Abstract
Myeloperoxidase (MPO) is a vital component of the innate immune system, which produces the potent oxidant hypochlorous acid (HOCl) to kill invading pathogens. However, an overproduction of HOCl during chronic inflammatory conditions causes damage to host cells, which promotes disease, including atherosclerosis. As such, there is increasing interest in the use of thiocyanate (SCN−) therapeutically to decrease inflammatory disease, as SCN− is the favoured substrate for MPO, and a potent competitive inhibitor of HOCl formation. Use of SCN− by MPO forms hypothiocyanous acid (HOSCN), which can be less damaging to mammalian cells. In this study, we examined the ability of SCN− to modulate damage to macrophages induced by HOCl, which is relevant to lesion formation in atherosclerosis. Addition of SCN− prevented HOCl-mediated cell death, altered the extent and nature of thiol oxidation and the phosphorylation of mitogen activated protein kinases. These changes were dependent on the concentration of SCN− and were observed in some cases, at a sub-stoichiometric ratio of SCN−: HOCl. Co-treatment with SCN− also modulated HOCl-induced perturbations in the expression of various antioxidant and inflammatory genes. In general, the data reflect the conversion of HOCl to HOSCN, which can induce reversible modifications that are repairable by cells. However, our data also highlight the ability of HOSCN to increase pro-inflammatory gene expression and cytokine/chemokine release, which may be relevant to the use of SCN− therapeutically in atherosclerosis. Overall, this study provides further insight into the cellular pathways by which SCN− could exert protective effects on supplementation to decrease the development of chronic inflammatory diseases, such as atherosclerosis.
Keywords: Myeloperoxidase, Thiocyanate, Hypochlorous acid, Inflammation, Atherosclerosis
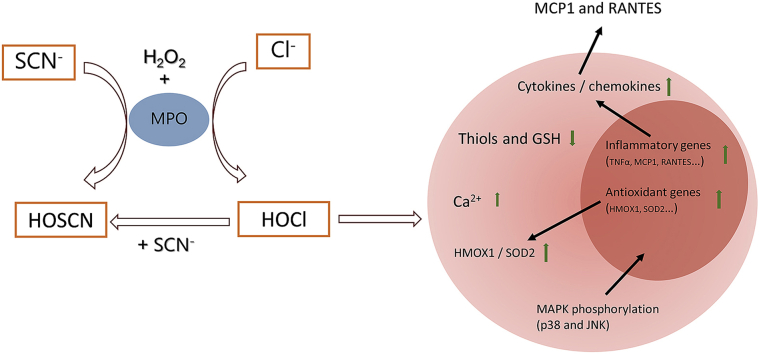
Graphical abstract
Highlights
-
•
Thiocyanate (SCN−) influences the extent and nature of HOCl-induced macrophage damage.
-
•
SCN− converts HOCl to HOSCN to give reversible modifications repairable by cells.
-
•
SCN− modulates HOCl perturbations of MAPK phosphorylation and antioxidant defences.
-
•
Both HOCl and HOSCN induce pro-inflammatory signalling and cytokine expression.
Abbreviations
- 18S
18S ribosomal RNA
- BSA
bovine serum albumin
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular signal regulated kinase
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCLc
glutamate – cysteine ligase catalytic subunit
- GCLm
glutamate – cysteine ligase modifier subunit
- GPx1
glutathione peroxidase 1
- GSTP1
glutathione S-transferase Pi 1
- HBSS
Hanks balanced salt solution
- HMOX1
heme oxygenase 1
- HOBr
hypobromous acid
- HOCl
hypochlorous acid
- HOSCN
hypothiocyanous acid
- IL-1β
interleukin-1β
- IL-18
interleukin 18
- JNK
c-Jun N-terminal kinase
- LDL
low-density lipoprotein
- LPO
lactoperoxidase
- MAPK
mitogen activated protein kinase
- MCP-1
monocyte chemoattractant protein 1
- MPO
myeloperoxidase
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
- NFκB
nuclear factor κB
- PTP
protein tyrosine phosphatases
- RANTES
Regulated on Activation Normal T cell Expressed and Secreted
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- SCN−
thiocyanate
- SOD2
mitochondrial superoxide dismutase 2
- TBP
TATA-box binding protein
- TNFα
tumour necrosis factor α
- TrxR
thioredoxin reductase
- TXNIP
thioredoxin interacting protein
1. Introduction
Myeloperoxidase (MPO), an important heme peroxidase enzyme released by immune cells, generates various oxidants, which have potent antibacterial properties and play an important role in innate immunity [1,2]. The main oxidants produced by MPO under normal physiological conditions are hypochlorous acid (HOCl) and hypothiocyanous acid (HOSCN) from the reaction of hydrogen peroxide (H2O2) with chloride (Cl−) and thiocyanate (SCN−) ions, respectively [3,4]. HOCl is a reactive and indiscriminate oxidant, which induces oxidative damage to proteins and many other biological molecules [1,5]. The overproduction of HOCl and the associated oxidative damage is strongly linked to the development of many chronic inflammatory pathologies [1].
The evidence linking MPO and production of HOCl with cardiovascular disease is particularly compelling. MPO is enriched in human atherosclerotic plaques and involved in all stages of atherosclerosis, from the initiation stage of low-density lipoprotein (LDL) oxidation, to inducing endothelial dysfunction and weakening of the fibrous cap (reviewed [[6], [7], [8]]). In addition, it is well established that MPO is a key risk factor and prognostic marker for the development of cardiovascular disease and associated complications (reviewed [9]). Evidence for elevated levels of HOCl-specific biomarkers in human atherosclerotic tissue implicates this oxidant as playing a role in MPO-mediated cellular damage [10,11]. In contrast, whether HOSCN plays a role in the development of chronic inflammatory pathologies is less clear [4,12].
Given the damaging nature of HOCl and its role in numerous chronic inflammatory pathologies, there is significant interest in developing strategies to mitigate the extracellular production of this oxidant by MPO. HOSCN is a less potent oxidant compared to HOCl, and reacts by targeting free thiol (and selenol) residues with high selectivity [[13], [14], [15], [16]]. This results in the formation of a range of reversible oxidation products, including sulfenyl thiocyanates, sulfenic acids and disulfides, which can potentially be repaired [[17], [18], [19], [20]]. In addition, unlike HOCl, HOSCN can be detoxified by cellular antioxidant systems, particularly thioredoxin reductase (TrxR) [21]. Therefore, HOSCN could be considered to be less damaging and more tolerated by host tissues than HOCl. This raises the possibility that supplementation with SCN− to favour HOSCN production by MPO could have therapeutic benefit in chronic inflammatory pathologies [4,22].
SCN− is ubiquitous in human plasma (around 30–50 μM) [23], and can be markedly elevated by smoking, drugs and diet [16,24,25]. Extracellular fluids, such as saliva and airway secretions, are enriched with SCN− with levels up to mM, but the concentrations vary widely depending on individuals and physiologic conditions [[26], [27], [28]]. Compared to Cl−, SCN− has a much higher specificity constant (ca. 730 fold) for MPO, which results in the preferential formation of HOSCN on increasing plasma SCN− [3,25]. Supplementation with SCN− to achieve levels of 400 μM are predicted to completely mitigate HOCl production by MPO under normal, physiological concentrations of Cl− [22]. Moreover, the direct reaction of SCN− with HOCl also produces HOSCN in physiological fluids (Reaction 1) [29]. However, it has been reported that an excess of SCN− is required to cleanly produce HOSCN from HOCl or related species, owing to the ability of HOSCN to react further with HOCl to form over-oxidized products such as HO2SCN (Reaction 2) [30]. The biological reactivity of these over-oxidation products has not been characterised, but sulfate (SO42−) and cyanate (OCN−) are the reported end products of this reaction (Reaction 3, where Cl+ is from HOCl) [30].
| HOCl + SCN− → OSCN− + Cl− + H+ | (1) |
| HOCl + OSCN− → O2SCN− + Cl− + H+ | (2) |
| 4 Cl+ + SCN− + 5 H2O → 4 Cl- + SO42− + OCN− + 10 H+ | (3) |
Previous studies have indicated that elevated concentrations of SCN− can decrease the extent of HOCl-induced protein oxidation [31], cell death in model in vitro systems [[32], [33], [34]] and the development of disease in vivo [[34], [35], [36]]. Thus, in a cystic fibrosis infection model, nebulisation with SCN− was shown to effectively decrease the infiltration of neutrophils into the airway, together with inflammation and pro-inflammatory cytokine production and reduced the bacterial load [34]. In atherosclerosis-prone mice that over-express human MPO, supplementation with SCN− in the drinking water resulted in a 30% decrease in lesion formation [35]. SCN− supplementation also reduced plaque size in ApoE−/- mice, and decreased serum levels of the pro-inflammatory cytokine interleukin 6 (IL-6), while increasing interleukin 10 (IL-10), which is associated with the resolution of inflammation [36]. Evidence was also obtained in this study for reduced oxidative damage and improved endothelial function [36]. In humans, it has been shown that elevated plasma SCN− correlates with decreased long-term mortality in patients after a first myocardial infarction [37].
However, in general, the underlying mechanism responsible for these effects are poorly defined. This is significant, in light of the body of data from in vitro studies with different cellular models, for a detrimental and damaging effect of HOSCN. Studies show that both HOCl and HOSCN result in oxidative damage, enzyme inactivation, altered cytosolic Ca2+ accumulation, activation of pro-inflammatory signalling to culminate in cell death by various pathways (reviewed [1,38]). In some cases, HOSCN has been reported to be more damaging than HOCl on account of its ability to selectively target free Cys residues, which can result in a greater extent of enzyme inactivation (e.g. Ref. [39,40]). However, these effects are highly dependent on the specific treatment conditions, as there is evidence that cells can recover from oxidative insult following exposure to HOSCN [41].
In this study, we examined the ability of SCN− to influence the extent and nature of HOCl-induced damage to macrophages, which are a key target for MPO-derived oxidants in the vasculature [42], and play a critical role in the development of atherosclerosis [43,44]. We focus on the role of HOCl compared to HOSCN in the oxidation of intracellular thiols, perturbation of cytosolic Ca2+ and activation of pro-inflammatory signalling cascades, and assess how the presence of SCN− influences these pathways. These studies provide further insight into the pathways by which SCN− could influence lesion development, given the aberrant pro-inflammatory signalling and macrophage dysfunction that is prevalent in atherosclerosis.
2. Materials and methods
2.1. Reagents and materials
All aqueous solutions were prepared using nano-pure H2O from a MilliQ system (Millipore). The concentration of HOCl was determined by UV absorbance at 292 nm at pH 11 using an extinction coefficient of 350 M−1 cm−1 [45]. HOSCN was prepared enzymatically using lactoperoxidase (LPO; from bovine milk) as described previously [39] with the concentration determined by quantifying the consumption of 5-thio-2- nitrobenzoic acid (TNB) at 412 nm [46] using an extinction coefficient of 14,150 M−1 cm−1 [47]. All chemicals and reagents were of the highest purity available and purchased from Sigma-Aldrich/Merck unless stated otherwise.
