Abstract
Angiogenesis plays a critical role within the human body, from the early stages of life (i.e., embryonic development) to life-threatening diseases (e.g., cancer, heart attack, stroke, wound healing). Many pharmaceutical companies have expended huge efforts on both stimulation and inhibition of angiogenesis. During the last decade, the nanotechnology revolution has made a great impact in medicine, and regulatory approvals are starting to be achieved for nanomedicines to treat a wide range of diseases. Angiogenesis therapies involve the inhibition of angiogenesis in oncology and ophthalmology, and stimulation of angiogenesis in wound healing and tissue engineering. This review aims to summarize nanotechnology-based strategies that have been explored in the broad area of angiogenesis. Lipid-based, carbon-based and polymeric nanoparticles, and a wide range of inorganic and metallic nanoparticles are covered in detail. Theranostic and imaging approaches can be facilitated by nanoparticles. Many preparations have been reported to have a bimodal effect where they stimulate angiogenesis at low dose and inhibit it at higher doses.
Keywords: Nanotechnology, Nanoparticles, Angiogenesis, Vascularization, Cancer therapy, Imaging, Tissue engineering, Regenerative medicine
Graphical Abstract
The progress, opportunities, and challenges of nanotechnology-based strategies for angiogenesis inhibition, angiogenesis stimulation, theranostic and imaging purposes are summarized.

1. Introduction to Angiogenesis
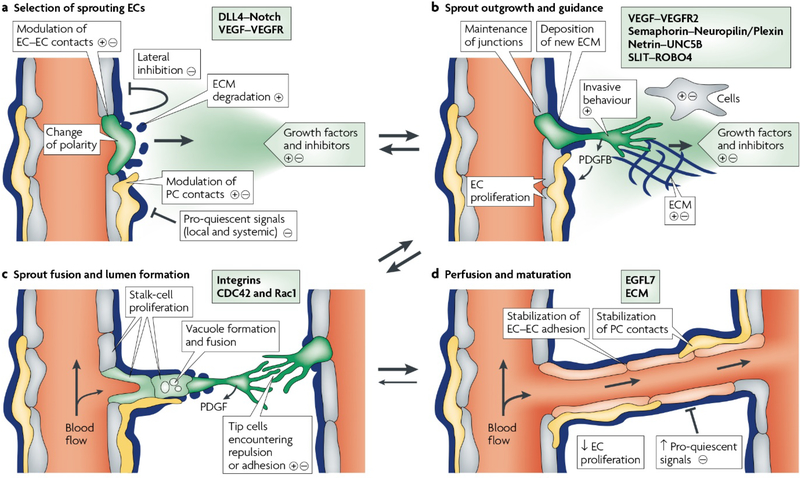
Any living mammalian tissue needs oxygen and nutrients to ensure cell survival under in vivo conditions; therefore, blood vessels play a pivotal role in sustaining life. Endothelial cells (ECs) form the main component of small blood vessels, while pericytes and smooth muscle cells (SMCs) surround larger vessels that are lined with ECs 1, 2. Formation of new blood vessels (neovascularization) within the human body can be achieved via two distinct biological processes. One is called vasculogenesis while the other is called angiogenesis. Vasculogenesis refers to the formation of new vessels de novo from ECs generated by differentiation of progenitor cells (e.g., angioblasts), which self-assemble into lumens and form primitive blood vessels. On the other hand, angiogenesis means the formation of new blood vessels by sprouting from preexisting vasculature 3. A series of molecular and cellular processes are involved in angiogenesis, which can be divided into different steps, including EC activation in response to pro-angiogenic factors, capillary wall degradation via the action of extracellular proteinase enzymes, and formation of a branch point in the vessel walls, ECs migrate into the extracellular matrix (ECM) towards the source of the angiogenic stimulus, and then form tubules with a central lumen that create a vessel network (anastomosis) via the interconnection of the new tubules (Fig. 1) 4.
Fig. 1.
Schematic representation of different steps of angiogenic sprouting. A) The balance between pro-angiogenic signals (+) (e.g., VEGF), and anti-angiogenic factors (–) (e.g., tight pericyte (PC; yellow) contact), certain ECM molecules and VEGF inhibitors can control the sprouting. Under the appropriate conditions of angiogenesis, ECs can sprout (green), while others inhibit this phenomenon (grey). It has been well documented that the sprouting process needs to flip the apical-basal EC polarity, induce motile and invasive activity, modulate cell-cell contacts and degrade the local ECM. B) Attractive (+) or repulsive (–) cues from cells in the tissue environment are responsible for the growing EC sprouts. C) The fusion of adjacent sprouts into vessels occurs after adhesive or repulsive interactions between the cells at the tip. The fusion of vacuoles facilitates lumen formation in stalk ECs. D) A continuous lumen results from the fusion processes at the EC–EC interface; blood flow enhances oxygen delivery and subsequently reduces the hypoxia-induced pro-angiogenic signals. Maturation processes (e.g., the stabilization of cell junctions, matrix deposition, and tight PC attachment) is likely promoted by increased perfusion. Reproduced with permission from (Nature reviews Molecular cell biology, 2007, 8, 464–478), Copyright 2007, Nature Publishing Group.
Angiogenesis is a critical process involved in embryogenesis and also in maintaining normal homeostasis, including repair and regeneration of injured tissues. Angiogenesis may be deregulated in many pathological conditions. Although angiogenesis remains quiescent during adulthood, it becomes physiologically active in normal conditions such as the cycling ovary and the placenta during pregnancy. Furthermore, angiogenesis regularly occurs via the activation of ECs in response to some specific stimuli (e.g., hypoxia) occurring during the wound healing process to accelerate tissue reconstruction 5. However, there is another story about unwanted angiogenesis that occurs in many diseases and disorders, i.e., an imbalance between angiogenic stimulators and inhibitors leads to triggering an angiogenic on-and-off switch. For instance, the angiogenesis process is switched on in the case of malignancies and some inflammatory disorders. On the contrary, insufficient angiogenesis is observed in other pathological conditions such as ischaemic heart tissue, in which healing and regeneration are impaired as a result of dysfunction of ECs, and vessel malformation or regression. To detect and evaluate angiogenesis process, a series of in vitro (e.g., a cell scratch wound), ex vivo (aortic ring assay), and in vivo (chick chorioallantoic membrane (CAM)) assays have been developed and applied that are considered as relible ways towards the translation of results from the laboratory to the clinic 6, 7.
An imbalance in angiogenesis is found in a series of diseases and disorders (e.g., retinopathy); however, this review paper mainly focuses on the importance of inhibiting angiogenesis to fight cancer, and stimulation of angiogenesis in tissue engineering and wound healing by using various types of nanoparticles, nanomaterials, and so on.
2. Angiogenesis mediators
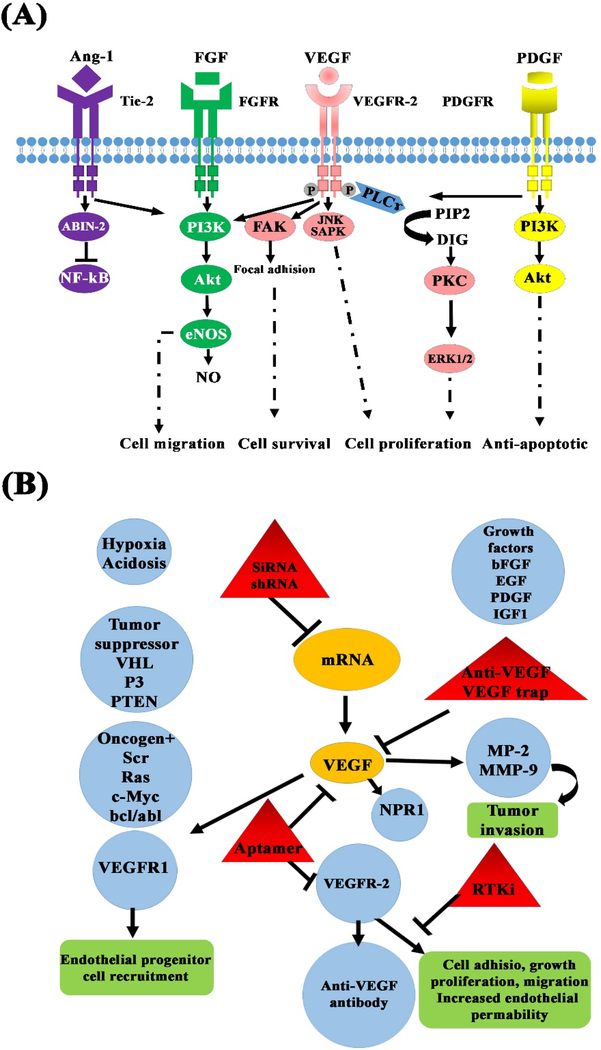
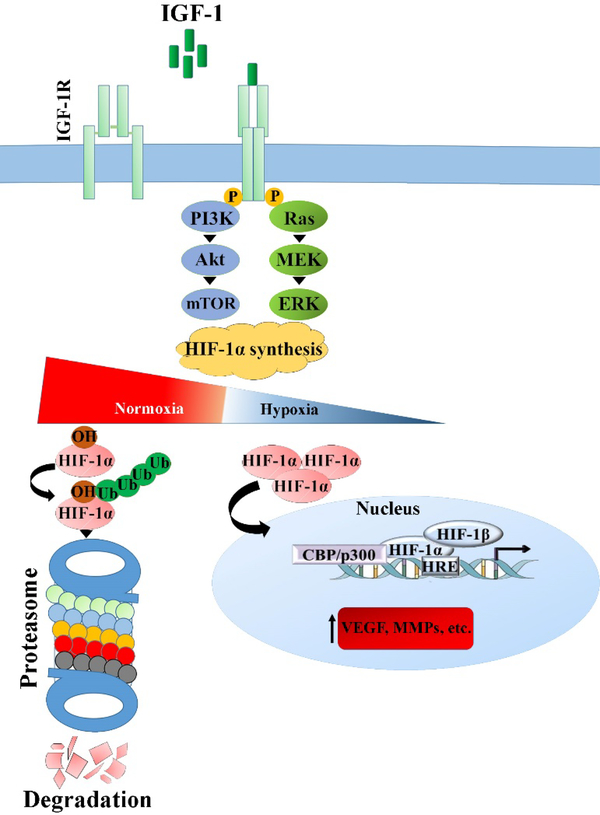
The molecular mediators of angiogenesis consist of different growth factors and cytokines (e.g., VEGF and FGF), matrix metalloproteinases (MMPs), and molecules involved in intracellular signaling pathways (Rho GTPases) (see Fig. 2 and Table. 1) 8. There are specific types of receptors on the surface of cells (e.g., ECs) responding to angiogenic biomolecules; receptor tyrosine kinases (RTKs) are among the largest and most well-known receptor families 9. VEGF receptors (VEGFR1–3), FGF receptors (FGFRs), PDGF receptors (PDGFRs), IGF receptors (IGFRs), and the Tie receptors (Tie1 and Tie2) are different classes of RTKs mediating angiogenesis through the activation of relevant signaling pathways after receiving the appropriate signals. For instance, the coupling the IGF to its receptor (IGFRs) triggers two distinct signaling pathways in the cells, resulting improved angiogenesis in the hypoxia condition (see Fig. 3).
Fig. 2.
Schematic illustration of (A) pro-angiogenic mediators and pathways involved in the activation of ECs and (B) the main clinical and preclinical factors involved in anti-angiogenic therapy.
Table. 1.
Pro- and anti-angiogenic factors and receptors. With some modifications from Ref 10.
| Category | Molecules | cognate receptor | Effects* |
|---|---|---|---|
| Growth factors | VEGF | Tyrosine kinase receptors (VEGFR1, VEGFR2, and VEGFR3) | PA |
| PDFG | Tyrosine kinase receptors (PDGFRα and β) | PA | |
| FGF | Tyrosine kinase receptors (FGFR1, FGFR2, FGFR3, and FGFR4) | PA | |
| EGF | Tyrosine kinase receptors: EGFR (ErbB1, HER1), ErbB2 (HER2), ErbB3 (HER3) and ErbB4 (HER4) | PA | |
| TGF | Serine/threonine kinase receptors (type I and type II) | PA | |
| TNF | Tyrosine kinase receptors (TNFRI and TNFRII) | PA | |
| Angiopoetin | Tyrosine kinase receptors (Tie-1 and Tie-2) | PA | |
| Cytokines | IL-8 | CXCR1 and CXCR2 and thereby VEGFR2 | PA |
| CSF-1 | CSFR1, CSFR 2, and CXCR4 | PA | |
| Bioactive lipids | PGE2 | EP1-4 receptors | PA |
| Matrix-degrading enzymes | MMPs | Low-density LRP | PA |
| Heparanases | HBP | PA | |
| Small mediators | NO | Tyrosine kinase receptors (VEGFR1, VEGFR2) | PA |
| Serotonin | 5- HT1 and 5-HT2 | PA | |
| Histamine | H1R and H2R | PA | |
| Chemotherapeutic agents | Cyclophosphamide | Induces EC apoptosis and decreases circulating EPC | AA |
| Paclitaxel | Microtubule | AA | |
| VEGF-targeted therapy | Bevacizumab | VEGF-A | AA |
| VEGF-Trap | VEGF-A, VEGF-B, and PlGF | AA | |
| Sunitinib | VEGFR1–3, PDGFR-α, PDGFR-β, c-Kit, CSF-1R and Flt-3 | AA | |
| Sorafenib | VEGFR1–3, PDGFR-β, Raf-1, B-Raf | AA | |
| Vatalanib | VEGFR1–3, PDGFR-β and c-Kit | AA | |
| Axitinib | VEGFRs, PDGFR-β, and c-Kit | AA | |
| SU6668 | VEGFR2, FGFR1 and PDGF-β | AA | |
| FGF-targeted therapy | AZD4547 | FGFR1–3 | AA |
| Ponatinib | FGFR1–4 | AA | |
| SSR | FGFRs | AA | |
| Brivanib | VEGFRs and FGFRs | AA | |
| Dovitinib | FEGFRs, VEGFRs, and PDGFR | AA | |
| Nintedanib | VEGFRs, FGFRs, and PDGFR | AA | |
| Oncogene-targeted therapy/signaling transduction-targeted therapy | Dasatinib | Src and indirectly VEGF, IL-8 | AA |
| Tipifarnib | MMP-1 | AA | |
| NVP-AUY922 | Hsp90 | AA | |
| Bortezomib | NF-κB-dependent release of VEGF and IL-8 | AA | |
| Gossypol | VEGF and IL-8 release | AA | |
| Dacinostat | Histone deacetylase | AA | |
| Matrix degrading and remodelling-targeted therapy | DX-2400 | MMP-14 | AA |
| PI-88 | Heparanase | AA | |
| Thrombospondins | CD36 and CD47 | AA | |
| Tumor-associated stromal cell-targeted therapy | JNJ-28312141 | CSF-1R | AA |
| Zoledronic acid | TAM-associated production of VEGF | AA | |
| Anti-BV8 antibody | Neutrophils recruitment | AA | |
| CAMs-targeted therapy | Cilengitide | αvβ3 and αvβ5 integrins ligation to matrix proteins | AA |
| Volociximab | αvβ1 integrin interaction with fibronectin | AA | |
| ADH-1 | N-cadherin | AA | |
| Inflammatory angiogenesis-targeted therapy | Ibuprofen | COX1/2 | AA |
| Celecoxib | COX-2 | AA | |
| Repertaxin | CXCR1 and CXCR2 | AA |
Note: PA and AA refer to pro-angiogenic and anti-aniogenic effect, respectively.
Fig. 3.
The binding the IGF, an angiogenic molecule, to IGF-1R receptor on the cell surface activates two cell signaling pathways, leading to increased synthesis of HIF-1α by which the production of VEGF and thereby improved angiogenesis occur in the hypoxia condition.
Angiogenesis inhibitors can be divided into two distinct classes, including those directly targeting the microvascular ECs, and those indirectly targeting the pro-angiogenic communication pathways between the cancer cells and ECs 10. A number of direct inhibitors (e.g., angiostatin) have been identified and used to inhibit angiogenesis in cancer treatment. The main action of these inhibitors is to prevent proliferation and migration of ECs stimulated by angiogenesis inducers (e.g., VEGF) 11. An inhibitory effect on integrin receptors and subsequent signaling pathways is another mechanism proposed for the action of direct angiogenic inhibitors by which they prevent the proliferation of ECs 12.
The U.S. FDA has approved several angiogenesis inhibitors for the treatment of cancer (see section 1.3). R. K. Jain reported that for both direct and indirect anti-angiogenic therapy, the balance between pro-angiogenic and anti-angiogenic factors could be restored through the reduction of vessel permeability and hypoxia, and enhancement of the homogeneity of blood flow and perivascular cell coverage 13.
3. Angiogenesis as a promising target in medicine
Nowadays, controlling unwanted vessel outgrowth is considered as an important therapeutic strategy in the medical setting. Accordingly, a large number of approaches have been developed and approved to suppress aberrant angiogenesis 14, 15. The molecular mechanisms (signaling pathways, mediators, and receptors) involved in the angiogenesis process, including VEGF/VEGFR, PDGFB/PDGFR-β, and the angiopoietins (Angs) are often considered potential targets 16, 17. As an example, bevacizumab (Avastin®), a recombinant humanized monoclonal antibody, targets the VEGF/VEGFR signaling pathway to suppress angiogenesis in glioblastoma, and the clinical data have shown improvement in both progression-free and overall survival of patients 18v19. However, it should be noted that development of resistance to anti-angiogenic therapies is common due to activation of alternative pro-angiogenic signaling pathways 20–24. It should be mentioned that cancer cells, which harbor many mutations, often activate compensatory signaling pathways in response to inhibition of a particular pathway, thus rendering cancer cells therapy-resistant. Therefore, there is a rationale for combination therapy (simultaneously targeting multiple pathways) in cancer treatment in order to reduce drug resistance and cancer recurrence 25, 26. In brief, the main antiangiogenic drugs developed to inhibit cancer progression in various types of malignancies include monoclonal antibodies, small-molecule tyrosine kinase inhibitors (TKIs), and non-TKI small-molecule inhibitors (Table. 2).
Table. 2.
FDA-approved anti-angiogenic drugs used to treat different cancers.
| Classification | Drug name | Chemical formulation | Mechanism of action | Clinical usage | Ref (s) |
|---|---|---|---|---|---|
| Monoclonal Antibodies | Bevacizumab (Avastin®, Genentech) | C6538H10034N1716O2033S44, Mw = 149 kDa | - Hinders the interaction between VEGF-A and VEGFR2 via targeting VEGF-A | A variety of cancers including CRC, BC, GBM, NSCLC, RCC, and EOC | 29, 30 |
| Ramucirumab (Cyramza®, ImClone Systems Incorporated) | C6374H9864N1692O1996S46, Mw = 143.6 kDa | - Hinders the interaction between VEGFR2 ligands (i.e., VEGF-A, VEGF-C, and VEGF-D) and VEGFR2 via targeting VEGFR2 | GAC, GEJAC and NSCLC | 31, 32 | |
| Aflibercept (Zaltrap®, Regeneron pharmaceuticals) | C4318H6788N1164O1304S32, Mw = 115 kDa | - Suppresses angiogenesis via hindering the interaction between VEGF isoforms, mainly VEGF-A, VEGF-B, and PIGF, and their cognate receptors VEGFR-1 and VEGFR-2 | Metastatic CRC and age-related macular degeneration | 33, 34 | |
| Olaratumab (Lartruvo®, Eli Lilly) | C6554H10076N1736O2048S40, Mw = 154 kDa | - Acts against the external domain of human PDGFR-α, blocking its ligand binding hindering activation of downstream signaling molecules protein kinase B (Akt) and MAPK | STS | 35, 36 | |
| Small-molecule tyrosine kinase inhibitors | Axitinib (Inlyta®, Pfizer) | C22H18N4OS, Mw = 386.47 Da | - Inhibits the VEGFR-1, VEGFR-2, VEGFR-3 | RCC | 37 |
| Cabozantinib (Cabometyx®, Exelixis) | C28H24FN3O5, Mw = 501.514 Da | - A multi-kinase TKI of various receptors including VEGFR-1, −2 and −3, KIT, FLT-3, AXL, RET, MET, and TIE-2 | RCCand MTC | 38 | |
| Lenvatinib (Lenvima®, Eisai) | C21H19ClN4O4, Mw = 426.86 Da | - A multi-kinase inhibitor of VEGFR 1, −2 and −3, fibroblast growth factor receptor FGFR 1, −2 and −3, PDGFRα, KIT, and RET | Radioiodine refractory differentiated TC, advanced RCC, and HCC | 39 | |
| Nintedanib (Ofev®, Boehringer Ingelheim Pharmaceuticals) | C31H33N5O4, Mw = 539.6248 Da | - A multi-kinase inhibitor of VEGFR 1, −2 and −3, FGFR 1, −2 and −3, PDGFRα/β, and FLT3 | IPF | 40 | |
| Pazopanib (Votrient®, GlaxoSmithKline) | C21H23N7O2S, Mw = 437.518 Da | - A multi-kinase inhibitor of several kinases including VEGFR 1, −2 and −3, FGFR 1, −2 and −3, PDGFRα/β, and KIT | Advanced RCC and advanced soft tissue sarcoma | 41 | |
| Ponatinib (Iclusig®, Ariad Pharmaceuticals) | C29H27F3N6O, Mw = 532.5595 Da | - A multi-kinase inhibitor of several kinases mainly BCR-ABL, BCR-ABL T315I, VEGFR2, PDGFRα, FGFR1, −2 and −3, ephrin receptor EPHR, SRC family kinases, KIT, RET, TIE2, and FLT3 | CML or Ph+ALL resistant to previous TKI therapies. The drug is also effective for CML or Ph+ ALL patients with positive T315I mutation | 42, 43 | |
| Regorafenib (Stivarga®, Bayer) | C21H15ClF4N4O3, Mw = 482.815 Da | - A multi-kinase inhibitor of several kinases including VEGFR 1, −2 and −3, FGFR 1, −2, PDGFRα/β, RET, KIT, TIE2, Eph2A, BCR-ABL, B-RAF, and B-RAF V600E | Metastatic CRC, locally advanced, unresectable or metastatic GIST previously treated with imatinib or sunitinib, and HCC | 44 | |
| Sorafenib (Nexavar®, Bayer) | C21H16ClF3N4O3, Mw = 464.825 Da | - A multi-kinase inhibitor of several kinases including BRAF, BRAF V600E, KIT, FLT-3, VEGFR-2, −3, and PDGFR-ß - Targets the Raf/Mek/Erk pathway inhibiting downstream signaling pathways leading to cancer hallmarks such as cell proliferation, apoptosis evasion, angiogenesis, invasion, and metastasis |
Unresectable HCC, advanced RCC and differentiated TC refractory to radioactive iodine | 45 | |
| Sunitinib (Sutent®, Pfizer) | C22H27FN4O2, Mw = 398.4738 Da | A multi-kinase inhibitor of several kinases including VEGFR 1, −2 and −3, PDGFRα/β, KIT, FLT3, colony stimulating factor receptor Type 1(CSF-1R), and RET | Advanced RCC, GIST resistant to imatinib and NETs | 46 | |
| Vandetanib (Caprelsa®, Genzyme Corporation) | C22H24BrFN4O2, Mw = 475.354 Da | - Multi-kinase inhibitor of several kinases mainly EGFR, VEGFR2, and RET | Locally advanced or metastatic MTC | 47 | |
| Non-TKI small-molecule Inhibitors | Thalidomide (Thalidomide®, Celgene) | C13H10N2O4, Mw = 258.2295 Da | - Inhibition of the production of TNF-α and VEGF | Multiple myeloma (MM) | 48 |
| Lenalidomide (Revlimid®, Celgene) | C13H13N3O3, Mw = 259.2606 | - Inhibiting the expression of COX-2 | MM, MDS and MCL, FL, and MZL | 49 | |
| Temsirolimus (Torisel®, Wyeth Pharmaceuticals) | C56H87NO16, Mw = 1030.2871 Da | - An inhibitor of mTOR - Inhibition of mTOR suppresses angiogenesis by reducing levels of the hypoxia-inducible factors HIF-1 and HIF-2, and the VEGF |
RCC | 50 | |
| Everolimus (Afinitor®, Novartis) | C53H83NO14, Mw = 958.24 Da | An inhibitor of mTOR. Inhibition of mTOR suppresses angiogenesis by reducing levels of the HIF-1 and HIF-2, and the VEGF | Some malignancies mainly RCC, advanced HR+ (hormone receptor), HER2- BC; progressive neuroendocrine tumors of pancreatic, gastrointestinal or lung origin | 51 |
The expression of many pro-angiogenic factors (including VEGF as a key factor) and their cognate receptors is upregulated in the tumor microenvironment. Anti-angiogenic monoclonal antibodies (mAbs) act by blocking the interaction between pro-angiogenic ligands and their cognate receptors hindering the downstream signaling pathways promoting angiogenesis 27. Small-molecule tyrosine kinase inhibitors (TKIs) as anti-angiogenic drugs act by blocking the ATP binding site in a pro-angiogenic receptor and, hence, inhibiting phosphorylation of the tyrosine residue of that receptor, which eventually hinders downstream pro-angiogenic signaling pathways. Compared to anti-angiogenic mAbs, TKI usually targets not only the VEGF/VEGFR pathway but also other pro-angiogenic pathways such as platelet-derived growth factor receptor (PDGFR), mesenchymal epithelial transition factor receptor (c-MET) and TIE-2 28.
4. The pivotal role of chemistry towards the angiogenic design of nanomaterials
Nowadays, the design and development of nanotechnology-based therapies by using organic and inorganic materials form a substantial part of the modern medicine; indeed, chemistry plays a central role in this sense 52. There are huge numbers of commercially available nanotechnology-based products (e.g., nanopharmaceuticals) on the market, which are used in a broad range of applications including cancer therapy 53. Still, more research is needed to progress towards novel and more efficient nanomaterials/nano-systems-based cancer therapies, which will be key to overcome the limitations of current treatments (e.g., drug resistance). Therefore, it is an undeniable evidence that the medicinal chemistry will play a critical role in imaginable achievments in the near future 54.
The pro- and anti-angiogenic potential of nanomaterials could be straightforwardly controlled by chimestry rules, from simple adjustments in the synthesis and structural manipulation to complicated surface modifications, self-assembly, processing and integration to make smart materials in the concept of advanced healthcare materials. As an illustration, making mesoporous bioactive glass (MBG) nanoparticles with the ability to carry biomolecules (e.g., pro- or anti-angiogenic agents) is simply applicable via a wet-chemical technique, i.e. the sol-gel process 55–57. Targeted cancer therapy is of utmost importance to reduce side effects of chemotherapy as well as to improve the clinical outcomes. Targeting angiogenesis via nano-structured materials is one of the most interesting issues in cancer therapy 58, 59. Chemical coupling of various biomolecules including antibodies, peptides (e.g. RGD), and peptidomimetics to nanomaterials has provided the opportunity to target vascular integrins (e.g., αvβ3 integrin) and subsequent targeted cancer therapy 60–63.
It is also worth pointing out that some nano(materials) are able to elicit an inherent pro- or anti-angiogenic effect associated to the release of therapeutic ions. In this regard, materials composition and chemistry strongly govern the biological response. However, there are some critical factors that limit the progress in the field of angiogenesis modulation utilizing ions. Essentially, this is because (i) ions can easily diffuse to other non-target cells or tissues and stimulate unwanted responses, and (ii) the biochemical/biomolecular effects elicited by such ions may be partly unpredictable. Hence, at least a couple of key questions in “ionic research” need to be addressed before clinical translation, namely: How can the non-specific side effects of ion-based therapeutics be minimized? What is the signaling cascade of these ions on angiogenesis? We cannot ignore that our current biochemical/biomolecular knowledge is still incomplete and unable to provide an exhaustive response to these questions; the goal of this review is to draw a structured picture of the relevant state-of-the-art, on which researchers can further build new knowledge and plan experiments to bridge the gaps.
5. Nanotechnology meets angiogenesis
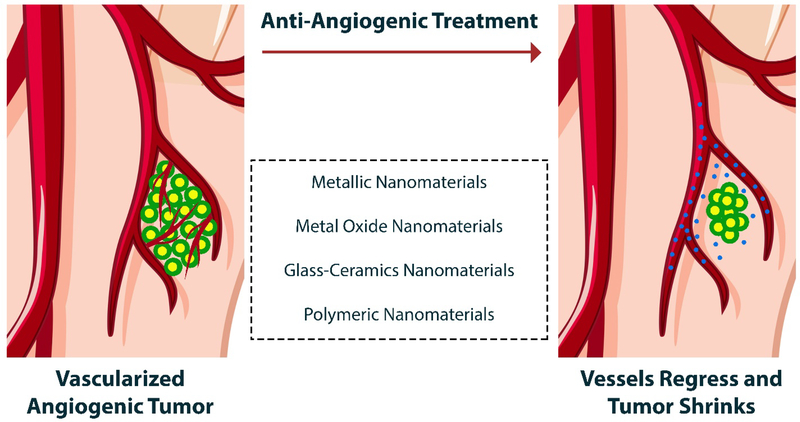
Loading and delivery of various natural and synthetic pro-angiogenic or anti-angiogenic substances by using nanostructured vehicles is recognized as one of the most promising approaches in medicine 64–66. There is strong evidence for the utility of nano-sized delivery systems for therapeutic drugs, since they can overcome the limited tissue diffusion of drugs, protect them in the blood circulation, and lower the risk of systemic toxicity. In other words, targeted therapy using nano-scale vehicles helps drug-loaded nanostructures more easily reach the desired sites in the body (cells, tissues, and organs) and the drug release profile occurs in a more controlled manner 67. In addition, it has been well-documented that organic and inorganic nanoparticles can display pro-angiogenic and anti-angiogenic characteristics depending on their nano-sized design (see Fig. 4) 68, 69. In the following sections we introduce and discuss the types of nano-sized particles (organic and inorganic), as well as nanotechnology-based systems that have been designed and developed for pro- and anti-angiogenic applications.
Fig. 4.
Different types of NPs that have been used as therapeutics for anti-angiogenesis and vessel regression.
6. Polymeric nanoparticles as carriers for the delivery of anti-angiogenic biomolecules
The most commonly-reported natural and synthetic materials for constructing nanoparticle carriers are polymers, liposomes, micelles, and inorganic nanoparticles 70. The use of polymers in drug delivery strategies (DDSs) has been proved to be a successful approach; some of them have been on the market since the early 1990s 71. However, polymeric nanoparticles used as drug delivery vehicles are considered to be newer members of DDSs, bringing new hope in medicine thanks to their properties, such as higher bioavailability, low toxicity, and controllable drug release kinetics 72, 73. Although polymeric nanoparticles have been used in the treatment of some diseases (e.g., arthritis, multiple sclerosis), the main focus is still on cancer therapy 74. In this sense, numerous nano-sized polymers have been used to load and deliver anti-angiogenic chemicals and drugs, in the fight against various cancers (see Table. 3) 69. Among them, PEG, PLA, PCL, PLGA, chitosan, heparin, gelatin, and albumin have extensively being using for therapeutic angiogenesis, either in bare or modified form.
Table. 3.
Summary of polymeric and carbon-based nano-sized drug delivery systems for pro- and anti-angiogenic therapeutic strategies.
| Nanotechnology platform | Chemical structure | Modification (s) | Therapeutic agent | Effects* | Remarks | Ref (s) |
|---|---|---|---|---|---|---|
| PEG | 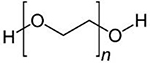 |
Cell adhesive (RGDS) and MMP-degradable (GGGPQGflIWGQGK) peptides conjugated PEG | - | PA | - PEG hydrogels could support the lumen formation, expression of ECs proteins (e.g., eNOS), and perivascular investment of PDGFR-β and α-SMA positive cells by 2 weeks of co-culture | 77 |
| PLA | 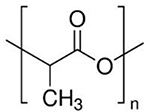 |
APTEDB peptide functionalized pegylated PLA NPs | PTX | AA | - Significantly elevated cellular accumulation of PTX loaded NPs via energy-dependent, caveolae and lipid raft-involved endocytosis. - In vitro tube formation assay and in vivo matrigel angiogenesis analysis confirmed a significant improvement in the antiangiogenic ability of PTX. |
81 |
| Pegylated PCL | 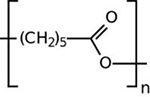 |
CGKRK peptide-functionalized pegylated PCL NPs | PTX | AA | - An enhanced accumulation via an energy-dependent, lipid raft/caveolae-mediated endocytosis with the involvement of microtubules in HUVECs. - An energy-dependent, lipid raft/caveolae-mediated endocytosis with the participation of Golgi apparatus in human U87MG cells. |
89 |
| PEGylated PLGA | 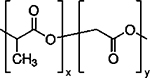 |
RGD peptide and RGD-peptidomimetic functionalized pEGylated PLGA NPs | PTX | AA | - Higher affinity to HUVECs by binding to αvβ3 integrin was observed in the functionalized NPs containing PTX - Successful in vivo targeting to transplantable liver tumors was obtained in the case of the functionalized NPs containing PTX, leading to prolonged survival times of mice |
92 |
| Chitosan |  |
Hyaluronic acid coated chitosan NPs | PLXDC1 small interfering siRNA | AA | - Significant inhibition of tumor growth in A2780 tumor-bearing mice - Significant decrease in microvessel density |
107 |
| - | Ursolic acid (UA) | AA | - Inhibition of the angiogenesis in CAM model and H22 xenograft model - Controlled release of UA and thereby its reduced side effects |
108 | ||
| Heparin | 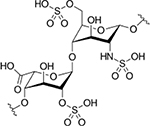 |
Cyclic RGD-modified heparin-lithocholic acid (HL) | - | AA | - Significantly inhibition of adhesion and migration of ECs - Prohibition of the formation of tubular structures of ECs |
122 |
| Heparin-surface modified polyurethane (PU) macroporous discs | VEGF165 | PA | - Accelerated neovascularization and tissue repair in tread animals with PU containing high (6.6 mg/g) heparin content immobilized VEGF165 | 123 | ||
| Albumin | 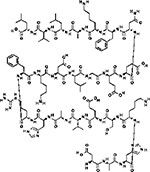 |
Abituzumab (DI17E6)-coupled NPs | DOX | AA | - DI17E6 coupled NPs specifically targeted αvβ3 integrin positive melanoma cells - DI17E6 coupled NPs containing DOX Inhibited angiogenesis by targeting of endothelial cells |
148 |
| Gelatin | Electrospun gelatin nanofibers | bFGF | PA | - Capillary formation was improved as a function of bFGF loaded aligned or random nanofibers | 133 | |
| PEG-modified thiolated gelatin NPs | sFlt-1 (VEGF-R1) plasmid DNA | AA | - Successful suppression of tumor growth and microvessel density | 131 | ||
| PAMAM dendrimer | 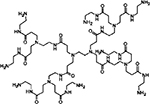 |
RGD-4C peptide conjugate | - | AA | - Taken up by cells expressing αVβ3 receptors, providing suitable imaging agents and/or chemotherapeutics to angiogenic tumor vasculature | 326 |
| Multi-walled carbon nanotubes (MWCNT) |  |
Polyethyleneimine (PEI) or polyamidoamine dendrimer (PAMAM) functionalized SWCNTs | miR-503 oligonucleotides | AA | - Reduced toxicity for both polymer-coated SWCNTs in comparison to the pristine counterparts - Efficiently delivery of miR-503 oligonucleotides to ECs - Providing the possibility to regulate ECs proliferation and in vitro and in vivo angiogenesis |
293 |
| Single-wall carbon nanotubes (SWCNTs) |  |
Polyethylenimine (PEI)-SWCNTs conjugations linked with candesartan | VEGF-targeted siRNA (siVEGF) | AA | - Highly inhibited tube formation of HUVECs - Inhibition of tumor growth and tumor-associated angiogenesis repression |
327 |
| Graphene oxide |  |
Bovine serum albumin-capped graphene oxide (BSA-GO) | VEGF165 | AA | - Showing high stability in physiological saline solution and having ultrastrong binding affinity to VEGF-A165 - Inhibiting the proliferation, migration and tube formation of HUVECs - Ability to strongly disturb the physiological process of angiogenesis in CAM model - The capability of blocking VEGF-A165-induced blood vessel formation in rabbit corneal neovascularization |
297 |
| Gelatin methacrylate (GelMA) containing GO | - | PA | - Increasing the proliferation and migration of keratinocytes - Improved wound healing via promoted angiogenesis |
328 | ||
| Nanodiamonds |  |
Arg-Gly-Asp-Ser (RGDS) conjugated anodiamonds | VEGF-siRNA | AA | - Prolonged the release time of VEGF-siRNA by 6 folds - Reducing the formation of the tubes and without any testable cytotoxicity |
306 |
| Fullerene |  |
Polyhydroxylated fullerenes | Doxorubicin (Dox) | AA | - Inhibiting ECs proliferation in vitro - Exhibiting antiangiogenic activity in zebrafish and murine tumor angiogenesis models |
329 |
PEG is a non-ionic water-soluble polymer, which has been extensively used in drug delivery applications due to its biocompatibility. More than 35 FDA-approved nanoparticles incorporating PEG are presently on the market, designed for both imaging and therapeutic purposes 75. There are some experimental studies in which PEG has been used in combination with other biocompatible polymers for targeted delivery of angiogenic substances 76, 77.
