Key Points
Question
Does endovascular treatment benefit patients with ischemic stroke due to emergent large vessel occlusion after 16 hours?
Findings
In this case-control study of 150 patients with emergent large vessel occlusion who arrived between 16 hours and 10 days after onset, endovascular treatment was associated with favorable outcomes in dichotomatized modified Rankin Scale scores and shift of strata in modified Rankin Scale scores at 3 months, with numerically more hemorrhagic complications. Target mismatch profiles were found in approximately one-third of the study participants.
Meaning
Endovascular treatment may benefit patients with emergent large vessel occlusion and target mismatch profiles regardless of the time from onset.
Abstract
Importance
Endovascular treatment (EVT) after ischemic stroke due to emergent large vessel occlusion is usually constrained by a specific window of less than 16 to 24 hours from the time the patient was last known well (LKW). Patients with slow progression and tenacious collateral circulation may persist beyond 16 hours.
Objectives
To estimate the prevalence of salvageable tissues 16 hours or more from LKW after ischemic stroke due to emergent large vessel occlusion and investigate the effectiveness of EVT in delayed large vessel occlusion.
Design, Setting and Participants
In this case-control study, from a total of 8032 patients with stroke or transient ischemic attack who were admitted between January 1, 2012, and December 31, 2018, to a single referral university hospital, 150 patients were retrospectively identified who had an acute ischemic stroke with internal carotid artery or middle cerebral artery occlusion, had a baseline National Institutes of Health Stroke Scale score of 6 or more, and arrived 16 hours or more from time LKW. The decision for EVT was made by a treating physician according to the institutional protocol.
Main Outcomes and Measures
Baseline ischemic core, collateral circulation status, and computed tomographic or magnetic resonance perfusion parameters were retrospectively quantified. Follow-up images, evaluated a median of 93 hours (interquartile range, 66-120 hours) after arrival, were used to assess the final infarct and hemorrhagic transformation. The main outcome was the modified Rankin Scale score at 90 days.
Results
For 150 patients (81 men [54%]; mean [SD] age at onset, 70.1 [13.0] years; median National Institutes of Health Stroke Scale score, 12 [interquartile range, 8-18]), the median ischemic core volume was 11.5 mL (interquartile range, 0-39.1 mL), the median penumbra volume (>6 seconds) was 55.0 mL (interquartile range, 15-128 mL), and the median mismatch ratio was 4.0 (interquartile range, 0.9-18.3). By the imaging inclusion criteria for EVT trials, there were 50 DAWN (DWI or CTP Assessment With Clinical Mismatch in the Triage of Wake-up and Late Presenting Strokes Undergoing Neurointervention With Trevo)–eligible patients (33%), 58 DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke)–eligible patients (39%), and 57 ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion With Emphasis on Minimizing CT to Recanalization Times)–eligible patients (38%). Endovascular treatment was performed for 24 patients (16%). In propensity score–matched analyses, EVT was associated with better odds of a 90-day modified Rankin Scale score of 0 to 2 (adjusted odds ratio, 11.08 [95% CI, 1.88-108.60]) and a 90-day modified Rankin Scale score shift (common adjusted odds ratio, 5.17 [95% CI, 1.80-15.62]). Type 2 parenchymal hemorrhage was seen in 3 of 24 patients (13%) who received EVT and in 4 of 126 patients (3%) who received medical management (adjusted odds ratio, 4.06 [95% CI, 0.63-26.30]). In a subgroup of 109 patients who were 24 hours from time LKW, EVT was associated with a favorable mRS shift (common adjusted odds ratio, 10.54 [95% CI, 2.18-59.34]).
Conclusions and Relevance
This study suggests that patients with anterior circulation large vessel occlusion presenting very late (>16 hours to 10 days) from the time they were LKW may benefit from EVT.
This case-control study examines the prevalence of salvageable tissues 16 hours or more from the time the patient was last known well after ischemic stroke due to emergent large vessel occlusion and investigates the effectiveness of endovascular treatment in delayed large vessel occlusion.
Introduction
Recanalization treatment for acute ischemic stroke, including both intravenous and endovascular approaches, is currently offered within specific time windows, even according to the most recent clinical guidelines.1 However, as the presence of potentially salvageable tissue is determined by the severity and duration of ischemia and the extent of collateral circulation,2 the speed of the “tissue clock” may differ in individuals.3,4,5 After the groundbreaking results from the DAWN (DWI or CTP Assessment With Clinical Mismatch in the Triage of Wake-up and Late Presenting Strokes Undergoing Neurointervention With Trevo)6 and DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trials,7 a “late-window paradox” was proposed: reperfusion treatments may be efficacious for patients with emergent large vessel occlusion (LVO) and slow progression with sustained ischemic penumbra, despite delayed initiation of reperfusion treatment.8 Variability in collateral circulation after emergent LVO is likely a major determinant of the speed of infarct evolution.9,10 Another often-ignored issue is the fact that the last known well (LKW) time is occasionally not when the stroke actually occurs. Patients living alone or waking up with stroke symptoms may have had their stroke within a short time window, although their time LKW may have been many hours before the stroke.11 These are reasons why recanalization treatments ought to be offered to patients after assessing the ischemic pathophysiology and not after assessing time from LKW.
Recent literature has suggested that a target mismatch profile persists up to several days after stroke onset in patients with LVO.12 The time window for recanalization, therefore, could be extended even beyond 16 to 24 hours from the time LKW, provided that imaging results show evidence of good collateral circulation and a salvageable brain. A randomized clinical trial to investigate the effectiveness of recanalization treatment after 16 to 24 hours from the time LKW does not seem feasible owing to the scarcity of patients in this very late time period.13 In this context, we analyzed a prospective stroke registry from a single hospital that contained records of patients with acute stroke who arrived beyond 16 hours from the time LKW and underwent endovascular treatment (EVT) beyond the current time-window thresholds. We used these data to estimate the frequency of target mismatch profiles in patients with LVO and the effectiveness of EVT in the very late period beyond 16 hours from the time LKW.
