Graphical abstract
Keywords: Ventilator, Pandemic, Ventilation, Influenza pandemic, Coronavirus, Coronavirus pandemic, Pandemic ventilator, Single-limb, Open source, Open hardware, COVID-19, Medical hardware, RepRap, 3-D printing, Open source medical hardware, Embedded systems, Real-time operating system
Abstract
This study describes the development of a simple and easy-to-build portable automated bag valve mask (BVM) compression system, which, during acute shortages and supply chain disruptions can serve as a temporary emergency ventilator. The resuscitation system is based on the Arduino controller with a real-time operating system installed on a largely RepRap 3-D printable parametric component-based structure. The cost of the materials for the system is under $170, which makes it affordable for replication by makers around the world. The device provides a controlled breathing mode with tidal volumes from 100 to 800 mL, breathing rates from 5 to 40 breaths/minute, and inspiratory-to-expiratory ratio from 1:1 to 1:4. The system is designed for reliability and scalability of measurement circuits through the use of the serial peripheral interface and has the ability to connect additional hardware due to the object-oriented algorithmic approach. Experimental results after testing on an artificial lung for peak inspiratory pressure (PIP), respiratory rate (RR), positive end-expiratory pressure (PEEP), tidal volume, proximal pressure, and lung pressure demonstrate repeatability and accuracy exceeding human capabilities in BVM-based manual ventilation. Future work is necessary to further develop and test the system to make it acceptable for deployment outside of emergencies such as with COVID-19 pandemic in clinical environments, however, the nature of the design is such that desired features are relatively easy to add using protocols and parametric design files provided.
1. Specifications table
| Hardware name | RepRapable Automated Open Source BVM-based Ventilator |
| Subject area |
|
| Hardware type |
|
| Open Source License | GNU General Public License (GPL) v3.0 and CERN Open Hardware License (OHL) v1.2 |
| Cost of Hardware | < $170 |
| Source File Repository | https://osf.io/fjdwz/ |
2. Hardware in context
Coronavirus disease 2019 (COVID-19) is increasing mortality rates by overwhelming medical infrastructure at the regional level [1], [2], [3], [4]. Mechanical ventilators, which are essential for treating both influenza and COVID-19 patients in severe acute respiratory failure [5], [6], are in critical short supply in some locations [7], [8], [9], [10]. During pandemics intensive care units (ICUs) do not have sufficient ventilators to treat all the patients requiring them [11], [12], [13], which forces triage and rationing [14], [15]. This is despite national stockpiles of proprietary, mass-manufactured ventilators, which are simply not numerous enough due to prohibitive costs to service society during an extreme pandemic [16], [17], [18], [19], [20].
Another approach to provide products uses the technically and economically-viable open source small-scale digital technologies and off-the-shelf components for distributed manufacturing [21], [22]. There has already been a concerted effort to apply open source hardware and 3-D printing during the COVID-19 pandemic [23], [24], [25], [26], [27], [28], [29]. In addition, challenges with supply chains during any type of pandemic can be partially offset by open source recyclebots [30], [31], [32], [33], [34] and direct recycling extrusion [35] to close the loop on material supplies with local waste converted into additive manufacturing feedstock [36], [37], [38], [39], [40], [41]. The distributed manufacturing of scientific equipment has been shown to provide custom, high-quality scientific tools for substantially lower costs than conventional proprietary products [42], [43], [44], [45], [46]. This is because a scientific tool can be developed once and then digitally replicated for approximately the cost of the materials [47] creating enormous distributed value [48] a high return on investment [49], and the ability to focus investments for strategic national goals [50], [51]. This same open source hardware design approach [52] can be applied to medical equipment [53], [54], [55], [56] to overcome supply shortages [57], [58], [59], [60], [61].
There has already been some effort in developing low-cost ventilators in the literature [62], [63], [64], [65], [66], [67], [68], [69], [70] as well as in the maker community; however, the former failed to provide full source code and the latter (as of March 2020) was unvalidated and largely untested [71]. To both fill the current critical need for ventilators as well as provide a basis for future pandemics, this article provides the full source code for a fully-functional low-cost 3-D printable open-source pandemic ventilator and includes validation testing using an artificial lung.
3. Hardware description.
The open-source pandemic single-limb (with one hose for the respiratory circuit; exhalation occurs through a single orifice located at the distal end of the circuit) ventilator (Fig. 1) was designed to be highly reproducible, simple in fabrication, maintenance and use for epidemics, pandemics and in developing and under-resourced communities. The design of the device and software was governed by ISO standards [72], the British Medicines & Healthcare Products Regulatory Agency’s rapidly manufactured ventilator system [73] and the Key Ventilation Specifications developed by the E-Vent project [74] along with consultation with health care professionals. The system can be fabricated from readily accessible components, open source Arduino microcontrollers [75], [76] open source electronics that can be made with open source mills [77], [78], [79] and custom parts with a RepRap-class material extrusion-based 3-D printer [80], [81], [82], [83], [84]. Mechanical ventilation, which can be easily controlled by a simple user input, was chosen to be most effective at treating the largest number of people.
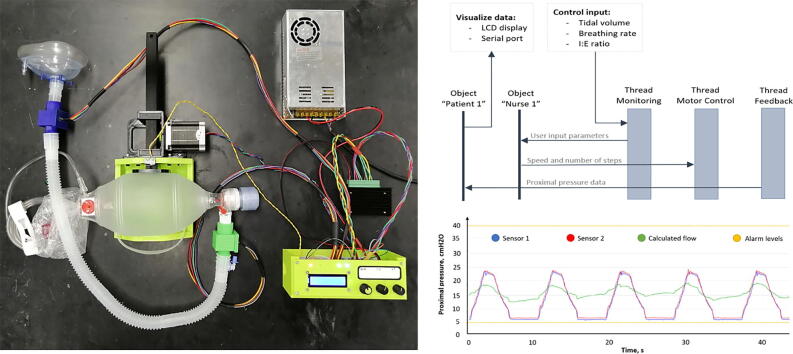
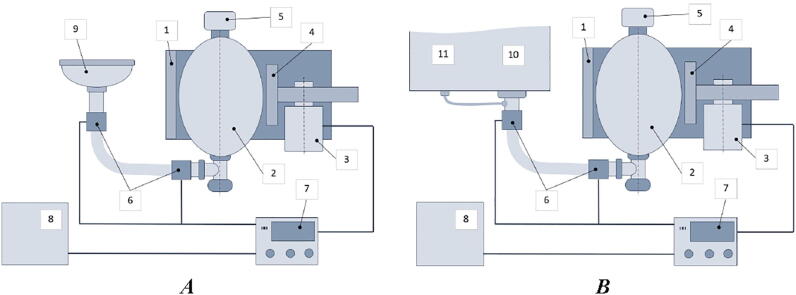
Fig. 1.
RepRapable Ventilator System: A) standalone automated BVM-based resuscitation system, B) testing procedure, 1) bag mounting system, 2) self-inflating bag, 3) motor setup, 4) compression mechanism (pusher), 5) Positive End Expiratory Pressure (PEEP) valve [85], 6) feedback pressure sensors, 7) control system, 8) power supply with backup battery, 9) air mask, 10) mechanical lung, 11) airway pressure sensor.
The system implements two following modes: controlled mechanical ventilation (CMV) and inverse ratio ventilation (IRV). A user can control breathing rates (breaths per minute or BPM), tidal volume (VT, air volume pushed into the lungs), inspiratory/expiration time ratio (I/E ratio). All the mechanical components (Fig. 1: components 1, 4, 7) were developed in open-source CAD systems. The use of a parametric OpenSCAD generator of 3-D printable components (junction boxes for the feedback pressure sensors (Fig. 1) allows to fit any tubing system. A backup battery enables short-term patient mobility and safety protocols in software provide alarm signals when the monitored proximal pressure exceeds the permissible range, or the pressure sensors are disconnected.
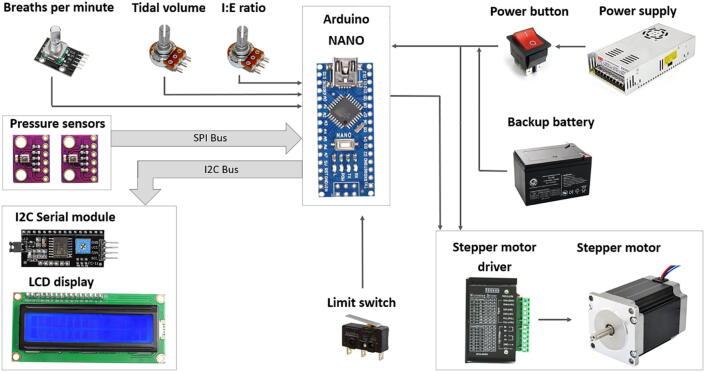
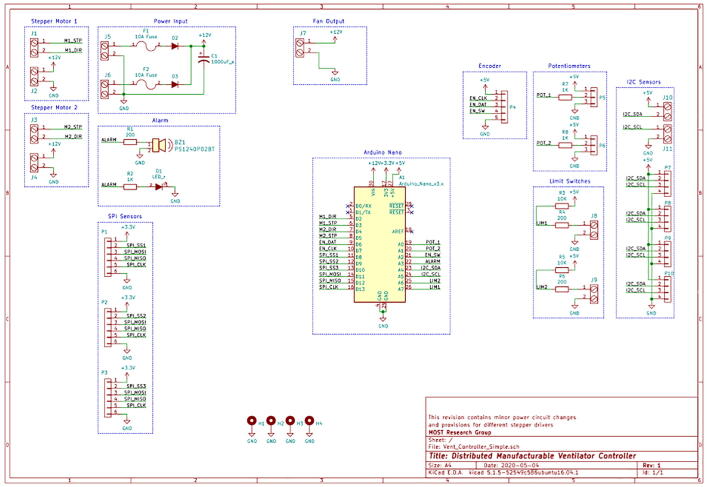
The electrical architecture is illustrated in Fig. 2. The development process of a medical device as an embedded real-time system can be divided into the main following steps:
-
1.
System design
-
2.
Schematic development
-
3.
Fabrication and assembly
-
4.
Software development
-
5.
Testing
Fig. 2.
Electrical system architecture.
Each of the above steps undergoes numerous iterations, starting with a concept passing the basic and detailed engineering stages, and ending with a finished product [86], [87], [88], [89], [90], [91].
This study of ventilator systems is based on fundamental works [92], [93], [94], [95], [96], [97]. In addition to the technical difficulties with the development of an embedded real-time system, there are also a significant number of details associated with the fabrication of parts that are used in contact with the patient.
The developed system has three control inputs for the variables: tidal volume (VT), breathing rate per minute (BPM), and inspiratory-to-expiratory ratio (I/E). BPM and I/E are controlled by rotary potentiometers, and BPM is controlled with a rotary encoder. Having a rotary encoder with an additional button may allow developers to upgrade the system in the future (for example, add a menu to select another mode).
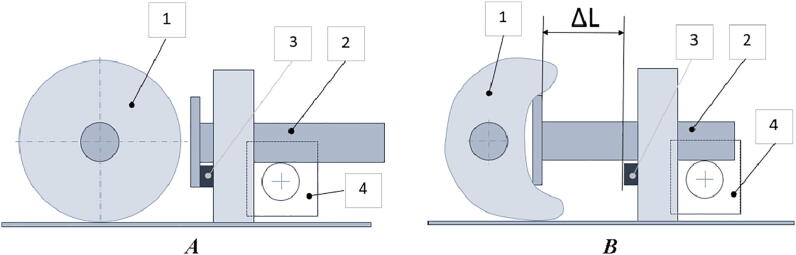
The self-inflating bag compression process is shown in Fig. 3. At the beginning of the operation, the pusher reaches the home position by hitting the limit switch. From this point, the tidal volume can be adjusted by the amplitude of the movement of the pusher (ΔL), and the breathing rate can be adjusted by a pusher frequency.
Fig. 3.
The process of compression of a self-inflating bag: A) initial homing position of the pusher, B) compression stage, 1) self-inflating bag, 2) pushing rod, 3) limit switch, and 4) stepper motor.
A breathing control diagram is presented in Fig. 4. According to the stepper motors datasheets [98], [99], both the widely used NEMA-17 and NEMA-23 stepper motors have 1.8 degrees per step, which would give N = 365/1.8 ≈ 203 steps per one revolution of the shaft. With specified micro-stepping multiplier, k = 2…16, it is possible to increase the number of steps per one revolution and provide a more smooth and stable rotation of the motor shaft.
Fig. 4.
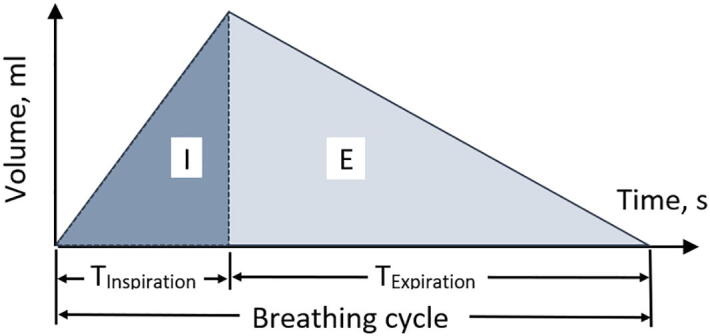
Breathing control diagram: the tidal volume depends on the length of extension of the pusher, and the timings for the inspiratory and expiratory phases – are functions of stepper motor delays between its successive steps.
