Abstract
BACKGROUND:
Cancer treatment costs are not routinely addressed in shared decisions for breast cancer surgery. Thus, we sought to characterize cost awareness and communication among surgeons treating breast cancer.
METHODS:
We conducted a self-administered, confidential electronic survey among members of the American Society of Breast Surgeons from July 1-September 15, 2018. Questions assessed surgeon demographics, cost sensitivity, and communication. Descriptive summaries and cross-tabulations with chi-square statistics were used, with exact tests where warranted, to assess findings.
RESULTS:
Of those surveyed (N=2293), 598 (25%) responded. Surgeons reported that “risk of recurrence” (70%), “appearance of the breast” (50%), and “risks of surgery” (47%) were the most influential on patients’ decisions for breast cancer surgery; 6% cited out-of-pocket costs as significant. Over half (53%) of surgeons agreed that doctors should consider patient costs when choosing cancer treatment, yet (58%) reported “infrequently” (43%) or “never” (15%) consider patient costs in medical recommendations. The overwhelming majority (87%) of surgeons believed that patients should have access to the costs of their treatment before making medical decisions. Surgeons treating a higher percentage of Medicaid or uninsured patients were more likely to consistently consider costs (p<0.001). Participants reported that insufficient knowledge or resources (61%), a perceived inability to help with costs (24%), and inadequate time (22%) impeded cost discussions. Notably, 20% believed that discussing costs might impact the quality of care patients receive.
CONCLUSIONS:
Cost transparency remains rare, yet, in shared decisions for breast cancer surgery, improved cost awareness by surgeons has the potential to reduce financial hardship.
INTRODUCTION
In the United States, approximately 250,000 women are diagnosed with breast cancer each year. [1–3] Since the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06, longstanding randomized trial data and contemporary observational series have demonstrated that lumpectomy with radiation and mastectomy result in comparable local recurrence rates and equivalent survival. [4–7] Women consider many factors when weighing each surgical choice, including but not limited to, their desire for breast preservation, options for reconstruction, aesthetic results, expected surveillance, risk of recurrence, and peace of mind.[8, 9] Ultimately, decisions for breast cancer surgery are highly preference-sensitive, and guided by patient values with recommendations from their oncology team.[8, 10]
In parallel, contemporary healthcare costs continue to rise, and high deductibles, co-payments and premiums have resulted in treatment-related financial hardship for up to 70% of cancer patients. [11–14] Financial hardship after cancer has been associated with poor quality of life, a greater risk of treatment non-adherence, bankruptcy, and, more recently, early death. [15–19] In 2009, the American Society of Clinical Oncology formally recognized treatment-related financial hardship as a major side effect of cancer care, and, more recently, personal spending burden has been proposed as national measure of high quality healthcare.[20–22] The oncology community has become increasingly aware that medical expenditures related to cancer treatment may have substantial financial implications for our patients.
Nevertheless, as women face decisions for breast cancer surgery, a setting that has otherwise epitomized patient-centered care, the financial consequences of surgical choice are not routinely addressed. [23, 24] A growing body of research suggests that comparably effective surgical treatments for breast cancer differ significantly in their risk of financial harm; bilateral mastectomy has been associated with higher patient-reported out-of-pocket costs, greater incurred debt, and disrupted or altered employment when compared to breast conservation.[23–26] Notably, past literature has demonstrated that surgeons strongly influence women’s choice for breast cancer surgery, and may contribute to the growing national trend of contralateral prophylactic mastectomy. [4, 23, 27, 28] Thus, we conducted a national survey of breast cancer surgeons to examine surgeon perspectives about the costs of cancer care, including their cost-awareness and practices with regards to cost communication, when counseling women with breast cancer.
