Abstract
Thyroid storm is a potentially fatal intensification of thyrotoxicosis normally marked by tachycardia, hyperthermia, impaired mental status, and severe agitation. It can be initiated by numerous causes. Failure to promptly diagnose the condition may lead to high mortality. Early diagnosis and treatment of thyroid storm are essential to prevent further life-threatening complications. A 10-year-old girl was admitted to our emergency center for intensive care. The patient presented tachypnea with stridor, paradoxical abdominal breathing, and “barking” cough. The patient was diagnosed as upper airway obstruction complicated by thyroid storm associated with influenza infection. Following immediate airway management, the patient was administered a short-acting beta-blocker, hydrocortisone, thiamazole, and saturated solution of potassium iodide was initiated. The patient was extubated on day 8 and transferred to a local hospital on day 11 without adverse complications. When examining patients with influenza infection, emergency doctors should be more attentive not to miss other critical diagnoses. The present case was initially diagnosed as croup due to influenza infection. Sharing our experience may help emergency physicians treat similar cases of pediatric airway compromise due to thyroid storm.
Keywords: Thyroid storm, Influenza A virus, Airway obstruction, Case report
Abbreviations: EEG, Electroencephalography; ICU, Intensive care unit; MRI, Magnetic resonance imaging; NR, Normal range; TRAb, Thyroid stimulating hormone receptor antibody
1. Introduction
Thyroid storm, defined as a potentially fatal intensification of thyrotoxicosis, is rare but a clinical emergency with a high mortality rate. The condition can be initiated by numerous causes, and is commonly marked by tachycardia, hyperthermia, impaired mental status, and severe agitation [1].
Herein, we present a 10-year-old patient with thyroid storm presenting airway obstruction who was initially diagnosed with influenza infection with croup. Unexplained tachycardia, goiter, and elevated circulating thyroid hormones led us to an accurate diagnosis. Rapid medical management brought about complete resolution. This case report may highlight the very important fact that physicians should be very vigilant when evaluating cases of influenza infection and should consider other diagnoses. The patient gave consent for these studies and their publication.
2. Case report
A 10-year-old girl (height 140 cm, weight 50.4 kg) was admitted to our emergency center for intensive care. The patient's mother had a history of Graves' disease. The patient had a medical history of Kawasaki disease at two years old; however, she had been healthy and had steadily grown without further management since then. School teachers noticed her attention deficit and hyperactivity in school; however, no treatment was required. One day prior to admission to our department, the patient visited a local clinic with fever, sore throat, and rhinorrhea. As a rapid influenza test conducted using a nasopharyngeal swab was positive for the influenza A virus, baloxavir was administered. A few hours later, the patient presented tachypnea, restlessness, hypoxia (oxygen saturation 86%, ambient air) and drowsiness. The patient presented tachypnea with stridor, paradoxical abdominal breathing, and “barking” cough. Arterial blood gas analysis indicated carbon dioxide narcosis: pH; 7.11, pCO2; 84 mmHg, pO2;110 mmHg, HCO3−; 21.1 mmol/L.
The patient was diagnosed with croup and influenza infection and was given nebulized racemic epinephrine and dexamethasone 8 mg intravenously. Since the patient's breathing remained difficult with stridor and her work of breathing was followed by clonic convulsion, intravenous thiamylal (40 mg) was administered and intratracheal intubation using a 5 mm tracheal tube for mechanical ventilation was required. As influenza encephalopathy and airway compromise due to croup with influenza infection was suspected, the patient was transferred to our department for intensive care. In our emergency department, physical examination revealed a body temperature of 40.0 °C, blood pressure of 115/68 mmHg, heart rate of 150 bpm, respiratory rate of 12 breaths/min, oxygen saturation of 97% (FIO2 0.8), and Glasgow Coma Scale score of E4VTM4. Her clinical examination revealed a diffuse elastic goiter with bilateral exophthalmoses (Fig. 1A). The patient had normal heart sounds without murmur or gallop. Her lungs were clear when auscultated. Her abdomen was soft and non-tender. Her skin was warm and wet. She had no lower extremity edema. A chest radiograph was normal. The electrocardiogram showed marked arterial tachycardia. Neurological examination showed normal reactive pupils without lateralization signs or neck stiffness.