PLA is an FDA and EMA-approved material thanks to its excellent properties such as biocompatibility, biodegradability and lack of any toxic by-products. Several FDA-approved DDSs based on PLA or PGA/PLA copolymers are available on the market, used in nanoparticle or microparticle formulations for the treatment of different cancers 78. The use of nano-sized PLA particles has achieved much attention in drug delivery applications 79; one of the first reports on the use of this nano-sized particles in an anti-angiogenic strategy was published by Burt et al. in 1995 80. The use of nanoparticles containing co-polymers made of PCL and other biocompatible polymers (e.g., PEG) is suggested to improve anti-angiogenic efficacy and thereby anti-cancer potential in vivo compared to PLA 79, 81. In contrast, it has been reported that electrospun PLA nanofibers could increase the proliferation of ECs in vitro 82. Moreover, surface functionalization of PLA has been proposed as an approach to increase its pro-angiogenic properties; polyethylenelmine (PEI) and polyacrylic acid (PAC)-coated electrospun PLA nanofibers significantly promoted angiogenesis both in vitro and in vivo 83. PLA may be a suitable platform for delivery of a range of pro-angiogenic molecules, such as VEGF 84.
As an FDA-approved substance, PCL in different formulations has received much attention in controlled drug delivery and tissue engineering applications 85, 86. For example, Niza et al. prepared micro- and nano-sized vehicles based on PCL for doxorubicin delivery to glioblastoma 87. However, some limitations have restricted the use of PCL in biomedicine, as compared to PLGA, such as its slow biodegradability 88. There are few studies in the literature in which PCL was used for pro- and anti-angiogenic applications 89, 90; Jiang et al. could successfully prepare PCL nanofibers containing VEGF-encapsulated gelatin particles to enhance MSCs differentiation and angiogenesis of ECs 90.
PLGA is another FDA-approved pharmaceutical product, extensively used as a DDS in imaging, diagnostics, and therapy due to its favorable properties, such as biocompatibility, as well as controlled and sustained release of drugs 91. In several studies, researchers have demonstrated the applicability of PLGA nanoparticles (pristine, chemically modified, or hybrids) to load and deliver anti-angiogenic molecules 81, 92–94. A tumor-vessel-recognizing and tumor-penetrating system was developed based on iNGR-modified PEG-PLGA nanoparticles for treating glioma in mice 95. The modified nanoparticles could penetrate into the tumor parenchyma and showed good cellular uptake in HUVECs, resulting in enhanced anti-proliferative and anti-capillary tube formation activities of paclitaxel in vitro. Moreover, the results showed improved anti-angiogenic acticity of the drug-loaded nano-carriers. It is worth noting that several research groups have used PLGA nanoparticles to load pro-angiogenic biomolecules (e.g., aptamers) and other chemicals to improve angiogenesis and subsequently accelerate tissue healing 96–98.
As an FDA-approved product, chitosan in micro- and nano-sized formulations is commonly used in a broad range of biomedical applications, from wound healing to drug delivery 99, 100. The biological activities of chitosan can be summarized as antimicrobial, antioxidant, and anti-cancer 101–103. Furthermore, it has been reported that chitosan nanoparticles can inhibit angiogenesis in a dose- and time-dependent manner in cancer models in vivo 104. The suppression of VEGFR-2 and subsequent blockage of VEGF is proposed to explain the anti-angiogenic activity of chitosan nanoparticles. Anti-angiogenic activity was also observed in the case of depolymerized chitosan products, i.e., water-soluble low-molecular-weight chitosan (LMWC) and chito-oligosaccharides (COs) 105, 106. Furthermore, it should be stated that chitosan has also been used as a drug delivery system in pro- and anti-aniogenic applications 107–109.
Heparin is a natural water-soluble polysaccharide with a high negative surface charge used for a broad range of applications, from the treatment of thromboembolism to anti-cancer strategies. Although heparin exhibits anticancer effects by inhibition of angiogenesis 110, the side effects of thrombocytopenia and heart arrhythmias restrict its long-term administration in humans 111–113. Chemical modification using deoxycholate or lithocholate could reduce the anticoagulant activity of heparin, encouraging its broader use as an anti-tumor drug carrier. The use of nano-sized heparin as a conjugate carrier for delivery of a wide range of pro-angiogenic and anti-angiogenic substances has also been proposed 114–123.
Gelatin is extensively used in biomedical products due to its versatile characteristics, including biocompatibility, biodegradability, non-antigenicity, cost-effectiveness, and easy availability 124. A number of experimental studies showed the utility of cationic gelatin in drug delivery strategies, either as pristine or surface-modified forms 125. However, the use of nano-sized gelatin in the development of DDSs has been encouraged by several surface modifications to improve the targeted and sustained release of therapeutic genes, drugs, and chemicals 126–130. The study published by Kommareddy and Amiji is one of the first reports using gelatin nanoparticles as an antiangiogenic strategy 131. They used gelatin, thiolated gelatin (SHGel), and PEG-modified gelatin (PEG-Gel) nanoparticles to encapsulate and deliver plasmid DNA encoding the VEGF receptor-1 (VEGFR1 or sFlt-1) in order to entrap excess VEGF produced by tumor cells and thereby reduce the angiogenesis process. On the other hand, gelatin nanoparticles have been used in pro-angiogenic strategies; such as, the sustained release of pro-angiogenic factors (e.g., VEGF and bFGF) loaded into gelatin-based nanoparticles to improve neo-vascularization 132, 133.
Albumin is one of the most important components of human blood with a half-life of 19 days on average, which is extensively used in various biomedical applications such as drug delivery 134. In order to improve the inherent properties of albumin, nano-sized albumin systems can be prepared via different procedures including desolvation (coacervation), emulsification, thermal gelation, nano spray drying, and self-assembly 135–141. The FDA approved a nanoparticle formulation (130-nm) of albumin-bound paclitaxel called ABI-007 (Abraxane®; Abraxis BioScience and AstraZeneca), which is used to treat cancers such as breast, non-small-cell lung carcinoma (NSCLC), and pancreatic cancer 142, 143. Albumin nanoparticles (either in pristine or modified forms) have been studied as anti-angiogenic strategies for treating various types of solid tumors in experimental models 144–147. In addition, albumin could be applied as a suitable platform to deliver anti-angiogenic cargos to tumoric sites 148.
7. Regulation of angiogenesis by chemicals and drugs
7.1. Herbs and Phytochemicals
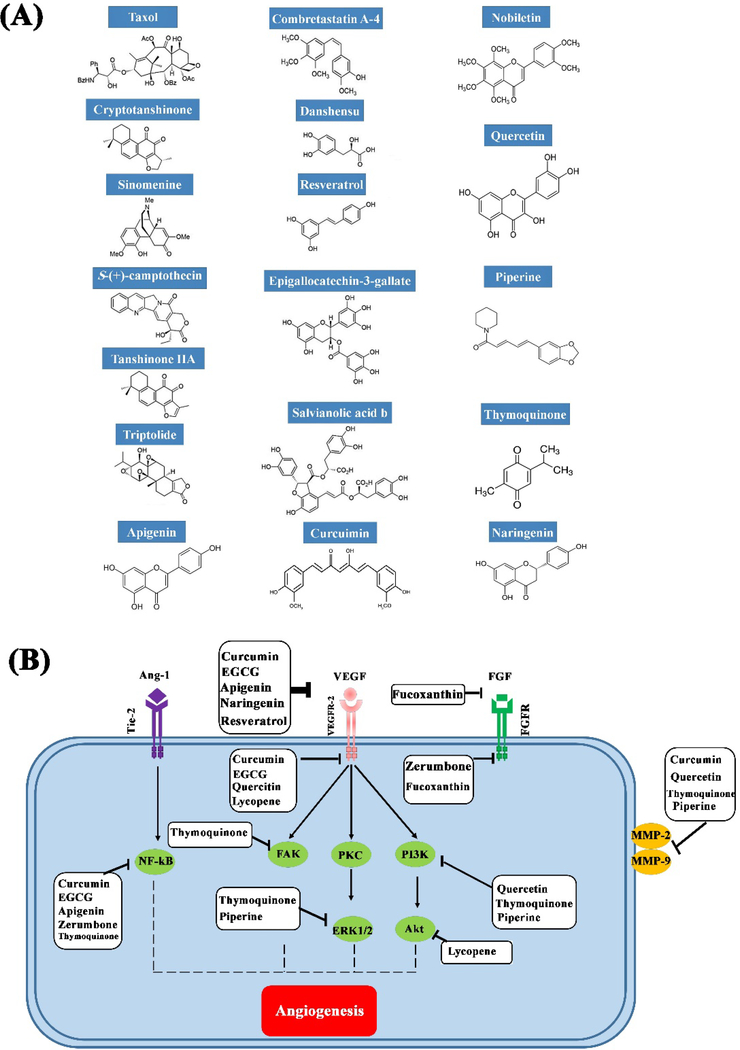
The use of plant-derivd chemicals and drugs for pro-angiogenic and anti-angiogenic strategies has a long history, especially in traditional Chinese medicine. With the emergence of modern technology, chemical optimization of these compounds has led to a substantial improvement in their effectiveness to modulate angiogenesis 149, 150. These natural products affect the angiogenesis process via distinct molecular pathways (Fig. 5) 151. In the following sections, we introduce and discuss the pro- and anti-angiogenic activities of the most commonly-used plant-derived components, and then show their effectiveness when used in nano-sized format, including nano-carriers.
Fig. 5.
(A) Chemical structures of some of the most well-known pro- and anti-angiogenic substances derived from medicinal plants, and (B) the main signaling pathways of angiogenesis.
7.2. Curcumin
Curcumin is the principal polyphenolic compound present in turmeric, and is among the most studied organic compounds in biomedical applications. There are conflicting data regarding the potential of curcumin in the new vessel formation; however, most reports seem to suggest the anti-angiogenic activity of this biomolecule. The anti-angiogenic properties of curcumin result from its interaction with multiple cell signaling proteins and pathways 152. As an illustration, curcumin shows inhibitory effects on the expression or synthesis of some of the most important proteins involved in angiogenesis in solid tumors, including HIF-1 α, VEGF, CD31, and bFGF 153, 154. The inhibitory effects of curcumin on angiogenesis is also related to its activity against cell signal transduction pathways involving PKC and the transcription factors NF-κB and AP-1. Furthermore, curcumin could affect proteinases (MMP and uPA families), which are involved in the angiogenesis process. Some studies showed that curcumin can act as a blocker of cell adhesion molecules that are upregulated in active angiogenesis 155. The use of nano-formulated curcumin shows promise for overcoming some limitations of curcumin such as its low aqueous solubility, rapid systemic clearance, and low cellular uptake. Although the preparation of curcumin nanoparticles has been previously reported by a process based on a wet-milling technique 156, most research has been focused on using different nanocarriers (e.g., liposome/lipid nanoparticles, micelles, polymer conjugates, etc.) to efficiently encapsulate and then deliver curcumin to target sites 157. In the cancer therapy setting, various nano-formulations of curcumin, including micelles and liposomes, have exhibited a significant improvement in anti-angiogenic efficacy 158–161; Mukerajee et al. introduced targeted nanocurcumin therapy as an effective approach in inhibiting neovascularization 162. The anti-angiogenic and subsequent anti-cancer effects of liposomal curcumin have also been evaluated in vitro and in vivo against human pancreatic cancer 163. Intraperitoneal injection of 20 mg/kg liposome-encapsulated curcumin into tumor-bearing mice (three times a week for one month) could reduce tumor growth up to 42% in comparison to untreated animals. The histological and immunohistological assessment showed a significant decrease in the formation of blood vessels, as well as expression of VEGF in animals treated with liposomal curcumin.
7.3. Icariin
Icariin is a prenylated flavonol glycoside and one of the main bioactive components of Epimedium (family Berberidaceae), which is used in a broad range of medical applications, including cancer therapy 164. Icaritin is another bioactive chemical found in the ethyl acetate fraction of Epimedii extract. There are several studies in the literature revealing anti-cancer activities mediated by these organic compounds, including apoptosis, cell cycle arrest, anti-angiogenesis and anti-metastasis, as well as immunomodulation 164. It has been proposed that their anti-angiogenic effects could be mediated via inhibition of the ERK signaling pathway 165. Icariin and icaritin have inhibitory effects on the proliferation, migration, and tube formation of human umbilical vein endothelial cells 166 and could attenuate angiogenesis in a chick embryo model in a dose-dependent manner 167, 168. In vivo experiments have also shown that both icariin and icaritin exhibit anti-angiogeneic effects in xenograft models of tumors, including hepatocellular and renal carcinoma 169, 170. The inhibition of the VEGF signaling pathway via reduction of the transcriptional activity of HIF-1α was reported to explain the anti-angiogenic effects of icariin and icaritin in vitro and in vivo 171. On the contrary, there are a few studies claiming that icariin can stimulate angiogenesis by activating relevant signaling pathways 172, 173. For example, Chung et al. reported icariin at a concentration of 5 μM could activate the MEK/ERK and PI3K/Akt/eNOS-dependent signaling pathways in human endothelial cells; however, it did not affect VEGF signaling pathway. The authors showed that this pro-angiogenic concentration (5 μM) was comparable to that of 10 ng/ml VEGF. The results of an ex vivo experiments on rat aortic rings showed that 5 μM icariin increased vessel sprouting at the cut edge, three times more than controls. Other research groups also reported that icariin (at concentrations of 7.5, 15 and 30 μM) via activating eNOS increased the number of sprouting tubules in endothelial progenitor cells (EPCs) 174. Although icariin has been used in both pro- and anti-angiogenic strategies, there are few experimental studies concerning the use of its nanoformulation 175–177.
7.4. Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene) is a non-flavonoid polyphenolic compound found in a number of plants, including grapes, peanut roots, and the heartwood of mulberry trees 178, 179. This compound has been shown to be a cancer chemopreventive agent, as it could inhibit angiogenesis in various tumors 180–182. It has been previously well-documented that systemic delivery of resveratrol at concentrations of 2.5–100 mg/kg inhibits tumor-induced neovascularization in animal models 183. However, in vitro studies showed that the anti-angiogenic activity of resveratrol was dose-dependent, so that it could completely inhibit tube formation and cell migration of HUVECs at concentrations of 50, 100 and 500 μM, while it showed pro-angiogenic activity at lower concentrations (e.g., 5 μM) 184. The molecular mechanisms involved in the pro- and anti-angiogenic activities of resveratrol have explored in several experimental studies. These include altering endothelial morphology and subsequently causing cytoskeletal rearrangements in both β-catenin and VE-cadherin; activating PI3-K/Akt and MAPK/ERK signaling pathways followed by upregulation of endothelial NOS and increased levels of NO, leading to over-expression of VEGF and MMPs 185. On the other hand, resveratrol at high doses can bind to VEGF thus interfering with its binding to VEGF receptors, resulting in a decrease in VEGF receptor-2 phosphorylation and JNK phosphorylation as well as inhibiting the VEGF-mediated phosphorylations of eNOS, Akt and Erk 186, 187. Like other anti-angiogenic substances, targeted delivery of resveratrol to tumor sites could be conducted using a variety of nano-based DDSs, including solid lipid nanoparticles (Fig. 6) 188, 189. For example, Pund et al. successfully used a lipid-based nanoemulsion delivery system of resveratrol, and showed its good anti-angiogenic activity in vivo using a CAM assay 190. The nanoemulsification included Acrysol K 150 as a lipid and a mixture of Labrasol and Transcutol HP as a surfactant system to form emulsion particles with a size of 85 nm to 120 nm. A few studies have explored resveratrol nanoparticles; Kim et al. reported the successful preparation of trans-resveratrol (t-RVT) in nanoparticles via temperature-controlled anti-solvent precipitation with hydroxypropyl methylcellulose as the stabilizer 191.
Fig. 6.
Pharmacokinetics and pharmacodynamics of resveratrol including bioavailability, anti-oxidant and inflammatory, anticancer, as well as healing properties, are enhanced when administered by nanocarriers in vivo. Reproduced with permission from (Colloids and Surfaces B: Biointerfaces, 2019, 180, 127–140), Copyright 2019, Elsevier Ltd.
7.5. Paclitaxel
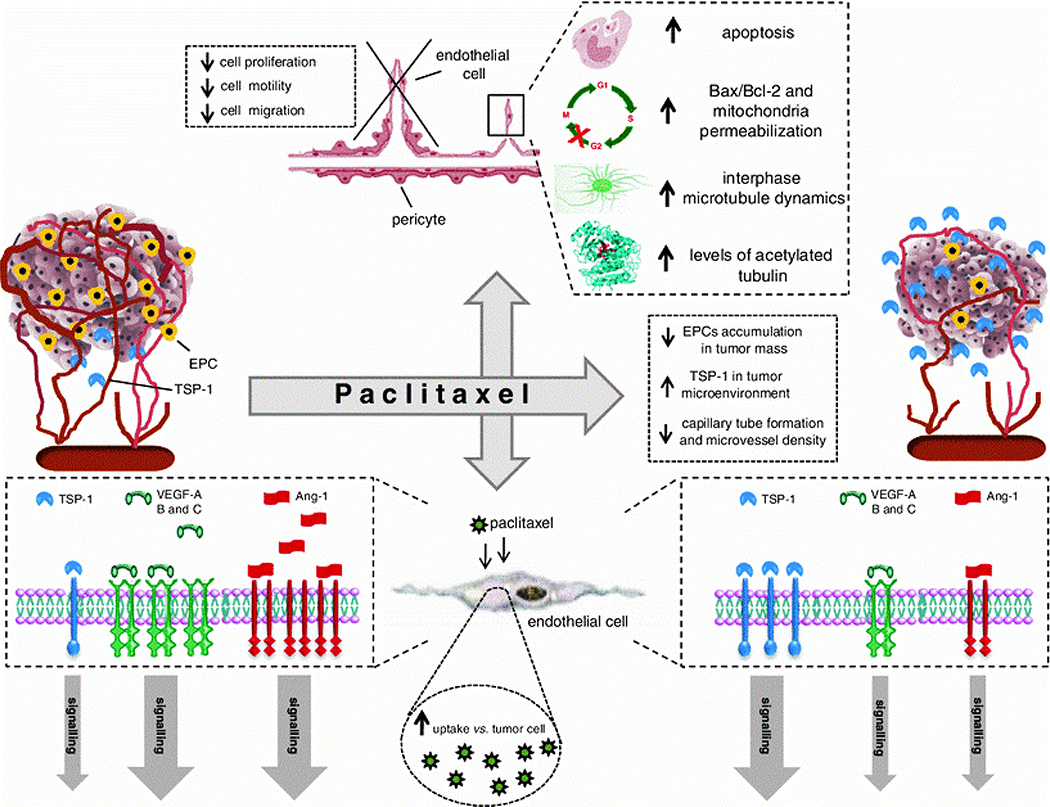
Paclitaxel (Taxol®) is a naturally occurring diterpene alkaloid, which was firstly isolated from the bark of Pacific Yew, Taxus brevifolia Nutt. (Taxaceae) in the 1960s, and since then has been commonly used clinically as first-line chemotherapy for many different cancers (e.g., lung and breast) 192. As a member of the taxane family, paclitaxel binds to the beta-subunit of polymerized tubulin in the cytoskelton and prevents the dissociation of the tubulin subunits from the tubule, leading to the formation of microtubule bundles, and subsequent cell cycle arrest inhibiting mitosis 193, 194. The first reports on the anti-angiogenic activity of paclitaxel were published by Dordunoo et al. in 1995 195 and Belotti et al. in 1996 196. Paclitaxel can inhibit angiogenesis at a broad dose range, from ultra-low to high concentrations. For instance, Wang et al. reported that paclitaxel inhibited the proliferation of human ECs at ultra-low concentrations of 0.1–100 pM, with an IC50 (the half maximal inhibitory concentration) of 0.1 pM 197. The anti-angiogenic activity of paclitaxel at low concentrations was also observed using in vivo models of neovascularization (CAM model), in which paclitaxel inhibited angiogenesis at doses of 4, 8, and 12 nM 198. Several studies (in vitro, ex vivo, and in vivo) showed that paclitaxel hinders proliferation, motility, and migration of ECs by interfering with a series of molecular cellular signaling pathways involved in angiogenesis (Fig. 7) 199. Two of the most important and well-defined target proteins of paclitaxel are VEGF and FGF-2 in HUVECs, as reported by several studies 200, 201. Moreover, paclitaxel can down-regulate the expression of Ang-1, a potent pro-vasculogenic and angiogenic factor, in vitro 202. The induced expression of TSP-1, a potent endogenous inhibitor of angiogenesis, is another route by which paclitaxel could elicit its anti-angiogenic activity 203.
Fig. 7.
Different molecular and cellular mechanisms of the antiangiogenic activity of paclitaxel. Reproduced with permission from (Angiogenesis, 2013, 16, 481–492), Copyright 2013, Springer Nature.
Nano-based systems designed for paclitaxel delivery have shown an enhanced transvascular permeability and increased accumulation in tumors causing increased cancer cell death 203. Moreover, the use of nano-based DDSs could also be effective in the treatment of multidrug-resistant cancers 204. Up to date, several carrier systems have been developed and tested, including liposomes, solid lipid nanoparticles, lipid nanocapsules, and nano-emulsions 205. Banerjee et al. prepared Tyr-3-octreotide (TOC)-modified solid lipid nanoparticles (SLN) containing paclitaxel to improve anti-cancer efficacy via the inhibition of angiogenesis in glioblastoma-bearing rats 206. The anti-angiogenic potential of this system was confirmed via analysis of tube formation and CD31 staining, and its anti-glioma efficacy was proven by histopathological assessment of the treated animals. Furthermore, the use of pure paclitaxel nanoparticles for treating cancer has also been reported. As one illustration, Wu et al. prepared pure paclitaxel nanoparticles using an electrostatic spraying method and showed their anti-cancer effect on human liver cancer SMMC-7721 cells 207.
7.6. Camptothecin
20-(S)-Camptothecin (CPT) is a natural pentacyclic alkaloid first isolated by Wall et al. from the bark of the Chinese tree Camptotheca acuminata 208. This compound is a topoisomerase-I (Top1) inhibitor with the ability to inhibit DNA replication, thus subsequently killing tumor cells as well as inhibiting EC proliferation 209. Over the years, medicinal chemists have succeeded in synthesizing several CPT derivatives, including topotecan (TPT, 3), irinotecan (CPT-11, 4), and belotecan (CKD-602, 5) which have received FDA-approval for various cancers such as ovarian and small-cell lung cancer 210. Furthermore, a series of water-soluble and non-water-soluble analogs are being tested in preclinical and clinical trials 211–215. One of the first reports on the anti-angiogenic activity of CPT was published by Clements et al. 216. They aimed to determine the inhibitory effects of sub-cytotoxic doses of CPT and TPT on angiogenesis both in vitro and in vivo, in comparison to other anti-angiogenic compounds (i.e., TNP-470 and cisplatin). Their results showed that treatment with 50 nM CPT or TPT led to growth inhibition in HUVECs without any cytotoxicity. Furthermore, CPT or TPT effectively inhibited angiogenes in an in vivo disc model comparable to TNP-470. Similar results have been reported in other experimental studies, clarifying the anti-angiogenic potential of CPT at various doses and formulations against different cancers 217–219. The use of nanotechnology for targeted delivery of CPT is a promising approach to overcome its limitations (e.g., low bioavailability and poor water solubility); therefore, various CPT-based nanodrug platforms (e.g., liposomes and nanosponges) have been tested in cancer therapy. It is worth mentioning that, although nano-structured delivery systems developed for CPT have been extensively studied, their widespread use is limited due to the side effects of the nanomaterials used. Therefore, the application of CPT nanodrugs prepared by self-assembled drug molecules is preferred to delivery systems based on nanocarriers 220. From an anti-angiogenic point of view, targeted delivery of CPT can be achieved by nano-structured platforms; Gigliotti et al. used CPT-containing nanosponges to enhance the cytotoxic effect against anaplastic thyroid cancer cells in vitro, and suppress angiogenesis in orthotopic xenograft tumors in vivo 221. CRLX101 is a nanoparticle preparation containing a cyclodextrin-based polymer and camptothecin, and is in phase II clinical trials for treating metastatic castration-resistant prostate cancer and small cell lung cancer. Preclinical studies have revealed that this nanoformulation could improve cancer (e.g., gastric and breast) chemoradiotherapy via inhibiting DNA repair (apoptosis) and HIF1α (anti-angiogenesis) 222–225.
7.7. Combretastatin
Combretastatin A-4 (CA4) is a dihydrostilbenoid used as a chemotherapy drug for the treatment of a variety of solid tumors, such as ovarian, and colon cancer 226, 227. This compound is extracted from the bark of the South African bush willow tree, i.e., Combretum caffrum 228. CA4 exerts its anti-cancer activity via inhibiting polymerization of tubulin via attachment to the colchicine-binding site of the β-tubulin subunit in mammalian cells 229, 230,229, 230. It has been shown that CA4 exhibits cytotoxicity (doses below 4 nM) against bladder cancer cells through inducing G2-M phase arrest with sub-G1 formation 231. CA4 can induce apoptosis in cancer cells by activating caspase-3 and decreasing BubR1/Bub3 231. CA4 could cause the disruption of tubular organization inside HUVECs followed by inhibition of the branching outgrowth 232. Therefore, the CA4 acts as a vascular disrupting agent, which is considered to be a new class of anti-angiogenic drugs. Recent studies have demonstrated that the anti-angiogenic activity of CA4 could suppress microvessel formation at a dose of 5 nM and completely block microvessel sprouting at a dose of 20 nM in the aortic ring model embedded in Matrigel 233. The attenuation of the VEGF/VEGFR-2 signaling pathway is considered to explain the anti-angiogenic activity of CA4 233. Ren et al. had previously proposed the Raf-MEK-ERK and Rho/Rho-kinase signaling pathways for the anti-angiogenic activity of CA4 234. Poor water-solubility, low bioavailability, and rapid metabolism are the main limitations that could be overcome by nano-formulations of CA4. Up to now, a series of nano-based systems (nanoliposomes and oil nanodroplets) have been developed to enhance the bioavailability of CA4 235, 236. Co-delivery of CA4 with other chemotherapy agents (e.g., DOX) using iRGD-grafted mesoporous silica nanoparticles was studied to destroy and kill tumor cells and vasculature 237. Recently, Wang et al. tested co-administration of CA4 nanoparticles and sorafenib to treat hepatocellular carcinoma 238. The authors developed nanoparticles of poly(L-glutamic acid)-graft-methoxy poly(ethylene glycol)/CA4 sodium salt (CA4-NPs) combined with sorafenib. The rationale was that the CA4-NPs could disrupt established tumor blood vessels and result in extensive tumor necrosis, while sorafenib could reduce VEGF-A-induced angiogenesis (induced by CA4-NP) and lead to the inhibition of tumor proliferation (see Fig. 8). The results showed that the combination therapy with sorafenib 30 mg/kg + CA4-NPs 30 mg/kg (on the CA4 basis) could lead to a significant tumor suppression (over 90%) in an orthotopic hepatic H22 xenograft mouse model; and 5 out of 7 mice receiving the combination therapy survived tumor-free for 96 days.
Fig. 8.
Schematic representation of the combined mechanism of CA4-NPs and sorafenib to treat hepatocellular carcinoma (HCC). As shown, although the disruption of established tumor blood vessels and extensive tumor necrosis are achieved by systemic administration of CA4-NPs, the overexpression of VEGF-A and thereby angiogenesis occurs in response to hypoxia. On the other hand, sorafenib can decrease the expression of VEGF-A and hence subsequently inhibit angiogenesis and tumor proliferation. This strategy could be considered as a potential approach to completely eradicate the whole tumor. Reproduced with permission from (Acta biomaterialia, 2019, 92, 229–240), Copyright 2019, Elsevier Ltd.
8. Lipid-based nanosystems
Liposomes, solid-lipid nanoparticles (SLNs), self-emulsifying drug delivery systems (SEDDSs), and micelles are the major types of lipid nanoparticles with the ability to load and deliver various chemicals, drugs, and genes used in cancer diagnosis and therapy 239. They exhibit some attractive properties as DDS, such as biocompatibility, biodegradability, capacity to self-assemble, as well as the ability to entrap both hydrophobic and hydrophilic drugs 240. In addition, it is easy to tailor their size, functionality, and surface charge via simple approaches 241, 242. Liposomes are FDA-approved self-assembled phospholipid vesicles composed of lipid bilayers surrounding an aqueous core, and they can be produced in a size range of 30 nm to 3000 nm 243. The loading of bioactive substances with various chemical structures into liposomes can include: (1) hydrophilic drugs in the aqueous core; (2) lipophilic drugs inside the lipid bilayer; and (3) amphiphilic drugs partitioned at the surface of the inner or outer bilayer 244, 245. Active targeting using liposomes is achievable using surface modification with target-specific ligands or antibodies 246. In addition, stimuli-responsive liposomal DDSs are under investigation. ThermoDox is a temperature-responsive nano-liposome used for un-resectable hepatocellular carcinoma in Phase III clinical trials 247. Doxil®, the first FDA-approved nano-drug, is a liposomal doxorubicin formulation used for the treatment of various cancers, like Kaposi’s sarcoma 248. Moreover, there are additional FDA-approved liposomal drug formulations for cancer therapy on the market, including Myocet™, Lipo-dox®, DaunoXome®, and Marqibo® 249–252. With respect to anti-angiogenic applications, several research groups have shown the ability of liposomes. For example, Pont et al. showed the effectiveness of Fumagillin (an anti-angiogenic drug)-loaded liposomal nanoparticles to treat early atherosclerotic lesions in mice 253.
SLNs were firstly introduced in 1991 with the goal to create a carrier system as an alternative to traditional colloidal carriers (e.g., emulsions and liposomes) 254–256. However, there are some limitations to use of the SLNs as DDSs, including their rapid clearance, serum instability, as well as nonspecific uptake by the mononuclear phagocytic system 256. In this regard, functionalizing the SLNs using a variety of bioactive molecules, including ligands and antibodies, has been suggested to improve their potential in targeted drug delivery 257–259. Recently, Bayón-Cordero et al. reviewed the application of SLNs in anti-cancer drug delivery, with the advantages of biocompatibility, high bioavailability of encapsulated drugs, possible loading of many hydrophilic and lipophilic molecules, and relatively easy large-scale production 260. The use of SLNs for the loading and delivery of anti-angiogenic agents has been confirmed by several research groups. As one example, VEGF antisense oligonucleotides were successfully loaded into SLNs, and tested in vitro and in vivo rat glioma models showing down-regulation of VEGF expression levels 261.
SEDDSs are multi-component systems composed of an oil phase, surfactants, co-surfactants, emulsifying agents, and co-solvents 262. Based on their size, two types of these systems are self-nano-emulsifying agents (SNEDDS) and self-micro-emulsifying agents (SMEDDS) 262, 263. Up to now, various chemicals and drugs have been successfully loaded into SEDDSs, including anti-cancer agents, and there are more than four such commercialized drug products on the market 262, 264, 265. In 2015, Valicherla et al. prepared docetaxel (DCT) loaded SEDDSs (D-SEDDS) to improve the oral bioavailability and therapeutic efficacy of the drug. The results showed a 3.19-fold increase in bioavailability of the D-SEDDS in rats and a 25-fold increase in vitro cytotoxic activity compared to free DCT 266. In order to obtain more effective anti-angiogenic formulations, several groups have incorporated anti-angiogenic substances (e.g. curcumin) into SEDDSs, and the results have been promising 267–269.
9. Polymeric nanofibers
Polymeric nanofibers are among the most widely-applied constructs in biomedicine, from anti-tumor strategies to tissue healing. Nanofibers exhibit some attractive properties, including large specific surface area, controllable pore size, and tunable drug release profiles, making them highly-promising candidates for anti-cancer applications 270. Recently, Abid et al. reviewed the anti-cancer applications of electrospun polymeric nanofibers loaded with various chemicals and drugs including, doxorubicin, paclitaxel, and curcumin 271. Apart from electrospun nanofibers, several studies showed the utility of synthetic nanofibrous peptide scaffolds to mimic the pro-angiogenic and anti-angiogenic activity of small molecules, including heparin, and maspin 272–274. For instance, Fan et al. investigated docetaxel- and curcumin-loaded nanofibrous microspheres made of PLA-PEO-PPO-PEO-PLA polymers as an injectable and sustained-release system for enhancing anti-colon cancer activity 275. The results of the combined nanofibrous microsphere treatment showed a significant increase in the inhibition of angiogenesis and subsequent inhibition of colon cancer in mice. On the contrary, there are a number of publications in which pro-angiogenic cargos were delivered using polymeric nanofibrous scaffolds produced by both electrospinning and self-assembly procedures 133, 276–279. Most of the pro-angiogenic nanofibers have been applied to accelerate tissue repair and regeneration, especially to promote wound healing 280–282.
