Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected more than 8 million people worldwide, becoming a pandemic. Detecting antibodies against SARS-CoV-2 is of utmost importance and a good indicator of exposure and circulation of the virus within the general population. Two serological tools based on a double recognition assay [enzyme-linked immunosorbent assay (DR-ELISA) and lateral flow assay (DR-LFA)] to detect total antibodies to SARS-CoV-2 have been developed based on the recombinant nucleocapsid protein. A total of 1065 serum samples, including positive for COVID-19 and negative samples from healthy donors or infected with other respiratory pathogens, were analyzed. The results showed values of sensitivity between 91.2% and 100%, and specificity of 100% and 98.2% for DR-LFA and DR-ELISA, respectively. No cross-reactivity against seasonal coronavirus (HCoV-NL63, HCoV-229E, HCoV-HKU1, HCoV-OC43) was found. These results demonstrate the importance of serology as a complementary tool to polymerase chain reaction for follow-up of recovered patients and identification of asymptomatic individuals.
Keywords: COVID-19, SARS-CoV-2, Diagnosis, Serology, ELISA, LFA
1. Introduction
In December 2019, a novel coronavirus of animal origin [the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] emerged in the city of Wuhan, China, with the ability for human-to-human transmission (Zhu et al., 2020). The associated disease, now named COVID-19, spread rapidly all over the world and was declared a pandemic by the World Health Organization (WHO) on March 11, 2020. Infection due to SARS-CoV-2 induces high rates of morbidity and mortality as described by the WHO (2020). A significant concern is how rapidly the virus spreads due in major part to the high number of asymptomatically infected individuals which could be an important source of viral dissemination (Bai et al., 2020; Du et al., 2020; Hu et al., 2020). The main preventive ways to avoid the spread of the virus are hygiene measures together with keeping social distance, as there are no vaccine available, neither efficient treatment. Serological studies can be used to collect epidemiological information on the prevalence of SARS-CoV-2. Moreover, in cases of COVID-19 not detected by reverse-transcription polymerase chain reaction (RT-PCR), the serological assays should be considered as a supplementary diagnostic tool, especially from the second week of illness when the sensitivity of the current molecular tests decreases (Pan et al., 2020; Zou et al., 2020). Therefore, the aim of the present work was the development of serological tools to determine the presence of antibodies against SARS-CoV-2 in the population as an indicator of an ongoing or previous infection.
As with many other coronaviruses, one of the main structural proteins of SARS-CoV-2 is the nucleocapsid (N) protein. The N protein shows high immunogenic activity and is abundantly expressed during infection (Che et al., 2004; Meyer et al., 2014; Narayanan et al., 2003). These features make the N protein a potential target for serodiagnosis of SARS-CoV-2 infection. To date, some diagnostic methods have been developed based on the N protein, although validated methods are still lacking to better understand the epidemiology of SARS-CoV-2. In the current study, a double recognition enzyme-linked immunosorbent assay (DR-ELISA) was developed to determine the presence of immunoglobulins of different classes (IgG, IgM and IgA) to SARS-CoV-2 in human serum to support the diagnosis of COVID-19. In parallel with this screening tool, a point-of-care test, based also on a double recognition format [a double recognition lateral flow assay (DR-LFA)] and using the N protein as the target antigen, was produced to be used immediately and on site when there is suspicion for infection. A double recognition assay is based on the use of the same protein (in this case, the N protein) as the target antigen and detection molecule, using the principle that antibodies possess multiple antigen binding regions (2 for IgG, 4 for IgA, and 10 for IgM), allowing their binding to both the target and detection antigen. Double recognition tests have the advantage that they screen for all SARS-CoV-2 antibodies, regardless if it is IgA, IgG, or IgM.
To carry out this study, a total of 1065 samples were analyzed with 380 samples from positive patients to COVID-19 and 685 negative samples collected before 2019 or from patients negative to COVID-19. Finally, a cohort of samples from patients infected with common-cold coronavirus or respiratory pathogens that could potentially cross-react with SARS-CoV-2 was included in the study.
