Abstract
Background:
Emerging evidence suggests osteoarthritis (OA) and related symptom burden may increase risk for Alzheimer’s disease and related dementias (ADRD). However, longitudinal studies are sparse, and none have examined the potential mediating effects of mood or sleep disorders.
Objective:
To determine the association of OA and related pain to incident ADRD in U.S. elders.
Methods:
In this retrospective cohort study, we used baseline and two-year follow-up data from linked Medicare claims and Medicare Current Beneficiary Survey files (11 pooled cohorts, 2001–2013). The study sample comprised 16,934 community-dwelling adults ≥ 65 years, ADRD-free at baseline and enrolled in fee-for-service Medicare. Logistic regression was used to assess the association of OA and related pain (back, neck, joint, neuropathic) to incident ADRD, explore the mediating influence of mood and insomnia-related sleep disorders, and (sensitivity analyses) account for potential survival bias.
Results:
Overall, 25.5% of beneficiaries had OA at baseline (21.0% with OA and pain); 1149 elders (5.7%) were subsequently diagnosed with ADRD. Compared to beneficiaries without OA, those with OA were significantly more likely to receive a diagnosis of incident ADRD after adjustment for sociodemographics, lifestyle characteristics, comorbidities, and medications (adjusted odds ratio (AOR) = 1.23 (95% confidence interval (CI) 1.06, 1.42). Elders with OA and pain at baseline were significantly more likely to be diagnosed with incident ADRD than were those without OA or pain (AOR = 1.31, CI 1.08, 1.58). Sensitivity analyses yielded similar findings. Inclusion of depression/anxiety, but not sleep disorders, substantially attenuated these associations.
Conclusion:
Findings of this study suggest that: OA is associated with elevated ADRD risk, this association is particularly pronounced in those with OA and pain, and mood disorders may partially mediate this relationship.
Keywords: Alzheimer’s disease and related dementias, arthritis, cognition, dementia, medicare current beneficiaries survey, mood, pain, sleep
INTRODUCTION
Prevalence of Alzheimer’s disease and related dementias (ADRD), which encompass a range of progressive and devastating neurodegenerative disorders, is increasing dramatically worldwide, imposing an enormous clinical, societal, and economic burden and constituting a serious and rapidly growing global public health crisis. For example, Alzheimer’s disease (AD), the most common form of dementia and a leading cause of mortality in higher income countries and many developing nations [1–3], affects over 46 million adults worldwide, with prevalence projected to reach over 75 million by 2030 and 135.5 million by 2050 [4]. In the U.S., at least 5.8 million are currently living with AD, at an estimated annual direct cost of $290 billion for those over age 65 [3], figures that are projected to rise steeply in coming years. Even in high-income countries, the economic costs and associated societal burden of dementia threaten to overwhelm existing resources as the population ages and longevity increases [5].
With effective treatments for cognitive impairment or ADRD still lacking after decades of clinical trials and billions of dollars invested, research is shifting increasingly to prevention and early intervention [6, 7], with large-scale risk reduction trials now underway in multiple countries [7–11], including the U.S. [7, 12]. However, while there has been considerable progress in identifying modifiable risk factors for ADRD, major knowledge gaps remain. Important among these is our still limited understanding regarding the contribution of common chronic pain conditions to the development of cognitive impairment. The former include osteoarthritis (OA), a condition which, like ADRD, continues to increase rapidly in prevalence worldwide, and that itself poses a looming global public health crisis [13–16].
OA is the most common form of arthritis, affecting an estimated 31–52 million (12–20%) U.S. adults [13,17,18]. The global prevalence of OA is expected to rise steeply in coming years due to aging of the population and increasing rates of obesity and physical inactivity [13, 16, 19]. A leading cause of disability worldwide [16, 19, 20], OA is associated with substantial clinical, economic, and societal burden [21, 22], with excess expenditures considerably greater than those for diabetes, hypertension, and other common chronic disorders [21]. OA is also associated with increased risk for medical comorbidity [23, 24], falls [25], and mortality [19, 23, 26, 27]. The second most common cause of chronic pain worldwide [13], OA is linked to significant reductions in physical function [13], productivity [13, 18, 28], mood [13, 29, 30], sleep quality [29, 31], and quality of life (QOL) [21, 26]. In the U.S., 80% of those with OA suffer some degree of mobility limitation due to chronic pain, and at least 25% are restricted in major activities of daily living [20].
While studies remain sparse, emerging evidence from cross-sectional and longitudinal studies by our group and others suggests that OA and OA symptom burden may also be linked to increased ADRD risk. In our recent cross-sectional study of a large Appalachian population, OA and related joint pain showed a strong, dose-response relation to reported memory loss; this association appeared to be partially mediated by impaired sleep and mood, factors strongly and reciprocally associated with OA and linked to increased ADRD risk [32]. Likewise, in our cross-sectional study of a large, representative sample of U.S. elders, we found OA accompanied by moderate to severe pain interference to be strongly and positively associated with ADRD after adjustment for multiple potential confounders [33, 34]. Consistent with these findings, the results of two large nested case-control studies of Taiwanese nationals suggested those with diagnosed OA at baseline were at significantly elevated risk for incident dementia within the maximum 4 [35] to 10 [36] year follow-up period [35, 36].
However, although findings from existing studies suggest OA and related symptom burden may increase risk for incident dementia, interpretation is hampered by some important limitations. These include cross-sectional design [32, 34], potential self-report bias [32, 34]; and failure to assess the influence of OA-associated pain [35, 36], evaluate the potential role of psychosocial factors [35, 36] or survival bias [32, 34–36], or control for several potential confounders known to affect cognition, including certain: lifestyle and demographic factors [34–36], chronic health conditions [35, 36], and medications [35, 36]. In addition, longitudinal studies in U.S. and other western populations are lacking. Clearly, additional rigorous investigations are needed. In this large retrospective cohort study of U.S. community-dwelling fee-for-service (FFS) Medicare beneficiaries, we examine: the relation of OA to incident ADRD; assess the influence of pain symptoms on this association; and explore the potential mediating role of mood and insomnia-related sleep disorders.
METHODS
Study design and data source
We used a retrospective cohort design to assess the association of baseline OA and related pain to incident ADRD using linked, longitudinal data from FFS Medicare Claims and Medicare Current Beneficiaries Survey (MCBS). Initiated in 1991, MCBS is a continuous, in-person, multi-purpose survey of a nationally representative sample of adults enrolled in Medicare, a U.S. government health insurance program for U.S. citizens and permanent residents who are at least 65 years of age or have eligible disabilities. Survey participants are recruited using a complex stratified, three-stage probability sampling design described in detail elsewhere [37, 38]. Structured in-person interviews are administered at baseline and follow-up rounds each year for up to three years.
MCBS generates comprehensive cross-sectional and longitudinal data on healthcare cost and utilization of survey participants using a combination of survey, administrative, and claims-based records. Our study used the MCBS cost and use files (MCBS-CU). These files include information on demographics, lifestyle factors, access to care, insurance coverage, and health status, linked to Medicare FFS claims data, which contain information on medical services, spending, and utilization.
Study sample
The study sample comprised 16,934 community-dwelling adults who were aged 65 years or over, were continuously enrolled in FFS Medicare, and were still alive at end of study, defined as either diagnosis of incident ADRD or end of follow-up period (see below). Participants with diagnosed ADRD at baseline (5.3%) were excluded from the sample, as were institutionalized adults. Based on the recommendations by MCBS investigators [39] and sampling strategies documented in prior published studies using MCBS [40,41], we combined multiple three-year MCBS cohorts (2001–2013) in order to minimize relative standard errors and to enhance the reliability and precision of our study estimates. As illustrated in the sample selection flow chart (Fig. 1), application of all a priori exclusion criteria yielded a final sample size of 16,934 adults (Ns by cohort = 1874 (2001–2003); 1768 (2002_2004); 1884 (2003_2005); 1766 (2004_2006); 1735 (2005_2007); 1650 (2006_2008); 1495 (2007_2009); 1278 (2008_2010); 1034 (2009_2011); 1159 (2010_2012); and 1291 (2011_2013)). All MCBS data received were deidentified.
Fig. 1.