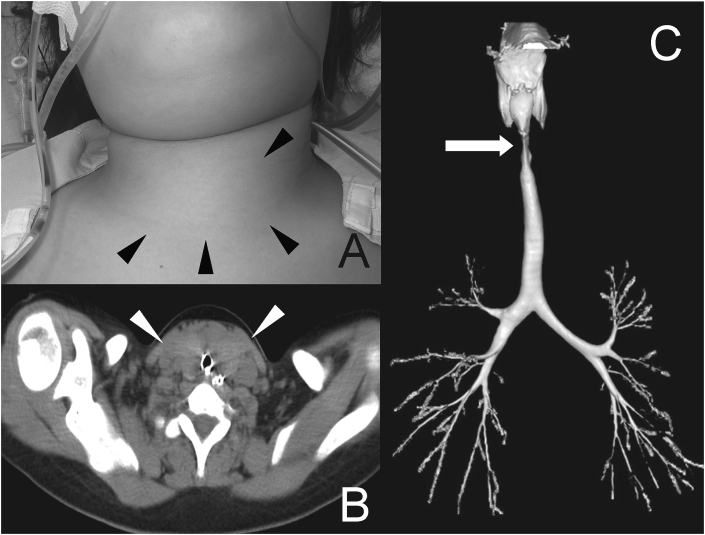
Fig. 1.
(A) Appearance of the patient's neck. A large goiter was located (black arrowhead). (B) Computed tomography showed the trachea was narrowed by the nodular goiter (white arrowhead). (C) Reconstructed computed tomography of the chest revealed a narrowing of the trachea.
Laboratory results were as follows: white blood cell count 14.66 × 109/L; hemoglobin, 11.5 g/dL; serum aspartate aminotransferase 66 U/L (normal range [NR], 10–35 U/L); serum alanine aminotransferase 39 U/L (NR, 0–40 U/L); blood urea nitrogen, 11.3 mg/dL (NR, 8–20 mg/dL); creatinine, 0.37 mg/dL (NR, 0.46–0.79 mg/dL); blood sugar, 142 mg/dL (NR, 73–109 mg/dL); and serum sodium 125 mEq/L (NR, 138–145 mEq/L). Blood lactate level was 0.8 mg/dL. Sputum culture grew oral organisms, but blood culture did not grow any organisms.
She had nasal flaring, inspiratory stridor, accessory respiratory muscle use, and paradoxical abdominal breathing. As there was no swelling on the epiglottis and glottis, the 5 mm diameter tracheal tube was exchanged for one 6 mm in diameter. While slight difficulty in intubation was noted due to subglottic stenosis, airway management was successfully performed, and the patient was sedated with midazolam and fentanyl. Computed tomography showed the trachea was narrowed by the nodular goiter (Fig. 1B). Reconstructed computed tomography of the chest revealed a narrowing of the trachea (Fig. 1C). Electroencephalography (EEG) revealed no slow waves or paroxysmal waves. Brain computed tomography was unremarkable. Brain magnetic resonance imaging (MRI) was performed on the second hospital day; a splenium of the corpus callosum lesion appeared with high intensity on diffusion weighted image (Fig. 2). Initial thyroid function tests revealed hyperthyroidism; free-T4; 6.46 ng/dL (normal range NR 0.9–1.4 ng/dL), free T3; 7.51 pg/mL, thyroid-stimulating hormone under the threshold of sensitivity (0.3–5.0 μIU/mL), thyroglobulin under the threshold of sensitivity (NR < 33.70 mg/mL), and TRAb (thyroid stimulating hormone receptor antibody) 39.30 IU/L (NR ≤ 2).
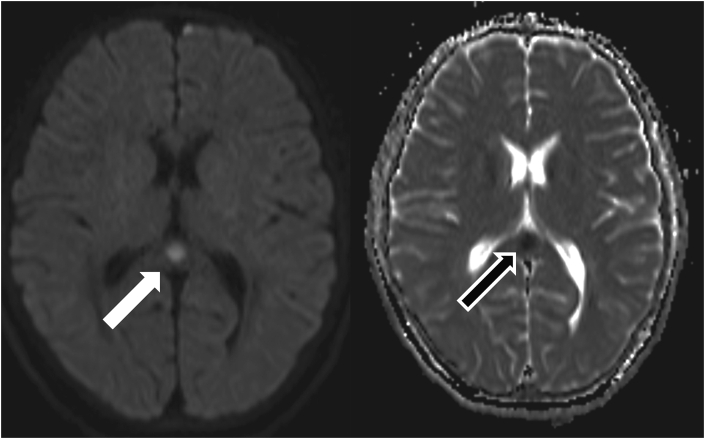
Fig. 2.
Brain magnetic resonance imaging on day 2 demonstrated a round lesion with high intensity on diffusion weighted image (right panel, white arrow) and low intensity in apparent diffusion coefficient (left panel, black arrow) in the splenium of the corpus callosum.