The results shown in this paper reinforce the potential utility of serological testing as a complementary tool for interpretation of results in different scenarios of infection with SARS-CoV-2, including the identification of asymptomatic individuals.
2. Materials and methods
2.1. Serum samples
A total of 1065 human serum samples were used in this study. Eighty-seven serum samples were provided by the Hospital General Universitario Gregorio Marañón in Madrid (Spain), 140 serum samples by the Instituto de Salud Carlos III (Madrid, Spain), 665 serum samples from the “Program of Surveillance and Early Detection Program of COVID19 in essential services personnel of the city of Madrid” given by the Institute of Public Health of the Madrid City Council (Spain), 109 serum samples by the Amsterdam University Medical Center in Amsterdam (the Netherlands), and 64 serum samples already available in the lab from a previous European project, RespViruses (EU FP6-2005-LIFESCHEALTH-7). The samples were classified as follows: 163 serum samples of patients positive to COVID-19 by PCR (all the PCRs described in this study were RT-PCRs done in respiratory material) and confirmed by a commercial assay [NovaLisa® SARS-CoV-2 IgG and IgM ELISAs (Novatec) or 2019-nCoV IgG/IgM Rapid Test (T&D Diagnostics Canada)], 43 serum samples of patients positive to COVID-19 by PCR but negative in the serological assays, 174 serum samples of patients negative to COVID-19 by PCR but positive in a commercial serological assay, 452 serum samples of patients negative to COVID-19 both by PCR and serological assay, and 233 negative sera collected before 2019. A summary of these data is shown in Table 1 .
Table 1.
Serum classification by PCR and serological assays.
| Serum samples | |
|---|---|
| PCR+/antibody+ | 163 |
| PCR+/antibody− | 43 |
| PCR−/antibody+ | 174 |
| PCR−/antibody− | 452 |
| Samples prior 2019 | 233 |
| Total | 1065 |
A collection of sera positive to other infectious diseases which can provoke pneumonia (5 sera positive to Chlamydia trachomatis, 17 positive to Mycoplasma pneumoniae, and 21 to Legionella pneumophila) was tested and classified by the Department of Serology of the Spanish National Center of Microbiology and was included in our study.
The 64 samples from the RespViruses project were collected from blood donors and people requesting serological tests and other virological investigations at the University Hospital Bonn, and 62 were found positive to human respiratory syncytial virus (hRSV) in different assays (Sastre et al., 2010).
Finally, the collection provided by the Amsterdam University Medical Center included a total of 20 serum samples from the Amsterdam Cohort Studies on human immunodeficiency virus infection and AIDS (van Bilsen et al., 2020). The sera were obtained shortly (within 6 months) after infection by a seasonal Alphacoronavirus (HCoV-NL63 or HCoV-229E) or a seasonal Betacoronavirus (HCoV-HKU1 or HCoV-OC43) and contained high concentrations of antibodies to these common-cold coronaviruses.
For all the human serum samples collected, the participation was voluntary and without incentive. Formal agreements with the institutions providing the serum samples or written informed consent of each participant at enrollment was obtained.
