilled water (10 ml/kg, p.o.) while group III received silymarin (100 mg/kg/day, p.o.) as a standard control group; group IV–VI served as extract-treated groups and received three different doses of extract of R. abyssinicus (125, 250, and 500 mg/kg/day, p.o.). All treatments were given daily for 7 days. On the 7th day of treatments, animals in groups II–VI were fasted overnight and administered with a single oral dose of 1 ml/kg of a freshly prepared CCl4 in olive oil (1:1 v/v). After 24 h of CCl4 administration, all the mice were anesthetized by halothane and blood was collected from each mouse from the jugular vein. Serum was separated by centrifugation at 3000 rpm for 5 min and aspirated into test tubes for biochemical analysis.34 Subsequent to blood collection, each mouse was sacrificed by using the inhaled anesthetic halothane and liver slices were taken for histopathological examination.
Post-Treatment Model
In this model, the hepatoprotective activity of R. abyssinicus crude extract and the butanol fraction was investigated by using the method described by Wills and Asha (2006)35 with certain modifications in the dose of CCl4 and silymarin. Animals were grouped randomly into five groups of six mice. The first group (group I) served as a normal control group and received 1 ml/kg of olive oil on day 1, and distilled water on wards in the rest of the treatment days. Mice in group II (CCl4 or toxic control group) were administered with a single dose of CCl4 (1 ml/kg of 1:1 mixture in olive oil) on day 1, and distilled water on wards in the rest of the treatment days. Groups III–V received an oral single dose of CCl4 (1 ml/kg of 1:1 a mixture in olive oil) on the first day and received R. abyssinicus crude extract (500 mg/kg), butanol fraction (500 mg/kg), and silymarin (100 mg/kg), respectively, at 2, 24, and 48 h after CCl4 administration. All the animals were sacrificed 72 h after CCl4 administration. Blood was collected from the jugular vein and serum was separated by centrifugation at 3000 rpm for 5 min.36 After finishing all the experiments, each mouse was sacrificed under halothane anesthetic.
Serum Biochemical Analysis
Serum was prepared by centrifugation of blood collected from each mouse. The separated serum was used to measure the liver enzyme markers, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) using blood chemistry analyzer (Humalyzer, Germany).
Histopathological Examination
Liver tissue was carefully dissected out, washed with 0.9% normal saline solution, and preserved in a 10% formalin solution for fixation. Dipper sections of the tissue were cut using microtome and stained with hematoxylin and eosin dye for microscopic examination with low power field (10X or 40X), the stained sections were examined and photographed under a light microscope (Olympus CHS six headed).
Preliminary Phytochemical Screening
Preliminary phytochemical screening of R. abyssinicus rhizome extract was carried using the method described by Trease and Evans, (1989) and Jones and Kinghorn, (2006) for the presence or absence of phytoconstituents such as anthraquinones, alkaloids, saponins, phenols, flavonoids, tannins, and terpenoids.37,38
Statistical Analysis
For the in vivo hepatoprotective models the result was calculated using the Statistical Package for the Social Science (SPSS) program (Version 21.0) and reported as mean ± standard error of the mean (SEM). The data were analyzed by one-way analysis of variance (ANOVA) and post hoc Tukey’s test. P<0.05 was considered statistically significant and the down shown formula was employed to compute the percentage protection of the extracts:
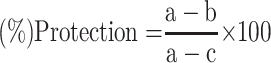 |
(1) |
where a is the mean value of the marker produced by hepatotoxin; b is the mean value of the marker produced by toxin plus test sample; c is the mean value of the marker produced by the vehicle control.36 While for the in vitro DPPH radical scavenging activity assay, the result was expressed as 50% inhibitory concentration (IC50) values, representing the concentration of sample required to scavenge 50% DPPH free radicals, which were calculated from a concentration versus % inhibition graph of each sample.33 The following formula was used to calculate percentage inhibition:
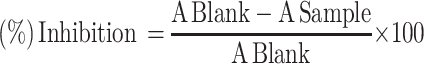 |
(2) |
where Ablank is the absorbance of the DPPH, and Asample is the absorbance of test samples.
Results
Extract and Fractions Yield
The 80% methanol extraction method resulted in a yellowish water-soluble crude extract with a percentage yield of 14% (w/w) while the percentage yields (w/w) of the solvent fractions were 0.6% (petroleum ether), 1.8% (chloroform), 13% (ethyl acetate), 10.5% (butanol), and 47.5% (aqueous).
Acute Oral Toxicity Study
Administration of the 80% methanolic extract of the dried rhizomes of R. abyssinicus at a dose of 2000 mg/kg did not produce any gross behavioral changes, such as loss of appetite, hair erection, lacrimation, tremors, convulsions, salivation, diarrhea, and mortality during the 14 days observation period. Hence, the extract was considered to be safe to proceed in further experimentation.