2.2. Cell culture
Murine macrophage-like J774A.1 cells (ATCC No. 91051511) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; ThermoFisher), 2 mM l-glutamine and 100 units mL−1 penicillin (Invitrogen) at 37 °C in a 5% CO2 incubator. For experiments, cells were seeded in 12-, 24- or 96-well plates at a density of 1 × 106 cells mL−1 using a volume of 1000 μL, 500 μL or 100 μL, respectively, and allowed to adhere overnight. Before treatments, cells were washed with warm Hanks buffered salt solution (HBSS) at 37 °C. The same volumes of treatment media were used to ensure that the ratio of oxidant: cell remains constant in each case.
2.3. Cell viability
Cell viability was measured using a commercial MTS (3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay kit (Promega). J774A.1 cells (0.1 × 106 cells/well in 96-well plates) were treated with HOCl (0–200 μM) or HOSCN (0–200 μM), or were co-treated with HOCl (100 or 200 μM) and SCN− (0–200 μM) for 1 h, with or without subsequent re-incubation in DMEM for 4–24 h. Following treatment, cells were washed with HBSS and re-incubated in DMEM (100 μL) containing the MTS reagent (10 μL) for 4 h. The absorbance change was measured at 490 nm using a Spectra Max i3x microplate reader (Molecular Devices).
2.4. Quantification of cellular thiols
J774A.1 cells (0.5 × 106 cells/well in 24-well plates) were treated with HOSCN or co-treatments of HOCl with SCN− as described above. To assess whether thiol oxidation could be reversed, total cellular thiols were measured after 1 h treatment and following re-incubation in DMEM for 2, 4 or 24 h. The cellular thiol concentrations were assessed using the ThioGlo-1 assay, as described previously, with fluorescence measured at λex 384 nm and λem 513 nm [46]. Thiol concentrations were quantified using a standard curve constructed with GSH. Total protein concentrations were quantified by BCA assay (Pierce™ BCA Protein Assay Reagent A, ThermoFisher) assay to normalize thiol concentrations, as previously [41].
2.5. Cellular GSH quantification by HPLC
J774A.1 cells (0.5 × 106 cells/well in 24-well plates) were treated with HOCl, HOSCN or HOCl/SCN− for 1 h, with and without re-incubation in DMEM (4 and 24 h). Cells were washed with PBS and lysed in water (200 μL) before analysis of GSH. Cellular GSH was quantified after derivatization with monobromobimane using HPLC separation, as described previously [41]. Briefly, an equal volume of KPBS buffer (50 mM potassium phosphate buffer, 17.5 mM EDTA, 50 mM serine, 50 mM boric acid, pH 7.4) was added to cell lysate, and samples were derivatized by addition of 10 μL monobromobimane (3 mM in acetonitrile), and incubated for 30 min in the dark. The reaction was terminated by the addition of 10 μL perchloric acid (70% v/v). Mobile phase A consisted of 1% (v/v) acetic acid and 5% (v/v) acetonitrile, and mobile phase B consisted of 1% (v/v) acetic acid and 20% (v/v) acetonitrile. The pH of each mobile phase was adjusted to 4.25 using ammonium hydroxide. GSH was quantified after separation using a Shimadzu HPLC system equipped with a fluorescence detector (RF-20Axs, Shimadzu; using λex 390 nm and λem 480 nm), with a Synergi 4-mm Hydro-RP C-18 column (150 × 4.6 mm; Phenomenex) and a flow rate of 1 mL min−1 at 30 °C. Gradient elution used tfor the separation as follows: 0% B for 0–5 min; 0–100% B over 5–10 min; 100% B for 10–15 min; 100-0% B over 15–17 min; 0% B re-equilibration for 17–25 min. Total protein concentrations in the cell lysates were quantified by BCA assay to normalize GSH concentrations to cell protein in each case.
2.6. Quantitative real-time polymerase chain reaction (qPCR)
J774A.1 cells (0.5 × 106 cells/well in 24-well plates) were treated with HOCl (50 or 100 μM), HOSCN (100 or 200 μM) or a co-treatment of HOCl (100 μM) with SCN− (50 or 200 μM) for 1 h, before re-incubation in DMEM (3 h for NF-κB1 and p65, 24 h for other genes). Total RNA was extracted using an RNeasy Kit (Qiagen), and genomic DNA removed using RNAse Free DNase (Qiagen) before reverse transcription using a SensiFAST cDNA Synthesis Kit (Nordic Biosite). Real-time PCR was performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems) under the following thermal cycling conditions: 95 °C for 5 min, then 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s for 40 cycles, followed by 95 °C for 1 min and 55 °C for 1 min. A melt curve step consisting of step-wise temperature increases of 0.5 °C every 5 s beginning at 65 °C and ending at 95 °C was performed. The primer sequences are shown in Table S1. Relative mRNA concentrations of the genes of interest were normalized to 18S ribosomal RNA (18S) and TATA-box binding protein (TBP) housekeeping genes. Data analysis was carried out using the 2−ΔΔCT method.
2.7. Western blotting
For the expression of mitochondrial superoxide dismutase (SOD2) and heme oxygenase 1 (HMOX1), J774A.1 cells (1 × 106 cells/well in 12-well plates) were treated with oxidants as for the qPCR experiments and re-incubated in DMEM for 3 h and 24 h. The cell lysates were harvested in RIPA buffer (Sigma-Aldrich) containing 1% protease inhibitors (Sigma-Aldrich). To assess phosphorylation indicative of mitogen activated protein kinase (MAPK) signalling, the cells were treated with HOCl (100 μM) or HOSCN (200 μM) for different treatment time points (5–120 min), or treated with oxidants for 1 h and then re-incubated for different treatment time points (0–180 min) in DMEM. Cell lysates were harvested in RIPA buffer containing 1% protease and phosphatase (PhosSTOP) inhibitors (Sigma-Aldrich). Lysates were centrifuged (10,000 g, 10 min, 4 °C) to remove cell debris. The total protein concentration was determined by BCA assay to make sure an equal amount of protein was loaded.
Protein (10 μg) was then separated by SDS-PAGE using NuPAGE 4–12% Bis-Tris gels (ThermoFisher) at 200 V for 35 min, and transferred onto a polyvinylidene fluoride (PVDF) membrane (ThermoFisher) at 20 V for 7 min. Membranes were blocked with 1% (w/v) BSA in TBST (0.1% Tween-20 in TBS) for 1 h at 21 °C, washed three times in TBST for 5 min, and then incubated with the following primary antibodies (dilution 1:1000) overnight at 4 °C: anti-HMOX-1 (No. ab13243, Abcam), anti-SOD2 (No. ab68155, Abcam), anti-p-ERK (No. 44-680G, ThermoFisher), anti-ERK (No. 44-654G, ThermoFisher), anti-p-JNK (No. 4668s, CST/BioNordika), anti-JNK (No. 9252s, CST/BioNordika), anti-p-p38 (No. 4511s, CST/BioNordika) or anti-p38 (No. 8690s, CST/BioNordika). The membranes were washed three times in TBST and incubated in HRP-conjugated anti-mouse (1:2000, No. NXA931, VWR) or anti-rabbit (1:2000, No. 7074S, BioNordika) IgG secondary antibodies for 1 h at 21 °C. The membrane was washed a further three times in TBST and imaged using SuperSignal™ West Pico PLUS Chemiluminescent Substrate (ThermoFisher) using a Sapphire Biomolecular Imager (Azure Biosystems). Band densities were normalized to the loading control protein β-actin.
2.8. Quantification of cytokines using ELISA
J774A.1 cells (1 × 106 cells/well in 12-well plates) were treated for 1 h and re-incubated in cell media for 3 and 24 h, as described above. Cell media (supernatant) was collected after centrifugation (1000 g, 10 min) at 21 °C. Cell lysates were harvested in RIPA buffer containing 1% protease inhibitors, as described above. Levels of extracellular and intracellular MCP-1/CCL2 and CCL5/RANTES were quantified using a commercial MCP-1/CCL2 Mouse ELISA Kit (ThermoFisher) and Mouse CCL5/RANTES DuoSet ELISA (R&D) according to the manufacturer's instructions. For cell lysates, the concentrations of each cytokine was normalized to total protein measured by BCA assay.
2.9. Measurement of intracellular Ca2+
Cytosolic Ca2+ was assessed using the calcium indicator Fluo-4 AM (Life technologies) coupled with flow cytometry. J774A.1 cells (1 × 106 cells/well in 12-well plates) were treated with oxidants for 1 h in either Ca2+-supplemented HBSS or Ca2+-free HBSS, followed by the addition of Fluo-4 AM (5 μM) for 15 min, and washing and lifting into suspension by gentle scraping. Flow cytometry analysis was performed on a FACSVerse flow cytometer (BD Biosciences). To further investigate the alterations in cytosolic Ca2+, cells were pre-treated for 15 min with 1) Ru 360 (25 μM), to inhibit mitochondrial calcium uptake, 2) thapsigargin (25 μM), to inhibit SERCA (sarco/endoplasmic reticulum Ca2+ ATPase), or 3) nisoldipine (25 μM), to inhibit L-type (dihydropyridine) calcium channels, before treatment with oxidants.
2.10. Statistical analyses
Statistical analyses were performed using GraphPad Prism (versions 7 and 8; GraphPad Software, San Diego, USA) using 1-way or 2-way ANOVA with p < 0.05 taken as significant. Data represent mean ± S.E.M. from at least 3 independent experiments in each case, with the details of the specific multiple comparison tests used outlined in the Figure Legends.