10. Other carbon-based nanomaterials and nano-systems
Nano-sized carbon-based materials are among the most promising DDSs and include several members, including carbon nanotubes, nanodiamonds, nanohorns, graphene, fullerenes, and nanofibers 283. These nanomaterials show attractive properties; for example, they typically possess high mechanical strength and large specific surface area, and thus provide numerous sites for chemical or physical conjugation; moreover, they are relatively easy to manufacture on a large scale 284, 285. These nanomaterials in either pristine or functionalized formats can be suitable platforms for conjugation, loading and release of a wide range of bioactive molecules 286–288. Additionally, some carbon nanomaterials especially carbon nanotubes and graphene are being studied in laser-induced hyperthermia of different types of solid tumors 289.
The use of carbon-based nanomaterials in anti-angiogenic cancer therapy is growing. One of the first reports was published by Muruges et al. who showed that 100 μg of graphite, multi-walled carbon nanotubes (MWCNT), and fullerenes could significantly inhibit angiogenesis induced by FGF2 or VEGF in vivo in a CAM model assay 290. In a comprehensive study, Wierzbicki et al. evaluated the anti-angiogenic properties of diamond nanoparticles, graphite nanoparticles, graphene nanosheets, MWCNT, and C60 fullerenes at a concentration of 500 mg/L in a CAM assay, 291. Their results revealed the anti-angiogenic effects of diamond nanoparticles and MWCNTs. However, graphite nanoparticles and graphene showed no anti-angiogenesis activity, and interestingly fullerenes exhibited pro-angiogenic activity.
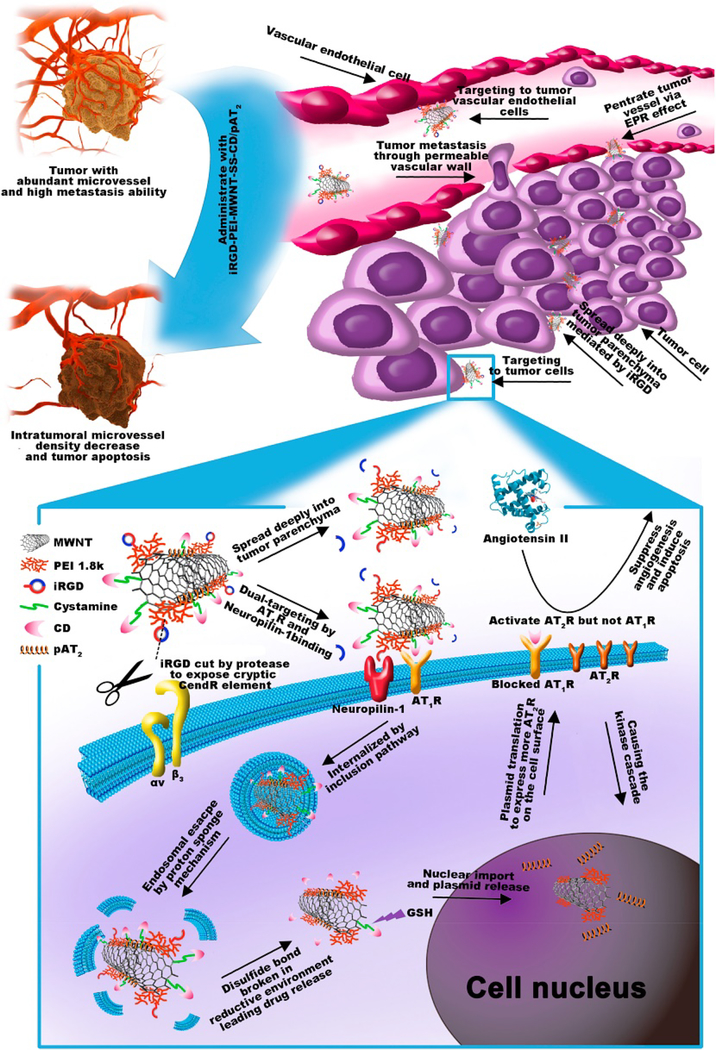
With respect to the interactions of single-wall carbon nanotubes (SWCNTs) with endothelial cells, Albini et al. concluded that these nano-sized carbon materials could be useful vehicles for targeting the vasculature and potential carriers of anti-angiogenic agents 292. Masotti et al. in 2016 reported that polyethyleneimine (PEI) and polyamidoamine dendrimer (PAMAM)-coated carbon nanotubes (CNTs) were appropriate delivery systems for microRNAs (miR-503 oligonucleotides) for angiogenesis regulation 293. More recently, Su et al. designed and developed a dual-targeted co-delivery system based on iRGD-modified MWCNTs for use in anti-angiogenic therapy of lung cancer 294. For this aim, polyethyleneimine (PEI) and cystamine (SS) were used to attach iRGD and the chemotherapy drug candesartan (CD) to MWCNTs, respectively. Then, the authors assembled functionalized MWCNTs with the plasmid AT2 (pAT2) and prepared iRGD-PEI-MWNT-SS-CD/pAT2 complexes. The results obtained from in vivo experiments in nude mice demonstrated that co-delivery of CD and pAT2 synergistically increased anti-angiogenic effects through down-regulation of VEGF (see Fig. 9). However, some reports showed that SWCNTs could promote angiogenesis through an indirect pathway in which SWCNTs enhanced fibrogenesis in mammalian cells (e.g., CRL-1490) via reactive oxygen species (ROS)-mediated phosphorylation of p38MAPK and, thereby, overexpression of pro-angiogenic molecules TGF-β1 and VEGF 295. However, other researchers have reported conflicting results; for example, Roman et al. observed that SWCNTs inhibited angiogenesis in vivo and was harmful to the normal embryonic development due to deregulation of important genes involved in cell proliferation, apoptosis, survival, and angiogenesis in brain and liver tissues 296.
Fig. 9.
Schematic representation of the use of iRGD-PEI-MWNT-SS-CD/pAT2 for the inhibition of tumor angiogenesis. Intravenous administration of iRGD-PEI-MWNT-SS-CD/pAT2 complexes results in specific accumulation at tumor tissues via EPR effect; angiotensin II type 1 receptor (AT1R) and integrin receptor-mediated binding. Reproduced with permission from (Biomaterials, 2017, 139, 75–90), Copyright 2017, Elsevier Ltd.
There are several reports in the literature on the use of modified graphene oxide (GO) in anti-angiogenic strategies; for example, Lai et al. prepared bovine serum albumin-capped GO (BSA-GO) which was able to entrap and block VEGF-A165 (a potent pro-angiogenic molecule), and thereby inhibit angiogenesis 297. Another example was provided by a study conducted by Shi et al. who conjugated reduced GO (rGO) with 64Cu, 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA), and the anti-CD105 antibody TRC105 to produce an appropriate system for theranostics 298. On the other hand, there have been several reports reporting the use of GO nanoparticles to promote angiogenesis. As one example, Mukherjee et al. showed that low amounts of GO (10 ng/mL) and rGO (50 ng/mL) could improve angiogenesis via the formation and activation of ROS and reactive nitrogen species (RNS) and consequent activation of Akt and eNOS signaling pathways 299. Moreover, Chen et al. showed that SrTiO3 CNTs could be used as a delivery system for Ag2O nanoparticles to exert antibacterial, osteogenic, and pro-angiogenic activities simultaneously 300.
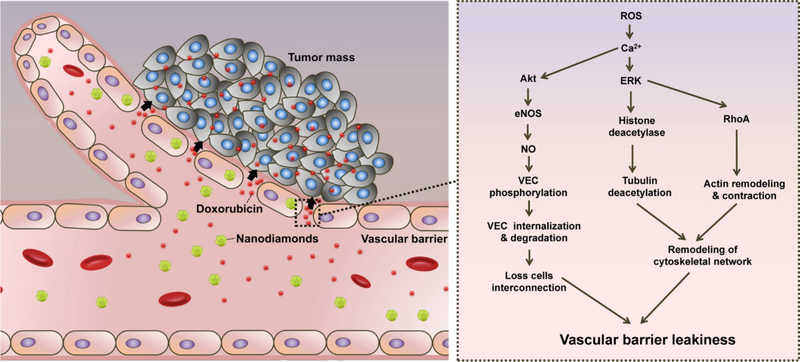
Nanodiamonds are another type of carbon-based nanomaterials that can act as platforms in cancer nanomedicine, both for therapy, and imaging. Nanodiamonds are biocompatible, and show efficacy as carriers for various cancer therapeutic drugs, and possess tunable surface structures 301–303. For example, Setyawati et al. used surface-modified nanodiamonds to induce endothelial permeability 304. They functionalized the samples with –COOH and –NH2 groups and showed that these derivatives could induce endothelial leakiness in a surface-dependent manner, resulting in increased delivery of doxorubicin to tumors. The mechanism proposed for this phenomenon (i.e., leakiness of the vascular barrier) was based on an increase of intracellular ROS and Ca2+, which facilitated the loss of cell-cell interconnections in the vascular barrier caused hy cytoskeletal remodeling (see Fig. 10). Zhang et al. used lipid-coated nanodiamonds to enhance the bioavailability and efficacy of an anti-angiogenic drug, sorafenib, to combat metastasis of gastric cancer 305. The authors successfully prepared sorafenib-loaded nanodiamonds with a size of 127.6 ±12.9 nm. The drug-loaded nanodiamonds led to increased bioavailability (up to 7.64 fold) and a higher concentration of sorafenib in the tumor (up to 14.95 fold) in vivo compared to control groups. These improvements showed a significant suppression of the metastasis of gastric cancer to distant organs (liver and kidney). Furthermore, other research groups have studied nanodiamonds in pro-angiogenic strategies, for the loading and delivery of a broad range of pro-angiogenic molecules 306–308.
Fig. 10.
Schematic representation of nanodiamond (ND)-induced vascular barrier leakiness. ND-induced vascular barrier leakiness leads to higher accumulation of doxorubicin in the tumor site. The increase of intracellular ROS and Ca2+ account for the ND-induced vascular barrier leakiness through the loss of cell-cell interconnection in the vascular barrier and cytoskeletal remodeling. Reproduced with permission from (ACS nano, 2016, 10, 1170–1181), Copyright 2016, American Chemical Society.
Carbon nanohorns have a conical structure, and are used in drug delivery strategies, both in pristine and functionalized formats 309, 310. The main member of nanohorn family is the single-walled carbon nanohorn (SWNH), which is a tubular unit with a size of 2–5 nm in diameter and 40–50 nm in length 311, 312. Although SWNHs have some properties in common with the CNTs, they exhibit possess more uniform and controllable morphology, and easier large-scale production without metal contamination, making them preferable in the clinical setting 313, 314. Different morphologies of SWNHs have been identified, including “dahlia-like” type, “bud-like” type or “seed-like” type. The dahlia-like SWNHs are most commonly-used type for cancer theranostic applications 312. Several reports have shown the applicability of modified SWNHs as DDSs for the delivery of anti-cancer drugs in vitro and in vivo 315–317. For example, Li et al. reported the use of oxidized SWNHs (oxSWNHs) as an effective DDS for transporting higher doses of vincristine to tumors 318.
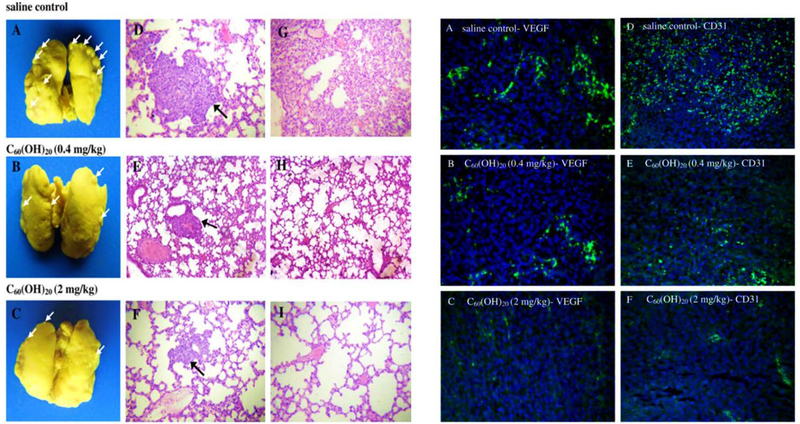
Fullerenes is the first symmetric closed-cage type of the carbon nanomaterial family and have been extensively used in a variety of forms (number of C-atoms, pristine, surface-modified, and hybrid compounds) in different industrial and biomedical areas, including cancer imaging and therapy 319–322. It has been reported that fullerenes can act as anti-cancer agents on their own; for example, Prylutska et al. reported that water-soluble C60 fullerenes were effective in the treatment of transplanted malignant tumors. They believed that the anti-cancer activity of C60 fullerenes might be related to their high antioxidant activity, and their ability to block some specific cell receptorssuch as EGFRs. The anti-tumor activity of other fullerene derivatives has also been verified in other studies. Jiao et al. studied the anti-tumor and anti-metastatic potential of fullerenol in a mouse breast cancer model 323. They injected 0.1 mL saline solution containing fullerenol C60(OH)20 (0.08 and 0.4 mg/ml) daily for a period of 16 days and histopathologically evaluated the anti-tumor and anti-metastatic activities of the samples. The results showed that injection of fullerenol modulated oxidative stress and down-regulated the expression of multiple angiogenic factors (e.g., CD31) in tumors, leading to inhibition of tumor growth and metastasis in vivo (Fig. 11).
Fig. 11.
The images on the left side belong to anti-tumor metastasis activity of C60(OH)20; (A–C) Macroscopic observations of mice lungs soaked in Bouin’s solution exhibit spontaneous pulmonary breast cancer metastases (white arrows); (D–I) Pulmonary histology in mice received saline (D and G), 0.4 mg/kg (E and H) and 2 mg/kg C60(OH)20 (F and I). Note that black arrows indicate pulmonary metastases (original magnification: D–F ×100; G–I ×200). The images on the right side present the immunohistochemical staining of VEGF and CD31 expression to clarigy the effect of C60(OH)20 on EMT-6 tumor microvessel density; (A and D) Tumor tissues harvested from mice treated with saline; (B and E) 0.4 mg/kg C60(OH)20; and(C and F) 2 mg/kg C60(OH)20. Note that cells positive for VEGF and CD31 expression are in green and cell nuclei are blue (stained with DAPI). (×200 original magnification). Reproduced with permission from (Carbon, 2010, 48, 2231–2243), Copyright 2010, Elsevier Ltd.
Various surface-functionalized fullerenes (e.g., Gd@C82(OH)22, C60(OH)22 and C60(C(COOH)2)2) also showed ROS scavenging properties and hence were potentially applicable in cancer therapy 324. Moreover, fullerene derivatives have exhibited potent anti-angiogenic activity; Meng et al. reported that the multiple hydroxyl group-functionalized surface of Gd@C82(OH)22 fullerene-based nanoparticles (f-NPs) exhibited the ability to simultaneously down-regulate more than 10 pro-angiogenic factors ar both the mRNA and protein levels 325. These researchers evaluated the in vivo efficacy of the functionalized NPs, and found that the surface-modified samples could reduce tumor microvessel density by > 40% as well as efficiently decrease the speed of blood flow to tumors by up to 40% at 2 weeks post-injection compared to the effect of paclitaxel alone. Moreover, the functionalized NPs had no pronounced toxic side-effects in nude mice. Based on these results, the authors concluded that this nano-sized compound holds great promise for use in cancer treatment.
11. Inorganic ions, nanoparticles, and nano-systems for anti-angiogenic and pro-angiogenic applications
Many inorganic metallic elements are delivered to humans via normal nutrition or by therapeutic diets since they are known to have specific effects on cell metabolism and biological functions. Some of these elements have also been embedded in implantable/injectable nanomaterials, nano-systems for advanced nanotechnology-based therapies to control angiogenesis. This section deals with the chemical and biological functions produced by inorganic elements with regard to promoting or suppressing angiogenesis; furthermore, a description of the various biomaterials used (e.g., nanoparticles, nanotextured surfaces, hierarchical systems) is provided. Inorganic elements usually perform their angiogenesis-related functions after being released as solble ions; however, direct interaction between the surface of metallic nanoparticles and cells/biomolecules has also been reported in some cases (e.g. gold and silver nanoparticles) (see Tables. 4 and 5). Elements having an effect on angiogenesis, but exhibiting severe toxicity to animals and humans (e.g. arsenic, lead and mercury contained in industrial waste nano-particulates) have not been included in this section due to the lack of therapeutic significance.
Table. 4.
Pro-angiogenic biochemical and biological functions elicited by inorganic elements and nanomaterials (the elements are listed in alphabetical order).
| Element | Notes/Biochemical and biological functions | Ref (s) |
|---|---|---|
| B | Borate ((BO3)3−) ions can induce: - Stimulation of endothelial cell migration and proliferation - Increased secretion of VEGF and other pro-angiogenic factors - Tubule formation |
334, 539, 540 |
| Ca | Ca2+ ions induce: - Endothelial cell proliferation - Overexpression of PDGF, EGF, IGF-I, bFGF, VEGF |
340, 341, 344 |
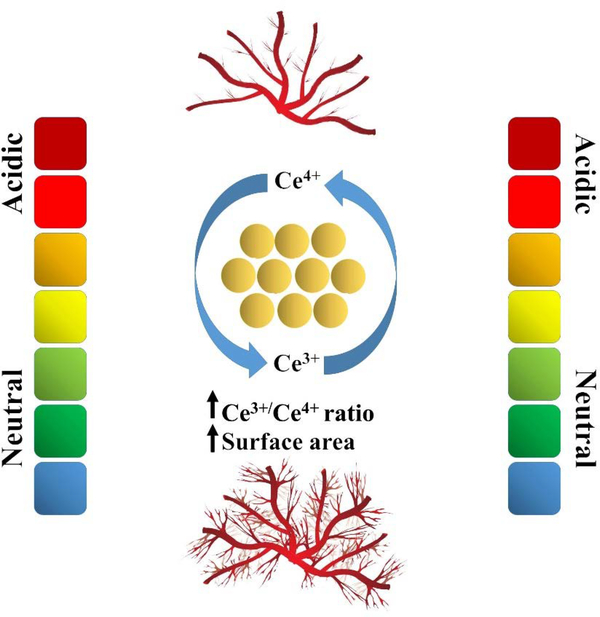
| Ce | - Nanoceria could stabilize HIF-1α in ECs and up-regulate VEGF expression, resulting in induced pro-angiogenesis - High surface area and increased Ce3+/Ce4+ ratio make nanoceria a robust inducer of angiogenesis |
351 |
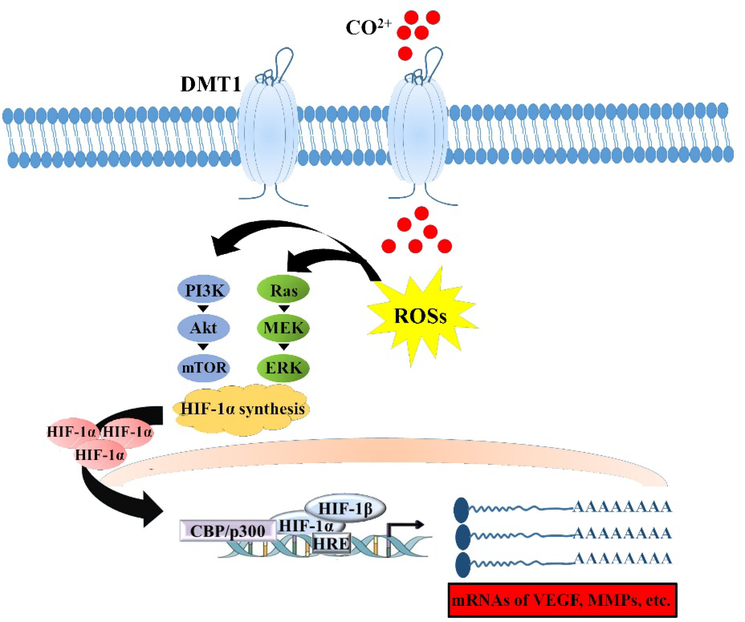
| Co | Co2+ ions induce: - Activation of the HIF-1 pathway - Overexpression of angiogenic factors VEGF and bFGF - Enhanced tubule formation |
363, 364 |
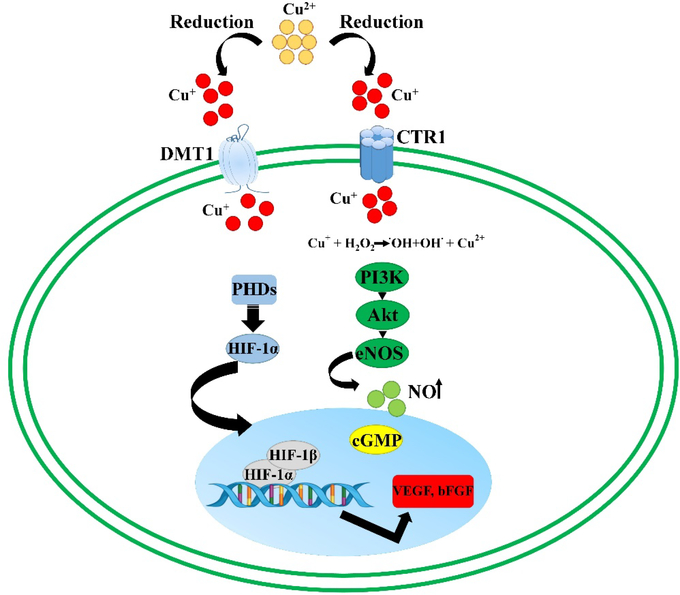
| Cu | Cu+/Cu2+ ions and copper nanoparticles induce: - Activation of the HIF-1 pathway - MAPK signaling pathway - Activation of VEGF, bFGF, TNF, IL-1β, IL-6, and IL-8 - Endothelial cell proliferation |
387, 389, 390, 403 |
| Eu | Eu3+ ions induce: - Overexpression of angiogenic genes CD31, MMP9, VEGFR1/2 and PDGFRα/β of HUVECs - Promotion of endothelial cell proliferation in vitro and vascular sprouting in vivo (CAM model and mice) Eu(OH)3 nanorods/nanoparticles promote angiogenesis mediated by ROS production (especially H2O2). |
413, 415 |
| Fe | Fe2+/Fe3+ ions induce: - ROS generation - Stabilization of HIF - VEGF increase |
431, 432, 441 |
| Li | Li+ ions promote: - VEGF secretion - Vasculogenesis |
444, 447, 448 |
| Mg | Mg2+ ions released from silicate bioceramics and glasses induce: - Stimulation of proliferation and migration of microvascular cells - Enhancement of the mitogenic response to angiogenic factors |
454 |
| Nb | Nb5+ ions released from bioactive glasses promote angiogenesis in vitro through enhancing VEGF secretion | 458 |
| P | Phosphate ((PO4)2−) ions induce: - Stimulation of pro-angiogenic bFGF, VEGF, FOXC2, and osteopontin - Stimulation of migration and tube formation in the HUVEC model |
347, 464 |
| S | Different sulphur compounds can exert pro-angiogenic (H2S, NaHS, Na2S) | 503, 504 |
| Si | - Pro-angiogenic effect elicited by silicate ((SiO4)4−) ions (induction of endothelial cell homing, polarization and migration; induction of angiogenic differentiation and new blood vessel sprouting) | 455, 486 |
| Tb | Tb(OH)3 nanorods stimulate NOX-mediated generation of ROS, with activation of the PI3K/Akt/MAPK signaling cascade and formation of intracellular NO, which is a key signaling molecule for angiogenesis | 508 |
| Ti | - Pro-angiogenic effect elicited by hydrophilic and relatively smooth titanium surfaces | 514–519 |
| Y | Y2O3 nanoparticles stimulate VEGF and EGFR secretion | 529 |
| Zn | - Pro-angiogenic effect elicited by ZnO nanoparticles through ROS generation and upregulation of bFGF and VEGF | 532 |
Table. 5.
Anti-angiogenic biochemical and biological functions elicited by inorganic elements and nanomaterials (the elements are listed in alphabetical order).
| Element | Notes/Biochemical and biological functions | Ref (s) |
|---|---|---|
| Ag | Silver nanoparticles act on the PI3K/Akt signaling pathway | 304 |
| Au | Gold nanoparticles induce: - Inhibition of the MAPK pathway - Inhibition of pro-angiogenic factors (e.g., VEGF, bFGF, PlGF) |
417, 423 |
| Ce | The antiangiogenic effect was found to occur at high concentrations and in the presence of rod-shaped nanoceria. | 359, 541 |
| S | Sulphur compounds such as heparan sulfonate can exert antiangiogenic effects | 507 |
| Se | - Inhibition of VEGF secretion - Apoptosis of endothelial cells |
472, 473, 475 |
| Si | - Anti-angiogenic effect elicited by pure silica nanoparticles or at a high dosage of silicate materials (cytotoxicity) | 525 |
| Ti | - Anti-angiogenic effect elicited by titania nanoparticles via suppression of VEGF/MAPK pathways | 542 |
| Zn | - Anti-angiogenic effect elicited by Zn2+ ions (e.g., activation of endostatin, reverse effect on hypoxia-modulated genes) | 533, 534 |
11.1. Boron
Boron is a trace element playing diverse and vitally-important roles in many biological functions ranging from bone metabolism to anti-inflammatory activity, as comprehensively discussed by Pizzorno in a valuable review 330. The first evidence of the role of boron in the context of angiogenesis was reported in 2002 by Dzondo-Gadet et al. 331, who examined the action of boric acid at the molecular level using cell-free transcription systems (isolated placenta nuclei) and translation systems (wheat germ extracts). It was found that 10 mM of H3BO3 greatly increased mRNA synthesis associated with the translation of pro-angiogenic proteins like VEGF and TGF-β1.
Based on these early results, boron-releasing bioactive glasses have been intensively investigated over the last two decades, and have been proposed as therapeutic implantable (nano)biomaterials for accelerating wound healing in tissue engineering applications 332. Bioactive melt-derived B2O3-CaO-based glasses are more reactive than silicate glasses upon contact with aqueous solutions and were found to rapidly release a large amount of Ca2+ ions into biological fluids, which is beneficial for skin regeneration because calcium promotes the migration of epidermal cells to the wound site 333. Melt-derived borate glasses with the composition 1605 (6Na2O-12K2O-5MgO-20CaO-4P2O5-51.6B2O3-0.4CuO-1ZnO wt.%) and 13–93B3 (53B2O3–6Na2O–12K2O–5MgO–20CaO–4P2O5 wt.%) were shown to stimulate VEGF secretion in vitro 334. Furthermore, Durand et al. 335 doped 45S5 Bioglass® with 2 wt% of B2O3 and reported that the presence of boron in the ionic dissolution products stimulated the proliferation and migration of HUVECs, in vitro tubule formation, and the secretion of IL-6 and bFGF to a greater extent compared to the B-free control glass, thereby demonstrating the pro-angiogenic potential of borate ions. These in vitro results were confirmed in vivo by comparing the vascularization induced by the same materials in an embryonic quail chorioallantoic membrane (CAM) model 336. Higher expression of integrin αvβ3 and greater blood vessel density were observed in response to implanted B-doped 45S5 glass. Researchers from Missouri University developed 13–93B3 nano-fibers (diameter in the range of 300 nm to 2 μm) which, after being organized in a “cotton-candy” morphology, could be used as a dressing material to treat full-thickness cutaneous wounds 337. An interesting mechanism was observed to explain the promotion of in vivo angiogenesis by this nanomaterial 338, i.e. the newly-formed blood vessels were attached to the hydroxyapatite micro-clusters that originated during the nanofiber degradation due to the glass bioactivity 339. After implantation in rats for 22 days, significant regeneration of dermal, epidermal and subcutaneous tissues was reported. Seven out of 12 diabetic patients involved in a clinical study experienced complete healing of their chronic wounds with less scarring and equal or faster-wound closure rate (from 0.3 to 0.8 mm/day depending on the type of injury) compared to other more expensive wound treatments, such as vacuum-assisted systems 338. These 13–93B3 borate glass nano-fibers, trade-named DermaFuse®, received Food and Drug Administration (FDA) approval for medical applications in 2016 and are currently marketed for treating wound injuries in animals (“RediHeal” veterinary product) as well as acute/chronic wounds in humans (Mirragen® Advanced Wound Matrix). At present, these commercial products are the only ones based on nano-bioactive glasses for use in soft tissue engineering and also as stimulators of angiogenesis. Further research is needed to fully elucidate all the biomolecular and biochemical aspects behind the pro-angiogenic effect of boron as well as its synergistic action with other relevant ions (e.g., Ca2+) released from these glasses on the complex process of wound healing.
11.2. Calcium
Calcium is one of the most important elements involved in the biological functions of mammals, such as participation in building the mineral phase of hard tissues (bone and teeth) and regulating bone homeostasis via various cell signaling pathways 340. Some proteins, e.g., parvalbumin and calbindin-D, can bind to Ca2+ ions and store them, thus acting as calcium stores or buffers, and limiting free calcium diffusion in the intracellular environment 341. Pro-angiogenic factors like platelet-derived growth factor (PDGF), EGF, IGF-I, bFGF and VEGF are known to trigger a significant increase in the level of Ca2+ ions in different cell types 342–344. In this regard, it was shown that bFGF and VEGF (the most potent pro-angiogenic endogenous factors) could bind to different families of receptor tyrosine kinases (RTKs) which trigger intracellular calcium increases in endothelial cells 345. Fang et al. studied the role of calcium stored in fibroblasts isolated from pterygium, and reported that calcium-related signaling pathways were associated with persistent fibroblast proliferation and angiogenesis, as shown by the high density of blood vessels 346. Ca2+ ions can be typically released from all forms of calcium phosphate implants as well as melt-derived and sol-gel bioactive glasses (e.g., micro- and nanoparticles, scaffolds, coatings, fibers) upon contact with biological fluids in vitro and in vivo. The angiogenic properties of calcium phosphates in the context of bone regeneration have been recently discussed by Malhotra and Habibovic 347. The pro-angiogenic effect of bioactive silicate glasses in contact with both hard and soft tissues is well-known, but it is typically considered to be due to all the ionic dissolution products released from the glass, including silicate ions 348.
The bioactive borate glass 13–93B3, with a high CaO content, was recently used to fabricate nanofibrous scaffolds that significantly accelerated wound healing when implanted in both animals and humans 338. A possible explanation for this beneficial effect relied on the release of Ca2+ ions which stimulate angiogenesis and accelerate the migration of keratinocytes, thereby promoting skin regeneration. These cotton-candy borate glass nanofibres were also found to impressively help the healing of long-term venous stasis ulcers in diabetic patients, who were unresponsive to conventional pharmacological treatment 349.
11.3. Cerium
Cerium is a rare-earth metal that usually does not participate in biological functions; however, cerium oxide nanoparticles (nanoceria) have recently attracted interest in biomedicine due to their antioxidant properties and ability to act as a free radical scavenger in cells and tissues. Many chemical processes can be used to produce ceria nanoparticles, including hydrothermal methods, sol-gel, and polymer-assisted synthesis; these routes have been comprehensively reviewed by Kargozar et al. 350. Researchers have also found interesting dual properties (stimulatory or antagonistic effect) of nanoceria in the context of angiogenesis. The oxygen-buffering capacity of nanoceria can be exploited to stabilize HIF-1α, thereby promoting angiogenesis in vitro and in vivo 351. It was shown that the pro-angiogenic potential of ceria nanoparticles is markedly dependent on the surface valence states of cerium: specifically, high surface area and a high Ce3+/Ce4+ ratio make nanoceria more catalytically active for regulating the intracellular oxygen content, which leads to a stronger pro-angiogenic effect (see Fig. 12)352. Ceria nanoparticles were also found capable of stimulating the migration and proliferation of endothelial cells in vitro 353. Functionalization strategies have been carried out to further enhance the pro-angiogenic properties of nanoceria. Nethi et al. 354 synthesized nanoconjugates of organosilane-functionalized cerium oxide nanoparticles by using an ammonia-catalysed ethylene glycol-assisted precipitation method in an aqueous suspension of samarium-doped nanoceria conjugated with hydrophilic triethoxysilane (6-{2-[2-(2-methoxy-ethoxy)-ethoxy]-ethoxy}55-hexyl) moieties. The ceria/polymer nanoconjugates promoted endothelial cell viability and proliferation without eliciting any significant cytotoxicity and induced in vivo blood vessel formation in a chick embryo chorioallantoic membrane model. The p38-MAPK/HIF-1α signaling pathway was proposed to be the mechanism governing the pro-angiogenic effect induced by these functionalized nanoparticles, which was greater compared to that associated with “conventional” nanoceria.
Fig. 12.
Schematic illustration of the effect of environment on the pro-angiogenesis and anti-angiogenesis properties of nanoceria. The pH, reactive oxygen species (ROSs) generation, and intracellular oxygen concentration are identified as the main determinants of angiogenic behavior of nanoceria.
Ceria nanoparticles, synthesized by using gelatin as a stabilizing agent, retained their pro-angiogenic properties when embedded in electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) membranes 355 and PCL scaffolds 356, as demonstrated by accelerated wound healing in rat models.
Conversely however, cerium oxide nanoparticles can exhibit an anti-angiogenic effect depending on the surrounding environmental conditions. There are some parameters that influence this dual behavior including pH, ROS generation, intracellular oxygen concentration and concentration of the nanoparticles 352. In fact, high concentrations of nanoceria exhibit anti-angiogenic effects 357. For example, the proliferation of HUVECs is reduced if the nanoceria concentration exceeds 8.6 mg/mL 358. It has also been shown that the anti-angiogenic effect is more pronounced when nanoceria is functionalized by heparin 359: hence, heparin-functionalized and pristine nanoceria at high concentrations have been proposed as therapeutic agents for reducing endothelial cell growth and vascularization in tumors, thereby acting as an adjuvant in anticancer approaches.
Interestingly, there are some “shape and size effects” associated with ceria nanostructures. Das et al. 351 showed that exposure to ceria nanorods led to a slight reduction in endothelial cell proliferation, whereas spherical ceria nanoparticles or nanostars elicited no toxic effect in HUVECs. Furthermore, only ceria nanoparticles with size < 15 nm showed the potential to induce tubule formation, whereas micrometer-sized ceria particles even inhibited tube formation in HUVECs. This different behavior is probably due to the higher reactivity of smaller particles, due to a higher specific surface area. Cerium oxide nanoparticles are non-absorbable, but a controlled release of Ce3+/Ce4+ ions from soluble (nano)biomaterials should be studied in future research as a potential means to promote angiogenesis. At present, Ce2O3 has already been incorporated in gel-derived silicate MBGs, but only its physico-chemical role in modulating glass dissolution kinetics and its biological effect in improving bone cell activity have been studied so far 360–362.
11.4. Cobalt
Controlled release of cobalt ions (Co2+) has been shown to promote angiogenesis in vitro and in vivo, via the creation of hypoxia-mimicking conditions. Specifically, Co2+ ions can activate the hypoxia-inducible factor-1 (HIF-1) pathway independently of the overall cellular oxygen level 363. The HIF-1 pathway is the main regulator of cell response to variations in oxygen tension by triggering the expression of about 100 hypoxia-targeted genes 364. HIF-1 is a heterodimeric transcription factor comprising two subunits, i.e. the oxygen tension-regulated HIF-1α and the constitutively-expressed HIF-1β subunits 365. Activation of the HIF-1 pathway is strongly related to the concentration of HIF-1α in the cytoplasm. Specifically, two scenarios are possible: (i) under normoxic conditions, HIF-1α is continuously produced and then degraded through the ubiquitin proteasome pathway, or (ii) under hypoxic conditions, HIF-1α is stabilized and can accumulate, translocate to the cell nucleus and then dimerize with HIF-1β to induce the expression of its target genes. The role of Co2+ ions is to “artificially” stabilize HIF-1α concentration by blocking the protein degradation regardless of the oxygen levels (see Fig. 13). As a result, broad transcriptional responses occur, including the upregulation of pro-angiogenic factors (e.g. VEGF) that subsequently lead to angiogenesis and improvement of the oxygen supply 366.