METHODS
Following Institutional Review Board (IRB) Approval, the study was reviewed by the American Society of Breast Surgeons (ASBrS) Research Committee and approved by the Board of Directors. The American Society of Breast Surgeons (ASBrs) is a leadership organization for surgeons treating breast disease, and advocates to promote excellence in the care of breast patients through education, research and the development of advanced surgical techniques. The ASBrS membership received an electronic link to the 10-item anonymous survey, which took approximately 5 minutes to complete. The invitation to participate included a brief overview about the growing problem of cancer-related financial hardship, and national endorsements of patient-provider cost communication by professional oncologic societies. The survey also included questions evaluating surgeon demographics (i.e. age, gender, training, and practice setting), self-reported patient population, and perspectives and practices around cost awareness and communication in shared decisions for breast cancer surgery (Appendix 1). Additionally, costs were differentiated as patient (i.e. deductibles, co-payments, work absenteeism, and overall debt) versus societal or health system costs. The request to participate was sent on July 1, 2018, and three additional reminders (7/19/2018, 8/24/2018, and 9/12/2018) were sent prior to closing on September 15, 2018.
Descriptive statistics were used to summarize surgeon demographics and perspectives regarding patient costs. Surgeons were dichotomized according to their response to “How often do you consider patient out-of-pocket costs (i.e. deductibles and co-pays) when making treatment recommendations for breast cancer?” Those who responded “Most of the time” or “All of the time” were categorized as “Cost Sensitive”. All other responses, “Never” to “Sometimes”, were categorized as “Cost Insensitive”. Where appropriate, chi-squared and Fisher’s exact tests were performed to evaluate differences between these categories. All analyses were performed using SAS software version 9.4. Two-sided p-values less than 0.05 were deemed statistically significant.
RESULTS
Participant characteristics
Overall, 2434 members of the ASBrS were invited by email to participate. Twenty-nine invitations were un-receivable and 112 surgeons declined participation. Of the remaining 2293, 598 (25%) responded to the survey. The majority (65%) of participants were female and 34% were male. Twenty-eight percent was ≤45 years old (n=165), 31% (n=188) ranged from 46-55 years old, and 41% (n=245) were ≥56 years old. Thirty-one percent (n=187) reported practicing in an academic setting, 37% (n=221) as breast-only private practice surgeons, and 31% (n=188) as a general surgeons or surgical oncologists performing breast surgery in a private setting. Over half (51%) reported having practiced for greater than 20 years (n=302). Participant characteristics are outlined in Table 1.
Table 1.
Participant characteristics (N=598)
| n(%) | |
|---|---|
| Age (years) | |
| ≤45 | 165(28%) |
| 46-55 | 188(31%) |
| 56+ | 245(41%) |
| Gender | |
| Female | 391(65%) |
| Male | 206(34%) |
| Years in practice | |
| ≤10 | 127(31%) |
| 10-20 | 169(28%) |
| >20 | 302(51%) |
| Clinical setting | |
| Academic | 187(31%) |
| Private-breast only | 221(37%) |
| Private- general/surgical oncology | 188(31%) |
| Uninsured/Medicaid patients | |
| <20% | 328(55%) |
| 20-40% | 173(29%) |
| 40-100% | 96(16%) |
Cost consideration: perspectives and practices
Participants were asked to choose the three factors they believed were the most important variables to women facing decisions for breast cancer surgery (Figure 1). “Risk of recurrence” (70%), “appearance of the breast” (50%), and “risks of surgery” (47%) were the most heavily selected. Six percent of surgeons identified patient “out-of-pocket costs” as a priority for women facing surgical decisions. Overall, 53% (n=316) of surgeons agreed (44%) or strongly agreed (9%) that doctors should consider patient costs when choosing cancer treatments, while 24% (n=146) disagreed (17%) or strongly disagreed (7%). Nearly half (49%) believed that personal out-of-pocket and indirect costs were “infrequently” (45%) or “never” (4%) considered by women facing breast cancer treatment decisions. Similarly, the majority of surgeons (58%) reported “infrequently” (43%) or “never” (15%) considering patient out-of-pocket costs (i.e. deductibles and co-payments) themselves, when making medical recommendations. Ninety-two percent of surgeons believed that they considered cost about as much as or more than their patients (Figure 2). Overall, 92% of surgeons believed that they considered costs at a similar level (89%) or more than (3%) their patients, defined as reporting within one level of their patients or at least 2 levels higher, respectively.
Figure 1.