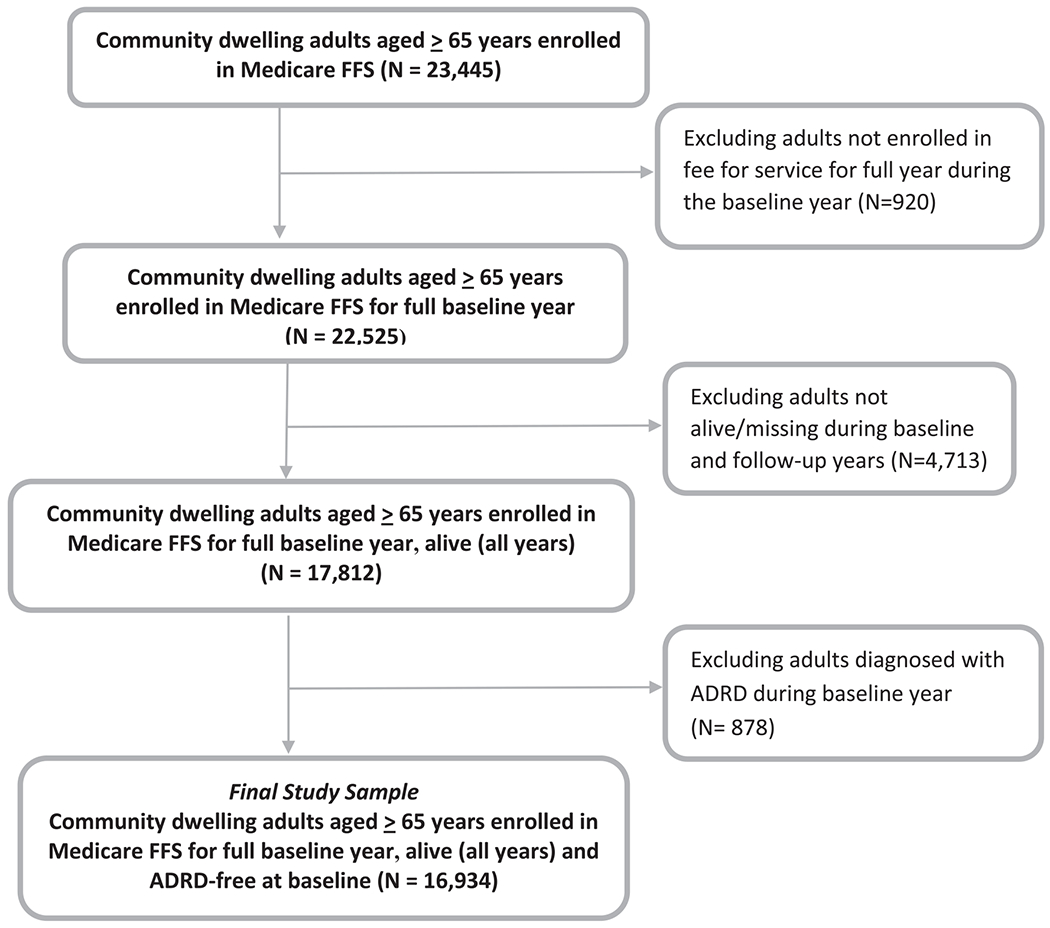
Study Sample Selection Diagram (linked Medicare Current Beneficiary Survey and Medicare claims, 11 pooled cohorts, 2001–2013). FFS, fee-for-Service; ADRD, Alzheimer’s disease and related dementias.
Measures
Outcome variable: Incident ADRD at follow-up
ADRD status at baseline (year 1) and follow-up (years 2 and 3) were ascertained using the 2001–13 Medicare FFS claims for inpatient (IP), skilled nursing facility (SNF), outpatient (OT), home health agency (HHA), and physician office (PO), as well as MCBS CU self-reported Health Status and Functioning files. To help increase capture of diagnosed ADRD, both claims and survey data were used to identify ADRD [42]. Specifically, the presence of ADRD was defined as 1) one or more FFS claims with any of the following International Classification of Diseases, ninth Edition, clinical modification (ICD-9-CM) diagnostic codes: 290.0–290.3, 331.0–331.2, 331.7, and 331.8 [43, 44]; or 2) an affirmative response to the self-reported Health Status question “Has a doctor ever told you that you had Alzheimer’s?’.
Key independent variable: OA and related chronic pain symptoms
Baseline OA, related chronic pain symptoms (joint, back, and neck pain) were identified using ICD9 codes from FFS Medicare claims; each condition was defined as the presence of two or more outpatient claims, at least 90 days apart, for the specific condition or any one inpatient or skilled nursing facility claim. Eligible claims were identified using clinical classification codes software (CCS) for ICD-9-CM diagnostic codes as follows: CCS code 203 (OA); 201, 204, 211, 212, 54, 225 (Joint pain); 205, 209, 217, 231, 232 (back and neck pain); and 84, 95 (neuropathic pain).
To determine the relation of OA to incident ADRD, we first assessed OA as a binary variable (OA versus no OA). To evaluate the potentially differential associations of OA and related symptom burden to incident ADRD, we also created a composite variable from the above, categorized as follows: no OA, no pain (joint, neck, back, or neuropathic) (referent category); pain present but no OA; OA without pain; and OA with pain.
Covariates
To control for the influence of potential confounding, specific baseline characteristics known or suspected to be associated with ADRD risk and/or OA were selected for inclusion as covariates in our multivariable models. These included biological factors, i.e., age group (assessed as a categorical variable), sex (female/male), and race/ethnicity (Non-Hispanic White, African American, Hispanic, and other); other demographic characteristics, i.e., education (less than high school, high school, some college, college), marital status (married, widowed, divorce/separated, other), family income, measured as the percentage of federal poverty line (FPL) (poor/low-income (<200%), (middle to high-income (≥200%)); health insurance status (Medicaid (yes/no); private insurance (yes/no)); rurality (residence in a metropolitan area (yes/no)); and lifestyle factors, i.e., smoking status (current smoker, past smoker, never smoked) and body mass index (BMI, kg/m2, categorized as underweight (BMI < 18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25–29.9), and obese (BMI ≥ 30)).
We also included as covariates other chronic health conditions reported at baseline (yes/no), including: hypertension, diabetes, heart disease, chronic respiratory illness, history of stroke or traumatic brain injury (TBI), cancer, and certain auto-immune conditions associated with chronic pain, including rheumatoid arthritis (RA, yes/no), and systemic lupus erythematosus (SLE, yes/no), as well as other chronic pain conditions (headache, migraine). As for OA, other chronic health conditions were ascertained using Medicare FFS claims. In analyses of OA as a binary variable, we also assessed the influence of back, neck, joint and neuropathic pain as covariates on the association of OA to incident ADRD. In addition, certain commonly used medications associated with ADRD risk and/or used in chronic pain management were also evaluated as covariates, including opioid analgesics and nonsteroidal anti-inflammatory drugs (NSAIDS).
To examine the potential mediating influence of mood and sleep disorders on the relation of OA and associated pain symptoms to incident ADRD, we also evaluated the effects of diagnosed depression and anxiety disorders, as well as insomnia and related sleep disorders both separately and in combination. As detailed above, these disorders were determined using Medicare FFS claims (for ICD-9 codes, see the Supplementary Material)
Statistical analysis
Potential differences in baseline characteristics by OA and ADRD status were assessed using Rao-Scott chi-square tests (categorical variables). Logistic regression was used to assess the unadjusted and independent relation of OA and related pain burden to incident ADRD and to evaluate the potential mediating effects of mood and sleep disorders. All multivariable logistic regression models included demographic and socioeconomic factors; additional models also adjusted for lifestyle factors, chronic physical health conditions, access to care, and medications, including NSAIDs and opioid analgesics. In addition, we also included baseline diagnoses of depression, anxiety, and insomnia, both individually and in combination, in the fully adjusted models to assess the potential mediating influence of these factors on the relation of OA and related pain to ADRD risk. Specifically, to assess the potential mediating influence of these factors, we first determined the associations of sleep and mood disorders both to incident ADRD and to baseline OA and related pain symptoms in adjusted logistic regression models. Following confirmation of these associations, we then included sleep and mood disorders, both separately and together, in the fully adjusted models to determine the influence of these factors on the associations of OA and related pain symptoms to incident ADRD.
To account for potential survival bias, we also examined, in sensitivity (competing risk of death) analyses, the relation of OA and pain to incident ADRD using multinomial logistic regression; eligible participants who died during the follow-up period (N = 1479, including 259 with incident ADRD) were included in these models, with the outcomes categorized as: 1) died, no incident ADRD; 2) died, incident ADRD; 3) alive, incident ADRD; 4) alive, no ADRD.
All bivariate and MVLR analyses were performed using MCBS complex survey design sampling weights [37,38]. We used 3-year backward longitudinal weights for analysis of our pooled cohorts to yield up to two years of continuous follow-up. All analyses were conducted using SAS survey procedure (SAS version 9.4, SAS Institute, Inc.).
RESULTS
Study population characteristics
Of the 16,934 FFS Medicare beneficiaries included in the study sample, the majority were female (57%), white (83%), married (56.5%), and from metropolitan areas (71%); most reported a family income of at least 200% of the FPL (54.1%). Baseline prevalence of OA was 25.5% (weighted percentage). OA prevalence was similar by cohort year (p = 0.95) and did not differ significantly by census region or rurality (p’s > 0.05). As illustrated in Table 1, a significantly higher percentage of beneficiaries with OA were older, female, widowed, lower income, more poorly educated, on Medicaid, and overweight or obese relative to those without OA (p’s <0.0001). A significantly greater proportion of those with OA also reported a history of chronic physical health conditions associated with ADRD, including hypertension (82.7% versus 70.4%), heart disease (30.8% versus 21.8%), diabetes (22.4% versus 20.2%), chronic respiratory disease (16.2% versus 13.5%), and history of stroke (13.4% versus 10.9%) and TBI (2.1% versus 0.9%), as well as other chronic pain conditions, including RA (6.5 versus 1.6%), lupus (1.5% versus 0.5%), and headache/migraine (9.4% versus 4.5%) (p’s <0.0001). Presence of chronic pain symptoms, including back and neck, joint, and/or neuropathic pain, were significantly more common in beneficiaries with a history of OA, with 82.5% of the latter indicating pain versus 45.7% of those without OA (Table 1).