The thrust of the motor depends on the motor torque and the diameter of the gear according to the following equation: F = 2 T/R, where R – is the gear radius and T – is the motor torque. Therefore, by varying the motor current and the size of the gear, it was experimentally found that the herringbone gear (double helical gear) with a diameter of 15 mm will provide reasonable thrust and consistency of contacts between the gear teeth.
As can be seen from Fig. 4, VT, BPM, and I/E are functions of the number of steps and the speed of the stepper motor. To provide the desired breathing parameters, the number of motor steps should be calculated as follows:
| (1) |
where D is the gear diameter in millimeters, ΔL is the desired pusher length in millimeters, and N is the number of steps per one full revolution. At the same time, N = k ∙ 365/1.8 steps, where k is the micro-stepping multiplier (usually k varies from 2 to 16).
A greater number of steps per revolution of the motor shaft allows smooth rotation and prevents unwanted vibration of the entire apparatus. It is worth noting, however, that the use of micro-stepping higher k values reduces the overall torque of the motor. Thus, a balance was experimentally found between the number of motor steps and the permissible vibration of the bag support system with a micro-stepping coefficient of 4, which corresponds to ~800 steps per single revolution of the shaft.
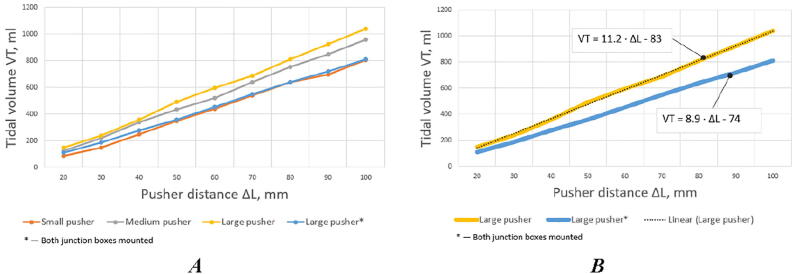
The volume of air or gas mixture provided by the self-inflating bag is largely due to the shape and size of the pusher. The experiments with three pushers with a total area of 14, 42, and 74 square centimeters (Fig. 5) revealed linear relationships between the volume of air supplied to the lungs and the pusher travel distance (Fig. 6, A). The linear dependency between the pusher travel distance and provided tidal volume equals to ΔL = (83 + VT)/11.2 mm.
Fig. 5.
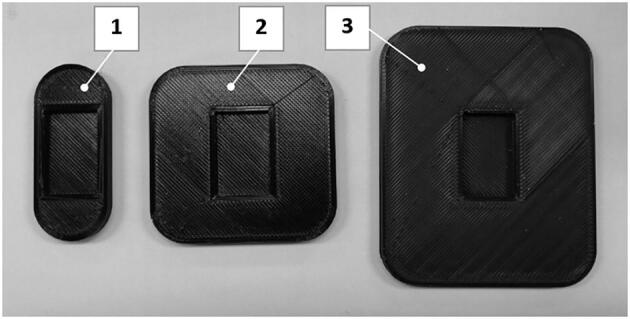
Pushers used for the experiments: 1) 14 cm2, 2) 42 cm2, 3) 74 cm2. The recess shown is the press-fit attachment point for the rack printed part.
Fig. 6.
Correspondence of tidal volume and pusher travel distance: A) pushing rod with different pushers and the whole system is without feedback pressure sensors (junction boxes dismounted), B) pushing rod with the large pusher with (*) and without junction boxes mounted.
It should also be noted that air leakage [100], [101] due to the mounting design of the pressure sensors can lead to a decrease in the angle of inclination of the calibration curve (Fig. 6, B).
Thus, the number of motor steps to push the plunger in order to provide the desired air volume can be expressed as follows:
| (2) |
where VT is the tidal volume in milliliters, k is the micro-stepping multiplier and D is the gear diameter in millimeters.
Manipulating the BPM and IE control knobs (Fig. 2), it is possible to set the specified breathing parameters by adjusting the time delays between successive motor steps:
| (3) |
where Δti is the time delays (in seconds) during the inspiratory phase of the breathing cycle, BPM is the breathing rate (breaths per minute), I/E is the inspiratory-to-expiratory ratio. The time delays for the expiratory phase will be equal to Δte = Δti∙(I/E)−1 s.
Two pressure sensors located at the edges of the air duct are used to calculate proximal airflow using the simplified Bernoulli equation (4) [102], [103]. Sensitive elements of pressure sensors are based on piezo-resistive technology [104], which ensures accuracy, linearity, and stability during long-term operation. Healthcare devices and applications represent the typical use of the given devices. The value of the airflow is not used as a feedback signal and is meant for illustrative purposes only.
| (4) |
where Q is the flow rate in liters per minute, Δp is the pressure difference (pressure drop) between two sensor readings in pascals, and m is the calibrated scaling factor.
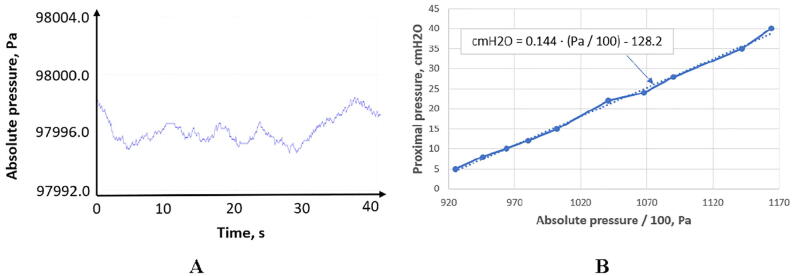
The BMP280 sensor measures the absolute pressure in the range of 300 to 1100 hPa. Therefore, it is necessary to calibrate the system each time the ventilator is used to determine the level of normal ambient pressure (Fig. 7). For these purposes, an additional sensor can also be used to isolate atmospheric pressure so that a pair of BMP280 sensing elements will allow measuring the relative proximal pressure in the airways.
Fig. 7.
Proximal pressure calibration: A) absolute pressure of the laboratory environment (zero-level for proximal pressure), B) BMP280 calibration curve for proximal pressure.
To suppress the noise of the signal from the pressure sensors, an exponential filter is used [105]. This smooths the curve without using significant memory resources. When a new measured value pt is provided, the exponential filter updates a smoothed observation, St:
| (5) |
where St-1 is the previous output value of the filter in pascals, pt is the new measured value in pascals, and α is the smoothing constant ().
Since the BMP80 pressure sensors are located in the junction boxes (Fig. 1), and not directly in the airflow path, their readings must be brought to real proximal pressure values based on the results of experiments with the mechanical lung [106].
A calibration curve coerces the sensors values to proximal airway pressure can be described by the following equation:
| (6) |
where Pproximal – proximal pressure in cmH2O, Pabsolute – absolute BMP280 pressure in pascals.
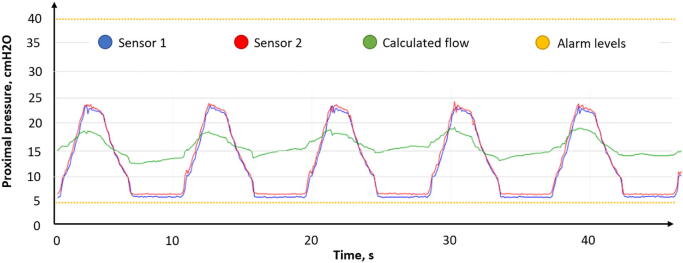
Thus, the signals from pressure sensors located at opposite ends of the airway can be interpreted as proximal pressure. Based on the above Eq. (6), it is possible to determine the readings of the sensors corresponding to the minimum allowable PEEP pressure and the maximum critical pressure of 40 cmH2O (Fig. 8).
Fig. 8.
Pressure sensors feedback.
The control system is based on the Arduino controller and a stepper motor setup (NEMA-23 motor). The Arduino Nano board was chosen as a controller due to low relative expense while having sufficient digital and analog pins.
A significant number of medical software development standards contain information and requirements regarding software design, validation, and certification [107], [108], [109], [110], [111], [112]. However, in the global pandemic, meeting all requirements can be difficult. The main guidelines for emergency ventilation systems is the use of real-time operating systems and a serial peripheral interface for connecting sensing devices [113].
The use of an open-source real-time operating system (FreeRTOS) library [114] for Arduino considerably expands the possibilities of the controller. A real-time operating system provides essential functions to software tasks, such as scheduling, dispatching, inter-task communication, and synchronization [115].
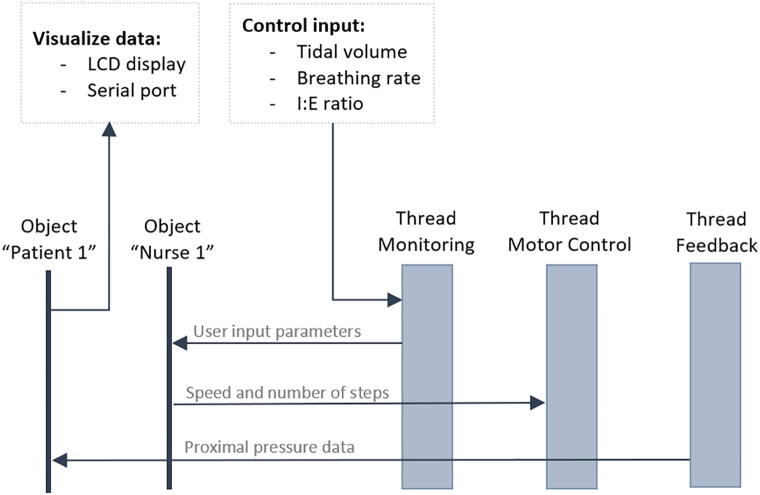
The software system architecture is shown in Fig. 9. There are three parallel tasks with equal priorities communicating with the two instances of the patient and nurse classes, which provide scalability (there may be more “patients” and “nurses”, as well as threads with other functions) and possibility of transition to another hardware background since FreeRTOS supports most popular processors and microcontrollers [114].
Fig. 9.
Software system architecture.
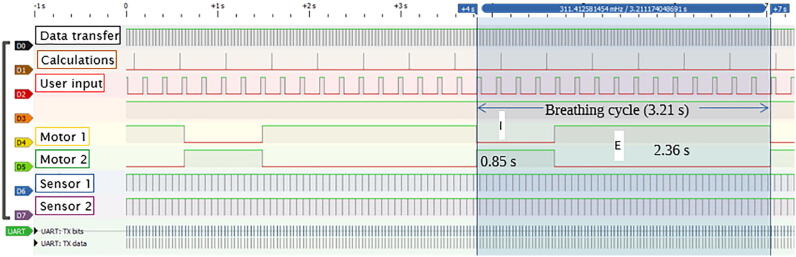
The software trace (Fig. 10) obtained using a logic analyzer can visualize the execution of the algorithm in terms of the frequency and duration of existing tasks. The main utilities are presented in Table 1.
Fig. 10.
Algorithm trace: example for 455 mL of tidal volume, 20 breaths per minute, and 1:3 inspiration-to-expiration ratio.
Table 1.
Software tracing summary.
| Utility | Frequency, Hz | Duration, ms |
|---|---|---|
| Serial data transfer | 14 | 2.5 |
| Motor parameters recalculation | 2 | 0.1 |
| Reading user input and LCD display update | 4.5 | 50 |
| Two pressure sensors readings | 14 | 1.5 |
To summarize the main characteristics of the ventilator can be represented as follows:
-
•
Low cost (~$20 for 3-D printed mechanical components, ~$120 for electronic components, and ~$23 for BVM and single-limb ventilator circuit). Note that this is solely the cost of materials.
-
•
Availability of components for assembly and ease of fabrication.
-
•Providing a controlled breathing mode with the following parameters:
-
1.Tidal volume in the range from 100 to 800 mL
-
2.Breathing rate in the range from 5 to 40 BPM
-
3.Inspiratory-to-expiratory ratio in the range from 1:1 to 1:4
-
1.
-
•
Software reliability through the use of the real-time operating system
-
•
Reliability and scalability of measurement circuits through the use of the serial peripheral interface (SPI)
-
•
Ability to connect additional hardware due to the object-oriented algorithmic approach
4. Design files
4.1. Design files summary
| No. | Design file name | Image | File type | Open source license | Location of the file |
|---|---|---|---|---|---|
| Bag mounting system | |||||
| 1 | BagSupport.FCStd |  |
FreeCAD | GNU GPL v3 | https://osf.io/fjdwz/ |
| 2 | MotorMount.FCStd |  |
FreeCAD | GNU GPL v3 | https://osf.io/fjdwz/ |
| 3 | Rack.FCStd |  |
FreeCAD | GNU GPL v3 | https://osf.io/fjdwz/ |
| 4 | Pinion.FCStd |  |
FreeCAD | GNU GPL v3 | https://osf.io/fjdwz/ |
| 5 | Rod_head_pusher.scad |  |
OpenSCAD | GNU GPL v3 | https://osf.io/fjdwz/ |
| Junction boxes for pressure sensors | |||||
| 6 | Junction_box_generator.scad | OpenSCAD | https://osf.io/fjdwz/ | ||
| 7 | Pressure_sensor_junction_box.stl |  |
STL | GNU GPL v3 | https://osf.io/fjdwz/ |
| 8 | Pressure_sensor_ junction_box_plate.stl |  |
STL | GNU GPL v3 | https://osf.io/fjdwz/ |
| Control box | |||||
| 9 | Control_box_panel.FCStd |  |
FreeCAD | GNU GPL v3 | https://osf.io/fjdwz/ |
| 10 | BreadBoxBase.FCStd |  |
FreeCAD | GNU GPL v3 | https://osf.io/fjdwz/ |
| Electrical schematics and printed circuit board | |||||
| 11 | Vent_Controller_Simple.pdf | — | GNU GPL v3 | https://osf.io/fjdwz/ | |
| 12 | Vent_Controller_Simple.pro | — | KiCAD Project | GNU GPL v3 | https://osf.io/fjdwz/ |
| 13 | Vent_Controller_Simple.sch | — | KiCAD Schematic | GNU GPL v3 | https://osf.io/fjdwz/ |
| 14 | Vent_Controller_Simple.kicad_pcb | — | KiCAD PCB File | GNU GPL v3 | https://osf.io/fjdwz/ |
| 15 | /gerbers | — | Gerber | GNU GPL v3 | https://osf.io/fjdwz/ |
| Arduino firmware | |||||
| 12 | arduino_firmware.ino | — | Arduino sketch | GNU GPL v3 | https://osf.io/fjdwz/ |
Both the FreeCAD and OpenSCAD files were designed to be parametric to allow future developers to replicate this system for different core components (e.g. different sizes of bags).