Methods
Study Design
This was an observational, single-center, retrospective case-control study of prospectively acquired data performed to evaluate the status of the target mismatch profile and the outcome of EVT in consecutive patients with emergent LVO after 16 hours from their time LKW. The present study and relevant analyses were approved by the Seoul National University Bundang Hospital institutional review board. The included patients or their next of kin provided written informed consent for the registration of their data in the prospective stroke registry.
Study Population and Stroke Care
Between January 1, 2012, and December 31, 2018, a total of 8032 admissions were recorded in the Cerebrovascular Center of the Seoul National University Bundang Hospital, which participates in a prospective multicenter stroke registry.14 Among these admissions, patients with (1) ischemic stroke documented through relevant neuroimaging (n = 6408), (2) anterior circulation LVO (n = 1122), (3) arrival more than 16 hours after the time LKW (n = 482), and (4) baseline National Institutes of Health Stroke Scale (NIHSS) score of 6 points or more (range, 0-42, where 0 indicates no discernable neurologic deficits and 42 indicates death or equivalent state) (n = 199) were included. Exclusion criteria were (1) readmission (n = 2), (2) misclassification or clinically irrelevant chronic occlusions (n = 15), (3) mainly posterior circulation infarction (n = 6), (4) presenting 10 or more days after the time LKW (n = 16), (5) recanalization treatment before arrival (n = 3), (6) poor-quality images (n = 3), and (7) missing the 3-month modified Rankin Scale (mRS) score (n = 4). A total of 150 participants were included for analysis (eFigure 1 in the Supplement).
Acute stroke management was performed according to the institutional protocol based on local and international guidelines at the time of practice and at the discretion of the individual physicians.15,16 The protocol recommended taking collateral circulation or perfusion images for patients with confirmed LVO, even after the conventional time window. Decisions about EVT were made by individual interventionists and vascular neurologists, usually based on the core-penumbra mismatch from visual inspection of machine-generated images and clinical considerations. Quantified parameters for ischemic core and perfusion delay from an automated software were not available at the time of stroke care.
Data Collection and Image Analyses
Baseline clinical information and follow-up functional recovery data for all study participants were retrieved from the prospective stroke registry. Data on the mRS score were prospectively collected during a regular clinic visit or through a structured telephone interview conducted by a trained research nurse.
In the present study, computed tomography (CT) or magnetic resonance (MR) images and cerebral angiographies that were performed for routine stroke care were collected and analyzed. As per the institutional protocol, MR imaging including diffusion-weighted images (DWIs), perfusion scans, and MR angiography is the recommended imaging choice for patients with suspected stroke who arrive 6 or more hours from the time LKW. However, a noncontrast brain CT scan and perfusion CT scan are additionally requested when LVO is detected on an initial MR scan without perfusion images or when the institution receives prenotification that a patient with LVO is referred from another hospital.
Only images acquired at our center were analyzed. Perfusion parameters were retrospectively estimated using the RAPID system (iSchemaView). Infarct core volume was defined as a regional cerebral blood flow less than 30% on perfusion CT scan or an apparent diffusion coefficient less than 620 μm2/s on DWI. If both DWI and CT scans were taken before EVT, DWI images were used to measure the ischemic core. ASPECTS (the Alberta Stroke Program Early CT score; range, 0-10, where 0 indicates overt infarction over the cerebral hemisphere and 10 indicates no discernable ischemia) was evaluated using CT images whenever available (62 [41%]), but DWI was used when CT was not available (n = 88). Moderate to good collateral circulation was defined as filling 50% or more of the middle cerebral artery pial arterial circulation on the source images of CT scan or MR angiography.17 ASPECTS and collateral circulation status were quantified by an interventional neurologist (J.Y.K.) and by a stroke neurologist (D.-W.S.), with disagreements resolved by another stroke neurologist (B.J.K.); all of these raters were blinded to clinical information.
Follow-up images are routinely acquired at our center 3 to 5 days after arrival or at the time of neurologic deterioration to evaluate hemorrhagic complications and final infarct volume. Magnetic resonance is the recommended image modality, but CT scans are accepted based on clinical circumstances. Hemorrhagic transformation was evaluated on follow-up imaging using the European Cooperative Acute Stroke Study II criteria.18 The final infarct volume was measured in a semiautomated manner using Analyze 12.0 (AnalyzeDirect Inc). The volume of reperfused penumbra was calculated as the difference between the follow-up infarct core volume and the baseline penumbra lesion volume, as previously suggested.19
Statistical Analysis
Baseline characteristics were summarized and compared using χ2 tests for categorical variables and independent t tests for interval variables. To adjust for baseline imbalances between treatment groups, we derived a propensity score (PS) for receiving EVT for each individual from clinically relevant variables. The PS is a good method of reducing potential bias when a relatively large set of confounders exists.20 Patients who received EVT and controls who received best medical management were matched 1:2 with the PS. Binary and ordinal logistic regression models were used to test the independent associations between EVT and functional status at 3 months after stroke using the matched data set (eMethods 1 in the Supplement). Covariates with standardized mean differences of 0.10 or more after matching were further incorporated into logistic regression models.21 A separate PS was generated and matched in the same way from patients who arrived more than 24 hours after the time LKW.
Binary logistic regression models without PS matching were also applied for post hoc analyses. Clinically relevant covariates were prespecified, including age, baseline NIHSS score, location of occlusion, delay from time LKW to arrival, and the volume of the ischemic core.
Significance levels were set at P < .05 for 2-tailed tests. All statistical analyses were performed using R, version 3.5.3 (R Foundation for Statistical Computing).