Table 1.
Study sample baseline characteristics stratified by osteoarthritis in a representative sample of community-dwelling Medicare fee-for-service beneficiaries aged ≥ 65 years (N = 16,934; linked Medicare Current Beneficiary Survey (MCBS) and fee-for-service Medicare claims, 2001–2013)
| Diagnosed Osteoarthritis |
p | ||||
|---|---|---|---|---|---|
| Yes |
No |
||||
| N | Weighted % | N | Weighted % | ||
| Total | <0.0001 | ||||
| Incident ADRD | 4,545 | 25.5 | 12,389 | 74.5 | |
| Yes | 386 | 7.5 | 763 | 5.1 | |
| No | 4,159 | 92.5 | 11,626 | 94.9 | |
| Sociodemographics | <0.0001 | ||||
| Sex | <0.0001 | ||||
| Female | 3,047 | 67.2 | 6,622 | 53.4 | |
| Male | 1,498 | 32.8 | 5,767 | 46.6 | |
| Age in Years | <0.0001 | ||||
| 65–69 | 848 | 23.9 | 3,340 | 34.5 | |
| 70–74 | 861 | 21.8 | 2,623 | 22.8 | |
| 75–79 | 1,030 | 23.5 | 2,644 | 20.7 | |
| 80 + | 1,806 | 30.7 | 3,782 | 22.1 | |
| Mean (SE) | 75.90(0.110) | 74.14 (0.079) | <0.0001 | ||
| Race/Ethnicity | 0.262 | ||||
| Non-Hispanic White | 3,739 | 82.4 | 10,346 | 83.4 | |
| Black/African American | 344 | 7.4 | 815 | 6.5 | |
| Hispanic | 264 | 5.8 | 692 | 5.6 | |
| Other | 194 | 4.4 | 515 | 4.5 | |
| Marital Status | <0.0001 | ||||
| Married | 2,255 | 52.4 | 6,884 | 58.0 | |
| Widowed | 1,832 | 36.6 | 3,971 | 28.5 | |
| Divorce/separated | 322 | 7.7 | 1,133 | 10.2 | |
| Other | 135 | 3.4 | 397 | 3.3 | |
| Education | <0.0001 | ||||
| <High School | 1,066 | 27.6 | 3,264 | 24.1 | |
| High School | 1,329 | 35.6 | 4,553 | 36.7 | |
| Some College | 532 | 15.1 | 1,784 | 15.0 | |
| College | 766 | 21.7 | 2,752 | 24.2 | |
| Household Income | <0.0001 | ||||
| <200% Federal Poverty Level | 2,374 | 50.1 | 5,886 | 44.5 | |
| ≥200% Federal Poverty Level Insurance | <0.0001 | ||||
| Insurance | |||||
| Medicaid | 2,171 | 49.9 | 6,503 | 55.5 | |
| Yes | 739 | 15.9 | 1,408 | 10.5 | |
| No | 3,806 | 84.1 | 10,981 | 89.5 | |
| Private Insurance | 0.23 | ||||
| Yes | 3,646 | 80.1 | 9,772 | 79.1 | |
| No | 899 | 19.9 | 2,615 | 20.9 | |
| Rurality | 0.07 | ||||
| Metro | 3,127 | 72.3 | 8,248 | 70.0 | |
| Rural | 1,418 | 27.7 | 4,140 | 30.0 | |
| Lifestyle Characteristics | |||||
| Body Mass Index (kg/m2) | <0.0001 | ||||
| Underweight (<18.5) | 66 | 1.4 | 246 | 1.8 | |
| Normal (18.5–24.9) | 1,361 | 29.1 | 4,506 | 35.6 | |
| Overweight (25–29.9) | 1,768 | 38.7 | 4,941 | 40.5 | |
| Obese (≥30) | 1,295 | 30.9 | 2,586 | 22.1 | |
| Mean (SE) | 28.09 (0.086) | 26.91 (0.056) | <0.0001 | ||
| Smoking Status | <0.0001 | ||||
| Current | 291 | 6.9 | 1,303 | 11.3 | |
| Past | 2,072 | 46.5 | 5,904 | 47.8 | |
| Never | 2,173 | 46.6 | 5,153 | 40.9 | |
| Baseline Health History | |||||
| Physical Health Conditions Hypertension | <0.0001 | ||||
| Yes | 3,791 | 82.7 | 8,922 | 70.4 | |
| No | 754 | 17.3 | 3,467 | 29.6 | |
| Ischemic Heart disease | <0.0001 | ||||
| Yes | 1,449 | 30.8 | 2,908 | 21.8 | |
| No | 3,096 | 69.2 | 9,481 | 78.2 | |
| History of Stroke | <0.0001 | ||||
| Yes | 650 | 13.4 | 1,489 | 10.9 | |
| No | 3,895 | 86.6 | 10,900 | 89.1 | |
| Diabetes | 0.003 | ||||
| Yes | 996 | 22.4 | 2,499 | 20.2 | |
| No | 3,549 | 77.6 | 9,890 | 79.8 | |
| Chronic Respiratory disease | <0.0001 | ||||
| Yes | 719 | 16.2 | 1,704 | 13.5 | |
| No | 3,826 | 83.8 | 10,685 | 86.5 | |
| History of Cancer | <0.0001 | ||||
| Yes | 2,017 | 43.2 | 5,084 | 39.4 | |
| No | 2,528 | 56.8 | 7,305 | 60.6 | |
| History of Traumatic Brain Injury | <0.0001 | ||||
| Yes | 100 | 2.1 | 121 | 0.9 | |
| No | 4,445 | 97.9 | 12,268 | 99.1 | |
| Other Chronic Pain Conditions | |||||
| Rheumatoid Arthritis | <0.0001 | ||||
| Yes | 284 | 6.5 | 224 | 1.6 | |
| No | 4,261 | 93.5 | 12,165 | 98.4 | |
| Lupus | <0.0001 | ||||
| Yes | 70 | 1.5 | 66 | 0.5 | |
| No | 4,475 | 98.5 | 12,323 | 99.5 | |
| Headache/Migraine | <0.0001 | ||||
| Yes | 422 | 9.4 | 567 | 4.5 | <0.0001 |
| No | 4,123 | 90.6 | 11,822 | 95.5 | |
| Chronic pain symptoms | |||||
| Back/neck pain | <0.0001 | ||||
| Yes | 2,051 | 45.4 | 2,782 | 21.9 | |
| No | 2,494 | 54.6 | 9,607 | 78.1 | |
| Joint Pain | <0.0001 | ||||
| Yes | 3,422 | 76.0 | 4,967 | 39.5 | |
| No | 1,123 | 24.0 | 7,422 | 60.5 | |
| Neuropathic Pain | <0.0001 | ||||
| Yes | 332 | 7.2 | 439 | 3.5 | |
| No | 4,213 | 92.8 | 11,950 | 96.5 | |
| Presence of any chronic pain symptoms | <0.0001 | ||||
| Yes | 3,731 | 82.5 | 5,773 | 45.7 | |
| No | 814 | 17.5 | 6,616 | 54.3 | |
| Sleep and Mood Disorders | |||||
| Insomnia and related sleep disorders | <0.0001 | ||||
| Yes | 1,055 | 23.2 | 1,673 | 13.3 | |
| No | 3,490 | 76.8 | 10,716 | 86.7 | |
| Depression | <0.0001 | ||||
| Yes | 491 | 10.8 | 667 | 5.6 | |
| No | 4,054 | 89.2 | 11,722 | 94.4 | |
| Anxiety | <0.0001 | ||||
| Yes | 346 | 7.7 | 516 | 4.2 | |
| No | 4,199 | 92.3 | 11,873 | 95.8 | |
| Analgesic medication use | |||||
| NSAIDs | <0.0001 | ||||
| Yes | 1,612 | 35.7 | 1,629 | 12.8 | |
| No | 2,933 | 64.3 | 10,760 | 87.2 | |
| Opioid Analgesics | <0.0001 | ||||
| Yes | 1,491 | 33.2 | 1,862 | 14.6 | |
| No | 3,054 | 66.8 | 10,527 | 85.4 | |
In addition, use of prescription analgesics was significantly higher in beneficiaries with than without OA, including NSAIDs (35.5% versus 12.8%) and opioids (33.2% versus 14.6%) (p’s < 0.0001). Elders with prevalent OA were also significantly more likely to report mood disorders, including depression (10.8% versus 5.6%) and anxiety (7.7% versus 4.2%), as well as sleep disorders (23.2% versus 13.3%) relative to those without OA (p’s < 0.0001 (Table 1).