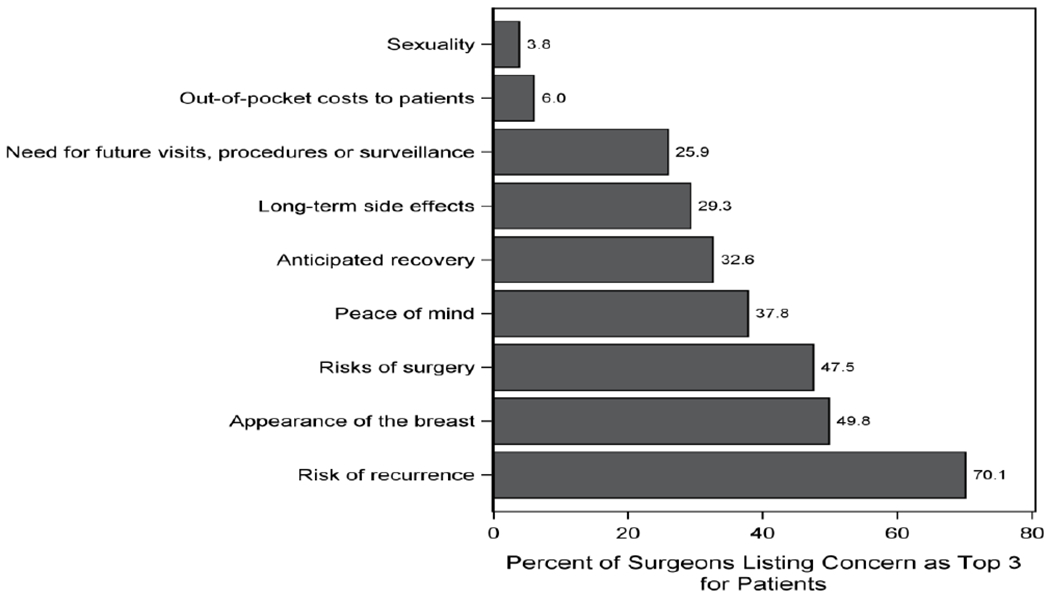
Surgeon perceptions of the factors that are most important to women facing decisions for breast cancer surgery (N= 598)
Figure 2.
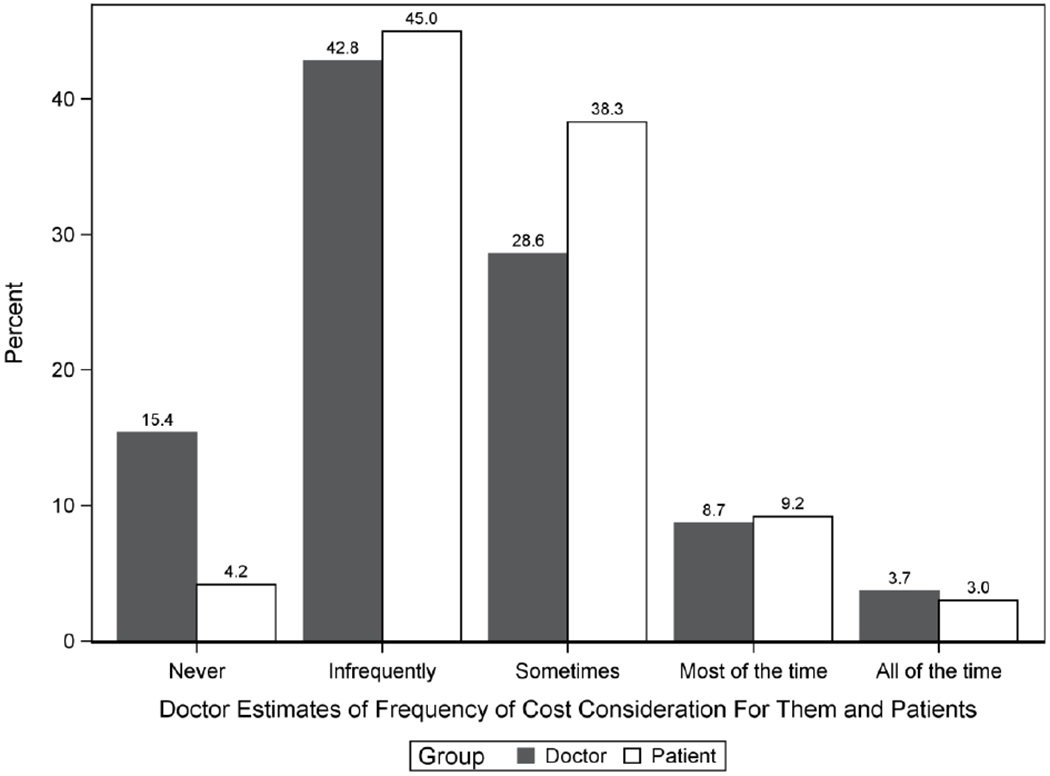
Participants’ cost consideration compared to their estimates of how frequently patients consider costs.
