Abstract
This study evaluates the proportion of patients with myelin oligodendrocyte glycoprotein IgG–associated disorders who fulfill the seronegative neuromyelitis optica spectrum disorder criteria.
The 2015 international consensus diagnostic criteria for neuromyelitis optica spectrum disorder (NMOSD) was created to identify adult patients with similar clinical and radiological phenotypes, distinct from multiple sclerosis.1 It includes patients who are seropositive for aquaporin-4 immunoglobulin G (AQP4-IgG) and those who are seronegative. These criteria were created prior to the current understanding of myelin oligodendrocyte glycoprotein–IgG (MOG-IgG1)–associated disorders (MOGAD). Although MOGAD has some phenotypic overlap with AQP4-IgG and NMOSD, there are many clinical, radiologic, serologic, and prognostic differences.2,3,4,5,6 There is inconsistent use of diagnostic MOG-IgG1 terminology for MOGAD, with many neurologists using the term seronegative NMOSD. We sought to evaluate the proportion of patients with MOGAD who fulfill the seronegative NMOSD criteria.1
Methods
This is a retrospective study approved by the institutional review board of Mayo Clinic (08-00647) using clinical and radiological data from Mayo Clinic patients with MOGAD (MOG-IgG1+ by flow cytometric assay) who provided written consent for research. The 2015 NMOSD diagnostic criteria were applied.1 Descriptive statistics included Fisher exact and Wilcoxon rank sum tests (2-sided P value <.05 was considered statistically significant).
Results
Demographic and clinical characteristics are shown in the Table. Among adults, 26 (23%) met seronegative NMOSD criteria (median time, 7.1 months; interquartile range [IQR], 2.3-23.3). Core clinical characteristics included optic neuritis (ON) and longitudinally extensive transverse myelitis (LETM) (n = 20 [77%]), ON and diencephalon lesions with diencephalon attack in the context of acute disseminated encephalomyelitis (ADEM) (n = 4 [15%]), and LETM and brainstem syndrome with brainstem periependymal lesions (n = 2 [8%]) (Figure).
Table. Application of Seronegative NMOSD Criteria to MOGAD Cohort.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| Adults (n = 115) | Children (n = 55) | Total (N = 170) | ||
| Sex | ||||
| Female | 60 (52.2) | 35 (63.6) | 95 (55.9) | .191 |
| Male | 55 (47.8) | 20 (36.4) | 75 (44.1) | |
| Age at onset, median (IQR) | 39.0 (30.0-53.5) | 10.0 (6.0-13.0) | 30.0 (14.0-48.0) | <.0012 |
| Follow-up, median (IQR), y | 1.8 (0.9-4.1) | 5.0 (1.9-7.8) | 2.4 (1.1-5.6) | <.0012 |
| Relapsing course | 64 (55.7) | 39 (70.9) | 103 (60.6) | .071 |
| Clinical attacks, median (IQR) | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | .242 |
| Meets seronegative NMOSD criteria | ||||
| No | 89 (77.4) | 38 (69.1) | 127 (74.7) | .261 |