-
1.
“Bag support” provides support for the bag to keep it stabilized in the transverse and longitudinal directions. Major modifications may involve changing the entire geometry to fit a different self-inflating bag. Minor modifications may include changes to the attachment points to the motor mount part or additional support for the bag.
-
2.
“Motor mount” provides a mounting point and support for NEMA-23, it also provides a sliding path for the rack. Major modifications may involve changing the geometry for use with a different motor. Minor modifications may involve changing the attachment points to the bag support, changes to the sliders.
-
3.
“Rack” and “Pinion” use the motor power to compress the bag. Both the FreeCAD source files were created with the “FCGear” add-on that generates gear profiles. Steps are named for ease of use. Major modifications involve changes to the gear (gear specifications are accessible within the file) which requires “FCGear” workbench. Minor modification involves changes to the geometry of the hole for the motor shaft, changes to the nut-trap, as well as tolerance adjustments.
-
4.
“Rod head pusher” is attached to the rack and serves both to compress the bag and to close the limit switch during the homing process.
-
5.
“Junction box generator” is the master file for rendering the junction box and plate in order to create a press-fit between two tubes with a sensor epoxied inside.
-
6.
“Pressure sensor junction box” and “Pressure sensor junction box plate” are the current precise geometries for the ventilator design described in this work. Import into a slicer to use.
-
7.
“Control box panel” and “Breadboard box base” are the parts of the control system housing with user input.
-
8.
“Schematic” is a control system wiring diagram that can be implemented using both a breadboard and a printed circuit board.
-
9.
“Arduino firmware” is a program that reads user input and implements motor control in accordance with user-defined breathing parameters.
5. Bill of materials
The complete Bill of Materials is available in the OSF repository (https://osf.io/ugt3e/).
5.1. Breathing system Bill of materials
| Designator | Number | Cost per unit – USD | Total cost – USD | Source of materials |
|---|---|---|---|---|
| Adult Bag-Valve-Mask Ambu SPUR II | 1 | 15.95 | 15.95 | https://www.heartsmart.com/ambu-adult-spur-ii-adult-bvm-p |
| Single-limb ventilator circuit | 1 | 6.71 | 6.71 | https://www.saveritemedical.com/products/adult-single-limb-portable-ventilator-circuit?variant=32484935052&gclid=EAIaIQobChMIzsvWlbye6gIVDtbACh2DswWiEAQYAiABEgL0FPD_BwE |
5.2. Mechanical system Bill of materials
| Designator | Component | Mass in grams | Cost per unit – USD | Total cost – USD | Source of materials | Material type |
|---|---|---|---|---|---|---|
| PLA – 3-D printer filament | BagSupport.stl, MotorMount.stl, Rack.stl, Pinion.stl, BreadBoardBase.stl, BreadBoardCover.stl |
700 | $25/kg | $17.50 | https://us.polymaker.com/product/polylite-pla/ | Hard thermoplastic |
| Ninjaflex – 3-D printer filament | Junction_box and plate STLs | 40 | $85/kg | $3.40 | https://www.fennerdrives.com/product-lines/_/3d/ | Flexible polymer |
5.3. Control system Bill of materials
6. Build instructions
The installation of the device consists of three stages: 1) bag holder assembly, 2) breathing system assembly, and 3) control system assembly. To print all components, a RepRap-class 3-D printer with a minimum printing area of 230x230x100mm is needed. Fabrication of all parts takes from 25 to 34 h, depending on print settings. Printing material can be polylactic acid (PLA) or glycol modified polyethylene terephthalate (PETG). For junction boxes with pressure sensors, thermoplastic polyurethane (TPU, NinjaFlex in this work) material was chosen to minimize air leakage.
6.1. Bag holder assembly
1. Obtain any RepRap-class 3-D printer with build dimension of at least 230 × 230 × 100 mm.
2. Obtain a minimum of 500 g of 3-D printer filament such as PLA or PETG.
3. Download the files from https://osf.io/fjdwz
4. Make modifications to FCStd file if necessary and export STLs or use provided STLs
5. Import the STL file into a slicing software, such as Cura.
6. Use default printing parameters for the material. Change orientation of the model if necessary. Use a layer height of 0.1–0.25 mm, at least 20% infill and a minimum top/bottom thickness of 0.5 mm and wall thickness of 1 mm. No supports are necessary.
7 Print time and mass of filament used:
-
•
The print time would be approximately 12–15 h, with around 170–250 g of filament used for bag support.
-
•
The print time would be approximately 7–10 h, with 100–150 g of filament used for motor mount.
-
•
The print time would be approximately 5–7 h, with 70–100 g of filament used for Rack
-
•
The print time would be approximately 1–2 h, with 5–10 g of filament used for Pinion
8. Assemble the parts as shown in Fig. 11. All nine bolts and nuts are M4. It can be modified in the FreeCAD files if necessary.
Fig. 11.
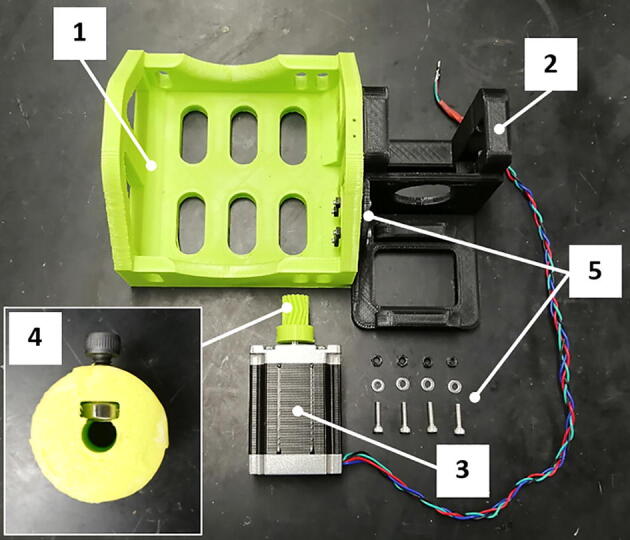
Bag holder assembly: 1) bag support, 2) motor mount, 3) stepper motor, 4) pinion gear, 5) fasteners.
9. Print and assemble pusher.
-
•
Obtain a common rigid polymer-based filament such as PLA or PETG
-
•
If it is necessary to alter the design Download the Rod_head_pusher.scad from https://osf.io/fjdwz/. Alter the X-Y dimensions and pusher grip length parameters in the design using the variable list as desired.
-
•
If there is no need to alter the design download the Rod_head_pusher.stl from https://osf.io/fjdwz/ and import into Cura.
-
•
Download the material file from https://osf.io/fjdwz/ and import into Cura and set the appropriate print parameters individually or by importing the associated material file from https://osf.io/fjdwz/. 50 min are needed to print the 10 g out of PLA at 100% infill, at 30 mm/s as determined by Cura.
-
•
Once printed as seen in Fig. 12, slot the pusher onto the rod as it is installed in the ventilator. The pusher should press-fit tightly onto the rod.
Fig. 12.
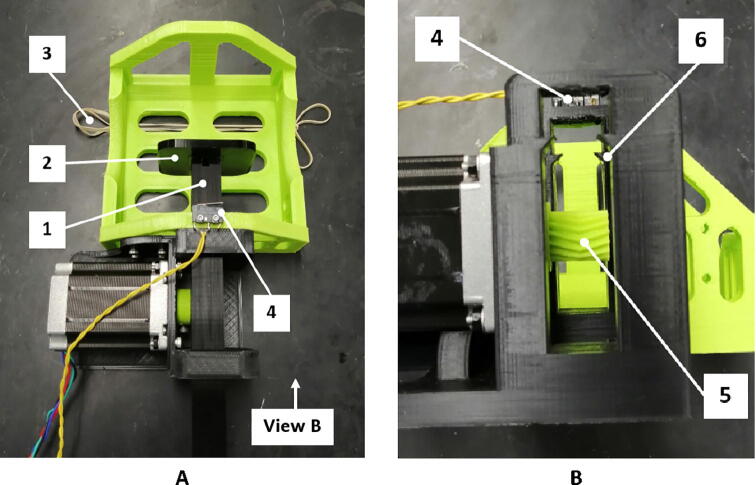
Bag support and motor mount assembly: A) top view, B) view from the side of the pushing rod groove, 1) pushing rod, 2) pusher, 3) rubber band for bag support, 4) limit switch, 5) pinion gear, 6) pushing rod groove.
6.2. Breathing system assembly
For fabrication the junction boxes an open source RepRap Lulzbot TAZ 3-D printer was used with Ninjaflex filament and the open source Lulzbot Cura slicer (edition 3.6.8).
1. Obtain Ninjaflex filament
2. Obtain a RepRap-class 3-D printer capable of printing flexible filament
3. If it is necessary to alter the design Download the Junction_box_generator.scad from https://osf.io/fjdwz/ and download OpenSCAD from https://www.openscad.org/
4. Alter the input and output parameters in the design using the variable list as desired to fit the tubing sizes available
5. If there is no need to alter the design download the Pressure_sensor_junction_box_plate.stl and Pressure_sensor_junction_box.stl from https://osf.io/fjdwz/ and import into Cura or other open source slicer.
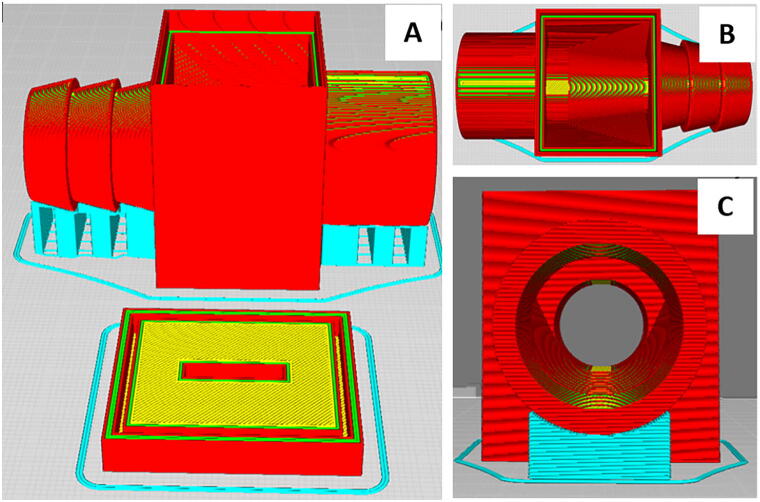
6. Download the material file from https://osf.io/fjdwz/ and import into Cura and set the appropriate print parameters individually or by importing the associated material file from https://osf.io/fjdwz/. 5 h and 36 min are needed to print the 39 g out of Ninjaflex at 50% infill, at 30 mm/s as determined by Cura (Fig. 13).
Fig. 13.
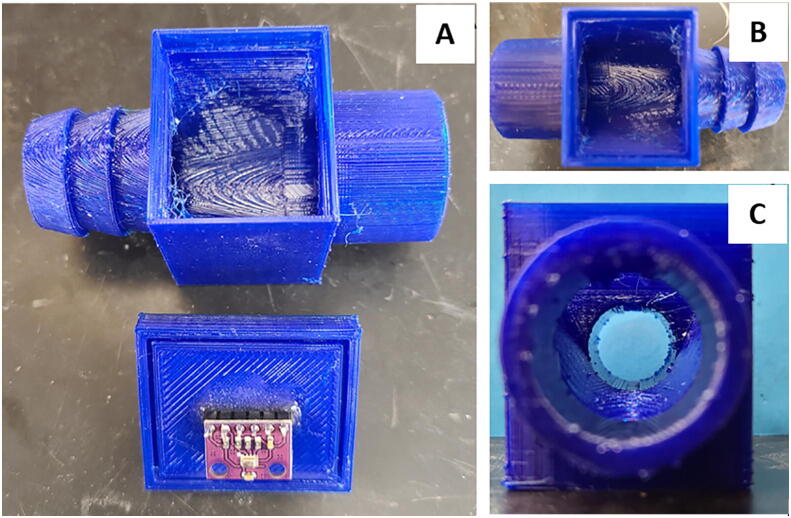
Junction box: Cura line types with support material in blue, shell in red, top/bottom in yellow, and inner wall in green. Overview of both the junction box and plate (A), top down view with a barbed input and a straight output for different tube diameters (B), side view down the output (C).
7. Using epoxy mount a 6-prong attachment for connection to the pressure sensor as seen in Fig. 13, then install the pressure sensor (Fig. 14). Fig. 15 shows the completed printed junction box assembly ready for use.