Fig. 13.
Representative schematic of cobalt roles in activating two signaling pathways involved in angiogenesis progress, i.e., the PI3K/Akt/mTOR and Ras/MEK/ERK pathway. It is assumed that cobalt via Akt activation could trigger transcription factors including SP1, NF-κB, RTEF and NFAT and thereby result in enhanced the transcription of HIF-1/2. Moreover, cobalt through activation of the MEK/ERK pathway could leads to the phosphorylation of 4E-BP and subsequent enhanced HIF-1 α translation.
Altrhough it might have potential for inducing angiogenesis, the therapeutic use of cobalt is still under debate among researchers. Caution is suggested by the occurrence of systemic (and lethal in some cases) toxicity of cobalt ions released from Co-Cr alloys used in hip joint replacement prostheses. Systemic cobalt toxicity was reported to lead to neurotoxicity and heart failure; furthermore, local accumulation of Co2+ ions at the implant site could contribute to tumor formation 367–369.
At present, there have been some reports about the incorporation of cobalt as a biological modifier in bioactive glasses. Most of these studies have been concerned with improving bone tissue repair and regeneration. Wu et al. 370 reported the fabrication of Co-doped multiscale macro-mesoporous scaffolds by a co-templating sol-gel-like procedure where a surfactant (Pluronic P123) was used as a surface-directing agent to produce a mesoporous texture (average diameter 4.1 nm) and an open-cell polyurethane sponge as a template for the macropores (300–500 μm). These hierarchical MBG scaffolds showed promise as multifunctional systems for the synergistic delivery of antibiotics (ampicillin) and Co2+ ions, which stimulated VEGF over-expression in bone marrow mesenchymal stem cells (BMSCs). The relatively low amount (<5 mol.%) of cobalt incorporated in the MBGs was non-toxic to the BMSCs, but no in vivo studies have been reported on these materials so far.
An international research team led by Stevens and Hill synthesized a series of melt-derived Co-doped silicate bioactive glasses with up to 4 mol.% of cobalt and demonstrated their hypoxia-mimicking function on human mesenchymal stem cells (hMSCs) 371, 372. The ionic dissolution products released from these Co-doped glasses (particle size <38 μm) successfully increased HIF-1α activity after 8 h of incubation with hMSCs and promoted VEGF expression.
Given that hypoxia plays a key signaling role during cartilage formation, the same research group investigated the influence of Co-doped glasses on inducing hMSC chondrogenesis, in an attempt to develop a novel approach for cartilage tissue engineering 373. It was shown that reduced oxygen tension could aid chondrogenic differentiation 374, 375. Because cartilage is a non-vascular tissue, oxygen must diffuse from the surface of the joint facing the synovial fluid into the cartilage due to the gradient of oxygen tension from the surface of the articular cartilage (partial pressure of oxygen 5%) to the subchondral bone (partial pressure of oxygen 0.1%) 376. Enhanced chondrogenesis in low-oxygen conditions is mainly mediated through HIF-1α by inducing the expression of pro-chondrogenic genes (e.g. Sox9) 377, 378. Interestingly, the ionic dissolution products released from melt-derived Co-doped bioactive glass particles were reported to increase the level of HIF-1α in hMSCs in a cobalt concentration-dependent manner but on the other hand, prolonged exposure to Co-containing solutions reduced cell proliferation and metabolic activity, as well as inhibited chondrogenic differentiation 373. This study suggested that the exposure time to cobalt needs to be taken into careful account and tailored to the specific application, since it can markedly influence the biological and biochemical processes of cells and tissues.
Kargozar et al. showed that incorporation of low amounts of CoO (up to 0.5 mol.%) in melt-derived silicate bioactive glasses increased the expression of angiogenesis-related genes during in vitro tests with HUVECs and Saos-2 cells, while causing minimal cytotoxicity after 21 days of culture 379, and the overall bone healing was improved in rabbits at 4 and 12 weeks post-implantation compared to Co-free glass particles. The same research group also showed the importance of the size effect, i.e., fine Co-doped glass particles (9 μm) were more effective in promoting angiogenesis compared to large particles (725 μm), but were also associated with higher cytotoxicity due to more release of Co2+ ions 380. This effect must be taken into account if the use of nano-sized Co-doped glass particles is envisaged, because the higher specific surface area of the nanoparticles would be expected to make them more toxic.
The toxicity of Co-containing nanoparticles has been reported in many studies. It has been demonstrated that the inhalation of tungsten carbide (WC)/Co nano-powder, consisting of 80 to 90% of WC and 5 to 10% of metallic cobalt, could cause interstitial pulmonary disease and lung cancer, the mechanism involving the generation of ROS and DNA damage 381–383. In a recent study using normal human bronchial epithelial cells (BEAS-2B) and human lung adenocarcinoma cells (A549), Liu et al. 384 reported that WC/Co nanoparticles at a concentration of 5 μg/cm2 induced ROS production which activated the Akt and ERK1/2 signaling pathways, and greatly increased the transcriptional activation of AP-1, NF-κB, and VEGF, thereby promoting pathological angiogenesis. This suggests further caution in proposing cobalt nanomaterials for controlling angiogenesis due to the risks associated with their toxicity and carcinogenicity.
11.5. Copper
Copper is an essential cofactor in various enzymatic activities in animals and humans. It was demonstrated that Cu+/Cu2+ ions modulate the activity of several proteins and factors involved in angiogenesis (see Fig. 14), such as VEGF, fibronectin, angiogenin, collagenase, prostaglandin E-1, ceruloplasmin, FGF-1 and −2, which play important roles in the initiation (vasodilation and vascular permeabilization), maturation (endothelial cell proliferation, migration and morphogenesis), and regulation of blood vessel formation (ECM remodelling) 385. It was also reported that endothelial cells are stimulated to proliferate in vitro upon exposure to copper ions regardless of the VEGF levels, which demonstrates the inherent pro-angiogenic effect of copper 386.
Fig. 14.
Schematic representation of the angiogenesis regulation by copper ions. As seen, the entrance of copper into the cells is mediated by the copper transporter Ctr-1 and DMT1 proteins. Copper’s delivery to intracellular proteins is regulated by copper transport proteins (chaperones) such as Atox-1. By inhibiting PHD-mediated hydroxylation of HIF-1α, copper facilitates the translocation of the factor into the nucleus, leading to its dimerization with HIF-1 β and subsequent interactions to hypoxia-responsive elements and VEGF gene over-expression. Moreover, copper could activate the molecular signaling pathways resulting in increased NO and thereby promote angiogenesis.
There are two main signaling pathways involved in Cu-induced angiogenesis, i.e. (i) the hypoxia-inducible HIF-1 pathway (similar to cobalt); and (ii) the MAPK signaling pathway. The first mechanism is involved in the initiation of the angiogenesis process 387, while the latter plays a role in the endothelial cell proliferative phase 388.
Since copper is normally involved in physiological vascularization processes 389, its reduction or removal in patients suffering from cancer is being studied to combat malignancies as an antiangiogenic treatment 390. In fact, the reduction of copper levels by following a Cu-deficient diet or administering a Cu-chelating drug (e.g., penicillamine) can inhibit angiogenesis by “switching” endothelial cells back into the G0 phase or triggering apoptosis 391. Furthermore, copper reduction attenuates the pro-angiogenic activity of VEGF, bFGF, TNF, IL-1β, IL-6 and IL-8 392, 393.
The use of copper ions as pro-angiogenic dopants in implantable (nano)biomaterials has recently been proposed to promote better vascularization of tissues in regenerative medicine. Since the goal is to achieve a sustained release of copper ions over time, biocompatible matrices with a relatively low dissolution rate, such as bioactive glasses, are often selected to deliver the copper ions. Bührer et al. 394 showed that melt-derived 45S5 Bioglass® doped with 1 wt.% of Cu increased angiogenesis in a rat arteriovenous loop model when compared to Cu-free 45S5 parent glass. Zhao et al. 395 synthesized Cu-doped borate glass microfibers and showed that their ionic dissolution products stimulated the migration of HUVECs, tubule formation, and VEGF secretion, along with the fibroblast expression of angiogenic genes, to a greater extent as compared to the Cu-free parent glass. This pro-angiogenic effect was proportional to the amount of copper in the glass composition, and the Cu-doped fibers were found to accelerate the healing of full-thickness skin wounds in rats. The pro-angiogenic effect of Cu-doped borate materials was also studied by Bi et al. 396 in a rat calvarial defect model. Specifically, melt-derived bioactive borate glass (13–93B3 composition) was doped with 0.4 wt.% of copper and used to fabricate porous scaffolds with trabecular, unidirectional or fibrous microstructures. It was reported that the percentage of new blood vessels at 12 weeks post-implantation was higher for Cu-doped 13–93B3 scaffolds compared to Cu-free 13–93B3 control implants with the same porous architecture. The glass fibrous scaffolds exhibited the best pro-angiogenic effect of all these porous microstructures. Similar results were obtained by the same research group in a dorsal skin window model in mice implanted with Cu-doped or Cu-free 13–93B3 glasses 397.
Wu et al. 398 prepared Cu-doped MBG scaffolds with hierarchical porosity (interconnected large pores within 100–500 μm and well-ordered mesoporous channels around 5 nm) and reported that their ionic dissolution products (mainly Cu2+ ions) stimulated HIF-1α and VEGF expression in human BMSCs, thus further supporting the suitability of mesoporous materials as platforms for the controlled release of pro-angiogenic ions.
Although most applications of copper for promoting angiogenesis are in the field of bone regeneration, Baino first suggested in 2015 399 that Cu-doped MBGs could also be used to accelerate the vascularization of porous orbital implants for ophthalmic socket surgery. This hypothesis was actually confirmed in vivo in 2018 by Wang et al. 400, who performed primary angiogenic tests in a panniculus carnosus muscle model in rabbits and reported that Cu-doped MBG coating significantly promoted the vascularization of porous hydroxyapatite orbital implants compared to Cu-free materials. Incorporation of copper in glass-ceramic orbital implants was also reported using a nearly-inert alumino-silicate glass-ceramic as a base material 401. In a first approach, melt-derived Cu-doped macroporous scaffolds were produced by sponge replication, but the release of copper ions was insufficient to elicit any pro-angiogenic effect. The second strategy, involving the deposition of a thin Cu-doped MBG nano-textured layer on the struts of silicate glass-ceramic foams, permitted a more sustained release of copper to be achieved. At present, none of these Cu-releasing (nano)systems have been tested in preclinical studies, but the early results achieved so far are promising and require further research.
A few studies have addressed the therapeutic properties of metallic copper nanoparticles. Chen et al. 402 reported that the lethal dose (LD50) of copper nanoparticles (size 23.5 nm), micro-sized particles (17 μm) and Cu ions in mice were 413, over 5000 and 110 mg/kg body weight, respectively. Since copper nanoparticles showed higher in vivo biocompatibility than copper ions, Mroczek-Sosnowska et al. 403 extended the investigation to understand if this also applied to pro-angiogenic properties (Fig. 15). It was found that the pro-angiogenic effect of commercial colloidal copper nanoparticles in a chick embryo chorioallantoic membrane model was actually more potent than that elicited by CuSO4 salt, thus confirming the hypothesis.
Fig. 15.
Pro-angiogenic effect of copper nanoparticles: images of implants maintained in the chicken embryo chorioallantoic membrane (CAM) for 2 days, soaked with (1) control (non-soaked); (2) control (PBS); (3) CuSO4; (4) nano-copper, evaluated at day 12 of incubation. Scale bars, 2000 μm. Reproduced with permission from (International journal of molecular sciences, 2015, 16, 4838–4849), Copyright 2015, MDPI AG.
11.6. Europium
Europium is a lanthanides element, with applications in the context of bioimaging due to its long-life fluorescence properties. In fact, Eu3+ ions were found to possess good luminescence with suitable brightness and prolonged signal intensity for use as biolabelling agents 404. Most Eu-containing (nano)materials have therefore been used for luminescent imaging of cells and tissues 405, as well as for tracking and monitoring the kinetics of drug release from mesoporous biomaterials 406. There are few studies about the role of europium in regenerative medicine, but it was reported that Eu3+ ions could functionally mimic Ca2+ ions, thus influencing bone remodelling and being potentially useful to treat bone density disorders (e.g. osteoporosis) 407, 408. Patra et al. 409 first showed that Eu(OH)3 nanorods, synthesized via a microwave-assisted method, could enhance the proliferation of HUVECs in vitro and stimulate vascular sprouting in vivo in a chick CAM model. Similar results were also achieved when Eu(OH)3 nanorods were embedded in electrospun nanofibrous PCL scaffolds 410, which could be applied in tissue engineering (e.g., soft patches). The pro-angiogenic properties of these nanorods at low concentrations were associated to the production of ROS (especially H2O2) both in vitro (HUVECs) and in vivo (zebrafish model) (Fig. 16) 411.
Fig. 16.
Redox signaling mechanism proposed for the pro-angiogenic effect induced by Eu(OH3) nanorods in endothelial cells (EC). ROSs (especially H2O2) are generated by the nanorods in the cytosolic part of the ECs, thus functioning as signaling molecules.
This signaling mechanism was confirmed, also using a zebrafish model, in another study dealing with Eu(OH)3 nanorods and nanoparticles produced by hydrothermal treatment 412.
Eu(OH)3 nanorods show great promise for therapeutic applications also considering their safety, as suggested by the absence of genotoxicty in a mouse model 413.
Ma et al. 414 prepared Eu-doped hydroxyapatite nanorods (length 40–60 nm, width 20–40 nm) by a precipitation method followed by annealing at 600 °C and found that the nanoparticles had a dose- and time-dependent inhibitory effect on HUVECs in vitro. However, this effect could be related to the needle-like morphology of the nano-hydroxyapatite (inherent “shape effect”) rather than to a toxic release of europium ions.
Other Eu-doped crystalline nanomaterials (e.g., NaYF4) exhibit poor biodegradability and can evoke adverse responses in cells and tissues (e.g., necrosis of bone cells) 415, thus requiring the need for removal once they have performed their function in the body.
Clear evidence of the safety and pro-angiogenic effect of Eu3+ ions in vitro and in vivo was reported by Shi et al., who synthesized Eu-doped mesoporous silica nanoparticles (Eu-MSNs, size 280–300 nm) by adding TEOS and Eu(NO3)3·6H2O) to a water/ethanol mixture, followed by a sol-gel process where cetyltrimethylammonium bromide (CTAB) was used as a structure-directing agent. It was found that some angiogenesis-related genes (i.e., CD31, MMP9, VEGFR1/2, and PDGFRα/β) were significantly upregulated in HUVECs by Eu-MSNs. Results from in vivo experiments carried out in diabetic rats revealed that the Eu-MSNs could increase formation of blood vessels and capillary network in chronic skin wounds, showing superior pro-angiogenic ability compared to Eu-free MSNs (Fig. 17). As a result of enhanced neovascularization at the wound site, collagen deposition and re-epithelialization were also promoted. The same study also reported that Eu-MSNs underwent partial degradation in cell culture media – which could help to overcome the need for removal in the long term – and the release of Eu3+ ions stimulated new bone formation at critical-sized cranial defects in rats.
Fig. 17.
Overview of the size change of the large excision wounds made in the dorsal skin of diabetic mice with different periods (a) and relevant statistical analysis (b). Eu-MSNs-polymer film (Eu-P) significantly accelerated the wound healing compared to other groups. Masson’s Trichrome staining images (c) of wounds treated with different groups of films (blank control indicated as Ctrl, pure polymer film as Poly, MSNs-Polymer composite films as M-P and Eu-MSNs-Polymer composite films with as Eu-P). Green arrows indicates the newly formed epithelium at the wound site (scale bar 500 μm, *p < 0.05; **p < 0.01). Reproduced with permission from (Biomaterials, 2017, 144, 176–187), Copyright 2017, Elsevier Ltd.
In summary, europium is a highly-promising metallic element to be incorporated into implantable nanospheres with multifunctional properties (angiogenesis and osteogenesis) for potential use in both bone and skin tissue engineering.
11.7. Gold
Gold nanoparticles, collectively called “nano-gold,” are known to elicit an anti-angiogenic effect, and therefore have recently received attention to combat cancer. In fact, neovascularization outgrowth from preexisting blood vessels is well known to be the key to allowing the growth and progression of tumors 3.
The anti-angiogenic property of nano-gold was first reported by Mukherjee and coworkers who investigated the effects on HUVECs in vitro 416 and in mice 417. Gold nanoparticles (size <220 nm) were prepared by mixing sodium borohydride with an aqueous solution of tetrachloroauric acid under vigorous agitation 418. It was shown that nano-gold could interact with heparin-binding growth factors (HB-GFs) and inhibit their activity. These nanoparticles bound to vascular permeability factor (VPF)/VEGF-165 and bFGF, which resulted in inhibition of: (i) endothelial/fibroblast cell proliferation in vitro; and (ii) VEGF-induced permeability and angiogenesis in vivo. Gold nanoparticles also significantly inhibited VEGF receptor-2 phosphorylation, intracellular Ca release and RhoA activation in vitro but did not reduce the expression of VEGF-121 and epidermal growth factor, which is not a HB-GF. The ability of gold nanoparticles to inhibit the function of VEGF-165 (the most potent of the VEGF isoforms) 419 and placental growth factor (PlGF) was confirmed in another study by the same research team 420. Fig. 18 provides molecular mechanisms by which nano-gold affect angiogenesis process 421.
Fig. 18.
Schematic illustration of molecular pathways affected by the anti-angiogenic effects of nano-gold (AuNPs). The main angiogenic pathways suppressed by nano-gold include VEGFR2, Tie2R, FGFR, and their downstream signaling pathways. As depicted, VEGF-165-mediated intracellular calcium release is suppressed by AuNPs. Moreover, AuNPs upregulate E-cadherin and downregulate vimentin, which results in reduced epithelial-to-mesenchymal transition (EMT) attenuating angiogenesis. Another anti-angiogenenic mechanism proposed for AuNPs is related to its ability to reduce ILs, MMPs, and TNF-α expression and inhibit neovascularization via induction of autophagy. Reproduced from (International Journal of Nanomedicine, 2019, 14, 7643), Copyright 2019, Dove Medical Press.
The inhibitory effect on pro-angiogenic factors VEGF-165, bFGF, and PlGF is related to the strong affinity of nano-gold for thiols, phosphines, disulfides and amines which are groups commonly present in HB-GFs 416, 417.
An in vitro study with HUVECs revealed that the ability of gold nanoparticles to selectively disrupt the functions of pro-angiogenic HB-GFs was dependent on the particle size (inhibitory effect: 20 nm > 10 nm > 5 nm size of nano-gold) 422. Surface charge and chemistry of the nanoparticles were also reported to play an important role; naked nano-gold exhibited the maximum inhibitory effect towards HB-GFs as compared to the same nanoparticles functionalized with various charged ligands. This effect was mediated through direct binding of nano-gold to cysteine residues in HB-GFs. The resulting ionic/pseudo-covalent chemical bonds between the gold surface and HB-GFs induced a conformational change in HB-GFs mediating the inhibition of their function. On the contrary, no alternation was observed in the conformation of non-HB-GFs.
The literature strongly supports the suitability and potential of gold nanoparticles for cancer treatment, due to their biocompatibility, and ability to selectively interact with the biomolecular and biochemical processes of cancer cells and not healthy cells 423. Moreover, another useful property of gold (nano)compounds is the strong inhibitory effect on the enzyme thioredoxin reductase (anti-mitochondrial activity), which is involved in cancer cell proliferation 424.
Preclinical studies have been highly promising. Naked gold nanoparticles were reported to inhibit tumor growth and metastasis in a mouse model of ovarian cancer 425. This effect was due to the inhibition of the MAPK pathway (a key pathway for cancer cell proliferation) because nano-gold disrupts the function of HB-GFs secreted by cancer cells. HB-GFs are also involved in the epithelial–mesenchymal transition (EMT), which is one of the main mechanisms behind cancer metastasis 426, 427. A study carried out using colorectal cancer cells confirmed that treatment with 20-nm gold nanoparticles could actually reverse EMT, thus inhibiting tumor metastasis 428. This was possible as nano-gold reduced the expression of EMT-associated proteins, and up-regulated E-cadherin and down-regulated Snail,.
Furthermore, nano-gold can be used for the treatment of B-cell lymphoblastic leukemia and prostate cancer. B-cell leukemia is a generally incurable disease characterized by resistance against apoptosis, partly because the leukemic cells continuously secrete VEGF and express VEGF receptors. Mukherjee et al. 429 reported that gold nanoparticles coated with anti-VEGF antibodies (Avastin or bevacizumab) increased the level of apoptosis in leukemic cells, and naked nano-gold was also able to induce the same effect to a more limited extent. An application of nano-gold for the theranostic treatment of prostate cancer was reported. Prostate-specific membrane antigen-targeted gold nanoparticles were loaded with a fluorescent photodynamic therapy drug and successfully tested in vitro and in vivo 430. This system aimed to provide surgical guidance for accurate resection of prostate cancer and additional photodynamic therapy when surgery was insufficient.
11.8. Iron
Iron plays a key roles as an enzymatic cofactor and as the central metal in heme in many physiological functions, the most important of which is oxygen transport. In fact, about 70% of the iron available in the body is stored in the blood in the form of hemoglobin, a metalloprotein 431. Iron deficiency can induce the stabilization of HIF and the increase in VEGF secretion, thus stimulating angiogenesis 432. In this regard, induction of iron deficiency was proposed to be an anticancer strategy to reduce the resistance of tumor cells to antiangiogenic therapies. It was reported that Fe2+ ions at concentrations within 10–100 μM exhibit a relatively weak pro-angiogenic activity 433, which is less pronounced than that of other transition metallic ions (e.g. Cu2+) 434. On the contrary, Fe3+ ions have a stronger pro-angiogenic effect at the same concentrations, but are highly toxic to the cells, due to the oxidative stress and ROS generation associated with ferric ions 433. This is probably the main reason why iron-releasing (nano)biomaterials have not been much investigated as implantable systems to promote local angiogenesis. However, some Fe-doped phosphate glass compositions have been suggested for use in tissue engineering as the incorporation of iron allows tailoring the phosphate glass dissolution rate to match that of tissue regeneration 435, 436.
At present, iron is mainly used in the form of magnetite (Fe3O4) nanoparticles that are employed as a diagnostic contrast agent in magnetic resonance imaging, and for cell labeling and tracking 437. Superparamagnetic iron oxide nanoparticles (SPIONs) have also recently emerged as a promising clinical option to treat some types of tumors via magnetic hyperthermia after being injected into the patient’s bloodstream 438. Furthermore, there is interest in the development of multifunctional SPION-based systems with: (i) a magnetite core functioning as a contrast agent; (ii) a biocompatible coating; and (iii) a therapeutic outer layer conjugated to targeting ligands such as, nucleic acids, small molecules, or antibodies 439, 440. However, possible toxicity associated with the non-degradable magnetite core of SPIONs is still a matter of debate, including problems related to biodistribution, local accumulation and long-term fate of Fe3O4 nanoparticles in vivo 441.
11.9. Lithium
Lithium supplementation is a commonly-used clinical approach for the treatment of several psychiatric diseases including bipolar disorder, unipolar depression, and schizophrenia 442. In this context, Li+ ions act on the regulation of neurotransmitters and mitochondrial function, attenuating the expression of genes associated with signaling pathways such as protein kinases A and C (pKA/pKC) in hyperexcitable neurons, thereby favoring mood stabilization 201, 443.
Lithium is also known to activate the Wnt/β-catenin pathway to inhibit the glycogen synthase kinase (GSK)-3β signaling pathway 444. The latter is involved in the suppression of nuclear factor-kB (NF-κB) and activation of IκBα kinase, c-Jun-N-terminal kinase, p44/p42 MAPK, and Akt via tumor necrosis factor (TNF) 445. In fact, it was observed that NF-κB-regulated gene products such as, cyclooxygenase (COX)-2, cyclin D1, matrix metalloproteinase (MMP)-9, survivin, inhibitor-of-apoptosis protein (IAP) 1 and 2, Bcl-xL, Bfl-1/A1, and TNF receptor-associated factor 1 were generally increased upon exposure to Li+ ions.
While lithium was once thought only to be involved in vasculogenesis, but not in angiogenesis 446, recent studies have clearly demonstrated its pro-angiogenic effect as well. Lithium was reported to increase VEGF secretion in rat brain endothelial cells by a mechanism involving the PI3K and GSK-3β signaling pathways 447. The proliferation, migration, and viability of endothelial cells were also shown to be stimulated by Li+ ions in vitro and in vivo through the activation of the Wnt/β-catenin canonical pathway 448.
To the best of our knowledge, incorporation of lithium in implantable biomaterials in order to promote angiogenesis has been carried out in only one study. Haro Durand et al. 449 replaced up to 5 wt.% of Na2O with Li2O in melt-derived 45S5 Bioglass® microparticles, and reported that HUVECs showed a greater migratory/proliferative response and ability to form tubules in vitro upon exposure to Li-doped glasses compared to the Li-free parent material. In agreement with previous studies, they also observed the activation of the Wnt/β-catenin signaling pathway with an increase in expression of the pro-angiogenic cytokines, insulin-like growth factor-1 (IGF-1) and transforming growth factor-β (TGF-β).
Most studies have been devoted to evaluating the physico-chemical and structural properties of Li-doped biomaterials and the biological effect of Li+ ions for stimulating osteoblast activity and, hence, osteogenesis 450–452. Han et al. 453 also demonstrated that Li+ ions released from silicate MBGs could promote the differentiation of periodontal ligament cells into cementoblasts and increase cementogenesis. The multifunctional properties of lithium are promising for new advanced therapies and indeed deserve further investigation in coming years.
11.10. Magnesium
There is no study dealing directly with the use of magnesium nanostructures to stimulate angiogenesis. However, Mg2+ ions are known to play a direct role in modulating inflammatory responses and microvascular functions. In this regard, Bernardini et al. 454 reported that Mg2+ deficiency inhibited the growth and migration of microvascular 1G11 cells while increasing some inflammatory markers such as interleukins 1a and 6, nitric oxide, which is a mediator of inflammatory responses, and VCAM which mediates monocyte/endothelial interactions. On the contrary, high levels of Mg2+ ions stimulate the proliferation and migration of microvascular cells, and hence stimulate angiogenesis.
A couple of studies apparently support the stimulatory effect of Mg-releasing bioceramics and bioactive glasses on angiogenesis. Zhai et al. 455 reported that ionic extracts from akermanite, a Si-, Ca- and Mg-containing biocompatible ceramic, up-regulated the expression of genes encoding the receptors for pro-angiogenic cytokines and increased the expression level of genes encoding the pro-angiogenic downstream cytokines, as well as nitric oxide synthase and increased nitric oxide synthesis. It was also shown that akermanite implants promoted neovascularization in a rabbit femoral condyle model at both 8 and 16 weeks post-implantation. Spontaneous angiogenesis and tubule formation in human endometrial stem cells cultured with sol-gel SiO2-CaO-MgO-P2O5 bioactive glass extracts were also reported by Shamosi et al. 456. However, in both studies the specific role of Mg2+ ions could not be isolated from that of other ionic dissolution products due to the lack of control experiments using Mg-free bioceramic/bioactive glass; therefore, the overall pro-angiogenic effect could be due to the synergistic effects of silicate, Ca2+ and Mg2+ ions that were collectively released by both materials.
11.11. Niobium
Niobium is used as an alternative to vanadium in Ti-based alloys for metallic orthopedic implants (Ti-6Al-7Nb vs. Ti-6Al-4V). Niobium was found to be less toxic to cells than vanadium, with lower inhibitory effects on the proliferation and viability of human osteoblasts, fibroblasts, and lymphocytes 457. No reports were found on the use of niobium nanostructures in the context of angiogenesis; however, there is one study dealing with the pro-angiogenic properties of Nb5+ ions released from bioactive melt-derived silicate glass-ceramic granules. Miguez-Pacheco et al. 458 reported the results of cell culture tests using bone marrow stromal cells (ST-2) in contact with Nb-doped glass-ceramic extracts, which revealed enhanced VEGF secretion induced by the ionic dissolution products released from the Nb-doped material as compared to the parent glass. A complete picture of the biomolecular and biochemical mechanisms behind the pro-angiogenic action of Nb5+ ions is still to be obtained; nevertheless, incorporation of niobium in MBG platforms allowing a controlled ion release and modulation of angiogenesis deserves investigation in the future.
11.12. Phosphorus
Phosphorus is known to be important, along with calcium, in constructing the mineral phase (mainly hydroxyapatite) of bones and teeth 459. Moreover, the pro-angiogenic role exerted by phosphate ions has been shown in some studies. In this regard, phosphate ions were reported to increase the expression of MMP-2 and bFGF in the lungs of developing mice, thereby stimulating angiogenesis 460. It was also observed that high levels of phosphate ions could increase the expression of key pro-angiogenic genes such as forkhead box protein C2 (FOXC2, a regulator of vascular formation and remodeling 461, osteopontin (OPN, a cytokine-like factor associated with tumor angiogenesis 462 and VEGF in pre-osteoblastic cells 463, 464. On the other hand, hyperphosphatemia can induce human endothelial cell apoptosis resulting from increased ROS generation and mitochondrial dysfunction 465.
Phosphate ions can be released from calcium phosphate implants, the effects of which on angiogenesis have been recently reviewed in the context of bone tissue engineering 347. Furthermore, most silicate bioactive glasses, such as 45S5 Bioglass®, contain a moderate amount of P2O5; therefore, phosphate ions released from these materials could indeed contribute to their well-established pro-angiogenic potential 466.
In vitro studies using Cu- or Co-doped P2O5-based glasses revealed the pro-angiogenic properties of phosphate glasses 467, 468. However, it cannot be ignored that both glass formulations incorporated metallic dopants (cobalt and copper) with potent pro-angiogenic effects, so the phosphate ions could have just supplied an adjuvant effect.
11.13. Selenium
Selenium is needed in normal nutrition or can be supplied as a dietary supplement in either elemental form or in inorganic salts or water-soluble organic compounds 469. Elemental selenium is contained in a number of different enzymes and proteins with wide distribution and broad physiological functions throughout the body 470. Selenium was found to elicit an anti-angiogenic effect in vitro and in vivo, which suggested its suitability as an adjuvant in anticancer therapeutic strategies. In this regard, the beneficial activity of selenium against cancer was first reported in the 1980s in a couple of animal studies in rodents 471, 472. Since then, different metabolites of selenium have been shown to play a key role to protect cells against free radicals (glutathione peroxidase), regulate energy use (tri-iodothyronine deiodinase) and modulating the intracellular redox potential (thioredoxin reductase) 473.
Jiang et al. 474 reported that monomethyl selenium could inhibit the transformation of healthy prostatic epithelial cells into cancerous cells due to different effects including, decreased cell proliferation, apoptosis, and inhibition of angiogenesis. Selenium, administered in the form of inorganic salt (sodium selenite) or organic compound (methyl selenocysteine), was also shown to be effective to decrease the density of blood capillaries in mammary cancer in rats 475. However, the biomolecular and biochemical mechanisms behind this experimental evidence still remain unclear. In general, selenium is thought to be associated with: (i) decreased VEGF secretion by cancer cells; and (ii) direct apoptosis of the endothelial cells via inhibition of matrix metalloproteinase (MMP) activity 475. It is reasonable to hypothesize that both effects may be due to Se-induced redox regulation of the activity of transcription factors, or redox modification of the functional state/activity of redox-sensitive enzymes and proteins. In fact, VEGF secretion is stimulated by hypoxic conditions through HIF-1 and activator protein-1 (AP-1) 476, the activity of which are both redox regulated 477. Therefore, it is possible that selenium inhibits hypoxia-induced VEGF expression by modulating the redox state of thioredoxin, a critical redox mediator for HIF-1 and AP-1, via its effect on the selenoprotein, thioredoxin reductase 478. Cell culture studies revealed that AP-1 was significantly inhibited by exposure to high levels of selenium, which binds to cysteine residues forming Se–S mixed disulfides or selenotrisulfides, thereby causing a conformational change in the protein 479, 480.
At present, there are few studies dealing with Se-containing nanomaterials for biomedical applications related to angiogenesis. Aksakal and Boccaccini 481 demonstrated the feasibility of depositing a selenium coating on metallic implants using electrophoretic deposition (EPD). EPD was used in that study to produce selenium coatings with thickness in the range of tens of micrometers, although the method is potentially suitable to obtain thin nanoscale coatings. The difficulty of obtaining stable selenium powder suspensions using a mixture of sulfuric acid, ethanol, and distilled water for EPD procedures needs to be overcome.
Selenium/PLGA composite nanoparticles were incorporated in 45S5 Bioglass® foams by Stevanovic et al. 482 who were interested in the antibacterial properties of the scaffolds, rather than investigating the effects on angiogenesis. Furthermore, selenium was incorporated in mesoporous bioactive glasses (MBGs) to be used as a carrier for the controlled release of doxorubicin, an anticancer drug 483. However in this case, the anti-angiogenic potential of selenium was not investigated, and thus the possible multifunctional properties of Se-doped MBGs (Se-induced antiangiogenic effect + anticancer drug effect) still remain to be fully determined.
11.14. Silicon
The available literature suggests that the effects of pure silica and silicate nanomaterials on angiogenesis strongly depend on the form/embodiment in which they are used. Duan et al. 484 reported that silicon oxide (silica) nanoparticles could induce dose- and time-dependent cytotoxicity through the production of ROS and generation of oxidative stress, thereby inhibiting angiogenesis and inducing apoptosis of endothelial cells. Specifically, the presence of silica nanoparticles interferes with the formation and development of the heart in zebrafish embryos by inhibiting the activation of ERK1/2 and VEGFR-2, and by down-regulating the expression of homeoboxprotein NKX-2.5 and myocyte-specific enhancer factor 2C. The same research group studied the role of ultrafine silica particulates (size <100 nm) in inducing heart ischemia and cardiovascular disease in mice 485. They synthesized pure-silica nanoparticles (average particle size 62 nm) by the Stober method, and observed that the cardiovascular toxicity triggered by the injected nanoparticles occurred mainly in the vascular endothelium rather than cardiomyocytes. The SiO2 nanoparticles were able to disrupt the cell cytoskeletal organization, activate endothelial cell autophagy, cause mitochondrial damage, and partially inhibit the expression of cell-adhesion biomolecules. As a result, the endothelial cell homeostasis was disturbed and angiogenesis was eventually impaired. Cardiovascular toxicity was associated with SiO2 nanoparticle-induced VEGFR2/PI3K/Akt/mTOR and VEGFR2/MAPK/Erk1/2/mTOR signaling pathways; crosstalk was also observed between the VEGFR2-mediated autophagy signaling pathway and the angiogenesis signaling pathway (Fig. 19).
Fig. 19.
Schematic model of the molecular mechanisms on VEGFR2-mediated crosstalk between autophagy and angiogenesis signaling pathways triggered by silica nanoparticles (SiNPs).