A total of 1,149 beneficiaries (5.7%, weighted percentage) were diagnosed with incident ADRD during the 2-year follow-up period. Incident ADRD rates did not differ by study cohort (range 4.9 to 6.2 per 100 participants, p > 0.8), but were significantly higher in women (6.2% versus 5.0% in men), and in elders who were African American (7.4% versus 5.5% in Non-Hispanic whites), widowed (8.2% versus 4.5% in married), and more poorly educated (p’s < 0.0001) (Supplementary Table 1). Incident ADRD increased strongly with age, from 1.9% in adults 65–69 years to 12% in those 80 and older. The proportion of participants diagnosed with incident ADRD was also significantly higher in those who reported incomes under 200% of the federal poverty level (7.4% versus 4.3%), were on Medicaid (9.1% versus 5.2%) and who lacked private insurance (7.2% versus 5.3%) (p’s < 0.0001). In addition, the percentage diagnosed with incident ADRD was significantly greater in those who indicated a history of specific physical health conditions at baseline, including hypertension, heart disease, stroke, and TBI, as well as in those with certain other chronic pain conditions (including migraine and non-migraine headache) and who reported use of opioid analgesics (p’s < 0.0001). Incident ADRD rates were also significantly higher among those who were diagnosed with depression, anxiety disorders, or insomnia and related sleep disorders at baseline, and notably, were elevated in elders with a history of OA or chronic pain symptoms (p’s < 0.0001).
Association of OA to incident ADRD
As illustrated in Table 2A, OA was strongly and positively associated with likelihood of incident ADRD in unadjusted analyses (odds ratio (OR) = 1.5, 95% confidence interval (CI) 1.31, 1.73, p < 0.0001). Adjustment for sociodemographics, lifestyle factors, other chronic conditions (including RA and lupus), and medications attenuated but did not eliminate this association (adjusted OR (AOR) = 1.23, CI 1.06, 1.42, p = 0.005). Further adjustment for other pain conditions, including those commonly associated with OA (joint, back, neck, and neuropathic) only modestly reduced this risk estimate (AOR = 1.19, CI 1.02, 1.38, p = 0.026), suggesting that accompanying pain only partially explained the observed association of OA to incident ADRD.
Table 2A.
Relation of OA to incident ADRD in 16,934 Medicare fee-for-service beneficiaries ≥ 65 years (linked Medicare Current Beneficiary Survey (MCBS) and fee-for-service Medicare claims, 11 pooled cohorts, 2001–13)
| Unadjusted model |
Adjusted for sociodemographics¥ |
Also adjusted for lifestyle factor†, chronic conditions¥¥, medications |
Also adjusted for other chronic pain conditions‡ |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| No OA (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| OA | 1.50(1.31,1.73) | <0.00001 | 1.24 (1.07, 1.43) | 0.004 | 1.23 (1.06, 1.42) | 0.005 | 1.19(1.02,1.38) | 0.026 |
Including sex, age, race/ethnicity, education, income, supplemental insurance, marital status, region.
Including smoking status, BMI.
Including chronic physical health conditions (including RA, lupus), history of stroke, TBI.
Including joint, back, neck, or neuropathic pain, headache, migraine.
Association of OA and pain symptoms to incident ADRD
Table 2B depicts the association of OA and pain, alone and in combination, to incident ADRD. Again, in the unadjusted analysis, OA at baseline, in both the presence and absence of pain, was strongly and positively associated with likelihood of incident ADRD, with the magnitude of risk greatest in those with OA accompanied by pain (ORs for OA with and without pain, respectively = 1.75 (CI 1.46, 2.09) and 1.58 (CI 1.19, 2.09), p’s ≤ 0.001). Baseline back, neck, or joint pain in the absence of OA was likewise associated, albeit more modestly, with elevated ADRD risk (OR = 1.32, CI 1.12, 1.54) (Table 2B). Adjustment for demographic and socioeconomic characteristics attenuated these associations, but did not eliminate the relation of incident ADRD to either OA with pain (AOR = 1.40, CI 1.16–1.68, p = 0.0004) or pain in the absence of OA (AOR = 1.21, CI 1.03, 1.42, p = 0.02). The magnitude of these latter associations was further reduced after additional adjustment for lifestyle factors, other physical health conditions (including other pain conditions), and medications, with only OA accompanied by pain retaining a significant, positive association with incident ADRD (AOR = 1.31, CI 1.08–1.58, p = 0.006).
Table 2B.
Relation of OA and associated pain† to incident ADRD in 16,934 Medicare fee-for-service beneficiaries ≥ 65 years (linked Medicare Current Beneficiary Survey (MCBS) and fee-for-service Medicare claims, 11 pooled cohorts, 2001–13)
| Unadjusted model |
Adjusted for sociodemographics¥ |
Also adjusted for lifestyle factors ‡, chronic conditions¥¥, medications |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| No OA, no paini† | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| No OA, paini† | 1.32(1.12, 1.54) | 0.0006 | 1.21 (1.03, 1.42) | 0.02 | 1.13 (0.96, 1.33) | 0.15 |
| OA without paini† | 1.58(1.19,2.09) | 0.001 | 1.22 (0.90, 1.66) | 0.21 | 1.23 (0.94, 1.66) | 0.16 |
| OA with paini† | 1.75 (1.46, 2.09) | <0.00001 | 1.40(1.16, 1.68) | 0.0004 | 1.31 (1.08, 1.58) | 0.006 |
Including joint, back, neck, or neuropathic pain.
Adjusted for sex, age, race/ethnicity, education, income, supplemental insurance, marital status, region.
Including smoking status, BMI.
Including diagnosed headache, migraine, and other physical health conditions, history of stroke, TBI.
Potential mediating effects of sleep and mood disorders
As illustrated in Table 3, inclusion of mood disorders attenuated the relation of OA (both with and without pain) to incident ADRD after adjustment for sociodemographics, lifestyle factors, and medical history (AORs for OA versus no OA = 1.14 (0.98, 1.32) and OA with pain versus no OA, no pain = 1.20 (0.99, 1.45). In contrast, the inclusion of insomnia-related disorders alone only modestly affected these associations (AORs for OA versus no OA = 1.18 (1.01, 1.37) and OA with pain versus no OA, no pain = 1.26 (1.05, 1.52) (Table 3). Depression and anxiety disorders, as well as insomnia and related disorders remained significantly and positively associated with incident ADRD (AORs for depression, anxiety, and sleep disorders, respectively = 2.35 (1.86, 2.96), 1.33 (1.04, 1.71), and 1.31 (1.09, 1.58)), p’s ≤ 0.03) and baseline OA (AORs, respectively = 1.46 (1.26, 1.70), 1.38 (1.18, 1.62), 1.44 (1.29. 1.61), p’s < 0.0001)) in the fully adjusted analyses.
Table 3.
Relation of osteoarthritis (OA) and related pain to incident ADRD in 16,934 community-dwelling Medicare beneficiaries ≥ 65 years (linked Medicare Current Beneficiary Survey (MCBS) and fee-for-service Medicare claims, 11 pooled cohorts, 2001–13). Potential mediating influence of diagnosed mood and sleep disorders
| Fully adjusted model¥ |
Adjusted model including depression, anxiety |
Adjusted model including sleep disorders |
Adjusted model including sleep disorders, depression, and anxiety |
|||||
|---|---|---|---|---|---|---|---|---|
| AOR (95% CI) | p | AOR (95% CI) | p | AOR (95% CI) | p | AOR (95% CI) | p | |
| Diagnosed OA | ||||||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| Yes | 1.23 (1.06, 1.42) | 0.005 | 1.14 (0.98, 1.32) | 0.09 | 1.18 (1.01, 1.37) | 0.03 | 1.13 (0.97, 1.31) | 0.11 |
| Diagnosed OA, stratified by presence of pain | ||||||||
| No OA, no pain† | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| No OA, pain† | 1.13 (0.96, 1.33) | 0.15 | 1.09 (0.92, 1.28) | 0.06 | 1.11 (0.94, 1.31) | 0.21 | 1.08 (0.91, 1.27) | 0.21 |
| OA without pain† | 1.23 (0.94, 1.66) | 0.16 | 1.18 (0.90, 1.59) | 0.28 | 1.22 (0.90, 1.65) | 0.19 | 1.18(0.87, 1.59) | 0.29 |
| OA with pain† | 1.31 (1.08, 1.58) | 0.006 | 1.20 (0.99, 1.45) | 0.06 | 1.26 (1.05, 1.52) | 0.01 | 1.18(0.97, 1.43) | 0.09 |
Adjusted for sex, age, race/ethnicity, education, income, insurance, marital status, region; lifestyle factors (smoking, BMI); other chronic conditions, history of TBI, stroke; and medications.
Including joint, back, neck, or neuropathic pain.
Sensitivity analyses
Analyses using competing risk of death models yielded results strongly consistent with findings from our primary analyses (Tables 4A,B). As detailed in Table 4A, relative to elders without baseline OA, those with OA were significantly more likely to be living with incident ADRD at follow-up after adjustment for sociodemographic and lifestyle factors, chronic health conditions, and medications (AOR = 1.22, CI 1.05, 1.41); further adjustment for pain conditions only modestly attenuated this association. In contrast, OA was unrelated to death during the follow up period, either with or without a diagnosis of incident ADRD. Likewise, sensitivity (competing risk of death) analyses regarding the potential mediating influence of mood and sleep disorders yielded findings consistent with those described above (Table 4B). These findings suggest survival bias is unlikely to explain the positive associations observed between baseline OA and incident ADRD, or the apparent partial mediating effects of depression and anxiety disorders.