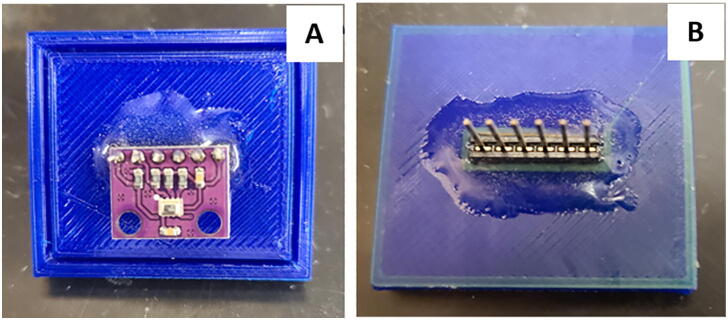
Fig. 14.
Pressure sensor cover: pressure sensor location (A) on bottom of junction box plate and epoxied 6 prong wire connection (B) on top of junction box plate.
Fig. 15.
Junction box assembly: overview of printed junction box with press-fit plate and epoxied 6 prong attachment for pressure sensor wiring (A), top down view with a barbed input and a straight output for different tube diameters (B), side view down the output (C).
8 Connect cables and, junction boxes and tubes as shown on Fig. 16. Connect tubes to the BVM-bag (Fig. 17).
Fig. 16.
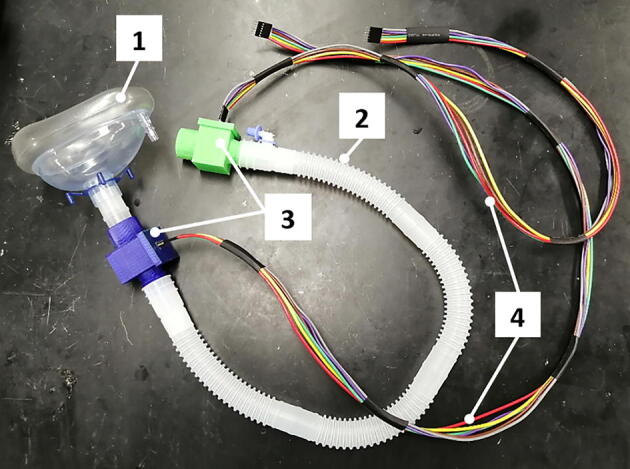
Breathing system assembly: 1) breathing mask, 2) 22 mm air tube, 3) junction boxes with pressure sensors inside, 4) cables for connecting pressure sensors.
Fig. 17.
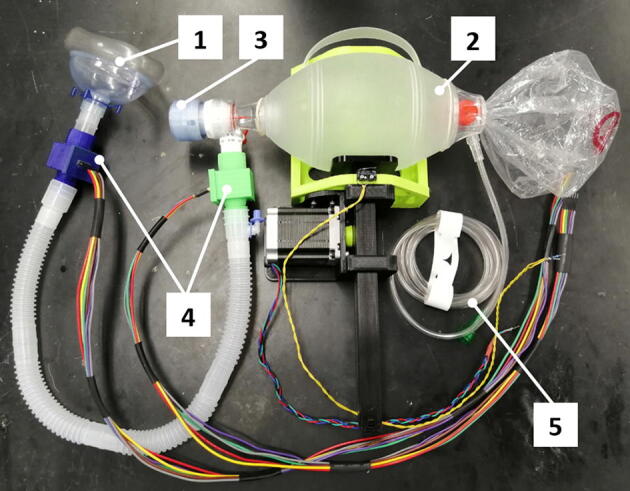
Breathing system connections: 1) breathing mask, 2) self-inflating bag, 3) PEEP valve, 4) junction boxes with pressure sensors inside, 5) oxygen hose (not involved in this work).
6.3. Control system assembly
9 Print and assemble the case for the control system according to Fig. 18.
Fig. 18.
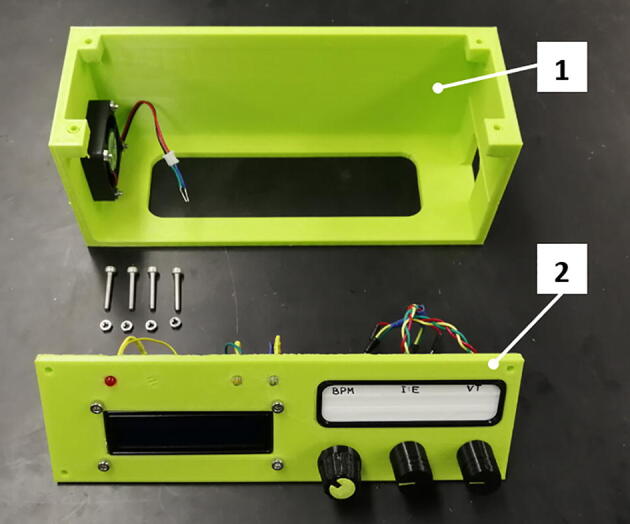
Assembly of the control system box: 1) base, 2) cover.
10. Connect the components according to the schematics (Fig. 19) and build a breadboard (Fig. 20, A). Note that the fuses in the schematic cannot be installed into the breadboard and must be omitted. Additionally, there are some sets of redundant or extra connections that are not needed for this specific implementation. Install the breadboard into the case (Fig. 20, B).
Fig. 19.
Electrical schematics of the control system.
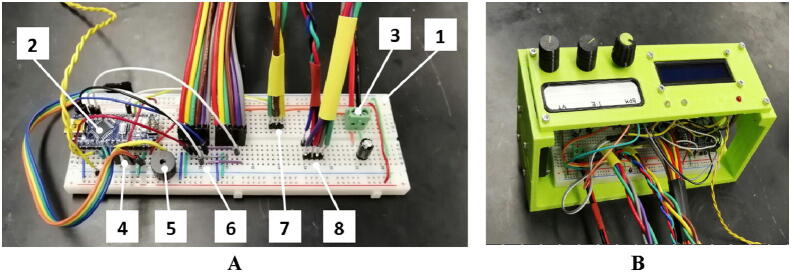
Fig. 20.
Breadboard assembly: A) breadboard with the components, B) breadboard installed inside the case, 1) breadboard, 2) Arduino Nano, 3) power supply connection, 4) LCD display connection via I2C bus, 5) alarm buzzer, 6) pressure sensors connection via the serial peripheral interface (SPI), 7) stepper motor control, and 8) stepper motor coils.
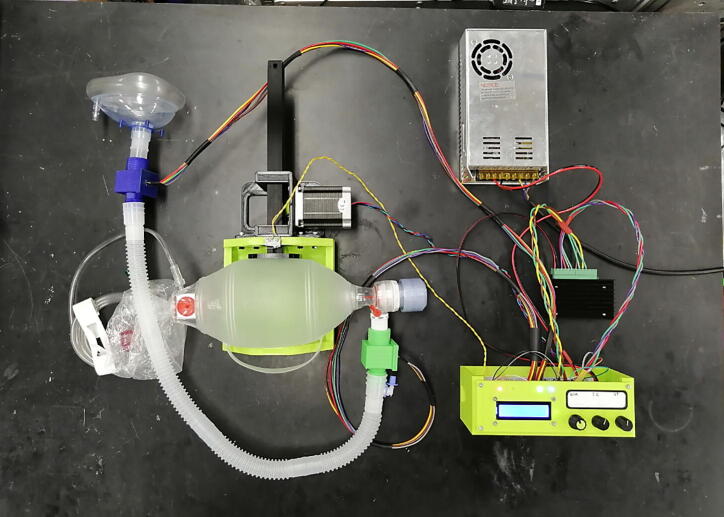
The complete system is then assembled as shown in Fig. 21.
Fig. 21.
Complete assembly of the standalone automated BVM-based resuscitation system.
10 Install the firmware by uploading the “arduino_firmware.ino” file to the Arduino Nano controller via Arduino IDE (https://www.arduino.cc/en/main/software).
7. Operation instructions
Using the control knobs on the top panel (Fig. 22, component 1), a user must set the desired breathing mode and connect the patient to the mask.
Fig. 22.
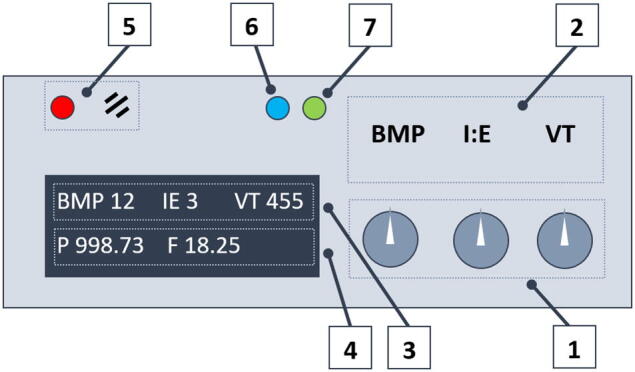
Control panel: 1) control knobs for breath rate, I:E ratio, and tidal volume, 2) text field, 3) input parameters displayed on LCD display, 4) feedback parameters (airway pressure and airflow), 5) alarm section (LED and buzzer), 6) inspiration indicator (motor pushes the self-inflated bag), 7) motor step command indicator.
The LCD (Fig. 22, components 3 and 4) displays the input parameters (VT, BPM, and I/E) and feedback (proximal airway pressure and estimated airflow). LEDs 6 and 7 (Fig. 22) reflect the motor operation mode. LED 5 signals an alarm when the proximal airway pressure exceeds the permissible range.
When using the device, there may be a danger of electric shock. Performed incorrectly, BVM-based ventilation can accelerate hypoxia and aggravate airway obstruction [116]. This can result in serious injury or death [117], [118], [119], [120], [121], [122], [123], [124], [125], [126]. According to the international “Medical Device Software” standard IEC 62,304 [127], [128], ventilators are class C medical equipment that can lead to patient death.
8. Validation and characterization
The mechanical design was experimentally tested for consistency, accuracy, and reliability using a Michigan Instruments Lung Simulator [106] as shown in Fig. 23. Table spreadsheets were created that compared values from outputs from Michigan Instruments Test and Training Lung software, PneuView3, [129] with target values. These variables included, peak inspiratory pressure (PIP), respiratory rate (RR), positive end-expiratory pressure (PEEP), I:E ratio, and tidal volume. A sample spreadsheet used for tests is illustrated on Fig. 24. The spreadsheets are included in the OSF repository.
Fig. 23.
Testing procedure set up.
Fig. 24.
Example of the values collected for each test at a given tidal volume, respiratory rate, and an I:E ratio: values highlighted in blue represent the required metrics.
Each experimental test was conducted at a set tidal volume (starting from 100, increasing to 800 at an interval of 100), a set respiratory rate (starting at 5 BPM, increasing to 15 at an interval of 5), and a set I:E ratio (1:2). The airway resistance was kept at a constant Rp5 [106] with a compliance of 0.05 to simulate a healthy adult lung. The PEEP valve was not touched to determine if it was consistent for all tests.
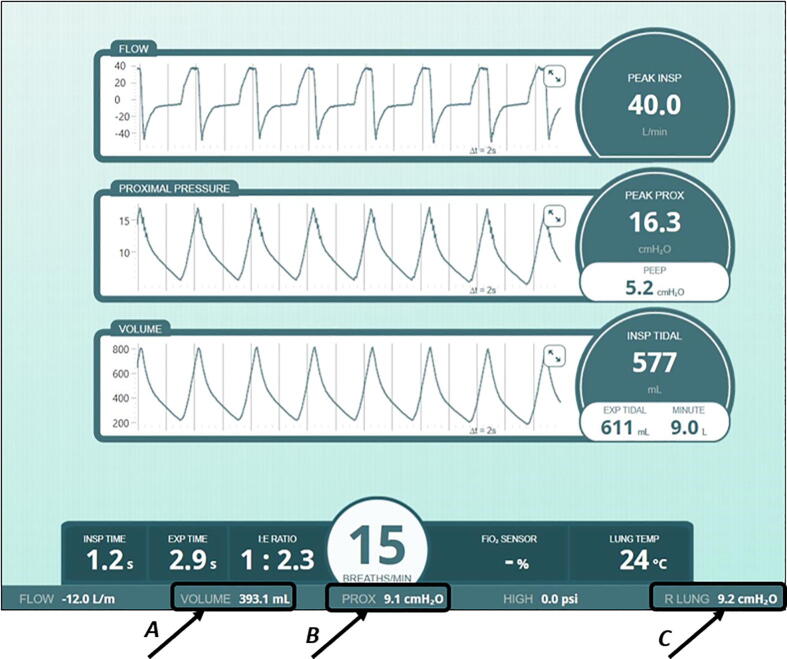
The values for every measurement, excluding the flow, oxygen concentration, and FiO2 percentage, were recorded through the PneuView3 (Fig. 25) by taking a screenshot of the software screen once it became constant. While waiting for the data to become constant, the maximum values for tidal volume, proximal pressure, and lung pressure were recorded in real time.
Fig. 25.
PneuView software, values recorded in real time: A) tidal volume. B) proximal (airway) pressure, and C) lung pressure.
Due to the data being recorded in real time, the values fluctuated as the tests went on. Statistical analysis was completed by calculating the standard error between each test using the built in STDEV.P function in Excel. Since the tests were run for three trials (N = 3) to determine repeatability, the standard error (SE) was found by the equation (7). This was completed for the most important values such as PIP, PEEP, tidal volume, proximal pressure, and lung pressure.
| (7) |
where σ is the standard deviation of the parameter distribution and N is the number of observations.