The toxicity was related to the persistence of the insoluble (or poorly soluble) pure silica nanoparticles in contact with cells and tissues, and could thus be supposed to be related to “nano-shape aspects.” The situation is markedly different when SiO2-based materials are biodegradable and thus, undergo progressive dissolution in contact with biological fluids, thereby releasing silicate ions with biological and biochemical significance. Silicate ions delivered from calcium silicate bioceramics (concentrations within 0.7–1.8 μg/mL) were shown to play a key role in stimulating angiogenesis in co-cultures of human dermal fibroblasts (HDFs) and HUVECs 486. Specifically, calcium silicate extracts stimulated VEGF expression in HDFs, and enhanced the expression of VEGF receptor 2 in HUVECs. Angiogenesis was initiated by the activation of endothelial nitric oxide synthase and nitric oxide production in these co-cultures. The expression of vascular endothelial cadherin in co-cultured HUVECs was up-regulated and was concentrated at the cell junctions to facilitate endothelial tubule formation. A similar pro-angiogenic mechanism was observed in vitro by the same research group 455 when human aortic endothelial cells were cultured with dissolved extracts from akermanite, containing silicate, Ca2+, and Mg2+ ions. Furthermore, akermanite promoted neo-vascularization after 2 and 4 months in vivo after being implanted in a rabbit femoral condyle model.
Silica can also be used as a network-forming oxide in the production of silicate bioactive glasses, which are highly versatile candidates for producing tissue-engineering implants. The pro-angiogenic potential of bioactive glasses has been comprehensively discussed by Kargozar et al. in a recent review 466.
In general, the size of the glass particles plays a role in evoking the angiogenic response, as the higher the specific surface area, the more reactive the material, and hence more ion release. In order to address this issue, an international research team led by Boccaccini investigated the pro-angiogenic potential of poly(D, L-lactic acid) (PDLLA) scaffolds embedding 45S5 Bioglass® (45SiO2-24.5CaO-24.5Na2O-6P2O5 wt.%) particles produced either by conventional melt-quenching (0.1–25 μm) or by flame synthesis (35–40 nm) 487. The in vitro experiments using human colon (CD-18CO) fibroblasts showed that composite scaffolds containing 20 wt.% of micro- or nano-sized glass increased the expression level of VEGF up to 5 times in comparison to pure PDLLA. These results were confirmed by in vivo studies (rats) that revealed higher vascularization and blood vessel-to-tissue percentage in glass-filled PDLLA. The percentage of newly-formed blood vessels was 37% and 78% higher in the scaffolds with micrometric and nanometric glass particles, respectively, as compared to the controls at 8 weeks postoperative.
The same particle size-dependent trend for glasses on angiogenesis was observed at larger size scales. Detsch et al. 488 reported that smaller particles (1–2 mm) of melt-derived S53P4 glass (53SiO2-23Na2O-20CaO-4P2O5 mol.%) stimulated a higher secretion of VEGF in human CD-18CO fibroblasts when compared to larger particles (2–3.15 mm). The pro-angiogenic potential of nano-sized sol-gel bioactive glasses 58S (58.2SiO2-32.6CaO-9.2P2O5 wt.%) and 80S (80SiO2-15CaO-5P2O5 wt.%) was investigated in vitro by Mao et al. 489, who reported that both materials could accelerate endothelial cell migration and up-regulate the expression of VEGF and bFGF), which resulted in enhanced tubule formation.
The dependence of the pro-angiogenic effect on glass concentration was reported by Day et al. 490, who seeded fibroblasts on 45S5 Bioglass®-coated polymeric implants and observed that VEGF secretion was suppressed at high glass concentrations (>0.1 wt.%). This early evidence was further confirmed by other experimental studies 491 and the concentration is now considered to be a key factor for designing therapeutic bioactive glasses, because the same glass formulation can have different effects (angiogenic at relatively low dosage or osteogenenic at relatively high dosage) depending on the concentration of glass particles at the implantation site.
High doses of silicate material can also induce cytotoxicity due to increased concentration of ionic dissolution products, which may also increase the pH of the culture medium, producing excessive alkalinity to allow cell survival 492. This trend was also observed in vivo, where it was shown that the volume of blood vessels formed at the defect site around silica/collagen composite implants was inversely proportional to the biomaterial volume 493. It cannot be ignored that, when multicomponent SiO2-based bioactive glasses are used, it is impossible to separate the biological effects of silicate ions from those of the other ions released from the material: therefore, the beneficial or adverse effects observed could be the result of a synergistic combination of the various ions. It is also worth underlining that, although silicate ions can indeed elicit a pro-angiogenic effect on their own, incorporation of metallic dopants with more potent angiogenic effects into SiO2-based glass matrices, especially mesoporous silica nanostructures 494 may be a better strategy to promote vascular sprouting.
11.15. Silver
Silver is well known for its antimicrobial properties both in the form of free ions and nanoparticles 495. Some recent studies have also demonstrated the activity of silver nanoparticles to modulate angiogenesis; however, the picture is still incomplete, and the mechanisms involved are yet to be fully understood.
On one hand, it was reported that silver nanoparticles (average size 500 nm) biosynthesized in Bacillus licheniformis biomass can inhibit the proliferation and migration of bovine retinal endothelial cells supplemented with exogenous VEGF, as well as micro-vessel formation in mice 496, 497 (Fig. 20). The inhibitory effect on angiogenesis was attributed to the inactivation of the PI3K/Akt signaling pathway by the metallic nanoparticles (see Fig. 21). This was confirmed using biosynthesized silver nanoparticles with a smaller size (average diameter 16.5 nm) in a chick CAM model 498. These experimental findings suggest the potential of silver nanoparticles for treating diseases where suppressing pathological angiogenesis is a goal, such as age-related macular degeneration.
Fig. 20.
Anti-angiogenic activity of Ag nanoparticles in vivo (rat model). Top panel: Gross photographs of Day 7 Matrigel implants with skin vessel background. Representative figures show (a) Streptozotocin without Ag nanoparticles, (b) Streptozotocin + Ag nanoparticles. Bottom panel: Histologic sections and hematoxylin and eosin-stained cross-sections showing representative photographs obtained from the sections of retina stained by hematoxylin and eosin in rats (c,d). Significant differences from control group were observed (p < 0.05). Reproduced with permission from (Biomaterials, 2009, 30, 6341–6350), Copyright 2009, Elsevier Ltd.
Fig. 21.
Schematic representation of the effect of AgNPs on angiogenesis process in cancer cells. AgNPs could enter the cells and prevent HIF-1α accumulation in the cytoplasm, followed by the suppression of HIF-1 target gene expression such as VEGF.
On the other hand, silver nanoparticles (diameter below 100 nm) produced using plant extracts from Azadirachta indica were suggested as potential agents for stimulating in vivo angiogenesis, since they could stimulate the closure of thermally-induced wounds in rats 499. Specifically, the wounds decreased in size over time, achieving closure at 2 weeks in healthy mice and at 3 weeks in diabetic animals. However, the pro-angiogenic effect proposed to be elicited by silver nanoparticles was questionable in this study, as no clear evidence was provided. On the contrary, it could be suggested that wound healing was actually favored by the antimicrobial properties of Ag+ ions, which may have played a predominant role that overcame the anti-angiogenic (i.e., anti-healing) effect of the nanoparticles.
A recent study showed that poly(vinyl pyrrolidone)-coated silver nanoparticles (average size 2.3 nm) exhibited pro-angiogenic properties both in vitro and in vivo (mouse model) 500. In fact, polymer-coated nanoparticles induced tube formation by endothelial cells, generation of ROS, and production of angiogenic factors like VEGF and nitric oxide (NO). From a biomolecular viewpoint, the silver nanoparticles promoted the activation of FAK, Akt, ERK1/2, and p38, which are all involved in the VEGFR-mediated signaling pathway. Silver-induced angiogenesis has also been observed to occur in vivo around melanomas in mice. The pro-angiogenic effect observed in this study could be attributed to the presence of the surface polymer coating; a size effect could also play a role as the silver nanoparticles were significantly smaller than those used in other studies reporting an anti-angiogenic effect, but this issue remains to be elucidated.
11.16. Sulfur
Sulfur is delivered to humans via normal nutrition, for example by allium vegetables which are known to possess antioxidant, anti-inflammatory and even anticancer effects 501. S-containing compounds may be used as drugs in medical applications as they have pro- or anti-angiogenic properties depending on the type and concentration of the specific molecules. H2S was reported to directly increase endothelial cell migration and growth as well as the formation of tubular structures in vitro 502, 503. NaHS and Na2S promote significant angiogenesis in vitro via the release of H2S that activates the KATP channel/MAPK pathway 504, and NaHS was also found able to improve regional blood flow in mice 505. Heparan sulfate proteoglycans facilitate cell signaling by acting as co-receptors for key pro-angiogenic factors like bFGF and VEGF 506. On the contrary, high-molecular-weight sulfonated polysaccharides show promise as anti-angiogenic agents in anti-cancer treatment as they can carry out metal chelation, or a competitive process (which is still unclear) with heparan sulfate proteoglycans, thereby inhibiting tubule formation 507.
A novel nanotechnology approach involving sulfur was recently published by Cacciotti et al. who functionalized electrospun poly(lactic acid) nanofibrous patches (average diameter of the fibers around 700 nm) with organosulphur compounds extracted from garlic. These low-cost H₂S-releasing membranes could find potential applications in various biomedical sectors, primarily as wound dressings, to combat oxidative stress in cells and improve tissue regeneration, although their specific pro-angiogenic properties still remain to be confirmed.
11.17. Terbium
Apart from europium, terbium is another element of the class of lanthanides which has recently received attention in the context of therapeutic angiogenesis. Zhao et al. reported the synthesis of Tb(OH)3 nanorods and spherical nanoparticles via hydrothermal method and observed that their pro-angiogenic properties, as well as their ROS-mediated mechanism of action, are analogous to those of Eu(OH)3 nanomaterials in a zebrafish model 412.
Following its own previous studies on Eu(OH)3 nanorods, Patra’s group 508 reported the synthesis of Tb(OH)3 nanorods assisted by microwave irradiation. These nanorods exhibited pro-angiogenic properties in vitro towards endothelial cells (HUVECs and EA.hy926) as well as the ability to stimulate blood vessel growth in the chick CAM in vivo assay. The authors tried elucidating the biochemical mechanism behind angiogenesis and assessed that Tb(OH)3 nanorods stimulated NOX-mediated generation of ROS, which then activated the PI3K/Akt/MAPK signaling cascade: this resulted in the formation of intracellular NO, which is a key signaling molecule for angiogenesis 509. Enhanced wound healing induced by Tb(OH)3 nanorods was also observed in a punch biopsy mouse model.
11.18. Titanium
Titanium has been widely used for the production of load-bearing orthopedic and dental implants for decades due to its high biocompatibility and good mechanical properties 510, 511. Despite may studies published on this topic, the biophysical, biochemical and biomolecular mechanisms behind the activity of titanium-based materials on angiogenesis are still to be fully understood 512. In general, it was shown that titanium implants could be favorable to angiogenesis under certain circumstances (high hydrophilicity and various micro-/nanostructures), whereas titanium oxide (TiO2, titania) nanoparticles typically exert an anti-angiogenic effect.
It is well known that the surface characteristics (e.g., ionic charge, chemistry, topography) of implantable biomaterials are key to determining the biological response of cells and tissues. The effect of the surface properties of titanium and titanium alloys in the context of bone tissue engineering has been recently reviewed by Spriano et al. 513. As regards angiogenesis, it was shown that titanium surfaces with high surface energy and micro-roughness can promote the secretion of pro-angiogenic growth factors (primarily VEGF) by osteoblasts, as well as the migration and differentiation of human aortic endothelial cells cultured in contact with titanium implant extracts 514. These results are consistent with other in vitro studies showing that hydrophilic titanium surfaces increased the absorption of plasma fibronectin 515, improved osteoblast differentiation and up-regulated osteoblast-related growth factors 516. The micro-/nano-roughened surface of titanium dental screws was also found to promote osteointegration and neovascularization in vivo 517.
The role played by hydrophilicity and surface topography is more complex regarding endothelial cells. Shi et al. 518 reported that the expression levels of some angiogenesis-related proteins (e.g., EPCR and E-selectin) were higher when osteoblast/endothelial cells were co-cultured on a hydrophobic smooth titanium surface compared to a rougher one. The favorable effect of the smooth surface was explained by the tendency of endothelial cells to spread and attach onto smooth biological surfaces typical of blood vessels. However, these findings were not consistent with the results reported by Au et al. 519 using only HUVECs, which revealed a higher expression of pro-angiogenic genes when cultured on hydrophilic rough titanium surfaces. This apparent inconsistency can be explained taking into account that the cell behavior might be affected by cell-cell interactions and cross-talk in the co-culture experiment 518. In fact, many other authors have observed an increase of VEGF secretion by endothelial progenitor cells in contact with micro-rough titanium surfaces, which were also capable of accelerating vascularization in human patients 520–522. Endothelial progenitor cells can affect neovascularization by secreting paracrine factors (e.g. cytokines and VEGF) and forming a primary cell network after differentiating into endothelial cells or perivascular supporting cells. Ziebart et al. 522 showed that rough titanium surfaces promoted an undifferentiated rounded phenotype with low proliferation rate and lower endothelial nitric oxide (NO) synthase and/or inducible NO synthase activities; however, these cells showed a high rate of VEGF secretion, thus eventually promoting angiogenesis.
Comparison between different studies is difficult due to the differing experimental conditions used, cell types and titanium topographies, which prevent firm conclusions from being drawn at this stage. Functionalization strategies have also been recently carried out on titanium implants to impart them with clear pro-angiogenic properties. For example, Chen et al. 523 modified the surface of titanium substrates by depositing a composite coating (nanofibers of chitosan-catechol, gelatin, and hydroxyapatite) that improved angiogenesis in vitro and in vivo; of course, this effect cannot solely be attributed to titanium in itself.
Doping titanium-based surfaces with other metals having a potent pro-angiogenic (e.g., copper) is another interesting approach recently reported by Zong et al. 524, who applied an anodization treatment through magnetron sputtering to TiCu layers previously deposited on pure titanium foils. The resulting Cu-doped titania-based nanotubular surfaces were capable of up-regulating VEGF secretion by endothelial cells as compared to Cu-free titania nanotubes due to the release of Cu2+ ions. On the contrary, pure titania nanoparticles were found to exert an anti-angiogenic effect apparently associated with their specific ability to inhibit the angiogenic processes, rather than to a “general” nanosize-dependent cytotoxicity. Interestingly, Jo et al. 525 observed that titania nanoparticles inhibited VEGF-induced tube formation and migration of human retinal microvascular endothelial cells via the suppression of VEGFR2 and MAPK. This property potentially suggests therapeutic applications in which the suppression of angiogenesis is a goal, including age-related macular disease and tumor treatment.
A very recent study by Augustine et al. 526 showed that, interestingly, titania nanorods produced via hydrothermal treatment exhibit a pro-angiogenic effect. This finding was assessed both in a CAM model and in rats, where electrospun PCL meshes loaded with titania nanorods provided a faster wound healing compared to bare PCL fibres.
Hence, the comparison between the above-mentioned studies suggests a “shape effect” related to nano-titania (pro-angiogenic nanorods vs. anti-angiogenic spherical nanoparticles), which deserves to be more comprehensively investigated in the future.
11.19. Yttrium
Yttrium oxide (Y2O3) has been used for decades to stabilize the tetragonal phase of zirconia in ceramic components for joint prostheses 527. More recently, Y2O3 nanoparticles have been proved to have antioxidant and radical scavenging ability 528, which are of great interest in advanced tissue engineering applications. Investigation of the potential of Y2O3 nanoparticles in the specific context of angiogenesis is in its very beginning, but the existing evidence shows promise. Following the approach reported in their previous studies on nanoceria, Augustine et al. 529 produced Y2O3 nanoparticles using gelatin as a stabilizer and incorporated them in electrospun PCL scaffolds. An amount of 1 wt.% Y2O3 nanoparticles was found to be the most effective to promote the proliferation of fibroblasts (L-929) and osteoblast-like cells (UMR-106), as well as to support the highest blood vessel formation in a chick CAM model. Gene expression study following subcutaneous implantation in rats demonstrated that the presence of Y2O3 nanoparticles in the polymeric scaffolds could upregulate the expression of cell proliferation and angiogenesis-related biomolecules, such as VEGF and EGFR.
11.20. Zinc
Zinc is an essential element in human metabolism because Zn2+ ions are included in a number of proteins and are involved in many biological processes 530. Zinc can have anti- or pro-angiogenic effects depending on the form under which it is available, i.e., Zn2+ cations or zinc oxide (ZnO) nanoparticles, respectively. It was observed that the ability of Zn2+ ions to bind to endostatin is essential for the potent anti-angiogenic activity of this angiogenesis inhibitor that can convert malignant cancer cells into a “quiescent” tumor phenotype unable to induce angiogenesis, and thus unable to grow 531. The effect of zinc on cancer cells was also related to a conformational change in the p53 protein, resulting in halting the progression of cancer cell mitosis and promoting cell death. As regards the effect of Zn2+ ions on healthy cells, it was observed that zinc concentrations in the range of 10 to 500 μM neither inhibited nor stimulated the growth of HUVECs 530; however, zinc could enhance the proliferation of bovine aortic endothelial cells in the presence of exogenous bFGF, suggesting a role of zinc ions in amplifying the bFGF-dependent proliferation of the cells 532.
An in vitro study published by Shiah et al. 533 reported that the drug bis(diethylthiocarbamoyl) disulfide (disulfiram), which is used for treating alcoholism, could directly interact with MMP-2 and MMP-9, inhibiting their proteolytic activity through a zinc-chelating mechanism. As a result, reduction of angiogenesis was predicted using disulfuram in vivo, with therapeutic benefits in cancer treatment. Zinc is also capable of reversing the expression of many genes modulated by hypoxia, thus reducing the activity of HIF-1 with an associated decrease in VEGF secretion and reduction of angiogenesis 534, 535.
On the contrary, pro-angiogenic effects were observed in vitro and in vivo when zinc was used in the form of ZnO nano-sized structures like nanoparticles 536 and nanoflowers 537 (Fig. 22), alone or embedded in a polymeric scaffold (e.g. electrospun poly(vinylidene fluoride-trifluoroethylene) fibres 538). In both cases, generation of ROS by the ZnO nanomaterials resulted in upregulation of bFGF and VEGF, which ultimately led to improved vascularization.
Fig. 22.
The possible molecular mechanisms and signaling pathways involved in zinc nanoflower-induced angiogenesis.
12. Nanoparticles for imaging of angiogenesis
Visualization of tumor angiogenesis provides invaluable information to assess the biologic aggressiveness and allow monitoring of tumor response to anti-angiogenic therapies. Scientists have taken advantage of nanotechnology for imaging of tumor angiogenesis since nanoparticles show several advantages, such as the ability to carry high payloads of diagnostic or imaging agents, with an increased signal-to-noise ratio, longer circulation times, and enhanced image contrast 58. Successful imaging of angiogenesis leads to gaining valuable information, which can be useful in determining the optimal dose and schedule of an anti-angiogenic therapeutic, as well as measuring early signs of tumor relapse/recurrence 543. Magnetic (e.g. Gd), fluorescent and radiolabeled nanoparticles (e.g., Tc-99m and I-123) have commonly been used to detect the angiogenesis process. Positron-emission tomography (PET), single-photon emission computed tomography (SPECT), computerized tomography (CT), magnetic resonance imaging (MRI), optical imaging, and ultrasounds imaging have all been investigated as techniques to measure the progress of angiogenesis in vivo 544–549. Targeting αvβ3 –integrin and VEGFR2 has been proposed for targeted imaging of tumor angiogenesis 550–553. The important parameters measured are microvessel density (the so-called “hot-spots”) and circulating markers of angiogenesis 543. The latter are comprised of soluble circulating protein markers such as angiogenic GFs and their receptors (e.g., VEGF), cell adhesion and ECM molecules (VCAM-1), circulating EPCs and their precursors 543. However, there are other biomarkers that are over-expressed on the tumor cell surface, which could be potential targets for detection of tumor angiogenesis. As an illustration, Wu et al. functionalized gold nanoparticles with a tumor-homing cyclized asparagine–glycine–arginine peptide (SH–cNGR) and carboxylpoly(ethylene glycol)thiol (SH–PEG–COOH) via Au–S bonds to target the aminopeptidase-N (APN/CD13) over-expressed on the endothelium of tumor angiogenesis 554. CT imaging and immunohistochemistry results showed that the surface-functionalized nanoparticles showed significantly higher and faster tumor uptake post-intravenous injection in comparison to unmodified samples. The authors suggested that this nano-based imaging system could be a promising contrast agent for targeted angiogenesis imaging using CT.
Organic based nanoparticles have also been used to detect angiogenesis in vivo. Ryu et al. developed a HSA-based nanoprobe for non-invasive optical imaging of MMP activity in a small rodent hindlimb ischemia model 555. For this aim, the authors covalently conjugated MMP-specific fluorogenic peptide probes to HSA for preparation of self-quenched MMP eHSA nanoprobes 36 nm in diameter. It has been proven that MMP-2 and MMP-9 are two essential mediators in the angiogenesis process. The nanoprobes showed enhanced fluorescence emission in the presence of MMP-2 and MMP-9 without any cytotoxicity. Moreover, the authors showed longer blood circulation half-life for this system compared to control groups after intravenous injection in a mouse hindlimb ischemia model, as well as successful optical imaging of MMP activity during angiogenesis. It should be mentioned that the use of viral nanoparticles (e.g., cowpea mosaic virus nanoparticles) as next-generation imaging agents may be a new approach to non-invasive monitoring of angiogenesis, and could serve as suitable therapy and imaging probes 556–558.
Multimodality (e.g., bimodal, trimodal, and four-modal) imaging has emerged as an approach for imaging of the angiogenesis process due to its potential to provide complementary information that can be co-registered 559–561. For instance, lanthanide-based nanoprobes have been used for imaging of tumor angiogenesis thanks to their favorable optical, magnetic, radioactive, and X-ray attenuation properties. In 2013, Sun et al. optimized core-shell lanthanide upconversion nanophosphors with an enhanced imaging ability for tumor angiogenesis in small animals (mice) 562. NaLuF4: Yb,Tm nanocrystals and 153Sm3+ doped NaGdF4 were the core and the shell of the nanophosphors, respectively. The recorded lifetime for upconversion luminescence (UCL) was 1044 μs at 800 nm, and its relaxation rate (1/T1) was 1044 μs and 18.15 s−1 .mM−1. The detailed information obtained by four-modal imaging techniques, i.e., X-ray, CT, MRI, and SPECT (see Fig. 23), showed the effectiveness and applicability of this system for monitoring tumor angiogenesis in vivo.
Fig. 23.
The use of NaLuF4: Yb,Tm@NaGdF4(153Sm) for four-modal imaging of tumor-bearing nude mice at 60 min post intravenous injection. A-D represent images obtained from upconversion luminescence (UCL), X-ray CT, SPECT, and MR of tumor, respectively. E exhibits UCL confocal image of the paraffin section of tumor tissue, and F is actually a schematic illustration of tumor angiogenesis imaging by applying the nanoparticles as the probe. Reproduced from (ACS Nano, 2013, 7, 11290–11300), Copyright 2013, American Chemical Society.
Carbon-based nanoparticles (e.g., nanographene oxide and quantum dots) are other promising candidates for targeted angiogenesis imaging in vivo 563, 564. The use of quantum dots for in vivo imaging purposes was first reported by Dubertret and colleagues in 2002 565. Today, paramagnetic QD-micelles have been used for MR and optical-based molecular imaging in vivo 566–568. QDs in pristine and modified forms were suggested to be tools in multimodal molecular imaging of tumor angiogenesis 569, 570.
13. Summary and future perspectives
Because angiogenesis is indispensible for tumor growth (beyond a certain relatively small size) and also for cancer metastasis which is the leading cause of cancer death, therefore, inhibiting angiogenesis has been an important part of cancer therapy in the clinical setting for some decades. VEGF signaling pathway plays a central role in both physiological and pathological processes of angiogenesis; therefore, its regulation has been considered to be one of the main targets in cancer therapy 571. Recent studies have revealed the critical role of angiogenesis in the failure of initially successful immunotherapy approached to solid tumors. Therefore combining antiangiogenic therapy with modern immunotherapy has been proposed to improve the effectiveness of immunotherapy and also to diminish the risk of autoimmune-related adverse effects.
Many cancers could be resistant to anti-angiogenic approaches targeting the VEGF signaling pathway either inherently or by acquired resistance. In the case of acquired resistance, cancer cells upregulate different pro-angiogenic molecules which might be connected with the genetic instability of cancers 572. Also, this resistance could be related to the ability of cancer cells to receive nutrients from existing adjacent blood vessels 573. The invention of nanotechnology has opened up new horizons in many areas of biomedical science, and inhibiting angiogenesis using different types of nanoparticles is now a promising approach especially in the case of resistant cancers. Up to now, a large number of studies have been published dealing with nanoparticles that inhibit angiogenesis. Better outcomes may be obtained when organic and inorganic nanoparticles are combined. There has been a series of anti-angiogenic nano-sized drugs (e.g., humanized anti-VEGF monoclonal antibody Avastin™) gaining marketing approval to treat various types of cancer 18, 29. In addition, it should be mentioned that many attempts are being made to develop nano-sized particles incorporating previously used anti-angiogenic commercial drugs, as well as substances originating from herbs and phytochemicals. The high efficacy of Taxol® (generic name paclitaxel) nanoparticles has been reported in several studies 207, 574. The use of nano-based DDSs (e.g., lipid-based and carbon-based nanosystems) provide another possibility for researchers to take advantage of nanotechnology to inhibit angiogenesis in cancers both in vitro and in vivo. Doxil®, Myocet™, Lipo-dox®, DaunoXome®, and Marqibo® are well-known examples of FDA-approved nanosystems containing drugs with additional anti-angiogenic properties, which are currently used in cancer therapy. Targeted therapy of angiogenesis by surface-modified nanoparticles and nanosystems is currently under investigation to improve the efficacy of anti-angiogenic based cancer therapy. The anti-angiogenic effect of nanoparticles depends on many factors, including their size and shape 575. Although there are several reported in vitro and in vivo experiments concerning the size effects of nanoparticles on angiogenesis, some conflicting results make it difficult to draw firm conclusions about this parameter 542, 576, 577. However, the use of smaller particles is generally suggested to improve the results of anti-angiogenesis based cancer therapy. It is worth underlining that metallic and metal oxide nanoparticles can exhibit either pro-angiogenic or anti-angiogenic properties depending on various parameters, including not only the size but also the surface properties (e.g. wettability, charge) and effective concentration. All these factors were shown to be key determinants for the production of ROS, which have a deep impact on the angiogenic properties of nanomaterials 578. In the last decade, redox signalling-based nanomedicine has indeed emerged as a fascinating approach for the treatment of angiogenesis-related diseases, where nano-sized materials may promote angiogenesis via the controlled production of ROS or antiangiogenesis by triggering excessive ROS formation 579–581. A generally-valid “set of rules” on how ROS production can be actually controlled does not exist, also considering the high number of factors involved (e.g. type of nanomaterial, shape, size, concentration, specific environment/model etc.) and their interlocking – which may even be unpredictable: hence, the need for detailed and individual studies on every material/system embedding nanoparticles is recommended before exploitation for therapeutic purpose.
Imaging and monitoring of angiogenesis by nanotechnology-based probes may lead to faster and more accurate diagnosis of cancer progression; while multimodality imaging of angiogenesis for instance using lanthanide-based nanoprobes has shown good efficacy.
The issue of toxicity of nanoparticle-based molecules, chemicals, and drugs requires to be carefully evaluated; some nanoparticulate elements with the ability to modulate angiogenesis (e.g., arsenic, lead, and mercury) have severe toxicity to mammalian cells and are never used in therapeutic applications. Moreover, the selection of solvents and reagents used for preparation of nanoparticles is of great importance when aiming to use them in the human body. Green chemistry offers better approaches to reduce toxicity and also to improve the stability of nanomaterials 582, 583.
On the other side, improving angiogenesis is critical in wound healing, tissue engineering and reconstructive strategies, since it can facilitate the growth and repair of damaged tissues and organs 466. A large number of studies have investigated the pro-angiogenic potential of various organic and inorganic nanomaterials both in vitro and in vivo. The results obtained so far can be regarded as quite promising, and much attention has been given to this direction. The critical issues for the use of nanotechnology in promoting angiogenic strategies are similar to the above-mentioned factors affecting anti-angiogenic strategies.
One interesting theme that has emerged in serveral of the approaches covered in this review, is the bimodal effects of many nanostructures, that can stimulate angiogenesis at low doses or concentrations, while the same material can inhibit angiogenesis at higher doses or concentrations. This may even allow the same preparation to be used for opposite goals at different doses depending on the disease or condition to be treated.
Taken together, nanotechnology has had and will continue to have a major impact on the therapeutic tools and imaging approaches targeting the neovascularization process in both pro-angiogenic and anti-angiogenic strategies. In addition, the quality of recorded images of angiogenesis process is really improved by applying nanotechnology-based methods. Based on the current knowledge of nanotechnology, it can be assumed that novel chemical formulations will be invented and developed into different formats (e.g., small molecules) affecting angiogenesis in a more effective manner, even in the case of cancer resistances.
Acknowledgments
Funding and conflicts of interest
MRH was supported by US NIH Grants R01AI050875 and R21AI121700. MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiThera Inc, Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc, Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX; Mitonix, Newark, DE. The other authors declare there are no conflicts of interest.