Results
Baseline Characteristics
Among the 6408 patients with ischemic stroke who underwent neuroimaging studies and were admitted to the comprehensive stroke center over a 7-year period, 150 patients met the prespecified selection criteria of anterior circulation LVO, a baseline NIHSS score of 6 points or more, admission at least 16 hours after their time LKW, and available 3-month mRS scores (eFigure 1 in the Supplement). The mean (SD) age at onset was 70.1 (13.0) years, with 81 male patients (54%), a median NIHSS score of 12 (interquartile range [IQR], 8-18), and a median duration between the time LKW to onset time of 43.5 hours (IQR, 22.8-76.9 hours). Seventy-three patients (49%) had unwitnessed onset of stroke. Mortality within 3 months was observed in 18 patients (12%) (eTable 1 in the Supplement). The median ASPECT score was 9 (IQR, 6-10) and did not differ according to whether it was determined via CT or MRI. Interrater reliability was acceptable for the ASPECT score (κ = 0.68) and dichotomized pial collateral circulation status (κ = 0.78). Middle cerebral artery pial collateral circulation of 50% or more compared with the contralateral hemisphere was documented in 79 of 127 available patients (62.2%).
Target Mismatch Profiles and Trial Eligibility in the Very Late Time Window
Perfusion images from CT or MR scans were taken in 111 cases (74%) and collateral circulation status was evaluated in 127 cases (85%) (eTable 1 in the Supplement). Median ischemic core volume of 11.5 mL (IQR, 0-39.1 mL), median penumbra volume (>6 seconds) of 55.0 mL (IQR, 15-128 mL), and a median mismatch ratio of 4.0 (IQR, 0.9-18.3) were noted. Acute imaging parameters representing irreversible ischemia, salvageable tissues, or final infarction were comparable across the 16 to 240 hours of inclusion duration (Table 1; eTable 2 in the Supplement). When the image eligibility criteria for selected trials were applied (eMethods 2 in the Supplement), 50 patients (33%) met the requirements for the DAWN trial, 58 (39%) met the requirements for the DEFUSE 3 trial, and 57 (38%) met the requirements for the ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion With Emphasis on Minimizing CT to Recanalization Times) trial.17 The proportions of patients with LVO who were eligible for trials were not different according to the delay since the time LKW, and the proportions of patients receiving EVT were consistently lower than the proportions of patients eligible for imaging (eFigure 2 in the Supplement).
Table 1. Imaging Parameters According to the Delay From Time LKW.
| Parameter | Time from time LKW, h | P value for trend | ||||
|---|---|---|---|---|---|---|
| 16-24 (n = 41) | 24-48 (n = 39) | 48-72 (n = 27) | 72-120 (n = 23) | 120-240 (n = 20) | ||
| Ischemic core, median (IQR), mLa | 10.0 (0 to 33.0) | 16.7 (4.0 to 88.0) | 10.8 (0 to 44.5) | 18.9 (7.5 to 44.5) | 10.5 (2.5 to 19.3) | .50 |
| ASPECTS, median (IQR)a | 9 (8 to 10) | 8 (5 to 9) | 9 (6 to 10) | 9 (6 to 10) | 9 (7 to 10) | .36 |
| MCA pial collateral ≥50%, No./total No. (%)b | 21/30 (70) | 19/33 (58) | 15/22 (68) | 15/23 (65) | 9/19 (47) | .29 |
| Penumbra volume, median (IQR), mLc | ||||||
| >10 s | 9.0 (0 to 29.0) | 12.0 (4.0 to 82.0) | 4.0 (0 to 31.5) | 10.0 (4.0 to 37.0) | 2.8 (0 to 9.0) | .22 |
| >6 s | 46.0 (16.0 to 81.0) | 44.0 (7.0 to 169.0) | 78.0 (5.5 to 184.5) | 78.5 (40.0 to 120.0) | 44.5 (19.0 to 101.0) | .95 |
| Mismatch ratio, median (IQR)c,d | 2.7 (1.2 to 15.0) | 4.0 (1.1 to 7.0) | 6.0 (0.7 to 24.8) | 4.6 (0.6 to 17.0) | 5.2 (1.6 to 32.0) | .81 |
| Volume of penumbra, median (IQR), mLc | 35.0 (1.0 to 58.0) | 24.0 (3.0 to 69.0) | 23.0 (0 to 157) | 24.0 (0 to 87) | 28.0 (7.0 to 101) | .94 |
| Infarct growth, median (IQR), mLe,f | 10.5 (0.8 to 51.4) | 7.5 (−1.0 to 22.7) | 9.3 (−0.4 to 27.0) | 2.2 (−15.3 to 14.2) | 3.7 (−2.0 to 11.4) | .09 |
| Final infarct volume, median (IQR), mLe | 23.6 (7.5 to 87.0) | 29.8 (9.0 to 82.9) | 17.4 (4.4 to 73.1) | 21.9 (7.5 to 63.5) | 15.2 (5.1 to 32.4) | .34 |
Abbreviations: ASPECTS, Alberta Stroke Program Early CT score; IQR, interquartile range; LKW, last known well; MCA, middle cerebral artery.
For 150 patients.
For 127 patients.
For 109 patients.
Ratio of volume of penumbra volume greater than 6 seconds over volume of core.
For 138 patients.
Difference between final infarct volume and the baseline ischemic core volume.
Benefit From EVT in the Very Late Time Window
Of the included 150 patients, 24 (16%) received EVT. The clinical profiles and medical treatments of patients who received EVT were statistically comparable, but those who received EVT were numerically more frequent in the subgroup of patients with unwitnessed stroke onset (15 [63%] vs 126 [46%]), had less prestroke dependency (4 [17%] vs 37 [29%]), and had a shorter median delay from their time LKW to presentation (median, 26.3 hours [IQR, 19.1-48.2 hours] vs 48.0 hours [IQR, 25.2-86.3 hours]; Table 2). An mRS score of 0 to 2 at 3 months after stroke occurred in 55 patients (37%): 13 of 24 patients who received EVT (54%) and 42 of 126 patients who received medical treatment (33%).