Echocardiogram revealed tachycardia >150/min and deteriorated left ventricle ejection fraction (40%), although the patient had no congenital heart disease. As the diagnosis of thyroid storm associated with concomitant high-output cardiac failure was considered, administration of a short-acting beta-blocker (landiolol 1 μg/kg/min), hydrocortisone (300mg/day), thiamazole (30 mg/day), and saturated solution of potassium iodide (170 mg/day) was initiated. Tachycardia and hemodynamics significantly improved after these interventions. Her level of consciousness and thyroid gland swelling causing airway obstruction improved. Thyroid function tests on day 5 demonstrated significantly improved thyroid hormones, free T3 of 3.91 pg/mL and free T4 of 2.84 ng/dL. On day 8, the patient was extubated and transferred to a local hospital on day 11.
3. Discussion
Without management, the mortality of thyroid storm ranges from 80% to 100%. However, with management, mortality ranges between 10% and 50%. Early cardiogenic shock and multiple organ failure seem to notably affect the prognosis. One study found respective in-intensive care unit (ICU) and six-month postadmission mortality rates of 17% and 22%. ICU non-survivors were more likely to need extracorporeal membrane oxygenation, vasopressors, therapeutic plasmapheresis, mechanical ventilation, and/or renal replacement therapy [2]. Thus, thyroid storm is a potentially fatal condition that usually requires rapid identification and aggressive management [3]. Prevalence has recently been estimated at 0.4% among teenagers and young adults, and the annual incidence has been estimated as 3/100,000 in adolescents [[4], [5], [6]].
Infections are frequently cited as a precipitant of thyroid storms in patients with thyrotoxicosis. Other causes include hypoglycemia, diabetic ketoacidosis, pulmonary embolism, hyperosmolar coma, iodinated contrast medium ingestion, thyroid hormone overdose, withdrawal of antithyroid medications, surgery, vascular accidents, parturition, stress, trauma, eclampsia, and myocardial infarction [3]. In our patient, influenza infection may have triggered thyroid storm. While the parainfluenza virus has previously been associated with one case of thyroid storm, influenza infection causing thyroid storm has rarely been described. As far as we know, very few cases of thyroid storm have been related to H1N1 influenza infection [1]. The mechanism of thyroid storm induced by influenza infection has not fully elucidated. A possible explanation is direct involvement of the thyroid gland (suppurative thyroiditis) with acute or subacute infection. Another explanation is that the stress of systemic infections such as urinary tract infections, endocarditis, and respiratory involvement can precipitate a crisis [7].
Diagnosing thyroid storm is challenging without a diagnosis of preexisting hyperthyroidism, particularly in children. Although we noticed goiter, elevated thyroid hormones, and unexplained tachycardia later, our patient's initial diagnosis was croup due to influenza virus infection and concomitant neurological symptoms. Croup, also known as laryngotracheobronchitis, is a common pediatric illness. Croup can be caused by a number of viruses, including parainfluenza and influenza, and commonly occurs in children six to 36 months old [8,9]. As children grow, like our patient, the airway becomes bigger and more rigid, making it less susceptive to inhalation's negative pressure effects. Burch and Wartofsky suggested a rating scale to diagnose thyroid storm, where a score of 45 or higher likely indicates thyroid storm [7]. Our patient scored 95 on this proposed scale. The diagnosis of thyroid storm was supported by her definitive quick response to antithyroid medication, iodine, and steroid therapy [4,10]. In retrospect, our patient was not a typical case of croup or thyroid storm.
Our patient required urgent management for difficult airway, which is sometimes underestimated. The literature has described acute airway obstruction due to retrosternal benign goiters or thyroid malignancies; however, thyroid crisis causing airway obstruction is very rare. Sizable goiters with obstructive symptoms including dyspnea and shortness of breath is a clear indication for surgical intervention [11]. Benign goiter located mostly in the neck can acutely obstruct the airway, even if the nodules are small. As our initial diagnosis was croup associated with influenza, first line interventions for our patient included oxygen, nebulized epinephrine, and corticosteroids. However, immediately after issuing the diagnosis of thyroid storm, management was initiated, consisting of restricting new hormone synthesis (thionamide drugs), thyroid hormone release (iodine), and T4 to T3 conversion (corticosteroids). In our patient, initiating short-acting selective beta-1 blockade may have effectively controlled symptoms and reduced the risk of cardiovascular collapse. Plasmapheresis may be applicable for removal of surplus circulating thyroid hormone. Early diagnosis of cardiac failure with a comprehensive echocardiographic examination is absolutely required before the development of irreversible multiple organ dysfunction.