Abbreviations in alphabetic order
- ABIN-2
A20-binding inhibitor of NF-kappaB 2
- AKT
Protein kinase B (PKB)
- Ang
Angiopoietin
- BAD
Bcl-2 associated agonist of cell death
- BAX
Bcl-2-associated X protein
- BC
Breast cancer
- Bcl-2
B-cell lymphoma 2
- bFGF
Basic fibroblast growth factor
- BMSCs
Bone marrow mesenchymal stem cells
- BSA
Bovine serum albumin
- CAM assay
Chick Chorioallantoic Membrane Assay
- CA4
Combretastatin A-4
- CML
Chronic myeloid leukemia
- COX
Cyclooxygenase
- COX-2
Cyclooxygenase-2
- CNTs
Carbon nanotubes
- CPT
20-(S)-Camptothecin
- CRC
Colorectal cancer
- CSF-1
Colony stimulating factor 1
- CSFR1
Colony stimulating factor 1 receptor
- CT
Computerized tomography
- DAG
Diacylglycerol
- DDSs
Drug delivery systemes
- EC
Endothelial cells
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EGCG
Epigallocatechin gallate
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial–mesenchymal transition
- eNOS
Endothelial nitric oxide synthase
- EOC
Epithelial ovarian cancer
- EPC
Endothelial progenitor cell
- EPCR
Endothelial cell protein C receptor-dependent
- ERK
Extracellular-signal-regulated kinase
- EPR
Enhanced permeability and retention
- EP1
Prostaglandin E2 receptor 1
- ERK
Extracellular signal-regulated kinase
- FAK
Focal adhesion kinase
- FDA
Food and drug administration
- FGF
Fibroblast growth factors
- FGFR1
fibroblast growth factors 1
- FL
Follicular lymphoma
- FLT-3
Fms-like tyrosine kinase 3
- GAC
Gastric adenocarcinoma
- GBM
Glioblastoma
- GEJAC
Gastroesophageal junction adenocarcinoma
- GF
Growth factor
- GIST
Gastrointestinal stromal tumor
- GLUTs
Glucose transporters
- HBP
Heparanase-binding protein
- HB-GFs
Heparin-binding growth factors
- HCC
Hepatocellular carcinoma
- HER2
Human epidermal growth factor receptor 2
- HIF-1α
Hypoxia-inducible factor 1-alpha
- hMSCs
Human mesenchymal stem cells
- HREs
Hypoxia-responsive elements
- HSA
Human serum albumin
- Hsp90
Heat shock protein 90
- HUVEC
Human umbilical vein endothelial cells
- H1R
Histamine receptor 1
- IPF
Idiopathic pulmonary fibrosis
- IGFs
Insulin-like growth factors
- IL-6
Interleukin 6
- IL-8
Interleukin 8
- iNGR
CRNGRGPDC peptide
- JNKs
c-Jun N-terminal kinases
- KATP channel
ATP-sensitive potassium channel
- LRP
Lipoprotein receptor-related protein
- MAPK
Mitogen-activated protein kinase
- MBGs
Mesoporous bioactive glasses
- MCL
Mantle cell lymphoma
- MDS
Myelodysplastic syndromes
- MEK
Mitogen-activated protein kinase kinase
- MM
Multiple myeloma
- MMPs
Matrix metallopeptidases
- MRI
Magnetic resonance imaging (MRI)
- MTC
Medullary thyroid cancer
- mTOR
Mammalian target of rapamycin
- MWCNTs
Multi-walled carbon nanotubes
- MZL
Marginal zone lymphoma
- NETs
Pancreatic neuroendocrine tumors
- NO
Nitric oxide
- NPs
Nanoparticles
- NSCLC
Non-small cell lung cancer
- NF-Κb
Nuclear factor kappa-light-chain-enhancer of activated B cells
- OPN
Osteopontin
- OVA
Ovalbumin
- PAC
Polyacrylic acid
- PAMAM
Polyamidoamine
- PC
Pericyte
- PCL
Polycaprolactone
- PDGF
Platelet-derived growth factor
- PDGFRα
Platelet-derived growth factor receptor α
- PDGFRβ
Platelet-derived growth factor receptor β
- PDLLA
Poly(D, L-lactic acid)
- PEG
Polyethylene glycol
- PEI
Polyethyleneimine
- PEO
Poly(ethylene oxide)
- PET
Positron-emission tomography
- PGE2
Prostaglandin E2
- PHD
Proline hydroxylases
- PIGF
Placenta growth factor
- PIP2
Phosphatidylinositol biphosphate
- PKC
Protein Kinase C
- PLA
Poly(D, L-lactic acid)
- PLC
Phospholipase C
- PLGA
Poly(lactic-co-glycolic acid)
- PPO
Poly(p-phenylene oxide)
- PTEN
Phosphatase and tensin homolog
- Ph+ALL
philadelphia chromosome positive acute lymphoblastic leukemia
- pVHL
Von Hippel-Lindau tumor suppressor protein
- RCC
Renal cell carcinoma
- RET
Glial cell-line derived neurotrophic factor receptor
- RGD
Arginine (Arg)- Glycine (Gly)- Aspartic acid (Asp)
- ROS
Reactive oxygen species
- rGO
Reduced graphene oxide
- SEDDSs
Self-emulsifying drug delivery systems
- SEM
Scanning electron microscope
- SLNs
Solid-lipid nanoparticles
- SMCs
Smooth muscle cells
- SMEDDS
Self-micro-emulsifying agents
- Sox2
Sex determining region Y-box 2
- SPECT
Single-photon emission computed tomography
- SPIONs
Superparamagnetic iron oxide nanoparticles
- STS
Soft tissue sarcoma
- SWCNTs
Single-wall carbon nanotubes
- SWNH
Single-walled carbon nanohorn
- TC
Thyroid cancer
- TEM
Transmission Electron Microscope
- TEOS
Tetraethyl orthosilicate
- TGF
Transforming growth factor
- TIE2
Tyrosine-protein kinase receptor for ANGPT1–2 and 4
- TKIs
Tyrosine kinase inhibitors
- TNF
Tumor necrosis factor
- TNP-470
O-(chloroacetylcarbamoyl)fumagillol
- Ub
ubiquitin
- VCAM
Vascular cell adhesion molecule 1
- VEGF
Vascular endothelial growth factor
- VEGFR1
Vascular endothelial growth factor 1
- VEGFR2
Vascular endothelial growth factor 2
- VEGFR3
Vascular endothelial growth factor 2
- VPF
Vascular permeability factor
- 5-HT
5-hydroxytryptamine receptors
References
- 1.Jain RK, Nature medicine, 2003, 9, 685. [DOI] [PubMed] [Google Scholar]
- 2.Conway EM, Collen D and Carmeliet P, Cardiovascular research, 2001, 49, 507–521. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P and Jain RK, Nature, 2000, 407, 249–257. [DOI] [PubMed] [Google Scholar]
- 4.Adams RH and Alitalo K, Nature reviews Molecular cell biology, 2007, 8, 464. [DOI] [PubMed] [Google Scholar]
- 5.Schito L and Semenza GL, Trends in cancer, 2016, 2, 758–770. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach R, Lewis R, Shinners B, Kubai L and Akhtar N, Clinical chemistry, 2003, 49, 32–40. [DOI] [PubMed] [Google Scholar]
- 7.Irvin MW, Zijlstra A, Wikswo JP and Pozzi A, Experimental Biology and Medicine, 2014, 239, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ucuzian AA, Gassman AA, East AT and Greisler HP, Journal of Burn Care & Research, 2010, 31, 158–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yunus M, Jansson PJ, Kovacevic Z, Kalinowski DS and Richardson DR, Biochimica et Biophysica Acta (BBA) - General Subjects, 2019, 1863, 1217–1225. [DOI] [PubMed] [Google Scholar]
- 10.El-Kenawi AE and El-Remessy AB, British journal of pharmacology, 2013, 170, 712–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribatti D, Leukemia research, 2009, 33, 638–644. [DOI] [PubMed] [Google Scholar]
- 12.Eikesdal HP, Sugimoto H, Birrane G, Maeshima Y, Cooke VG, Kieran M and Kalluri R, Proceedings of the National Academy of Sciences, 2008, 105, 15040–15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain RK, Nature medicine, 2001, 7, 987. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J, New england journal of medicine, 1971, 285, 1182–1186. [DOI] [PubMed] [Google Scholar]
- 15.Ronca R, Benkheil M, Mitola S, Struyf S and Liekens S, Medicinal research reviews, 2017, 37, 1231–1274. [DOI] [PubMed] [Google Scholar]
- 16.Jayson GC, Kerbel R, Ellis LM and Harris AL, The Lancet, 2016, 388, 518–529. [DOI] [PubMed] [Google Scholar]
- 17.Ellis LM and Hicklin DJ, Nature reviews cancer, 2008, 8, 579. [DOI] [PubMed] [Google Scholar]
- 18.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR, Journal of clinical oncology, 2012, 30, 2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P and Bamias A, Obstetrical & Gynecological Survey, 2014, 69, 402–404. [Google Scholar]
- 20.Bergers G and Hanahan D, Nature Reviews Cancer, 2008, 8, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiménez-Valerio G, Martínez-Lozano M, Bassani N, Vidal A, Ochoa-de-Olza M, Suárez C, García-del-Muro X, Carles J, Viñals F and Graupera M, Cell reports, 2016, 15, 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cascone T, Xu L, Lin HY, Liu W, Tran HT, Liu Y, Howells K, Haddad V, Hanrahan E and Nilsson MB, Clinical Cancer Research, 2017, 23, 5489–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D and Casanovas O, Cancer cell, 2009, 15, 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotink KJ, Broxterman HJ, Labots M, De Haas RR, Dekker H, Honeywell RJ, Rudek MA, Beerepoot LV, Musters RJ and Jansen G, Clinical Cancer Research, 2011, 17, 7337–7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SA and Minn AJ, Immunity, 2018, 48, 417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A and Moon JJ, Nature communications, 2018, 9, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong D-H, Kim M, Jang J, Na H-J and Lee S. J. I. j. o. m. s., 2017, 18, 1786. [Google Scholar]
- 28.Rajabi M and Mousa SJB, 2017, 5, 34. [Google Scholar]
- 29.Ferrara N, Hillan KJ, Gerber H-P and Novotny W. J. N. r. D. d., 2004, 3, 391. [DOI] [PubMed] [Google Scholar]
- 30.Braghiroli MI, Sabbaga J and Hoff P. M. J. E. r. o. a. t., 2012, 12, 567–580. [DOI] [PubMed] [Google Scholar]
- 31.Spratlin JL, Mulder KE and Mackey JRJFO, 2010, 6, 1085–1094. [DOI] [PubMed] [Google Scholar]
- 32.Poole RM and Vaidya AJD, 2014, 74, 1047–1058. [DOI] [PubMed] [Google Scholar]
- 33.Ciombor KK, Berlin J and Chan EJCCR, 2013, 19, 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaya A and Tse V. J. C. t. r., 2012, 38, 484–493. [DOI] [PubMed] [Google Scholar]
- 35.Shirley MJD, 2017, 77, 107–112. [Google Scholar]
- 36.Andrick BJ and Gandhi A. J. A. o. P., 2017, 51, 1090–1098. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Tortorici MA, Garrett M, Hee B, Klamerus KJ and Pithavala Y. K. J. C. p., 2013, 52, 713–725. [DOI] [PubMed] [Google Scholar]
- 38.Grüllich C, in Small Molecules in Oncology, Springer, 2014, pp. 207–214. [Google Scholar]
- 39.Zschäbitz S and Grüllich C, in Small Molecules in Oncology, Springer, 2018, pp. 187–198. [Google Scholar]
- 40.Wind S, Schmid U, Freiwald M, Marzin K, Lotz R, Ebner T, Stopfer P and Dallinger C. J. C. p., 2019, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verheijen RB, Beijnen JH, Schellens JH, Huitema AD and Steeghs N. J. C. p., 2017, 56, 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shamroe CL and Comeau J. M. J. A. o. P., 2013, 47, 1540–1546. [DOI] [PubMed] [Google Scholar]
- 43.Hoy SMJD, 2014, 74, 793–806. [Google Scholar]
- 44.Rimassa L, Pressiani T, Personeni N and Santoro A. J. E. r. o. a. t., 2017, 17, 567–576. [DOI] [PubMed] [Google Scholar]
- 45.Rini B. I. J. E. o. o. p., 2006, 7, 453–461. [DOI] [PubMed] [Google Scholar]
- 46.Debiec-Rychter MJNRCO, 2007, 4, 342. [DOI] [PubMed] [Google Scholar]
- 47.Fallahi P, Ferrari SM, Baldini E, Biricotti M, Ulisse S, Materazzi G, Miccoli P and Antonelli A. J. E. r. o. a. t., 2016, 16, 1109–1118. [DOI] [PubMed] [Google Scholar]
- 48.Zhu YX, Kortuem KM, Stewart AKJL and lymphoma, 2013, 54, 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu L, Payvandi F, Wu L, Zhang L-H, Hariri RJ, Man H-W, Chen RS, Muller GW, Hughes CC and Stirling D. I. J. M. r., 2009, 77, 78–86. [DOI] [PubMed] [Google Scholar]
- 50.Patard J-J, Pouessel D, Bensalah K and Culine S. J. W. j. o. u., 2008, 26, 135–140. [DOI] [PubMed] [Google Scholar]
- 51.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T and Franz D. N. J. N. E. J. o. M., 2010, 363, 1801–1811. [DOI] [PubMed] [Google Scholar]
- 52.Yina Y and Talapin D, Chemical Society Reviews, 2013, 42, 2484–2487. [DOI] [PubMed] [Google Scholar]
- 53.Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M and Hamblin MR, Nanomedicine, 2019, 14, 93–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kargozar S, Ramakrishna S and Mozafari M, Current Opinion in Biomedical Engineering, 2019, 10, 181–190. [Google Scholar]
- 55.Shi M, Xia L, Chen Z, Lv F, Zhu H, Wei F, Han S, Chang J, Xiao Y and Wu C, Biomaterials, 2017, 144, 176–187. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M and Jiang L, Journal of biomedical nanotechnology, 2016, 12, 1975–1986. [DOI] [PubMed] [Google Scholar]
- 57.Kargozar S, Mozafari M, Hamzehlou S, Kim H-W and Baino F, Materials Letters, 2019, 251, 241–246. [Google Scholar]
- 58.Banerjee D, Harfouche R and Sengupta S, Vascular cell, 2011, 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paris JL, Villaverde G, Gómez-Graña S and Vallet-Regí M, Acta biomaterialia, 2020, 101, 459–468. [DOI] [PubMed] [Google Scholar]
- 60.Danhier F, Le Breton A and Préat V. r., Molecular pharmaceutics, 2012, 9, 2961–2973. [DOI] [PubMed] [Google Scholar]
- 61.Graf N, Bielenberg DR, Kolishetti N, Muus C, Banyard J, Farokhzad OC and Lippard SJ, ACS nano, 2012, 6, 4530–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majumder P, Bioengineering, 2018, 5, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rüegg C and Alghisi GC, in Angiogenesis Inhibition, Springer, 2010, pp. 83–101. [Google Scholar]
- 64.Clavreul A, Pourbaghi-Masouleh M, Roger E and Menei P, International journal of nanomedicine, 2019, 14, 2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taneja G, Sud A, Pendse N, Panigrahi B, Kumar A and Sharma AK, Cardiovascular toxicology, 2019, 19, 1–12. [DOI] [PubMed] [Google Scholar]
- 66.Sanchez-Moreno P, Ortega-Vinuesa JL, Peula-Garcia JM, Marchal JA and Boulaiz H, Current drug targets, 2018, 19, 339–359. [DOI] [PubMed] [Google Scholar]
- 67.Bahrami B, Hojjat-Farsangi M, Mohammadi H, Anvari E, Ghalamfarsa G, Yousefi M and Jadidi-Niaragh F, Immunology Letters, 2017, 190, 64–83. [DOI] [PubMed] [Google Scholar]
- 68.Vargas GE, Durand LAH, Cadena V, Romero M, Mesones RV, Mačković M, Spallek S, Spiecker E, Boccaccini AR and Gorustovich AA, Journal of Materials Science: Materials in Medicine, 2013, 24, 1261–1269. [DOI] [PubMed] [Google Scholar]
- 69.Abdalla AM, Xiao L, Ullah MW, Yu M, Ouyang C and Yang G, Theranostics, 2018, 8, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ali I, Salim K, A Rather M, A Wani W and Haque A, Current cancer drug targets, 2011, 11, 135–146. [DOI] [PubMed] [Google Scholar]
- 71.Gaspar R and Duncan R, Advanced drug delivery reviews, 2009, 61, 1220–1231. [DOI] [PubMed] [Google Scholar]
- 72.Yadavalli T, Ramasamy S, Chandrasekaran G, Michael I, Therese HA and Chennakesavulu R, Journal of magnetism and Magnetic Materials, 2015, 380, 315–320. [Google Scholar]
- 73.Hughes GA, in Nanomedicine in Cancer, Pan Stanford, 2017, pp. 47–72. [Google Scholar]
- 74.Li Y, Thambi T and Lee DS, Advanced healthcare materials, 2018, 7, 1700886. [DOI] [PubMed] [Google Scholar]
- 75.Jokerst JV, Lobovkina T, Zare RN and Gambhir SS, Nanomedicine, 2011, 6, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qiu M, Wang C, Chen D, Shen C, Zhao H and He Y, Applied Sciences, 2016, 6, 290. [Google Scholar]
- 77.Peters EB, Christoforou N, Leong KW, Truskey GA and West JL, Cellular and molecular bioengineering, 2016, 9, 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee BK, Yun Y and Park K, Advanced drug delivery reviews, 2016, 107, 176–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu Q, Gao X, Gu G, Kang T, Tu Y, Liu Z, Song Q, Yao L, Pang Z and Jiang X, Biomaterials, 2013, 34, 5640–5650. [DOI] [PubMed] [Google Scholar]
- 80.Burt HM, Jackson JK, Bains SK, Liggins RT, Oktaba AMC, Arsenault AL and Hunter WL, Cancer letters, 1995, 88, 73–79. [DOI] [PubMed] [Google Scholar]
- 81.Gu G, Hu Q, Feng X, Gao X, Menglin J, Kang T, Jiang D, Song Q, Chen H and Chen J, Biomaterials, 2014, 35, 8215–8226. [DOI] [PubMed] [Google Scholar]
- 82.François S, Chakfé N, Durand B and Laroche G, Acta Biomaterialia, 2009, 5, 2418–2428. [DOI] [PubMed] [Google Scholar]
- 83.Gigliobianco G, Chong CK and MacNeil S, Journal of biomaterials applications, 2015, 30, 50–60. [DOI] [PubMed] [Google Scholar]
- 84.Kanczler J, Barry J, Ginty P, Howdle S, Shakesheff K and Oreffo R, Biochemical and biophysical research communications, 2007, 352, 135–141. [DOI] [PubMed] [Google Scholar]
- 85.de Jesus Sousa-Batista A, Cerqueira-Coutinho C, do Carmo FS, de Souza Albernaz M and Santos-Oliveira R, American journal of therapeutics, 2019, 26, e12–e17. [DOI] [PubMed] [Google Scholar]
- 86.Bao C, Chong MS, Qin L, Fan Y, Teo EY, Sandikin D, Choolani M and Chan JKY, Materials Technology, 2019, 1–7. [Google Scholar]
- 87.Niza E, Castro-Osma JA, Posadas I, Alonso-Moreno C, Bravo I, Garzón A, Canales-Vázquez J, Ceña V, Lara-Sánchez A and Albaladejo J, International journal of pharmaceutics, 2019, 558, 110–119. [DOI] [PubMed] [Google Scholar]
- 88.Filipović N, Veselinović L, Ražić S, Jeremić S, Filipič M, Žegura B, Tomić S, Čolić M and Stevanović M, Materials Science and Engineering: C, 2019, 96, 776–789. [DOI] [PubMed] [Google Scholar]
- 89.Hu Q, Gao X, Kang T, Feng X, Jiang D, Tu Y, Song Q, Yao L, Jiang X, Chen H and Chen J, Biomaterials, 2013, 34, 9496–9508. [DOI] [PubMed] [Google Scholar]
- 90.Jiang YC, Wang XF, Xu YY, Qiao YH, Guo X, Wang DF, Li Q and Turng LS, Biomacromolecules, 2018, 19, 3747–3753. [DOI] [PubMed] [Google Scholar]
- 91.Lü J-M, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q and Chen C, Expert review of molecular diagnostics, 2009, 9, 325–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, Jérôme C, Marchand-Brynaert J, Feron O and Préat V, Journal of Controlled Release, 2009, 140, 166–173. [DOI] [PubMed] [Google Scholar]
- 93.d’Angelo I, Parajó Y, Horváth A, Kéri G, La Rotonda MI and Alonso MJ, Journal of microencapsulation, 2010, 27, 57–66. [DOI] [PubMed] [Google Scholar]
- 94.Duan T, Xu Z, Sun F, Wang Y, Zhang J, Luo C and Wang M, Biomedicine & Pharmacotherapy, 2019, 117, 109121. [DOI] [PubMed] [Google Scholar]
- 95.Kang T, Gao X, Hu Q, Jiang D, Feng X, Zhang X, Song Q, Yao L, Huang M and Jiang X, Biomaterials, 2014, 35, 4319–4332. [DOI] [PubMed] [Google Scholar]
- 96.Chereddy KK, Her C-H, Comune M, Moia C, Lopes A, Porporato PE, Vanacker J, Lam MC, Steinstraesser L and Sonveaux P, Journal of Controlled Release, 2014, 194, 138–147. [DOI] [PubMed] [Google Scholar]
- 97.Park JS, Yang HN, Yi SW, Kim J-H and Park K-H, Biomaterials, 2016, 76, 226–237. [DOI] [PubMed] [Google Scholar]
- 98.Parajó Y, d’Angelo I, Horváth A, Vantus T, György K, Welle A, Garcia-Fuentes M and Alonso MJ, European Journal of Pharmaceutical Sciences, 2010, 41, 644–649. [DOI] [PubMed] [Google Scholar]
- 99.Ahsan SM, Thomas M, Reddy KK, Sooraparaju SG, Asthana A and Bhatnagar I, International journal of biological macromolecules, 2018, 110, 97–109. [DOI] [PubMed] [Google Scholar]
- 100.Ali A and Ahmed S, International journal of biological macromolecules, 2018, 109, 273–286. [DOI] [PubMed] [Google Scholar]
- 101.Ngo D-H and Kim S-K, in Advances in food and nutrition research, Elsevier, 2014, vol. 73, pp. 15–31. [DOI] [PubMed] [Google Scholar]
- 102.Karagozlu MZ and Kim S-K, in Advances in food and nutrition research, Elsevier, 2014, vol. 72, pp. 215–225. [DOI] [PubMed] [Google Scholar]
- 103.Zheng L-Y and Zhu J-F, Carbohydrate polymers, 2003, 54, 527–530. [Google Scholar]
- 104.Xu Y, Wen Z and Xu Z, Anticancer Research, 2009, 29, 5103–5109. [PubMed] [Google Scholar]
- 105.Prashanth KH and Tharanathan R, Biochimica et Biophysica Acta (BBA)-General Subjects, 2005, 1722, 22–29. [DOI] [PubMed] [Google Scholar]
- 106.Wu H, Aam BB, Wang W, Norberg AL, Sørlie M, Eijsink VG and Du Y, Carbohydrate polymers, 2012, 89, 511–518. [DOI] [PubMed] [Google Scholar]
- 107.Kim GH, Won JE, Byeon Y, Kim MG, Wi TI, Lee JM, Park YY, Lee JW, Kang TH, Jung ID, Shin BC, Ahn HJ, Lee YJ, Sood AK, Han HD and Park YM, Drug Delivery, 2018, 25, 1394–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin H, Pi J, Yang F, Wu C, Cheng X, Bai H, Huang D, Jiang J, Cai J and Chen ZW, Applied Microbiology and Biotechnology, 2016, 100, 6643–6652. [DOI] [PubMed] [Google Scholar]
- 109.Zahid AA, Ahmed R, ur Rehman SR, Augustine R, Tariq M and Hasan A, International journal of biological macromolecules, 2019, 136, 901–910. [DOI] [PubMed] [Google Scholar]
- 110.Shen X, Fang J, Lv X, Pei Z, Wang Y, Jiang S and Ding K, Journal of Biological Chemistry, 2011, 286, 26616–26627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harenberg J, Leber G, Dempfle C, Heene D, Zimmermann R and Kübler W, Nouvelle revue francaise d’hematologie, 1989, 31, 363–369. [PubMed] [Google Scholar]
- 112.Group R, The Lancet, 1990, 336, 827–830. [PubMed] [Google Scholar]
- 113.Walenga JM and Bick RL, Medical Clinics of North America, 1998, 82, 635–658. [DOI] [PubMed] [Google Scholar]
- 114.Alam F, Al-Hilal TA, Chung SW, Seo D, Mahmud F, Kim HS, Kim SY and Byun Y, Biomaterials, 2014, 35, 6543–6552. [DOI] [PubMed] [Google Scholar]
- 115.Chung HJ, Kim HK, Yoon JJ and Park TG, Pharmaceutical research, 2006, 23, 1835–1841. [DOI] [PubMed] [Google Scholar]
- 116.Kemp MM, Kumar A, Mousa S, Dyskin E, Yalcin M, Ajayan P, Linhardt RJ and Mousa SA, nanotechnology, 2009, 20, 455104. [DOI] [PubMed] [Google Scholar]
- 117.Lee DY, Kim SK, Kim YS, Nam JH, San Kim I, Park RW, Kim SY and Byun Y, Journal of Controlled Release, 2007, 118, 310–317. [DOI] [PubMed] [Google Scholar]
- 118.Rodriguez-Torres MDP, Acosta-Torres LS and Diaz-Torres LA, Journal of Nanomaterials, 2018, 2018. [Google Scholar]
- 119.Park I-K, Tran TH, Oh I-H, Kim Y-J, Cho KJ, Huh KM and Lee Y.-k., European Journal of Pharmaceutical Sciences, 2010, 41, 148–155. [DOI] [PubMed] [Google Scholar]
- 120.Khatun Z, Nurunnabi M, Cho KJ, Byun Y, Bae YH and Lee Y.-k., Journal of controlled release, 2014, 177, 64–73. [DOI] [PubMed] [Google Scholar]
- 121.Liang Y and Kiick KL, Acta biomaterialia, 2014, 10, 1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park K, Kim YS, Lee GY, Park RW, Kim IS, Kim SY and Byun Y, Pharmaceutical Research, 2008, 25, 2786–2798. [DOI] [PubMed] [Google Scholar]
- 123.McLuckie M, Schmidt CA, Oosthuysen A, Sanchez-Macedo N, Merker H, Bezuidenhout D, Hoerstrup SP and Lindenblatt N, Journal of Biomedical Materials Research Part A, 2017, 105, 2543–2550. [DOI] [PubMed] [Google Scholar]
- 124.Su K and Wang C, Biotechnology letters, 2015, 37, 2139–2145. [DOI] [PubMed] [Google Scholar]
- 125.Foox M and Zilberman M, Expert opinion on drug delivery, 2015, 12, 1547–1563. [DOI] [PubMed] [Google Scholar]
- 126.Sahoo N, Sahoo RK, Biswas N, Guha A and Kuotsu K, International journal of biological macromolecules, 2015, 81, 317–331. [DOI] [PubMed] [Google Scholar]
- 127.Kuo W-T, Huang H-Y, Chou M-J, Wu M-C and Huang Y-Y, Journal of Nanomaterials, 2011, 2011, 28. [Google Scholar]
- 128.Balthasar S, Michaelis K, Dinauer N, von Briesen H, Kreuter J and Langer K, Biomaterials, 2005, 26, 2723–2732. [DOI] [PubMed] [Google Scholar]
- 129.Lin SF, Jiang PL, Tsai JS, Huang YY, Lin SY, Lin JH and Liu DZ, Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2019, 107, 1228–1237. [DOI] [PubMed] [Google Scholar]
- 130.ElMasry SR, Hathout RM, Abdel-Halim Mand Mansour S, Journal of Drug Delivery Science and Technology, 2018, 48, 30–39. [Google Scholar]
- 131.Kommareddy S and Amiji M, Cancer gene therapy, 2007, 14, 488. [DOI] [PubMed] [Google Scholar]
- 132.Xie J, Wang H, Wang Y, Ren F, Yi W, Zhao K, Li Z, Zhao Q, Liu Z and Wu H, Cardiovascular therapeutics, 2013, 31, e12–e18. [DOI] [PubMed] [Google Scholar]
- 133.Montero RB, Vial X, Nguyen DT, Farhand S, Reardon M, Pham SM, Tsechpenakis G and Andreopoulos FM, Acta biomaterialia, 2012, 8, 1778–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Elzoghby AO, Samy WM and Elgindy NA, Journal of controlled release, 2012, 157, 168–182. [DOI] [PubMed] [Google Scholar]
- 135.Lin W, Coombes A, Davies M, Davis S and Illum L, Journal of Drug Targeting, 1993, 1, 237–243. [DOI] [PubMed] [Google Scholar]
- 136.Jun JY, Nguyen HH, Chun HS, Kang B-C and Ko S, Food chemistry, 2011, 127, 1892–1898. [Google Scholar]
- 137.Patil GV, Drug development research, 2003, 58, 219–247. [Google Scholar]
- 138.Sundar S, Kundu J and Kundu SC, Science and Technology of Advanced Materials, 2010, 11, 014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hao H, Ma Q, Huang C, He F and Yao P, International journal of pharmaceutics, 2013, 444, 77–84. [DOI] [PubMed] [Google Scholar]
- 140.Lee SH, Heng D, Ng WK, Chan H-K and Tan RB, International journal of pharmaceutics, 2011, 403, 192–200. [DOI] [PubMed] [Google Scholar]
- 141.Choi SH, Byeon HJ, Choi JS, Thao L, Kim I, Lee ES, Shin BS, Lee KC and Youn YS, Journal of controlled release, 2015, 197, 199–207. [DOI] [PubMed] [Google Scholar]
- 142.Miele E, Spinelli GP, Miele E, Tomao F and Tomao S, International journal of nanomedicine, 2009, 4, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kratz F, Journal of controlled release, 2014, 190, 331–336. [DOI] [PubMed] [Google Scholar]
- 144.Noorani L, Stenzel M, Liang R, Pourgholami MH and Morris DL, Journal of nanobiotechnology, 2015, 13, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kushwah V, Katiyar SS, Dora CP, Agrawal AK, Lamprou DA, Gupta RC and Jain S, Acta biomaterialia, 2018, 73, 424–436. [DOI] [PubMed] [Google Scholar]
- 146.Gonzalez-Villasana V, Rodriguez-Aguayo C, Arumugam T, Cruz-Monserrate Z, Fuentes-Mattei E, Deng D, Hwang RF, Wang H, Ivan C and Garza RJ, Molecular cancer therapeutics, 2014, 13, 2583–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Son S, Song S, Lee SJ, Min S, Kim SA, Yhee JY, Huh MS, Kwon IC, Jeong SY and Byun Y, Biomaterials, 2013, 34, 9475–9485. [DOI] [PubMed] [Google Scholar]
- 148.Wagner S, Rothweiler F, Anhorn MG, Sauer D, Riemann I, Weiss EC, Katsen-Globa A, Michaelis M, Cinatl J Jr and Schwartz D, Biomaterials, 2010, 31, 2388–2398. [DOI] [PubMed] [Google Scholar]
- 149.Fan T-P, Yeh J-C, Leung KW, Yue PY and Wong RN, Trends in Pharmacological Sciences, 2006, 27, 297–309. [DOI] [PubMed] [Google Scholar]
- 150.Majnooni MB, Fakhri S, Smeriglio A, Trombetta D, Croley CR, Bhattacharyya P, Sobarzo-Sánchez E, Farzaei MH and Bishayee A, Molecules, 2019, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rajasekar J, Perumal MK and Vallikannan B, The Journal of Nutritional Biochemistry, 2019, 71, 1–15. [DOI] [PubMed] [Google Scholar]
- 152.Kunnumakkara AB, Anand P and Aggarwal BB, Cancer letters, 2008, 269, 199–225. [DOI] [PubMed] [Google Scholar]
- 153.Shan B, Schaaf C, Schmidt A, Lucia K, Buchfelder M, Losa M, Kuhlen D, Kreutzer J, Perone M and Arzt E, Journal of Endocrinology, 2012, 214, 389. [DOI] [PubMed] [Google Scholar]
- 154.Arbiser JL, Klauber N, Rohan R, Van Leeuwen R, Huang MT, Fisher C, Flynn E and Byers HR, Molecular Medicine, 1998, 4, 376–383. [PMC free article] [PubMed] [Google Scholar]
- 155.Bhandarkar SS and Arbiser JL, in The molecular targets and therapeutic uses of curcumin in health and disease, Springer, 2007, pp. 185–195. [Google Scholar]
- 156.Basniwal RK, Buttar HS, Jain V and Jain N, Journal of agricultural and food chemistry, 2011, 59, 2056–2061. [DOI] [PubMed] [Google Scholar]
- 157.Yallapu MM, Nagesh PKB, Jaggi M and Chauhan SC, The AAPS journal, 2015, 17, 1341–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li L, Braiteh FS and Kurzrock R, Cancer: Interdisciplinary International Journal of the American Cancer Society, 2005, 104, 1322–1331. [DOI] [PubMed] [Google Scholar]
- 159.Gong C, Deng S, Wu Q, Xiang M, Wei X, Li L, Gao X, Wang B, Sun L and Chen Y, Biomaterials, 2013, 34, 1413–1432. [DOI] [PubMed] [Google Scholar]
- 160.Yang X, Li Z, Wang N, Li L, Song L, He T, Sun L, Wang Z, Wu Q and Luo N, Scientific reports, 2015, 5, 10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ding Q, Niu T, Yang Y, Guo Q, Luo F and Qian Z, Journal of biomedical nanotechnology, 2014, 10, 632–641. [DOI] [PubMed] [Google Scholar]
- 162.Mukerjee A, Ranjan AP and Vishwanatha JK, Journal of biomedical nanotechnology, 2016, 12, 1374–1392. [DOI] [PubMed] [Google Scholar]
- 163.Ranjan AP, Mukerjee A, Helson L, Gupta R and Vishwanatha JK, Anticancer research, 2013, 33, 3603–3609. [PubMed] [Google Scholar]
- 164.Tan H-L, Chan K-G, Pusparajah P, Saokaew S, Duangjai A, Lee L-H and Goh B-H, Frontiers in pharmacology, 2016, 7, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu X, Tong Y, Han XQ, Kwok HF, Yue GGL, Bik-San Lau C and Ge W, Phytotherapy Research, 2013, 27, 1368–1375. [DOI] [PubMed] [Google Scholar]
- 166.Da Z, Journal of International Translational Medicine, 2014, 2, 385–388. [Google Scholar]
- 167.Hong J, Zhang Z, Lv W, Zhang M, Chen C, Yang S, Li S, Zhang L, Han D and Zhang W, PLoS One, 2013, 8, e71347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.YE Y.-h., HU F.-h., Zou J.-p., Zhan Y, Wang S.-q. and Liu J.-y., Chinese Academy of Medical Sciences, 2015, 37, 264–268. [Google Scholar]
- 169.Yang J-X, Fichtner I, Becker M, Lemm M and Wang X-M, The American journal of Chinese medicine, 2009, 37, 1153–1165. [DOI] [PubMed] [Google Scholar]
- 170.Li S, Priceman SJ, Xin H, Zhang W, Deng J, Liu Y, Huang J, Zhu W, Chen M and Hu W, PLoS One, 2013, 8, e81657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Choi HJ, Eun J-S, Kim DK, Li RH, Shin T-Y, Park H, Cho N-P and Soh Y, European journal of pharmacology, 2008, 579, 58–65. [DOI] [PubMed] [Google Scholar]
- 172.Jing X, Yin W, Tian H, Chen M, Yao X, Zhu W, Guo F and Ye Y, Life sciences, 2018, 202, 52–60. [DOI] [PubMed] [Google Scholar]
- 173.Wu Y, Xia L, Zhou Y, Ma W, Zhang N, Chang J, Lin K, Xu Y and Jiang X, Journal of Materials Chemistry B, 2015, 3, 4871–4883. [DOI] [PubMed] [Google Scholar]
- 174.Tang Y, Jacobi A, Vater C, Zou L, Zou X and Stiehler M, Stem cells, 2015, 33, 1863–1877. [DOI] [PubMed] [Google Scholar]
- 175.Zheng Y, Lu L, Yan Z, Jiang S, Yang S, Zhang Y, Xu K, He C, Tao X and Zhang Q, Artificial cells, nanomedicine, and biotechnology, 2019, 47, 801–811. [DOI] [PubMed] [Google Scholar]
- 176.Han L-Y, Wu Y-L, Zhu C-Y, Wu C-S and Yang C-R, Pharmaceutics, 2019, 11, 51. [Google Scholar]
- 177.Gong M, Chi C, Ye J, Liao M, Xie W, Wu C, Shi R and Zhang L, Colloids and Surfaces B: Biointerfaces, 2018, 170, 201–209. [DOI] [PubMed] [Google Scholar]
- 178.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD and Mehta RG, Science, 1997, 275, 218–220. [DOI] [PubMed] [Google Scholar]
- 179.Baur JA and Sinclair DA, Nature reviews Drug discovery, 2006, 5, 493. [DOI] [PubMed] [Google Scholar]
