Abstract
Previous studies have reported hypo-glycosylated FSH and fully-glycosylated FSH to be naturally occurring in humans, and these glycoforms exist in changing ratios over a woman’s lifespan. The precise cellular and molecular effects of recombinant human FSH (hFSH) glycoforms, FSH21 and FSH24, have not been documented in primary granulosa cells. Herein, biological responses to FSH21 and FSH24 were compared in primary porcine granulosa cells. Hypo-glycosylated hFSH21 was significantly more effective than fully-glycosylated hFSH24 at stimulating cAMP accumulation and protein kinase A (PKA) activity, leading to the higher phosphorylation of CREB and β-Catenin. Compared to fully-glycosylated hFSH24, hypo-glycosylated hFSH21 also induced greater levels of transcripts for HSD3B, STAR and INHA, and higher progesterone production. Our results demonstrate that hypo-glycosylated hFSH21 exerts more robust activation of intracellular signals associated with steroidogenesis than fully-glycosylated hFSH24 in primary porcine granulosa cells, and furthers our understanding of the differing bioactivities of FSH glycoforms in the ovary.
Keywords: Ovary, Follicle, Cell signaling, protein kinase A, β-catenin, Steroidogenesis
1. Introduction
Follicle-stimulating hormone(FSH)is produced and secreted by the anterior pituitary gland and plays an important role in the control of reproductive processes in mammals. In females, FSH exerts its biological actions by binding to the G protein-coupled FSH receptor (FSHR) located on the surface of granulosa cells (George et al., 2011), thereby regulating ovarian follicle growth, steroidogenic activity and oocyte maturation (Howles et al., 2000; Rimon et al., 2016). However, the biological response of the ovary to FSH varies depending on extent of glycosylation of the FSH molecule (Bishop et al., 1994; Smitz et al., 2016; Wide and Eriksson, 2018). In humans, FSH is released as a mixture composed of fully-glycosylated hFSH, which possesses all four N-glycans and hypo-glycosylated hFSH which lacks one of two FSHβ subunit N-glycans (Walton et al., 2001; Bousfield et al., 2014a; Bousfield et al., 2014b). The fully-glycosylated and hypo-glycosylated forms of FSH are referred to as FSH24 and FSH21, based on their FSHβ molecular weights (Bousfield et al., 2014b; Davis et al., 2014; Butnev et al., 2015). It has been reported that the ratios of different glycoforms of FSH varies under physiological conditions, with the hypo-glycosylated hFSH more abundant in younger women, while fully-glycosylated hFSH is more abundant in older women (Bousfield et al., 2014a; Bousfield et al., 2014b; Bousfield et al., 2018;Wide and Eriksson,2018). Additionally, changes in glycoform abundance are also observed during the natural menstrual cycle (Wide and Eriksson, 2018).
A previous study that compared the biological actions of FSH glycoforms in KGN cells, a steroidogenic human granulosa-like tumor cell line, found that hypo-glycosylated hFSH21 had greater bioactivity than fully-glycosylated hFSH24. This study demonstrated that FSH24 was significantly less effective than FSH21 at stimulating the cAMP-protein kinase A (PKA) signaling pathway and synthesis of estradiol and progesterone (Jiang et al., 2015). Wang et al. (2016) evaluated the bioactivity of FSH glycoforms in Fshb null mice and observed that injection of hFSH21 and hFSH24 glycoforms differentially regulated 20 genes via distinct biological pathways in the ovary after 2 h of treatment. More recently, Simon et al., (2019) evaluated the direct actions of FSH glycoforms on the development of murine follicles in vitro; and observed that high concentrations (100 ng/mL) of hFSH21 and hFSH24 exhibited differential abilities to increase phosphorylated PKA substrates and regulate expression of FSH-responsive genes. Based on the above observations, it is clear that FSH glycoforms exert differential bioactivities at the level of the ovary. Because FSH is known to rapidly engage growth factor signaling pathways in intact follicles (Taymor, 1996; El-Hayek et al., 2014) and KGN is a FOXL2-mutated granulosa tumor cell line (Nishi et al., 2001; Schrader et al., 2009), it is essential to compare the actions of FSH glycoforms in primary granulosa cells, which maintain important functions of granulosa cells in vivo.
The pig is a polyovular species, with both ovaries undergoing ovarian follicle development at similar stages during the estrus cycle (LaVoie et al., 2017), and has proven to be an excellent model to study human reproductive events due to physiological similarities to human (Mordhorst and Prather, 2017). Therefore, the aim of the present study was to evaluate the biological effects of fully-glycosylated hFSH24 and hypo-glycosylated hFSH21 preparations in primary porcine granulosa cells to increase our understanding of the principal responses of primary granulosa cells to hFSH glycoforms and the underlying regulatory signaling pathways. To achieve this aim we evaluated cAMP accumulation and PKA activity, phosphorylation of the transcription regulators CREB and β-catenin, expression of FSH-responsive genes, and progesterone synthesis.
2. Material and Methods
2.1. Reagents
Hypo-glycosylated hFSH21 and fully-glycosylated hFSH24 were expressed and purified from transformed rat pituitary GH3 cells. The characterization of recombinant hFSH glycoforms was as previously described (Butnev et al., 2015; Jiang et al., 2015). For the sake of simplicity, hFSH21/18 was specified as hFSH21, which is the predominant glycoform in the FSH21/18 mixture examined in this study. The estimated masses for FSH24 and FSH21 masses are 31,497 and 29,743, respectively. Thus, 10 ng/ml of FSH24 equals 317.5 pM and 10 ng/ml FSH21 equals 336.2 pM, a difference of 5.8%.
Information on the source and use of antibodies and reagents are listed in Table 1. A reporter gene assay was used to study CREB-response element (CRE)-mediated transcription as previously described (Jiang et al., 2015). The assay employed adenoviruses expressing a canonical CRE-luciferase reporter (Ad.CRE-Luc), a control construct (Ad. MCS-Luc, firefly luciferase), and an internal control (Ad. pRL-Luc, Renilla luciferase) (Vector Biolabs). Unless otherwise stated, all chemicals used in this study were purchased from either Sigma-Aldrich (St. Louis, MO, USA) or Fisher Scientific (Carlsbad, CA, USA).
Table 1.
Antibodies used for Western blotting and microscopy.
| Dilution ratio | Species Specificit y |
Source | Supplier | Cat. No | |
|---|---|---|---|---|---|
| CREB | 1:1000 | Mouse | Rabbit mAB | Cell Signaling | 9197L |
| Phospho-CREB | 1:10001/1:2002 | Mouse | Rabbit pAB | Cell Signaling | 9198S |
| (Ser133) | |||||
| Phospho-PKA | 1:1000 | Mouse | Rabbit pAB | Cell Signaling | 9624S |
| Substrate(RRXS */T *) | |||||
| β-catenin | 1:1000 | Mouse pAB | Cell Signaling | 2698S | |
| Phospho-P-catenin (Ser552) | 1:1000 | Mouse | Rabbit mAB | Cell Signaling | 9566S |
| Phospho-P-catenin (Ser675) | 1:1000 | Mouse | Rabbit mAB | Cell Signaling | 4176S |
| HSD3B1 | 1:1000 | Mouse | Rabbit mAB | A gift from Dr. Ian Mason | |
| Actin | 1:5000 | Bovine | Mouse mAB | Sigma-Aldrich | A5441 |
| α-tubulin | 1:200 | Bovine | Mouse mAB | Abcam | Ab7291 |
| HRP-linked | 1:10000 | Anti-rab bit |
Jackson ImmunoResearch | 111035144 | |
| HRP-linked | 1:10000 | Anti-mo use |
Jackson ImmunoResearch | 115035205 | |
| Alexa Fluro 488 | 1:500 | Anti-mo use |
Invitrogen | A21202 | |
| Alexa Fluro 594 | 1:500 | Anti-rab bit |
Invitrogen | A11032 |
Dilution used for Western blotting.
Dilution used for Confocal Microscopy.
2.2. Cell culture
Porcine granulosa cells were isolated from healthy 1–4 mm ovarian follicles obtained from pre-pubertal gilt ovaries removed at slaughter, as described elsewhere (Keel et al., 1999). Briefly, cells (4 X 104 cells/cm2) were plated and cultured in DMEM/F12 medium supplemented with 10% (v/v) fetal bovine serum, 100 IU/mL penicillin and 100 μg/mL streptomycin, and incubated in a humidified 5% CO2 atmosphere at 37 °C. When the cultures were 80–90% confluent the cultures were washed with DPBS and incubated for 2 h with fresh serum-free DMEM/F12 medium prior to treatment with hFSH glycoforms. For experiments determining FSH-responsive signaling pathways, granulosa cells were treated with 0–100 ng/mL of hFSH21 or hFSH24 for 30 minutes. For experiments measuring steroid synthesis and gene expression patterns, granulosa cells were treated with increasing concentration of hFSH glycoforms for 48 hours. To determine estradiol synthesis the medium was supplemented with 200 nM 4-androstene-3,17-dione.
2.3. cAMP accumulation assay
After treatment with increasing concentrations of hFSH glycoform preparations for 30 min, cAMP accumulation in the medium was measured using a cAMP chemiluminescent immunoassay kit (Arbor, Michigan, USA) according to the manufacturer’s protocol.
2.4. Western blot analysis
Briefly, western blot procedures were as described (Jiang et al., 2015; Plewes et al., 2019). Granulosa cells were harvested with ice-cold cell lysis buffer [20 mM Tris-HCl (pH 7.0), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100 and protease and phosphatase inhibitor cocktails]. Protein content was estimated by Bio-Rad protein assay (Hercules, CA, USA). Cell extracts (15 μg) were separated by 10% SDS-PAGE and proteins transferred onto nitrocellulose membranes. The membranes were blocked with 5% BSA for 1 h and then incubated at 4 °C overnight with primary antibodies as listed in Table 1. Immuno-reactivity was detected using HRP-conjugated secondary antibodies (Table 1). Bands were visualized using an enhanced chemiluminescence (ECL) detection system (UVP, Upland, CA, USA).