2.2. Production of SARS-CoV-2 nucleocapsid protein
The cDNA encoding for the full-length nucleocapsid protein of the SARS-CoV-2 (GenBank accession NC_045512) was kindly provided by Dr. Volker Thiel. The forward primer N (5′-TCT GAT AAT GGA CCC CAA AAT C-3′) and reverse primer N (5′-TTA GGC CTG AGT TGA GTC AGC-3′) were used to amplify this gene. The N protein was cloned in the pCR™8/GW/TOPO™ and subsequently in the Gateway™ pDEST™17 Vector (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA), carrying a polyhistidine tag, according to the manufacturer's instructions. Resulting plasmid was verified by sequence analysis and used to transform BL21-AI™ One Shot™ (Invitrogen; Thermo Fisher Scientific, Inc.). The N protein was produced in Escherichia coli; an overnight culture was grown in lysogeny broth medium, supplemented with ampicillin (100 μg/mL) and incubated at 37 °C, 220 rpm. The overnight culture was diluted in fresh medium and incubated at 37 °C until reaching an optical density at 600 nm between 0.6 and 0.8. The culture was then induced with arabinose 0.2% and incubated overnight at 18 °C, with shaking at 220 rpm. The cells were harvested by centrifugation and resuspended in lysis buffer [300 mM NaCl; 20 mM Tris–HCl, pH 7.4; 20 mM imidazole; 1 mM MgCl2; 0.1% Triton X-100; 1 mg/mL lysozyme; 1 tablet cOmplete™, EDTA-free Protease Inhibitor Cocktail (Roche Applied Science)]. Finally, the N protein was purified using High-Density Metal Free resin (Agarose Bead Technologies) coupled to nickel according to the manufacturer's instructions. Protein gel electrophoresis followed by Coomassie staining was used to assess the purity and molecular size of the N protein.
The SARS-CoV-2 N protein was labeled with peroxidase according to the method described by Nakane and Kawaoi (1974) to be used as detector molecule in the DR-ELISA described below.
2.3. DR-ELISA (INgezim COVID 19 DR)
A DR-ELISA was developed as previously described (Venteo et al., 2012). Briefly, the N protein was used to coat 96-well plates and incubated overnight at 4 °C in carbonate buffer, pH 9.6. After washing the wells with phosphate buffered saline pH 7.4 with 0.05% Tween 20 (PBST), a blocking step was performed with StabilZyme® SELECT Stabilizer (SurModics, Inc.) for 1 h at room temperature (RT). The plate was incubated with serum samples diluted 1:5 in PBST with 2.5% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) for 30 min at RT. Duplicates of positive (rabbit polyclonal antibody to N protein of SARS-CoV-2 produced in-house) and negative (dilution buffer) controls were included in each plate. The wells were washed as described above and incubated with the HRP-conjugated N protein for 30 min at RT. Finally, after a washing step, the plate was incubated for 15 min with the substrate (TMB-MAX, Neogen Corporation), and the reaction was stopped by addition of 0.5 M sulfuric acid. The absorbance was measured at 450 nm using a SpectraMax M5 plate reader (Molecular Devices, LLC).
2.4. DR-LFA (INgezim COVID 19 CROM)
2.4.1. Capture reagents
Recombinant N protein was diluted to 0.2 mg/mL in 20 mM Tris–HCl buffer at pH 8.5 to be used as the test line capture reagent. As control line capture reagent, a monoclonal antibody against the control protein (Probumin® Bovine Serum Albumin, Merck Millipore) at 1 mg/mL was used. Both reagents were dispensed in 2 parallel lines on nitrocellulose membrane (HF120, Merck Millipore). After drying for 5 min at 45 °C, the membranes were sealed and stored at room temperature.
2.4.2. Detector reagents
Black latex beads (Merck Millipore) were activated with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxysuccinimide and then coupled to N protein at a surface concentration of 1 mg/m2, and blue latex beads were conjugated with the control protein.
To prepare the conjugate solution, the N-latex and control-latex particles were diluted at a concentration of 0.15% each in a 25-mM Tris–HCl pH 9.5 buffer. The mixture was dispensed onto the conjugate pad, dried for 30 min at 45 °C, and stored at room temperature.
2.4.3. Preparation of chromatographic strips
To assemble the 30-cm master card, nitrocellulose membrane, conjugate pad, sample pad (Cytosep 1662, Ahlstrom-Munksjö), and wicking pad were pasted on a plastic backing with adhesive and covered with a protector film. The master card was then cut into strips of 4.2-mm width.
2.4.4. Test procedure
The test was designed to be used with serum, plasma, and blood samples.