Surgeons reported selective cost sensitivity, with 36% who “agreed” or “strongly agreed” that patients’ insurance status and socioeconomic background influenced their consideration of patient costs. As a general statement, when choosing cancer treatment for an individual patient, 47% of respondents agreed that doctors should consider costs to society, while 34% disagreed. In response to the statement “If two treatments are equally effective, I believe doctors should recommend the less expensive one,” a 38% (n=226) agreed (24%) and strongly agreed (14%). Only 12% (n=74) reported consistently considering costs, while 87% (n=519) were admittedly inconsistent at best with cost consideration. Notably, there were no statistically discernible differences between surgeons who consistently considered costs and those who did not in age, gender, practice setting, or years since completion of training. However, consideration of patient costs was greater among surgeons treating a higher percentage of Medicaid or uninsured patients (Table 2). Importantly, respondents who reported consistently considering costs were more likely to endorse cost communication than surgeons who did not. This included beliefs that: a) doctors should explain to patients the costs of their cancer care (54% vs 20%, p<0.0001), b) doctors should consider costs to the patient when choosing cancer treatment (82% vs 49%, p<0.0001), and c) doctors should consider costs to society when choosing cancer treatment (62% vs. 45%, p=0.005).
Table 2.
Surgeon demographics and cost sensitivity.
| Characteristic | Cost Sensitive n=74 | Cost Insensitive n=519 | p-value |
|---|---|---|---|
| Age (years) | |||
| ≤45 | 19 (26%) | 146 (28%) | |
| 46-55 | 18 (24%) | 168 (32%) | 0.21 |
| ≥56 | 37 (50%) | 205 (40%) | |
| Gender | |||
| Female | 50 (68%) | 337 (65%) | 0.74 |
| Male | 24 (32%) | 181 (35%) | |
| Years in practice | |||
| ≤10 | 14 (19%) | 113 (22%) | |
| 10-20 | 22 (30%) | 146 (28%) | 0.88 |
| >20 | 38 (51%) | 260 (50%) | |
| Clinical setting | |||
| Academic | 26 (35%) | 158 (30%) | |
| Private- breast only | 29 (39%) | 192 (37%) | 0.61 |
| Private- general/surgical oncology | 19 (26%) | 167 (32%) | |
| Uninsured/Medicaid patients | |||
| <20% | 27 (37%) | 300 (58%) | |
| 20-40% | 27 (37%) | 142 (27%) | <0.01 |
| 40-100% | 20 (27%) | 76 (15%) | |
Barriers to cost communication
Regardless of their personal beliefs and practices around cost consideration, 87% (n=521) of surgeons agreed (53%) or strongly agreed (34%) that patients should have access to the costs of their cancer treatment prior to making oncologic treatment decisions; only 3% (n=18) disagreed. Despite this, just 20% of surgeons reported feeling prepared to discuss cancer treatment costs with patients. While feeling prepared to discuss costs was more common among those who reported consistently considering costs (49% vs. 16%, p<0.0001), it is notable that even in this group approximately half felt unprepared for these discussions. We identified several barriers to cost communication: insufficient knowledge or resources (61%), inability to help with costs (24%), and inadequate time (22%). Thirty two percent reported that nothing prevented them from discussing costs with patients. (Table 3).