3. Results
3.1. SCN− prevents HOCl-induced cell death and alters the pattern of cellular thiol oxidation
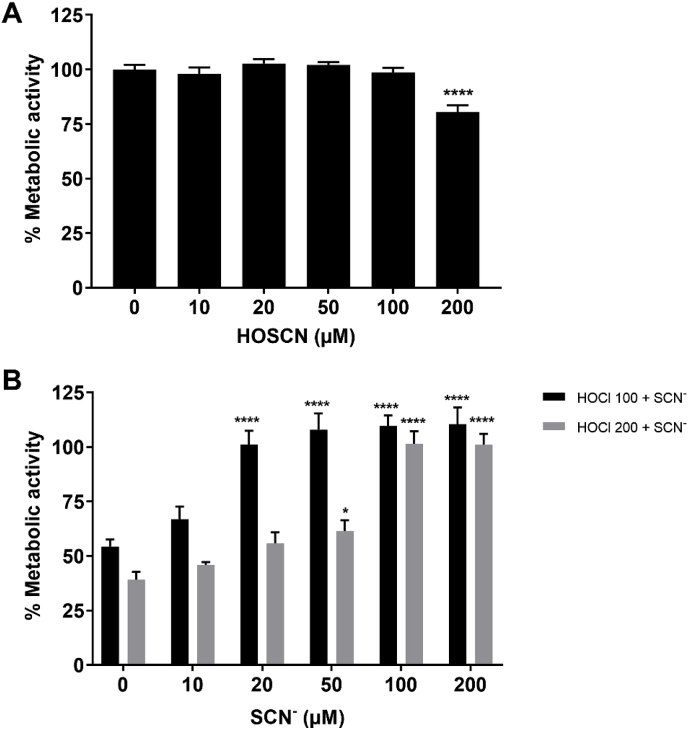
Initial studies were performed to examine the ability of SCN− to prevent HOCl-induced J774A.1 cell death, as reported for other cell types [33,34]. J774A.1 cells were exposed to HOCl (100 or 200 μM) for 1 h in the absence or presence of SCN− (10–200 μM), prior to examining the metabolic activity of the cells using the MTS assay. Exposure of the cells to HOCl caused a significant decrease in their metabolic activity, consistent with a loss in viability to ≤50%, when exposed to 100 or 200 μM HOCl, compared to that seen in the non-treated cells (Fig. 1B). The loss in metabolic activity was less extensive or prevented, on addition of HOCl to the cells in the presence of increasing concentrations of SCN− (Fig. 1B). This protective effect was dependent on the concentration of both HOCl and SCN−. A more pronounced protective effect of SCN− was seen in experiments with 100 μM HOCl, where the addition of a sub-stoichiometric concentration of SCN− (20 μM) led to a significant increase in the metabolic activity to levels comparable to that of the non-treated cells (Fig. 1B).
Fig. 1.
SCN−decreases HOCl-induced cell death. J774A.1 cells (1 × 105) were exposed to (A) HOSCN (0–200 μM) or (B) HOCl (100 μM, black bars; 200 μM, grey bars) in the absence and presence of SCN− (0–200 μM) for 1 h at 37 °C in HBSS before washing and re-incubation in cell media with MTS for 4 h. The metabolic activity of the treated cells is shown as a percentage compared to the non-treated control group. In (A) **** shows a significant difference (p < 0.0001) compared to the non-treated control group by a 1-way ANOVA with a Dunnett's multiple comparison test. In (B) *, and **** show a significant difference (p < 0.05 and 0.0001, respectively) compared to the respective HOCl-treated group without addition of SCN− by a 2-way ANOVA with a Sidak's multiple comparison test.
The metabolic activity of the cells exposed to 100 μM HOCl in the presence of >20 μM SCN− was greater (over 100%) than that seen in the non-treated cells (Fig. 1B). This effect was not apparent in cells exposed to SCN− in the absence of HOCl (data not shown). This effect was also not observed in the corresponding experiments with HOSCN, which is formed on reaction of HOCl with SCN− [29,30]. With HOSCN, evidence was obtained for a loss in metabolic activity on exposure of J774A.1 cells to 200 μM oxidant, which was much less extensive than that seen with HOCl (Fig. 1A). These data conflict with previous studies performed with HOCl and HOSCN in this cell type, where a greater degree of toxicity was apparent with HOSCN [39]. This discrepancy may be attributed to the measurement of MTS metabolism following washing and re-incubation of the cells over 4 h in cell media, which could allow for alterations in gene expression, or the repair of reversible modifications to thiols, which are key targets for HOSCN [16]. Therefore, total cellular thiols were measured using ThioGlo-1, to further investigate cellular toxicity induced by HOCl and HOSCN.
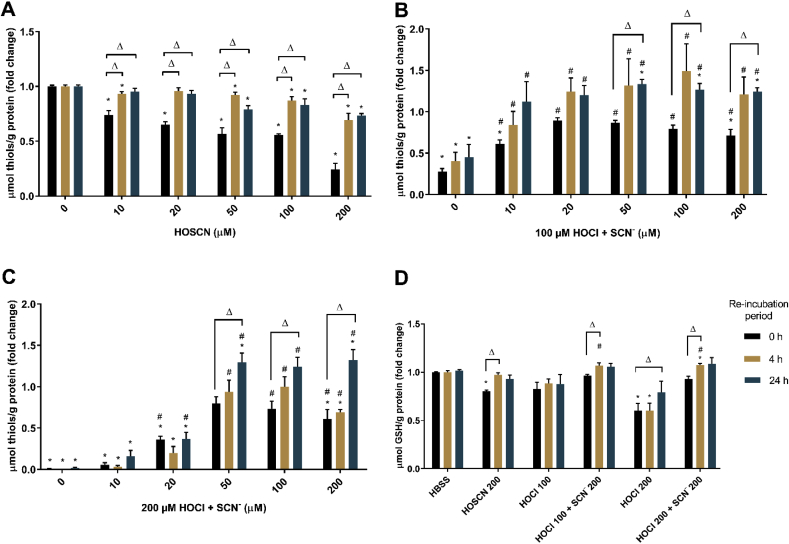
Exposure of J774A.1 cells to HOSCN (Fig. 2A) and HOCl (Fig. 2B and C) for 1 h resulted in a significant, dose-dependent, decrease in intracellular thiols compared to that seen in the non-treated, control cells, when measured immediately following treatment. This dose-dependent decrease in cellular thiols was not as marked on re-incubation of the cells in media for 4 or 24 h, following initial HOSCN exposure (Fig. 2A). In each case, the cell thiol concentrations have been normalized to the intracellular protein concentration to account for any loss of thiols due to cell lysis. The data are therefore consistent with reversible thiol oxidation under these treatment conditions, particularly with ≤100 μM HOSCN.
Fig. 2.
SCN−influences the extent and nature of HOCl-induced thiol oxidation in J774A.1 cells. Thiol concentration was assessed by the ThioGlo-1 assay after treatment of the J774A.1 cells (0.5 × 106 cells/well) with (A) HOSCN (0–200 μM), (B) co-treatment with HOCl (100 μM) and SCN− (0–200 μM) or (C) co-treatment with HOCl (200 μM) and SCN− (0–200 μM) for 1 h at 37 °C, followed by re-incubation in cell media for 0 h (black bars), 4 h (yellow bars) and 24 h (blue bars). (D) shows the GSH concentration in the cells after treatment with HOSCN (200 μM), HOCl (100 or 200 μM) or co-treatment of HOCl (100 or 200 μM) and SCN− (200 μM) for 1 h before cell lysis and derivatization of GSH with monobromobimane and HPLC separation as described above. Cellular thiols and GSH are normalized by total protein and shown as fold changes compared to the non-treated control groups. * shows a significant difference (p < 0.05) compared to the non-treated control groups with same re-incubation time period; # shows a significant difference (p < 0.05) compared to HOCl group without addition of SCN−; Δ shows a significant difference (p < 0.05) compared to counterparts (with same treatments) without re-incubation by 2-way ANOVA with a Tukey's multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In contrast, although exposure to 100 or 200 μM HOCl for 1 h resulted in a decrease in intracellular thiols, there was no significant increase in intracellular thiols on re-incubation of cells for 4 or 24 h following initial exposure to HOCl (Fig. 2B and C), consistent with non-reversible thiol oxidation. The HOCl-induced decrease in intracellular thiols was significantly less extensive on addition of the oxidant in the presence of SCN− (10–200 μM), particularly in experiments with 100 μM HOCl, where the concentration of thiols was not significantly different to non-treated, control cell levels, in the presence of ≥20 μM SCN− (Fig. 2B and C). In the co-treatment experiments, a further increase in thiol concentration to levels greater than that seen in the non-treated cells was also apparent on re-incubation of the cells, which was significant at 24 h following the initial oxidant exposure with 50, 100 and 200 μM SCN− (Fig. 2B and C).
These studies were extended to determine the effect of HOCl, HOSCN and the co-treatment of HOCl with SCN− on intracellular GSH, using HPLC following derivatization of the GSH with monobromobimane. A significant loss of GSH was observed on exposure of the cells to HOCl or HOSCN (200 μM) for 1 h, which was reversed on re-incubation of the cells with media for 4 h (HOSCN) or 24 h (HOCl) (Fig. 2D). The concentration of GSH in the cells was greater on co-treatment of the cells with HOCl and SCN− (both 200 μM) compared to that seen with HOCl (Fig. 2D). Under these co-treatment conditions, a further increase in intracellular GSH was observed on re-incubation of the cells for 4 and 24 h following the initial oxidant treatment, analogous to that seen in the experiments with HOSCN (Fig. 2D).
3.2. Effect of HOCl, HOSCN and SCN− on the expression of antioxidant enzymes in J774A.1 cells
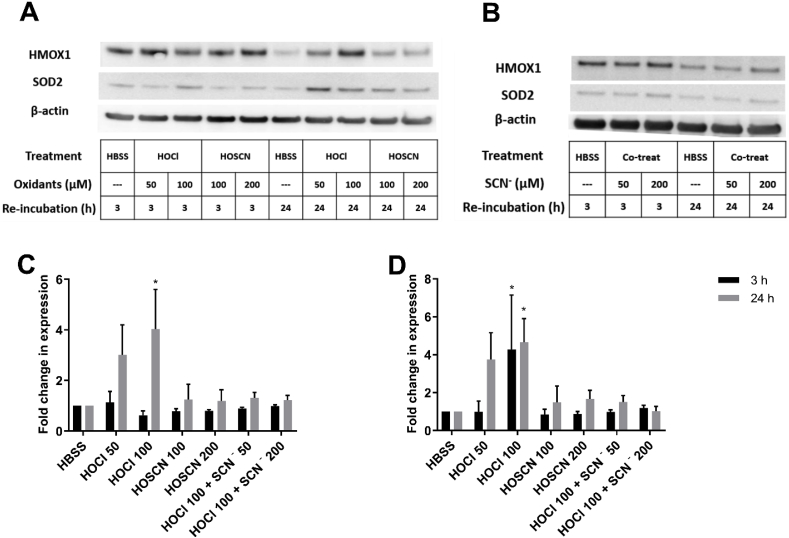
Given the effects of SCN− on the extent and nature of HOCl-induced thiol oxidation, studies were performed to examine the effect of the oxidants on the expression of a range of antioxidant enzymes, including Nrf2-related genes involved in GSH synthesis and turnover. J774A.1 cells were treated with HOCl (50–100 μM), HOSCN (100–200 μM) or a co-treatment of HOCl (100 μM) with SCN− (50 or 200 μM) for 1 h, before washing and re-incubation of the cells for a further 24 h and qPCR to determine alterations in mRNA expression. A significant increase in the gene expression of thioredoxin interacting protein (TXNIP), heme oxygenase 1 (HMOX1), superoxide dismutase 2 (SOD2), and glutathione S-transferase Pi 1 (GSTP1) but not glutathione peroxidase 1 (GPx1), was detected in the cells exposed to HOCl (Fig. 3, red bars). Western blotting experiments performed under the same treatment conditions showed that the protein expression of HMOX1 and SOD2 was also increased on exposure of the cells to HOCl (Fig. 4). In the case of HMOX1 and TXNIP, but not SOD2, a significant increase in mRNA expression was also observed following 3 h re-incubation of the cells after the initial HOCl treatment (Suppl. Fig. S1). With SOD2, the changes in gene expression with 3 h re-incubation following HOCl treatment, are not consistent with the corresponding Western blotting experiments, where an increase in protein expression is seen (Fig. 4).