Radical Scavenging Activity (DPPH Assay)
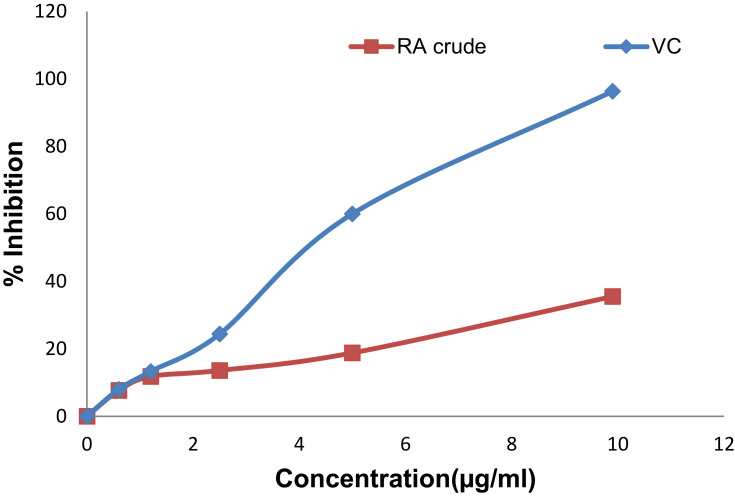
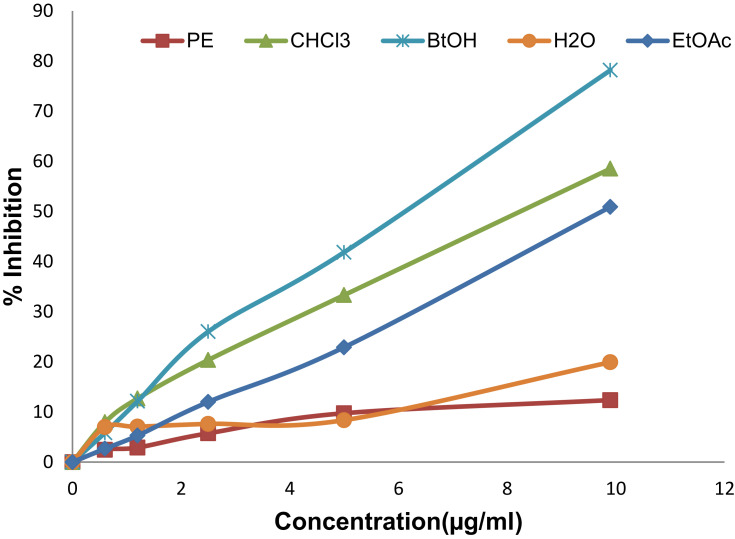
The crude extract and solvent fractions of rhizomes of R. abyssinicus exhibited concentration-dependent radical scavenging activity over the tested concentration ranges (Figures 1 and 2). The IC50 value of the crude extract was 13.1 μg/ml and that of ascorbic acid was 4.9 μg/ml. Similarly, the different solvent fractions showed different degrees of radical scavenging activities with IC50 values as shown in Table 1. Of all fractions, the butanol fraction (6.1 μg/ml) was associated with the highest scavenging activity.
Figure 1.
DPPH radical scavenging activity of different concentrations of dried rhizomes of R. abyssinicus crude extract (RA-crude) and vitamin C (VC).
Figure 2.
DPPH radical scavenging activity of different concentrations of solvent fractions: petroleum ether (PE), chloroform (CHCl3), ethyl acetate (EtOAc), butanol (BtOH), and aqueous (H2O) fractions from R. abyssinicus extract.
Table 1.
IC50 Values of DPPH Radical Scavenging Activity of the Solvent Fractions of Rumex abyssinicus in Comparison with Vitamin C
| Test Samples | IC50 (µg/ml) |
|---|---|
| Vitamin C | 4.8 |
| Petroleum ether fraction | 34.4 |
| Chloroform fraction | 8.0 |
| Ethyl acetate fraction | 9.9 |
| Butanol fraction | 6.1 |
| Aqueous fraction | 24.1 |
Hepatoprotective Activity
Serum Biochemical Analysis in the Pretreatment Model
Hepatoprotective activity of the 80% methanolic extract of R. abyssinicus at 125, 250, and 500 mg/kg was determined by estimating levels of ALT, AST, and ALP (Table 2) and histopathological examinations (Figure 3). As shown in Table 2, the mean value of ALT of normal animals was 44.7 ± 8.9 U/L, while in the CCl4 (1 ml/kg)-treated groups it was 145.3 ± 11.4 U/L which was a statistically significant (p <0.001) elevation when compared to the normal control groups. Pretreatment with 80% methanolic extract of R. abyssinicus at doses of 125, 250, and 500 mg/kg brought the level of this enzyme to 121.7 ± 9.3U/L, 97.6 ± 7.2U/L (p <0.01) and 93.7 ± 7.5 U/L (p <0.01) respectively, while silymarin (100 mg/kg) reduced the level to 65.5 ± 7.5 U/L (p<0.001). The effects of the extract at 250 and 500 mg/kg were statistically significant (p<0.01) and comparable with silymarin. A similar pattern was reflected with AST and ALP (Table 2). Furthermore, in the 500 mg/kg pre-treated mice, the levels of ALT and AST were significantly (p <0.01) reduced but there was no statistically significant difference in the level of ALP. Overall administration of the crude extract of R. abyssinicus at a dose of 500 mg/kg reduced the serum ALT (51.3%), ALP (63.8%) as well as the level of AST (73.9%) better than silymarin (69%). On the other hand, pre-treating mice with a dose of 125 mg/kg do not show a statistically significant difference in the reduction of the levels of all enzyme markers. Similarly, in the 250 mg/kg pre-treated mice, no statistically significant difference was seen in the levels of AST and ALP as compared to CCl4-treated group.
Table 2.