Twenty microliters of blood or 10 mL of serum/plasma was applied to the sample pad followed by 110 μL of running buffer (Tris–HCl pH 7.5, NaCl, casein, and NaN3). Results were interpreted 10 min after running buffer addition. A scale of the intensity of the signal of the test line from 1 to 10 was used in order to give a semiquantitative value for statistical purposes.
2.5. Statistical analysis
Data were statistically analyzed by a receiver–operator characteristics (ROC) curve analysis using the MedCalc® 10 software to establish the optimal cutoff value for each assay. Prior to the analyses, the samples were classified into positive or negative by PCR or by other commercial serological assays. Using the same software, Fisher's exact test was performed to determine the statistical dependence between the 2 assays developed.
3. Results
3.1. Expression of the SARS-CoV-2 N protein
The complete SARS-CoV-2 N protein was cloned in the pDEST17 vector, expressed in E. coli, and further purified by immobilized metal affinity chromatography. The highly purified N protein was analyzed by gel electrophoresis followed by Coomassie staining. A band of the expected molecular mass of the N protein (around 45 kDa) was observed (Fig. 1 ).
Fig. 1.
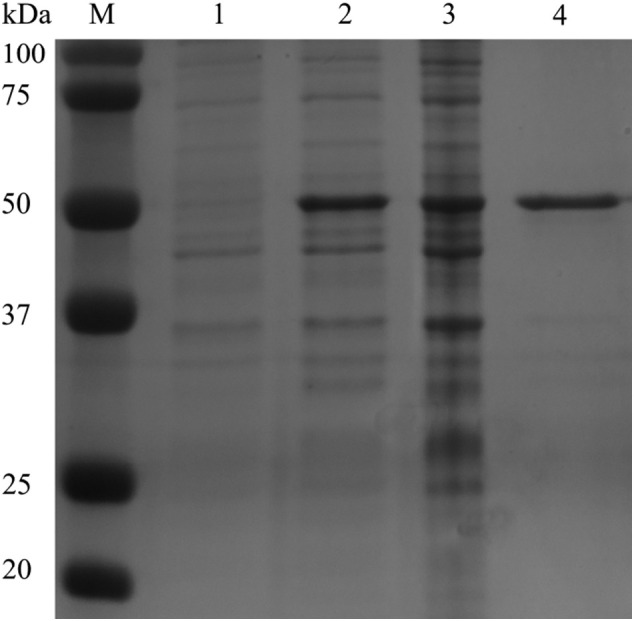
Polyacrylamide gel electrophoresis of SARS-CoV-2 N protein expressed and purified from bacterial cultures, and visualized following staining with Coomassie brilliant blue. From left to right: uninduced culture (lane 1), induced culture (lane 2), N protein before purification (lane 3), and N protein after purification by immobilized metal affinity chromatography (lane 4, MW: 46.5 kDa).
3.2. Validation of the newly developed tools: DR-ELISA and DR-LFA with samples from positive patients to COVID-19 and healthy donors
DR-ELISA and DR-LFA were developed based on the N protein of SARS-CoV-2. The DR-ELISA's and DR-LFA's conditions were assessed to obtain the optimal conditions for detection of total antibodies in the sera and blood against SARS-CoV-2.
After optimization of each assay (data not shown), a total of 1065 samples were tested in the DR-ELISA, classified as described in “Material and Methods.” To determine the performance characteristics of the newly developed DR-ELISA, an ROC analysis was performed. Since there is currently no serological gold standard, the samples were considered positive or negative according to the results obtained by PCR (in respiratory material) and a commercial serological assay (in serum). Moreover, the group of serum samples collected before 2019 was considered true-negative samples. The correlation between the results obtained in the DR-ELISA and the classification of the samples as positive (positive in PCR and serological assay) and negative (negative in PCR and serological assay or collected prior 2019) was determined. To calculate the cutoff of the assay, the sample to positive control (S/P) ratio was calculated using:
Based on this S/P value, samples were considered negative when the S/P ratio was under 5.6 and positive when its S/P ratio was equal to or above 5.6. With these values, the diagnostic sensitivity of the DR-ELISA was 100% with a 95% confidence interval (97.7–100%), and the diagnostic specificity was 98.2% with a 95% confidence interval (97–99.1%) (Fig. 2A).