A few changes were made between trials to gain more accurate data. This included attaching the rack pusher to the pinion, securing the valve bag with rubber bands, and switching out the gear used to push the rack forward. However, the data remained slightly inaccurate after the modifications. Future work should focus on designing a more stable mechanical set-up that will not need to be adjusted after a few hours.
Another metric that was analyzed by this protocol was to determine if adding a junction box was going to cause failures within the system, or if there was a specific location that the box should not be installed. The oxygen was not measured due to the ventilator using room air, thus, assuming that the O2 concentration and FiO2 percent were up to standards, it was also assumed that the hospital themselves would be able to observe these values using their technology and resources.
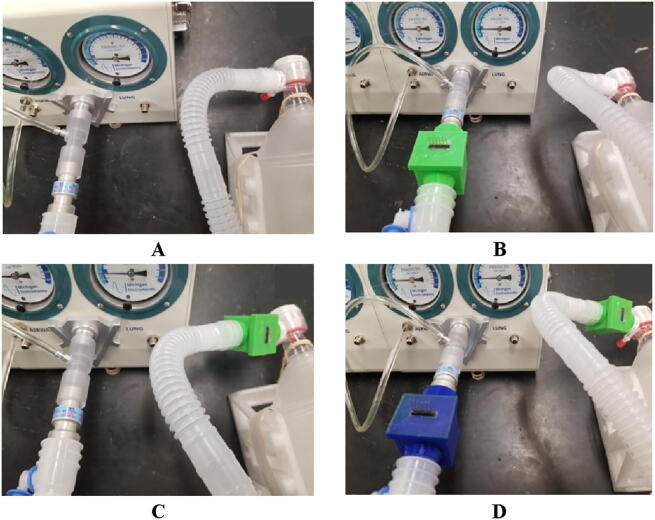
There was a total of four tests that were completed using the previously mentioned protocols. These included tests where there was no junction box attached to the ventilator connection tube, with a junction box at each end of the tube separately, and finally with a junction box at both ends of the tube (Fig. 26). The wires connected to the pressure sensors were not used during the testing process. These were also completed a total of three time (N = 3).
Fig. 26.
The placements of the junction boxes for testing: A) no junction box attached (control), B) junction box attached at the lung end of the connection tube, C) junction box connected at the ventilator end of the connection tube, and D) junction box connected at both ends of the connection tube.
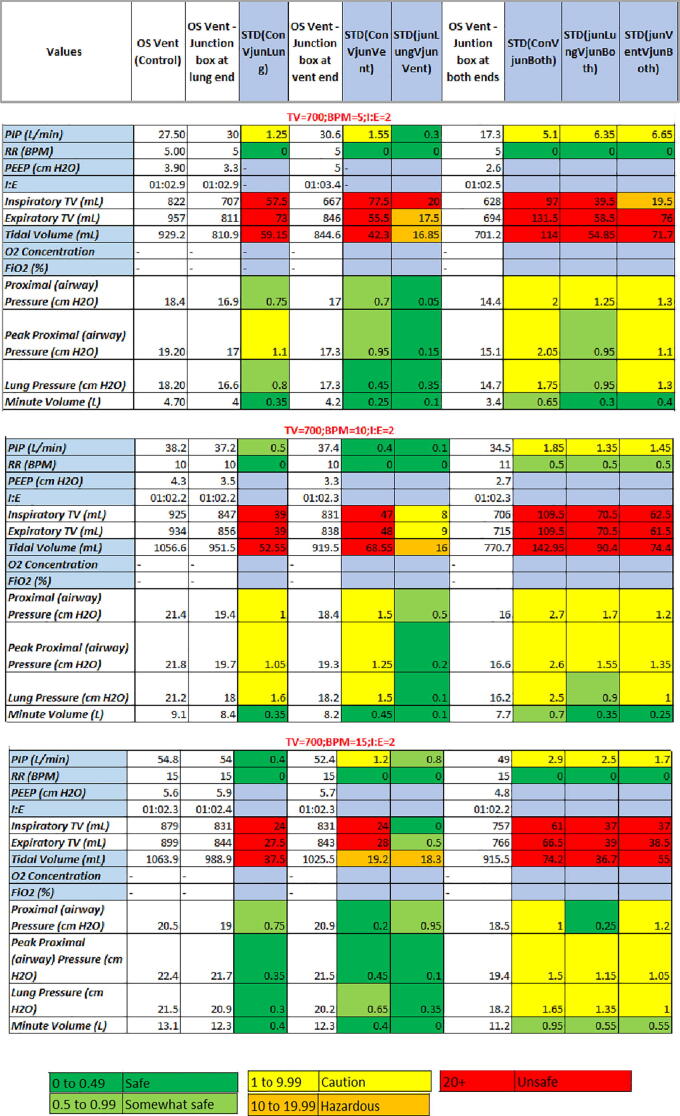
Both the green and blue boxes were epoxied between the pressure sensor and the lid. The meaningful difference being, the blue box did not contain super glue to hold the pressure sensor to the ports on the underside of the lid, whereas the green box did. After completing these tests, it was found that adding a junction box could cause significant changes in the data if the box was not assembled correctly. It was also seen that at low tidal volumes the lung was unable to calculate the majority of values, thus half the data could not be collected. In some instances the lung struggled to maintain consistent data causing values to be estimated. However, the main significant difference for each test, and trial, was the tidal volume. For the majority of tests, the tidal volume recorded was different than the volume manually set on the user interface. The standard deviations were also incredibly unsafe with the majority being over 60 mL for all three trials. A representative data set from trial 1 was created to show the similarities and differences between each test condition (Fig. 27).
Fig. 27.
Representative data gathered for the junction box testing: set tidal volume of 700 mL, respiratory rate of 5, 10, and 15 BPM, and an I:E ratio of 1:2 for trial 1.
The difference in tidal volume may simple be due to the design of the valve bag, undetected motor slippage, or the mechanical set-up of the ventilator. It can also be assumed that it was from the addition of junction boxes. In regard to having two boxes, the proximal and lung pressures were also not reliable. By looking, however, at the comparison between the single junction box tests, there was little to no standard deviation. Future work will delve into how to make the junction boxes more reliable, as well as how to maintain tidal volume.
Another investigation was based off the respiratory rate itself. It can be seen that the standard deviations between tests at 15 BPM are slightly lower than those of the 5 BPM test. This makes sense since there was a higher sample rate for calculations, and less time between breaths to let air escape through any leaks. The 10 BPM data could be a considered an outlier because the majority of values are above both the 5 and 15 BPM tests. This could have been caused by a shift in the valve bag, a slip of the motor, or inaccurate data gathered from the lung itself. The mechanical issues associated with this design should be addressed in future work to confidently confirm that the ventilator is consistent, accurate, and reliable.
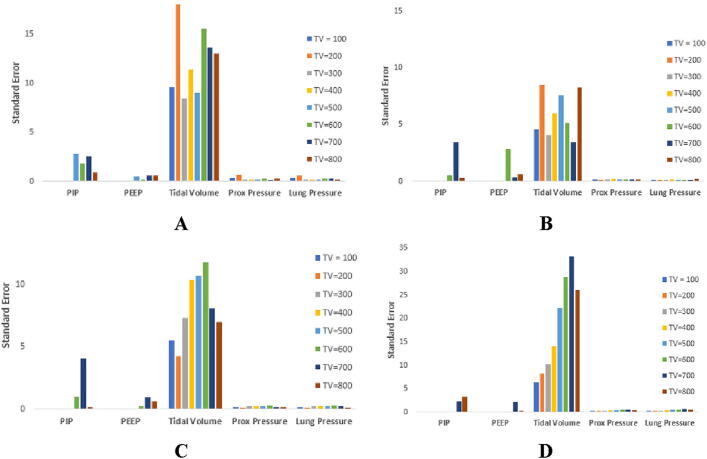
The standard error results (Fig. 28) indicate, notably, significant difference between the means of the targeted tidal volumes.
Fig. 28.
Representative standard error between each test for each position of junction box at a respiratory rate of 5 BPM: A) no junction boxes attached, B) junction box at lung end, C) junction box at ventilator end, D) junction box at both ends. Legend shows the tidal volumes that were input by the user.
This tidal volume was not what was manually input by the user, but the volume that was outputted by the PneuView software. It can be seen that for every tidal volume, and trial, the output TV experienced a high standard error. This is especially true with the addition of two junction boxes where the highest value was at a standard error of about 35 cm H2O, almost 20 cm H2O greater than the control trial. The PIP and PEEP values also showed inconsistency between trials however they were not as severe as the tidal volume values. The pressure metrics were relatively constant for each trial, with the exception of a few outliers. These outliers can be seen in the control trial at an input tidal volume of 200 cm H2O.
The open source ventilator here had alarms for 1) low pressure, 2) high pressure and 3) wire disconnect. Future work could consider adding oxygen concentration alarms, oxygen tube disconnection alarms, battery backup alarms and a mechanical failure alarm. The low/high pressure alarm was tested by manually squeezing, and releasing, the valve bag so that the pressure sensors detected pressures above 40 cm H2O, and below 5 cm H2O. The wire disconnection alarm was tested by manually unplugging the wires that were connected to the pressure sensors themselves.
The ventilator was then assessed on if it was able to pass the key ventilation specifications developed by the E-Vent Key Ventilation Specifications (v 27 March 2020) [74]. The first three tests were completed by adjusting the respiratory rate, tidal volume, and I:E ratio using the potentiometers installed on the circuit board from the minimum value to the maximum. The proximal pressure was limited by programming the pressure alarms to go off above 40 cm H2O. A plateau pressure limited to 30 cm H2O can be added by introducing an adjustable pause after the end of the inspiratory phase.
The PEEP values were confirmed by adjusting the PEEP valve connected to the exhale port of the valve bag. However, since the mechanical spring underlying the PEEP valve operation is a very sensitive part, even slight valve adjustment can lead to deviations of positive end expiratory pressure up to 4 cmH2O. The PEEP values depend on the tidal volume and respiratory rate. Thus, the PEEP could be stationary at one spot, but be different for a respiratory rate of 5 BPM than for a respiratory rate of 10 BPM. It should also be noted that various materials and printing parameters can lead to tolerance deviations, which makes the calibration of a mechanical PEEP valve a non-trivial task.
HEPA filters can be added in the future to determine the effect that a filter will have on the data. The e-Vent minimum requirements are met as shown in Table 2.
Table 2.
Determination of meeting E-Vent Key Ventilation Technical Specifications [74].
| Key Ventilation Specifications | E-Vent Recommendations | RepRapable OS Design |
|---|---|---|
| 2.1. Control of Breathing Rate (breaths per minute) | 8–30 BPM | 5–45 BPM, controlled by user interface |
| 2.2 Control of Tidal Volume (air volume pushed into lung in mL) | 200–800 mL based on patient weight | 100–846 mL, controlled by user interface |
| 2.3 Control of I/E Ratio (inspiratory/expiration time ratio) | best if adjustable between range of 1:1–1:4 | Adjustable between range of 1:1–1:4, controlled by user interface |
| 2.4 Assist detection pressure. When a patient tries to inspire, they can cause a dip on the order of 1–5 cm H2O, with respect to PEEP pressure (not necessarily = atmospheric). | Required | Can be added based on pressure [132] or temperature [66] feedback |
| 3. Airway pressure must be monitored | Required | Contains two pressure sensors connected between the ventilator and patient via an airway tube, pressures are shown on the LCD screen |
| 3.1.1 Pressure limits: Max pressure | 40 cm H2O | Alarms sound if pressure exceeds 40 cmH2O |
| 3.1.2 Pressure limits: Plateau pressure | 30 cm H2O | Can be added by introducing a pause at the end of the inspiratory phase [132] |
| 3.2 Passive mechanical blow-off valve | 40 cm H2O | Can be purchased together with the self-inflating bag kit |
| 3.3.1 Monitor plateau pressure | Clinician viewable | Can be added by modifying the control algorithm |
| 3.3.2 Monitor PEEP | Clinician viewable | Can be added, need software to see the quantitative value |
| 3.4 PEEP | 5–15 cm H2O | 2–11 cm H2O based on observed data during testing |
| 4. Manual clinician override | Failure of automatic ventilation allows conversion to immediate ventilation. | Yes |
| 5. Ability to use ventilation on room air. Implemented with an oxygen/air gas blender that some hospitals already have. |
Required for emergencies | Yes |
| 6. HEPA filtration on the patient’s exhalation | Required because COVID-19 can be aerosolized | Can be added, HEPA filters can usually be purchased alongside manual resuscitator bags. |
| 8. Failure conditions result in alarm | Required | Alarms sound if pressure exceeds the allowed limits from 5 to 40 cmH2O |
Based on the trials the capabilities of the open source ventilator design include:
-
1.
Maintaining a steady tidal volume, respiratory rate, and I:E ratio.
-
2.
Containing multiple tidal volume values ranging from 100 mL to 846 mL at intervals of one.
-
3.
Maintaining constant motor speed with no slippage below any tidal volume of 800 mL for a respiratory rate less than, or equal to, 15 BPM.
-
4.
Creating consistent data graphs for flow, pressure, and volume.
-
5.
Motors are able to quickly adjust to changes in tidal volume, respiratory rate, and I:E ratio.
-
6.
Rack pusher increases total tidal volume and pressure that can be achieved.
-
7.
All parts are 3-D printable on any RepRap-class printer excluding the electronics.
-
8.