Effects of Different Concentrations of R. abyssinicus Crude Extract and Silymarin on Serum Biochemical Parameters
| Group | Dose | ALT (U/L) | AST (U/L) | ALP (U/L) |
|---|---|---|---|---|
| DW | 10 ml/kg | 44.7 ± 8.9 | 89.4 ± 9.8 | 92.8 ± 14.1 |
| CCl4 | 1 ml/kg | 145.3 ± 11.4b** | 226.7 ± 26.2b** | 236.3 ± 34.8b* |
| Silymarin + CCl4 | 100 mg/kg | 65.5 ± 7.5a** | 131.8 ± 11.5a* | 124.3 ± 21.5 |
| RA+ CCl4 | 125 mg/kg | 121.7 ± 9.3 | 226.7 ± 15.1 | 200.6 ± 27.8 |
| RA + CCl4 | 250 mg/kg | 97.6 ± 7.2a* | 195.4 ± 18.5 | 191.2 ± 25.0 |
| RA + CCl4 | 500 mg/kg | 93.7 ± 7.5a* | 125.2 ± 6.1a* | 144.7 ± 31.8 |
Notes: Data presented as mean ± S.E.M. aCompared to CCl4, bCompared with normal control (DW), *p< 0.01, **p< 0.001. One-way ANOVA followed by Tuckey test; n = 6.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; RA, Rumex abyssinicus; DW, distilled water; CCl4, carbon tetrachloride.
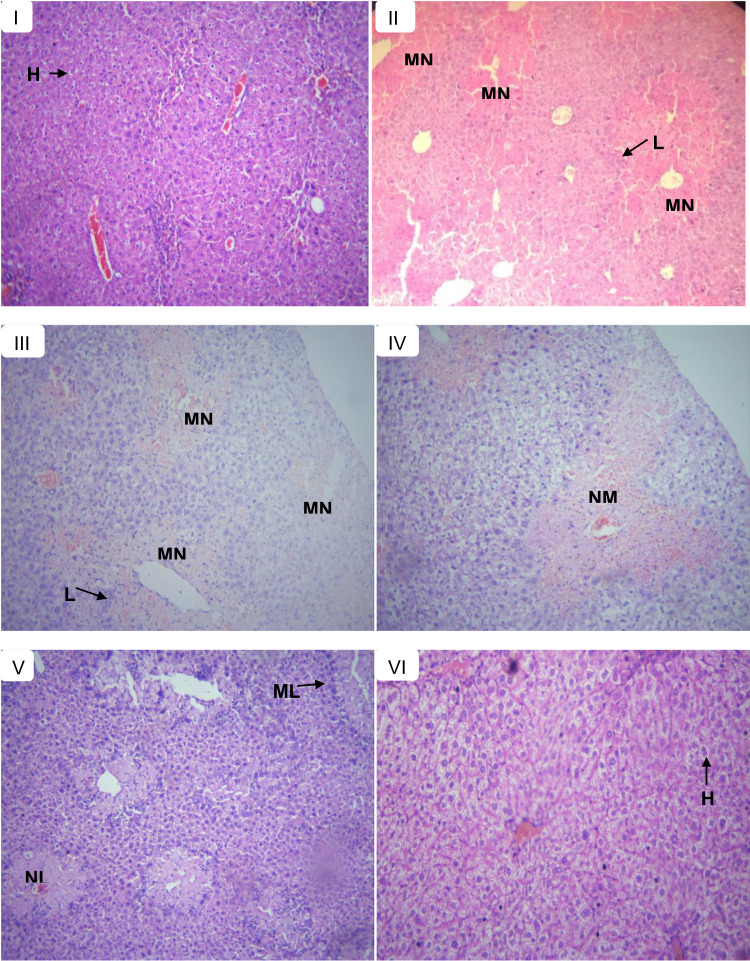
Figure 3.
Histopathological section of liver tissues in control and experimental groups of mice in the pre-treatment model; showing normal hepatic cells (H), lymphocytic infiltrates (L), multifocal necrosis (MN), moderate necrosis (NM), mild lymphocytic infiltrates (ML), and mild necrosis (NL). (A) Normal control received with distilled water, (B) toxic control received CCl4, (C) treated with 125 mg/kg extract, (D) treated with 250 mg/kg extract, (E) treated with 500 mg/kg extract, and (F) treated with silymarin 100 mg/kg.
Serum Biochemical Analysis in the Post-Treatment Model
In this study, CCl4 groups showed significant (p<0.001) increase in serum ALT, AST, and ALP levels compared with the normal control group while treatment of mice with 500 mg/kg of crude extract, butanol fraction, and silymarin significantly lowered (p<0.01 to p <0.001) these alteration (Table 3). The mean value of ALT of normal control mice was 67.2 ± 6.2 U/L while the level of CCl4 (1 ml/kg) received group was 205.6 ± 28.1 U/L which is a statistically significant (p<0.001) elevation. Post-treatment with 80% methanolic crude extract of R. abyssinicus and its butanol fraction, which has shown a strong radical scavenging activity at the dose of 500 mg/kg, brought the level of this enzyme to 106.8 ± 13U/L (p<0.01) and 103 ± 12.6U/L (p<0.01), respectively. These reductions were statistically significant and comparable to silymarin (100 mg/kg), which reduced the level to 97.1 ± 9.2 U/L (p<0.001). For instance, mice treated with butanol fraction of R. abyssinicus at a dose of 500 mg/kg had a reduced level of ALT by 74.1% while silymarin reduced ALT levels by 78.4%. Likewise, levels of AST and ALP showed a similar pattern of reduction in the treatment group as compared to CCl4-treated group.
Table 3.