- 180.Tseng S-H, Lin S-M, Chen J-C, Su Y-H, Huang H-Y, Chen C-K, Lin P-Y and Chen Y, Clinical Cancer Research, 2004, 10, 2190–2202. [DOI] [PubMed] [Google Scholar]
- 181.Garvin S, Öllinger K and Dabrosin C, Cancer letters, 2006, 231, 113–122. [DOI] [PubMed] [Google Scholar]
- 182.Fouad M, Agha A, Merzabani MA and Shouman S, Human & experimental toxicology, 2013, 32, 1067–1080. [DOI] [PubMed] [Google Scholar]
- 183.Kimura Y and Okuda H, The Journal of nutrition, 2001, 131, 1844–1849. [DOI] [PubMed] [Google Scholar]
- 184.Wong JC and Fiscus RR, Anticancer research, 2015, 35, 273–281. [PubMed] [Google Scholar]
- 185.Simão F, Pagnussat AS, Seo JH, Navaratna D, Leung W, Lok J, Guo S, Waeber C, Salbego CG and Lo EH, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 2012, 32, 884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hu W-H, Duan R, Xia Y-T, Xiong Q-P, Wang H-Y, Chan GK-L, Liu S-Y, Dong TT-X, Qin Q-W and Tsim KW-K, Journal of agricultural and food chemistry, 2018, 67, 1127–1137. [DOI] [PubMed] [Google Scholar]
- 187.Chen P-L and S Easton A, Current neurovascular research, 2011, 8, 14–24. [DOI] [PubMed] [Google Scholar]
- 188.Santos AC, Pereira I, Pereira-Silva M, Ferreira L, Caldas M, Collado-González M, Magalhães M, Figueiras A, Ribeiro AJ and Veiga F, Colloids and Surfaces B: Biointerfaces, 2019, 180, 127–140. [DOI] [PubMed] [Google Scholar]
- 189.Arora D and Jaglan S, Environmental chemistry letters, 2018, 16, 35–41. [Google Scholar]
- 190.Pund S, Thakur R, More U and Joshi A, Colloids and Surfaces B: Biointerfaces, 2014, 120, 110–117. [DOI] [PubMed] [Google Scholar]
- 191.Kim S, Ng WK, Dong Y, Das S and Tan RB, Journal of Food Engineering, 2012, 108, 37–42. [Google Scholar]
- 192.Kingston DG, Journal of Natural Products, 2000, 63, 726–734. [DOI] [PubMed] [Google Scholar]
- 193.Damber J-E, Vallbo C, Albertsson P, Lennernäs B and Norrby K, Cancer Chemotherapy and Pharmacology, 2006, 58, 354–360. [DOI] [PubMed] [Google Scholar]
- 194.Dai Y, Cai X, Bi X, Liu C, Yue N, Zhu Y, Zhou J, Fu M, Huang W and Qian H, European Journal of Medicinal Chemistry, 2019, 171, 104–115. [DOI] [PubMed] [Google Scholar]
- 195.Dordunoo SK, Jackson JK, Arsenault LA, Oktaba AMC, Hunter WL and Burt HM, Cancer chemotherapy and pharmacology, 1995, 36, 279–282. [DOI] [PubMed] [Google Scholar]
- 196.Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, Giavazzi R and Taraboletti G, Clinical Cancer Research, 1996, 2, 1843–1849. [PubMed] [Google Scholar]
- 197.Wang J, Lou P, Lesniewski R and Henkin J, Anti-cancer drugs, 2003, 14, 13–19. [DOI] [PubMed] [Google Scholar]
- 198.Vacca A, Ribatti D, Iurlaro M, Merchionne F, Nico B, Ria R and Dammacco F, Journal of hematotherapy & stem cell research, 2002, 11, 103–118. [DOI] [PubMed] [Google Scholar]
- 199.Bocci G, Di Paolo A and Danesi R, Angiogenesis, 2013, 16, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Klauber N, Parangi S, Flynn E, Hamel E and D’Amato RJ, Cancer research, 1997, 57, 81–86. [PubMed] [Google Scholar]
- 201.Merchan JR, Jayaram DR, Supko JG, He X, Bubley GJ and Sukhatme VP, International journal of cancer, 2005, 113, 490–498. [DOI] [PubMed] [Google Scholar]
- 202.Hata K, Osaki M, Dhar DK, Nakayama K, Fujiwaki R, Ito H, Nagasue N and Miyazaki K, Cancer Chemotherapy and Pharmacology, 2004, 53, 68–74. [DOI] [PubMed] [Google Scholar]
- 203.Danhier F, Danhier P, De Saedeleer CJ, Fruytier A-C, Schleich N, des Rieux A, Sonveaux P, Gallez B and Préat V, International journal of pharmaceutics, 2015, 479, 399–407. [DOI] [PubMed] [Google Scholar]
- 204.Kang KW, Chun M-K, Kim O, Subedi RK, Ahn S-G, Yoon J-H and Choi H-K, Nanomedicine: Nanotechnology, Biology and Medicine, 2010, 6, 210–213. [DOI] [PubMed] [Google Scholar]
- 205.Ma P and Mumper RJ, Journal of nanomedicine & nanotechnology, 2013, 4, 1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Banerjee I, De K, Mukherjee D, Dey G, Chattopadhyay S, Mukherjee M, Mandal M, Bandyopadhyay AK, Gupta A and Ganguly S, Acta biomaterialia, 2016, 38, 69–81. [DOI] [PubMed] [Google Scholar]
- 207.Wu C, Gao Y, Liu Y and Xu X, International Journal of Nanomedicine, 2018, 13, 6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wall ME, Wani M, Cook C, Palmer KH, McPhail A. a. and Sim G, Journal of the American Chemical Society, 1966, 88, 3888–3890. [Google Scholar]
- 209.Martino E, Della Volpe S, Terribile E, Benetti E, Sakaj M, Centamore A, Sala A and Collina S, Bioorganic & medicinal chemistry letters, 2017, 27, 701–707. [DOI] [PubMed] [Google Scholar]
- 210.Liu YQ, Li WQ, Morris-Natschke SL, Qian K, Yang L, Zhu GX, Wu XB, Chen AL, Zhang SY and Nan X, Medicinal research reviews, 2015, 35, 753–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Koshkina NV, Kleinerman ES, Waldrep C, Jia S-F, Worth LL, Gilbert BE and Knight V, Clinical Cancer Research, 2000, 6, 2876–2880. [PubMed] [Google Scholar]
- 212.Verschraegen C, Gilbert B, Huaringa A, Newman R, Harris N, Leyva F, Keus L, Campbell K, Nelson-Taylor T and Knight V, Annals of the New York Academy of Sciences, 2000, 922, 352–354. [DOI] [PubMed] [Google Scholar]
- 213.Takimoto CH and Thomas R, Annals of the New York Academy of Sciences, 2000, 922, 224–236. [DOI] [PubMed] [Google Scholar]
- 214.Pratesi G, Beretta GL and Zunino F, Anti-cancer drugs, 2004, 15, 545–552. [DOI] [PubMed] [Google Scholar]
- 215.Pommier Y, Cushman M and Doroshow JH, Oncotarget, 2018, 9, 37286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Clements MK, Jones CB, Cumming M and Daoud SS, Cancer Chemotherapy and Pharmacology, 1999, 44, 411–416. [DOI] [PubMed] [Google Scholar]
- 217.Gigliotti CL, Minelli R, Cavalli R, Occhipinti S, Barrera G, Pizzimenti S, Cappellano G, Boggio E, Conti L and Fantozzi R, Journal of biomedical nanotechnology, 2016, 12, 114–127. [DOI] [PubMed] [Google Scholar]
- 218.Dai M, Xu X, Song J, Fu S, Gou M, Luo F and Qian Z, Cancer letters, 2011, 312, 189–196. [DOI] [PubMed] [Google Scholar]
- 219.Jayasooriya RGPT, Park SR, Choi YH, Hyun J-W, Chang W-Y and Kim G-Y, Environmental toxicology and pharmacology, 2015, 39, 1189–1198. [DOI] [PubMed] [Google Scholar]
- 220.Wen Y, Wang Y, Liu X, Zhang W, Xiong X, Han Z and Liang X, Cancer biology & medicine, 2017, 14, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gigliotti CL, Ferrara B, Occhipinti S, Boggio E, Barrera G, Pizzimenti S, Giovarelli M, Fantozzi R, Chiocchetti A and Argenziano M, Drug delivery, 2017, 24, 670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lin C-J, Lin Y-L, Luh F, Yen Y and Chen R-M, Oncotarget, 2016, 7, 42408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tian X, Nguyen M, Foote HP, Caster JM, Roche KC, Peters CG, Wu P, Jayaraman L, Garmey EG and Tepper JE, Cancer research, 2017, 77, 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gaur S, Chen L, Yen T, Wang Y, Zhou B, Davis M and Yen Y, Nanomedicine: Nanotechnology, Biology and Medicine, 2012, 8, 721–730. [DOI] [PubMed] [Google Scholar]
- 225.Conley SJ, Baker TL, Burnett JP, Theisen RL, Lazarus D, Peters CG, Clouthier SG, Eliasof S and Wicha MS, Breast cancer research and treatment, 2015, 150, 559–567. [DOI] [PubMed] [Google Scholar]
- 226.Doura T, Takahashi K, Ogra Y and Suzuki N, ACS medicinal chemistry letters, 2017, 8, 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Seguin J, Mignet N, Ossa HL, Tanter M and Gennisson J-L, Ultrasound in medicine & biology, 2017, 43, 2352–2361. [DOI] [PubMed] [Google Scholar]
- 228.Pettit GR, Singh SB, Niven ML, Hamel E and Schmidt JM, Journal of natural products, 1987, 50, 119–131. [DOI] [PubMed] [Google Scholar]
- 229.Tozer GM, Kanthou C, Parkins CS and Hill SA, International journal of experimental pathology, 2002, 83, 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.West CM and Price P, Anti-cancer drugs, 2004, 15, 179–187. [DOI] [PubMed] [Google Scholar]
- 231.Shen CH, Shee JJ, Wu JY, Lin YW, Wu JD and Liu YW, British journal of pharmacology, 2010, 160, 2008–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Griggs J, Metcalfe JC and Hesketh R, The lancet oncology, 2001, 2, 82–87. [DOI] [PubMed] [Google Scholar]
- 233.Su M, Huang J, Liu S, Xiao Y, Qin X, Liu J, Pi C, Luo T, Li J and Chen X, Scientific reports, 2016, 6, 28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ren X, Dai M, Lin LP, Li PK and Ding J, British journal of pharmacology, 2009, 156, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nik ME, Momtazi-Borojeni AA, Zamani P, Navashenaq JG, Iranshahi M, Jaafari MR and Malaekeh-Nikouei B, Journal of Cellular Physiology, 2019, 234, 14721–14733. [DOI] [PubMed] [Google Scholar]
- 236.Mico V, Charalambous A, Peyman SA, Abou-Saleh RH, Markham AF, Coletta PL and Evans SD, International journal of pharmaceutics, 2017, 526, 547–555. [DOI] [PubMed] [Google Scholar]
- 237.Li X, Wu M, Pan L and Shi J, International journal of nanomedicine, 2016, 11, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang Y, Yu H, Zhang D, Wang G, Song W, Liu Y, Ma S, Tang Z, Liu Z and Sakurai K, Acta biomaterialia, 2019, 92, 229–240. [DOI] [PubMed] [Google Scholar]
- 239.Kumar R, Singh A, Garg N and Siril PF, Ultrasonics sonochemistry, 2018, 40, 686–696. [DOI] [PubMed] [Google Scholar]
- 240.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R and Langer R, Nature nanotechnology, 2007, 2, 751. [DOI] [PubMed] [Google Scholar]
- 241.Pozzi D, Colapicchioni V, Caracciolo G, Piovesana S, Capriotti AL, Palchetti S, De Grossi S, Riccioli A, Amenitsch H and Laganà A, Nanoscale, 2014, 6, 2782–2792. [DOI] [PubMed] [Google Scholar]
- 242.Hatakeyama H, Akita H and Harashima H, Advanced drug delivery reviews, 2011, 63, 152–160. [DOI] [PubMed] [Google Scholar]
- 243.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M and Nejati-Koshki K, Nanoscale research letters, 2013, 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Torchilin VP, Nature reviews Drug discovery, 2005, 4, 145. [DOI] [PubMed] [Google Scholar]
- 245.Pattni BS, Chupin VV and Torchilin VP, Chemical reviews, 2015, 115, 10938–10966. [DOI] [PubMed] [Google Scholar]
- 246.Torchilin V, Critical reviews in therapeutic drug carrier systems, 1985, 2, 65–115. [PubMed] [Google Scholar]
- 247.Mura S, Nicolas J and Couvreur P, Nature materials, 2013, 12, 991. [DOI] [PubMed] [Google Scholar]
- 248.Barenholz YC, Journal of controlled release, 2012, 160, 117–134. [DOI] [PubMed] [Google Scholar]
- 249.Gardikis K, Tsimplouli C, Dimas K, Micha-Screttas M and Demetzos C, International journal of pharmaceutics, 2010, 402, 231–237. [DOI] [PubMed] [Google Scholar]
- 250.Chou H-H, Wang K-L, Chen C-A, Wei L-H, Lai C-H, Hsieh C-Y, Yang Y-C, Twu N-F, Chang T-C and Yen M-S, Gynecologic oncology, 2006, 101, 423–428. [DOI] [PubMed] [Google Scholar]
- 251.Fassas A and Anagnostopoulos A, Leukemia & lymphoma, 2005, 46, 795–802. [DOI] [PubMed] [Google Scholar]
- 252.Rahimi-Rad M, Alizadeh E and Samarei R, Pneumologia (Bucharest, Romania), 2011, 60, 85–86. [PubMed] [Google Scholar]
- 253.Pont I, Calatayud-Pascual A, López-Castellano A, Albelda EP, García-España E, Martí-Bonmatí L, Frias JC and Albelda MT, PloS one, 2018, 13, e0190540–e0190540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Üner M and Yener G, International journal of nanomedicine, 2007, 2, 289. [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang N, Ping Q, Huang G, Xu W, Cheng Y and Han X, International journal of pharmaceutics, 2006, 327, 153–159. [DOI] [PubMed] [Google Scholar]
- 256.Rostami E, Kashanian S, Azandaryani AH, Faramarzi H, Dolatabadi JEN and Omidfar K, Chemistry and physics of lipids, 2014, 181, 56–61. [DOI] [PubMed] [Google Scholar]
- 257.Costa A, Sarmento B and Seabra V, European Journal of Pharmaceutical Sciences, 2018, 114, 103–113. [DOI] [PubMed] [Google Scholar]
- 258.Muga JO, Gathirwa JW, Tukulula M and Jura WG, Malaria journal, 2018, 17, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gaspar DP and Almeida AJ, in Surface Modification of Nanoparticles for Targeted Drug Delivery, Springer, 2019, pp. 73–98. [Google Scholar]
- 260.Bayón-Cordero L, Alkorta I and Arana L, Nanomaterials, 2019, 9, 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Brioschi AM, Calderoni S, Pradotto LG, Guido M, Strada A, Zenga F, Benech CA, Benech F, Serpe L and Zara GP, Journal of Nanoneuroscience, 2009, 1, 65–74. [Google Scholar]
- 262.Rani S, Rana R, Saraogi GK, Kumar V and Gupta U, AAPS PharmSciTech, 2019, 20, 129. [DOI] [PubMed] [Google Scholar]
- 263.Singh B, Beg S, Khurana RK, Sandhu PS, Kaur R and Katare OP, Critical Reviews in Therapeutic Drug Carrier Systems, 2014, 31, 121–185. [DOI] [PubMed] [Google Scholar]
- 264.Cho HY, Kang JH, Ngo L, Tran P and Lee YB, Journal of Nanomaterials, 2016, 2016. [Google Scholar]
- 265.Huang W, Su H, Wen L, Shao A, Yang F and Chen G, Journal of Drug Delivery Science and Technology, 2018, 48, 266–273. [Google Scholar]
- 266.Valicherla GR, Dave KM, Syed AA, Riyazuddin M, Gupta AP, Singh A, Mitra K, Datta D and Gayen JR, Scientific reports, 2016, 6, 26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ramshankar YV, Suresh S and Devi K, Clinical Research and Regulatory Affairs, 2008, 25, 213–234. [Google Scholar]
- 268.Nazari-Vanani R, Azarpira N, Heli H, Karimian K and Sattarahmady N, Colloids and Surfaces B: Biointerfaces, 2017, 160, 65–72. [DOI] [PubMed] [Google Scholar]
- 269.Pund S, Borade G and Rasve G, Phytomedicine, 2014, 21, 307–314. [DOI] [PubMed] [Google Scholar]
- 270.Goyal R, Macri LK, Kaplan HM and Kohn J, Journal of Controlled Release, 2016, 240, 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Abid S, Hussain T, Raza ZA and Nazir A, Materials Science and Engineering: C, 2019, 97, 966–977. [DOI] [PubMed] [Google Scholar]
- 272.Mammadov R, Mammadov B, Toksoz S, Aydin B, Yagci R, Tekinay AB and Guler MO, Biomacromolecules, 2011, 12, 3508–3519. [DOI] [PubMed] [Google Scholar]
- 273.Kumar VA, Taylor NL, Shi S, Wang BK, Jalan AA, Kang MK, Wickremasinghe NC and Hartgerink JD, ACS nano, 2015, 9, 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zha RH, Sur S, Boekhoven J, Shi HY, Zhang M and Stupp SI, Acta biomaterialia, 2015, 12, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fan R, Li X, Deng J, Gao X, Zhou L, Zheng Y, Tong A, Zhang X, You C and Guo G, Scientific reports, 2016, 6, 28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zigdon-Giladi H, Khutaba A, Elimelech R, Machtei EE and Srouji S, Journal of Biomedical Materials Research Part A, 2017, 105, 2712–2721. [DOI] [PubMed] [Google Scholar]
- 277.Lai H-J, Kuan C-H, Wu H-C, Tsai J-C, Chen T-M, Hsieh D-J and Wang T-W, Acta biomaterialia, 2014, 10, 4156–4166. [DOI] [PubMed] [Google Scholar]
- 278.Li W, Wu D, Zhu S, Liu Z, Luo B, Lu L and Zhou C, Chemical Engineering Journal, 2019, 365, 270–281. [Google Scholar]
- 279.Ren X, Han Y, Wang J, Jiang Y, Yi Z, Xu H and Ke Q, Acta biomaterialia, 2018, 70, 140–153. [DOI] [PubMed] [Google Scholar]
- 280.Gao W, Sun L, Fu X, Lin Z, Xie W, Zhang W, Zhao F and Chen X, Journal of Materials Chemistry B, 2018, 6, 277–288. [DOI] [PubMed] [Google Scholar]
- 281.Satish A and Korrapati PS, AAPS PharmSciTech, 2019, 20, 110. [DOI] [PubMed] [Google Scholar]
- 282.Zhang D, Li L, Shan Y, Xiong J, Hu Z, Zhang Y and Gao J, Journal of Drug Delivery Science and Technology, 2019, 52, 272–281. [Google Scholar]
- 283.Cha C, Shin SR, Annabi N, Dokmeci MR and Khademhosseini A, ACS nano, 2013, 7, 2891–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Bianco A, Kostarelos K and Prato M, Expert opinion on drug delivery, 2008, 5, 331–342. [DOI] [PubMed] [Google Scholar]
- 285.Lim D-J, Sim M, Oh L, Lim K and Park H, Archives of pharmacal research, 2014, 37, 43–52. [DOI] [PubMed] [Google Scholar]
- 286.Caoduro C, Hervouet E, Girard-Thernier C, Gharbi T, Boulahdour H, Delage-Mourroux R and Pudlo M, Acta biomaterialia, 2017, 49, 36–44. [DOI] [PubMed] [Google Scholar]
- 287.Hu X, Liu S, Zhou G, Huang Y, Xie Z and Jing X, Journal of controlled release, 2014, 185, 12–21. [DOI] [PubMed] [Google Scholar]
- 288.Wang R, Cui H, Wang J, Li N, Zhao Q, Zhou Y, Lv Z and Zhong W, RSC Advances, 2016, 6, 47272–47280. [Google Scholar]
- 289.Singh R and Torti SV, Advanced drug delivery reviews, 2013, 65, 2045–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Murugesan S, Mousa SA, O’Connor LJ, Lincoln DW and Linhardt RJ, FEBS letters, 2007, 581, 1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wierzbicki M, Sawosz E, Grodzik M, Prasek M, Jaworski S and Chwalibog A, Nanoscale research letters, 2013, 8, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Albini A, Mussi V, Parodi A, Ventura A, Principi E, Tegami S, Rocchia M, Francheschi E, Sogno I and Cammarota R, Nanomedicine: Nanotechnology, Biology and Medicine, 2010, 6, 277–288. [DOI] [PubMed] [Google Scholar]
- 293.Masotti A, Miller MR, Celluzzi A, Rose L, Micciulla F, Hadoke PW, Bellucci S and Caporali A, Nanomedicine: Nanotechnology, Biology and Medicine, 2016, 12, 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Su Y, Hu Y, Wang Y, Xu X, Yuan Y, Li Y, Wang Z, Chen K, Zhang F and Ding X, Biomaterials, 2017, 139, 75–90. [DOI] [PubMed] [Google Scholar]
- 295.Azad N, Iyer AKV, Wang L, Liu Y, Lu Y and Rojanasakul Y, Nanotoxicology, 2013, 7, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Roman D, Yasmeen A, Mireuta M, Stiharu I and Al Moustafa A-E, Nanomedicine: Nanotechnology, Biology and Medicine, 2013, 9, 945–950. [DOI] [PubMed] [Google Scholar]
- 297.Lai P-X, Chen C-W, Wei S-C, Lin T-Y, Jian H-J, Lai IP-J, Mao J-Y, Hsu P-H, Lin H-J and Tzou W-S, Biomaterials, 2016, 109, 12–22. [DOI] [PubMed] [Google Scholar]
- 298.Shi S, Yang K, Hong H, Valdovinos HF, Nayak TR, Zhang Y, Theuer CP, Barnhart TE, Liu Z and Cai W, Biomaterials, 2013, 34, 3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mukherjee S, Sriram P, Barui AK, Nethi SK, Veeriah V, Chatterjee S, Suresh KI and Patra CR, Advanced healthcare materials, 2015, 4, 1722–1732. [DOI] [PubMed] [Google Scholar]
- 300.Chen Y, Gao A, Bai L, Wang Y, Wang X, Zhang X, Huang X, Hang R, Tang B and Chu PK, Materials Science and Engineering: C, 2017, 75, 1049–1058. [DOI] [PubMed] [Google Scholar]
- 301.Zhu Y, Li J, Li W, Zhang Y, Yang X, Chen N, Sun Y, Zhao Y, Fan C and Huang Q, Theranostics, 2012, 2, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lien Z-Y, Hsu T-C, Liu K-K, Liao W-S, Hwang K-C and Chao J-I, Biomaterials, 2012, 33, 6172–6185. [DOI] [PubMed] [Google Scholar]
- 303.Xiao J, Duan X, Yin Q, Zhang Z, Yu H and Li Y, Biomaterials, 2013, 34, 9648–9656. [DOI] [PubMed] [Google Scholar]
- 304.Setyawati MI, Mochalin VN and Leong DT, ACS nano, 2015, 10, 1170–1181. [DOI] [PubMed] [Google Scholar]
- 305.Zhang Z, Niu B, Chen J, He X, Bao X, Zhu J, Yu H and Li Y, Biomaterials, 2014, 35, 4565–4572. [DOI] [PubMed] [Google Scholar]
- 306.Cui C, Wang Y, Yang K, Wang Y, Yang J, Xi J, Zhao M, Wu J and Peng S, Journal of biomedical nanotechnology, 2015, 11, 70–80. [DOI] [PubMed] [Google Scholar]
- 307.Pacelli S, Acosta F, Chakravarti AR, Samanta SG, Whitlow J, Modaresi S, Ahmed RPH, Rajasingh J and Paul A, Acta biomaterialia, 2017, 58, 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Schimke MM, Stigler R, Wu X, Waag T, Buschmann P, Kern J, Untergasser G, Rasse M, Steinmüller-Nethl D and Krueger A, Nanomedicine: Nanotechnology, Biology and Medicine, 2016, 12, 823–833. [DOI] [PubMed] [Google Scholar]
- 309.Chen D, Dougherty CA, Zhu K and Hong H, Journal of controlled release, 2015, 210, 230–245. [DOI] [PubMed] [Google Scholar]
- 310.Guerra J, Herrero MA, Carrion B, Pérez-Martínez FC, Lucío M, Rubio N, Meneghetti M, Prato M, Ceña V and Vázquez E, Carbon, 2012, 50, 2832–2844. [Google Scholar]
- 311.Murata K, Kaneko K, Kokai F, Takahashi K, Yudasaka M and Iijima S, Chemical physics letters, 2000, 331, 14–20. [Google Scholar]
- 312.Karousis N, Suarez-Martinez I, Ewels CP and Tagmatarchis N, Chemical reviews, 2016, 116, 4850–4883. [DOI] [PubMed] [Google Scholar]
- 313.Zhang M, Yamaguchi T, Iijima S and Yudasaka M, Nanomedicine: Nanotechnology, Biology and Medicine, 2013, 9, 657–664. [DOI] [PubMed] [Google Scholar]
- 314.Azami T, Kasuya D, Yuge R, Yudasaka M, Iijima S, Yoshitake T and Kubo Y, The Journal of Physical Chemistry C, 2008, 112, 1330–1334. [DOI] [PubMed] [Google Scholar]
- 315.Yang J, Su H, Sun W, Cai J, Liu S, Chai Y and Zhang C, Theranostics, 2018, 8, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ajima K, Murakami T, Mizoguchi Y, Tsuchida K, Ichihashi T, Iijima S and Yudasaka M, ACS nano, 2008, 2, 2057–2064. [DOI] [PubMed] [Google Scholar]
- 317.Zhang M, Murakami T, Ajima K, Tsuchida K, Sandanayaka AS, Ito O, Iijima S and Yudasaka M, Proceedings of the National Academy of Sciences, 2008, 105, 14773–14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Li N, Zhao Q, Shu C, Ma X, Li R, Shen H and Zhong W, International journal of pharmaceutics, 2015, 478, 644–654. [DOI] [PubMed] [Google Scholar]
- 319.Goodarzi S, Da Ros T, Conde J, Sefat F and Mozafari M, Materials Today, 2017, 20, 460–480. [Google Scholar]
- 320.Xu J, Wang H, Hu Y, Zhang YS, Wen L, Yin F, Wang Z, Zhang Y, Li S, Miao Y, Lin B, Zuo D, Wang G, Mao M, Zhang T, Ding J, Hua Y and Cai Z, Advanced Science, 2019, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Grebinyk A, Prylutska S, Grebinyk S, Prylutskyy Y, Ritter U, Matyshevska O, Dandekar T and Frohme M, Nanoscale research letters, 2019, 14, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kazemzadeh H and Mozafari M, Drug Discovery Today, 2019, 24, 898–905. [DOI] [PubMed] [Google Scholar]
- 323.Jiao F, Liu Y, Qu Y, Li W, Zhou G, Ge C, Li Y, Sun B and Chen C, Carbon, 2010, 48, 2231–2243. [Google Scholar]
- 324.Yin J-J, Lao F, Fu PP, Wamer WG, Zhao Y, Wang PC, Qiu Y, Sun B, Xing G and Dong J, Biomaterials, 2009, 30, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Meng H, Xing G, Sun B, Zhao F, Lei H, Li W, Song Y, Chen Z, Yuan H and Wang X, ACS nano, 2010, 4, 2773–2783. [DOI] [PubMed] [Google Scholar]
- 326.Shukla R, Thomas TP, Peters J, Kotlyar A, Myc A and Baker JR Jr, Chemical communications, 2005, 5739–5741. [DOI] [PubMed] [Google Scholar]
- 327.Ding X, Su Y, Wang C, Zhang F, Chen K, Wang Y, Li M and Wang W, ACS Applied Materials and Interfaces, 2017, 9, 23353–23369. [DOI] [PubMed] [Google Scholar]
- 328.Ur Rehman SR, Augustine R, Zahid AA, Ahmed R and Hasan A, 2019. [Google Scholar]
- 329.Chaudhuri P, Harfouche R, Soni S, Hentschel DM and Sengupta S, ACS Nano, 2010, 4, 574–582. [DOI] [PubMed] [Google Scholar]
- 330.Pizzorno L, Integrative Medicine: A Clinician’s Journal, 2015, 14, 35. [PMC free article] [PubMed] [Google Scholar]
- 331.Dzondo-Gadet M, Mayap-Nzietchueng R, Hess K, Nabet P, Belleville F and Dousset B, Biological Trace Element Research, 2002, 85, 23–33. [DOI] [PubMed] [Google Scholar]
- 332.Balasubramanian P, Buettner T, Pacheco VM and Boccaccini AR, Journal of the European Ceramic Society, 2018, 38, 855–869. [Google Scholar]
- 333.Liu X, Rahaman MN and Day DE, Journal of Materials Science: Materials in Medicine, 2013, 24, 583–595. [DOI] [PubMed] [Google Scholar]
- 334.Chen S, Yang Q, Brow RK, Liu K, Brow KA, Ma Y and Shi H, Materials Science and Engineering: C, 2017, 73, 447–455. [DOI] [PubMed] [Google Scholar]
- 335.Durand LAH, Góngora A, López JMP, Boccaccini AR, Zago MP, Baldi A and Gorustovich A, Journal of Materials Chemistry B, 2014, 2, 7620–7630. [DOI] [PubMed] [Google Scholar]
- 336.Durand LAH, Vargas GE, Romero NM, Vera-Mesones R, Porto-López JM, Boccaccini AR, Zago MP, Baldi A and Gorustovich A, Journal of Materials Chemistry B, 2015, 3, 1142–1148. [DOI] [PubMed] [Google Scholar]
- 337.Jung SB and Day DE, Journal, 2012. [Google Scholar]
- 338.Wray P, Am. Ceram. Soc. Bull, 2011, 90, 24–31. [Google Scholar]
- 339.Hench LL, Annals of the New York academy of sciences, 1988, 523, 54–71. [DOI] [PubMed] [Google Scholar]
- 340.Clapham DE, Cell, 1995, 80, 259–268. [DOI] [PubMed] [Google Scholar]
- 341.Berridge MJ, Lipp P and Bootman MD, Nature reviews Molecular cell biology, 2000, 1, 11. [DOI] [PubMed] [Google Scholar]
- 342.Munaron L, Distasi C, Carabelli V, Baccino FM, Bonelli G and Lovisolo D, The Journal of physiology, 1995, 484, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wu H. m., Yuan Y, Zawieja DC, Tinsley J and Granger HJ, American Journal of Physiology-Heart and Circulatory Physiology, 1999, 276, H535–H542. [DOI] [PubMed] [Google Scholar]
- 344.Munaron L and Fiorio Pla A, Journal of cellular physiology, 2000, 185, 454–463. [DOI] [PubMed] [Google Scholar]
- 345.Cross MJ and Claesson-Welsh L, Trends in pharmacological sciences, 2001, 22, 201–207. [DOI] [PubMed] [Google Scholar]
- 346.Liang K, Jiang Z, Zhao B, Shen J, Huang D and Tao L, British Journal of Ophthalmology, 2012, 96, 1246–1251. [DOI] [PubMed] [Google Scholar]
- 347.Malhotra A and Habibovic P, Trends in biotechnology, 2016, 34, 983–992. [DOI] [PubMed] [Google Scholar]
- 348.Hench LL, Journal of the European Ceramic Society, 2009, 29, 1257–1265. [Google Scholar]
- 349.Jung S, Day D, Day T, Stoecker W and Taylor P, Wound Repair and Regeneration, 2011, 19. [Google Scholar]
- 350.Kargozar S, Baino F, Hoseini SJ, Hamzehlou S, Darroudi M, Verdi J, Hasanzadeh L, Kim H-W and Mozafari M, Nanomedicine, 2018, 13, 3051–3069. [DOI] [PubMed] [Google Scholar]
- 351.Das S, Singh S, Dowding JM, Oommen S, Kumar A, Sayle TX, Saraf S, Patra CR, Vlahakis NE and Sayle DC, Biomaterials, 2012, 33, 7746–7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Das S, Chigurupati S, Dowding J, Munusamy P, Baer DR, McGinnis JF, Mattson MP, Self W and Seal S, MRS Bulletin, 2014, 39, 976–983. [Google Scholar]
- 353.Chigurupati S, Mughal MR, Okun E, Das S, Kumar A, McCaffery M, Seal S and Mattson MP, Biomaterials, 2013, 34, 2194–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Nethi SK, Nanda HS, Steele TW and Patra CR, Journal of Materials Chemistry B, 2017, 5, 9371–9383. [DOI] [PubMed] [Google Scholar]
- 355.Augustine R, Hasan A, Patan NK, Dalvi YB, Varghese R, Antony A, Unni RN, Sandhyarani N and Moustafa A-EA, ACS Biomaterials Science & Engineering, 2020, 6, 58–70. [DOI] [PubMed] [Google Scholar]
- 356.Augustine R, Dalvi YB, Dan P, George N, Helle D, Varghese R, Thomas S, Menu P and Sandhyarani N, ACS Biomaterials Science & Engineering, 2018, 4, 4338–4353. [DOI] [PubMed] [Google Scholar]
- 357.Saghiri MA, Orangi J, Asatourian A, Sorenson CM and Sheibani N, Critical reviews in oncology/hematology, 2016, 98, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Dowding JM, Das S, Kumar A, Dosani T, McCormack R, Gupta A, Sayle TX, Sayle DC, von Kalm L and Seal S, ACS nano, 2013, 7, 4855–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Lord MS, Tsoi B, Gunawan C, Teoh WY, Amal R and Whitelock JM, Biomaterials, 2013, 34, 8808–8818. [DOI] [PubMed] [Google Scholar]
- 360.Salinas A, Shruti S, Malavasi G, Menabue L and Vallet-Regí M, Acta biomaterialia, 2011, 7, 3452–3458. [DOI] [PubMed] [Google Scholar]
- 361.Shruti S, Salinas AJ, Malavasi G, Lusvardi G, Menabue L, Ferrara C, Mustarelli P and Vallet-Regì M, Journal of Materials Chemistry, 2012, 22, 13698–13706. [Google Scholar]
- 362.Shruti S, Andreatta F, Furlani E, Marin E, Maschio S and Fedrizzi L, Applied Surface Science, 2016, 378, 216–223. [Google Scholar]
- 363.Chachami G, Simos G, Hatziefthimiou A, Bonanou S, Molyvdas P-A and Paraskeva E, American journal of respiratory cell and molecular biology, 2004, 31, 544–551. [DOI] [PubMed] [Google Scholar]
- 364.Bracken C, Whitelaw M and Peet D, Cellular and Molecular Life Sciences CMLS, 2003, 60, 1376–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Wang GL, Jiang B-H, Rue EA and Semenza GL, Proceedings of the national academy of sciences, 1995, 92, 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Carmeliet P and Jain RK, Nature, 2011, 473, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Peters RM, Willemse P, Rijk PC, Hoogendoorn M and Zijlstra WP, Case reports in orthopedics, 2017, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Green B, Griffiths E and Almond S, BMC psychiatry, 2017, 17, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Jantzen C, Jørgensen HL, Duus BR, Sporring SL and Lauritzen JB, Acta orthopaedica, 2013, 84, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Wu C, Zhou Y, Fan W, Han P, Chang J, Yuen J, Zhang M and Xiao Y, Biomaterials, 2012, 33, 2076–2085. [DOI] [PubMed] [Google Scholar]
- 371.Azevedo MM, Tsigkou O, Nair R, Jones JR, Jell G and Stevens MM, Tissue Engineering Part A, 2014, 21, 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Azevedo M, Jell G, O’donnell M, Law R, Hill R and Stevens M, Journal of Materials Chemistry, 2010, 20, 8854–8864. [Google Scholar]
- 373.Littmann E, Autefage H, Solanki A, Kallepitis C, Jones J, Alini M, Peroglio M and Stevens M, Journal of the European Ceramic Society, 2018, 38, 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ren H, Cao Y, Zhao Q, Li J, Zhou C, Liao L, Jia M, Zhao Q, Cai H and Han ZC, Biochemical and biophysical research communications, 2006, 347, 12–21. [DOI] [PubMed] [Google Scholar]
- 375.Lee H-H, Chang C-C, Shieh M-J, Wang J-P, Chen Y-T, Young T-H and Hung S-C, Scientific reports, 2013, 3, 2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zhou S, Cui Z and Urban JP, Arthritis & Rheumatism, 2004, 50, 3915–3924. [DOI] [PubMed] [Google Scholar]
- 377.Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS and Zelzer E, Development, 2007, 134, 3917–3928. [DOI] [PubMed] [Google Scholar]
- 378.Zhang C, Yang F, Cornelia R, Tang W, Swisher S and Kim H, Bone, 2011, 48, 507–513. [DOI] [PubMed] [Google Scholar]
- 379.Kargozar S, Lotfibakhshaiesh N, Ai J, Samadikuchaksaraie A, Hill RG, Shah PA, Milan PB, Mozafari M, Fathi M and Joghataei MT, Journal of Non-Crystalline Solids, 2016, 449, 133–140. [Google Scholar]