Fig. 3.
Exposure of J774A.1 cells to HOCl increases the expression of antioxidant genes. J774A.1 cells (0.5 × 106 cells) were treated with HOCl (50, 100 μM; red bars), HOSCN (100, 200 μM; blue bars), 100 μM HOCl with SCN− (50, 200 μM; orange bars), and SCN− (50, 200 μM; green bars) for 1 h at 37 °C before re-incubation in cell media for 24 h. Expression of antioxidant genes, TXNIP (A), HMOX1 (B), SOD2 (C), GPx1 (D) and GSTP1 (E) was measured using qPCR. The data are expressed as the fold change compared to the non-treated control group following normalization to the housekeeping genes TBP and 18s. *, **. *** and **** show a significant difference (p < 0.05, 0.01, 0.001 and 0.0001, respectively) compared to non-treated control groups, by 1-way ANOVA with a Dunnett's multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Exposure of J774A.1 cells to HOCl increased the protein expression of HMOX1 and SOD2. J774A.1 cells (1 × 106 cells) were treated with (A) HOCl (50 and 100 μM) or HOSCN (100 and 200 μM), or (B) co-treatment of HOCl (100 μM) and SCN− (50 and 200 μM) in HBSS for 1 h at 37 °C, before re-incubation in cell media for 3 h (black bars) or 24 h (grey bars) and probing for HMOX1 and SOD2 by Western blotting with β-actin used as a loading control. Images are representative of 3 independent experiments. Panels C and D show the densitometry analysis of HMOX1 (C) and SOD2 (D) following normalization to β-actin. Data are expressed as the fold change compared to the respective non-treated control group. * shows a significant difference (p < 0.05) compared to the non-treated control groups by 2-way ANOVA with a Tukey's multiple comparison test.
These experiments were extended to examine expression of other Nrf-2 related genes, including glutamate – cysteine ligase catalytic subunit (GCLc), glutamate – cysteine ligase modifier subunit (GCLm), glutathione synthetase (GS) and NAD(P)H:quinone oxidoreductase (NQO1) in the HOCl-treated cells. However, in this case, no changes were observed in the mRNA expression (data not shown). Similarly, the changes in mRNA expression of TXNIP, HMOX1, SOD2, GPx1 and GSTP1 were not observed in the corresponding experiments with HOSCN or on co-treatment of the cells with HOCl and SCN− (Fig. 3). These data are consistent with the conversion of HOCl to HOSCN following reaction with SCN−, with sub-stoichiometric molar excesses of SCN− compared to HOCl shown to have a significant effect on gene expression (Fig. 3). Experiments were also performed with cells exposed to SCN− for 1 h in the absence of HOCl, with subsequent re-incubation for 24 h. Treatment with SCN− alone resulted in a small, but non-significant, increase in the expression of TXNIP, SOD2, GPx1 and GSTP1, compared to the non-treated, control cells (Fig. 3, green bars).
3.3. Effect of HOCl, HOSCN and SCN− on the expression of inflammatory genes and chemokines in J774A.1 cells
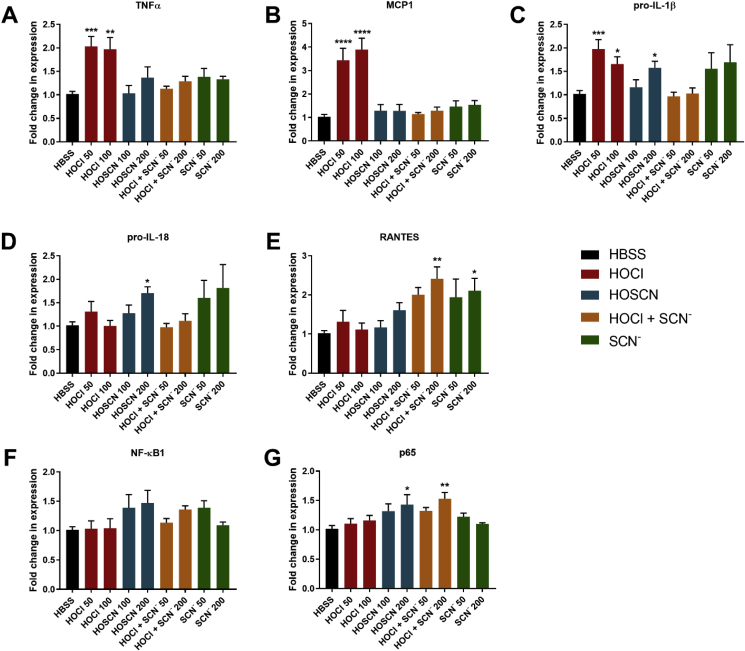
As HOCl and HOSCN have been reported to influence the expression of pro-inflammatory genes in various cell types (reviewed [1,38]), experiments were performed to examine the expression of a range of pro-inflammatory genes, including nuclear factor κB1 (NF-κB1), p65, tumour necrosis factor α (TNFα), pro-interleukin-18 (IL-18) and pro-interleukin-1β (IL-1β), monocyte chemotactic protein 1 (MCP1) and RANTES (Regulated on Activation Normal T cell Expressed and Secreted). Exposure of the J774A.1 cells to HOCl for 1 h followed by 24 h re-incubation, resulted in an up-regulated expression of TNFα, MCP1 and pro-IL-1β (Fig. 5A–C). This increase in gene expression was not observed on treatment of the cells with HOCl in the presence of SCN− (Fig. 5A–C). An increase in TNFα and pro-IL-1β gene expression was also observed in cells exposed to HOCl with a 3 h re-incubation period (Suppl. Fig. S2). There was no increase in the expression of pro-IL-18, RANTES, NF-κB1 or p65 in experiments with HOCl (Fig. 5D–G). However, a significant increase in the expression of RANTES and p65 was observed on co-treatment of J774A.1 cells with HOCl and SCN−, compared to that seen in the control or HOCl-treated cells (Fig. 5E–G).
Fig. 5.
Exposure of J774A.1 cells to HOCl and HOSCN increases the expression of inflammatory genes. J774A.1 cells (0.5 × 106 cells) were treated with HOCl (50, 100 μM; red bars), HOSCN (100, 200 μM; blue bars), 100 μM HOCl with SCN− (50, 200 μM; orange bars), and SCN− (50, 200 μM; green bars) for 1 h at 37 °C before re-incubation in cell media for 3 or 24 h. Expression of inflammatory genes TNFα (A), MCP-1 (B), pro-IL-1β (C), pro-IL-18 (D) and RANTES (E) were measured after 24 h re-incubation. As transcription factors can be modified in a short period of time, gene expression of NF-κB1 (F) and p65 (G) were measured after 3 h re-incubation. The data are expressed as the fold change compared to the non-treated control group following normalization to the housekeeping genes TBP and 18s. *, **. *** and **** show a significant difference (p < 0.05, 0.01, 0.001 and 0.0001, respectively) compared to non-treated control groups, by 1-way ANOVA with a Dunnett's multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Further experiments were performed to examine whether the changes seen on co-treatment of the cells with HOCl and SCN− reflect HOSCN formation, or are a result of a direct effect of SCN− on the cells. A different pattern of reactivity was observed on exposure of the J774A.1 cells to HOSCN compared to HOCl. There were no changes in the expression of TNFα or MCP1 (Fig. 5A and B), but an increase in the expression of pro-IL-1β, pro-IL-18 and RANTES was seen following 24 h re-incubation (Fig. 5C–E). Similarly, with HOSCN, there was an increase in the expression of NF-κB1 and p65 after 3 h of re-incubation (Fig. 5F and G). Changes in gene expression were also seen in experiments with SCN− added in the absence of HOCl, which was significant with RANTES (Fig. 5E). However, the release of RANTES into the cellular supernatant was not affected by SCN− supplementation (data not shown).
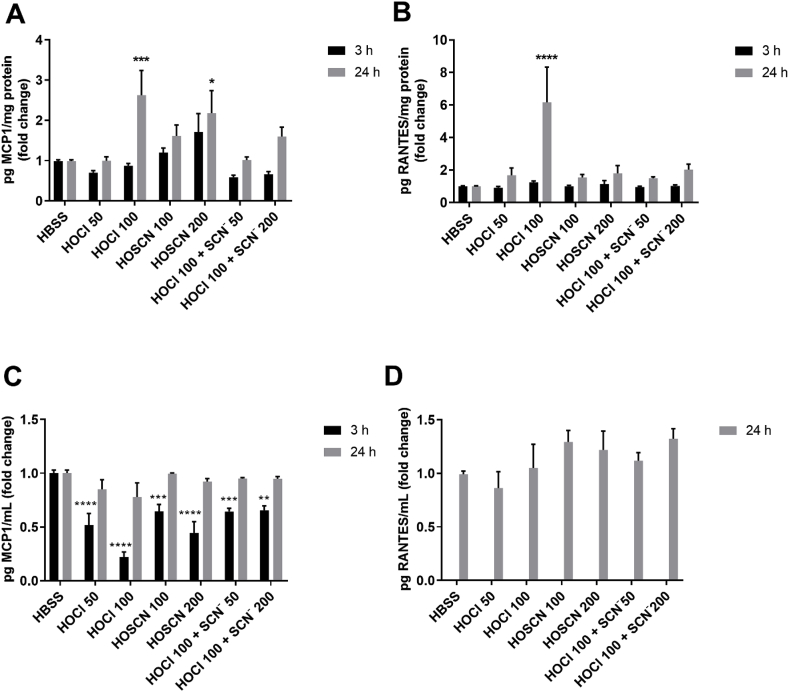
ELISA experiments were performed to examine the effects of each oxidant treatment on the intracellular concentration and release of MCP1 and RANTES, given the changes seen in the respective gene expression. Exposure of the J774A.1 cells to HOCl (100 μM) for 1 h with re-incubation in cell media for 24 h resulted in a significant increase in the intracellular concentration (in cell lysate), but not the extracellular release (in cell supernatant), of both MCP1 and RANTES (Fig. 6, grey bars). With HOSCN, there was a greater concentration of intracellular MCP1 but not RANTES, and no change in the extracellular release of either chemokine (Fig. 6, grey bars). Addition of HOCl to the cells in the presence of SCN− decreased the intracellular concentration of MCP1 and RANTES compared to that seen with HOCl, though in each case, the levels remained higher than those seen in the non-treated control cells (Fig. 6, grey bars).
Fig. 6.