Effect of the 80% Methanolic Extract and Butanol Fraction Obtained from the Dried Rhizomes of R. abyssinicus and Silymarin Against CCl4-Induced Hepatotoxicity on Serum Biochemical Parameters
| Group | Dose | ALT (U/L) | AST (U/L) | ALP (U/L) |
|---|---|---|---|---|
| DW | 10 ml/kg | 67.2 ± 6.2 | 116.6 ± 11.1 | 128.2 ± 5.8 |
| CCl4 | 1ml/kg | 205.6 ± 28.1b** | 347.3 ±18.8b** | 359.8 ± 41.7b** |
| RA + CCl4 | 500 mg/kg | 106.8 ± 13a* | 232.6 ± 14.3a** | 231.6 ± 10.2a* |
| BU-RA + CCl4 | 500 mg/kg | 103 ±12.6a* | 211.1 ± 6.8a** | 224.1 ±3.3a* |
| Silymarin + CCl4 | 100 mg/kg | 97.1 ± 9.2a** | 220.1 ± 11.6a** | 180.4 ± 16.6a** |
Notes: Data presented as mean ± S.E.M. aCompared to CCl4, bCompared with normal control (DW), *p < 0.01, **p< 0.001. One-way ANOVA followed by Tuckey test; n = 6.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; RA, R. abyssinicus; BU-RA, butanol fraction of R. abyssinicus; DW, distilled water; CCl4, carbon tetrachloride.
Histopathological Studies in the Pre-Treatment Model
Histopathological examination of the liver sections under a light microscope revealed that normal control-treated group showed normal liver histology (Figure 3A) whereas the CCl4-treated group showed extensive multifocal necrosis, disorganization of the hepatic plate, degenerated nuclei, severe lymphocytic infiltrates, marked cellular swelling, and fatty change (Figure 3B). The liver histopathology of the 125 mg/kg extract pre-treated mice was similar to that of CCl4-treated mice (Figure 3C). The liver section from 250 mg/kg extract pre-treated mice showed moderate necrosis and lymphocytic infiltrates (Figure 3D) while the extract at 500 mg/kg resulted in maintained architecture, mild necrosis and mild lymphocytic infiltrates (Figure 3E), which was quite similar to that of silymarin pre-treatments that showed normal hepatic architecture, no necrosis and mild lymphocytic infiltrates in most of the mice (Figure 3F).
Histopathological Studies in the Post-Treatment Model
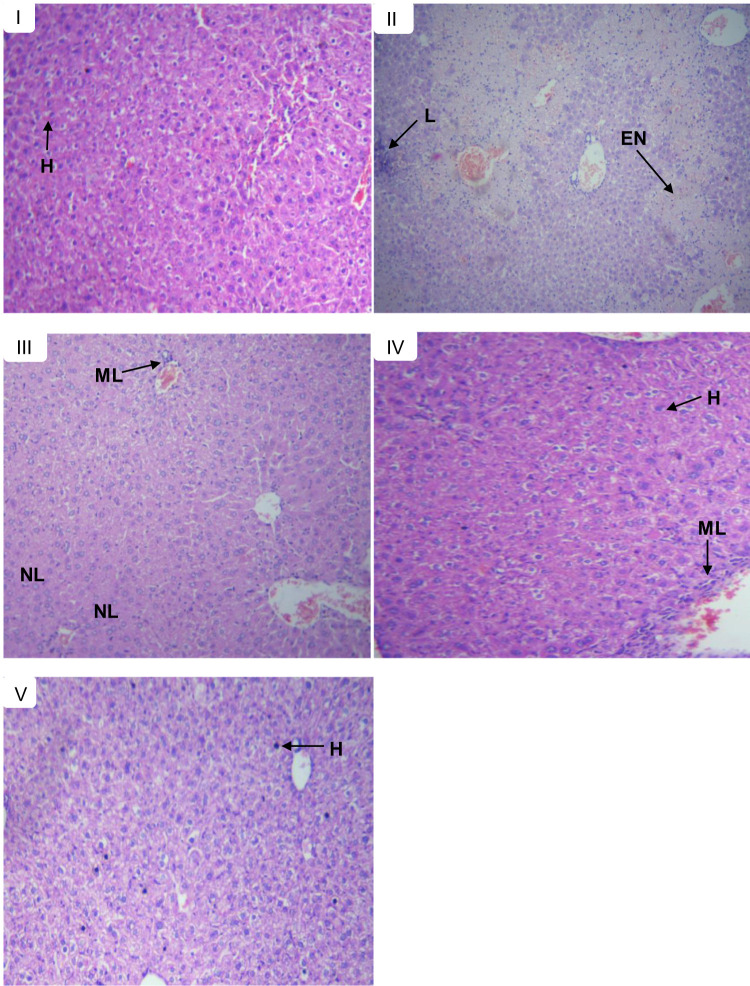
Like the pre-treatment model, liver sections from the control group, mice showed normal histology of the liver (Figure 4A) unlike the CCl4 received group that showed severe periportal necrosis, hepatocyte swelling, degenerated nuclei, marked (severe) lymphocytic infiltration, congested portal vein, and inflammation (Figure 4B). On the other hand, the 500 mg/kg extract treatments showed mild necrosis, mild periportal hepatocytes swelling, mild lymphocyte infiltrate and mild cellular swelling (Figure 4C) while the butanol fraction at a dose of 500 mg/kg prevented necrosis in almost all mice although there were congested blood vessels, mild periportal hepatocyte swelling, and mild lymphocytic infiltrates in the liver sections of some mice (Figure 4D). The results obtained from both extract and butanol fraction were similar to silymarin which showed no necrosis, mild lymphocytic infiltrates, dilated portal vein, periportal hepatocytes (Figure 4E).