Fig. 2.
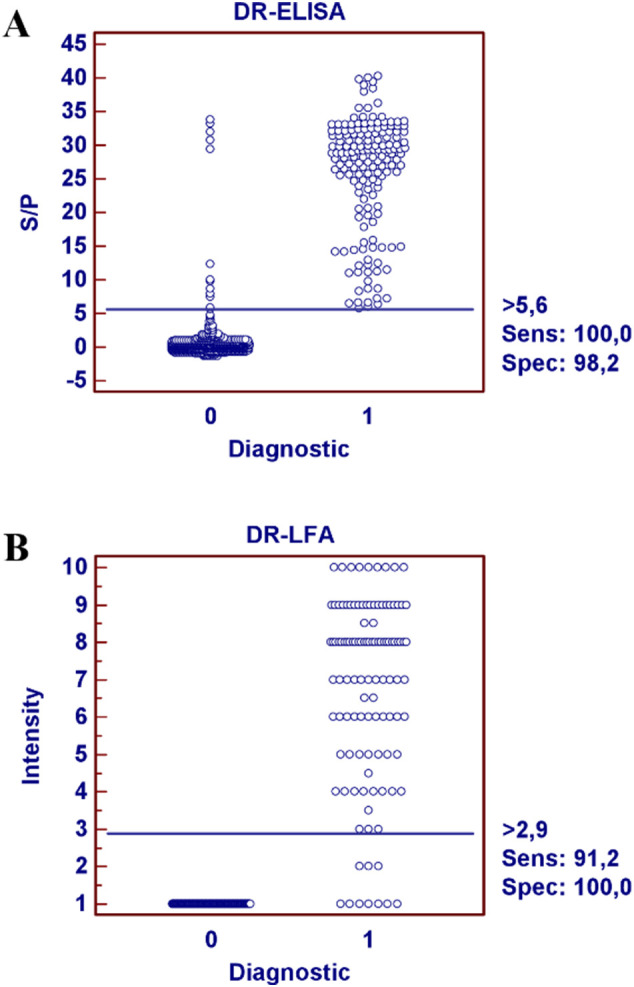
Validation of the DR-ELISA and DR-LFA. Dot plot diagrams where each dot represents an individual sample: results obtained for DR-ELISA (A) and DR-LFA (B). The horizontal solid line corresponds to the cutoff values in each assay, according to the MedCalc® 10 software. X-axis shows the positive (1) or negative (0) classification of samples according to the PCR and serological assay, and Y-axis shows S/P ratio and intensity obtained in the DR-ELISA and DR-LFA, respectively.
An ROC analysis was also performed to determine the performance characteristics of the DR-LFA and displayed in an interactive dot plot diagram (Fig. 2B). A lower number of samples were tested in the DR-LFA: 113 serum samples positive by PCR and a serological assay, 14 negative by PCR and a serological assay, and 120 serum samples collected prior 2019. The diagnostic sensitivity of the DR-LFA was 91.2% with a 95% confidence interval (84.7–95.7%), and the diagnostic specificity was 100% with a 95% confidence interval (97.3–100%).
For both assays developed, the null hypothesis was the area under the ROC curve is equal to 0.5. The P values obtained were 0.0001; therefore, there is evidence that the 2 assays developed can distinguish between the positive and negative samples tested.