Parts can be easily changed in case of a failure.
-
9.
Parts can be cleaned and sanitized.
9. Limitations and future work
The limitations of the final ventilator design include:
-
1.
Incorporating the possibility of self-inflating bag displacement, as well as the accuracy of the pusher rod travel distance calibration, the tidal volume may differ from the set value within the standard error, which is approximately 35 mL.
-
2.
During the tests, the NEMA-23 stepper motor was operated with the maximum current in the windings to cover the working range of the tidal volume and respiratory rate. These conditions lead to the excess heat buildup in the motor and the need for heat dissipation after several hours of continuous operation. Thus, to ensure ventilation modes with a tidal volume of more than 500 mL and a respiratory rate of more than 15 breaths per minute, it is necessary to use a motor cooling system in the form of a heat sink and/or active airflow.
-
3.
It is recommended that spring washers be used in the motor mounting system to prevent possible bolt loosening due to motor vibrations.
-
4.
During compression, the self-inflating bag may shift and rotate in the bag support, which will lead to a deviation of the set ventilation parameters. An elastic band is used as a fixing component, however, in the future, it is necessary to redesign the system to make bag movement physically impossible.
-
5.
Valve bag is limited to the amount of airway pressure that can be achieved in a cycle.
-
6.
Valve bag could be cause of shifting, and inconsistent, tidal volumes between tests.
-
7.
Ventilator has to be taped down to a stable surface (i.e. piece of wood or clamp) to avoid vibrations that cause movement.
-
8.In the process of bag compression, there is a possibility of the pinion gear steps slippage, both due to insufficient motor torque and due to the fastening of the pinion to the motor shaft. In the future, this problem will need to be solved as follows:
-
a.Add an extra screw securing the pinion to the motor shaft
-
b.Provide software protection against slippage by returning the pushing rod to its “home” position (hitting the limit switch) every N number of steps.
-
c.Implement an alarm signal in the event of the motor steps skipping (unexpected closure of the limit switch).
-
a.
-
9.
A large pusher can mechanically separate from the pushing rod due to the force exerted by the self-inflating bag at high tidal volumes. This problem can be solved by using a metal screw to secure the pusher.
-
10.
It is hard to maintain a positive end-expiratory pressure control due to the difficulty of calibrating the PEEP valve. In the future, it is necessary to implement a software calibration procedure of the PEEP valve or to use a ready-made calibrated industrial design.
-
11.
Junction boxes can cause leaks if not sealed correctly, reducing values drastically for low breathing rates.
In the future, the developed device can be improved by including the following modifications:
9.1. Electrical and software
-
•
Create an assistant mode based on feedback from the pressure sensors
-
•
Add alarms such as “Power disconnect”, “Gear slippage”, and “Critical PEEP”
-
•
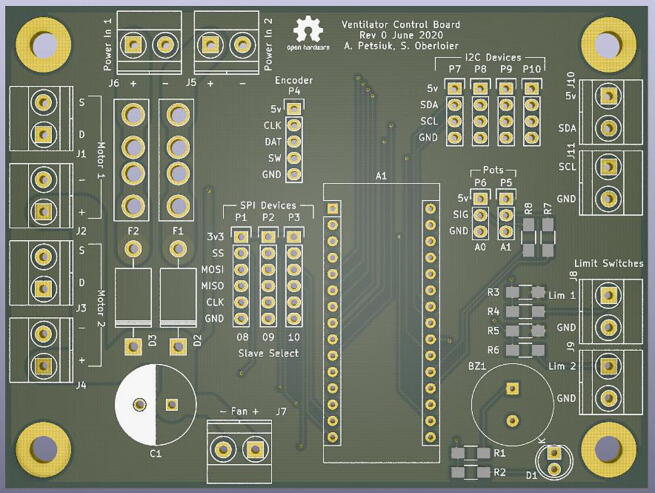
Replace the breadboard with a printed circuit board (PCB) (Fig. 29), which is provided in the OSF repository. The implementation of a PCB will reduce the cost of the system, as the board will cost $2.37USD per unit. The PCB replaces the $7.90USD breadboard, while adding robustness, clear labeling and a more compact design.
Fig. 29.
Printed circuit board design.
9.2. Mechanical part
-
•
Develop more efficient support for self-inflating bags
-
•
Improve PEEP valve performance or replace it with a ready-made calibrated industrial part
-
•
Add HEPA filter
-
•
Add a mechanical pressure relief valve as the default option
-
•
Add cooling system for the motor
-
•
Work towards a more completely distributed-manufacturable device (e.g. replace all the current purchased components for those that can be manufactured on site from feedstock).
9.3. Medical functionality and testing capabilities
-
•
Conduct longevity validation to ensure long-term reliability for multiple patients and determine the lifetime of the device
-
•
Add FiO2 and O2 sensors
-
•
Conduct medical validation with a clinician specialist to ensure the device is clinician friendly
-
•
Complete sterilization and testing to ensure that the device can be cleaned using conventional methods and chemicals. The chemical compatibility of commercial 3-D printing materials is well known [84] and this provides several chemical sterilization pathways that would need to be tested.
Finally, it should be noted that this device was designed for distributed manufacturing, which is currently discouraged by the nature of some regulations (e.g. the FDA certifies a device and a manufacturer as one). This device is not yet approved for clinical use. Future work is needed to develop integrated testing facilities for the open source ventilator to enable rapid manufacturer certification as well as full regulatory approval of the device. This will involve meeting medical device standards such as ISO 80601-2-12:2020 - Medical electrical equipment, ISO 5367:2014 - Anaesthetic and respiratory equipment, and IEC 62304:2006 - Medical device software.
10. Conclusions
The comparative characteristics of modern ventilators under development [113], [130], as well as the medical recommendations of experienced anesthesiologists [131], allow determining the main advantages and disadvantages of the developed system. Ventilators created by developers around the world can be divided into two main groups: 1) ventilators based on self-inflating bags [132], [69], [133], [134], [135], [136], [137], [138], and 2) ventilators based on compressors and pumps [139], [140], [141], [142], [143]. The main drawback of most existing projects is that the main stages of the design process, such as calculating of the required motor power, developing a mechanical compression system, feedback signal processing algorithms (pressure, temperature, flow, etc.), developing a cooling system based on temperature parameters of motors, are not well documented. Ventilators based on pumps often have advanced functionality that allows preparing gas mixtures, moisturizing the circulated atmosphere, and providing an autonomous assistance mode. The main disadvantage of such systems is the complexity of manufacturing, expensive and sometimes inaccessible components, as well as the difficulty in configuring and calibrating, which requires considerable expertise and experience from the user. BVM-based ventilators are easy to replicate and consist of low-cost, readily available components. The advantage of these systems is the ability to release a clinical specialist for a certain period of time to work with other patients. Such an automated apparatus significantly surpasses manual compression in accuracy and stability. Some of the considered BVM-based models, however, have a complex design with expensive components (personal computer, programmable logic controller, etc.) that may demand complex software algorithms. Many of these projects also did not put enough stress on testing.
In order to compare the development of open source ventilators, a five-point validation system has been developed for all types of ventilators, based on criteria such as openness, buildability, community support, functionality, reliability, COVID-19 suitability, clinician amiability [144]. Based on applying this metric the following can be concluded about the developed system described in this study:
-
•
Fully open source and well-documented
-
•
Easily reproducible
-
•
Has been tested for pressure and volume limits with respiratory rate and tidal volume control
-
•
Has critical emergency alarms
-
•
Consists of standard components and connection blocks
Although the developed ventilation system is inferior to certified medical ventilators in the number of available modes, the open source device is far less costly and is able to be deployed by means of distributed manufacturing. In addition, the open source ventilator described and tested here surpasses the capabilities of manual BVM-based ventilation in the accuracy of reproducing predetermined breathing modes, as well as in the stability of the repetition of respiratory cycles. Future work is necessary to further develop the system tested in this work for acceptable deployment in clinical environments, however, the nature of the design is such that desired features are relatively easy to add and test using protocols and parametric design files provided by this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to thank helpful contributions from Mukaram Shahid.
This research was supported by the Witte Endowment, Aleph Objects, and Michigan Instruments.
Contributor Information
Aliaksei Petsiuk, Email: apetsiuk@mtu.edu.
Nagendra G. Tanikella, Email: ngtanike@mtu.edu.
Samantha Dertinger, Email: scdertin@mtu.edu.
Adam Pringle, Email: ampringl@mtu.edu.
Shane Oberloier, Email: swoberlo@mtu.edu.
Joshua M. Pearce, Email: pearce@mtu.edu.
References
- 1.World Health Organization, Critical preparedness, readiness and response actions for COVID-19: interim guidance, 7 March 2020 (No. WHO/COVID-19/Community_Actions/2020.1). World Health Organization, 2020.
- 2.M. Silv, COVID-19: too little, too late?. The Lancet 10.1016/S0140-6736(20)30522-5. [DOI]
- 3.Fisher D., Heymann D. Q&A: The novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18(1):1–3. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.L. Ramsey, Hospitals could be overwhelmed with patients and run out of beds and ventilators as the coronavirus pushes the US healthcare system to its limits. Mar 11, 2020, 8:53 AM Business Insider. https://www.businessinsider.com/coronavirus-intensive-care-unit-shortages-of-ventilators-staff-space-2020-3?op=1.
- 5.Huang H.C., Araz O.M., Morton D.P., Johnson G.P., Damien P., Clements B., Meyers L.A. Stockpiling ventilators for influenza pandemics. Emerg. Infect. Dis. 2017;23(6):914. doi: 10.3201/eid2306.161417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLaren G., Fisher D., Brodie D. Preparing for the most critically ill patients with cOVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020 doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 7.Rubinson L., Vaughn F., Nelson S., Giordano S., Kallstrom T., Buckley T., Burney T., Hupert N., Mutter R., Handrigan M., Yeskey K. Mechanical ventilators in US acute care hospitals. Disaster Med. Public Health Preparedness. 2010;4(3):199–206. doi: 10.1001/dmp.2010.18. [DOI] [PubMed] [Google Scholar]
- 8.J. Miller, Germany, Italy rush to buy life-saving ventilators as manufacturers warn of shortages. Reuters, 2020. https://www.reuters.com/article/us-health-coronavirus-draegerwerk-ventil-idUSKBN210362.
- 9.P. Neighmond, As The Pandemic Spreads, Will There Be Enough Ventilators?,” 2020. NPR. https://www.npr.org/sections/health-shots/2020/03/14/815675678/as-the-pandemic-spreads-will-there-be-enough-ventilators.
- 10.J. Lagasse, COVID-19: Ventilators are in short supply, but so are medications for ventilator patients [Internet]. Healthcare Finance News. [cited 2020 Jun 4]. Available from: https://www.healthcarefinancenews.com/news/covid-19-ventilators-are-short-supply-so-are-medications-ventilator-patients.
- 11.Smetanin P., Stiff D., Kumar A., Kobak P., Zarychanski R., Simonsen N., Plummer F. Potential intensive care unit ventilator demand/capacity mismatch due to novel swine-origin H1N1 in Canada. Can. J. Infect. Dis. Med. Microbiol. 2009;20(4):e115–e123. doi: 10.1155/2009/808209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ercole A., Taylor B.L., Rhodes A., Menon D.K. Modelling the impact of an influenza A/H1N1 pandemic on critical care demand from early pathogenicity data: the case for sentinel reporting. Anaesthesia. 2009;64(9):937–941. doi: 10.1111/j.1365-2044.2009.06070.x. [DOI] [PubMed] [Google Scholar]
- 13.Stiff D., Kumar A., Kissoon N., Fowler R., Jouvet P., Skippen P., Smetanin P., Kesselman M., Veroukis S. Potential pediatric intensive care unit demand/capacity mismatch due to novel pH1N1 in Canada. Pediatr. Crit. Care Med. 2011;12(2):e51–e57. doi: 10.1097/PCC.0b013e3181e2a4fe. [DOI] [PubMed] [Google Scholar]
- 14.Kim K.M., Cinti S., Gay S., Goold S., Barnosky A., Lozon M. Triage of mechanical ventilation for pediatric patients during a pandemic. Disaster Med. Public Health Preparedness. 2012;6(2):131–137. doi: 10.1001/dmp.2012.19. [DOI] [PubMed] [Google Scholar]
- 15.Kanter R.K. Would triage predictors perform better than first-come, first-served in pandemic ventilator allocation? Chest. 2015;147(1):102–108. doi: 10.1378/chest.14-0564. [DOI] [PubMed] [Google Scholar]
- 16.Malatino E.M. Strategic national stockpile: overview and ventilator assets. Respir. Care. 2008;53(1):91–95. [PubMed] [Google Scholar]
- 17.Meltzer M.I., Patel A., Ajao A., Nystrom S.V., Koonin L.M. Estimates of the demand for mechanical ventilation in the United States during an influenza pandemic. Clin. Infect. Dis. 2015;60(suppl_1):S52–S57. doi: 10.1093/cid/civ089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowder L. Ventilator blues: infectious disease expert Tom Inglesby on the next major pandemic. Bull. Atom. Sci. 2018;74(5):340–347. [Google Scholar]
- 19.K. Folmer, 'We’ll take them all': Demand for ventilators spikes as coronavirus looms. ABC News March 14, 2020 https://abcnews.go.com/Health/demand-ventilators-spikes-coronavirus-looms/story?id=69597233.