Figure 4.
Histopathological section of liver tissues in control and experimental groups of mice in the post-treatment model; showing normal hepatic cells (H), lymphocytic infiltrates (L), mild lymphocytic infiltrates (ML), mild necrosis (NL), and extensive necrosis (EN). (A) Normal control received with distilled water, (B) toxic control received CCl4, (C) treated with 500 mg/kg extract, (D) treated with 500 mg/kg butanol fraction, and (E) treated with silymarin 100 mg/kg.
Preliminary Phytochemical Screening
The preliminary phytochemical screening of R. abyssinicus crude extracts revealed the possible presence of alkaloids, anthraquinones, phenols, flavonoids, saponins, tannins, and terpenoids. Moreover, previous phytochemical studies on R. abyssinicus showed the isolation of methyl gallate (a phenolic acid),39 anthraquinones (chrysophanol, emodin, emodin-8-O-β-D-glucopyranoside, helminthosporin, physcion, and physcion-8-O-β-D-glucopyranoside),39,40 flavanols (epicatechin, epicatechin-3-O-gallate, and epicatechin-3-O-(4′′methyl) gallate),39 betulone (a triterpenoid), and oleic acid (a fatty acid) from this plant.29
Discussion
In the present study, an attempt has been made to find out the hepatoprotective activity of R. abyssinicus extract using pre- and post-treatment mice models of CCl4 induced hepatotoxicity. Pre-treatment studies were done to investigate the preventive effects while post-treatment studies were done to investigate the efficacy of the test samples in curing liver damage. The dose which produced the greatest effect in the pre-treatment model (500 mg/kg) and the fraction (butanol fraction) which showed the highest DPPH radical scavenging activity was chosen for the post-treatment study. During studies of both pre-treatment and post-treatment models, exposure to CCl4 (1 ml/kg) resulted in substantial increase in levels of ALT, AST, and ALP within 24 h, and this increment is a hallmark to damage of structural integrity of the liver. As these enzymes occur in the cytoplasm, their release to circulation indicates cellular damage or hepatic injury.41 In both models, the R. abyssinicus extracts decreased ALT, AST, and ALP levels. These results are in line with previous reports on different Rumex species including R. vesicular,8 R. hastatus,.21 R. pictus,42 and R. dentatus.43 Interestingly, in the pre-treatment model, the dried rhizomes of R. abyssinicus at a dose of 500 mg/kg showed comparable hepatoprotective activity to that produced by 100 mg/kg of silymarin (a well-known plant preparation used as standard positive control and potent hepatoprotective agent in preventing liver pathologies in chemical-induced models). Reversal of serum enzymes by the extracts is an indication for the protection of the liver from CCl4-induced damage. Most of the time, it is regarded as proper when the raised amount of the transaminases restores to normal owing to the healing of hepatic parenchyma and the regeneration of hepatocytes.44 The mechanism by which R. abyssinicus extract produced anti-hepatotoxic effects needs further studies. Yet, one possibility could be related to its free radical scavenging activities. Free radicals are associated with various groups of ailments and are responsible for causing heterogeneous pathological events. Antioxidants neutralize the effect of free radicals and thereby protect us from various ailments including liver disease. These agents exert their action either by scavenging reactive free radicals or by enhancing the endogenous antioxidant defense mechanisms.45 In the DPPH radical scavenging activity assay, the results indicated that R. abyssinicus crude extract and its solvent fractions show concentration-dependent radical scavenging activity which was comparable to ascorbic acid. A similar study on extracts of R. crispus leaves and seeds reported concentration-dependent antioxidant activity.46
In the current study, the histological studies also showed the hepatoprotective potential of R. abyssinicus extracts in addition to the serum enzyme markers. R. abyssinicus crude extract at doses of 125 and 250 mg/kg resulted in weak and moderate hepatoprotective activity, respectively, but treatment with the higher dose (500 mg/kg) and silymarin (100 mg/kg) revealed substantial protective effect as compared to the CCl4-treated group. Girma et al (2015) showed that R. abyssinicus is rich in a number of anthraquinones that showed Cyclooxygenase-2 (COX-2) inhibitory activity and strong anti-inflammatory property which in turn may help the regeneration of hepatocytes.27 In line with our findings; this report supports the hepatoprotective potential of R. abyssinicus extracts as the pharmacological activity produced by plant extracts is usually attributed to the presence of secondary metabolites within them.47 In the present study, preliminary phytochemical screening of R. abyssinicus extract showed the possible presence of anthraquinones, alkaloids, saponins, phenols, flavonoids, tannins, and terpenoids which were also consistent with previously reported studies.28,39,48 Especially, flavonoids possess an antioxidant property, which may be useful in the treatment of liver disease.49 For example, the (-)-epicatechin, a flavonol50 has been demonstrated to possess hepatoprotective activity. Also, previous studies reported different bioactive anthraquinones in R. abyssinicus like chrysophanic acid, chrysophanol, emodin, and physcion;29,39,51 of which chrysophanol,52 emodin,53,54 and physcion55 did show the ability to scavenge free-radicals implying that may serve as natural antioxidant compounds. In line with this notion, emodin, chrysophanol, and physcion were reported to have a potential liver-protecting activity55 which could be due to their antioxidant activity.56 In addition to this, the hepatoprotective activity of emodin has been reported in a number of studies.56–59 Taken together, these findings indicate that phytoconstituents individually or synergistically may initiate a host of responsible mechanisms for the hepatoprotective activity, which in turn can give a potential clue for the use of this plant in the treatment of liver disease.