Finally, a comparison was done between the DR-ELISA and DR-LFA with the samples tested in both assays. Out of the 114 positive samples in the DR-ELISA, 92.3% were positive in the DR-LFA, and out of the 133 negative samples tested in the DR-ELISA, 100% were also negative in the DR-LFA. Using the MedCalc® 10 software, Fisher's exact test was performed to examine the relation between the results obtained in the DR-ELISA and in the DR-LFA. The null hypothesis being that the variables are independent, P < 0.000000001, meaning that there is a significant relationship between the 2 assays. The comparison between the 2 techniques is summarized in Table 2 .
Table 2.
Comparison between the DR-LFA and the DR-ELISA.
| DR-ELISA |
||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| DR-LFA | Positive | 106 | 0 | 106 |
| Negative | 8 | 133 | 141 | |
| Total | 114 | 133 | 247 | |
3.3. Cross-reactivity by antibodies directed to common-cold Alpha- and Betacoronavirus and other respiratory pathogens
To fully validate the DR-ELISA and DR-LFA, the potential cross-reactivity by antibodies induced by infection with seasonal coronaviruses (HCoV-NL63, HCoV-229E, HCoV-HKU1, and HCoV-OC43) was examined by both assays. Moreover, the assays were tested with sera containing antibodies to pathogens that can induce pneumonia in infected patients such as C. trachomatis, M. pneumoniae, L. pneumophila, and hRSV, the latter only tested in the DR-ELISA. Neither of the assays showed cross-reactivity against any of the other coronaviruses, neither any other respiratory pathogen. We found one false-positive signal in the DR-ELISA to M. pneumoniae (see Table 3 ).
Table 3.
Cross-reactivity with other respiratory pathogens.
| DR-ELISA |
DR-LFA |
|||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Alphacoronavirus (HCoV-NL63/ HCoV-229E) (n = 11) | 0 | 11 | 0 | 11 |
| Betacoronavirus (HCoV-HKU1/HCoV-OC43) (n = 9) | 0 | 9 | 0 | 9 |
| Pneumovirus (hRSV) (n = 62) | 0 | 62 | ND | ND |
| C. trachomatis (n = 5) | 0 | 5 | 0 | 5 |
| M. pneumoniae (n = 17) | 1 | 16 | 0 | 17 |
| L. pneumophila (n = 21) | 0 | 21 | 0 | 21 |
ND = not determined.
4. Discussion
For the new emerging virus SARS-CoV-2, the routinely used technique for testing patients is the RT-PCR, which detects the RNA of the virus at early stages of the infection (Zou et al., 2020). Fully validated serological tests are still missing as many of the commercial serological tests currently available for SARS-CoV-2 are poorly validated or display low sensitivity or specificity (Krüttgen et al., 2020). In order to determine the prevalence of antibodies in the population and to complement the nucleic acid detection assays, especially at later days after the onset of the symptoms, serological assays are required (Meyer et al., 2014). Detection of antibodies is the most valuable indicator of the immune status of a person, identifying patients that have had COVID-19 infection and providing more accurate data related to risk of infection.
In the present study, we developed 2 serological assays using the recombinant N protein of SARS-CoV-2 as the target antigen: a DR-ELISA which is aimed to be used for screening of large numbers of samples and could be used in epidemiological studies and a DR-LFA for point-of-care testing of individual patients, which could be used by physicians without any laboratory setting required. The double recognition method was previously used by our laboratory to develop a DR-ELISA and 2 DR-LFAs for detection of antibodies to other infectious diseases affecting swine (Fresco-Taboada et al., 2019; Sastre et al., 2016; Venteo et al., 2012).