- 20.M. Heal, Ventilators for coronavirus patients are in short supply. How scientists might pivot [Internet]. Los Angeles Times. 2020 [cited 2020 Jun 4]. Available from: https://www.latimes.com/science/story/2020-04-07/researchers-look-for-ways-to-divert-patients-from-ventilators-as-shortage-looms.
- 21.Wittbrodt B.T., Glover A.G., Laureto J., Anzalone G.C., Oppliger D., Irwin J.L., Pearce J.M. Life-cycle economic analysis of distributed manufacturing with open-source 3-D printers. Mechatronics. 2013;23(6):713–726. [Google Scholar]
- 22.Srai J.S., Kumar M., Graham G., Phillips W., Tooze J., Ford S., Beecher P., Raj B., Gregory M., Tiwari M.K., Ravi B. Distributed manufacturing: scope, challenges and opportunities. Int. J. Prod. Res. 2016;54(23):6917–6935. [Google Scholar]
- 23.Chesbrough H. To recover faster from Covid-19, open up: managerial implications from an open innovation perspective. Ind. Market. Manage. 2020 Apr 16 [Google Scholar]
- 24.Lai B.K., Erian J.L., Pew S.H., Eckmann M.S. Emergency open-source three-dimensional printable ventilator circuit splitter and flow regulator during the COVID-19 pandemic. Anesthesiol.: J. Am. Soc. of Anesthesiol. 2020 Apr 13 doi: 10.1097/ALN.0000000000003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavallo L., Marcianò A., Cicciù M., Oteri G. 3D printing beyond dentistry during COVID 19 epidemic: A technical note for producing connectors to breathing devices. Prosthesis. 2020 Jun;2(2):46–52. [Google Scholar]
- 26.Salmi M., Akmal J.S., Pei E., Wolff J., Jaribion A., Khajavi S.H. 3D Printing in COVID-19: productivity estimation of the most promising open source solutions in emergency situations. Appl. Sci. 2020 Jan;10(11):4004. [Google Scholar]
- 27.Pearce J.M. Distributed manufacturing of open source medical hardware for pandemics. J. Manuf. Mater. Process. 2020 Jun;4(2):49. [Google Scholar]
- 28.Maia Chagas A., Molloy J.C., Prieto-Godino L.L., Baden T. Leveraging open hardware to alleviate the burden of COVID-19 on global health systems. Plos Biol. 2020;18(4) doi: 10.1371/journal.pbio.3000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attaran M. 3D printing role in filling the critical gap in the medical supply chain during COVID-19 pandemic. Am. J. Ind. Bus. Manage. 2020 May 26;10(05):988. [Google Scholar]
- 30.Baechler C., DeVuono M., Pearce J.M. Distributed recycling of waste polymer into RepRap feedstock. Rapid Prototyping J. 2013;19(2):118–125. [Google Scholar]
- 31.F.A.C. Sanchez, S. Lanza, H. Boudaoud, S. Hoppe, M. Camargo, Polymer recycling and additive manufacturing in an open source context: optimization of processes and methods, in: Annual International Solid Freeform Fabrication Symposium, ISSF, 2015, pp. 1591–1600.
- 32.Sanchez F.A.C., Boudaoud H., Hoppe S., Camargo M. Polymer recycling in an open-source additive manufacturing context: mechanical issues. Addit. Manuf. 2017;17:87–105. [Google Scholar]
- 33.M.I. Mohammed, A. Das, E. Gomez-Kervin, D. Wilson, I. Gibson, EcoPrinting: investigating the use of 100% recycled acrylonitrile butadiene styrene (ABS) for additive manufacturing, in: Proceedings of the 28th Annual International Solid Freeform Fabrication Symposium, 2017, pp. 532–542.
- 34.M.I. Mohammed, D. Wilson, E. Gomez-Kervin, L. Rosson, J. Long, EcoPrinting: investigation of solar powered plastic recycling and additive manufacturing for enhanced waste management and sustainable manufacturing, in: 2018 IEEE Conference on Technologies for Sustainability (SusTech), IEEE, 2018, pp. 1–6.
- 35.Woern A.L., Byard D.J., Oakley R.B., Fiedler M.J., Snabes S.L., Pearce J.M. Fused particle fabrication 3-D printing: recycled materials’ optimization and mechanical properties. Materials. 2018;11(8):1413. doi: 10.3390/ma11081413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammed M.I., Wilson D., Gomez-Kervin E., Tang B., Wang J. Investigation of closed-loop manufacturing with acrylonitrile butadiene styrene over multiple generations using additive manufacturing. ACS Sustainable Chem. Eng. 2019;7(16):13955–13969. [Google Scholar]
- 37.Santander P., Sanchez F.A.C., Boudaoud H., Camargo M. Closed loop supply chain network for local and distributed plastic recycling for 3D printing: a MILP-based optimization approach. Resour. Conserv. Recycl. 2020;154 [Google Scholar]
- 38.Dertinger S.C., Gallup N., Tanikella N.G., Grasso M., Vahid S., Foot P.J., Pearce J.M. Technical pathways for distributed recycling of polymer composites for distributed manufacturing: Windshield wiper blades. Resour. Conserv. Recycl. 2020;157 [Google Scholar]
- 39.Hunt E.J., Zhang C., Anzalone N., Pearce J.M. Polymer recycling codes for distributed manufacturing with 3-D printers. Resour. Conserv. Recycl. 2015;97:24–30. [Google Scholar]
- 40.S. Pavlo, C. Fabio, B. Hakim, C. Mauricio, 3D-printing based distributed plastic recycling: a conceptual model for closed-loop supply chain design, in: 2018 IEEE International Conference on Engineering, Technology and Innovation (ICE/ITMC), IEEE, 2018, pp. 1–8.
- 41.Zhong S., Pearce J.M. Tightening the loop on the circular economy: Coupled distributed recycling and manufacturing with recyclebot and RepRap 3-D printing. Resour.Conserv. Recycl. 2018;128:48–58. [Google Scholar]
- 42.K.F. Daniel, J.G. Peter, Open-source hardware is a low-cost alternative for scientific instrumentation and research, Mod. Instrum. (2012).
- 43.Pearce J.M. Building research equipment with free, open-source hardware. Science. 2012;337(6100):1303–1304. doi: 10.1126/science.1228183. [DOI] [PubMed] [Google Scholar]
- 44.J.M. Pearce, Open-source lab: how to build your own hardware and reduce research costs, Elsevier, 2013.
- 45.Chagas A.M. Haves and have nots must find a better way: The case for open scientific hardware. PLoS Biol. 2018;16(9) doi: 10.1371/journal.pbio.3000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dryden M.D., Fobel R., Fobel C., Wheeler A.R. Upon the shoulders of giants: open-source hardware and software in analytical chemistry. Anal. Chem. 2017;89(8):4330–4338. doi: 10.1021/acs.analchem.7b00485. [DOI] [PubMed] [Google Scholar]
- 47.Coakley M., Hurt D.E. 3D printing in the laboratory: maximize time and funds with customized and open-source labware. J. Lab. Autom. 2016;21(4):489–495. doi: 10.1177/2211068216649578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearce J.M. Quantifying the value of open source hardware development. Mod. Econ. 2015;6:1–11. [Google Scholar]
- 49.Pearce J.M. Return on investment for open source scientific hardware development. Sci. Public Pol. 2016;43(2):192–195. [Google Scholar]
- 50.Heikkinen I.T.S., Savin H., Partanen J., Seppälä J., Pearce J.M. Towards national policy for open source hardware research: the case of Finland. Technol. Forecasting Social Change. 2020;155 [Google Scholar]
- 51.Pearce J. Impacts of open source hardware in science and engineering. Bridge. 2017:24–31. [Google Scholar]
- 52.Oberloier S., Pearce J.M. General design procedure for free and open-source hardware for scientific equipment. Designs. 2018;2(1):2. [Google Scholar]
- 53.Ventola C.L. Medical applications for 3D printing: current and projected uses. Pharm. Ther. 2014;39(10):704. [PMC free article] [PubMed] [Google Scholar]
- 54.Niezen G., Eslambolchilar P., Thimbleby H. Open-source hardware for medical devices. BMJ Innov. 2016;2(2):78–83. doi: 10.1136/bmjinnov-2015-000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michaels R.E., Pearce J.M. 3-D printing open-source click-MUAC bands for identification of malnutrition. Public Health Nutr. 2017;20(11):2063–2066. doi: 10.1017/S1368980017000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearce J.M. Maximizing returns for public funding of medical research with opensource hardware. Health Policy Technol. 2017;6(4):381–382. [Google Scholar]
- 57.Tatham P., Loy J., Peretti U. Three dimensional printing–a key tool for the humanitarian logistician? J. Humanitarian Logist. Supply Chain Manage. 2015 [Google Scholar]
- 58.S. Saripalle, H. Maker, A. Bush, N. Lundman, 3D printing for disaster preparedness: Making life-saving supplies on-site, on-demand, on-time, in: 2016 IEEE Global Humanitarian Technology Conference (GHTC), IEEE, 2016, pp. 205–208.
- 59.James E., James L. 3D printing humanitarian supplies in the field. Humanit. Exch. 2016;66:43–45. [Google Scholar]
- 60.Savonen B.L., Mahan T.J., Curtis M.W., Schreier J.W., Gershenson J.K., Pearce J.M. Development of a resilient 3-D printer for humanitarian crisis response. Technologies. 2018;6(1):30. [Google Scholar]
- 61.D. Kats, L. Spicher, B. Savonen, J. Gershenson, Paper 3D Printing to supplement rural healthcare supplies—What do healthcare facilities want?. In 2018 IEEE Global Humanitarian Technology Conference (GHTC), IEEE, 2018, pp. 1–8.
- 62.Kerechanin C.W., Cytcgusm P.N., Vincent J.A., Smith D.G., Wenstrand D.S. Development of field portable ventilator systems for domestic and military emergency medical response. John Hopkins Apl. Tech. Digest. 2004;25(3) [Google Scholar]
- 63.Jürß H., Degner M., Ewald H. A new compact and low-cost respirator concept for one way usage. IFAC-PapersOnLine. 2018;51(27):367–372. [Google Scholar]
- 64.Fuchs P., Obermeier J., Kamysek S., Degner M., Nierath H., Jürß H., Ewald H., Schwarz J., Becker M., Schubert J.K. Safety and applicability of a pre-stage public access ventilator for trained laypersons: a proof of principle study. BMC Emerg. Med. 2017;17(1):37. doi: 10.1186/s12873-017-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.M. Fogarty, J. Orr, D. Westenskow, L. Brewer, D. Sakata, Electric Blower Based Portable Emergency Ventilator, 2013. https://digitalcommons.usu.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=1016&context=spacegrant&fbclid=IwAR1EtJVcxXm82PjGWFCA0t7H_MxNVjuseAePRfxNORr9h4ZQLQ9sNdQjXhc.
- 66.M. Shahid, Prototyping of Artificial Respiration Machine Using AMBU Bag Compression. In 2019 International Conference on Electronics, Information, and Communication (ICEIC), IEEE, 2019, pp. 1–6.
- 67.Al Husseini A.M., Lee H.J., Negrete J., Powelson S., Servi A.T., Slocum A.H., Saukkonen J. Design and prototyping of a low-cost portable mechanical ventilator. Trans. ASME-W-J. Med. Dev. 2010;4(2) doi: 10.1115/1.3442790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.S.K. Powelson, Design and prototyping of a low-cost portable mechanical ventilator (Doctoral dissertation, Massachusetts Institute of Technology), 2010. https://dspace.mit.edu/handle/1721.1/59954. [DOI] [PMC free article] [PubMed]
- 69.Williams D., Flory S., King R., Thornton M., Dingley J. A low oxygen consumption pneumatic ventilator for emergency construction during a respiratory failure pandemic. Anaesthesia. 2010;65(3):235–242. doi: 10.1111/j.1365-2044.2009.06207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simon B.A., Mitzner W. Design and calibration of a high-frequency oscillatory ventilator. IEEE Trans. Biomed. Eng. 1991;38(2):214–218. doi: 10.1109/10.76389. [DOI] [PubMed] [Google Scholar]
- 71.J.M. Pearce, A review of open source ventilators for COVID-19 and future pandemics. F1000Res. 2020;9:218. https://f1000research.com/articles/9-218. [DOI] [PMC free article] [PubMed]
- 72.ISO 80601-2-12:2020. Medical electrical equipment — Part 2-12: Particular requirements for basic safety and essential performance of critical care ventilators. ISO. https://www.iso.org/standard/72069.html.
- 73.Medicines & Healthcare Products Regulatory Agency (MHRA), Rapidly Manufactured Ventilator System (RMVS) Document RMVS001 - Specification Issued by MHRA v 3.1, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/876167/RMVS001_v3.1.pdf
- 74.MIT E-Vent. Key Ventilation Specifications. V 27 March 2020. https://e-vent.mit.edu/clinical/key-ventilation-specifications/ (visited 3-28-2020).
- 75.M. Banzi, M. Shiloh, Getting started with Arduino: the open source electronics prototyping platform. Maker Media, Inc., 2014.