Conclusions
The results of measurements of serum biochemical markers and histopathological examinations from pre-treatment and post-treatment mice models, in the present study, revealed the possible hepatoprotective effects of the extracts of rhizomes of R. abyssinicus. These results may have an implicit association with the traditional use of the plant for the treatment of liver disease. The in vitro radical scavenging activity of the extracts may, at least in part, explain the possible hepatoprotective mechanism of the plant. Further work to establish bioactivity guided isolation of lead compound(s) responsible for hepatoprotective activities may be suggested.
Acknowledgments
The authors would like to express thanks to the Department of Pharmacognosy and Pharmacology, School of Pharmacy, Mekelle University for providing laboratory facilities for the research work. We also would like to thank Mr. Abiyu Enyew (botanist), for the authentication of the plant material. The principal investigator would like to thank the school of pharmacy, University of Gondar for covering her living expenses throughout the study period.
Abbreviations
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CCl4, carbon tetrachloride; DPPH, 2, 2-diphenyl-2-picrylhydrazyl hydrate; OECD, Organization of Economic Co-operation Development.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author up one request.
Ethical Approval
The ethical approval for this study was obtained from the Health Research Ethics Review Committee (HRERC) of College of Health Sciences, Mekelle University with ERC number 1024/2017. The experimental animals were handled according the Guide for the Care and Use of Laboratory Animals.
Author Contributions
All authors contribute to the conception, data analysis and interpretation, drafting and critical reviewing of the article, gave final approval upon which the final version of the article will be submitted, and agreed to take responsibility and to be accountable for all aspects of the work. BAA conceived the idea, drafted the proposal, and collected the plant materials. BAA and EMA carried out the actual experiments and statistical analysis. YKE and BAA prepared and critically reviewed the final manuscript for publication. MGH and GP were involved in the design of the study, involved at all implementation stage of the work, and revising the manuscript critically for important intellectual content. BS involved in developing and reviewing the proposal. All authors read and approved the final version of the manuscript.
Disclosure
The authors do not have any conflict of interest to disclose.
References
- 1.Suciu M, Ardelean A. Hepatoprotective and microbiological studies of three genera: equisetum, lycopodium, and gentiana. Analele Univ Din Oradea Fasc Biol. 2012;19(2):116–122. [Google Scholar]
- 2.Guan YS, He Q. A current update on the rule of alternative and complementary medicine in the treatment of liver diseases. Evidence-Based Complement Altern Med. 2013;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59(1):160–168. doi: 10.1016/j.jhep.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Zakaria ZA, Kamisan FH, Omar MH, et al. Methanol extract of Dicranopteris linearis L. leaves impedes acetaminophen-induced liver intoxication partly by enhancing the endogenous antioxidant system. BMC Complement Altern Med. 2017;17(1):271. doi: 10.1186/s12906-017-1781-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olokoba AB, Aderibigbe SA, Kayode OO. A community survey of practices related to risk factors for liver diseases among adults in Ilorin metropolis. Am J Sci Ind Res. 2010;1(2):118–121. [Google Scholar]
- 6.Tsega E. Current views on liver diseases in Ethiopia. Ethiop Med J. 1977;15(2):75–82. [PubMed] [Google Scholar]
- 7.Muriel P. Role of free radicals in liver diseases. Hepatol Int. 2009;3(4):526–536. doi: 10.1007/s12072-009-9158-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tukappa NK, Londonkar RL, Nayaka HB. Cytotoxicity and hepatoprotective attributes of methanolic extract of Rumex vesicarius L. Bio Res. 2015;48(1):19. doi: 10.1186/s40659-015-0009-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan RA, Khan MR, Ahmed M, et al. Hepatoprotection with a chloroform extract of Launaea procumbens against CCl4-induced injuries in rats. BMC Complement Altern Med. 2012;12:114. doi: 10.1186/1472-6882-12-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoyanovsky DA, Cederbaum AI. Thiol oxidation and cytochrome P450-dependent metabolism of CCl4 triggers Ca2+ release from liver microsomes. Biochemistry. 1996;35:15839–15845. doi: 10.1021/bi961295p [DOI] [PubMed] [Google Scholar]
- 11.Kaplowitz N, Tsukamoto H. Oxidative stress and liver disease. Progr Liver Dis. 1996;14:131–159. [PubMed] [Google Scholar]
- 12.Rector RS, Thyfault JP, Uptergrove GM, et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–736. doi: 10.1016/j.jhep.2009.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu R, Wang Y, Zhang L, Guo Q. Oxidative stress and liver disease. Hepatol Res. 2012;42:741–749. doi: 10.1111/j.1872-034X.2012.00996.x [DOI] [PubMed] [Google Scholar]