2.5. Immunofluorescence assay
We employed confocal microscopy to determine the effects of hFSH glycoforms on CREB phosphorylation and nuclear localization. Granulosa cells were seeded on No.1 glass coverslips in 6-well culture dishes. After culturing for 24 h, cells were stimulated with increasing concentrations (0–100 ng/mL) of hFSH21 or hFSH24 for 30 min. Cells were then washed with PBS and fixed with 200 μL 4% paraformaldehyde for 30 min at 4 °C. Following fixation, cells were washed, and biological membranes were permeabilized with 0.1% Triton-X for 10 min. Cells were washed and blocked with 1% BSA for 1 h. Appropriate antibodies (Table 1) were added to each coverslip and incubated for 24 h at 4 °C. Following incubation, cells were washed with PBS to remove unbound antibody and subsequently incubated with appropriate secondary antibodies (Table 1) for 60 min. Following labeling with antibodies, coverslips containing labeled cells were mounted to glass microscope slides using 10 μL ProLong® Gold Antifade Mountant with DAPI (Invitrogen, Carlsbad, CA, USA). Coverslips were sealed to glass microscope slides using clear nail polish and stored at −22 °C until imaging.
Images were collected using a Zeiss 800 confocal microscope. Approximately 40 cells were randomly selected from each slide and z-stacked images (0.33 μm) were generated from bottom to top of each cell. A 3-dimensional image was generated, and area of each cell was determined using Zen software. Images were converted to maximum intensity projections and processed utilizing Image J (National Institutes of Health) analysis software. Mean fluorescence intensity was determined as previously described by outlining the cell boundary and subtracting background fluorescence (Plewes et al, 2017).
2.6. CRE-luciferase reporter assay
An adenoviral CREB-response element (CRE)-driven luciferase (Luc) reporter construct Ad.CRE-Luc (firefly luciferase), the corresponding adenoviral control construct Ad.MCS-Luc (firefly luciferase) and internal control construct Ad.pRL-Luc (Renilla luciferase) were employed to determine the effect of hFSH glycoforms on transcriptional activity. Porcine granulosa cells were infected with 5×107 pfu/mL of Ad.CRE-Luc plus Ad.pRL-Luc or Ad.MCS-Luc plus Ad.pRL-Luc as previously described (Mao et al., 2013;Jiang et al., 2015). After infection for 24 h, cells were rinsed with DPBS and then treated with hFSH21 or hFSH24 for 6 h. The Dual-Luciferase Reporter Assay System Kit (Promega, WI, USA) was employed and luciferase signals detected using a FLUOstar OPTIMA plate reader (BMG Labtech). The results were presented as relative luminescence units (RLU).
2.7. RNA isolation, reverse transcription, and quantitative real-time PCR
After treatment with hFSH glycoforms at 3 or 30 ng/mL for 48 h, total RNA was extracted from porcine granulosa cells using a RNeasy Micro Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol, and RNA quantified and qualified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Carlsbad, CA, USA). Each 500 ng RNA sample was subjected to reverse transcription using the iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA, USA) in a total volume of 20 L. Real-time PCR was performed by adding 10 L of Sso Fast EvaGreen Supermix (Bio-Rad, Hercules, CA, USA), 200 ng of cDNA, 500 nM of each primer, and water to a final volume of 20 μL, and then subjected to the following conditions: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and 59 °C for 5 s in a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Forward and reverse primers were synthesized by Eurofins Genomics and listed in Table 2. A melting curve analysis was performed to ensure a single and expected product was amplified from each primer pair. Levels of gene expression were normalized to GAPDH levels using the 2−ΔΔCt method (Livak et al., 2001).
Table 2.
Primers used in Real-time PCR
| Gene | Primer sequences(5’−3’) | Length(bp) | Accession number |
|---|---|---|---|
| HSD3B1 | AGGGT T TCTGGGTCAGAGGAT C CGTTGACCACGTCGATGATAGAG | 236 | NM_001004049.2 |
| STAR | CATTACCATCTACTCCCAGC AACCCGTATCTTTCTTGTCAG |
109 | AY368628.1 |
| CYP19A | GCTGCTCATTGGCTTAC TCCACCTATCCAGACCC |
187 | NM_214431.2 |
| CYP11A1 | GGCTCCAGAGGCCATAAAGAACTCAAAGGCGAAGCGAAAC | 149 | NM_214427.1 |
| INHA | GCTCTGTTCCTGGATGCCTTCAGCGGGCACCTGTAGC | 173 | NM_214189.2 |
| INHBA | AGTCAGCTTGTCTGGTCTGCTTACTGGGTGGGAAAGTGCC | 106 | NM_214028.1 |
| INHBB | TCTTCATCTCCAACGAGGGTAA TTCAGGTCCACACGCTTCTC |
184 | NM_001164842.1 |
| LHCGR | AAAGCACAGCAAGGAGACCA T GAGGCAAT GAGT AGCAGGT AGA | 338 | NM_214449.1 |
| GAPDH | GGACTCATGACCACGGTCCAT TCAGATCCACAACCGACACGT |
220 | NM_001206359.1 |
2.8. Progesterone and estradiol ELISA
The concentrations of progesterone (P4) and estradiol (E2) in the culture medium of porcine granulosa cells were measured after treatment with hFSH glycoforms for 48 h by DRG® Progesterone and Estradiol ELISA kits (Springfield, NJ, USA) according to the manufacturer’s protocol, respectively. The assay sensitivity was 9.7 pg/mL for estradiol and 45 pg/mL for progesterone. Measurements of cell numbers/viability were performed with an MTT assay as previously described (Mao et al., 2013).
2.9. Statistical analysis
The data are presented as mean ± SEM. Data analysis was performed using GraphPad Prism 8.0 software and included Student’s t-test or one-way ANOVA followed by either Tukey’s or Bonferroni’s post hoc test.
3. Results
3.1. Hypo-glycosylated hFSH is more effective than fully glycosylated hFSH at increasing cAMP and stimulating PKA
To investigate the effects of hFSH glycoform preparations on cAMP accumulation in porcine granulosa cells, the cells were exposed to increasing concentrations of hFSH glycoforms for 30 min. At the highest concentration, hFSH21 stimulated 21.8-fold increases in cAMP accumulation (P < 0.01) compared to controls. In contrast, 100 ng/mL hFSH24 was much less effective, producing only 8.3-fold increases in cAMP accumulation (P < 0.01). hFSH21 at concentrations of 30 or 100 ng/mL stimulated significantly greater increases in cAMP levels when compared with comparable concentrations hFSH24 (P < 0.05 and P < 0.01, Fig. 1A).
Figure 1. (single column). Effect of FSH glycoform preparations on cAMP accumulation and PKA activation in porcine granulosa cells.
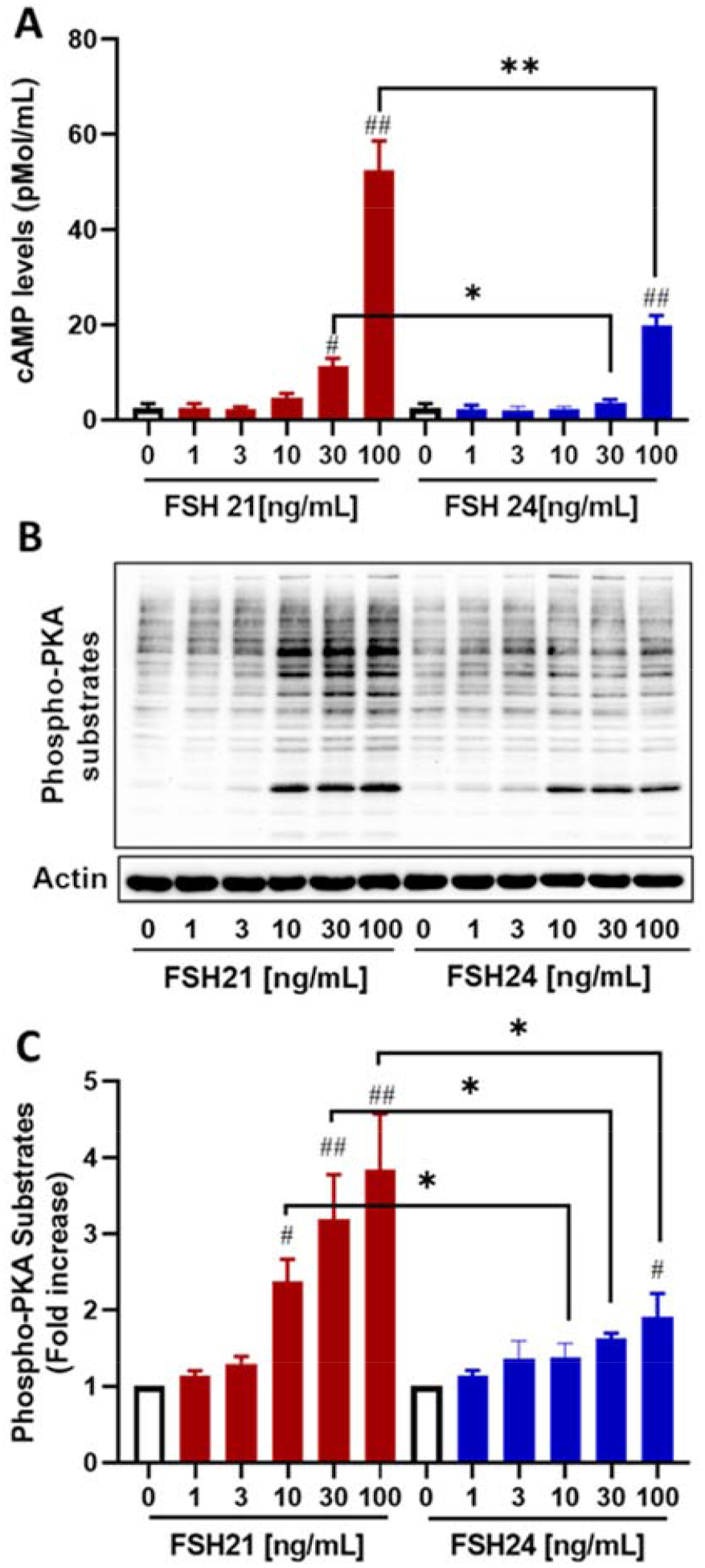
Granulosa cells were treated with increasing concentrations of hFSH21 or hFSH24 for 30 minutes. A, cAMP levels in the medium were measured by a cAMP chemiluminescent immunoassay kit as described in the Methods. B, representative Western blot result of phosphorylation of PKA-substrates after hFSH glycoform treatment. Actin was used as an internal control. C, fold increases in the phospho-PKA-substrates/Actin ratio. Results are presented as means ± SEM; n = 3 separate experiments. #P < 0.05 and ##P < 0.01 compared with respective control; *P < 0.05 and **P < 0.01 compared with the other glycoform at the same concentration.