Table 3.
Survey questions on cost awareness and communication in shared decisions for breast cancer surgery. (N=598)
| Which of the following are barriers to discussing costs of treatment with breast cancer patients? (select all that apply) | |
|---|---|
| I don’t know enough about the costs of care/lack resources | 366 (61%) |
| Nothing prevents me from discussing costs | 193 (32%) |
| I can’t help with the costs of care | 142 (24%) |
| Not enough time to discuss costs | 129 (22%) |
| Discussing costs might impact the quality of care patients receive | 118 (20%) |
| It is uncomfortable to discuss costs with patients | 57 (10%) |
| It’s not my place to discuss costs of care | 50 (8%) |
| Other | 37 (6%) |
DISCUSSION
In the United States, treatment-related financial hardship is a growing problem for the 1.7 million individuals diagnosed with cancer each year.[1–3] To address this issue, the American Society of Clinical Oncology has encouraged cost discussions, and more recently, patient-provider cost communication and personal spending burden have been proposed as metrics of quality cancer care.[21, 22, 29] Despite these high-level endorsements, our research and that of others, have shown that cost communication in clinical oncology is rare, and that the financial implications for patients are not routinely incorporated into therapeutic decisions. [30, 31] Decisions for breast cancer surgery are preference-sensitive, allowing for patient values and/or shared decision-making with women and their doctors to guide treatment plans. Yet, our survey demonstrates that breast cancer surgeons, a select group of physicians regularly engaged in intensive shared decision-making, still potentially underestimate the influence costs have on preference-sensitive surgical decisions and report feeling unequipped for cost discussions.
The literature demonstrates that over 75% of prompted oncologists believe that patients should have access to cost information related to their cancer treatment; however, only 30% routinely include cost communication as part of their clinical practice.[30–32] The majority of prior literature has focused on medical oncology, a subspecialty where treatment-related financial hardship has received significant attention. Results from our survey align with prior finding. In the limited prior literature including surgical oncologists, surgeons report similar barriers to cost discussions as other oncology subspecialists, including lack of access to accurate cost information and a perceived inability to intervene. [15, 30, 31] Importantly, one in five breast cancer surgeons believed that cost discussions may adversely impact the quality of care patients received.
There is limited evidence on outcomes of cost discussions, yet, published results are mixed. On retrospective analysis of 1,755 outpatient visits, Hunter et al found that cost conversations occurred in one-third of clinical encounters, and that 44% of such discussions resulted in cost-reducing strategies.[32, 33] Moreover, cost discussions were associated with improved patient satisfaction, reduced healthcare spending, and added only a median of 68 seconds to the clinic appointment.[34] The authors noted that physicians employed several common cost-reducing strategies, including: a) changing to less expensive alternative therapies (22%), b) altering the frequency of surveillance or interventions (5%), and c) finessing logistics of care (23%). In this setting, oncologists utilized relatively simple strategies to reduce patient costs, such as changing the location, source of received healthcare, or timing (i.e. providing expensive services after a deductible was met or before the end of the year). Additionally, approximately one-fourth (21%) of interventions involved financial navigation for co-payment assistance. Similarly, in a survey of adult cancer patients actively receiving treatment (N=300), Zafar et al found that over half (57%) of individuals who engaged in cost discussions had lower ensuing out-of-pocket costs.[35]
Other literature suggests that cost communication in oncology may negatively impact the receipt of care. In a comprehensive review of cost communication in cancer care, Shih et al found that although both cancer patients and their oncologists strongly desired accurate and transparent cost information, cost communication was associated with higher rates of medication non-adherence.[32] This association may not be causal; it is more likely that out-of-pocket expenditures have greater financial significance for the patients inquiring about treatment-related costs. There are concerns that cost communication has the potential to widen disparities, in that underinsured or impoverished individuals may elect to forego care to avoid undue financial burden they cannot afford; however, the research suggests that cost communication has benefits. Cost discussions are feasible, acceptable to cancer patients, and may improve treatment-related financial hardship in some settings.[34] Further research is needed on the outcomes of cost discussions in clinical oncology, specifically as women with breast cancer face equally effective surgical options.