In addition, protracted disturbance of consciousness, coma, convulsion, and abnormal brain MRI might have complicated the diagnostic process. Several reports have described thyrotoxic encephalopathy associated with reversible lesions on the corpus callosum, white matter, and cerebellum [12,13]. The possible causes of this diverse condition in thyrotoxic encephalopathy remain uncertain. Experts hypothesize that the regional brain metabolism alternation in hyperthyroidism is responsible. The myelin tissue water content is higher in the splenium, which may easily have disrupted the cellular fluid mechanics. Reversible intramyelinic edema because of myelin layer separation and the combination of the influx of inflammatory materials in thyrotoxic encephalopathy and related cytotoxic edema might have caused these reversible lesions on the splenium of the corpus callosum [12]. In our patient, based on clinical manifestations of rapid improvement of consciousness and normal electroencephalogram, abnormal finding seen on MRI was considered to be accompanied by thyrotoxic encephalopathy.
Additional history revealed that the patient had been symptomatic for several months. Her school teacher noticed that she presented attention deficit, restless, incontinence, and ataxia. Considering her increased serum TRAb levels, we assume that our patient may have had untreated and undetermined long-term hyperthyroidism. Influenza infection resulted in both development of thyroid storm and croup-like upper airway edema, leading to airway compromise.
In conclusion, emergency physicians must be aware that untreated and undetermined hyperthyroidism can cause airway obstruction associated with thyroid storm. Thyroid storm, sometimes accompanied by influenza infection, is an infrequent but potentially fatal result of hyperthyroidism. Prompt recognition and management of the condition as well as possible subsequent difficult airway is essential to reduce morbidity and mortality.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Baharoon S.A. H1n1 infection-induced thyroid storm. Ann. Thorac. Med. 2010;5:110–112. doi: 10.4103/1817-1737.62475. PMID: 20582177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourcier S., Coutrot M., Kimmoun A. Thyroid storm in the ICU: a retrospective multicenter study. Crit. Care Med. 2020;48:83–90. doi: 10.1097/CCM.0000000000004078. PMID:31714398. [DOI] [PubMed] [Google Scholar]
- 3.Idrose A.M. Acute and emergency care for thyrotoxicosis and thyroid storm. Acute Med Surg. 2015;2:147–157. doi: 10.1002/ams2.104. PMID: 29123713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akamizu T. Thyroid storm: a Japanese perspective. Thyroid. 2018;28:32–40. doi: 10.1089/thy.2017.0243. PMID: 28899229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hecht T., Brand J., Vlaho S. Encephalopathy and sinustachycardia in childhood--a possible differential diagnosis. J. Pediatr. Endocrinol. Metab. 2012;25:149–151. doi: 10.1515/jpem-2011-0436. PMID: 22570965. [DOI] [PubMed] [Google Scholar]
- 6.McLeod D.S., Cooper D.S., Ladenson P.W. Race/ethnicity and the prevalence of thyrotoxicosis in young Americans. Thyroid. 2015;25:621–628. doi: 10.1089/thy.2014.0504. PMID: 25744381. [DOI] [PubMed] [Google Scholar]
- 7.Burch H.B., Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab. Clin. N. Am. 1993;22:263–277. PMID: 8325286. [PubMed] [Google Scholar]
- 8.Johnson D.W. Croup. BMJ Clin Evid. 2014;2014 PMID: 25263284. [PMC free article] [PubMed] [Google Scholar]
- 9.Rajapaksa S., Starr M. Croup - assessment and management. Aust. Fam. Physician. 2010;39:280–282. PMID: 20485713. [PubMed] [Google Scholar]
- 10.Angell T.E., Lechner M.G., Nguyen C.T. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J. Clin. Endocrinol. Metab. 2015;100:451–459. doi: 10.1210/jc.2014-2850. PMID: 25343237. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita A., Hosokawa S., Mochizuki D. Emergent thyroidectomy with sternotomy due to acute respiratory failure with severe thyroid storm. Ann. R. Coll. Surg. Engl. 2018;e1-e3 doi: 10.1308/rcsann.2018.0145. PMID: 30286638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namatame C., Sonoo T., Fukushima K. A thyroid storm patient with protracted disturbance of consciousness and reversible lesion in the splenium of corpus callosum: a case report. Medicine (Baltim.) 2018;97:e9949. doi: 10.1097/MD.0000000000009949. PMID: 29443784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park M.H., Ryu J.K., Seo J.A. Reversible splenial abnormality in thyrotoxic encephalopathy. Eur. J. Neurol. 2007;14:e23–24. doi: 10.1111/j.1468-1331.2007.01848.x. PMID: 17594311. [DOI] [PubMed] [Google Scholar]