Quantification of MCP1 and RANTES in J774A.1 cells following treatment with HOCl and HOSCN. J774A.1 cells (1 × 106 cells) were treated with HOCl (50, 100 μM), HOSCN (100, 200 μM), and 100 μM HOCl with SCN− (50, 200 μM) for 1 h at 37 °C, before re-incubation in cell media for 3 h (black bars) or 24 h (grey bars). For intracellular cytokine determination, the concentrations of MCP1 (A) and RANTES (B) were measured in cell lysates by ELISA and normalized to total cell protein concentration. To assess extracellular release of cytokines, the concentrations of MCP1 (C) and RANTES (D) were measured in the cell media, after centrifugation to remove cell debris. The release of RANTES after 3 h re-incubation was below the limit of detection. Data are shown as the fold change compared to the non-treated control group. *, **, *** and **** show a significant difference (p < 0.05, 0.01, 0.001 and 0.0001, respectively) compared to the non-treated control (HBSS) group by 2-way ANOVA with a Dunnett's multiple comparison test.
For HOCl, the MCP1 protein concentrations match the pattern of mRNA expression, but this was not the case for RANTES, where no change in mRNA expression was seen (Fig. 5). Similarly, HOSCN treatment increased MCP1 protein but not mRNA, while the reverse is true for RANTES. Therefore, additional experiments were performed to examine the concentrations of MCP1 and RANTES following 3 h re-incubation of the oxidant-treated cells (Fig. 6, black bars). The pattern of MCP1 and RANTES protein expression was comparable to the 24 h experiments, though the intracellular and extracellular MCP1 and RANTES concentrations were generally lower in the 3 h experiments (Fig. 6).
3.4. Activation of MAPK pathway signalling by HOCl and HOSCN in J774A.1 cells
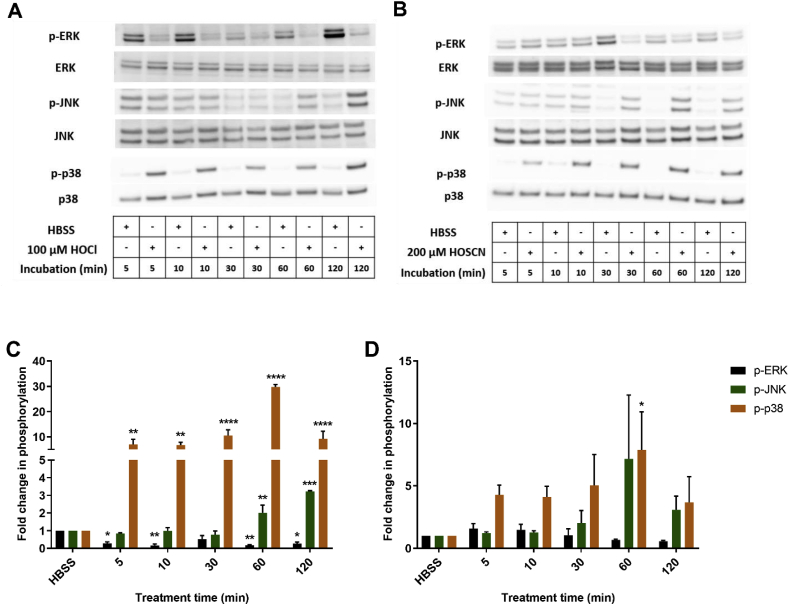
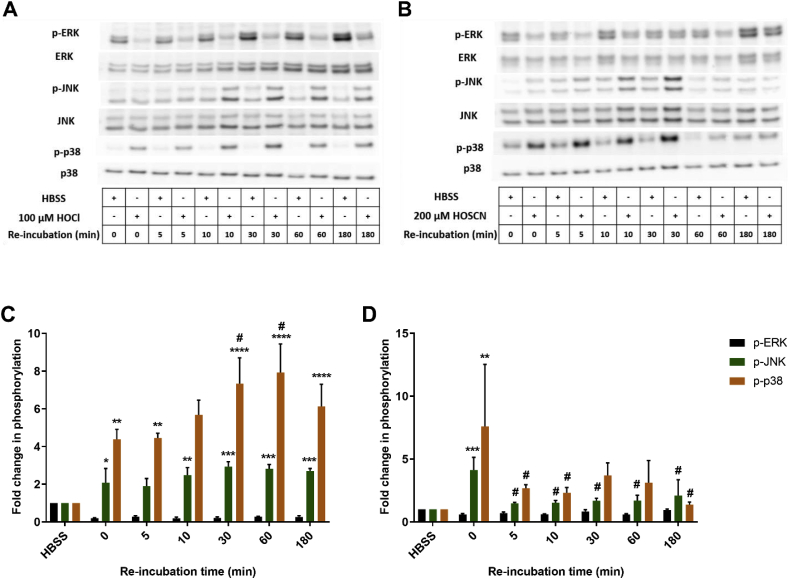
Mitogen activated protein kinases (MAPK) signalling pathways (ERK, JNK and p38) are involved in the induction of many genes, including those involved in inflammatory processes and cellular survival. Therefore, the phosphorylation of ERK, JNK and p38 was assessed by Western blotting in J774A.1 cells exposed to HOCl (100 μM) and HOSCN (200 μM) for different incubation times (5–120 min). Evidence was obtained for a rapid, time-dependent, increase in the phosphorylation of JNK and p38 on treatment of the cells with both HOCl and HOSCN (Fig. 7). In contrast, the phosphorylation of ERK1/2 decreased over time in cells treated with HOCl and HOSCN. The extent of p38 phosphorylation observed with HOCl was much greater than that seen with HOSCN treatment. In general, the extent of p38 and JNK phosphorylation reached a maximal value after 1 h exposure of the cells to each oxidant. Therefore, additional experiments were performed to examine the effect of re-incubation of the cells in media for varying times (5–180 min) following the initial 1 h HOCl or HOSCN treatments. With HOCl, the phosphorylation of p38 was increased further on re-incubation of the cells for an additional 30–180 min (Fig. 8A). This was not the case with JNK or ERK1/2, where no further alterations in phosphorylation were apparent with re-incubation (Fig. 8A). In contrast, the extent of phosphorylation of p38 decreased on re-incubation of cells exposed to HOSCN for 1 h (Fig. 8B). A similar effect was also observed with JNK, whereas an increase in the phosphorylation of ERK1/2 was seen following re-incubation for 180 min (Fig. 8B).
Fig. 7.
Exposure of J774A.1 cells to HOCl or HOSCN induces alterations in MAPK phosphorylation. J774A.1 cells (1 × 106 cells) were treated with 100 μM HOCl (A and C) or 200 μM HOSCN (B and D) at 37 °C for different treatment times (5–120 min). Panels A and B show representative Western blots for total and phosphorylated ERK1/2, JNK and p38. For the densitometry quantification shown in panels C and D, the density of bands corresponding to p-ERK1/2 bands (black bars), p-JNK (green bars) and p-p38 (orange bars) were normalized to the total (non-phosphorylated) ERK1/2, JNK, and p38 band density, respectively, with the data expressed as a fold change compared to the non-treated control group. *, **, *** and **** show a significant difference (p < 0.05, 0.01, 0.001 and 0.0001, respectively) compared to the non-treated control group at the same time point by 2-way ANOVA with a Dunnett's multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 8.
The extent of MAPK phosphorylation in J774A.1 cells exposed to HOCl or HOSCN is dependent on the re-incubation time. J774A.1 cells (1 × 106 cells) were treated with 100 μM HOCl (A and C) or 200 μM HOSCN (B and D) at 37 °C for 1 h before re-incubation in cell media for different times (0–180 min). Panels A and B show representative Western blots for total and phosphorylated ERK1/2, JNK and p38. For the densitometry quantification shown in panels C and D, the density of bands corresponding to p-ERK1/2 bands (black bars), p-JNK (green bars) and p-p38 (orange bars) were normalized to the total (non-phosphorylated) ERK1/2, JNK, and p38 band density, respectively, with the data expressed as a fold change compared to the non-treated control group. *, **, *** and **** show a significant difference (p < 0.05, 0.01, 0.001 and 0.0001, respectively) compared to the non-treated control group at the same time point, # shows a significant difference (p < 0.05) compared to the HOSCN treated group without re-incubation, by 2-way ANOVA with a Dunnett's multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In light of these differences particularly with p38, additional co-treatment experiments were performed with HOCl (100 μM) and SCN− (50–200 μM). No significant changes in the phosphorylation levels of ERK1/2, JNK or p38 were observed on addition of HOCl to the cells in the presence of SCN− compared to that seen in the non-treated controls (Suppl. Fig. S3). This suggests that SCN− is able to prevent the HOCl-induced phosphorylation of JNK and p38 and dephosphorylation of ERK1/2.
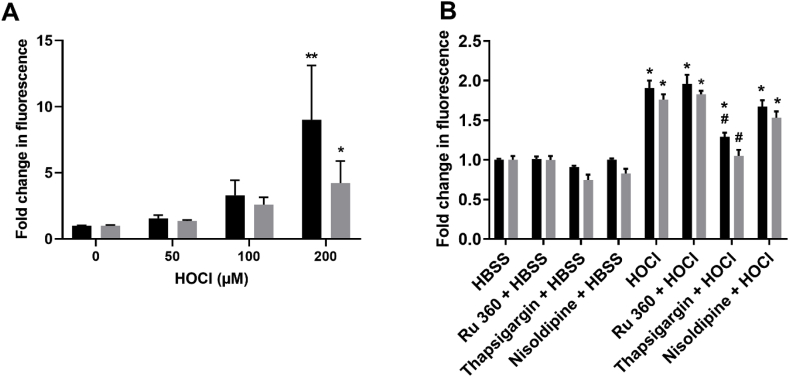
3.5. Exposure to HOCl but not HOSCN increases intracellular Ca2+ in J774A.1
Alterations in intracellular Ca2+ are strongly associated with oxidative stress and perturbation of stress-related signalling pathways. Thus, subsequent experiments were performed to examine changes in intracellular Ca2+ in the J774A.1 cells using the calcium fluorophore Fluo-4 AM and flow cytometry. Exposure of J774A.1 cells to HOCl (50–200 μM) in HBSS (with or without Ca2+) resulted in a significant increase in cytosolic Ca2+ compared to the non-treated cells (Fig. 9A, Suppl. Fig. S4). The change in cytosolic Ca2+ did not appear to be dependent on the presence of Ca2+ in the HBSS (Fig. 9A), suggesting that the Ca2+ is released from intracellular stores. To explore this possibility, cells were pre-treated with nisoldipine (L-type Ca2+ channel blocker), Ru360 (mitochondrial Ca2+ uptake inhibitor) or thapsigargin (non-competitive SERCA inhibitor) for 15 min, before the addition of HOCl (100 μM) and Fluo-4 AM staining. Pre-treatment with thapsigargin significantly attenuated the cytosolic Ca2+ increase mediated by HOCl, which was independent of the presence of extracellular Ca2+ (Fig. 9B).