Protein kinase A (PKA) is the best characterized downstream effector of cAMP in granulosa cells (Hunzicker-Dunn et al., 2006). To determine whether the increases in cAMP accumulation were coupled to PKA activation, immunoblot analysis was conducted on whole cell lysates using an antibody directed against PKA consensus phosphorylation sites (RRXS*/T*). Similar to findings with cAMP accumulation, hypo-glycosylated hFSH21 induced greater phosphorylation of PKA substrates than fully-glycosylated hFSH24 (Figs. 1B and 1C). We found that the lowest effective concentration of FSH21 was 10 ng/ml (2.4 ± 0.3 fold), whereas 100 ng/ml FSH24 was required to significantly increase phospho-PKA substrates (1.9 ± 0.3 fold).
3.2. Hypo-glycosylated hFSH is more effective than fully glycosylated hFSH at increasing CREB phosphorylation
Western blot and immunofluorescence experiments were performed to analyze the extent and subcellular localization of phosphorylated CREB, an important PKA substrate and transcriptional regulator in granulosa cells (Hunzicker-Dunn et al., 2006). Treatment of porcine granulosa cells with increasing concentrations of hFSH glycoform preparations for 30 minutes caused concentration-dependent increases in CREB phosphorylation at Ser133 (Figs. 2A and 2B). hFSH21 stimulated significantly greater phosphorylated CREB at 10, 30 and 100 ng/mL when compared with equal concentrations of hFSH24 (P < 0.05, P < 0.05 and P < 0.01, respectively). Significant increases in CREB phosphorylation were only observed at 100 ng/mL hFSH24 (P < 0.05). On average, 30 and 100 ng/mL hFSH21 stimulated 10.7- and 19.3-fold increases in CREB phosphorylation, whereas hFSH24 stimulated 3.2- and 8.9-fold increases, respectively.
Figure 2. (Single column). Effect of FSH glycoform preparations on CREB phosphorylation in porcine granulosa cells.
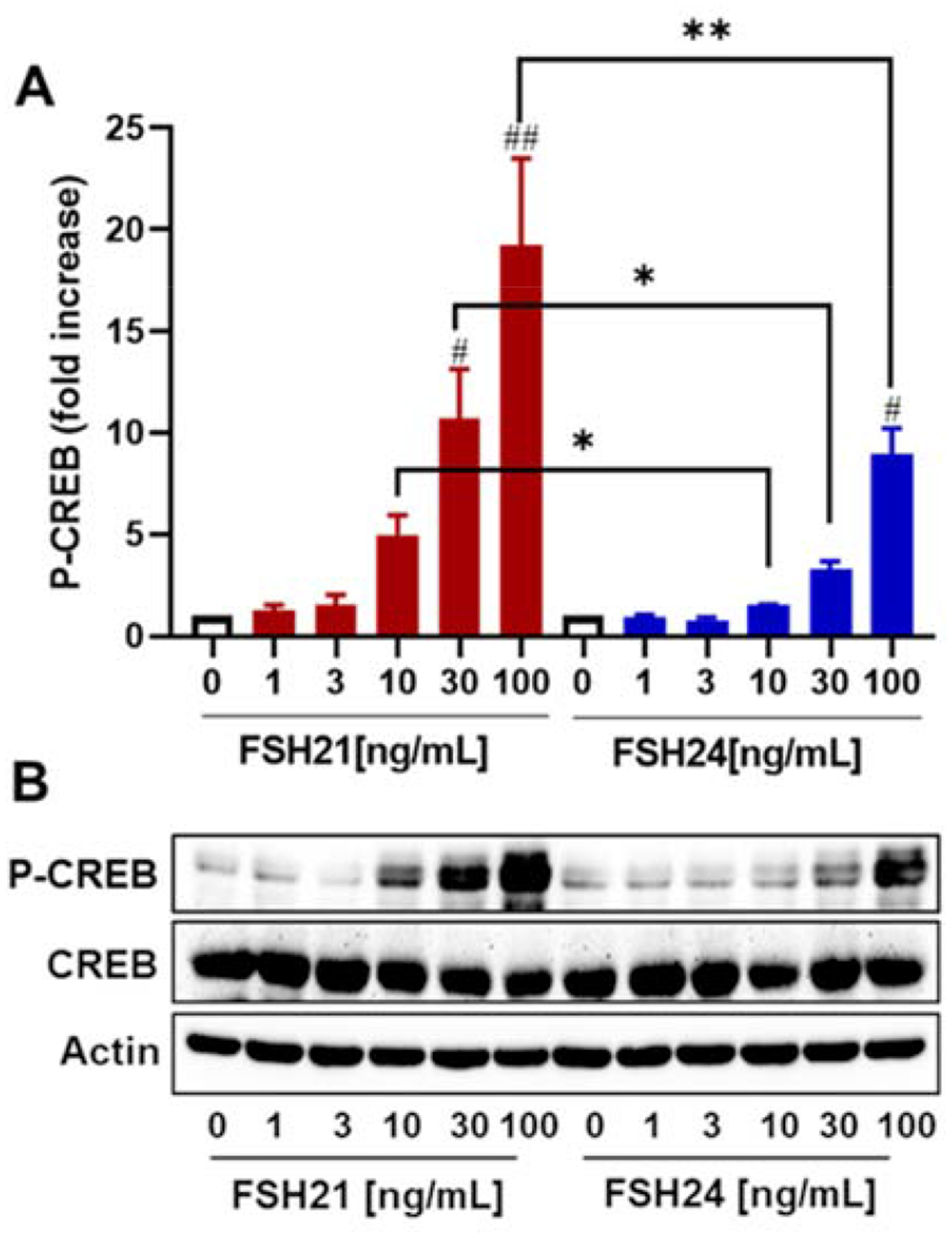
Granulosa cells were treated for 30 minutes with increasing concentrations of hFSH21 or hFSH24. Cell lysates were prepared and Western blot analysis was performed using phospho-Ser133-CREB (P-CREB) antibody, and β-actin antibodies. A, Fold increases in the phospho-CREB/Actin ratio; results are presented as means ± SEM; n = 3 separate experiments. #P < 0.05 and ##P < 0.01 compared with respective control; *P < 0.05 and **P < 0.01 compared with the other glycoform at the same concentration. B, representative Western blot analysis of CREB phosphorylation after hFSH glycoform treatment. Actin was used as an internal control.
Confocal microscopy was used to determine the cellular location of phosphorylated CREB following treatment with hFSH glycoforms. Consistent with the Western blot results, we observed that hFSH21 was more effective than hFSH24 at increasing the percentage of porcine granulosa cells with nuclear localized phosphorylated CREB (Fig. 3A). Interestingly, stimulation with either hFSH21 or hFSH24 resulted in an ‘all or nothing’ response in cells, leading to specific granulosa cell populations staining positively for phosphorylated CREB, rather than all cells uniformly expressing phosphorylated CREB (shown in Supplementary Fig). Results showed that the percentage of cells expressing phosphorylated CREB were greater for cells treated with hFSH21 compared with comparable concentrations of hFSH24 (P < 0.05).
Figure 3. (Single column). Effects of hFSH glycoform preparations on the number and intensity of nuclear phospho-CREB staining in porcine granulosa cells.
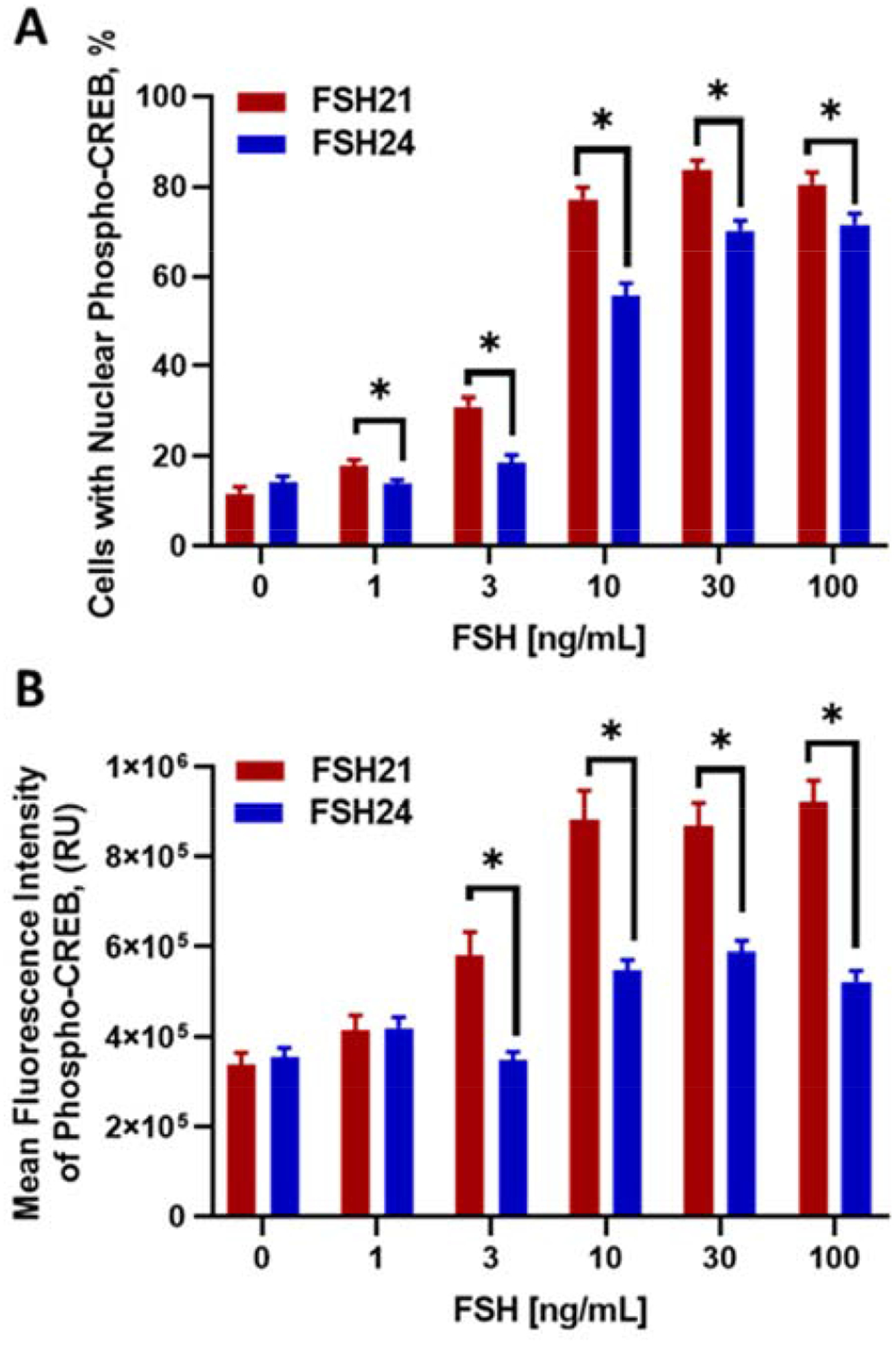
Granulosa cells were treated for 30 minutes with increasing concentrations of hFSH21 or hFSH24. A, the number of phospho-CREB-positive nuclei expressed as a percent of the total cells counted in each experiment. B, the mean fluorescence intensity of phospho-CREB in cell nuclei. *P < 0.05 and **P < 0.01 compared with the other glycoform at the same concentration.