Table 2. Clinical Profile of Patients With Emergent Large Vessel Occlusion With NIHSS Score of 6 or More Who Arrived Very Late (≥16 Hours) From the Time of LKW.
| Variable | Unmatched data set | Propensity score–matched data set | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | P value | SMD | No. (%) | P value | SMD | |||
| Medical management (n = 126) | EVT (n = 24) | Medical management (n = 48) | EVT (n = 24) | |||||
| Demographic variables | ||||||||
| Age, mean (SD), y | 70.2 (13.6) | 69.5 (9.7) | .83 | 0.054 | 71.1 (13.6) | 69.5 (9.7) | .61 | 0.136 |
| Male sex | 70 (56) | 11 (46) | .52 | 0.193 | 24 (50) | 11 (46) | .93 | 0.082 |
| Prestroke dependencya | 37 (29) | 4 (17) | .30 | 0.302 | 10 (21) | 4 (17) | .92 | 0.105 |
| Stroke information | ||||||||
| Delay from time LKW, median (IQR), h | 48.0 (25.2-86.3) | 26.3 (19.1-48.2) | .02 | 0.332 | 35.6 (20.9-64.3) | 26.3 (19.1-48.2) | .26 | 0.047 |
| Unwitnessed onset of stroke | 58 (46) | 15 (63) | .21 | 0.331 | 27 (56) | 15 (63) | .80 | 0.126 |
| Baseline NIHSS score, median (IQR) | 12 (7-17) | 14 (9.5-18.5) | .23 | 0.189 | 10 (7-16) | 14 (9.5-18.5) | .08 | 0.356 |
| Volume of ischemic core, median (IQR), mL | 13.2 (3.0-54.0) | 10.6 (0-24.4) | .20 | 0.517 | 5.2 (0-16.7) | 10.6 (0-24.4) | .46 | 0.223 |
| Stroke mechanism | ||||||||
| Large artery atherosclerosis | 85 (68) | 13 (54) | .39 | 0.203 | 32 (67) | 13 (54) | .76 | 0.222 |
| Cardioembolism | 27 (21) | 8 (33) | 12 (25) | 8 (33) | ||||
| Other determined etiology | 4 (3) | 0 | 0 | 0 | ||||
| Undetermined etiology | 10 (8) | 3 (13) | 12 (25) | 3 (13) | ||||
| Location of occlusion | ||||||||
| Proximal ICA or tandem occlusion | 34 (27) | 8 (33) | .35 | 0.057 | 13 (27) | 8 (33) | .76 | 0.090 |
| Distal ICA | 24 (19) | 1 (4) | 5 (10) | 1 (4) | ||||
| M1 | 47 (37) | 10 (42) | 18 (38) | 10 (42) | ||||
| M2 or distal tributaries | 21 (17) | 5 (21) | 12 (25) | 5 (21) | ||||
| Vascular risk factors | ||||||||
| Hypertension | 101 (80) | 16 (67) | .23 | 0.305 | 42 (88) | 16 (67) | .07 | 0.503 |
| Diabetes | 50 (40) | 6 (25) | .26 | 0.314 | 15 (31) | 6 (25) | .78 | 0.137 |
| Dyslipidemia | 36 (29) | 8 (33) | .82 | 0.102 | 14 (29) | 8 (33) | .93 | 0.089 |
| Habitual smoking | 53 (42) | 6 (25) | .18 | 0.363 | 14 (29) | 6 (25) | .93 | 0.092 |
| Atrial fibrillation | 30 (24) | 6 (25) | >.99 | 0.027 | 11 (23) | 6 (25) | >.99 | 0.048 |
| Laboratory test results, mean (SD) | ||||||||
| Hemoglobin, g/dL | 13.4 (2.2) | 12.9 (2.1) | .31 | 0.229 | 13.0 (2.5) | 12.9 (2.1) | .93 | 0.023 |
| Blood urea nitrogen, mg/dL | 19.6 (10.0) | 17.8 (14.1) | .57 | 0.141 | 19.5 (11.0) | 17.8 (14.1) | .59 | 0.128 |
| Creatinine, mg/dL | 0.9 (0.7) | 0.9 (0.4) | .72 | 0.066 | 1.0 (1.0) | 0.9 (0.4) | .60 | 0.116 |
| Glucose at arrival, mg/dL | 151 (102) | 133 (42) | .15 | 0.231 | 132 (39) | 133 (42) | .95 | 0.015 |
| Systolic blood pressure, mm Hg | 156 (28) | 143 (29) | .04 | 0.447 | 150 (28) | 143 (29) | .36 | 0.230 |
Abbreviations: EVT, endovascular treatment; ICA, internal carotid artery; IQR, interquartile range; LKW, last known well; M1, M1 portion of middle cerebral artery; M2, M2 portion of middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; SMD, standardized mean difference.
SI conversion factors: To convert hemoglobin to grams per liter, multiply by 10.0; blood urea nitrogen to millimoles per liter, multiply by 0.357; creatinine to micromoles per liter, multiply by 88.4; and glucose to millimoles per liter, multiply by 0.0555.
Prestroke dependency, modified Rankin Scale score of 1 or more before the index stroke.
To address the baseline imbalances between patients who received medical management and those who received EVT, each patients who received EVT was matched with 2 medical management controls by the PS. Endovascular treatment was associated with better functional recovery after ischemic stroke: the odds of having an mRS score of 0 to 2 at 3 months after stroke were increased (adjusted odds ratio [OR], 11.08 [95% CI, 1.88-108.60]), as were the odds of having a favorable mRS score shift (common adjusted OR, 5.17 [95% CI, 1.80-15.62]) from the matched data set that was further adjusted for covariates whose standardized mean difference was 0.10 or more (Figure 1A; eTables 3 and 4 in the Supplement). The benefits associated with EVT were maintained during the inclusion period (Figure 1B).
Figure 1. Effectiveness of Endovascular Treatment (EVT) in 3-Month Functional Recovery for Patients With Large Vessel Occlusion Who Presented in a Very Late Time Window .