- 14.Zhang A, Sun H, Wang X. Recent advances in natural products from plants for treatment of liver diseases. Eur J Med Chem. 2013;63:570–577. doi: 10.1016/j.ejmech.2012.12.062 [DOI] [PubMed] [Google Scholar]
- 15.Au JS, Navarro VJ, Rossi S. Review article, drug-induced liver injury-its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther. 2011;34:11–20. doi: 10.1111/j.1365-2036.2011.04674.x [DOI] [PubMed] [Google Scholar]
- 16.Madrigal-Santillan E, Madrigal-Bujaidar E, Alvarez-Gonzalez I, et al. Review of natural products with hepatoprotective effects. World J Gastroenterol. 2014;20:14787–14804. doi: 10.3748/wjg.v20.i40.14787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulla WA, Salunkhe VR, Bhise SB. Hepatoprotective activity of hydroalcoholic extract of leaves of Alocasia indica (Linn.). Indian J Exp Biol. 2009;47:816–821. [PubMed] [Google Scholar]
- 18.Soni RK, Dixit V, Irchhaiya R, et al. Potential herbal hepatoprotective plants: an overview. Int J Pharm Sci Res. 2014;5(3):774. [Google Scholar]
- 19.Roy A, Bhoumik D, Sahu RK. Medicinal plants used in liver protection-a review. UK J Pharm Biosci. 2014;2(1):23–33. doi: 10.20510/ukjpb/2/i1/91143 [DOI] [Google Scholar]
- 20.Mekonnen T, Urga K, Engidawork E. Evaluation of the diuretic and analgesic activities of the rhizomes of Rumex abyssinicus Jacq in mice. J Ethnopharmacol. 2010;127(2):433–439. doi: 10.1016/j.jep.2009.10.020 [DOI] [PubMed] [Google Scholar]
- 21.Vasas A, Orbán-Gyapai O, Hohmann J. The genus rumex: review of traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2015;175:198–228. doi: 10.1016/j.jep.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 22.Giday M, Asfaw Z, Woldu Z, et al. Medicinal plant knowledge of the Bench ethnic group of Ethiopia: an ethnobotanical investigation. J Ethnobiol Ethnomed. 2009;5(1):34. doi: 10.1186/1746-4269-5-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araya S, Abera B, Giday M. Study of plants traditionally used in public and animal health management in Seharti Samre District, Southern Tigray, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):22. doi: 10.1186/s13002-015-0015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abebe W. An overview of Ethiopian traditional medicinal plants used for cancer treatment. Eur J Med Plants. 2016;14(4):1–16. doi: 10.9734/EJMP/2016/25670 [DOI] [Google Scholar]
- 25.Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):65. doi: 10.1186/1746-4269-9-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raju NJ, Yesuf EA. Evaluation of anthelmintic activities of Rumex abyssinicus JACQ and Rumex nervosus Vahl. (Polygonaceae). Int J Pharm Sci Rev Res. 2010;5(2):55–57. [Google Scholar]
- 27.Girma B, Yimer G, Makonnen E. Effect of Rumex abyssinicus on preneoplastic lesions in dimethyl hydrazine induced colon carcinogenesis in rats. BMC Complement Altern Med. 2015;15(1):365. doi: 10.1186/s12906-015-0883-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulisa E, Asres K, Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J. (Polygonaceae) in mice. BMC Complement Altern Med. 2015;15(1):341. doi: 10.1186/s12906-015-0878-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fufa FM, Padmanabhan R, Gurmessa GT. Phytochemical investigation and in vitro antibacterial evaluation on root extracts of Rumex abyssinicus. Nat Prod Chem Res. 2016;4(6):1–14. [Google Scholar]
- 30.Amgen MB, Pre Labs PJR. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: The National Academies Press; 2010. [Google Scholar]
- 31.Otsuka H. Purification by solvent extraction using partition coefficient. Nat Prod Isola. 2006;20:269–273. [Google Scholar]
- 32.Organization for Economic Co-operation and Development. Guidance document on acute oral toxicity: up-and-down-procedures. Environmental health and safety monograph series on testing and assessment. J Environ Sci Health Saf Pub. 2008. No 425, OECD. [Google Scholar]
- 33.Braca A, De Tommasi N, Di Bari L, et al. Antioxidant principles from bauhinia tarapotensis. J Nat Prod. 2001;64(7):892–895. doi: 10.1021/np0100845 [DOI] [PubMed] [Google Scholar]
- 34.Bhat SH, Shrivastava R, Malla MY. Hepatoprotective activity of Argemone Mexicana linn against toxic effects of carbon tetrachloride in rats. World J Pharm Res. 2014;3(3):4037–4048. [Google Scholar]
- 35.Wills PJ, Asha VV. Protective effect of Lygodium flexuosum (L.) Sw. extract against carbon tetrachloride-induced acute liver injury in rats. J Ethnopharmacol. 2006;108(3):320–326. doi: 10.1016/j.jep.2006.05.032 [DOI] [PubMed] [Google Scholar]
- 36.Umer S, Asres K, Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants. Pharm Biol. 2010;48(4):461–468. doi: 10.3109/13880200903173593 [DOI] [PubMed] [Google Scholar]
- 37.Trease GE, Evans WC. Pharmacognosy. 13th ed. BailliereTindall Ltd; 1989:176–180. [Google Scholar]
- 38.Jones WP, Kinghorn AD. Extraction of plant secondary metabolites. Nat Prod Isola. 2006;323–351. [DOI] [PubMed] [Google Scholar]