Fig. 9.
Exposure of J774A.1 cells to HOCl influences Ca2+homeostasis. (A) J774A.1 cells (1 × 106 cells) were treated with HOCl (50, 100 and 200 μM) for 1 h in Ca2+ free (grey bars) or Ca2+ supplemented (black bars) HBSS. (B) Before exposure to HBSS or HOCl (100 μM) for 1 h, cells were pre-incubated with HBSS, Ru360, thapsigargin or nisoldipine (25 μM) for 15 min. Alterations in intracellular Ca2+ were assessed using Fluo-4 AM and flow cytometry with the data expressed as the fold change in fluorescence intensity in the treated compared to non-treated control groups. * shows a significant difference (p < 0.05) compared to the non-treated control group; # shows a significant difference (p < 0.05) compared to HOCl-treated group in HBSS only, by 2-way ANOVA with a Sidak's multiple comparison test.
4. Discussion
It has been proposed that SCN− may have therapeutic value in the context of inflammatory disease [4], with recent studies indicating that SCN− supplementation decreases the development of atherosclerosis in murine models [35,36]. Although this effect is postulated to be due to SCN− decreasing the HOCl production by MPO, the cellular mechanisms involved remain undefined. In this study, we show that the presence of sub-stoichiometric amounts of SCN− can inhibit HOCl-mediated cell death and influence the extent and nature of cellular thiol oxidation, MAPK signalling, and the expression of a range of antioxidant and inflammatory genes and cytokines in J774A.1 macrophages. The effects of SCN− on HOCl-induced cellular dysfunction are generally consistent with the formation of HOSCN, but SCN− itself could also increase the gene expression of RANTES in the macrophages.
Exposure of the J774A.1 macrophages to HOCl and HOSCN resulted in significant cell death and thiol loss in a concentration-dependent manner, with HOSCN inducing less cell death compared to HOCl, when assessed by the extent of MTS metabolism. A significant loss of thiols was observed on exposure of the cells to both HOCl and HOSCN. However, with HOSCN, the thiol oxidation appeared to be reversible, as an increase in the cellular thiol concentration was seen on removing any residual oxidant and further incubation of the cells for 4 or 24 h. These data are consistent with the formation of reversible oxidation products, such as sulfenyl thiocyanate (RS-SCN) and sulfenic acid (RS-OH) species, as reported previously in macrophages exposed to HOSCN [19,20,48]. The formation of reversible oxidation products with HOSCN also rationalise the discrepancy between the cell viability data in the current study compared with previous reports, where HOSCN was shown to be more damaging compared to HOCl on exposure to J774A.1 cells when assessed immediately following oxidant treatment [39]. Similar assay-dependent differences in the extent of cell death seen with HOSCN have been reported in studies with human coronary artery endothelial cells [41].
The extent of HOCl-induced cell death was decreased on treatment of the cells in the presence of SCN−. A significant protective effect of SCN− was apparent at sub-stoichiometric ratios of SCN− to HOCl. This is attributed to the formation of HOSCN, which can react further with residual HOCl forming over-oxidized products such as HO2SCN, and ultimately SO42− and OCN− [30]. Whether HO2SCN reacts with the cells or undergoes rapid decomposition to other products, such as OCN−, which could potentially be reactive if present in high concentration (e.g. Ref. [49]) is not known, and has not been examined, owing to the difficultly in detecting these products. Addition of SCN− also influenced the extent of HOCl-induced thiol oxidation, with a dose-dependent increase in thiol levels seen with ≤ equimolar concentrations of SCN− compared to HOCl. However, a small decrease in thiols is seen when the concentration of SCN− is in excess of that of HOCl, presumably due to the formation of higher concentrations of HOSCN. Supplementation with SCN− also promoted reversible thiol oxidation after 24 h re-incubation, which was not seen on treating the cells with HOCl alone, consistent with HOSCN formation.
Exposure of J774A.1 cells to HOCl decreased GSH, as reported previously studies for this, and other mammalian cell types (reviewed [38]). The results from the GSH measurements support the thiol data, further demonstrating a protective effect of SCN−, which decreased HOCl-induced GSH loss. Interestingly, a significantly higher thiol and GSH concentration was seen on co-treatment of the cells with HOCl and SCN− compared to the non-treated control group after re-incubation for 4 or 24 h. It is well established that oxidants can regulate redox homeostasis through Nrf2 signalling, which increases the expression of enzymes critical for GSH biosynthesis (e.g. Ref. [50,51]). It has been shown previously that exposure of THP1 macrophages to HOCl decreased intracellular GSH within the first hour of treatment, which was followed by a rebound, which surpassed the initial basal levels of GSH by up to 4-fold after 24 h [52]. This is attributed to the activation of Nrf2 and the downstream genes, HMOX1, NQO1, GCLc, and GS [52]. However, in the current study, HOCl induced irreversible loss of intracellular GSH, and the expression of Nrf2 associated genes was decreased on re-incubation of the J774A.1 cells co-treated with HOCl and SCN−.
The data obtained are consistent with the ability of HOCl, but not HOSCN, to upregulate cellular antioxidant responses, with treatment of the J774A.1 cells with HOCl resulting in an increase in the expression of mRNA for TXNIP, HMOX1, SOD2, GPx1 and GSTP1 in a time and concentration dependent manner. This may be a consequence of the induction of Nrf2, which has been reported on exposure of other types of macrophages and epithelial cells to HOCl [[52], [53], [54]]. The HOCl-induced activation of Nrf2 has been reported to involve oxidation of redox-sensitive Cys residues on kelch-like ECH-associated protein 1 (Keap1) to result in the formation of an intermolecular disulfide at Cys151 [55]. This inactivates the ubiquitin ligase activity of Keap1 by disrupting its interaction with cullin 3 (CUL3), thereby preventing the proteasomal degradation of Nrf2 [56].
A limitation of the current studies is that the ability of HOCl to activate Nrf2 in the J774A.1 cells was not directly assessed. It is possible that other cellular stress-related pathways could therefore be involved in the altered antioxidant gene expression, particularly as no evidence was obtained for any upregulation of antioxidant gene expression with HOSCN, which would also be expected to target redox-sensitive Cys residues, and potentially influence the ubiquitin ligase activity of Keap1. In addition, it is noted that while a significant increase in HMOX-1 mRNA and protein expression was seen in HOCl-treated cells, this occurred in the absence of the upregulation of several other Nrf2 genes studied, including NQO1, GCLc, GCLm and GS. Thus, MAPKs have also been implicated in the activation of signalling pathways that culminate in an increase of antioxidant enzyme expression [57]. For example, the upregulation of HMOX-1 is attenuated in vascular smooth muscle cells exposed to oxidized LDL following inhibition of p38 and JNK [58]. While exposure of J774A.1 cells to both HOCl and HOSCN induced a rapid increase in p38 and JNK phosphorylation, the extent of p38 phosphorylation seen in experiments with HOCl was particularly marked, which may influence the antioxidant gene expression observed.
Evidence for the perturbation of MAPK signalling on exposure of cells to MPO-derived oxidants has been reported previously (reviewed [1,38]). In J774A.1 cells, HOSCN has been shown to promote the phosphorylation of the mitogen activated protein kinase kinase MKK3/6 and p38, which was attributed to the reactivity of the oxidant with redox-sensitive Cys residues on protein tyrosine phosphatases (PTP) [59]. In the current study, HOCl and HOSCN both stimulated the phosphorylation of p38 and JNK, but attenuated the phosphorylation of ERK1/2, in a time-dependent manner in each case. Compared to HOSCN, HOCl induced a more rapid, non-reversible, and greater extent of phosphorylation, which was particularly marked in the case of p38. This could reflect the ability of HOCl to more extensively modify the redox-sensitive Cys residues on PTP for example, to sulfinic or sulfonic acid products. However, in previous work, the presence of SCN− was a key determinant in the extent of PTP inactivation observed in J774A.1 cells lysates exposed to the complete MPO/H2O2/Cl− system [59].
On the basis of these data, it might be expected HOSCN rather than HOCl would therefore favour altered MAPK phosphorylation. However, there is also a strong relationship between Ca2+ homeostasis and the regulation of MAPK signalling [60]. In particular, elevations in the concentration of cytosolic Ca2+ can result in increased p38 phosphorylation, owing to the activation of Ca2+/calmodulin-dependent protein kinases [61]. This could be important in the observed HOCl-induced alterations in MAPK signalling, as a marked increase in cytosolic Ca2+ was seen in cells exposed to HOCl but not HOSCN. The increase in cytosolic Ca2+ seen with HOCl occurred in a concentration-dependent manner, both in the presence and absence of extracellular Ca2+ and was attenuated on pre-treatment of the cells with thapsigargin but not Ru360 or nisoldipine. Given that thapsigargin inhibits SERCA and the re-uptake of cytosolic Ca2+ into the sarco/endoplasmic reticulum, these data suggest that the effect of HOCl on Ca2+ homeostasis is related to the removal of accumulated cytosolic Ca2+ released from other, unidentified, intracellular stores or Ca2+ channels, in accord with previous studies in endothelial cells [62]. That similar alterations in cytosolic Ca2+ are observed on exposure of the J774A.1 cells to HOCl in HBSS with and without Ca2+ suggests that the Ca2+ may originate from both extracellular and internal stores. This agrees well with data from human monocyte-derived macrophages, where perturbations in cytosolic Ca2+ could be prevented by pre-treatment of the cells with different L-type Ca2+ channel blockers and dantrolene, which selectively inhibits the ryanodine receptor (RyR) [63]. Alternatively, it is possible that the perturbation in Ca2+ homeostasis could reflect changes to mitochondrial membrane permeability or disruption to the ER membrane, which has been seen previously in several mammalian cell types on exposure to HOCl (reviewed [38]).
Unlike HOCl, the effects of HOSCN on the phosphorylation levels of p38, JNK and ERK1/2 appear to be reversible, with the extent of phosphorylation returning to basal, non-treated control cell levels, on re-incubation of the cells in media. This provides a further rationale for the lower toxicity of HOSCN compared to HOCl (as assessed by the MTS assay), considering the role of MAPK signalling pathways in apoptosis [64]. Overall, the data are consistent with an ability of SCN− to prevent the perturbations in MAPK phosphorylation seen with HOCl, certainly at sub-stoichiometric concentrations of SCN−, which remove HOCl in the absence of the formation of HOSCN. However, the extent of p38 phosphorylation is higher than in the non-treated cells with equimolar or concentrations of SCN− in excess of HOCl, further supporting a role for HOSCN in the activation of MAPK signalling.