Treatment of porcine granulosa cells with hFSH glycoform preparations also resulted in concentration-dependent increases in the mean fluorescence intensity (MFI) of phosphorylated CREB (Fig.3B). hFSH21 was more effective than hFSH24 at stimulating CREB phosphorylation as shown by increased mean fluorescence intensity. The intensity of phosphorylated CREB in cells treated with 3, 10, 30, or 100 ng/mL hFSH21 was significantly greater than phosphorylated CREB in cells treated with comparable concentrations of hFSH24 (P < 0.05).
3.3. Hypo-glycosylated hFSH is more effective than fully glycosylated hFSH at stimulating CRE-mediated transcriptional activity
CREB is an important transcription factor for the cAMP responsive-element (CRE) present at cAMP/PKA responsive genes. We examined CRE-mediated transcriptional activity in response to hFSH glycoforms using a cAMP-response element-driven luciferase reporter gene (CRE luc). As shown in Fig. 4, treatment with 30 ng/mL hFSH21 stimulated a significant, 11.5-fold increase in CRE-mediated transcription when compared to the control group (P < 0.01), whereas 30 ng/mL hFSH24 treatment provoked a smaller, non-significant increase. Treatment with 100 ng/mL hFSH21 and hFSH24 significantly increased CRE-mediated transcription 20.1- and 13.8-fold, respectively. Furthermore, CRE-mediated transcription activities in cells treated with 30 or 100 ng/mL hFSH21 were significantly greater than those treated with identical concentrations of hFSH24 (P < 0.05).
Figure 4. (Single column). Effects of hFSH glycoform preparations at stimulating CRE-mediated transcriptional activity.
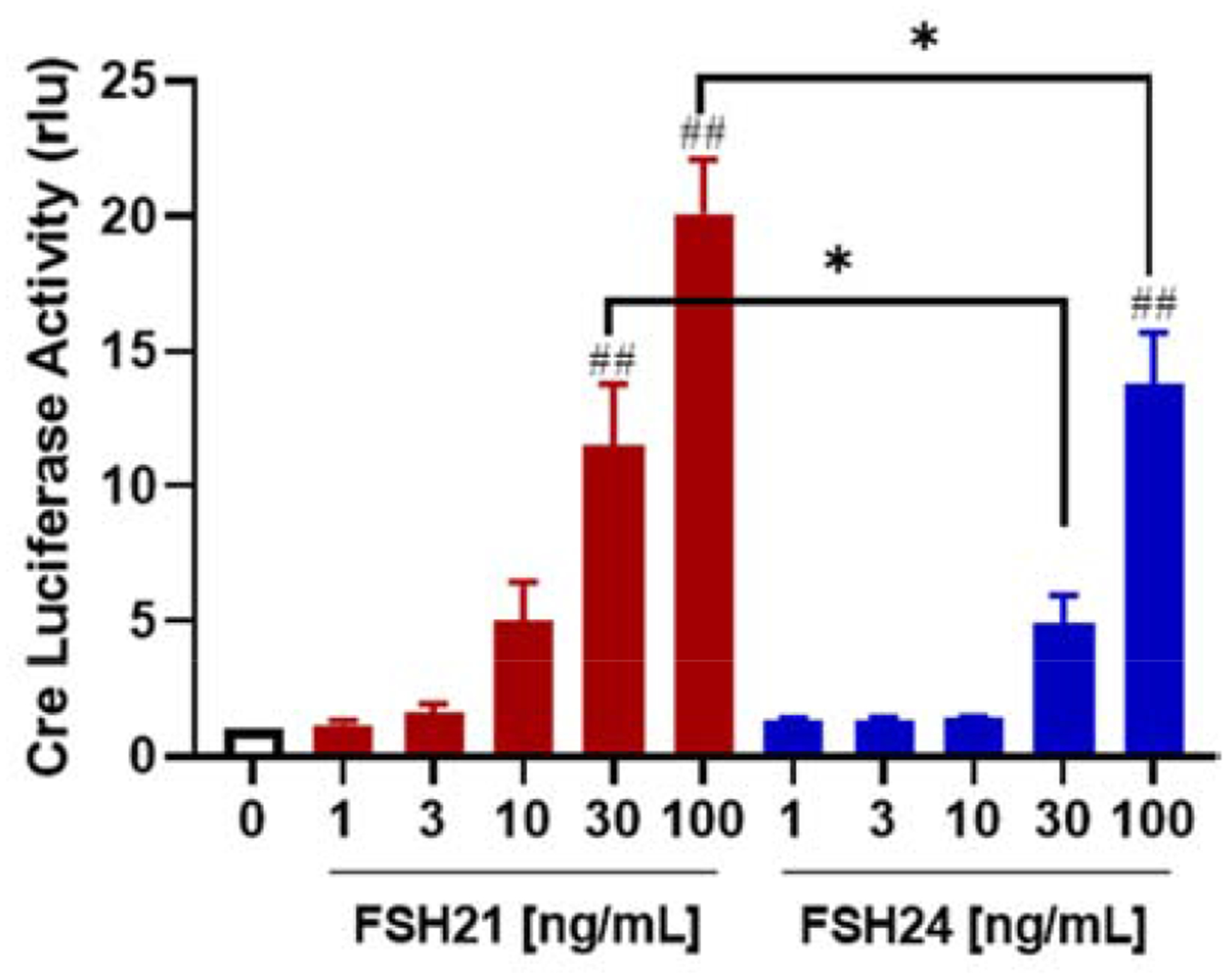
Granulosa cells were infected with an adenovirus expressing a CRE luciferase reporter (CRE-Luc) or pRL-Luc as an internal control. After 24 hours, granulosa cells were treated for 6 hours with either hFSH21or hFSH24 ranging from 0–100 ng/mL. Relative CRE-luciferase activity (rlu) was determined as described in the Methods. Bars represent means± SEM; n = 4 separate experiments. #P < 0.05 and ##P < 0.01 compared with control; *P < 0.05 and **P < 0.01 compared with the other glycoform at the same concentration.
3.4. Hypo-glycosylated hFSH is more effective than fully glycosylated hFSH at stimulating the phosphorylation of β-catenin
Apart from CREB, the transcription co-activator β-catenin (CTNNB1) is also regulated by PKA in various cells, including rat granulosa cells (Law et al., 2013) and bovine luteal cells (Roy et al, 2009). Activation of PKA stimulates phosphorylation of β-catenin on Ser552 and Ser675 residues (Law et al., 2013). Treatment of porcine granulosa cells with hFSH glycoform preparations for 30 min stimulated the phosphorylation of β-catenin at both Ser552 and Ser675 sites (Fig. 5). hFSH21 stimulated phosphorylation of β-catenin Ser552 in a concentration-dependent manner with significant increases observed at 10, 30 and 100 ng/mL (Fig. 5A), whereas peak phosphorylation of β-Catenin Ser675 was observed at 100 ng/mL (Fig. 5B). At the highest concentration, the levels of Ser552 and Ser675 phospho-β-catenin were significantly higher in cells treated with hFSH21 than in those treated with hFSH24 (P < 0.01 and P <0.05, respectively).
Figure 5. (Single column). Effect of FSH glycoform preparations on β-catenin phosphorylation in porcine granulosa cells.
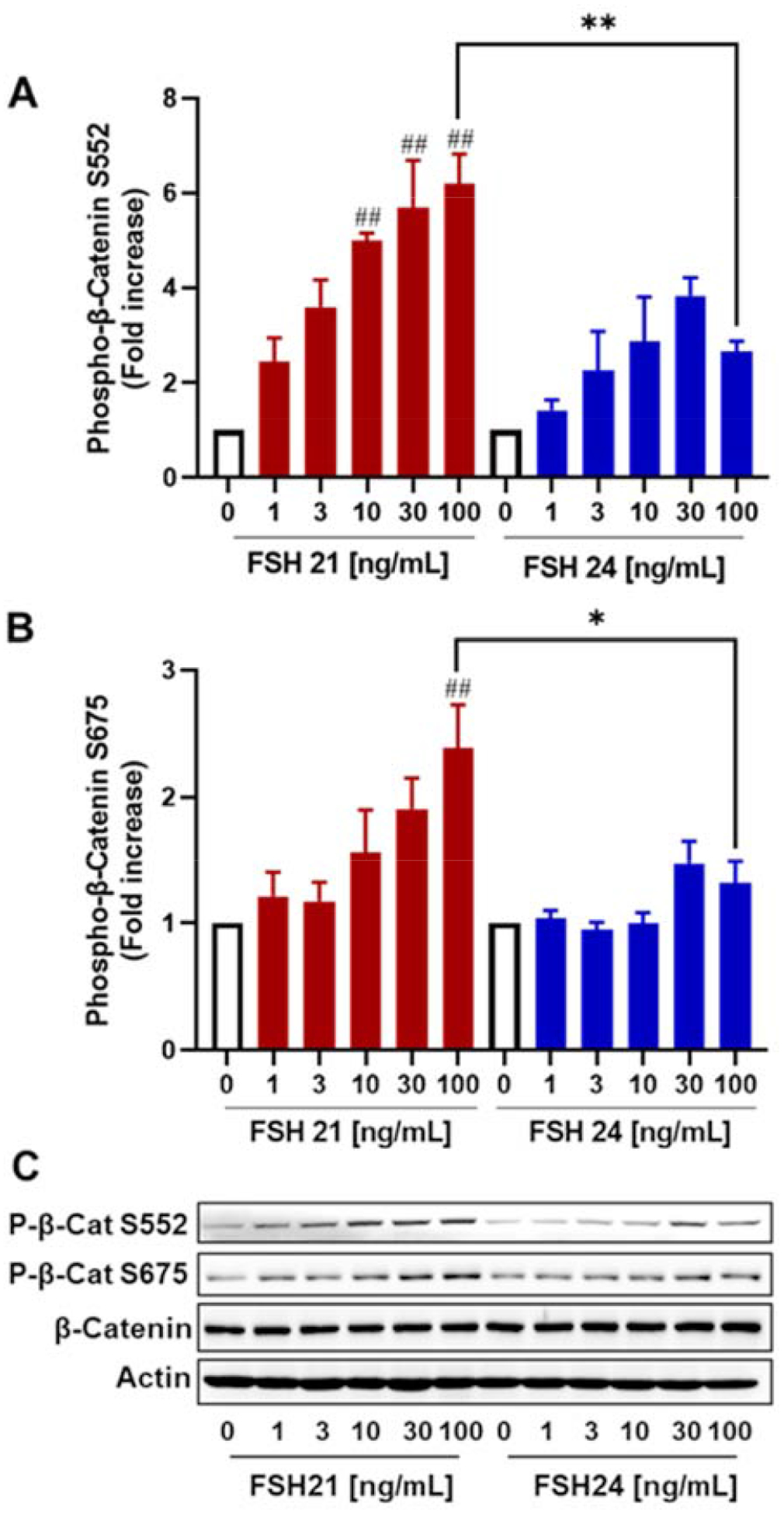
Cells were treated with increasing concentrations of hFSH21 or hFSH24 for 30 min. Cell lysates were prepared for Western blot analysis using phospho-Ser552 and phospho-Ser675-β-catenin (P-β-catenin) antibodies, and actin antibodies. A, fold changes in the phospho-β-catenin Ser552/Actin ratio; B, fold changes in the phospho-β-catenin Ser675/Actin ratio; C, representative Western blot analysis of phosphorylation of β-catenin after FSH glycoform treatment. Actin was used as an internal control. Data are expressed as means ± SEM; n = 3 separate experiments. #P < 0.05 and ##P < 0.01 compared with respective control; *P < 0.05 and **P < 0.01 compared with the other glycoform at the same concentration.