Regardless of an individual’s ability to pay, prior literature has suggested that physicians remain concerned about how cost conversations may potentially impact the doctor-patient relationship.[36–38] Perhaps this is based on the established culture that physicians should provide the best care to patients at all costs and without consideration of healthcare spending. Yet, as contemporary cancer-related expenditures are increasingly shifted to patients themselves, the oncology community must recognize that financial consequences of our treatment decisions, and that they cannot entirely be ignored. In our study, a portion of surgeons reported that cost discussions are uncomfortable, and believed that communicating treatment costs is not a doctor’s responsibility. However, Brick et al found that, overwhelmingly, cancer patients favored oncologists who openly discussed costs, and in actuality, had greater trust in physicians who accounted for their circumstances as a whole.[39] Kelly et al interviewed cancer patients about their perspectives around cost communication, and found that individuals going through treatment believed costs were a “normal part of life;” participants believed it was important to know what they were personally responsible for paying (80%), and simultaneously, had no negative feelings towards their oncologists who discussed the costs of care (81%). Thus, it seems as though oncologists may be unnecessarily apprehensive about engaging in cost discussions with patients. Future research is needed to determine how patients respond to this information, especially across varied socio-demographic, racial, and economic backgrounds.
Overall, surgeons in our study philosophically supported cost transparency. Participants agreed that physicians should proactively be thinking about patients’ financial burden when choosing cancer therapies, yet, almost 60% reported infrequently or never considering patient costs when making medical recommendations. Overwhelmingly, breast cancer surgeons believed that patients should have access to cost information prior to making medical decisions (87%). Nevertheless, cost discussions remain lacking in routine clinical care. Physicians lack access to accurate, personalized cost data for their patients, and many do not have the knowledge or time to provide financial navigation themselves. These services could be supported by other members of the healthcare team, with oncologists acting as liaisons. It is important to recognize that fee-for-service payment models incentivize medical intervention, which is particularly true for surgeons. In this setting, cost discussions may be even less likely to occur. As the U.S. healthcare system undergoes payment reform towards value-based care and bundled payments, the uptake of cost discussions, and their impact on financial hardship may change.
There are several limitations to our study that should be acknowledged. Overall, the response rate in the included survey was 25%. Although higher response rates approximating 60-65% are generally desirable, our findings are consistent with prior surveys of the ASBrS membership.[40] Additionally, surgical expenses comprise only a small proportion of treatment-related costs for women with breast cancer. Costlier aspects of cancer care, including imaging, chemotherapy, and radiation, may also be more significant contributors to overall healthcare spending than surgery itself. Yet, decisions for breast cancer surgery provide an unparalleled opportunity to address how cost discussions influence oncologic treatment choices, in a setting where all options result in excellent cancer outcomes.
Breast cancer surgeons regularly engage in shared decision-making with women facing treatment options. A growing body of research suggests that bilateral mastectomy is associated with greater out-of-pocket costs, incurred debt, and disrupted employment when compared to breast conservation.[23–25] Thus, differing surgical options with equivalent cancer outcomes vary in their risk of financial hardship. The American Society of Breast Surgeons and the Choosing Wisely Campaign have recommended that routine use of contralateral prophylactic mastectomy (CPM) be discouraged in average-risk women, recognizing that CPM lacks additional medical benefit and is associated with potentially greater harms. [41, 42] As surgeons guide women through shared decisions for breast cancer surgery, improved cost awareness may facilitate conversations around the financial implications of surgical choice. For some women, greater financial burden may impact preference-sensitives choices, and further influence these national trends.[27, 43]
Financial insecurity has been associated with treatment non-adherence and refusal of care.[32, 44] Notably, the initial surgical consultation is often a women’s first point of contact with her breast oncology team; thus, high treatment costs, lost productivity, and financial hardship from surgery have the potential to influence women’s receipt of subsequent therapies. Thus, in shared decisions for breast cancer surgery, cost transparency has the potential to be an effective early intervention, protecting women along the entire continuum of breast cancer care. Future research is needed to explore practical ways to improve cost transparency, evaluate the impact of cost discussions on surgical choice, and empower oncology teams to have cost conversations. Although financial considerations are only one of several important considerations in women’s surgical choice, it is an increasingly important factor in contemporary patient-centered breast cancer care.