The activation of MAPK signalling could be important in driving the altered gene expression of downstream inflammatory mediators, including TNFα, MCP1 and IL-1β [65], which are also elevated in J774A.1 cells following exposure to HOCl. Although some increase in inflammatory cytokines was also apparent with HOSCN, this occurred to a lesser extent than with HOCl. However, in contrast to HOCl, HOSCN induced an increase in the gene expression of RANTES, NF-κB1 and p65. Evidence for the activation of NF-κB has been presented in previous studies with THP-1 macrophages exposed to HOSCN [66]. Similarly, activation of NF-κB in endothelial cells has been implicated as a means to amplify inflammation, with exposure to HOSCN resulting in the increased expression of cellular adhesion molecules [67] and tissue factor [68].
With MCP1 and RANTES, additional experiments were performed to assess the protein concentration of each chemokine both within cells and released extracellularly. With MCP1, the intracellular protein concentrations match the pattern of mRNA expression in experiments with HOCl, with an elevation of intracellular MCP1 protein also apparent with HOSCN. Interestingly, similar results were obtained for RANTES, although no change in mRNA expression was seen with HOCl. The reason for the discrepancy between the mRNA and protein expression is not clear, but taken together, the data highlight the pro-inflammatory behaviour of both HOCl and HOSCN. Given this observation, it is perhaps not surprising that an elevation in several pro-inflammatory mediators is also apparent on treatment of cells HOCl in the presence of SCN−. In this case, it is noteworthy that supplementation of the cells with SCN− in the absence of HOCl could also elevate the mRNA expression of RANTES, suggesting that in this case, the effects could occur independently of HOSCN formation.
Whether the ability of SCN− to influence RANTES expression has relevance in vivo is not certain, and would be interesting to examine in future studies, given the association of RANTES to atherosclerosis [69]. In particular, there is a relationship between serum levels of RANTES, plaque vulnerability and the development of high-risk plaques [70,71]. The serum cytokine profile is reported to be altered to a less inflammatory phenotype in the murine apoE−/- atherosclerosis model on supplementation with SCN−, though serum RANTES levels were not assessed [36].
In summary, our data provide further insight into pathways by which supplementation with SCN− can influence the extent and nature of HOCl-induced cellular damage at a range of SCN− concentrations. The data are consistent with the conversion of HOCl to HOSCN, which can induce reversible modifications that are repairable by the cells on further incubation in the absence of oxidant. While SCN− could prevent HOCl-induced cell death and the perturbation of MAPK phosphorylation and antioxidant gene expression, the influence of SCN− on HOCl-induced pro-inflammatory signalling cascades is less clear. This is related to the capacity of HOSCN and to some extent, SCN− itself to also affect pro-inflammatory gene expression and cytokine/chemokine release, which may be important to consider in the context of diseases such as atherosclerosis. Overall, the data support the use of SCN− as a promising therapeutic agent to decrease the extent of MPO-induced cellular damage, and help to rationalise the observations from the in vivo supplementation studies [[34], [35], [36]].
Declaration of competing interest
None.
Acknowledgements
The authors are grateful for financial support from the Novo Nordisk Foundation (Laureate Research Grant NNF13OC0004294 to MJD) and the China Scholarship Council (PhD scholarship to CG).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101666.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Davies M.J., Hawkins C.L. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease, antioxid. Redox Signal. 2020;32(13):957–981. doi: 10.1089/ars.2020.8030. [DOI] [PubMed] [Google Scholar]
- 2.Day B.J. The science of licking your wounds: function of oxidants in the innate immune system. Biochem. Pharmacol. 2019;163:451–457. doi: 10.1016/j.bcp.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 3.van Dalen C.J., Whitehouse M.W., Winterbourn C.C., Kettle A.J. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997;327(2):487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandler J.D., Day B.J. Biochemical mechanisms and therapeutic potential of pseudohalide thiocyanate in human health. Free Radic. Res. 2015;49(6):695–710. doi: 10.3109/10715762.2014.1003372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins C.L. Hypochlorous acid-mediated modification of proteins and its consequences. Essays Biochem. 2020;64(1):75–86. doi: 10.1042/EBC20190045. [DOI] [PubMed] [Google Scholar]
- 6.Teng N., Maghzal G.J., Talib J., Rashid I., Lau A.K., Stocker R. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep. 2017;22(2):51–73. doi: 10.1080/13510002.2016.1256119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholls S.J., Hazen S.L. Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2005;25(6):1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 8.Karakas M., Koenig W. Myeloperoxidase production by macrophage and risk of atherosclerosis. Curr. Atherosclerosis Rep. 2012;14(3):277–283. doi: 10.1007/s11883-012-0242-3. [DOI] [PubMed] [Google Scholar]
- 9.Schindhelm R.K., van der Zwan L.P., Teerlink T., Scheffer P.G. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin. Chem. 2009;55(8):1462–1470. doi: 10.1373/clinchem.2009.126029. [DOI] [PubMed] [Google Scholar]
- 10.Hazen S.L., Heinecke J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalysed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 1997;99(9):2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeshita J., Byun J., Nhan T.Q., Pritchard D.K., Pennathur S., Schwartz S.M., Chait A., Heinecke J.W. Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue. A potential pathway for somatic mutagenesis by macrophages. J. Biol. Chem. 2006;281(6):3096–3104. doi: 10.1074/jbc.M509236200. [DOI] [PubMed] [Google Scholar]
- 12.Barrett T.J., Hawkins C.L. Hypothiocyanous Acid: benign or deadly? Chem. Res. Toxicol. 2012;25(2):263–273. doi: 10.1021/tx200219s. [DOI] [PubMed] [Google Scholar]
- 13.Nagy P., Jameson G.N., Winterbourn C.C. Kinetics and mechanisms of the reaction of hypothiocyanous acid with 5-thio-2-nitrobenzoic acid and reduced glutathione. Chem. Res. Toxicol. 2009;22(11):1833–1840. doi: 10.1021/tx900249d. [DOI] [PubMed] [Google Scholar]
- 14.Skaff O., Pattison D.I., Davies M.J. Hypothiocyanous acid reactivity with low-molecular-mass and protein thiols: absolute rate constants and assessment of biological relevance. Biochem. J. 2009;422:111–117. doi: 10.1042/BJ20090276. [DOI] [PubMed] [Google Scholar]
- 15.Skaff O., Pattison D.I., Morgan P.E., Bachana R., Jain V.K., Priyadarsini K.I., Davies M.J. Selenium-containing amino acids are targets for myeloperoxidase-derived hypothiocyanous acid: determination of absolute rate constants and implications for biological damage. Biochem. J. 2012;441(1):305–316. doi: 10.1042/BJ20101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattison D.I., Davies M.J., Hawkins C.L. Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 2012;46(8):975–995. doi: 10.3109/10715762.2012.667566. [DOI] [PubMed] [Google Scholar]
- 17.Ashby M.T., Aneetha H. Reactive sulfur species: aqueous chemistry of sulfenyl thiocyanates. J. Am. Chem. Soc. 2004;126(33):10216–10217. doi: 10.1021/ja048585a. [DOI] [PubMed] [Google Scholar]
- 18.Algunindigue Nimmo S.L., Lemma K., Ashby M.T. Reactions of cysteine sulfenyl thiocyanate with thiols to give unsymmetrical disulfides. Heteroat. Chem. 2007;18(5):467–471. [Google Scholar]
- 19.Barrett T.J., Pattison D.I., Leonard S.E., Carroll K.S., Davies M.J., Hawkins C.L. Inactivation of thiol-dependent enzymes by hypothiocyanous acid: role of sulfenyl thiocyanate and sulfenic acid intermediates. Free Radic. Biol. Med. 2012;52(6):1075–1085. doi: 10.1016/j.freeradbiomed.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Love D.T., Barrett T.J., White M.Y., Cordwell S.J., Davies M.J., Hawkins C.L. Cellular targets of the myeloperoxidase-derived oxidant hypothiocyanous acid (HOSCN) and its role in the inhibition of glycolysis in macrophages. Free Radic. Biol. Med. 2016;94:88–98. doi: 10.1016/j.freeradbiomed.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Chandler J.D., Nichols D.P., Nick J.A., Hondal R.J., Day B.J. Selective metabolism of hypothiocyanous acid by mammalian thioredoxin reductase promotes lung innate immunity and antioxidant defense. J. Biol. Chem. 2013;288(25):18421–18428. doi: 10.1074/jbc.M113.468090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandler J.D., Day B.J. Thiocyanate: a potentially useful therapeutic agent with host defense and antioxidant properties. Biochem. Pharmacol. 2012;84(11):1381–1387. doi: 10.1016/j.bcp.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foss O.P., Lund-Larsen P.G. Serum thiocyanate and smoking: interpretation of serum thiocyanate levels observed in a large health study. Scand. J. Clin. Lab. Invest. 1986;46(3):245–251. doi: 10.3109/00365518609083666. [DOI] [PubMed] [Google Scholar]
- 24.Vesey C.J., Saloojee Y., Cole P.V., Russell M.A. Blood carboxyhaemoglobin, plasma thiocyanate, and cigarette consumption: implications for epidemiological studies in smokers. Br. Med. J. 1982;284(6328):1516–1518. doi: 10.1136/bmj.284.6328.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan P.E., Pattison D.I., Talib J., Summers F.A., Harmer J.A., Celermajer D.S., Hawkins C.L., Davies M.J. High plasma thiocyanate levels in smokers are a key determinant of thiol oxidation induced by myeloperoxidase. Free Radic. Biol. Med. 2011;51(9):1815–1822. doi: 10.1016/j.freeradbiomed.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Lorentzen D., Durairaj L., Pezzulo A.A., Nakano Y., Launspach J., Stoltz D.A., Zamba G., McCray P.B., Jr., Zabner J., Welsh M.J., Nauseef W.M., Banfi B. Concentration of the antibacterial precursor thiocyanate in cystic fibrosis airway secretions. Free Radic. Biol. Med. 2011;50(9):1144–1150. doi: 10.1016/j.freeradbiomed.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz C.P., Ahmed A.H., Dawes C., Mantsch H.H. Thiocyanate levels in human saliva - quantification by Fourier transform infrared spectroscopy. Anal. Biochem. 1996;240:7–12. doi: 10.1006/abio.1996.0323. [DOI] [PubMed] [Google Scholar]
- 28.Minarowski L., Sands D., Minarowska A., Karwowska A., Sulewska A., Gacko M., Chyczewska E. Thiocyanate concentrations in saliva of cystic fibrosis patients. Folia Histochem. Cytobiol. 2008;46(2):245–246. doi: 10.2478/v10042-008-0037-0. [DOI] [PubMed] [Google Scholar]
- 29.Ashby M.T., Carlson A.C., Scott M.J. Redox buffering of hypochlorous acid by thiocyanate in physiologic fluids. J. Am. Chem. Soc. 2004;126:15976–15977. doi: 10.1021/ja0438361. [DOI] [PubMed] [Google Scholar]