- 39.Tala MF, Ansary MWR, Talontsi FM, et al. Anthraquinones and flavanols isolated from the vegetable herb Rumex abyssinicus inhibits motility of phytophthora capsici zoospores. S Afr J Bot. 2018;115:1–4. doi: 10.1016/j.sajb.2017.11.015 [DOI] [Google Scholar]
- 40.Augustin N, Nuthakki VK, Abdullaha M, et al. Discovery of helminthosporin, an anthraquinone isolated from Rumex abyssinicus Jacq as a dual cholinesterase inhibitor. ACS Omega. 2020;5(3):1616–1624. doi: 10.1021/acsomega.9b03693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahsan MR, Islam KM, Bulbul IJ. Hepatoprotective activity of methanol extract of some medicinal plants against carbon tetrachloride-induced hepatotoxicity in rats. Eur J Sci Res. 2009;37(2):302–310. [Google Scholar]
- 42.Gabr GA, Ansari MN, Abdulaziz SS, et al. Ameliorative effect of Rumex pictus extract on paracetamol induced hepatotoxicity in rats. Adv Biol Res. 2015;6(6):9–15. [Google Scholar]
- 43.Saleem M, Ahmed B, Karim M. Hepatoprotective effect of aqueous methanolic extract of Rumex dentatus in paracetamol induced hepatotoxicity in mice. Bangladesh J Pharmacol. 2014;9(3):284–289. doi: 10.3329/bjp.v9i3.18874 [DOI] [Google Scholar]
- 44.Anusha M, Venkateswarlu M, Prabhakaran V. Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian J Pharmacol. 2011;43(5):563. doi: 10.4103/0253-7613.84973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umamaheswari M, Chatterjee TK. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr J Tradit Complement Altern Med. 2008;5(1):61–73. [PMC free article] [PubMed] [Google Scholar]
- 46.Yıldırım A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agri Food Chem. 2001;49(8):4083–4089. doi: 10.1021/jf0103572 [DOI] [PubMed] [Google Scholar]
- 47.Mohammed N, Yaro AH, Nazifi AB. Evaluation of hepatoprotective activity of methanol stem bark extract of Haematostaphis barteri Hook. F. against paracetamol and carbon tetrachloride-induced liver injury in rats. Afri J Pharmacol Therapeut. 2017;6(2):88–95. [Google Scholar]
- 48.Mohammed SA, Panda RC, Madhan B, et al. Extraction of bio-active compounds from Ethiopian Plant material Rumex Abyssinicus (mekmeko) root-a study on kinetics, optimization, antioxidant and antibacterial activity. J Taiwan Inst Chem E. 2017:1–12. doi: 10.1016/j.jtice.2017.03.004. [DOI] [Google Scholar]
- 49.Oloyde GK, Onocha PA, Adaramoya OA, et al. Hepatoprotective activity and flavonoid of alchornea laxiflora leaf extracts. Res J Phytochem. 2011;5(4):190–200. doi: 10.3923/rjphyto.2011.190.200 [DOI] [Google Scholar]
- 50.Shanmugam B, Shanmugam KR, Ravi S, et al. Exploratory studies of (-)-Epicatechin, a bioactive compound of Phyllanthusniruri, on the antioxidant enzymes and oxidative stress markers in D-galactosamine-induced hepatitis in rats: a study with reference to clinical prospective. Pharmacogn Mag. 2017;13:56–62. doi: 10.4103/0973-1296.203973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Getie M, Gebre-Mariam T, Rietz R, et al. Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia. 2003;74(1–2):139–143. doi: 10.1016/S0367-326X(02)00315-5 [DOI] [PubMed] [Google Scholar]
- 52.Yusuf MA, Singh BN, Sudheer S, et al. Chrysophanol: a natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules. 2019;9:68. doi: 10.3390/biom902006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vargas F, Díaz Y, Carbonell K. Antioxidant and scavenging activity of emodin, aloe-emodin, and rhein on free-radical and reactive oxygen species. Pharm Biol. 2004;42(4–5):342–348. doi: 10.1080/13880200490519613 [DOI] [Google Scholar]
- 54.Cui YT, Liu B, Xie J, et al. The effect of emodin on cytotoxicity, apoptosis and antioxidant capacity in the hepatic cells of grass carp (ctenopharyngodon idellus). Fish Shellfish Immunol. 2014;38:74–79. doi: 10.1016/j.fsi.2014.02.018 [DOI] [PubMed] [Google Scholar]
- 55.Zhao YL, Wang JB, Zhou GD, et al. Investigations of free anthraquinones from rhubarb against α-naphthyl isothiocyanate-induced cholestatic liver injury in rats. Basic Clin Pharmacol Toxicol. 2009;104:463–469. doi: 10.1111/j.1742-7843.2009.00389.x [DOI] [PubMed] [Google Scholar]
- 56.Lee BH, Huang YY, Duh PD, et al. Hepatoprotection of emodin and Polygonum multiflorum against CCl4-induced liver injury. Pharm Biol. 2012;50(3):351–359. doi: 10.3109/13880209.2011.604335 [DOI] [PubMed] [Google Scholar]
- 57.Zhang SS. The research situation of emodin. Chin Her Med. 2006;23:12–14. doi: 10.3969/j.issn.1673-7210.2006.23.004 [DOI] [Google Scholar]
- 58.Dong MX, Jia Y, Zhang YB, et al. Emodin protects rat liver from CCl4-induced fibrogenesis via inhibition of hepatic stellate cells activation. World J Gastroenterol. 2009;15:4753–4762. doi: 10.3748/wjg.15.4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhadauria M. Dose-dependent hepatoprotective effect of emodin against acetaminophen-induced acute damage in rats. Exp Toxicol Pathol. 2010;62:627–635. doi: 10.1016/j.etp.2009.08.00 [DOI] [PubMed] [Google Scholar]