Table 4A.
Relation of baseline osteoarthritis (OA) to incident ADRD in 18,413 community-dwelling Medicare fee-for-service beneficiaries ≥ 65 years, including those who died prior to follow-up (linked Medicare Current Beneficiary Survey (MCBS) and fee-for-service Medicare claims, 11 pooled cohorts, 2001–13): Sensitivity (competing risk of death) analysis
| No OA (referent) versus OA | Alive at F/u, no diagnosis of ADRD |
Alive at F/u, diagnosed with ADRD |
Died with diagnosis of ADRD |
Died without diagnosis of ADRD |
||||
|---|---|---|---|---|---|---|---|---|
| Model | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| 1 (unadjusted) | 1.00 (referent) | - | 1.50(1.30, 1.72) | <0.00001 | 1.11 (0.86, 1.44) | 0.41 | 1.09 (0.94, 1.26) | 0.99 |
| 2 (including sociodemographics¥) | 1.00 (referent) | - | 1.23 (1.07, 1.42) | 0.0047 | 0.87 (0.67, 1.14) | 0.31 | 1.00 (0.85, 1.17) | 0.99 |
| 3 (+lifestyle factors†, medical hx‡) | 1.00 (referent) | - | 1.22 (1.05, 1.41) | 0.009 | 0.88 (0.66, 1.16) | 0.36 | 0.92 (0.78, 1.09) | 0.35 |
| 4 (+chronic pain conditions¥¥) | 1.00 (referent) | - | 1.17 (1.00, 1.36) | 0.046 | 0.82 (0.62, 1.09) | 0.18 | 0.88 (0.74, 1.05) | 0.15 |
Including sex, age, race/ethnicity, education, income, supplemental insurance, marital status, region.
Including smoking status, BMI.
Including chronic physical health conditions (including RA, lupus), history of stroke, TBI.
Including joint, back, and neck pain; neuropathic pain, headache, migraine.
Table 4B.
Relation of baseline osteoarthritis (OA) to incident ADRD in 18,413 community-dwelling Medicare fee-for-service beneficiaries ≥ 65 years (linked MCBS and fee-for-service Medicare claims, 11 pooled cohorts,2001–13). Potential mediating influence of diagnosed mood and sleep disorders: Sensitivity (competing risk of death) analysis
| No OA (referent) versus OA | Alive at F/u, no diagnosis of ADRD |
Alive at F/u, diagnosed with ADRD |
Died with diagnosis of ADRD |
Died without diagnosis of ADRD |
||||
|---|---|---|---|---|---|---|---|---|
| Model | AOR (95% CI) | p | AOR (95% CI) | p | AOR (95% CI) | p | AOR (95% CI) | p |
| Fully adjusted¥ | 1.00 (referent) | - | 1.22 (1.05, 1.41) | 0.009 | 0.82 (0.62, 1.09) | 0.18 | 0.88 (0.74, 1.05) | 0.15 |
| +depression, anxiety | 1.00 (referent) | - | 1.12 (0.97, 1.30) | 0.13 | 0.82 (0.61, 1.09) | 0.16 | 0.87 (0.73, 1.04) | 0.12 |
| +sleep disorders | 1.00 (referent) | - | 1.16 (0.99, 1.35) | 0.06 | 0.81 (0.61, 1.07) | 0.14 | 0.87 (0.73, 1.04) | 0.13 |
| + mood and sleep disorders | 1.00 (referent) | - | 1.11 (0.96, 1.29) | 0.16 | 0.81 (0.61, 1.07) | 0.13 | 0.86 (0.72, 1.03) | 0.10 |
Adjusted for sex, age, race/ethnicity, education, income, insurance, marital status, region; lifestyle factors (smoking, BMI); other chronic conditions, history of TBI, stroke; and medications.
Other covariates
Several demographic, lifestyle, and health-related factors remained significantly associated with incident ADRD in our fully adjusted model. These include: black (versus non-Hispanic white) race (AOR = 1.41, CI 1.04, 1.90); age (80+ versus 65–69 y, AOR 6.13, CI = 4.72, 7.95); poverty (<200% versus > 200%, AOR 1.16, CI 1.02, 1.34); enrollment in Medicaid (AOR 1.30, 95% CI 1.03, 1.65); and history of diabetes (AOR = 1.21, CI 1.02, 1.44), stroke (AOR 1.87, CI 1.59, 2.19) or TBI (AOR = 1.52, CI = 1.04, 2.23). In contrast, obesity was inversely associated with incident ADRD (AOR = 0.68, CI 0.56, 0.83). Use of neither prescription NSAIDs nor opioids were associated with risk for incident ADRD (AORs = 1.01–1.05, p’s > 0.5).
DISCUSSION
To our knowledge, this is the first study using longitudinal data to examine the association of OA to ADRD in a western population, to assess the potential influence of OA-associated pain on this relationship, and to explore the potential mediating effects of mood and sleep disorders on this association. This study is also among the first to account for the potential influence of survival bias on the association of any pain condition to ADRD. In this retrospective cohort study of a large sample of 16,934 Medicare beneficiaries, baseline OA was significantly and positively associated with likelihood of incident ADRD within the 3-year study period, an association that was most pronounced in those with OA accompanied by joint, neck, back, or neuropathic pain. However, OA-related pain did not appear to completely explain the link between OA and ADRD, and pain in the absence of OA was unrelated to incident ADRD after adjustment for multiple potential confounders. These findings are broadly consistent with those of our recent cross-sectional study of Appalachian adults [32]; in this investigation, OA and related pain demonstrated a strong, dose-response relation to reported memory loss, with OA retaining a significant, positive association to memory loss even in the absence of joint pain. Likewise, in our large, cross-sectional study of U.S. elders [33, 34], OA was associated with significantly elevated likelihood of ADRD, although only in the presence of pain interference [33, 34]. Our results are also in agreement with those of two large nested case-control studies of Taiwanese nationals indicating significantly elevated likelihood of incident dementia in those diagnosed with OA at baseline (ORs adjusted for age, sex, and certain comorbidities: 1.25 [35]-1.47 [36]), although these studies did not provide information on the relative contribution of OA-related pain or on the potential influence of other chronic pain conditions.
Similarly, our findings parallel those of a growing number of epidemiological studies suggesting certain chronic pain conditions, as well as nonspecific chronic pain symptoms may increase ADRD risk. Notably, recent large retrospective cohort studies in Taiwanese nationals [45–48] and prospective cohort studies Norwegian adults [49, 50] reported significantly increased risk for incident dementia in those diagnosed with fibromyalgia [45] and headache [46–50] after adjustment for demographics, comorbidities, and other factors. While longitudinal studies examining the relation of chronic pain symptoms to adverse cognitive outcomes remain few, and studies, all in U.S. [51–53] and British [54] adults, have varied widely in design, study population, measures, length of follow-up, and other factors, overall findings likewise suggest that persistent pain [51], severe chronic pain [53, 54], and/or reported pain interference [55] may predict subsequent deterioration in memory [53, 54], accelerated cognitive decline [51], and dementia [51, 55].
Potential mediating influence of mood and sleep impairment
OA pain and dysfunction have been bidirectionally linked to sleep impairment [29, 31, 56–58], psychological stress [30, 59–61], depression and anxiety [59, 62–64], and fatigue [30, 64, 65]. Sleep disturbances are common in OA, affecting over 70% of those with symptomatic OA [29, 66]. Likewise OA is often accompanied by serious mood disturbance; e.g., a recent meta-analysis estimated depression to affect 20% of OA patients overall [67], with prevalence rates well over 40% documented in some populations [67–70]. Current evidence from existing experimental, clinical, and epidemiologic studies suggest that the relationships between musculoskeletal pain, inadequate sleep, and psychological distress are strongly reciprocal [71–75]. Chronic pain can lead to significant disruption of both sleep and mood [64, 73, 75, 76]; conversely, growing research suggests that sleep deficits increase sensitivity to noxious stimuli and exacerbate both pain and affective symptoms [71, 74, 75, 77, 78]. Similarly, depression, anxiety, and other distressful states can lead to disordered sleep, as well as increased pain [73, 78–81]. In addition, a substantial body of evidence indicates that both mood and sleep impairment can significantly and adversely influence cognitive functioning, and are important independent predictors of subsequent cognitive decline, incident cognitive impairment, and ADRD [82–93]. Disruption of mood and sleep may thus in part mediate the documented negative effects of pain on memory and cognitive performance. Consistent with findings from investigations in other chronic pain populations, measures of sleep, depression, and anxiety disorder were significantly inter-related in this study (AORs = 1.7, p’s < 0.0001) and were strongly and positively associated with both OA (AORs = 1.4–1.5) and incident ADRD (AORs = 1.3–2.3) in the fully adjusted models (p’s < 0.0001).