A panel of standardized samples was included in the study to determine the performance characteristics of the tests. Positive samples from COVID-19 patients with a range of clinical presentations at multiple time points after onset of symptoms were analyzed to determine the sensitivity. Pre–COVID-19 outbreak samples and samples with antibodies to other respiratory pathogens were used to determine the specificity. The diagnostic sensitivity of the assays was 100% and 91.2% for the DR-ELISA and DR-LFA, respectively. The sensitivity of the DR-LFA was lower than that of the DR-ELISA, as it has been described in other cases (Parolo et al., 2013). The requirements of each assay are different, as the DR-LFA can be applied as a point-of-care test, whereas the DR-ELISA needs to be done in a laboratory by qualified technicians. Moreover, while the DR-LFA takes 10 min to give 1 result, the DR-ELISA takes 75 min to analyze 92 samples. Out of the PCR+/antibody− group from Table 1, a group of 14 serum samples from early days postinfection, positive to COVID-19 by respiratory-PCR yet still negative in the commercial serological assay (with seroconversion a few days later), was also tested in our assays. Four of these sera were positive in the DR-ELISA and 3 in the DR-LFA, indicating that the DR-assays we developed are highly sensitive.
Regarding the specificity of the newly developed assays, we only found 1 sample positive to M. pneumonia that gave a positive signal in the DR-ELISA. Interestingly, no cross-reactivity by antibodies directed to seasonal Alpha- or Betacoronavirus was observed in our DR assays, in contrast with regular SARS-CoV-2 antibody tests that do sometimes detect antibodies induced by HCoV-OC43 infection (Okba et al., 2020).
We tested samples from 452 individuals that were negative for the virus in respiratory material (PCR) and also negative in serological assays, yet they were collected in a high-risk group (personal communications). Eleven serum samples showed a positive signal in the DR-ELISA. Five had a very high S/P (>30), and the others were found with lower S/P (between 6 and 10). Three of the positives with high S/P were tested also for confirmation purposes in the DR-LFA, and also in this test, the samples showed positive signals. These results could indicate that our tests can give false positives or that the patients had experienced a previous infection, yet this was not diagnosed by the commercial assays, maybe not fully validated so far. The commercial tests used for classification only detect IgG and IgM, and the samples could contain IgA antibodies. This means these samples are considered negative in the commercial tests, but positive in our DR assays that detect all Ig isotypes (Béné et al., 2020).
The serological assays could be a great complementary tool to the nucleic acid detection assays as, in this study, out of 626 samples with a negative PCR in respiratory material, 174 serum samples could diagnose a SARS-CoV-2 infection via serology (see Table 1). In these 174 patients, 123 were positive only to IgG, 23 were positive only to IgM, and 28 were positive to IgG and IgM. An 89% correspondence was found between the DR-ELISA and the commercial serological assay for the 151 samples positive to IgG or positive to IgG and IgM, but only 1 of the IgM positive was found positive in the DR-ELISA. This could demonstrate the higher affinity of IgG compared to IgM (Murphy et al., 2008), which could lead to lower specificity of serological assays specifically targeting IgM.
Double recognition assays are sensitive tests, yet they also offer 2 additional advantages. First, it is a multispecies test, detecting antibodies in human serum but also in serum samples from other animal species since it uses the target antigen as the detector molecule instead of antispecies antibody, and secondly, it detects total antibodies in a given sample. Unlike the antibody response usually observed in other infectious diseases (first IgM followed by IgG), during COVID-19 infection, IgM and IgG antibody responses appear almost simultaneously (Long et al., 2020; Sethuraman et al., 2020). Similar results were described previously for SARS, where the IgM appeared at the same time as the IgA and IgG (Meyer et al., 2014). This shows the importance of having a test that detects total antibodies in serum.
5. Conclusions
Using the N protein of the SARS-CoV-2 as the coating antigen and as the enzyme-conjugated antigen instead of enzyme-conjugated secondary antibody provides a specific and sensitive serological assay for detection of total antibodies to SARS-CoV-2. The 2 assays developed in this study have been fully validated and received the CE marking. They can be used as complementary tools to nucleic acid detection assays, in epidemiological studies for screening of large populations (INgezim COVID 19 DR), and as point-of-care test (INgezim COVID 19 CROM), especially in later stages of the infection.