Supplementary Material
Acknowledgments
This work was presented at the 20th annual American Society of Breast Surgeons Meeting in May 2019, and supported by the National Institutes of Health Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Career Development Award, K12HD043446-11. Additional support was provided by the developmental funds of Duke Cancer Institute as part of the P30-CA014236 (Office of Cancer Centers, NCI).
REFERENCES
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2019. CA Cancer J Clin, 2019. 69(1): p. 7–34. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2018. CA Cancer J Clin, 2018. 68(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, and Jemal A, Cancer Statistics, 2017. CA Cancer J Clin, 2017. 67(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 4.Fisher CS, et al. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol, 2012. 19(10): p. 3246–50. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med, 2002. 347(16): p. 1233–41. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, et al. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. New England Journal of Medicine, 2002. 347(16): p. 1227–1232. [DOI] [PubMed] [Google Scholar]
- 7.Hwang ES, et al. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer, 2013. 119(7): p. 1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CN, et al. Decision Making about Surgery for Early-Stage Breast Cancer. Journal of the American College of Surgeons, 2012. 214(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins ED, et al. Can Women With Early-Stage Breast Cancer Make an Informed Decision for Mastectomy? Journal of Clinical Oncology, 2009. 27(4): p. 519–525. [DOI] [PubMed] [Google Scholar]
- 10.Lee C, et al. Quality of patient decisions about breast reconstruction after mastectomy. JAMA Surgery, 2017. 152(8): p. 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yabroff KR, et al. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev, 2011. 20(10): p. 2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariotto AB, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst, 2011. 103(2): p. 117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzoni L, Belloni A, and Sassi F, Flealth-care expenditure and health policy in the USA versus other high-spending OECD countries. The Lancet, 2014. 384(9937): p. 83–92. [DOI] [PubMed] [Google Scholar]
- 14.Elkin EB and Bach PB, Cancer’s next frontier: addressing high and increasing costs. JAMA, 2010. 303(11): p. 1086–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zafar Y, et al. Financial distress, communication, and cancer treatment decision making: Does cost matter? Journal of Clinical Oncology, 2013. 31(15_suppl): p. 6506–6506. [Google Scholar]
- 16.Zafar SY and Abernethy AP, Financial toxicity, Part I: a new name for a growing problem. Oncology (Williston Park), 2013. 27(2): p. 80–1, 149. [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker-Seeley RD and Yabroff KR, Minimizing the “Financial Toxicity” Associated With Cancer Care: Advancing the Research Agenda. JNCI: Journal of the National Cancer Institute, 2016. 108(5): p. djv410–djv410. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey SD, et al. Financial Insolvency as a Risk Factor for Early Mortality Among Patients With Cancer. Journal of Clinical Oncology, 2016. 34(9): p. 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kale HP and Carroll NV, Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer, 2016. 122(8): p. 283–9. [DOI] [PubMed] [Google Scholar]
- 20.Schnipper LE, et al. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J Clin Oncol, 2015. 33(23): p. 2563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meropol NJ, et al. American Society of Clinical Oncology Guidance Statement: The Cost of Cancer Care. Journal of Clinical Oncology, 2009. 27(23): p. 3868–3874. [DOI] [PubMed] [Google Scholar]
- 22.Blumenthal D and McGinnis JM, Measuring Vital Signs: an IOM report on core metrics for health and health care progress. JAMA, 2015. 313(19): p. 1901–2. [DOI] [PubMed] [Google Scholar]
- 23.Greenup RA, C. R, Fish L, Campbell BM, Tolnitch L, Hyslop T, Peppercorn J, Wheeler SB, Zafar SY, Myers ER, Hwang ES. , Financial Costs and Burden Related to Decisions for Breast Cancer Surgery. [DOI] [PMC free article] [PubMed]
- 24.Greenup RA, L. F Rushing CR, Peppercorn J, Hyslop T, Myers E, Zafar Y, Hwang ES. , The Costs of Breast Cancer Care: Patient-Reported Experiences and Preferences for Transparency. 2017.