| Yes | 26 (22.6) | 17 (30.9) | 43 (25.3) | |
| Clinical phenotype meeting seronegative NMOSD criteria | ||||
| LETM and ON | 20 (76.9) | 10 (58.8) | 30 (69.8) | NA |
| ON and diencephalon lesion with diencephalon attack (ADEM) | 4 (15.4) | 5 (29.4) | 9 (20.9) | NA |
| LETM and brainstem periependymal lesion with brainstem attack | 2 (7.7) | 1 (5.9) | 3 (7.0) | NA |
| LETM and diencephalon lesion and diencephalon attack (ADEM) | 0 (0.0) | 1 (5.9) | 1 (2.3) | NA |
| Time to meet seronegative NMOSD criteria, median (IQR), mo | 7.1 (2.3-23.3) | 12.1 (2.2-64.7) | 7.6 (2.3-25.0) | NA |
| Clinical phenotype not meeting seronegative NMOSD criteria | NA | |||
| rON | 26 (29.5) | 13 (38.2) | 39 (32.0) | NA |
| mON | 19 (21.6) | 7 (20.6) | 26 (21.3) | NA |
| LETM | 15 (17.0) | 0 | 15 (12.3) | NA |
| ON, TM (not LE) | 11 (12.5) | 2 (5.9) | 13 (10.7) | NA |
| TM (not LE) | 6 (6.8) | 0 | 6 (4.9) | NA |
| ADEM | 2 (2.3) | 4 (11.8) | 6 (4.9) | NA |
| ON, brainstem (not periependymal) | 2 (2.3) | 0 | 2 (1.6) | NA |
| TM (not LE), brainstem | 1 (1.1) | 0 | 1 (0.8) | NA |
| LETM, cerebellar | 1 (1.1) | 0 | 1 (0.8) | NA |
| LETM, ADEM | 1 (1.1) | 1 (2.9) | 2 (1.6) | NA |
| TM (not LE), brainstem, cerebral | 1 (1.1) | 0 | 1 (0.8) | NA |
| ON, ADEM | 1 (1.1) | 5 (14.7) | 6 (4.9) | NA |
| Recurrent APS | 1 (1.1) | 0 | 1 (0.8) | NA |
| TM (not LE), ADEM | 1 (1.1) | 0 | 1 (0.8) | NA |
| ADEM, LETM/ONa | 0 | 1 (2.9) | 1 (0.8) | NA |
| TM (not LE), ON, cerebellar | 0 | 1 (2.9) | 1 (0.8) | NA |
| Missing data | 1 | 4 | 5 | NA |
| Disease course of patients not meeting NMOSD criteria | ||||
| Monophasic | 50 (55.6) | 15 (39.5) | 65 (50.8) | NA |
| Relapsing | 40 (44.4) | 23 (60.5) | 63 (49.2) | NA |
| Subgroup: Relapsing Course | Adults (n = 64) | Children (n = 39) | Total (n = 103) | |
| Meets seronegative NMOSD criteria | ||||
| No | 40 (62.5) | 23 (59.0) | 63 (61.2) | .841 |
| Yes | 24 (37.5) | 16 (41.0) | 40 (38.8) | |
| Subgroup: ≥2-y Follow-up | Adults (n = 52) | Children (n = 40) | Total (n = 92) | |
| Meets seronegative NMOSD criteria | ||||
| No | 36 (69.2) | 28 (70.0) | 64 (69.6) | >.991 |
| Yes | 16 (30.8) | 12 (30.0) | 28 (30.4) | |
Abbreviations: ADEM, acute disseminated encephalomyelitis; APS, area postrema syndrome; IQR, interquartile range; LE, longitudinally extensive; LETM, longitudinally extensive transverse myelitis; mON, monophasic optic neuritis; NA, not applicable; NMOSD, neuromyelitis optica spectrum disorder; ON, optic neuritis; rON, recurrent optic neuritis; TM, transverse myelitis.
Not meeting criteria owing to optic neuritis criteria.
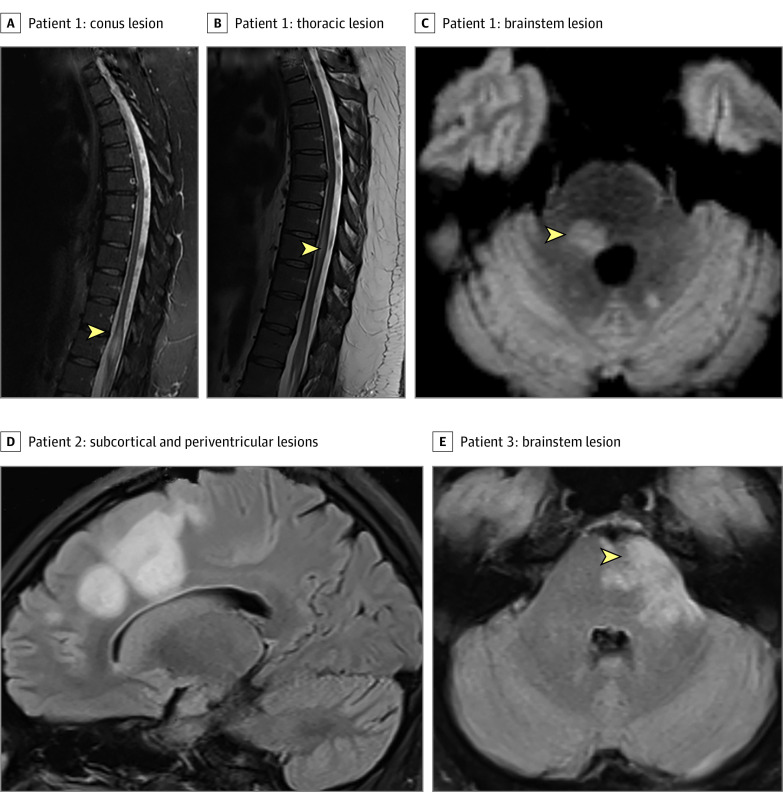
Figure. Magnetic Resonance Imaging Features of Patients With Myelin Oligodendrocyte Glycoprotein–IgG Associated Disorders Meeting and Not Meeting the Neuromyelitis Optica Spectrum Disorder (NMOSD) 2015 Seronegative Criteria.