Acknowledgments
Acknowledgments
We thank Dr. Belén Rebollo for technical support at Eurofins Ingenasa and María García and Alejandro Soler for technical assistance at INIA-CISA. We also thank Dr. John N. Barr for critical reading of the manuscript and assistance with the English language.
Funding
This work was supported by the EU Marie Skłodowska-Curie Actions Innovative Training Network HONOURs under grant no. 721367 (to Alexis C.R. Hoste).
Data availability
The datasets generated and analyzed during this work are available from the corresponding author on reasonable request.
References
- Bai Y., Yao L., Wei T., Tian F., Jin D., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béné M.C., de Carvalho Bittencourt M., Eveillard M., Le Bris Y. 2020. Good IgA bad IgG in SARS-CoV-2 infection? Clin Infect Dis ciaa426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bilsen W.P.H., Boyd A., van der Loeff M.F.S., Davidovich U., Hogewoning A., van der Hoek L. Diverging trends in incidence of HIV versus other sexually transmitted infections in HIV-negative MSM in Amsterdam. AIDS. 2020;34:301–309. doi: 10.1097/QAD.0000000000002417. [DOI] [PubMed] [Google Scholar]
- Che X.Y., Qiu L.W., Pan Y.X., Wen K., Hao W., Zhang L.Y. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J Clin Microbiol. 2004;42:2629–2635. doi: 10.1128/JCM.42.6.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Xu X., Wu Y., Wang L., Cowling B.J., Meyers L. Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis. 2020;26(6):1341–1343. doi: 10.3201/eid2606.200357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco-Taboada A., Risalde M.A., Gortázar C., Tapia I., González I., Venteo Á. A lateral flow assay for the rapid diagnosis of Mycobacterium bovis infection in wild boar. Transbound Emerg Dis. 2019;66:2175–2179. doi: 10.1111/tbed.13260. [DOI] [PubMed] [Google Scholar]
- Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüttgen A., Cornelissen C.G., Dreher M., Hornef M., Imöhl M., Kleines M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J Clin Virol. 2020;128:104394. doi: 10.1016/j.jcv.2020.104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Meyer B., Drosten C., Müller M.A. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. doi: 10.1016/j.virusres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Travers P, Walport M. 2008. The distribution and functions of immunoglobulin classes. Janeway's immunobiology, 7th ed. Garland Science, New York, pp. 400–401.
- Nakane P.K., Kawaoi A. Peroxidase-labeled antibody a new method of conjugation. J Histochem Cytochem. 1974;22:1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Narayanan K., Chen C.J., Maeda J., Makino S. Nucleocapsid-independent specific viral RNA packaging via viral envelope protein and viral RNA signal. J Virol. 2003;77:2922–2927. doi: 10.1128/JVI.77.5.2922-2927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okba N., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolo C., de la Escosura-Muñiz A., Merkoçi A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens Bioelectron. 2013;40:412–416. doi: 10.1016/j.bios.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Sastre P., Cusi M.G., Manoha C., Schildgen O., Ruiz T., Vela C. Serum antibody response to respiratory syncytial virus F and N proteins in two populations at high risk of infection: children and elderly. J Virol Methods. 2010;168:170–176. doi: 10.1016/j.jviromet.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Sastre P., Pérez T., Costa S., Yang X., Räber A., Blome S. Development of a duplex lateral flow assay for simultaneous detection of antibodies against African and classical swine fever viruses. J Vet Diagn Invest. 2016;28:543–549. doi: 10.1177/1040638716654942. [DOI] [PubMed] [Google Scholar]
- Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- Venteo A., Rebollo B., Sarraseca J., Rodriguez M.J., Sanz A. A novel double recognition enzyme-linked immunosorbent assay based on the nucleocapsid protein for early detection of European porcine reproductive and respiratory syndrome virus infection. J Virol Methods. 2012;181:109–113. doi: 10.1016/j.jviromet.2012.01.024. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) COVID-19 situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available at.
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during this work are available from the corresponding author on reasonable request.