Nonetheless, apart from our recent cross-sectional study of OA and reported memory loss [32], the potential mediating effects of mood and sleep impairment on the association of OA and related pain to ADRD remain little explored. In this study, inclusion of depression and anxiety disorders in the adjusted models attenuated but did not eliminate the association of OA to ADRD, suggesting the observed link between OA and incident ADRD may be partially mediated by these factors. However, in contrast to the findings of our cross-sectional study, we found little evidence for a mediating effect of insomnia and related sleep disorders, with inclusion of the latter only modestly attenuating the risk estimates. The apparent lack of a mediating effect of sleep disturbance in this study may in part reflect the increased prevalence of sleep problems with age; moreover, sleep disturbance and mood impairment are common in prodromal and early ADRD (conditions not captured or likely significantly under-ascertained, respectively, in our study), potentially diluting the effects of both mood and sleep disorders on the association of OA to ADRD, a possibility rendered more likely given the short follow-up period. As indicated above, depression, anxiety, and sleep disorders remained significantly and positively associated with increased ADRD risk after adjustment for pain conditions and multiple other factors.
Potential mechanisms of action
While the mechanisms underlying the observed link between OA and incident ADRD remain incompletely understood, OA may contribute to cognitive decline and the development of ADRD via several pathways. First, the chronic pain associated with OA can have profound effects on neurocognitive function, with potentially significant implications for the development of cognitive impairment and conversion to ADRD. In a recent review of 53 studies of cognitive performance in chronic pain populations, the authors concluded that chronic pain was related to deficits in several key neurocognitive domains, including memory, attention, and processing speed, and, albeit less consistently, executive functioning [94]. The neural systems involved in memory and cognition are closely linked to those involved in pain processing and likely affect one another reciprocally [95, 96], disrupting cognitive processing and contributing to a vicious cycle of continuing pain, adverse neurostructural changes, and deteriorating cognitive function [32]. Patients with chronic pain show changes in brain morphology paralleling those implicated in cognitive decline and cognitive impairment, including grey matter volume reduction in brain regions involved in attention, memory consolidation, and cognitive processing [32, 95–100]. Chronic pain has also been shown to disrupt the functioning of brain networks [99] essential to normal cognitive function. These alterations are thought to help explain the deterioration in cognitive function documented in many chronic pain populations, including those with OA [95,101], deterioration which may ultimately presage cognitive decline and the development of ADRD.
OA may contribute to ADRD pathogenesis via other pathways as well, e.g., OA has been associated with activation of central pain processing pathways [102] and CNS hypersensitivity [103], factors in turn, linked to disruption of cognitive functioning [95, 104]. Both systemic and peripheral inflammation may also play a role, e.g., systemic increases in Hs-CRP, IL-6, and other proinflammatory mediators have been implicated in the development and progression of both OA and cognitive impairment [105]. Likewise, local proinflammatory changes associated with OA have been linked to neuroinflammation [105]. cerebrovascular dysfunction [105], and adverse nociceptive [106] and structural [105] changes in the brain [106], alterations that may, in turn, impair cognitive processing and promote cognitive dysfunction [33, 105]. While some investigators have suggested the putative association between OA and ADRD may be due to use of NSAIDs [107], analgesics associated with reduced ADRD risk in some studies but not others [108–110], adjustment for use of NSAIDs and other prescription analgesics did not substantively alter risk estimates, and NSAID use was not independently associated with incident ADRD in this study.
Consistent with the findings of previous studies [3, 111–113], risk for incident ADRD rose strongly with age, and was increased in elders who were of African American descent, less educated, or poor, as well as those with a history of stroke, diabetes, or TBI in this sample. Likewise, the strong, positive associations between baseline depression, anxiety and sleep disorders, and subsequent likelihood of incident ADRD diagnosis observed in this study are in agreement with numerous prior investigations, including recent meta-analyses [93, 114–116]. Similarly, while midlife obesity has been associated with elevated risk for dementia [117], late life BMI has, in agreement with our findings, shown a generally consistent inverse association with likelihood of ADRD [118–121].
Strengths and limitations
Strengths of this study include the large sample of elderly U.S. FFS Medicare beneficiaries, longitudinal design, and rigorous ascertainment of OA, ADRD, and other chronic health conditions using claims data and validated algorithms. We were able to adjust for a wide array of sociodemographic characteristics, lifestyle factors, and health conditions, as well as use of medications linked to both OA and ADRD risk, reducing the possibility that confounding significantly affected our findings. Information on specific pain conditions commonly associated with OA also allowed us to assess the potential differing influence of OA with and without pain.
This study also carries some important limitations, including the relatively short follow-up period. The development of ADRD is generally insidious, with onset of clinical signs preceded years earlier by perceived and/or objective cognitive decline [122]. Thus, the two year follow up period was likely insufficient to capture the true magnitude of risk associated with OA, especially in light of the often delayed diagnosis of ADRD and the under-ascertainment of ADRD documented in many populations [123], including those on Medicare [124], which together may have biased estimates towards the null. On the other hand, those with chronic pain may be more likely to seek care, and thus to be diagnosed with dementia, potentially helping to explain the observed associations. However, pain in the absence of OA was not significantly associated with ADRD risk in this study, suggesting that such a detection bias is unlikely to have significantly influenced our findings. As noted above, mood and sleep impairment may have in part reflected signs of prodromal or early ADRD rather than independent riskfactors, potentially reducing our ability to detect a potential mediating role of these factors. Both chronic pain [125] and OA [27] have been linked to increased risk for mortality, raising the possibility that, relative to elders without OA, those with OA may be more likely to die before receiving an ADRD diagnosis and introducing potential survival bias. However, sensitivity analyses accounting for competing risk of death yielded findings consistent with those of our primary analyses, suggesting that survival bias is unlikely to have substantively affected our results. While we were able to adjust for a broad array of factors linked to both OA and ADRD, we lacked information on certain potential confounders, including physical activity and over the counter medications (including non-prescription NSAIDS). In addition, the study was not adequately powered to examine the effects of OA on specific dementia types. Insomnia and related sleep disorders, as well as depression and anxiety, were ascertained using claims data, precluding capture of mood or sleep problems that did not receive a clinical diagnosis and potentially leading to under-ascertainment of these conditions. Our sample was also restricted to community-dwelling elders enrolled in Medicare FFS, potentially limiting generalizability to institutionalized elders, adults enrolled in other insurance plans, younger Medicare beneficiaries, and other populations.
Conclusions
In this retrospective cohort study of community-dwelling Medicare FFS beneficiaries, baseline OA was significantly and positively associated with likelihood of incident ADRD at follow-up; this association was most pronounced in those with OA accompanied by pain and appeared to be partially mediated by depression and anxiety. Clearly, additional rigorous prospective studies are needed to confirm and extend these findings, and to explore potential underlying mechanisms. If confirmed in further longitudinal research, findings of this study could have important clinical and public health implications for the role of OA and associated symptom management in preventing, delaying, or slowing the development of ADRD.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 2U54GM104942-02, WVCTSI and the Alzheimer’s Research and Prevention Foundation (ARPF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the ARPF.
Footnotes
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-191311.
Authors’ disclosures available online http://www.j-alz.com/manuscript-disclosures/19-1311r1).