Meeting NMOSD 2015 Seronegative criteria: patient 1 (A) sagittal magnetic resonance imaging (MRI) T2 longitudinally extensive conus lesion (first attack), sagittal MRI T2 longitudinally extensive thoracic lesion from T7-T10 (B; second attack), and axial MRI with T2/fluid-attenuated inversion recovery periepenydmal brainstem lesion (C).
Not meeting NMOSD 2015 seronegative criteria: patient 2 (D) axial MRI T2/fluid-attenuated inversion recovery large bilateral subcortical and periventricular lesions in the setting of acute disseminated encephalomyelitis in patient with history of recurrent optic neuritis. Patient 3 (E) axial MRI with brainstem medullary T2 lesion (not peripendymal) in a patient with recurrent optic neuritis.
Among children, 17 (31%) met the seronegative NMOSD criteria (median time of 12.1 months; IQR, 2.2-64.7 months). Core clinical characteristics included ON and LETM (n = 10 [59%]), ON and diencephalon lesions with diencephalon attack in the context of ADEM (n = 5 [29%]), LETM and brainstem syndrome with brainstem peri-ependymal lesion (n = 1 [6%]), and LETM with diencephalon attack in the context of ADEM (n = 1 [6%]) (Table).
In the subgroup of patients who had a relapsing course (64 adults and 39 children), 24 adults (38%) and 16 children (41%) met the seronegative NMOSD criteria (Table). In the subgroup of patients with more than 2 years of follow-up from disease onset (52 adults and 10 children), 16 adults (31%) and 12 children (30%) met seronegative NMOSD criteria (Table).
Discussion
Among patients with MOGAD, 23% of adults and 31% of children fulfilled the seronegative NMOSD criteria. The proportion of cases meeting the seronegative NMOSD criteria increased slightly when subgroups restricted to more than 2-year follow-up or a relapsing course were analyzed.
The most common attacks in our MOGAD cohort were ON and myelitis. However, most had isolated ON (monophasic or recurrent) or isolated myelitis. Further, more than one-third of patients with MOGAD myelitis (38%) did not have LETM. Therefore, only 29 patients with MOGAD fulfilled the NMOSD criteria using the clinical and radiological criteria for ON and LETM. Optic neuritis not extending more than one-half of the optic nerve nor involving the chiasm and associated with large cerebral ADEM lesions does not meet the optic neuritis criteria due to the requirement of a normal brain magnetic resonance imaging (MRI) (Figure).1
Although nausea and vomiting can occur in MOGAD, the defined core clinical characteristic of area postrema syndrome (more than 48 hours of intractable nausea, vomiting, or hiccups) without a secondary cause and a discrete, isolated MRI lesion in the area postrema/dorsal medulla is rarely encountered.2 Brainstem lesions are common but do not necessarily correspond to a brainstem clinical attack and occur as part of multifocal demyelination in ADEM. Brainstem lesions are often diffuse and are not necessarily discrete periependymal lesions, as defined in the seronegative criteria (Figure).1
The limitations of this study include referral bias to Mayo Clinic for patients with severe and relapsing MOGAD, which may overestimate the number of patients who fulfill the seronegative NMOSD criteria. This study demonstrates that 2015 diagnostic criteria for seronegative NMOSD fail to capture most patients who have MOGAD and supports specific molecular biomarker-associated diagnostic criteria for inflammatory central nervous system disorders.
References
- 1.Wingerchuk DM, Banwell B, Bennett JL, et al. ; International Panel for NMO Diagnosis . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. doi: 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunchok A, Krecke KN, Flanagan EP, et al. . Does area postrema syndrome occur in myelin oligodendrocyte glycoprotein-IgG-associated disorders (MOGAD)? Neurology. 2020;94(2):85-88. doi: 10.1212/WNL.0000000000008786 [DOI] [PubMed] [Google Scholar]
- 3.Dubey D, Pittock SJ, Krecke KN, et al. . Clinical, radiologic, and prognostic features of myelitis associated with myelin oligodendrocyte glycoprotein autoantibody. JAMA Neurol. 2019;76(3):301-309. doi: 10.1001/jamaneurol.2018.4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. . Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. 2018;195:8-15. doi: 10.1016/j.ajo.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jitprapaikulsan J, Chen JJ, Flanagan EP, et al. . Aquaporin-4 and myelin oligodendrocyte glycoprotein autoantibody status predict outcome of recurrent optic neuritis. Ophthalmology. 2018;125(10):1628-1637. doi: 10.1016/j.ophtha.2018.03.041 [DOI] [PubMed] [Google Scholar]
- 6.Höftberger R, Guo Y, Flanagan EP, et al. . The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020;139(5):875-892. doi: 10.1007/s00401-020-02132-y [DOI] [PMC free article] [PubMed] [Google Scholar]