3.5. Hypo-glycosylated hFSH is more effective than fully glycosylated hFSH at regulating expression of FSH-responsive genes
The effects of hFSH glycoforms on expression of key steroidogenic enzymes and follicle differentiation markers were evaluated. As shown in Fig. 6A, hFSH21 stimulated concentration-dependent increases in 3β-hydroxysteroid dehydrogenase (HSD3B) and steroidogenic acute regulatory protein (STAR) transcripts. Additionally, the increases in HSD3B mRNA levels were greater in cells treated with hFSH21 compared to the levels in response to hFSH24 (P < 0.01). However, no changes were observed in cytochrome P450 side-chain cleavage enzyme (CYP11A1) expression in response to either hFSH21 or hFSH24. Interestingly, the expression of aromatase (CYP19A), a key enzyme for estrogen biosynthesis, was downregulated by 30 ng/mL concentrations of both hFSH glycoform preparations.
Figure 6. (1.5 column). Relative expression levels of steroidogenic-related genes after hFSH glycoform preparation treatment in porcine granulosa cells.
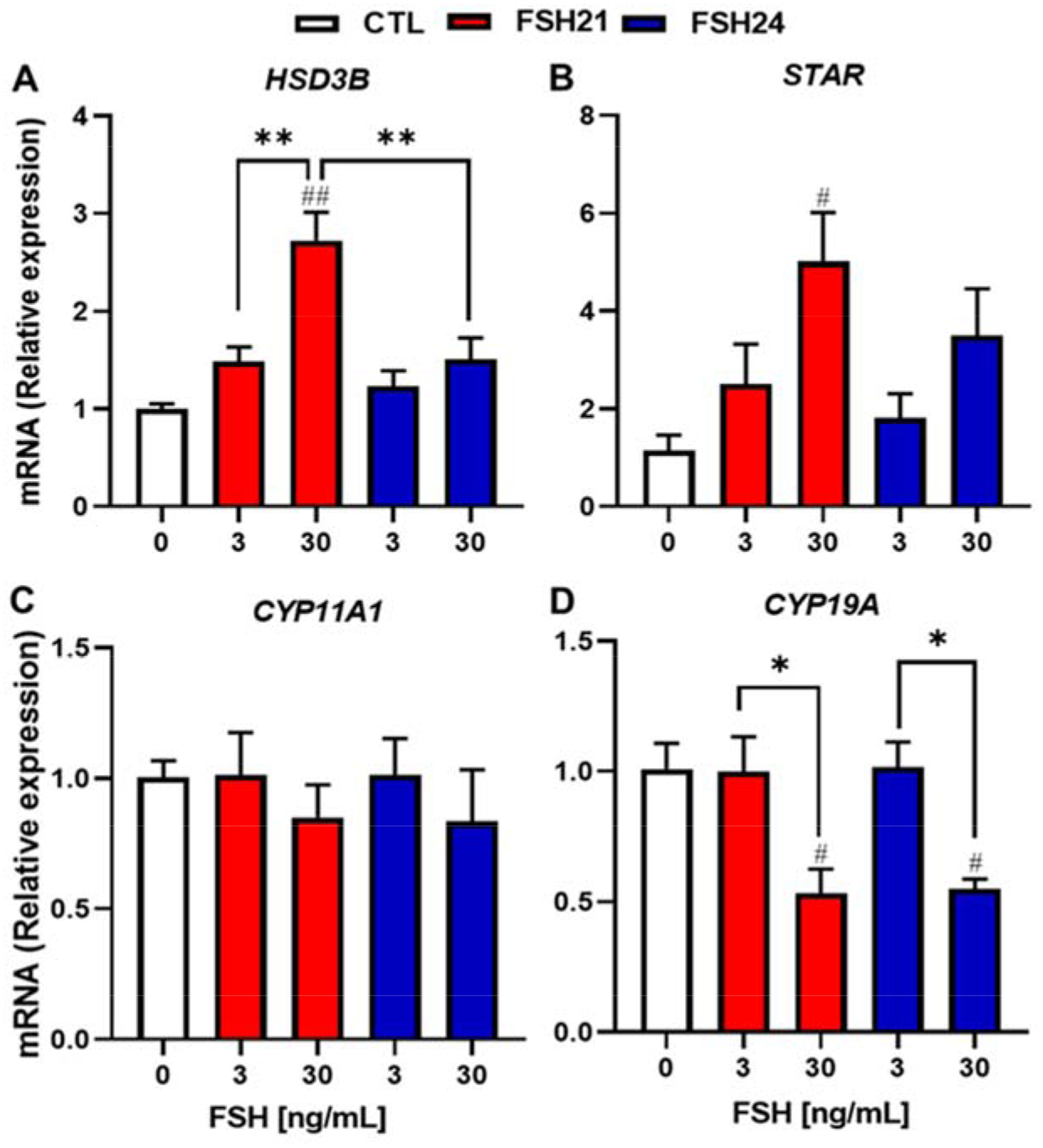
Granulosa cells were treated with hFSH21 or hFSH24 for 48 h. Real-time, quantitative PCR was performed. The results were evaluated as the relative ratio of the expression level of each mRNA to that of GAPDH. Data are expressed as means ± SEM; #P < 0.05 and ##P < 0.01 compared with control; *P < 0.05 and **P < 0.01 between the indicated experimental condition.
To confirm that the effect of hFSH glycoforms on steroidogenic-related genes was mediated by a cAMP-dependent mechanism, we investigated whether forskolin (FSK) could faithfully mimic the actions of hFSH glycoforms in porcine granulosa cells (Fig. 7A). Treatment with forskolin induced high expression of STAR and HSD3B genes (P < 0.01), with modest increases in the expression of CYP11A1 (P < 0.01). Similar to the hFSH glycoforms, forskolin reduced the expression of CYP19A (P < 0.01), suggesting porcine granulosa cells were undergoing differentiation under the experimental conditions.
Figure 7. (Single column). Relative expression levels of steroidogenic-related genes and differentiation-related genes after forskolin treatment in porcine granulosa cells.
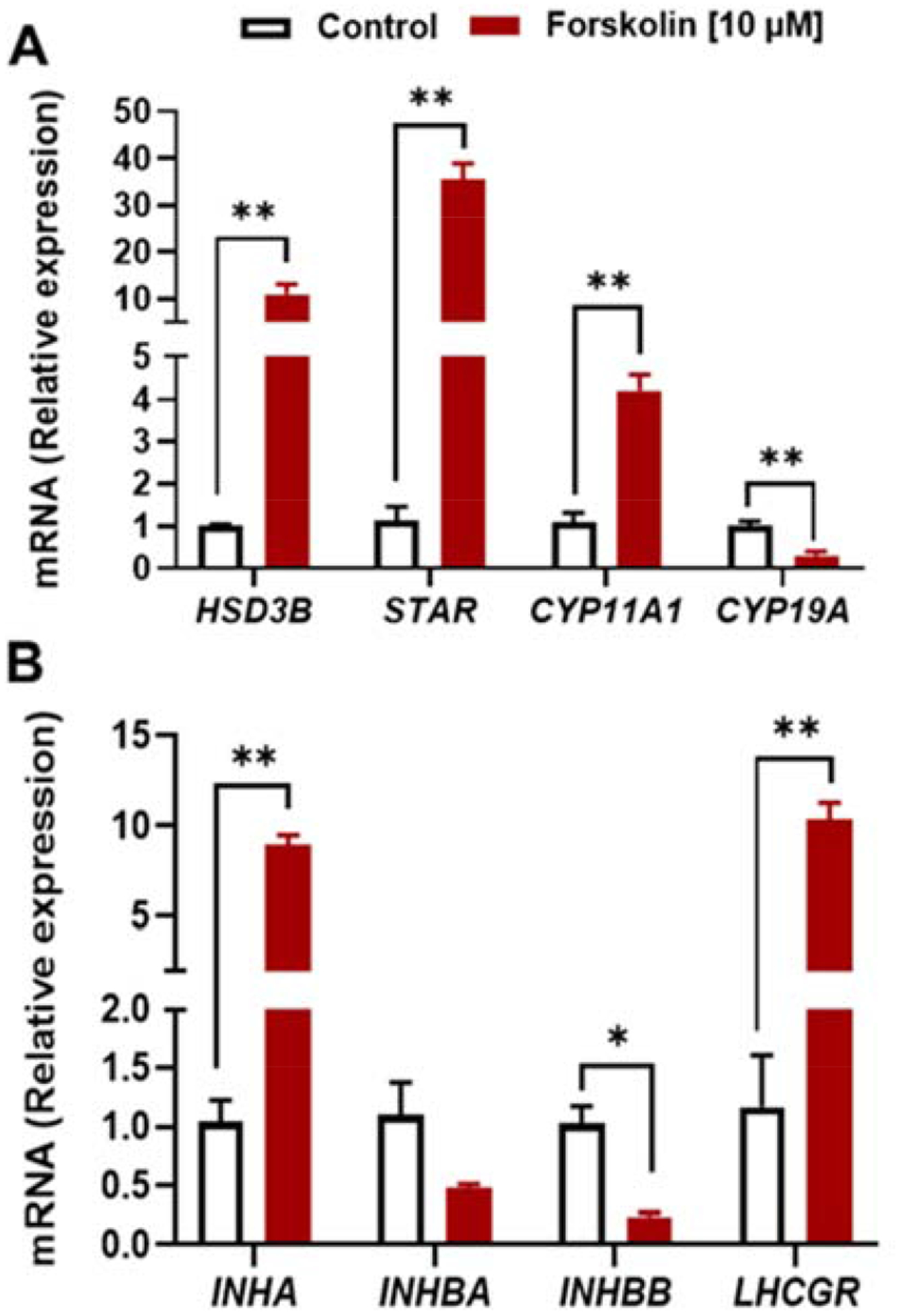
Granulosa cells were treated with 10 μM forskolin for 48 h. Real-time, quantitative PCR was performed. The results are expressed as the relative ratio of the expression level of each mRNA to that of GAPDH. Data are expressed as means ± SEM, n = 3 or 4 separate experiments; *P < 0.05 and **P < 0.01 compared with respective control.