- 30.Xulu B.A., Ashby M.T. Small molecular, macromolecular, and cellular chloramines react with thiocyanate to give the human defense factor hypothiocyanite. Biochemistry. 2010;49:2068–2074. doi: 10.1021/bi902089w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talib J., Pattison D.I., Harmer J.A., Celermajer D.S., Davies M.J. High plasma thiocyanate levels modulate protein damage induced by myeloperoxidase and perturb measurement of 3-chlorotyrosine. Free Radic. Biol. Med. 2012;53(1):20–29. doi: 10.1016/j.freeradbiomed.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Wagner B.A., Reszka K.J., McCormick M.L., Britigan B.E., Evig C.B., Burns C.P. Role of thiocyanate, bromide and hypobromous acid in hydrogen peroxide-induced apoptosis. Free Radic. Res. 2004;38(2):167–175. doi: 10.1080/10715760310001643302. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y., Szep S., Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc. Natl. Acad. Sci. U.S.A. 2009;106(48):20515–20519. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandler J.D., Min E., Huang J., McElroy C.S., Dickerhof N., Mocatta T., Fletcher A.A., Evans C.M., Liang L., Patel M., Kettle A.J., Nichols D.P., Day B.J. Antiinflammatory and antimicrobial effects of thiocyanate in a cystic fibrosis mouse model. Am. J. Respir. Cell Mol. Biol. 2015;53(2):193–205. doi: 10.1165/rcmb.2014-0208OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan P.E., Laura R.P., Maki R.A., Reynolds W.F., Davies M.J. Thiocyanate supplementation decreases atherosclerotic plaque in mice expressing human myeloperoxidase. Free Radic. Res. 2015;49(6):743–749. doi: 10.3109/10715762.2015.1019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zietzer A., Niepmann S.T., Camara B., Lenart M.A., Jansen F., Becher M.U., Andrie R., Nickenig G., Tiyerili V. Sodium thiocyanate treatment attenuates atherosclerotic plaque formation and improves endothelial regeneration in mice. PloS One. 2019;14(4) doi: 10.1371/journal.pone.0214476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nedoboy P.E., Morgan P.E., Mocatta T.J., Richards A.M., Winterbourn C.C., Davies M.J. High plasma thiocyanate levels are associated with enhanced myeloperoxidase-induced thiol oxidation and long-term survival in subjects following a first myocardial infarction. Free Radic. Res. 2014;48(10):1256–1266. doi: 10.3109/10715762.2014.947286. [DOI] [PubMed] [Google Scholar]
- 38.Rayner B.S., Love D.T., Hawkins C.L. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free Radic. Biol. Med. 2014;71:240–255. doi: 10.1016/j.freeradbiomed.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd M.M., Van Reyk D.M., Davies M.J., Hawkins C.L. HOSCN is a more potent inducer of apoptosis and protein thiol depletion in murine macrophage cells than HOCl or HOBr. Biochem. J. 2008;414(2):271–280. doi: 10.1042/BJ20080468. [DOI] [PubMed] [Google Scholar]
- 40.Arlandson M., Decker T., Roongta V.A., Bonilla L., Mayo K.H., MacPherson J.C., Hazen S.L., Slungaard A. Eosinophil peroxidase oxidation of thiocyanate - characterization of major reaction products and a potential sulfhydryl-targeted cytotoxicity system. J. Biol. Chem. 2001;276(1):215–224. doi: 10.1074/jbc.M004881200. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd M.M., Grima M.A., Rayner B.S., Hadfield K.A., Davies M.J., Hawkins C.L. Comparative reactivity of the myeloperoxidase-derived oxidants hypochlorous acid and hypothiocyanous acid with human coronary artery endothelial cells. Free Radic. Biol. Med. 2013;65:1352–1362. doi: 10.1016/j.freeradbiomed.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Daugherty A., Dunn J.L., Rateri D.L., Heinecke J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest. 1994;94(1):437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010;10(1):36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geovanini G.R., Libby P. Atherosclerosis and inflammation: overview and updates. Clin. Sci. (Lond.) 2018;132(12):1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 45.Morris J.C. Kinetics of reactions between aqueous chlorine and nitrogen compounds. In: Faust E.D., Hunter J.V., editors. Principles and Applications of Water Chemistry. John Wiley and Sons; New York: 1967. pp. 23–53. [Google Scholar]
- 46.Hawkins C.L., Morgan P.E., Davies M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009;46(8):965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Eyer P., Worek F., Kiderlen D., Sinko G., Stuglin A., Simeon-Rudolf V., Reiner E. Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal. Biochem. 2003;312(2):224–227. doi: 10.1016/s0003-2697(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 48.Love D.T., Guo C., Nikelshparg E.I., Brazhe N.A., Hawkins C.L. The role of the myeloperoxidase-derived oxidant hypothiocyanous acid (HOSCN) in the induction of mitochondrial dysfunction in macrophages. Redox Biol. 2020:101602. doi: 10.1016/j.redox.2020.101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Gamal D., Holzer M., Gauster M., Schicho R., Binder V., Konya V., Wadsack C., Schuligoi R., Heinemann A., Marsche G. Cyanate is a novel inducer of endothelial icam-1 expression, Antioxid. Redox Signal. 2012;16(2):129–137. doi: 10.1089/ars.2011.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H., Liu H., Zhou L., Yuen J., Forman H.J. Temporal changes in glutathione biosynthesis during the lipopolysaccharide-induced inflammatory response of THP-1 macrophages. Free Radic. Biol. Med. 2017;113:304–310. doi: 10.1016/j.freeradbiomed.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H., Davies K.J.A., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88(Pt B):314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pi J.B., Zhang Q., Woods C.G., Wong V., Collins S., Andersen M.E. Activation of Nrf2-mediated oxidative stress response in macrophages by hypochlorous acid. Toxicol. Appl. Pharmacol. 2008;226(3):236–243. doi: 10.1016/j.taap.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Woods C.G., Fu J., Xue P., Hou Y., Pluta L.J., Yang L., Zhang Q., Thomas R.S., Andersen M.E., Pi J. Dose-dependent transitions in Nrf2-mediated adaptive response and related stress responses to hypochlorous acid in mouse macrophages. Toxicol. Appl. Pharmacol. 2009;238(1):27–36. doi: 10.1016/j.taap.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu L., Pi J., Wachi S., Andersen M.E., Wu R., Chen Y. Identification of Nrf2-dependent airway epithelial adaptive response to proinflammatory oxidant-hypochlorous acid challenge by transcription profiling. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;294(3):L469–L477. doi: 10.1152/ajplung.00310.2007. [DOI] [PubMed] [Google Scholar]
- 55.Fourquet S., Guerois R., Biard D., Toledano M.B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 2010;285(11):8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rachakonda G., Xiong Y., Sekhar K.R., Stamer S.L., Liebler D.C., Freeman M.L. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chem. Res. Toxicol. 2008;21(3):705–710. doi: 10.1021/tx700302s. [DOI] [PubMed] [Google Scholar]
- 57.Kong A.N., Owuor E., Yu R., Hebbar V., Chen C., Hu R., Mandlekar S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab. Rev. 2001;33(3–4):255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- 58.Anwar A.A., Li F.Y., Leake D.S., Ishii T., Mann G.E., Siow R.C. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic. Biol. Med. 2005;39(2):227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Lane A.E., Tan J.T., Hawkins C.L., Heather A.K., Davies M.J. The myeloperoxidase-derived oxidant HOSCN inhibits protein tyrosine phosphatases and modulates cell signalling via the mitogen-activated protein kinase (MAPK) pathway in macrophages. Biochem. J. 2010;430(1):161–169. doi: 10.1042/BJ20100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White C.D., Sacks D.B. Regulation of MAP kinase signaling by calcium. Methods Mol. Biol. 2010;661:151–165. doi: 10.1007/978-1-60761-795-2_9. [DOI] [PubMed] [Google Scholar]
- 61.Wright D.C., Geiger P.C., Han D.H., Jones T.E., Holloszy J.O. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J. Biol. Chem. 2007;282(26):18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- 62.Cook N.L., Viola H.M., Sharov V.S., Hool L.C., Schoneich C., Davies M.J. Myeloperoxidase-derived oxidants inhibit sarco/endoplasmic reticulum Ca(2+)-ATPase activity and perturb Ca(2+) homeostasis in human coronary artery endothelial cells. Free Radic. Biol. Med. 2012;52(5):951–961. doi: 10.1016/j.freeradbiomed.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y.T., Whiteman M., Gieseg S.P. HOCl causes necrotic cell death in human monocyte derived macrophages through calcium dependent calpain activation. Biochim. Biophys. Acta. 2012;1823(2):420–429. doi: 10.1016/j.bbamcr.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 64.Wada T., Penninger J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23(16):2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 65.Haddad J.J. A redox microenvironment is essential for MAPK-dependent secretion of pro-inflammatory cytokines: modulation by glutathione (GSH/GSSG) biosynthesis and equilibrium in the alveolar epithelium. Cell. Immunol. 2011;270(1):53–61. doi: 10.1016/j.cellimm.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Pan G.J., Rayner B.S., Zhang Y., van Reyk D.M., Hawkins C.L. A pivotal role for NF-kappaB in the macrophage inflammatory response to the myeloperoxidase oxidant hypothiocyanous acid. Arch. Biochem. Biophys. 2018;642:23–30. doi: 10.1016/j.abb.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Wang J.G., Mahmud S.A., Nguyen J., Slungaard A. Thiocyanate-dependent induction of endothelial cell adhesion molecule expression by phagocyte peroxidases: a novel HOSCN-specific oxidant mechanism to amplify inflammation. J. Immunol. 2006;177(12):8714–8722. doi: 10.4049/jimmunol.177.12.8714. [DOI] [PubMed] [Google Scholar]
- 68.Wang J.G., Mahmud S.A., Thompson J.A., Geng J.G., Key N.S., Slungaard A. The principal eosinophil peroxidase product, HOSCN, is a uniquely potent phagocyte oxidant inducer of endothelial cell tissue factor activity: a potential mechanism for thrombosis in eosinophilic inflammatory states. Blood. 2006;107(2):558–565. doi: 10.1182/blood-2005-05-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zernecke A., Shagdarsuren E., Weber C. Chemokines in atherosclerosis: an update. Arterioscler. Thromb. Vasc. Biol. 2008;28(11):1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 70.Lv Y.B., Jing J., Li J.M., Zhong J.P., Fang L., Yang B. Assessment of RANTES levels as the indicators of plaque vulnerability in rabbit models of atherosclerosis. Pathol. Res. Pract. 2014;210(12):1031–1037. doi: 10.1016/j.prp.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 71.Virani S.S., Nambi V., Hoogeveen R., Wasserman B.A., Coresh J., Gonzalez F., 2nd, Chambless L.E., Mosley T.H., Boerwinkle E., Ballantyne C.M. Relationship between circulating levels of RANTES (regulated on activation, normal T-cell expressed, and secreted) and carotid plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Eur. Heart J. 2011;32(4):459–468. doi: 10.1093/eurheartj/ehq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.