- 76.D’Ausilio A. Arduino: a low-cost multipurpose lab equipment. Behav. Res. Methods. 2012;44(2):305–313. doi: 10.3758/s13428-011-0163-z. [DOI] [PubMed] [Google Scholar]
- 77.Oberloier S., Pearce J.M. Belt-driven open source circuit mill using low-cost 3-D printer components. Inventions. 2018;3(3):64. [Google Scholar]
- 78.Anzalone G.C., Wijnen B., Pearce J.M. Multi-material additive and subtractive prosumer digital fabrication with a free and open-source convertible delta RepRap 3-D printer. Rapid Prototyping J. 2015;21(5):506–519. doi: 10.1108/RPJ-09-2014-0113. [DOI] [Google Scholar]
- 79.Sathyakumar N., Balaji K.P., Ganapathi R., Pandian S.R. A build-your-own three axis CNC PCB milling machine. Mater. Today: Proc. 2018;5(11):24404–24413. [Google Scholar]
- 80.Jones R., Haufe P., Sells E., Iravani P., Olliver V., Palmer C., Bowyer A. RepRap–the replicating rapid prototyper. Robotica. 2011;29(1):177–191. [Google Scholar]
- 81.E. Sells, S. Bailard, Z. Smith, A. Bowyer, V. Olliver, RepRap: the replicating rapid prototyper: maximizing customizability by breeding the means of production, in: Handbook of Research in Mass Customization and Personalization, In 2 Volumes, 2010, pp. 568–580.
- 82.A. Bowyer, 3D printing and humanity's first imperfect replicator. 3D printing and additive manufacturing 1(1) (2014) 4–5.
- 83.Chen J.V., Dang A.B., Lee C.S., Dang A.B. 3D printed PLA Army-Navy retractors when used as linear retractors yield clinically acceptable tolerances. 3D Printing Med. 2019;5(1):1–9. doi: 10.1186/s41205-019-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heikkinen I.T., Kauppinen C., Liu Z., Asikainen S.M., Spoljaric S., Seppälä J.V., Savin H., Pearce J.M. Chemical compatibility of fused filament fabrication-based 3-D printed components with solutions commonly used in semiconductor wet processing. Addit. Manuf. 2018;23:99–107. [Google Scholar]
- 85.PANDApeep Gen2 PEEP valve. https://www.thingiverse.com/thing:4250354, 2020 (accessed 06.09.20).
- 86.Elecia White, Making Embedded Systems: Design Patterns For Great Software. O'Reilly Media; 1st ed., 2011.
- 87.M.B. Weinger, M.E. Wiklund, D.J. Gardner-Bonneau (Eds.), Handbook of Human Factors in Medical Device Design, 1st ed., CRC Press, 2010.
- 88.Gavriel Salvendy (Ed.), Handbook of Human Factors and Ergonomics, 4th ed., Wiley , 2012.
- 89.J.D. Bronzino (Ed.), Medical Devices and Systems, 1st ed., CRC Press, 2006.
- 90.A.D. Lantada (Ed.), Handbook on Advanced Design and Manufacturing Technologies for Biomedical Devices. Springer, 2013.
- 91.P. Ogrodnik. Medical Device Design: Innovation from Concept to Market, 1st ed., Academic Press, 2012.
- 92.Hooman Poor, Basics of Mechanical Ventilation, 1st ed., Springer, 2018.
- 93.D.R. Hess, R.M. Kacmarek. Essentials of Mechanical Ventilation, 3rd ed., McGraw-Hill Education/Medical, 2014.
- 94.R.L. Chatburn. Fundamentals of Mechanical Ventilation: A Short Course on the Theory and Application of Mechanical Ventilators, 2003.
- 95.E. Correger et al., Interpretation of ventilator curves in patients with acute respiratory failure, Med. Intensiva, v. 36(4), pp. 294–306, 2012. doi 10.1016/j.medine.2012.06.001, https://www.medintensiva.org/en-interpretation-ventilator-curves-in-patients-articuloS2173572712000732. [DOI] [PubMed]
- 96.J.-E. S. Kenny MD. ICU Physiology in 1000 Words, A series of articles, http://www.heart-lung.org/1000.html.
- 97.Daoud E.G., Farag H.L., Chatburn R.L. Airway pressure release ventilation: what do we know? Respir. Care. 2012;57(2):282–292. doi: 10.4187/respcare.01238. [DOI] [PubMed] [Google Scholar]
- 98.NEMA 17 Stepper motor. https://reprap.org/wiki/NEMA_17_Stepper_motor, 2020 (accessed 06.09.20).
- 99.NEMA-23 Stepper motor. https://www.cuidevices.com/product/resource/nema23-amt112s.pdf, 2020 (accessed 06.09.20).
- 100.Y. Ueno, N. Nakanishi, J. Oto, H. Imanaka, M. Nishimura. A bench study of the effects of leak on ventilator performance during noninvasive ventilation, Respir. Care 56 (11) (2011) 1758–1764. doi:https://doi.org/10.4187/respcare.01145, http://rc.rcjournal.com/content/56/11/1758. [DOI] [PubMed]
- 101.A. Berlinski, Pediatric Aerosol Therapy. Respiratory Care June 2017, 62 (6) 662-677; Doi:https://doi.org/10.4187/respcare.05298, http://rc.rcjournal.com/content/62/6/662. [DOI] [PubMed]
- 102.Flow Measurement. https://www.smar.com/en/technical-article/flow-measurement, (accessed 06.09.20).
- 103.Measuring Flow in Liquids with Pressure Transducers. https://www.validyne.com/blog/measuring-flow-rate-in-liquids-with-pressure-transducers, (accessed 06.09.20).
- 104.BMP280 Digital Pressure Sensor. https://cdn-shop.adafruit.com/datasheets/BST-BMP280-DS001-11.pdf, 2015 (accessed 06.09.20).
- 105.NIST/SEMATECH e-Handbook of Statistical Methods, https://www.itl.nist.gov/div898/handbook//pmc/section4/pmc431.htm (accessed 06.09.20).
- 106.Michigan Instruments: Adult Lung Simulators. https://www.michiganinstruments.com/lung-simulators/adult-test-lung-simulators, (accessed 06.09.20).
- 107.J. Jensen, I. Sandoval-Watt. Software in Medical Devices: Regulations, Guidance, and Standards (presentation), Advanced Medical Technology Association, https://www.advamed.org/sites/default/files/resource/software_in_medical_devices_-_module_1.pdf.
- 108.K. Fu. Trustworthy Medical Device Software. National Academies Press (US), 2011. https://spqr.eecs.umich.edu/papers/fu-trustworthy-medical-device-software-IOM11.pdf.
- 109.General Principles of Software Validation; Final Guidance for Industry and FDA Staff. U.S. Department Of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, 2002. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-principlessoftware-validation.
- 110.Standards Guide to Design Validation and Safe Medical Device Software. Medical Design Briefs, 2019, https://www.medicaldesignbriefs.com/.
- 111.Developing Medical Device Software to be compliant with IEC 62304-Amendment 1:2015. Liverpool Data Research Associates (LDRA), 2015, https://www.fuji-setsu.co.jp/files/EW_2018_62304Amd1_Paper.pdf.
- 112.Badr. A new approach to verifying and validating medical device development. IBM Software Group, 2009, https://www.softacus.com/images/files/A_new_approach_to_verifying_and_validating_medical_device_development_EN_2009_1.pdf.
- 113.R.L. Read, COVID-19 Ventilator Projects and Resources with FAQs. https://github.com/PubInv/covid19-vent-list, 2020 (accessed 06.09.20).
- 114.FreeRTOS: Real-time operating system for microcontrollers, https://www.freertos.org/index.html (accessed 06.09.20).
- 115.Phillip A. Laplante, Seppo J. Ovaska. Real-Time Systems Design and Analysis: Tools for the Practitioner, 4th ed., Wiley-IEEE Press, 2012.
- 116.M. Rock, The Dos and Don’ts of Bag-Valve Mask Ventilation. JEMS, 2014 https://www.jems.com/2014/08/08/dos-and-don-ts-bag-valve-mask-ventilatio (accessed 06.09.20).
- 117.Parker J.C., Hernandez L.A., Peevy K.J. Mechanisms of ventilator-induced lung injury. Crit. Care Med. 1993;21(1):131–143. doi: 10.1097/00003246-199301000-00024. [DOI] [PubMed] [Google Scholar]
- 118.Dreyfuss D., Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am. J. Respir. Crit. Care Med. 1998;157(1):294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 119.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 120.Ricard J.D., Dreyfuss D., Saumon G.J.I.C.M. Ventilator-induced lung injury. Eur. Respir. J. 2003;22(42 suppl):2s–9s. doi: 10.1183/09031936.03.00420103. [DOI] [PubMed] [Google Scholar]
- 121.Dos Santos C.C., Slutsky A.S. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J. Appl. Physiol. 2000;89(4):1645–1655. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 122.L.N. Tremblay, A.S. Slutsky, 2006. Ventilator-induced lung injury: from the bench to the bedside, in: Applied Physiology in Intensive Care Medicine, Springer, Berlin, Heidelberg, 2006, pp. 357–366.
- 123.Gattinoni L., Protti A., Caironi P., Carlesso E. Ventilator-induced lung injury: the anatomical and physiological framework. Crit. Care Med. 2010;38(10):S539–S548. doi: 10.1097/CCM.0b013e3181f1fcf7. [DOI] [PubMed] [Google Scholar]
- 124.Cressoni M., Gotti M., Chiurazzi C., Massari D., Algieri I., Amini M., Cammaroto A., Brioni M., Montaruli C., Nikolla K., Guanziroli M. Mechanical power and development of ventilator-induced lung injury. Anesthesiol.: J. Am. Soc. Anesthesiol. 2016;124(5):1100–1108. doi: 10.1097/ALN.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 125.Brochard L., Roudot-Thoraval F., Roupie E., Delclaux C., Chastre J., Fernandez-Mondejar E., Clementi E., Mancebo J., Factor P., Matamis D., Ranieri M. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1998;158(6):1831–1838. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- 126.Gattinoni L., Carlesso E., Cadringher P., Valenza F., Vagginelli F., Chiumello D. Physical and biological triggers of ventilator-induced lung injury and its prevention. Euro. Respir. J. 2003;22(47 suppl):15s–25s. doi: 10.1183/09031936.03.00021303. [DOI] [PubMed] [Google Scholar]
- 127.IEC 62304:2006 Medical device software – Software life cycle processes. https://www.iso.org/standard/38421.html, 2006 (accessed 06.09.20).
- 128.Developing Medical Device Software to be compliant with IEC 62304-Amendment 1:2015. https://www.fuji-setsu.co.jp/files/EW_2018_62304Amd1_Paper.pdf (accessed 06.09.20).
- 129.Michigan Instruments: PNEUVIEW® SOFTWARE. https://www.michiganinstruments.com/lung-simulators/pneuview-software (accessed 06.09.20).
- 130.J. Ebin, MD. Global Ventilator Projects. https://www.ebcore.io/global-ventilator-projects, https://docs.google.com/document/d/1FNRe2GS7ShhJAJu6Ogdl0OAjS0oLJZ-lkPjT1DSAhpI/edit?ts=5e79401f, 2020 (06.09.20).
- 131.E. Schulz, MBBS, FANZDA, A brief for engineers, by a doctor, on hacking a ventilator for surge capacity in Covid19 patients. https://docs.google.com/document/d/1sdrKYQ0mDOu4bJum6Fx6piRutIJovo7UqFKYHHxUD5A/edit#heading=h.zfqx8qqil1g1, 2020 (accessed 06.09.20).
- 132.MIT Emergency Ventilator (E-Vent) Project. https://e-vent.mit.edu, 2020 (accessed 06.09.20).
- 133.Ad Hoc ventilator. https://20100.be/ad-hoc-ventilator/, 2020 (accessed 06.09.20).
- 134.VentilatorPAL Pro. https://freebreathing.org/ventilatorpal-pro, 2020 (accessed 06.09.20).
- 135.AmboVent 1690.108. https://members.smoove.io//view.ashx?message=h44741568O122392748O219654O122323322&r=1009, 2020 (accessed 06.09.20).
- 136.ApolloBVM - Emergency Use Ventilator. http://oedk.rice.edu/apollobvm, 2020 (accessed 06.09.20).
- 137.VentilatorPAL. https://freebreathing.org, 2020 (accessed 06.09.20).
- 138.OPENBREATH. https://www.openbreath.it/en, 2020 (accessed 06.09.20).
- 139.O. Garmendia, M.A. Rodríguez-Lazaro, J. Otero, P. Phan, A. Stoyanova, A.T. Dinh-Xuan, D. Gozal, D. Navajas, J.M. Montserrat, R. Farré. Low-cost, easy-to-build non-invasive pressure support ventilator for under-resourced regions: open source hardware description, performance and feasibility testing, Eur Respir. J. (2020). doi:10.1183/13993003.00846-2020, https://erj.ersjournals.com/content/early/2020/04/16/13993003.00846-2020.short. [DOI] [PMC free article] [PubMed]
- 140.The Pandemic Ventilator. https://www.instructables.com/id/The-Pandemic-Ventilator, 2020 (accessed 06.09.2020).
- 141.The Ventilator Rex (V-Rex). https://github.com/hackadayrex/ventilator, 2020 (accessed 06.09.20).
- 142.Zephyr Open Source Ventilator. https://github.com/Julhh/Zephyr, 2020 (accessed 06.09.20).
- 143.RespiraWorks: Pandemic Ventilator. https://docs.google.com/document/d/1Dz7eMgXowFBtBA_0PKzfAXweHnNMbGlIAXPshCbI2Vk/edit, 2020 (accessed 06.09.20).
- 144.Evaluating Open Source Ventilator Projects. https://docs.google.com/document/u/1/d/e/2PACX-1vRl9yZ27KvslftcNvweHgH1A81pO8gHL62TWpY_VY-UELWdK9x-4-3hNw3DbkemClzExPsg8RfnxilP/pub, 2020 (accessed 06.09.20).