We also examined mRNA expression patterns for inhibin (INH) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR), two factors associated with follicular differentiation. The levels of INHA mRNA were upregulated with increasing concentrations of hFSH glycoform preparations (Fig. 8A), whereas the levels of INHBA and INHBB mRNA were unchanged (Figs. 8C and 8D). hFSH21 was more effective than hFSH24 at increasing INHA mRNA (2.8-fold vs. 1.7-fold, respectively, P < 0.01). In addition, both hFSH21 and hFSH24 upregulated the transcripts for LHCGR (Fig. 8B).
Figure 8. (1.5 column). Relative expression levels of differentiation-related genes after hFSH glycoform treatment in porcine granulosa cells.
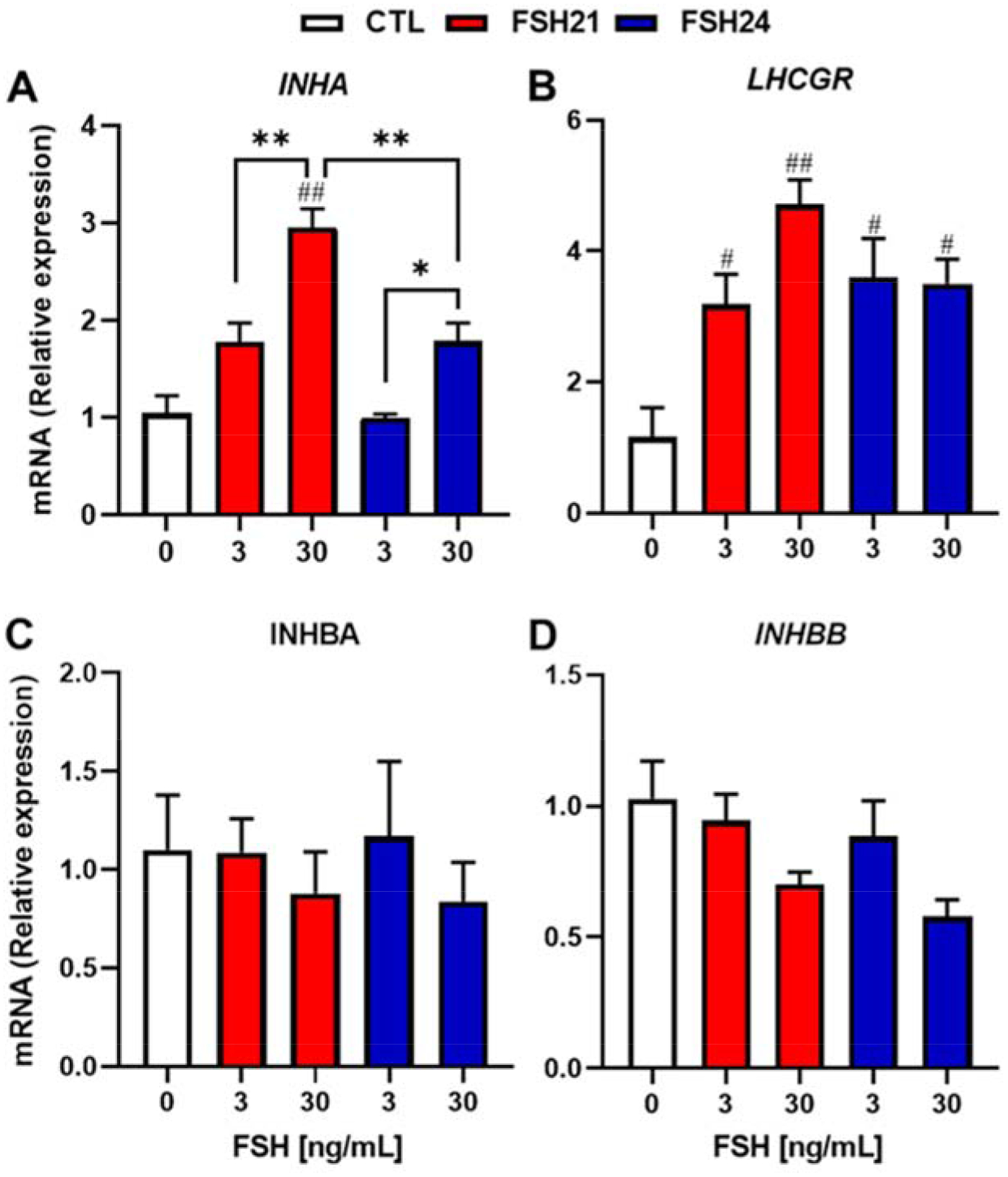
Granulosa cells were treated with hFSH21 or hFSH24 for 48 h. Real-time, quantitative PCR was performed. The expression level of each mRNA was normalized to that of GAPDH. Data are expressed as means ± SEM, n = 3 or 4 separate experiments; #P < 0.05 and ##P < 0.01 compared with control; *P < 0.05 and **P < 0.01 between the indicated experimental condition.
Stimulation with forskolin resulted in similar responses, transcripts for INHA and LHCGR were upregulated and transcripts for INHBA and INHBB were downregulated (Fig.7B)
3.6. Hypo-glycosylated hFSH is more effective than fully glycosylated hFSH at inducing progesterone synthesis
Progesterone concentrations in media were measured after treatment with hFSH glycoforms for 48 h. We observed significantly higher progesterone synthesis with hFSH21 treatment at 10, 30, 100 ng/mL in comparison with control, whereas 30 and 100 ng/mL fully-glycosylated hFSH24 were required to significantly increase progesterone (Fig. 9B). Progesterone levels in medium conditioned by cells treated with either 10 or 30 ng/mL hFSH21 were significantly greater than those in cells treated with identical concentrations of hFSH24. In keeping with the findings on progesterone synthesis, stimulation with hFSH glycoforms resulted in a concentration-dependent increase in the protein levels of HSD3B. The expression of HSD3B protein was significantly greater in cells treated with 30 ng/mL hFSH21 (3.9-fold increase) compared to levels in hFSH24 treated cells (1.9-fold increase) (Fig. 9A, P < 0.05). Estradiol levels in these cultures were very low and unresponsive to either hFSH21 or hFSH24 (Fig. 9C), possibly due to the downregulation of aromatase expression by hFSH glycoforms treatment. Neither hFSH21 nor hFSH24 had a significant effect on cell viability/cell numbers (Fig. 9D).
Figure 9. (Single column). Progesterone and estradiol synthesis in porcine granulosa cells.
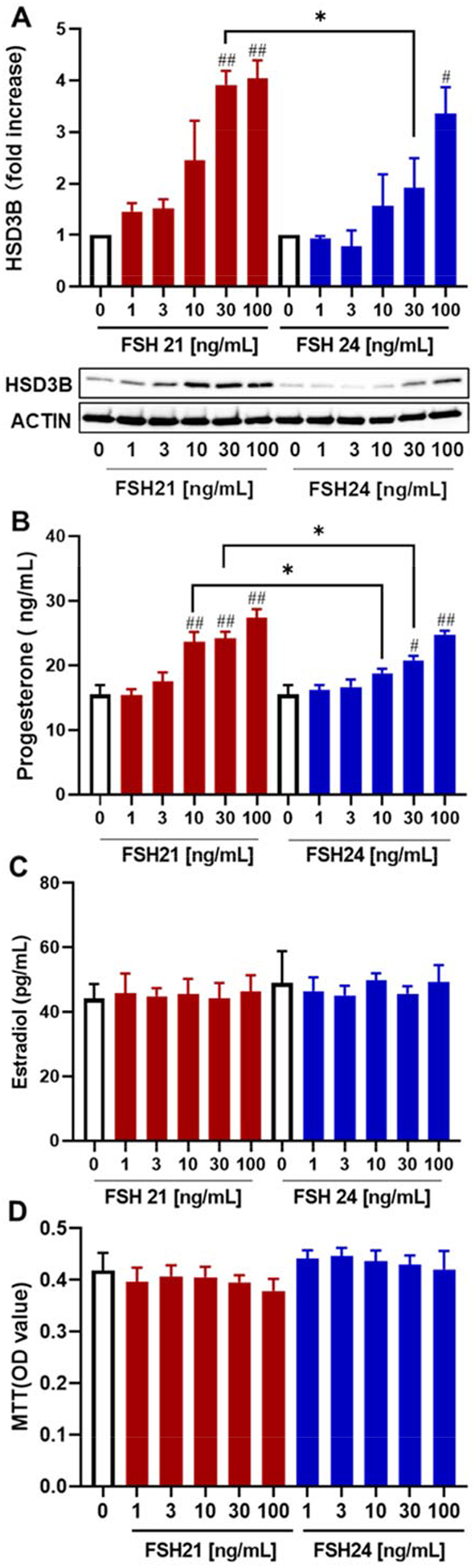
Granulosa cells were treated with increasing concentrations of hFSH21 or hFSH24 for 48 h. A, Cell lysates were prepared and Western blot analysis of HSD3B1 protein was performed, with actin as an internal control. B and C, progesterone and estradiol secretion in the culture medium, respectively. D, Cell viability assay (MTT). Data are expressed as means ± SEM; n = 3 separate experiments. #P < 0.05 and ##P < 0.01 compared with control; *P < 0.05 and **P < 0.01 compared with the other glycoform at the same concentration.