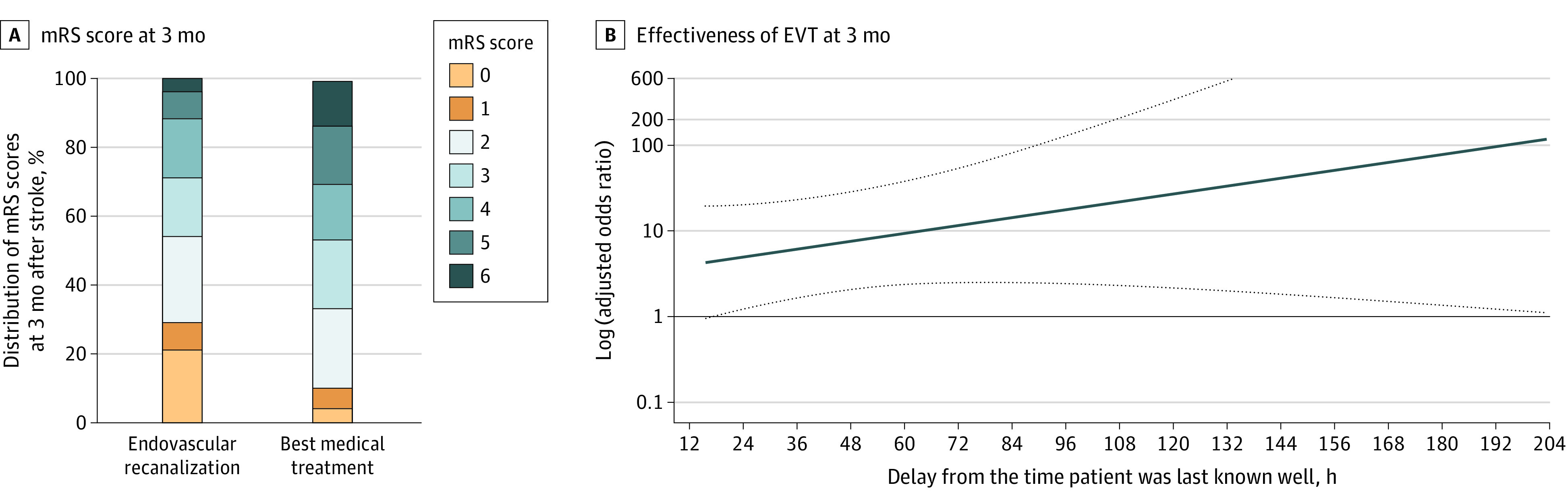
A, Distribution of modified Rankin Scale (mRS) scores at 3 months after stroke. B, Effectiveness of EVT at 3 months after stroke by delay from the time patient was last known well. Dotted lines indicate 95% CI.
Multivariable logistic regression models without PS matching also showed significant associations for having an mRS score of 0 to 2 (adjusted OR, 7.89 [95% CI, 2.25-32.42]) or a favorable shift in mRS score (common adjusted OR, 5.91 [95% CI, 2.42-14.77]), after adjustment for relevant covariates (eTable 5 in the Supplement).
Type 2 parenchymal hemorrhage was seen in 7 participants (5%): 3 of 24 patients (13%) in the EVT subgroup and 4 of 126 patients (3%) in the medical treatment subgroup (adjusted OR, 4.06 [95% CI, 0.63-26.30]). Symptomatic hemorrhage with an increase in the NIHSS score of 4 points or more occurred in 2 patients, both of whom had received EVT.
Additional Analyses
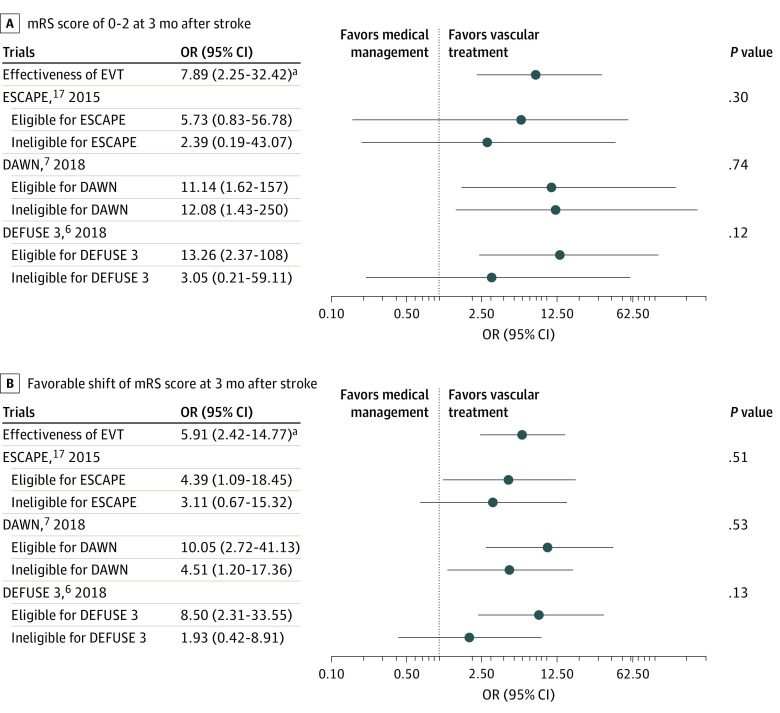
We performed several post hoc analyses to demonstrate the robustness of our findings. The image eligibility of the selected endovascular trials did not modify the effectiveness of EVT for patients with LVO who presented in the very late time window with an mRS score of 0 to 2 (Figure 2A) and a favorable shift in mRS scores (Figure 2B) at 3 months after stroke (eTable 6 in the Supplement). The image criteria of the DEFUSE 3 trial modestly modified the benefit from EVT without statistical significance.
Figure 2. Benefit of Endovascular Recanalization According to Imaging Criteria of the Selected Endovascular Treatment (EVT) Trials.
A, Modified Rankin Scale (mRS) score of 0-2 at 3 months after stroke. B, Favorable shift in mRS scores at 3 months after stroke. P values represent the statistical significance of the effect modifications according to trial eligibility. The eligibility according to the imaging criteria of selected trials was evaluated in 127 cases for the ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion With Emphasis on Minimizing CT to Recanalization Times) trial, 150 cases for the DAWN (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-up and Late Presenting Strokes Undergoing Neurointervention With Trevo) trial, and 109 cases for the DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trial.
aThe odds ratios (ORs) and 95% CIs of EVT were from the main model without the propensity score matching.