REFERENCES
- [1].Abubakar I, Tillmann T, Banerjee A (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF, Abyu GY, Ahmed MB, Aichour AN, Aichour I (2017) Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16, 877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alzheimer’s Association (2019) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15, 321–387. [Google Scholar]
- [4].Prince M, Albanese E, Guerchet M, Prina M (2014) World Alzheimer Report 2014. Dementia and Risk Reduction: An Analysis of Protective and Modifiable Factors. Alzheimer’s Disease International, London, UK. [Google Scholar]
- [5].Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H, Fratiglioni L, Frisoni GB, Gauthier S, Georges J, Graff C, Iqbal K, Jessen F, Johansson G, Jonsson L, Kivipelto M, Knapp M, Mangialasche F, Melis R, Nordberg A, Rikkert MO, Qiu C, Sakmar TP, Scheltens P, Schneider LS, Sperling R, Tjernberg LO, Waldemar G, Wimo A, Zetterberg H (2016) Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol 15, 455–532. [DOI] [PubMed] [Google Scholar]
- [6].Pickett J, Bird C, Ballard C, Banerjee S, Brayne C, Cowan K, Clare L, Comas-Herrera A, Corner L, Daley S, Knapp M, Lafortune L, Livingston G, Manthorpe J, Marchant N, Moriarty J, Robinson L, van Lynden C, Windle G, Woods B, Gray K, Walton C (2018) A roadmap to advance dementia research in prevention, diagnosis, intervention, and care by 2025. Int J Geriatr Psychiatry 33, 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kulmala J, Ngandu T, Kivipelto M (2018) Prevention matters: Time for global action and effective implementation. J Alzheimers Dis 64, S191–S198. [DOI] [PubMed] [Google Scholar]
- [8].Imtiaz B, Tolppanen AM, Kivipelto M, Soininen H (2014) Future directions in Alzheimer’s disease from risk factors to prevention. Biochem Pharmacol 88, 661–670. [DOI] [PubMed] [Google Scholar]
- [9].Kivipelto M, Mangialasche F, Ngandu T (2018) Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 14, 653–666. [DOI] [PubMed] [Google Scholar]
- [10].Brodaty H, Heffernan M, Fiatarone Singh MA, Valenzuela M, Andrews G, Lautenschlager NT, Anstey KJ, Maeder A, Jorm LR, McNeil J (2017) Maintain your brain: A randomised controlled trial of an internet-based multicomponent lifestyle intervention to prevent cognitive decline and dementia. Alzheimers Dement 13, P1216–P1216. [Google Scholar]
- [11].Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S, European Prevention of Alzheimer’s Dementia (EPAD) Consortium (2016) Development of interventions for the secondary prevention of Alzheimer’s dementia: The European Prevention of Alzheimer’s Dementia (EPAD) project. Lancet Psychiatry 3, 179–186. [DOI] [PubMed] [Google Scholar]
- [12].Bott N, Kumar S, Krebs C, Glenn JM, Madero EN, Juusola JL (2018) A remote intervention to prevent or delay cognitive impairment in older adults: Design, recruitment, and baseline characteristics of the Virtual Cognitive Health (VC Health) Study. JMIR Res Protocols 7, e11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arthritis Foundation (2018) Arthritis Statistics and Facts - Book of Trusted Facts & Figures. https://www.arthritis.org/about-arthritis/understanding-arthritis/arthritis-statistics-facts.php
- [14].Maiese K (2016) Picking a bone with WISP1 (CCN4): New strategies against degenerative joint disease. J Transl Med 1, 83–85. [PMC free article] [PubMed] [Google Scholar]
- [15].Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi M, Bruyere O, Guillemin F, Hochberg MC, Hunter DJ, Kanis JA, Kvien TK, Laslop A, Pelletier J-P, Pinto D, Reiter-Niesert S, Rizzoli R, Rovati LC, Severens JLH, Silverman S, Tsouderos Y, Tugwell P, Reginster J-Y (2013) Health economics in the field of osteoarthritis: An expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 43, 303–313. [DOI] [PubMed] [Google Scholar]
- [16].Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L (2014) The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann Rheum Dis 73, 1323–1330. [DOI] [PubMed] [Google Scholar]
- [17].Barbour KE, Helmick CG, Boring M, Brady TJ (2017) Vital Signs: Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2013-2015. MMWR Morb Mortal Wkly Rep 66, 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao X, Shah D, Gandhi K, Wei W, Dwibedi N, Webster L, Sambamoorthi U (2019) Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthritis Cartilage 27, 1618–1626. [DOI] [PubMed] [Google Scholar]
- [19].Palazzo C, Nguyen C, Lefevre-Colau M-M, Rannou F, Poiraudeau S (2016) Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med 59, 134–138. [DOI] [PubMed] [Google Scholar]
- [20].Ma VY, Chan L, Carruthers KJ (2014) Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 95, 986–995.e981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xie F, Kovic B, Jin X, He X, Wang M, Silvestre C (2016) Economic and humanistic burden of osteoarthritis: A systematic review of large sample studies. Pharma-coeconomics 34, 1087–1100. [DOI] [PubMed] [Google Scholar]
- [22].Zhao X, Shah D, Gandhi K, Wei W, Dwibedi N, Webster L, Sambamoorthi U (2018) Clinical, humanistic, and economic burden of osteoarthritis (OA) among noninstitutionalized adults in the United States (US). Value Health 21, S4–S4. [DOI] [PubMed] [Google Scholar]
- [23].Hawker GA, Croxford R, Bierman AS, Harvey PJ, Ravi B, Stanaitis I, Lipscombe LL (2014) All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: A population based cohort study. PloS One 9, e91286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kendzerska T, Juni P, King LK, Croxford R, Stanaitis I, Hawker GA (2017) The longitudinal relationship between hand, hip and knee osteoarthritis and cardiovascular events: A population-based cohort study. Osteoarthritis Cartilage 25, 1771–1780. [DOI] [PubMed] [Google Scholar]
- [25].Hoops ML, Rosenblatt NJ, Hurt CP, Crenshaw J, Grabiner MD (2012) Does lower extremity osteoarthritis exacerbate risk factors for falls in older adults? Womens Health (Lond) 8, 685–696; quiz 697–688. [DOI] [PubMed] [Google Scholar]
- [26].Hunter DJ, Schofield D, Callander E (2014) The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol 10, 437–441. [DOI] [PubMed] [Google Scholar]
- [27].Nuesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Juni P (2011) All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 342, d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sharif B, Garner R, Hennessy D, Sanmartin C, Flanagan WM, Marshall DA (2017) Productivity costs of work loss associated with osteoarthritis in Canada from 2010 to 2031. Osteoarthritis Cartilage 25, 249–258. [DOI] [PubMed] [Google Scholar]
- [29].Parmelee PA, Tighe CA, Dautovich ND (2015) Sleep disturbance in osteoarthritis: Linkages with pain, disability, and depressive symptoms. Arthritis Care Res (Hoboken) 67, 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Harris ML (2016) Psychological factors in arthritis: Cause or consequence? In Psychosocial Factors in Arthritis, Nicassio PM, ed. Springer International Publishing, Switzerland, pp. 53–77. [Google Scholar]
- [31].Pickering M-E, Chapurlat R, Kocher L, Peter-Derex L (2016) Sleep disturbances and osteoarthritis. Pain Pract 16, 237–244. [DOI] [PubMed] [Google Scholar]
- [32].Innes KE, Sambamoorthi U (2018) The association of perceived memory loss with osteoarthritis and related joint pain in a large Appalachian population. Pain Med 9, 1340–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ikram M, Innes K, Sambamoorthi U (2019) Association of osteoarthritis and pain with Alzheimer’s diseases and related dementias among older adults in the United States. Osteoarthritis Cartilage 27, 1470–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ikram M, Innes K, Sambamoorthi U (2019) Association of osteoarthritis and pain with Alzheimer’s diseases and related dementias among older adults in the United States. Value Health 22, S255–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang S-W, Wang W-T, Chou L-C, Liao C-D, Liou T-H, Lin H-W (2015) Osteoarthritis increases the risk of dementia: A nationwide cohort study in Taiwan. Sci Rep 5, 10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen KT, Chen YC, Fan YH, Lin WX, Lin WC, Wang YH, Lin L, Chiou JY, Wei JC (2018) Rheumatic diseases are associated with a higher risk of dementia: A nation-wide, population-based, case-control study. Int J Rheum Dis 21, 373–380. [DOI] [PubMed] [Google Scholar]
- [37].Adler GS (1994) A profile of the Medicare Current Beneficiary Survey. Health Care Financ Rev 15, 153–163. [PMC free article] [PubMed] [Google Scholar]
- [38].Centers for Medicare & Medicaid Services (CMS) (2018) 2015 Medicare Current Beneficiary Survey (MCBS) Methodology Report. Office of Enterprise Data and Analytics (OEDA), Baltimore, MD, pp. 134. [Google Scholar]
- [39].Centers for Medicare & Medicaid Services (CMS) (2019) Medicare Current Beneficiary Survey (MCBS). Office of Enterprise Data and Analytics (OEDA), Baltimore, MD. [Google Scholar]
- [40].Briesacher BA, Tjia J, Doubeni CA, Chen Y, Rao SR (2012) Methodological issues in using multiple years of the Medicare current beneficiary survey. Medicare Medicaid Res Rev 2, 002.001.a004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ciol MA, Hoffman JM, Dudgeon BJ, Shumway-Cook A, Yorkston KM, Chan L (2006) Understanding the use of weights in the analysis of data from multistage surveys. Arch Phys Med Rehabil 87, 299–303. [DOI] [PubMed] [Google Scholar]