- 25.Jagsi R, et al. Treatment decisions and employment of breast cancer patients: Results of a population-based survey. Cancer, 2017. 123(24): p. 4791–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagsi R, et al. Long-term financial burden of breast cancer: experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol, 2014. 32(12): p. 1269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz SJ, et al. Surgeon Influence on Variation in Receipt of Contralateral Prophylactic Mastectomy for Women With Breast CancerSurgeon Influence on Receipt of Contralateral Prophylactic MastectomySurgeon Influence on Receipt of Contralateral Prophylactic Mastectomy. JAMA Surgery, 2018. 153(1): p. 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venetis MK, et al. Social Network, Surgeon, and Media Influence on the Decision to Undergo Contralateral Prophylactic Mastectomy. American journal of clinical oncology, 2018. 41(6): p. 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging, P., S. Board on Health Care, and M. Institute of, in Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis, Levit L, et al. Editors. 2013, National Academies Press (US) Copyright 2013 by the National Academy of Sciences. All rights reserved.: Washington (DC). [PubMed] [Google Scholar]
- 31.Altomare I, et al. ReCAP: Physician Experience and Attitudes Toward Addressing the Cost of Cancer Care. Journal of Oncology Practice, 2016. 12(3): p. 247–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih YT and Chien CR, A review of cost communication in oncology: Patient attitude, provider acceptance, and outcome assessment. Cancer, 2017. 123(6): p. 928–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih YT, Nasso SF, and Zafar SY, Price Transparency for Whom? In Search of Out-of-Pocket Cost Estimates to Facilitate Cost Communication in Cancer Care. Pharmacoeconomics, 2018. 36(3): p. 259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter WG, et al. Discussing Health Care Expenses in the Oncology Clinic: Analysis of Cost Conversations in Outpatient Encounters. J Oncol Pract, 2017. 13(11): p. e944–e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zafar SY, et al. The utility of cost discussions between patients with cancer and oncologists. Am J Manag Care, 2015. 21(9): p. 607–15. [PubMed] [Google Scholar]
- 36.Hofstatter EW, Understanding patient perspectives on communication about the cost of cancer care: a review of the literature. J Oncol Pract, 2010. 6(4): p. 188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFarlane J, Riggins J, and Smith TJ, SPIKE$: a six-step protocol for delivering bad news about the cost of medical care. J Clin Oncol, 2008. 26(25): p. 4200–4. [DOI] [PubMed] [Google Scholar]
- 38.Schrag D and Hanger M, Medical oncologists’ views on communicating with patients about chemotherapy costs: a pilot survey. J Clin Oncol, 2007. 25(2): p. 233–7. [DOI] [PubMed] [Google Scholar]
- 39.Brick DJ, Scherr KA, and Ubel PA, The Impact of Cost Conversations on the Patient-Physician Relationship. Health Commun, 2019. 34(1): p. 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeSnyder SM, et al. American Society of Breast Surgeons’ Practice Patterns After Publication of the SSO-ASTRO-ASCO DCIS Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation. Ann Surg Oncol, 2018. 25(10): p. 2965–2974. [DOI] [PubMed] [Google Scholar]
- 41.Boughey JC, et al. Contralateral Prophylactic Mastectomy (CPM) Consensus Statement from the American Society of Breast Surgeons: Data on CPM Outcomes and Risks. Ann Surg Oncol, 2016. 23(10): p. 3100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boughey JC, et al. Contralateral Prophylactic Mastectomy Consensus Statement from the American Society of Breast Surgeons: Additional Considerations and a Framework for Shared Decision Making. Annals of Surgical Oncology, 2016. 23(10): p. 3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King TA, et al. Clinical Management Factors Contribute to the Decision for Contralateral Prophylactic Mastectomy. Journal of Clinical Oncology, 2011. 29(16): p. 2158–2164. [DOI] [PubMed] [Google Scholar]
- 44.Bestvina CM, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract, 2014. 10(3): p. 162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