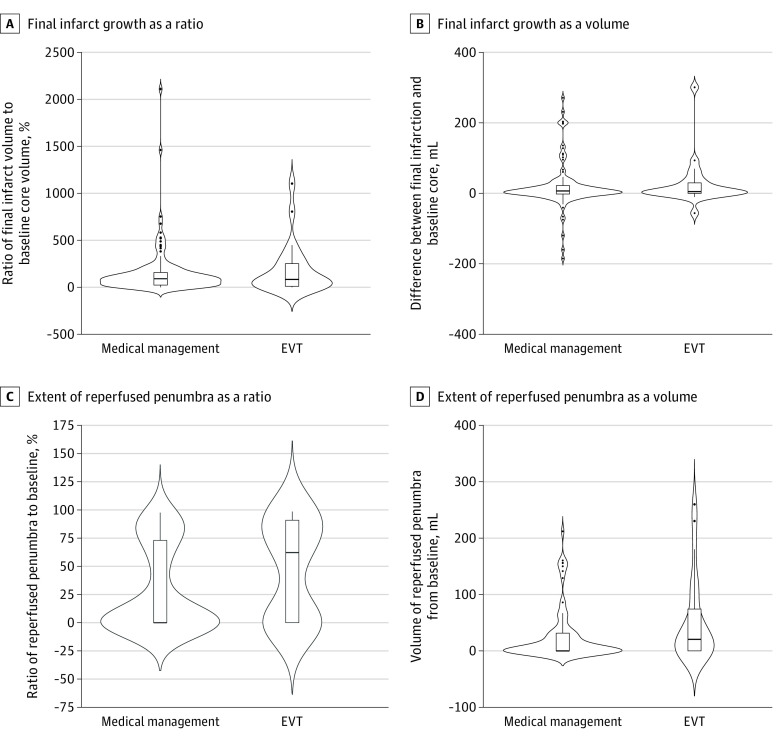
Final infarcts were measured in 138 patients (92%) using MR imaging (125 patients) or CT scan (13 patients), whichever was available, at a median of 93 hours (IQR, 66-120 hours) after arrival. The median volume of the final infarct was 21.0 mL (IQR, 6.8-74.1 mL). Forty patients had a smaller final infarct volume than the baseline ischemic core (6 of 24 [25%] in the EVT subgroup and 34 of 114 patients [30%] in the best medical management subgroup who had follow-up imaging performed; P = .82). The final infarct volume was not different from the baseline ischemic core according to the treatment subgroup (see Figure 3A for the ratio and Figure 3B for the absolute volume), but patients who received EVT showed a tendency toward a higher proportion of reperfused penumbra (see Figure 3C for the ratio and Figure 3D for the absolute volume). Unwitnessed onset of stroke did not modify the association of EVT with outcomes (eTable 10 in the Supplement).
Figure 3. Comparison of Tissue Fate After Medical Management or Endovascular Treatment (EVT) in the Very Late Time Period.
The final infarct growth from the baseline ischemic core as a ratio (A) and the final infarct growth from the baseline ischemic core as a volume (B) were similar. However, the extent of reperfused penumbra from the baseline penumbra volume greater than 6 seconds as a ratio (C) and the extent of reperfused penumbra from the baseline penumbra volume greater than 6 seconds as a volume (D) showed a trend toward a benefit of EVT. The thick horizontal line in the middle of the box denotes the median, boxes indicate the interquartile range, dots indicate individual extreme values out of the interquartile range, and curves indicate the probability density of the data at each y-axis, smoothed by a kernel density estimator.
In a subgroup analysis of 109 patients who presented more than 24 hours from their time LKW, EVT was associated with increased odds of having favorable functional recovery at 3 months (common adjusted OR, 10.54 [95% CI, 2.18-59.34]), but was nonsignificantly associated with an mRS score of 0 to 2 (adjusted OR, 7.53 [95% CI, 0.70-188.19]) in a separate PS-matched data set (eTables 7-9 in the Supplement).
Discussion
We selected 150 patients with anterior circulation LVO with moderate to severe neurologic deficits who arrived at least 16 hours after the time LKW among 8032 consecutive patients admitted for stroke or transient ischemic attack during a 7-year period. Approximately one-third of emergent LVO cases had met the image eligibility of EVT trials. Endovascular treatment was performed in 16% of patients and was associated with 11-fold higher odds of having independent functional status at 3 months after stroke. The benefit from EVT was maintained during the inclusion period of 16 to 240 hours after onset. Recanalization treatment was modestly associated with saving brain tissues at risk of infarction, but was not able to reverse the ischemic core. However, EVT had a tendency toward an increased risk of intracranial hemorrhages.