4. Discussion
Primary cultures of granulosa cells from pre-pubertal gilts were selected as a relevant model to elucidate the direct regulatory actions exerted by hFSH glycoforms on cell signaling and gene expression. The findings indicate that hypo-glycosylated hFSH21 has significantly greater ability to stimulate cAMP and PKA-dependent signaling compared to fully-glycosylated hFSH24. Furthermore, the results show that hFSH21 has greater ability to stimulate important transcription regulators (CREB and β-catenin), the expression of important FSH-responsive genes (HSD3B, STAR, INHA) and the induction of progesterone synthesis in granulosa cells. The results indicate that hFSH21 has greater bioactivity than hFSH24 on key parameters associated with granulosa cell function.
FSH exerts its actions on granulosa cells mainly through stimulation of the cAMP-PKA pathway (Richards, 2001; Hunzicker-Dunn et al., 2006; Puri et al., 2016; Casarini et al., 2019), although other signaling pathways may be activated downstream or parallel to cAMP signaling (Casarini et al., 2019; Coss, 2020). The results indicated that compared to fully-glycosylated hFSH24, hypo-glycosylated hFSH21 more robustly activated cAMP/PKA signaling with significant increases occurring at 10–30 ng/mL hFSH21. In contrast, 100 ng/ml FSH24 was required to effectively activate PKA signaling; nonetheless, the response to FSH24 was significantly less than the response to FSH21 at this relatively high concentration of hormone. The difference in FSH21 and FSH24 bioactivity cannot be explained based an alteration in mass due to variations in glycosylation. When used at 10 ng/ml fully glycosylated FSH24 is equivalent to 318 pM and 10 ng/ml hypoglycosylated FSH21 is equivalent to 336 pM. This difference of 5.8% cannot account for the observed differences in concentration responses and maximal responses to the FSH glycofroms. It is likely that the findings reflect a greater occupancy rate and receptor affinity for hFSH21 versus hFSH24 (Butnev et al., 2015), which account for the enhanced coupling to the cAMP/PKA signaling pathway. These findings are also consistent with a previous report showing that hFSH24 was much less effective than hFSH21 at stimulating cAMP/PKA signaling in the KGN immortalized granulosa tumor cell line (Jiang et al., 2015) and HEK293 cells stably expressing the human FSHR (Zariñán, et al., 2020). A recent result employing cultures of mouse follicles also showed differential PKA substrate phosphorylation responses to hFSH21 and hFSH24, with FSH21 being the more effective glycoform when used at concentration of 100 ng/mL (Simon et al., 2019). It seems that in vitro cultures of porcine granulosa cells are more sensitive to FSH glycoforms compared to in vitro incubation of pre-antral follicles.
The PKA substrate CREB is known to play an important role in regulating FSH responsive genes in granulosa cells (Hagiwara, et al., 1993; Mukherjee et al., 1996; Hunzicker-Dunn et al., 2006). The present results showed that porcine granulosa cells were more responsive to hFSH21 than to hFSH24 in terms of CREB phosphorylation (Ser133) and CRE-mediated transcription. The findings clearly showed that hFSH21 exerted greater concentration-dependent increases in the numbers and intensity of granulosa cells positive for nuclear CREB phosphorylation. Of importance are results showing that granulosa cells did not respond to hFSH24 when used at the lowest concentrations of hFSH21 that were effective for the induction of cAMP, PKA, CREB phosphorylation and CRE-mediated transcription. The results confirm a previous report in which hFSH21 was more effective than hFSH24 at activating the phosphorylation of CREB and CRE-mediated transcription in the KGN cell line (Jiang et al., 2015). The transcriptional co-activator β-catenin is implicated in FSH-mediated granulosa cell differentiation and steroidogenesis (Parakh et al., 2006, Castañon et al., 2012). Previous reports show that β-catenin is a direct target of PKA signaling, and phosphorylation at Ser552 and Ser675 is coupled to increases in transcriptional activity via a PKA-dependent, PI3K-independent pathway (Taurin et al., 2006; Law et al., 2013). Using porcine granulosa cells, we showed that either hypo-glycosylated hFSH21 or fully-glycosylated hFSH24 stimulated increases in the phosphorylation of β-catenin at both Ser552 and Ser675 residues. However, hFSH21 induced greater increases in phosphorylated β-catenin when compared with hFSH24, which presumably is attributed to higher levels of cAMP/PKA signaling in response to hFSH21.
It is likely that activation of CREB and β-catenin signaling contributes to the stimulatory actions of FSH on steroidogenic enzyme expression and ovarian differentiation factor expression (Parakh et al., 2006; Roy et al. 2009; Castañon et al., 2012; LaVoie, 2017). Treatment with hFSH21 or hFSH24 resulted in differential expression of FSH-responsive genes in porcine granulosa cells, including HSD3B, STAR, LHCGR, and INHA. The findings showed that transcripts for HSD3B and STAR genes were significantly upregulated in response to treatment with hFSH21. The upregulation of HSD3B transcripts correlates with the increases in HSD3B protein and progesterone secretion by granulosa cells. Studies in human granulosa cells show that FSH also stimulates progesterone production by increasing the enzymatic activity of HSD3B (Oktem et al., 2017), but the effect of hFSH glycoforms on HSD3B activity in porcine granulosa cells is unknown. However, the expression of CYP11A1 mRNA, the product of which converts cholesterol to pregnenolone, was unresponsive to either hFSH glycoform. As the molecular controls for CYP11A1 are not identical to those employed by the HSD3B and STAR genes, our culture conditions employing serum likely contributed factors that elevated basal CYP11A1 expression (Mizutani et al., 2015; Lavoie, 2017) and prevented FSH-responsiveness. Interestingly, the levels of estradiol showed no change after treatment with either hFSH glycoform, despite a significant downregulation of aromatase mRNA. This finding suggests that the culture conditions are not sufficient to support aromatase expression and/or the granulosa cells are acquiring characteristics of more differentiated follicles as evidenced by the upregulation of LHCGR and INHA transcripts (Picton et al., 1999). In the present study both hFSH glycoform preparations and forskolin repressed CYP19A mRNA expression, arguing for a role for cAMP/PKA in granulosa cell differentiation.
The expression of INHA mRNA was upregulated both by hFSH21 and hFSH24, showing significantly higher expression in cells treated with hFSH21, likely resulting from the greater CREB/CRE activity (Ito, et al., 2000). In contrast, neither hFSH21 nor hFSH24 affected the transcripts for INHBA and INHBB, although a trend for reduced transcripts was apparent at high glycoform concentrations. Our results are consistent with a previous report that revealed that both mRNA expression and production of INHA were clearly regulated by the different recombinant hFSH glycosylation variant preparations in the KGN cell line (Loreti et al., 2013). A study by Andreone et al. (2017) in Sertoli cells found that a less acidic/sialylated FSH preparation exerted a greater stimulatory effect on production of inhibin α in comparison to a more acidic/sialylated FSH preparation. Furthermore, they also observed differential stimulatory effects on INHB by hFSH glycosylation variants. Based on the differential expression of a few known FSH-responsive genes in this study, it would be valuable to determine global transcriptome responses elicited by each hFSH glycoform in primary granulosa cells.
5. Conclusions
Our investigation of the bioactivities of the hypo-glycosylated hFSH21 and fully-glycosylated hFSH24 preparations in porcine primary granulosa cells revealed that when compared to hFSH24, hFSH21 exhibited a greater ability to (1) stimulate cAMP/PKA-mediated signaling, (2) phosphorylate the transcription activators CREB and β-catenin, (3) induce expression of a set of FSH target genes, and (4) stimulate progesterone production. These findings extend our knowledge regarding the direct responses of primary granulosa cells to hFSH glycoforms. Whole genome scale differential gene expression analysis is currently being studied in murine (Espinal-Enriquez et al. 2020) and porcine granulosa cells (Liang and Davis, unpublished) and it will be of interest in the future to further test their regulatory actions in primary granulosa cells, eventually laying the foundation for clinical applications with hFSH glycoforms.
Supplementary Material
Highlights:
This is the first report documenting the cellular and molecular effects of recombinant human FSH (hFSH) glycoforms in primary granulosa cells.
Hypo-glycosylated hFSH21 exerted a more robust activation of cAMP accumulation and protein kinase A (PKA) activity than fully-glycosylated hFSH24.
Hypo-glycosylated hFSH21 stimulated greater phosphorylation of the transcription regulators CREB and β-Catenin
Compared to fully-glycosylated hFSH24, hypo-glycosylated hFSH21 induced greater levels of transcripts for HSD3B1, STAR and INHA, and higher progesterone production.
This study furthers our understanding of the differing bioactivities of FSH glycoforms in the ovary
Acknowledgements:
The authors thank advanced Microscopy Core Facility for their assistance with microscopy. The use of the microscope was supported by Center for Cellular Signaling CoBRE-P30GM106397 from the National Health Institutes.