- 380.Kargozar S, Baino F, Lotfibakhshaiesh N, Hill RG, Milan PB, Hamzehlou S, Joghataei MT and Mozafari M, Materials Today: Proceedings, 2018, 5, 15768–15775. [Google Scholar]
- 381.Lison D, Carbonnelle P, Mollo L, Lauwerys R and Fubini B, Chemical research in toxicology, 1995, 8, 600–606. [DOI] [PubMed] [Google Scholar]
- 382.Wild P, Perdrix A, Romazini S, Moulin J-J and Pellet F, Occupational and environmental medicine, 2000, 57, 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Tanaka J, Moriyama H, Terada M, Takada T, Suzuki E, Narita I, Kawabata Y, Yamaguchi T, Hebisawa A and Sakai F, BMJ open, 2014, 4, e004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Liu L-Z, Ding M, Zheng JZ, Zhu Y, Fenderson BA, Li B, Jing JY and Jiang B-H, Biological trace element research, 2015, 166, 57–65. [DOI] [PubMed] [Google Scholar]
- 385.Urso E and Maffia M, Journal of vascular research, 2015, 52, 172–196. [DOI] [PubMed] [Google Scholar]
- 386.Li Q.-f., Ding X.-q. and Kang YJ, The Journal of nutritional biochemistry, 2014, 25, 44–49. [DOI] [PubMed] [Google Scholar]
- 387.Rigiracciolo DC, Scarpelli A, Lappano R, Pisano A, Santolla MF, De Marco P, Cirillo F, Cappello AR, Dolce V and Belfiore A, Oncotarget, 2015, 6, 34158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D and Marshall CJ, Cancer cell, 2006, 9, 33–44. [DOI] [PubMed] [Google Scholar]
- 389.Finney L, Vogt S, Fukai T and Glesne D, Clinical and Experimental Pharmacology and Physiology, 2009, 36, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Nasulewicz A, Mazur A and Opolski A, Journal of Trace Elements in Medicine and Biology, 2004, 18, 1–8. [DOI] [PubMed] [Google Scholar]
- 391.Goodman V, Brewer G and Merajver S, Endocrine-related cancer, 2004, 11, 255–263. [DOI] [PubMed] [Google Scholar]
- 392.Brem S, Cancer Control, 1999, 6, 1–18. [PubMed] [Google Scholar]
- 393.Pan Q, Kleer CG, Van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM and Dick RD, Cancer research, 2002, 62, 4854–4859. [PubMed] [Google Scholar]
- 394.Bührer G, Rottensteiner U, Hoppe A, Detsch R, Dafinova D, Fey T, Greil P, Weis C, Beier JP, Boccacini AR, Horch RE and Arkudas A, Biomedical Glasses, 2016, 2, 111–117. [Google Scholar]
- 395.Zhao S, Li L, Wang H, Zhang Y, Cheng X, Zhou N, Rahaman MN, Liu Z, Huang W and Zhang C, Biomaterials, 2015, 53, 379–391. [DOI] [PubMed] [Google Scholar]
- 396.Bi L, Rahaman MN, Day DE, Brown Z, Samujh C, Liu X, Mohammadkhah A, Dusevich V, Eick JD and Bonewald LF, Acta biomaterialia, 2013, 9, 8015–8026. [DOI] [PubMed] [Google Scholar]
- 397.Watters RJ, Brown RF and Day DE, Biomedical Glasses, 2015, 1, 173–184. [Google Scholar]
- 398.Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J and Xiao Y, Biomaterials, 2013, 34, 422–433. [DOI] [PubMed] [Google Scholar]
- 399.Baino F, Journal of Biomedical Materials Research Part A, 2015, 103, 1259–1275. [DOI] [PubMed] [Google Scholar]
- 400.Wang C, Jin K, He J, Wang J, Yang X, Yao C, Dai X, Gao C, Gou Z and Ye J, Journal of Biomedical Nanotechnology, 2018, 14, 688–697. [DOI] [PubMed] [Google Scholar]
- 401.Baino F, Potestio I and Vitale-Brovarone C, Materials, 2018, 11, 1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Chen Z, Meng H, Xing G, Chen C, Zhao Y, Jia G, Wang T, Yuan H, Ye C and Zhao F, Toxicology letters, 2006, 163, 109–120. [DOI] [PubMed] [Google Scholar]
- 403.Mroczek-Sosnowska N, Sawosz E, Vadalasetty K, Łukasiewicz M, Niemiec J, Wierzbicki M, Kutwin M, Jaworski S and Chwalibog A, International journal of molecular sciences, 2015, 16, 4838–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Maloubier M, Shuh DK, Minasian SG, Pacold JI, Solari P-L, Michel H, Oberhaensli F. o. R., Bottein Y, Monfort M and Moulin C, Environmental science & technology, 2016, 50, 10730–10738. [DOI] [PubMed] [Google Scholar]
- 405.McMahon B, Mauer P, McCoy CP, Lee TC and Gunnlaugsson T, Journal of the American Chemical Society, 2009, 131, 17542–17543. [DOI] [PubMed] [Google Scholar]
- 406.Fan Y, Yang P, Huang S, Jiang J, Lian H and Lin J, The Journal of Physical Chemistry C, 2009, 113, 7826–7830. [Google Scholar]
- 407.Miao G, Chen X, Mao C, Li X, Li Y and Lin C, Journal of sol-gel science and technology, 2014, 69, 250–259. [Google Scholar]
- 408.Li G, Liang G, Zhao S, Ma K, Feng W, Zhou D and Liu X, Advances in Applied Ceramics, 2015, 114, 164–174. [Google Scholar]
- 409.Patra CR, Bhattacharya R, Patra S, Vlahakis NE, Gabashvili A, Koltypin Y, Gedanken A, Mukherjee P and Mukhopadhyay D, Advanced Materials, 2008, 20, 753–756. [Google Scholar]
- 410.Augustine R, Nethi SK, Kalarikkal N, Thomas S and Patra CR, Journal of Materials Chemistry B, 2017, 5, 4660–4672. [DOI] [PubMed] [Google Scholar]
- 411.Patra CR, Kim J-H, Pramanik K, d’Uscio LV, Patra S, Pal K, Ramchandran R, Strano MS and Mukhopadhyay D, Nano letters, 2011, 11, 4932–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zhao H, Osborne OJ, Lin S, Ji Z, Damoiseux R, Wang Y, Nel AE and Lin S, Small, 2016, 12, 4404–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Bollu VS, Nethi SK, Dasari RK, Rao SSN, Misra S and Patra CR, Nanotoxicology, 2016, 10, 413–425. [DOI] [PubMed] [Google Scholar]
- 414.Ma X, Liu Y, Wu XM, Wang C, Li Q and Fu T, Materials Technology, 2016, 31, 23–27. [Google Scholar]
- 415.Ge K, Sun W, Zhang S, Wang S, Jia G, Zhang C and Zhang J, RSC Advances, 2016, 6, 21725–21734. [Google Scholar]
- 416.Bhattacharya R, Mukherjee P, Xiong Z, Atala A, Soker S and Mukhopadhyay D, Nano Letters, 2004, 4, 2479–2481. [Google Scholar]
- 417.Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, Atala A, Mukhopadhyay D and Soker S, Clinical Cancer Research, 2005, 11, 3530. [DOI] [PubMed] [Google Scholar]
- 418.Maxwell DJ, Taylor JR and Nie S, Journal of the American Chemical Society, 2002, 124, 9606–9612. [DOI] [PubMed] [Google Scholar]
- 419.Ferrara N, Gerber H-P and LeCouter J, Nature Medicine, 2003, 9, 669–676. [DOI] [PubMed] [Google Scholar]
- 420.Bhattacharya R and Mukherjee P, Advanced Drug Delivery Reviews, 2008, 60, 1289–1306. [DOI] [PubMed] [Google Scholar]
- 421.Darweesh RS, Ayoub NM and Nazzal S, International Journal of Nanomedicine, 2019, 14, 7643–7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Arvizo RR, Rana S, Miranda OR, Bhattacharya R, Rotello VM and Mukherjee P, Nanomedicine : nanotechnology, biology, and medicine, 2011, 7, 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Jain S, Hirst DG and O’Sullivan JM, The British Journal of Radiology, 2012, 85, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Barnard PJ and Berners-Price SJ, Coordination Chemistry Reviews, 2007, 251, 1889–1902. [Google Scholar]
- 425.Arvizo RR, Saha S, Wang E, Robertson JD, Bhattacharya R and Mukherjee P, Proceedings of the National Academy of Sciences, 2013, 110, 6700–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Thiery JP, Acloque H, Huang RYJ and Nieto MA, Cell, 2009, 139, 871–890. [DOI] [PubMed] [Google Scholar]
- 427.Cooke Vesselina G., LeBleu Valerie S., Keskin D, Khan Z, O’Connell Joyce T., Teng Y, Duncan Michael B., Xie L, Maeda G, Vong S, Sugimoto H, Rocha Rafael M., Damascena A, Brentani Ricardo R. and Kalluri R, Cancer Cell, 2012, 21, 66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Conde J, Larguinho M, Cordeiro A, Raposo LR, Costa PM, Santos S, Diniz MS, Fernandes AR and Baptista PV, Nanotoxicology, 2014, 8, 521–532. [DOI] [PubMed] [Google Scholar]
- 429.Mukherjee P, Bhattacharya R, Bone N, Lee YK, Patra CR, Wang S, Lu L, Secreto C, Banerjee PC, Yaszemski MJ, Kay NE and Mukhopadhyay D, Journal of Nanobiotechnology, 2007, 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Mangadlao JD, Wang X, McCleese C, Escamilla M, Ramamurthy G, Wang Z, Govande M, Basilion JP and Burda C, ACS Nano, 2018, 12, 3714–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Abbaspour N, Hurrell R and Kelishadi R, Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences, 2014, 19, 164. [PMC free article] [PubMed] [Google Scholar]
- 432.Ohara T, Noma K, Urano S, Watanabe S, Nishitani S, Tomono Y, Kimura F, Kagawa S, Shirakawa Y and Fujiwara T, International journal of cancer, 2013, 132, 2705–2713. [DOI] [PubMed] [Google Scholar]
- 433.Le NT and Richardson DR, Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 2002, 1603, 31–46. [DOI] [PubMed] [Google Scholar]
- 434.Hu G. f., Journal of cellular biochemistry, 1998, 69, 326–335. [DOI] [PubMed] [Google Scholar]
- 435.Patel A and Knowles J, Journal of Materials Science: Materials in Medicine, 2006, 17, 937–944. [DOI] [PubMed] [Google Scholar]
- 436.Mohammadi MS, Ahmed I, Muja N, Almeida S, Rudd C, Bureau M and Nazhat S, Acta biomaterialia, 2012, 8, 1616–1626. [DOI] [PubMed] [Google Scholar]
- 437.Zhu L, Staley C, Kooby D, El-Rays B, Mao H and Yang L, Cancer letters, 2017, 388, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V and Jordan A, Journal of neuro-oncology, 2011, 103, 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Kievit FM and Zhang M, Accounts of chemical research, 2011, 44, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Yigit MV, Moore A and Medarova Z, Pharmaceutical research, 2012, 29, 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Dissanayake N, Current K and Obare S, International journal of molecular sciences, 2015, 16, 23482–23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Oruch R, Elderbi MA, Khattab HA, Pryme IF and Lund A, European journal of pharmacology, 2014, 740, 464–473. [DOI] [PubMed] [Google Scholar]
- 443.Chiu C-T, Wang Z, Hunsberger JG and Chuang D-M, Pharmacological reviews, 2013, 65, 105–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Mao CD, Hoang P and DiCorleto PE, Journal of Biological Chemistry, 2001, 276, 26180–26188. [DOI] [PubMed] [Google Scholar]
- 445.Takada Y, Fang X, Jamaluddin MS, Boyd DD and Aggarwal BB, Journal of Biological Chemistry, 2004, 279, 39541–39554. [DOI] [PubMed] [Google Scholar]
- 446.GILES JJ and BANNIGAN JG, The Journal of Anatomy, 1999, 194, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Guo S, Arai K, Stins MF, Chuang D-M and Lo EH, Stroke, 2009, 40, 652–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Zeilbeck LF, Müller B, Knobloch V, Tamm ER and Ohlmann A, PloS one, 2014, 9, e95546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Haro Durand L, Vargas G, Vera-Mesones R, Baldi A, Zago M, Fanovich M, Boccaccini A and Gorustovich A, Materials, 2017, 10, 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Vilarinho PM, Barroca N, Zlotnik S, Félix P and Fernandes MH, Materials Science and Engineering: C, 2014, 39, 395–402. [DOI] [PubMed] [Google Scholar]
- 451.Brückner R, Tylkowski M, Hupa L and Brauer DS, Journal of Materials Chemistry B, 2016, 4, 3121–3134. [DOI] [PubMed] [Google Scholar]
- 452.Miguez-Pacheco V, Büttner T, Maçon A, Jones J, Fey T, De Ligny D, Greil P, Chevalier J, Malchere A and Boccaccini A, Journal of Non-Crystalline Solids, 2016, 432, 65–72. [Google Scholar]
- 453.Han P, Xu M, Chang J, Chakravorty N, Wu C and Xiao Y, Biomaterials Science, 2014, 2, 1230–1243. [DOI] [PubMed] [Google Scholar]
- 454.Bernardini D, Nasulewicz A, Mazur A and Maier J, Front Biosci, 2005, 10, 1177–1182. [DOI] [PubMed] [Google Scholar]
- 455.Zhai W, Lu H, Chen L, Lin X, Huang Y, Dai K, Naoki K, Chen G and Chang J, Acta Biomaterialia, 2012, 8, 341–349. [DOI] [PubMed] [Google Scholar]
- 456.Shamosi A, Farokhi M, Ai J and Sharifi E, Journal of Medical Hypotheses and Ideas, 2015, 9, 94–98. [Google Scholar]
- 457.Hallab NJ, Anderson S, Caicedo M and Jacobs JJ, Journal of ASTM International, 2006, 3, 1–12. [Google Scholar]
- 458.Miguez-Pacheco V, De Ligny D, Schmidt J, Detsch R and Boccaccini A, Journal of the European Ceramic Society, 2018, 38, 871–876. [Google Scholar]
- 459.Takeda E, Taketani Y, Sawada N, Sato T and Yamamoto H, Biofactors, 2004, 21, 345–355. [DOI] [PubMed] [Google Scholar]
- 460.Jin H, Chang S-H, Xu C-X, Shin J-Y, Chung Y-S, Park S-J, Lee Y-S, An G-H, Lee K-H and Cho M-H, Toxicological sciences, 2007, 100, 215–223. [DOI] [PubMed] [Google Scholar]
- 461.Kume T, Trends in cardiovascular medicine, 2008, 18, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Hirama M, Takahashi F, Takahashi K, Akutagawa S, Shimizu K, Soma S, Shimanuki Y, Nishio K and Fukuchi Y, Cancer letters, 2003, 198, 107–117. [DOI] [PubMed] [Google Scholar]
- 463.Camalier CE, Yi M, Yu LR, Hood BL, Conrads KA, Lee YJ, Lin Y, Garneys LM, Bouloux GF and Young MR, Journal of cellular physiology, 2013, 228, 1536–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Lin Y, McKinnon KE, Ha SW and Beck GR Jr, Molecular carcinogenesis, 2015, 54, 926–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D and Pavenstadt H, American Journal of Physiology-Renal Physiology, 2008, 294, F1381–F1387. [DOI] [PubMed] [Google Scholar]
- 466.Kargozar S, Baino F, Hamzehlou S, Hill RG and Mozafari M, Trends in biotechnology, 2018, 36, 430–444. [DOI] [PubMed] [Google Scholar]
- 467.Stähli C, Muja N and Nazhat SN, Tissue Engineering Part A, 2012, 19, 548–557. [DOI] [PubMed] [Google Scholar]
- 468.Lee I-H, Yu H.-s., Lakhkar NJ, Kim H-W, Gong M-S, Knowles JC and Wall IB, Materials Science and Engineering: C, 2013, 33, 2104–2112. [DOI] [PubMed] [Google Scholar]
- 469.Ip C, The Journal of nutrition, 1998, 128, 1845–1854. [DOI] [PubMed] [Google Scholar]
- 470.Combs G Jr, Clark L and Turnbull B, Biofactors, 2001, 14, 153–159. [DOI] [PubMed] [Google Scholar]
- 471.El-Bayoumy K, Cancer research, 1985, 45, 3631–3635. [PubMed] [Google Scholar]
- 472.Jacobs MM, Shubik P and Feldman R, Cancer letters, 1980, 9, 353–357. [DOI] [PubMed] [Google Scholar]
- 473.Corcoran NM, Najdovska M and Costello AJ, The Journal of urology, 2004, 171, 907–910. [DOI] [PubMed] [Google Scholar]
- 474.Jiang C, Ganther H and Lu J, Molecular Carcinogenesis: Published in cooperation with the University of Texas MD Anderson Cancer Center, 2000, 29, 236–250. [DOI] [PubMed] [Google Scholar]
- 475.Jiang C, Jiang W, Ip C, Ganther H and Lu J, Molecular Carcinogenesis: Published in cooperation with the University of Texas MD Anderson Cancer Center, 1999, 26, 213–225. [DOI] [PubMed] [Google Scholar]
- 476.Levy AP, Levy NS, Wegner S and Goldberg MA, Journal of Biological Chemistry, 1995, 270, 13333–13340. [DOI] [PubMed] [Google Scholar]
- 477.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K and Yodoi J, Proceedings of the National Academy of Sciences, 1997, 94, 3633–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Gallegos A, Berggren M, Gasdaska JR and Powis G, Cancer research, 1997, 57, 4965–4970. [PubMed] [Google Scholar]
- 479.Spyrou G, Björnstedt M, Kumar S and Holmgren A, FEBS letters, 1995, 368, 59–63. [DOI] [PubMed] [Google Scholar]
- 480.Kim IY and Stadtman TC, Proceedings of the National Academy of Sciences, 1997, 94, 12904–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Aksakal B and Boccaccini A, Materials Letters, 2012, 76, 177–180. [Google Scholar]
- 482.Stevanović M, Filipović N, Djurdjević J, Lukić M, Milenković M and Boccaccini A, Colloids and Surfaces B: Biointerfaces, 2015, 132, 208–215. [DOI] [PubMed] [Google Scholar]
- 483.Wang X, Zhang Y, Ma Y, Chen D, Yang H and Li M, Ceramics International, 2016, 42, 3609–3617. [Google Scholar]
- 484.Duan J, Yu Y, Li Y, Yu Y and Sun Z, Biomaterials, 2013, 34, 5853–5862. [DOI] [PubMed] [Google Scholar]
- 485.Duan J, Yu Y, Yu Y, Li Y, Huang P, Zhou X, Peng S and Sun Z, Particle and fibre toxicology, 2014, 11, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Li H and Chang J, Acta biomaterialia, 2013, 9, 6981–6991. [DOI] [PubMed] [Google Scholar]
- 487.Gerhardt L-C, Widdows KL, Erol MM, Burch CW, Sanz-Herrera JA, Ochoa I, Stämpfli R, Roqan IS, Gabe S and Ansari T, Biomaterials, 2011, 32, 4096–4108. [DOI] [PubMed] [Google Scholar]
- 488.Detsch R, Stoor P, Grünewald A, Roether JA, Lindfors NC and Boccaccini AR, Journal of Biomedical Materials Research Part A, 2014, 102, 4055–4061. [DOI] [PubMed] [Google Scholar]
- 489.Mao C, Chen X, Miao G and Lin C, Biomedical materials, 2015, 10, 025005. [DOI] [PubMed] [Google Scholar]
- 490.Day RM, Boccaccini AR, Shurey S, Roether JA, Forbes A, Hench LL and Gabe SM, Biomaterials, 2004, 25, 5857–5866. [DOI] [PubMed] [Google Scholar]
- 491.Gorustovich AA, Roether JA and Boccaccini AR, Tissue Engineering Part B: Reviews, 2009, 16, 199–207. [DOI] [PubMed] [Google Scholar]
- 492.Keshaw H, Forbes A and Day RM, Biomaterials, 2005, 26, 4171–4179. [DOI] [PubMed] [Google Scholar]
- 493.Alt V, Kögelmaier DV, Lips KS, Witt V, Pacholke S, Heiss C, Kampschulte M, Heinemann S, Hanke T and Schnettler R, Acta biomaterialia, 2011, 7, 3773–3779. [DOI] [PubMed] [Google Scholar]
- 494.Wu C and Chang J, Journal of Controlled Release, 2014, 193, 282–295. [DOI] [PubMed] [Google Scholar]
- 495.Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G and Galdiero M, Molecules, 2015, 20, 8856–8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Gurunathan S, Lee K-J, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R and Eom SH, Biomaterials, 2009, 30, 6341–6350. [DOI] [PubMed] [Google Scholar]
- 497.Kalishwaralal K, Banumathi E, Pandian SRK, Deepak V, Muniyandi J, Eom SH and Gurunathan S, Colloids and Surfaces B: Biointerfaces, 2009, 73, 51–57. [DOI] [PubMed] [Google Scholar]
- 498.Baharara J, Namvar F, Mousavi M, Ramezani T and Mohamad R, Molecules, 2014, 19, 13498–13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Bhagavathy S and Kancharla S, Int J Herb Med, 2016, 4, 24–29. [Google Scholar]
- 500.Kang K, Lim D-H, Choi I-H, Kang T, Lee K, Moon E-Y, Yang Y, Lee M-S and Lim J-S, Toxicology letters, 2011, 205, 227–234. [DOI] [PubMed] [Google Scholar]
- 501.Milner JA, in Nutrition and Cancer Prevention, Springer, 2001, pp. 69–81. [Google Scholar]
- 502.Jiang B, Tang G, Cao K, Wu L and Wang R, Antioxidants & redox signaling, 2010, 12, 1167–1178. [DOI] [PubMed] [Google Scholar]
- 503.Szabó C and Papapetropoulos A, British journal of pharmacology, 2011, 164, 853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN and Wang R, Proceedings of the National Academy of Sciences, 2009, 106, 21972–21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Cai W-J, Wang M-J, Moore PK, Jin H-M, Yao T and Zhu Y-C, Cardiovascular research, 2007, 76, 29–40. [DOI] [PubMed] [Google Scholar]
- 506.van Wijk XM and van Kuppevelt TH, Angiogenesis, 2014, 17, 443–462. [DOI] [PubMed] [Google Scholar]
- 507.Raman K, Karuturi R, Swarup VP, Desai UR and Kuberan B, Bioorganic & medicinal chemistry letters, 2012, 22, 4467–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Nethi SK, Barui AK, Bollu VS, Rao BR and Patra CR, ACS Biomaterials Science & Engineering, 2017, 3, 3635–3645. [DOI] [PubMed] [Google Scholar]
- 509.Giustarini D, Milzani A, Colombo R, Dalle-Donne I and Rossi R, Clinica chimica acta, 2003, 330, 85–98. [DOI] [PubMed] [Google Scholar]
- 510.Osman R and Swain M, Materials, 2015, 8, 932–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Sun Y, Li Y, Wu B, Wang J, Lu X, Qu S, Weng J and Feng B, Acta biomaterialia, 2017, 58, 527–538. [DOI] [PubMed] [Google Scholar]
- 512.Saghiri M-A, Asatourian A, Garcia-Godoy F and Sheibani N, Medicina oral, patologia oral y cirugia bucal, 2016, 21, e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Spriano S, Yamaguchi S, Baino F and Ferraris S, Acta Biomaterialia, 2018, 79, 1–22. [DOI] [PubMed] [Google Scholar]
- 514.Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z and Boyan BD, Biomaterials, 2010, 31, 4909–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Rupp F, Scheideler L, Rehbein D, Axmann D and Geis-Gerstorfer J, Biomaterials, 2004, 25, 1429–1438. [DOI] [PubMed] [Google Scholar]
- 516.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL and Boyan B, Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 2005, 74, 49–58. [DOI] [PubMed] [Google Scholar]
- 517.Schwartz Z, Raz P, Zhao G, Barak Y, Tauber M, Yao H and Boyan BD, The Journal of Bone and Joint Surgery. American volume., 2008, 90, 2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Shi B, Andrukhov O, Berner S, Schedle A and Rausch-Fan X, Dental Materials, 2014, 30, 839–847. [DOI] [PubMed] [Google Scholar]
- 519.An N, Schedle A, Wieland M, Andrukhov O, Matejka M and Rausch-Fan X, Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 2010, 93, 364–372. [DOI] [PubMed] [Google Scholar]
- 520.Krenning G, van Luyn MJ and Harmsen MC, Trends in molecular medicine, 2009, 15, 180–189. [DOI] [PubMed] [Google Scholar]
- 521.Ziebart T, Yoon C-H, Trepels T, Wietelmann A, Braun T, Kiessling F, Stein S, Grez M, Ihling C and Muhly-Reinholz M, Circulation research, 2008, 103, 1327–1334. [DOI] [PubMed] [Google Scholar]
- 522.Ziebart T, Schnell A, Walter C, Kämmerer PW, Pabst A, Lehmann KM, Ziebart J, Klein MO and Al-Nawas B, Clinical oral investigations, 2013, 17, 301–309. [DOI] [PubMed] [Google Scholar]
- 523.Chen W, Xu K, Tao B, Dai L, Yu Y, Mu C, Shen X, Hu Y, He Y and Cai K, Acta biomaterialia, 2018, 74, 489–504. [DOI] [PubMed] [Google Scholar]
- 524.Zong M, Bai L, Liu Y, Wang X, Zhang X, Huang X, Hang R and Tang B, Materials Science and Engineering: C, 2017, 71, 93–99. [DOI] [PubMed] [Google Scholar]
- 525.Jo DH, Kim JH, Yu YS, Lee TG and Kim JH, Nanomedicine: Nanotechnology, Biology and Medicine, 2012, 8, 784–791. [DOI] [PubMed] [Google Scholar]
- 526.Augustine R, Hasan A, Patan NK, Augustine A, Dalvi YB, Varghese R, Unni RN, Kalarikkal N, Al Moustafa AE and Thomas S, Macromolecular bioscience, 2019, 1900058. [DOI] [PubMed] [Google Scholar]
- 527.Rahaman MN, Yao A, Bal BS, Garino JP and Ries MD, Journal of the American Ceramic Society, 2007, 90, 1965–1988. [Google Scholar]
- 528.Schubert D, Dargusch R, Raitano J and Chan S-W, Biochemical and biophysical research communications, 2006, 342, 86–91. [DOI] [PubMed] [Google Scholar]
- 529.Augustine R, Dalvi YB, Nath VY, Varghese R, Raghuveeran V, Hasan A, Thomas S and Sandhyarani N, Materials Science and Engineering: C, 2019, 103, 109801. [DOI] [PubMed] [Google Scholar]
- 530.Berg JM and Shi Y, Science, 1996, 271, 1081–1085. [DOI] [PubMed] [Google Scholar]
- 531.Boehm T, O’Reilly MS, Keough K, Shiloach J, Shapiro R and Folkman J, Biochemical and biophysical research communications, 1998, 252, 190–194. [DOI] [PubMed] [Google Scholar]
- 532.Kaji T, Fujiwara Y, Yamamoto C, Sakamoto M and Kozuka H, Life sciences, 1994, 55, 1781–1787. [DOI] [PubMed] [Google Scholar]
- 533.Shian S-G, Kao Y-R, Wu FY-H and Wu C-W, Molecular pharmacology, 2003, 64, 1076–1084. [DOI] [PubMed] [Google Scholar]
- 534.Nardinocchi L, Pantisano V, Puca R, Porru M, Aiello A, Grasselli A, Leonetti C, Safran M, Rechavi G and Givol D, PloS one, 2010, 5, e15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Sheffer M, Simon AJ, Jacob-Hirsch J, Rechavi G, Domany E, Givol D and D’Orazi G, Oncotarget, 2011, 2, 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Augustine R, Dominic EA, Reju I, Kaimal B, Kalarikkal N and Thomas S, RSC Advances, 2014, 4, 51528–51536. [Google Scholar]
- 537.Barui AK, Nethi SK and Patra CR, Journal of Materials Chemistry B, 2017, 5, 3391–3403. [DOI] [PubMed] [Google Scholar]
- 538.Augustine R, Dan P, Sosnik A, Kalarikkal N, Tran N, Vincent B, Thomas S, Menu P and Rouxel D, Nano Research, 2017, 10, 3358–3376. [Google Scholar]
- 539.Watters RJ, Brown RF and Day DE, Biomedical glasses, 2015, 1. [Google Scholar]
- 540.Mohamed S, Parayath NN, Taurin S and Greish K, Therapeutic delivery, 2014, 5, 1101–1121. [DOI] [PubMed] [Google Scholar]
- 541.Giri S, Karakoti A, Graham RP, Maguire JL, Reilly CM, Seal S, Rattan R and Shridhar V, PLoS ONE, 2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Jo DH, Kim JH, Son JG, Song NW, Kim Y-I, Yu YS, Lee TG and Kim JH, Nanomedicine: Nanotechnology, Biology and Medicine, 2014, 10, e1109–e1117. [DOI] [PubMed] [Google Scholar]
- 543.Pathak AP, Hochfeld WE, Goodman SL and Pepper MS, Angiogenesis, 2008, 11, 321. [DOI] [PubMed] [Google Scholar]
- 544.Tang L, Sun X, Liu N, Zhou Z, Yu F, Zhang X, Sun X and Chen X, ACS Applied Nano Materials, 2018, 1, 1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Wu T, Ding X, Su B, Soodeen-Lalloo A, Zhang L and Shi J-Y, Clinical and Translational Oncology, 2018, 20, 599–606. [DOI] [PubMed] [Google Scholar]
- 546.Yan F, Xu X, Chen Y, Deng Z, Liu H, Xu J, Zhou J, Tan G, Wu J and Zheng H, Ultrasound in medicine & biology, 2015, 41, 2765–2773. [DOI] [PubMed] [Google Scholar]
- 547.O’Donnell M, Physica C: Superconductivity and its Applications, 2018, 548, 103–106. [Google Scholar]
- 548.Loudos G, Kagadis GC and Psimadas D, European journal of radiology, 2011, 78, 287–295. [DOI] [PubMed] [Google Scholar]
- 549.Strijkers GJ, Kluza E, Van Tilborg GA, van der Schaft DW, Griffioen AW, Mulder WJ and Nicolay K, Angiogenesis, 2010, 13, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Yuan H.-x., Wang W.-p., Wen J.-x., Lin L.-w., Exner AA, Guan P.-s. and Chen X.-j., Ultrasound in medicine & biology, 2018, 44, 1460–1467. [DOI] [PubMed] [Google Scholar]
- 551.Debordeaux F, Chansel-Debordeaux L, Pinaquy J-B, Fernandez P and Schulz J, Nuclear medicine and biology, 2018, 62, 31–46. [DOI] [PubMed] [Google Scholar]
- 552.Chen Y.-l., Liu F.-q., Guo Y, Cheng J, Yang L, Lu M, Li P, Xu J, Yu T and Wang Z.-g., Biomaterials science, 2018, 6, 2130–2143. [DOI] [PubMed] [Google Scholar]
- 553.Giraudeau C, Geffroy F, Mériaux S, Boumezbeur F, Robert P, Port M, Robic C, Le Bihan D, Lethimonnier F and Valette J, Angiogenesis, 2013, 16, 171–179. [DOI] [PubMed] [Google Scholar]
- 554.Wu M, Zhang Y, Zhang Y, Wu M, Wu M, Wu H, Cao L, Li L, Li X and Zhang X, RSC advances, 2018, 8, 1706–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Ryu JH, Shin J-Y, Kim SA, Kang S-W, Kim H, Kang S, Choi K, Kwon IC, Kim B-S and Kim K, Biomaterials, 2013, 34, 6871–6881. [DOI] [PubMed] [Google Scholar]
- 556.Lewis JD, Destito G, Zijlstra A, Gonzalez MJ, Quigley JP, Manchester M and Stuhlmann H, Nature medicine, 2006, 12, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Steinmetz NF, Nanomedicine: Nanotechnology, Biology and Medicine, 2010, 6, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Beatty PH and Lewis JD, Advanced Drug Delivery Reviews, 2019, 145, 130–144. [DOI] [PubMed] [Google Scholar]
- 559.Li X, Wu M, Wang J, Dou Y, Gong X, Liu Y, Guo Q, Zhang X, Chang J and Niu Y, Nanomedicine: Nanotechnology, Biology and Medicine, 2019, 15, 252–263. [DOI] [PubMed] [Google Scholar]
- 560.Burke BP, Cawthorne C and Archibald SJ, Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 2017, 375, 20170261. [DOI] [PubMed] [Google Scholar]
- 561.Zhang XQ, Lam R, Xu X, Chow EK, Kim HJ and Ho D, Advanced materials, 2011, 23, 4770–4775. [DOI] [PubMed] [Google Scholar]
- 562.Sun Y, Zhu X, Peng J and Li F, Acs Nano, 2013, 7, 11290–11300. [DOI] [PubMed] [Google Scholar]
- 563.Shi S, Yang K, Hong H, Chen F, Valdovinos HF, Goel S, Barnhart TE, Liu Z and Cai W, Biomaterials, 2015, 39, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Yang Y, Chen S, Li H, Yuan Y, Zhang Z, Xie J, Hwang DW, Zhang A, Liu M and Zhou X, Nano letters, 2018, 19, 441–448. [DOI] [PubMed] [Google Scholar]
- 565.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH and Libchaber A, Science, 2002, 298, 1759–1762. [DOI] [PubMed] [Google Scholar]
- 566.Zhang J, Hao G, Yao C, Yu J, Wang J, Yang W, Hu C and Zhang B, ACS applied materials & interfaces, 2016, 8, 16612–16621. [DOI] [PubMed] [Google Scholar]
- 567.Xing X, Zhang B, Wang X, Liu F, Shi D and Cheng Y, Biomaterials, 2015, 48, 16–25. [DOI] [PubMed] [Google Scholar]
- 568.Ma Y, Shen H, Zhang M and Zhang Z, in Nanomaterials for Tumor Targeting Theranostics: A Proactive Clinical Perspective, World Scientific, 2016, pp. 85–141. [Google Scholar]
- 569.Stroh M, Zimmer JP, Duda DG, Levchenko TS, Cohen KS, Brown EB, Scadden DT, Torchilin VP, Bawendi MG and Fukumura D, Nature medicine, 2005, 11, 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Chen S and Imoukhuede PI, Analytical Chemistry, 2019, 91, 7603–7612. [DOI] [PubMed] [Google Scholar]
- 571.Kieran MW, Kalluri R and Cho Y-J, Cold Spring Harbor perspectives in medicine, 2012, 2, a006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Ebos JM and Kerbel RS, Nature reviews Clinical oncology, 2011, 8, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Auguste P, Lemiere S, Larrieu-Lahargue F and Bikfalvi A, Critical reviews in oncology/hematology, 2005, 54, 53–61. [DOI] [PubMed] [Google Scholar]
- 574.Park IH, Sohn JH, Kim SB, Lee KS, Chung JS, Lee SH, Kim TY, Jung KH, Cho EK and Kim YS, Cancer research and treatment: official journal of Korean Cancer Association, 2017, 49, 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Saeed BA, Lim V, Yusof NA, Khor KZ, Rahman HS and Samad NA, International journal of nanomedicine, 2019, 14, 5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Jo DH, Kim JH, Son JG, Piao Y, Lee TG and Kim JH, Nano research, 2014, 7, 844–852. [Google Scholar]
- 577.Guarnieri D, Malvindi MA, Belli V, Pompa PP and Netti P, Journal of nanoparticle research, 2014, 16, 2229. [Google Scholar]
- 578.Augustine R, Prasad P and Khalaf IMN, Materials Science and Engineering C, 2019, 97, 994–1008. [DOI] [PubMed] [Google Scholar]
- 579.Augustine R, Mathew AP and Sosnik A, Applied Materials Today, 2017, 7, 91–103. [Google Scholar]
- 580.Nethi SK, Barui AK, Mukherjee S and Patra CR, Antioxidants & redox signaling, 2019, 30, 786–809. [DOI] [PubMed] [Google Scholar]
- 581.Barui AK, Nethi SK, Haque S, Basuthakur P and Patra CR, ACS Applied Bio Materials, 2019, 2, 5492–5511. [DOI] [PubMed] [Google Scholar]
- 582.Bilecka I and Niederberger M, Nanoscale, 2010, 2, 1358–1374. [DOI] [PubMed] [Google Scholar]
- 583.Rajasekharreddy P, Huang C, Busi S, Rajkumari J, Tai M-H and Liu G, Current medicinal chemistry, 2019, 26, 1311–1327. [DOI] [PubMed] [Google Scholar]