From a consecutive series of patients with anterior circulation LVO who were assessed more than 16 hours after onset, salvageable tissues were identified in approximately one-third of patients. The prevalence of salvageable tissues was reported to be approximately 55% in earlier patients with emergent LVO evaluated at a tertiary referral hospital.22 The extent of leptomeningeal collateral circulation and the amount of supplanting flow may vary by individual, depending on age, anatomical variations, serum glucose level, and metabolic syndrome.23,24,25 Our study expands the prior knowledge of tenacious tissues resisting ischemic injury beyond 16 hours from the time LKW and concretizes the existence of “slow progressors,” even in this very late period. In addition, slow progressors in our data set consistently benefited from timely EVT during the 16- to 240-hour inclusion period, as demonstrated from a previous report.26
Irreversible infarction will occur when the unsteady balance between collateral perfusion and individual tissue vulnerability leads to ischemia as the supplanting blood flow decreases. With timely initiation of reperfusion treatment, the ischemic penumbra may be saved, regardless of the time passed since onset.27 The benefit of EVT in our study, mRS score 0 to 2 (seen in 54% of patients who received EVT), was also comparable to that identified in a multicenter observation study after 12 hours of stroke onset from Ireland.28 Bleeding complications increased only numerically in our study, similar to the findings from a French EVT registry.29
Endovascular treatment in our study showed a modest benefit in saving tissues with perfusion delay but failed to reverse baseline ischemic cores. This finding delineates the role of EVT in the very late time period to save the penumbral tissues,30 but is not involved in the so-called DWI reversal phenomenon.31,32
Although the absolute number of additional candidates for EVT in the very late period is small, approximately one-third of the patients with LVO presenting 16 hours or more from the time LKW may benefit from the recanalization. A consensus has not been reached on the best imaging criteria to identify treatable tissues in clinical practice.33 The image criteria for the DEFUSE 3 trial may have the potential to determine the treatment response based our subgroup analyses, which requires further study.34
Limitations
Our study has some limitations. The study is a retrospective analysis of a prospectively collected registry. Our results based on small and highly selected cases; the PS matching may not achieve sufficient balance between treatment groups and the subgroup analyses might be underpowered. The results from post hoc and image analyses need to be verified with future studies. Decisions for treatment strategy in this study were made individually by the treating physicians at their discretion, without quantified parameters from the RAPID system at the time of patient care. Endovascular treatment 16 hours or more after the time LKW was performed out of the clinical guidelines at the time of practice. The median penumbra volume of greater than 6-second lesions in our study was relatively smaller than that in previous trials.7,35 Treatment decisions, endovascular devices, and intervention techniques may have changed over the course of 7 years. Not all the included patients had perfusion imaging at baseline. The analysis of the tissue fate was based on the perfusion map from the RAPID system at baseline, and appropriate post-processing was not possible. Imaging modalities were heterogeneous owing to the observational nature of this study.
Conclusions
Patients with acute ischemic stroke and anterior circulation LVO may harbor substantial salvageable tissues in the very late period from 16 to 240 hours after the time LKW. Endovascular treatment in this population increased the chance of being independent after stroke, and the benefit remained constant over the inclusion period, but with a nonsignificant increase in hemorrhages. However, this was a small observational study. Future randomized clinical trials are warranted to address the effectiveness of EVT and to determine the best imaging features for determining treatment responses.
eMethods 1. Estimation of Propensity Scores for EVT and Case-Control Matching
eMethods 2. Image Eligibility Criteria for Selected Clinical Trials
eFigure 1. Study Profile
eFigure 2. Proportions of Image-Eligibilities for Selected Trials and EVT by Time Delay From Last Known Well
eTable 1. Baseline Characteristics of Included Patients
eTable 2. The Time Delay From LKW and Perfusion Images
eTable 3. Full Model Results From a Multivariable Binary Logistic Regression Model With Covariates Whose SMD>0.10
eTable 4. Full Model Results From a Multivariable Ordinal Logistic Regression Model With Covariates Whose SMD>0.10
eTable 5. Full Model Results From Multivariable Logistic Regression Models Without Propensity Score Matching
eTable 6. Functional Recovery at Three Months After Stroke Stratified by Acute Treatment and Imaging-Eligibility of Selected Clinical Trials
eTable 7. Baseline Characteristics of Patients Who Arrived >24 Hours From Last Known Well
eTable 8. Clinical Profile of Patients Who Arrived >24 Hours by Endovascular Recanalization Treatment
eTable 9. EVT and Functional Recovery at 3 Months After Stroke in Patients >24 Hours
eTable 10. Unwitnessed Onset and the Association of EVT With Outcomes
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2.Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol. 1983;14(3):294-301. doi: 10.1002/ana.410140307 [DOI] [PubMed] [Google Scholar]
- 3.Broocks G, Rajput F, Hanning U, et al. Highest lesion growth rates in patients with hyperacute stroke. Stroke. 2018;50(1):189-192. doi: 10.1161/STROKEAHA.118.023457 [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Christensen S, Tress BM, et al. ; EPITHET Investigators . Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 2013;33(8):1168-1172. doi: 10.1038/jcbfm.2013.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abou-Chebl A. Endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke. 2010;41(9):1996-2000. doi: 10.1161/STROKEAHA.110.578997 [DOI] [PubMed] [Google Scholar]
- 6.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albers GW. Late window paradox. Stroke. 2018;49(3):768-771. doi: 10.1161/STROKEAHA.117.020200 [DOI] [PubMed] [Google Scholar]
- 9.Kim BJ, Chung JW, Park HK, et al. CT angiography of collateral vessels and outcomes in endovascular-treated acute ischemic stroke patients. J Clin Neurol. 2017;13(2):121-128. doi: 10.3988/jcn.2017.13.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BJ, Kim H, Jeong HG, et al. Tenacity of collateral perfusion in proximal cerebral arterial occlusions 6-12 h after onset. Cerebrovasc Dis. 2018;45(5-6):263-269. doi: 10.1159/000489894 [DOI] [PubMed] [Google Scholar]