Grant Support:
This work was supported by the National Institutes of Health [1P01 AG029531], and the Olson Center for Women’s Health. This work was supported in part by Senior Research Career Scientist Award and Merit Review Award I01 BX004272 from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service. This material is the result of work supported with resources and the use of facilities at the VA NWIHCS, Omaha, NE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest:
None
References
- Andreone L, Ambao V, Pellizzari EH, Loreti N, Cigorraga SB, Campo S. Role of FSH glycan structure in the regulation of Sertoli cell inhibin production. Reproduction. 2017; 154(5):711–721. [DOI] [PubMed] [Google Scholar]
- Bishop LA, Robertson DM, Cahir N, Schofield PR. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol Endocrinol. 1994; 8(6):722–31. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Butnev VY, Rueda-Santos MA, Brown A, Hall AS, Harvey DJ. Macro- and micro-heterogeneity in pituitary and urinary follicle stimulating hormone glycosylation. J Glycomics Lipidomics 2014a; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfield GR, Butnev VY, Butnev VY, Hiromasa Y, Harvey DJ, May JV. Hypo-glycosylated human follicle-stimulating hormone (hFSH(21/18)) is much more active in vitro than fully-glycosylated hFSH (hFSH(24)). Mol Cell Endocrinol. 2014b; 382(2):989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfield GR, May JV, Davis JS, Dias JA, Kumar TR. In vivo and in vitro impact of carbohydrate variation on human follicle-stimulating hormone function. Front Endocrinol (Lausanne) 2018; 9: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butnev VY, Butnev VY, May JV, Shuai B, Tran P, White WK, Brown A, Smalter Hall A, Harvey DJ, Bousfield GR. Production, purification, and characterization of recombinant hFSH glycoforms for functional studies. Mol Cell Endocrinol. 2015. April 15; 405: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarini L, Crépieux P. Molecular mechanisms of action of FSH. Front Endocrinol (Lausanne) 2019. 14;10:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañon BI, Stapp AD, Gifford CA, Spicer LJ, Hallford DM, Hernandez Gifford JA. Follicle-stimulating hormone regulation of estradiol production: possible involvement of WNT2 and beta-catenin in bovine granulosa cells. Journal of Animal Science. 2012, 90: 3789–3797. [DOI] [PubMed] [Google Scholar]
- Coss D. Commentary on the recent FSH collection: Known knowns and known unknowns. Endocrinology. 2020; 161(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JS, Kumar TR, May JV, Bousfield GR. Naturally occurring follicle-stimulating hormone glycosylation variants. J Glycomics Lipidomics. 2014; 4(1):e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hayek S, Demeestere I, Clarke HJ. Follicle-stimulating Hormone regulates expression and activity of epidermal growth factor receptor in the murine ovarian follicle. Proc Natl Acad Sci U S A. 2014; 111(47):16778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinal-Enriquez J, De-Anda-Jáuregui G, Hernández-Montes G, Lira-Albarrán S, Zariñán T, Gutiérrez-Sagal R, Rebollar-Vega R, Bousfield GR, Butnev VY, Hernández-Lemus E, Ulloa-Aguirre A. MON-023 Dynamics of the transcriptome in rat granulosa cells exposed to different follicle-stimulating hormone (FSH) glycosylation variants as revealed by RNA-seq/new generation sequencing (NGS). J Endocr Soc 2020; 4 (Supplement_1) 10.1210/jendso/bvaa046.194 [DOI] [Google Scholar]
- George JW, Dille EA, Heckert LL. Current concepts of follicle stimulating hormone receptor gene regulation. Biol Reprod. 2011; 84(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M, Brindle P, Harootunian A, Armstrong R, Rivier J, Vale W, Tsien R, Montminy MR. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol. 1993;13(8):4852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howles CM. Role of LH and FSH in ovarian function. Mol Cell Endocrinol. 2000; 161(1–2):25–30. [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006; 18(9):1351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Park Y, Weck J, Mayo KE, Jameson JL. Synergistic activation of the inhibin alpha-promoter by steroidogenic factor-1 and cyclic adenosine 3’,5’-monophosphate. Mol Endocrinol. 2000; 14(1):66–81. [DOI] [PubMed] [Google Scholar]
- Keel BA, Davis JS. Epidermal growth factor activates extracellular signal-regulated protein kinases (ERK) in freshly isolated porcine granulosa cells. Steroids. 1999; 64(9):654–8. [DOI] [PubMed] [Google Scholar]
- LaVoie HA. Transcriptional control of genes mediating ovarian follicular growth, differentiation, and steroidogenesis in pigs. Mol Reprod Dev. 2017; 84(9):788–801. [DOI] [PubMed] [Google Scholar]
- Law NC, Weck J, Kyriss B, Nilson JH, Hunzicker-Dunn M. Lhcgr expression in granulosa cells: roles for PKA-phosphorylated β-Catenin, TCF3, and FOXO1 Mol Endocrinol. 2013; 27(8): 1295–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti N, Fresno C, Barrera D, Andreone L, Albarran SL, Fernandez EA, Larrea F, Campo S. The glycan structure in recombinant human FSH affects endocrine activity and global gene expression in human granulosa cells. Mol Cell Endocrinol. 2013; 366(1):68–80. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4):402–8. [DOI] [PubMed] [Google Scholar]
- Jiang C, Hou X, Wang C, May JV, Butnev VY, Bousfield GR, Davis JS. Hypoglycosylated hFSH has greater bioactivity than fully glycosylated recombinant hFSH in human granulosa cells. J Clin Endocrinol Metab. 2015; 100(6):E852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Hou X, Talbott H, Cushman R, Cupp A, Davis JS. ATF3 expression in the corpus luteum: possible role in luteal regression. Mol Endocrinol. 2013; 27(12):2066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T, Ishikane S, Kawabe S, Umezawa A, Miyamoto K Transcriptional regulation of genes related to progesterone production. Endocrine Journal 2015; 62(9): 757–763 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Park-Sarge OK, Mayo KE. Gonadotropins induce rapid phosphorylation of the 3’,5’-cyclic adenosine monophosphate response element binding protein in ovarian granulosa cells. Endocrinology. 1996; 137(8):3234–45. [DOI] [PubMed] [Google Scholar]
- Mordhorst BR, Prather RS. Pig Models of Reproduction In: Schatten H, Constantinescu GM, editors. Animal Models and Human Reproduction. John Wiley & Sons, Inc; 2017. [Google Scholar]
- Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, Haji M, Nawata H. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142(1):437–45. [DOI] [PubMed] [Google Scholar]
- Oktem O, Akin N, Bildik G, Yakin K, Alper E, Balaban B, Urman B. FSH Stimulation promotes progesterone synthesis and output from human granulosa cells without luteinization. Hum Reprod. 2017;32(3):643–652. [DOI] [PubMed] [Google Scholar]
- Parakh TN, Hernandez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ, Nilson JH. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proc Natl Acad Sci U S A. 2006. August 15; 103(33):12435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton HM, Campbell BK, Hunter MG. Maintenance of oestradiol production and expression of cytochrome P450 aromatase enzyme mRNA in long-term serum-free cultures of pig granulosa cells. J Reprod Fertil. 1999;115(1):67–77. [DOI] [PubMed] [Google Scholar]
- Plewes MR, Burns PD, Graham PE, Hyslop RM, Barisas BG. Effect of fish oil on lateral mobility of prostaglandin F2α (FP) receptors and spatial distribution of lipid microdomains in bovine luteal cell plasma membrane in vitro. Domest Anim Endocrinol. 2017; 58:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewes M, Burns P. Effect of fish oil on agonist-induced receptor internalization of the PG F2α receptor and cell signaling in bovine luteal cells in vitro. Domest Anim Endocrinol 2018; 63:38–47 [DOI] [PubMed] [Google Scholar]
- Plewes MR, Hou X, Zhang P, Liang A, Hua G, Wood JR, Cupp AS, Lv X, Wang C, Davis JS. Yes-associated protein 1 is required for proliferation and function of bovine granulosa cells in vitro. Biol Reprod. 2019; 101(5):1001–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Little-Ihrig L, Chandran U, Law NC, Hunzicker-Dunn M, Zeleznik AJ. Protein kinase A: a master kinase of granulosa cell differentiation. Sci Rep. 2016; 6:28132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS. New signaling pathways for hormones and cyclic adenosine 3′5′-monophosphate action in endocrine cells. Molecular Endocrinology. 2001;15(2): 209–218. [DOI] [PubMed] [Google Scholar]
- Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian Folliculogenesis. In: Piprek R. (eds) Molecular mechanisms of cell differentiation in gonad development. Results and Problems in Cell Differentiation, 2016;58:167–90. [DOI] [PubMed] [Google Scholar]
- Roy L, McDonald CA, Jiang C, Maroni D, Zeleznik AJ, Wyatt TA, Hou X, Davis JS. Convergence of 3’,5’-cyclic adenosine 5’-monophosphate/protein kinase A and glycogen synthase kinase-3beta/beta-catenin signaling in corpus luteum progesterone synthesis. Endocrinology. 2009; 150(11):5036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader KA, Gorbatcheva B, Senz J, Heravi-Moussavi A, Melnyk N, Salamanca C, Maines-Bandiera S. The specificity of the FOXL2 c. 402C> G somatic mutation: a survey of solid tumors. PLoS One. 2009; 4 : e7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon LE, Liu Z, Bousfield GR, Kumar TR, Duncan FE. Recombinant FSH glycoforms are bioactive in mouse preantral ovarian follicles. Reproduction. 2019; 158(6):517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitz J, Wolfenson C, Chappel S, Ruman J. Follicle-Stimulating Hormone: A Review of Form and Function in the Treatment of Infertility. Reprod Sci. 2016; 23(6):706–16. [DOI] [PubMed] [Google Scholar]
- Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006; 281(15):9971–6. [DOI] [PubMed] [Google Scholar]
- Taymor ML. The regulation of follicle growth: some clinical implications in reproductive endocrinology. Fertil Steril. 1996;65(2):235–47. [DOI] [PubMed] [Google Scholar]
- Walton WJ, Nguyen VT, Butnev VY, Singh V, Moore WT, Bousfield GR. Characterization of human FSH isoforms reveals a nonglycosylated beta-subunit in addition to the conventional glycosylated beta-subunit. J Clin Endocrinol Metab. 2001; 86(8):3675–85. [DOI] [PubMed] [Google Scholar]
- Wang H, May J, Butnev V, Shuai B, May JV, Bousfield GR, Kumar TR. Evaluation of in vivo bioactivities of recombinant hypo- (FSH21/18) and fully- (FSH24) glycosylated human FSH glycoforms in Fshb null mice. Mol Cell Endocrinol. 2016; 437:224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wide L, Eriksson K. Low-glycosylated forms of both FSH and LH play major roles in the natural ovarian stimulation. Ups J Med Sci. 2018; 123(2):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariñán T, Butnev VY, Gutiérrez-Sagal R, Maravillas-Montero JL, Martínez-Luis I, Mejía-Domínguez NR, Juárez-Vega G, Bousfield GR, & Ulloa-Aguirre A In vitro impact of FSH glycosylation variants on FSH receptor-stimulated signal transduction and functional selectivity. Journal of the Endocrine Society. 2020; 4(5), bvaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