- 11.Thomalla G, Simonsen CZ, Boutitie F, et al. ; WAKE-UP Investigators . MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611-622. doi: 10.1056/NEJMoa1804355 [DOI] [PubMed] [Google Scholar]
- 12.Christensen S, Mlynash M, Kemp S, et al. Persistent target mismatch profile >24 hours after stroke onset in DEFUSE 3. Stroke. 2019;50(3):754-757. doi: 10.1161/STROKEAHA.118.023392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KJ, Kim BJ, Kim DE, et al. ; Clinical Research Collaboration for Stroke in Korea (CRCS-K) Investigators . Nationwide estimation of eligibility for endovascular thrombectomy based on the DAWN trial. J Stroke. 2018;20(2):277-279. doi: 10.5853/jos.2018.00836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim BJ, Park JM, Kang K, et al. Case characteristics, hyperacute treatment, and outcome information from the Clinical Research Center for Stroke–Fifth Division Registry in South Korea. J Stroke. 2015;17(1):38-53. doi: 10.5853/jos.2015.17.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong KS, Ko SB, Yu KH, et al. Update of the Korean clinical practice guidelines for endovascular recanalization therapy in patients with acute ischemic stroke. J Stroke. 2016;18(1):102-113. doi: 10.5853/jos.2015.01655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powers WJ, Derdeyn CP, Biller J, et al. ; American Heart Association Stroke Council . 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020-3035. doi: 10.1161/STR.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 17.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 18.Larrue V, von Kummer R R, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001;32(2):438-441. doi: 10.1161/01.STR.32.2.438 [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Parsons MW, Clapham M, et al. Influence of penumbral reperfusion on clinical outcome depends on baseline ischemic core volume. Stroke. 2017;48(10):2739-2745. doi: 10.1161/STROKEAHA.117.018587 [DOI] [PubMed] [Google Scholar]
- 20.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158(3):280-287. doi: 10.1093/aje/kwg115 [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TL, Collins GS, Spence J, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17(1):78. doi: 10.1186/s12874-017-0338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocha M, Desai SM, Jadhav AP, Jovin TG. Prevalence and temporal distribution of fast and slow progressors of infarct growth in large vessel occlusion stroke. Stroke. 2019;50(8):2238-2240. doi: 10.1161/STROKEAHA.118.024035 [DOI] [PubMed] [Google Scholar]
- 23.Ribo M, Molina CA, Delgado P, et al. Hyperglycemia during ischemia rapidly accelerates brain damage in stroke patients treated with tPA. J Cereb Blood Flow Metab. 2007;27(9):1616-1622. doi: 10.1038/sj.jcbfm.9600460 [DOI] [PubMed] [Google Scholar]
- 24.Menon BK, Smith EE, Coutts SB, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013;74(2):241-248. doi: 10.1002/ana.23906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao YJ, Oyarzabal EA, Zhang H, Faber JE, Shih YI. Role of genetic variation in collateral circulation in the evolution of acute stroke: a multimodal magnetic resonance imaging study. Stroke. 2017;48(3):754-761. doi: 10.1161/STROKEAHA.116.015878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai SM, Haussen DC, Aghaebrahim A, et al. Thrombectomy 24 hours after stroke: beyond DAWN. J Neurointerv Surg. 2018;10(11):1039-1042. doi: 10.1136/neurintsurg-2018-013923 [DOI] [PubMed] [Google Scholar]
- 27.Hill MD, Goyal M, Demchuk AM, Fisher M. Ischemic stroke tissue-window in the new era of endovascular treatment. Stroke. 2015;46(8):2332-2334. doi: 10.1161/STROKEAHA.115.009688 [DOI] [PubMed] [Google Scholar]
- 28.Motyer R, Thornton J, Power S, et al. Endovascular thrombectomy beyond 12 hours of stroke onset: a stroke network’s experience of late intervention. J Neurointerv Surg. 2018;10(11):1043-1046. doi: 10.1136/neurintsurg-2017-013575 [DOI] [PubMed] [Google Scholar]
- 29.Boisseau W, Fahed R, Lapergue B, et al. ; ETIS Investigators . Predictors of parenchymal hematoma after mechanical thrombectomy: a multicenter study. Stroke. 2019;50(9):2364-2370. doi: 10.1161/STROKEAHA.118.024512 [DOI] [PubMed] [Google Scholar]
- 30.Okell TW, Harston GWJ, Chappell MA, Sheerin F, Kennedy J, Jezzard P. Measurement of collateral perfusion in acute stroke: a vessel-encoded arterial spin labeling study. Sci Rep. 2019;9(1):8181. doi: 10.1038/s41598-019-44417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsia AW, Luby M, Cullison K, et al. Rapid apparent diffusion coefficient evolution after early revascularization. Stroke. 2019;50(8):2086-2092. doi: 10.1161/STROKEAHA.119.025784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo J, Choi JW, Lee SJ, et al. Ischemic diffusion lesion reversal after endovascular treatment. Stroke. 2019;50(6):1504-1509. doi: 10.1161/STROKEAHA.118.024263 [DOI] [PubMed] [Google Scholar]
- 33.Chung JW, Kim BJ, Jeong HG, et al. Selection of candidates for endovascular treatment: characteristics according to three different selection methods. J Stroke. 2019;21(3):332-339. doi: 10.5853/jos.2019.01578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leslie-Mazwi TM, Hamilton S, Mlynash M, et al. DEFUSE 3 non-DAWN patients. Stroke. 2019;50(3):618-625. doi: 10.1161/STROKEAHA.118.023310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell BCV, Majoie CBLM, Albers GW, et al. ; HERMES collaborators . Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18(1):46-55. doi: 10.1016/S1474-4422(18)30314-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Estimation of Propensity Scores for EVT and Case-Control Matching
eMethods 2. Image Eligibility Criteria for Selected Clinical Trials
eFigure 1. Study Profile
eFigure 2. Proportions of Image-Eligibilities for Selected Trials and EVT by Time Delay From Last Known Well
eTable 1. Baseline Characteristics of Included Patients
eTable 2. The Time Delay From LKW and Perfusion Images
eTable 3. Full Model Results From a Multivariable Binary Logistic Regression Model With Covariates Whose SMD>0.10
eTable 4. Full Model Results From a Multivariable Ordinal Logistic Regression Model With Covariates Whose SMD>0.10
eTable 5. Full Model Results From Multivariable Logistic Regression Models Without Propensity Score Matching
eTable 6. Functional Recovery at Three Months After Stroke Stratified by Acute Treatment and Imaging-Eligibility of Selected Clinical Trials
eTable 7. Baseline Characteristics of Patients Who Arrived >24 Hours From Last Known Well
eTable 8. Clinical Profile of Patients Who Arrived >24 Hours by Endovascular Recanalization Treatment
eTable 9. EVT and Functional Recovery at 3 Months After Stroke in Patients >24 Hours
eTable 10. Unwitnessed Onset and the Association of EVT With Outcomes