- [42].Schussler-Fiorenza Rose SM, Xie D, Streim JE, Pan Q, Kwong PL, Stineman MG (2016) Identifying neuropsychiatric disorders in the Medicare Current Beneficiary Survey: The benefits of combining health survey and claims data. BMC Health Serv Res 16, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Centers for Medicare and Medicaid Services (2019) Chronic Conditions Data Warehouse. https://www2.ccwdata.org/web/guest/condition-categories
- [44].Lin PJ, Kaufer DI, Maciejewski ML, Ganguly R, Paul JE, Biddle AK (2010) An examination of Alzheimer’s disease case definitions using Medicare claims and survey data. Alzheimers Dement 6, 334–341. [DOI] [PubMed] [Google Scholar]
- [45].Tzeng NS, Chung CH, Liu FC, Chiu YH, Chang HA, Yeh CB, Huang SY, Lu RB, Yeh HW, Kao YC, Chiang WS, Tsao CH, Wu YF, Chou YC, Lin FH, Chien WC (2018) Fibromyalgia and risk of dementia-a nationwide, population-based, cohort study. Am J Med Sci 355, 153–161. [DOI] [PubMed] [Google Scholar]
- [46].Yang FC, Lin TY, Chen HJ, Lee JT, Lin CC, Kao CH (2016) Increased risk of dementia in patients with tension-type headache: A nationwide retrospective population-based cohort study. PLoS One 11, e0156097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tzeng N-S, Chung C-H, Lin F-H, Yeh C-B, Huang S-Y, Lu R-B, Chang H-A, Kao Y-C, Chiang W-S, Chou Y-C, Tsao C-H, Wu Y-F, Chien W-C (2017) Headaches and risk of dementia. Am J Med Sci 353, 197–206. [DOI] [PubMed] [Google Scholar]
- [48].Chuang C-S, Lin C-L, Lin M-C, Sung F-C, Kao C-H (2013) Migraine and risk of dementia: A nationwide retrospective cohort study. Neuroepidemiology 41, 139–145. [DOI] [PubMed] [Google Scholar]
- [49].Røttereng AKS, Bosnes O, Stordal E, Zwart J-A, Linde M, Stovner LJ, Hagen K (2015) Headache as a predictor for dementia: The HUNT Study. J Headache Pain 16, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hagen K, Stordal E, Linde M, Steiner TJ, Zwart J-A, Stovner LJ (2014) Headache as a risk factor for dementia: A prospective population-based study. Cephalalgia 34, 327–335. [DOI] [PubMed] [Google Scholar]
- [51].Whitlock EL, Diaz-Ramirez LG, Glymour MM, Boscardin WJ, Covinsky KE, Smith AK (2017) Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern Med 177, 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ezzati A, Wang C, Zammit A, Katz M, Zimmerman M, Derby C, Lipton R (2017) The temporal relationship between pain intensity and interference and incident dementia: A community-based prospective study Neurology 88, Supplement P3.092. [Google Scholar]
- [53].van der Leeuw G, Ayers E, Leveille SG, Blankenstein AH, van der Horst HE, Verghese J (2018) The effect of pain on major cognitive impairment in older adults. J Pain 19, 1435–1444. [DOI] [PubMed] [Google Scholar]
- [54].Veronese N, Koyanagi A, Solmi M, Thompson T, Maggi S, Schofield P, Mueller C, Gale CR, Cooper C, Stubbs B (2018) Pain is not associated with cognitive decline in older adults: A four-year longitudinal study. Maturitas 115, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ezzati A, Wang C, Katz MJ, Derby CA, Zammit AR, Zimmerman ME, Pavlovic JM, Sliwinski MJ, Lipton RB (2019) The temporal relationship between pain intensity and pain interference and incident dementia. Curr Alzheimer Res 16, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dautovich ND, Parmelee PA, Tighe CA (2015) Breaking the cycle: Sleep disturbance as a target for remedying the ‘cycle of distress’ in osteoarthritis. Int J Clin Rheumtol 10, 127–129. [Google Scholar]
- [57].Smith MT, Quartana PJ, Okonkwo RM, Nasir A (2009) Mechanisms by which sleep disturbance contributes to osteoarthritis pain: A conceptual model. Curr Pain Headache Rep 13, 447–454. [DOI] [PubMed] [Google Scholar]
- [58].Vitiello MV, McCurry SM, Shortreed SM, Baker LD, Rybarczyk BD, Keefe FJ, Von Korff M (2014) Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain 155, 1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Helminen E-E, Sinikallio SH, Valjakka AL, Vaisanen-Rouvali RH, Arokoski JP (2016) Determinants of pain and functioning in knee osteoarthritis: A one-year prospective study. Clin Rehabil 30, 890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tak SH, Laffrey SC (2003) Life satisfaction and its correlates in older women with osteoarthritis. Orthop Nurs 22, 182–189. [DOI] [PubMed] [Google Scholar]
- [61].Astin JA (2004) Mind-body therapies for the management of pain. Clin J Pain 20, 27–32. [DOI] [PubMed] [Google Scholar]
- [62].Zullig LL, Bosworth HB, Jeffreys AS, Corsino L, Coffman CJ, Oddone EZ, Yancy WS Jr, Allen KD (2015) The association of comorbid conditions with patient-reported outcomes in Veterans with hip and knee osteoarthritis. Clin Rheumatol 34, 1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Axford J, Butt A, Heron C, Hammond J, Morgan J, Alavi A, Bolton J, Bland M (2010) Prevalence of anxiety and depression in osteoarthritis: Use of the Hospital Anxiety and Depression Scale as a screening tool. Clin Rheumatol 29, 1277–1283. [DOI] [PubMed] [Google Scholar]
- [64].Hawker GA, Gignac MAM, Badley E, Davis AM, French MR, Li Y, Perruccio AV, Power JD, Sale J, Lou W (2011) A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care Res 63, 1382–1390. [DOI] [PubMed] [Google Scholar]
- [65].Stebbings S, Herbison P, Doyle TC, Trehame GJ, Highton J (2010) A comparison of fatigue correlates in rheumatoid arthritis and osteoarthritis: Disparity in associations with disability, anxiety and sleep disturbance. Rheumatology (Oxford) 49,361–367. [DOI] [PubMed] [Google Scholar]
- [66].Allen KD, Renner JB, Devellis B, Helmick CG, Jordan JM (2008) Osteoarthritis and sleep: The Johnston County Osteoarthritis Project. J Rheumatol 35, 1102–1107. [PMC free article] [PubMed] [Google Scholar]
- [67].Stubbs B, Aluko Y, Myint PK, Smith TO (2016) Prevalence of depressive symptoms and anxiety in osteoarthritis: A systematic review and meta-analysis. Age Ageing 45, 228–235. [DOI] [PubMed] [Google Scholar]
- [68].Küçükşen S, Yilmaz H, Karahan AY, Bağçaci SJCMR (2014) The prevalence of depression and its relevance to clinical and radiological characteristics among older adults with knee osteoarthritis. Clin Med Res 2, 25–30. [Google Scholar]
- [69].McCurry SM, Von Korff M, Vitiello MV, Saunders K, Balderson BH, Moore AL, Rybarczyk BD (2011) Frequency of comorbid insomnia, pain, and depression in older adults with osteoarthritis: Predictors of enrollment in a randomized treatment trial. J Psychosom Res 71, 296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Blixen CE, Kippes C (1999) Depression, social support, and quality of life in older adults with osteoarthritis. Image JNursSch 31,221–226. [DOI] [PubMed] [Google Scholar]
- [71].Moldofsky H (2010) Rheumatic manifestations of sleep disorders. Curr Opin Rheumatol 22, 59–63. [DOI] [PubMed] [Google Scholar]
- [72].Simons LE, Elman I, Borsook D (2014) Psychological processing in chronic pain: A neural systems approach. Neurosci Biobehav Rev 39, 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kroenke K, Wu JW, Bair MJ, Krebs EE, Damush TM, Tu WZ (2011) Reciprocal relationship between pain and depression: A 12-month longitudinal analysis in primary care. J Pain 12, 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Okifuji A, Hare BD (2011) Do sleep disorders contribute to pain sensitivity? Curr Rheumatol Rep 13, 528–534. [DOI] [PubMed] [Google Scholar]
- [75].Koffel E, Kroenke K, Bair MJ, Leverty D, Polusny MA, Krebs EE (2016) The bidirectional relationship between sleep complaints and pain: Analysis of data from a randomized trial. Health Psychol 35, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Karaman S, Karaman T, Dogru S, Onder Y, Citil R, Bulut YE, Tapar H, Sahin A, Arici S, Kaya Z, Suren M (2014) Prevalence of sleep disturbance in chronic pain. Eur Rev Med Pharmacol Sci 18, 2475–2481. [PubMed] [Google Scholar]
- [77].Lopresti AL, Hood SD, Drummond PD (2013) A review of lifestyle factors that contribute to important pathways associated with major depression: Diet, sleep and exercise. J Affect Disord 148, 12–27. [DOI] [PubMed] [Google Scholar]
- [78].Alvaro PK, Roberts RM, Harris JK (2013) A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep 36, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dominick CH, Blyth FM, Nicholas MK (2012) Unpacking the burden: Understanding the relationships between chronic pain and comorbidity in the general population. Pain 153, 293–304. [DOI] [PubMed] [Google Scholar]
- [80].Jordan KD, Okifuji A (2011) Anxiety disorders: Differential diagnosis and their relationship to chronic pain. J Pain Palliat Care Pharmacother 25, 231–245. [DOI] [PubMed] [Google Scholar]
- [81].Arola H-M, Nicholls E, Mallen C, Thomas E (2010) Self-reported pain interference and symptoms of anxiety and depression in community-dwelling older adults: Can a temporal relationship be determined? Eur J Pain 14, 966–971. [DOI] [PubMed] [Google Scholar]
- [82].Marin MF, Lord C, Andrews J, Juster RP, Sindi S, Arsenault-Lapierre G, Fiocco AJ, Lupien SJ (2011) Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem 96, 583–595. [DOI] [PubMed] [Google Scholar]
- [83].Chodosh J, Kado DM, Seeman TE, Karlamangla AS (2007) Depressive symptoms as a predictor of cognitive decline: MacArthur studies of successful aging. Am J Geriatr Psychiatry 15, 406–415. [DOI] [PubMed] [Google Scholar]
- [84].Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, Hyman BT, Locascio JJ, Johnson KA, Sperling RA, Marshall GA, Rentz DM (2014) Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry 22, 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Keage HAD, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE (2012) What sleep characteristics predict cognitive decline in the elderly? Sleep Med 13, 886–892. [DOI] [PubMed] [Google Scholar]
- [86].Blackwell T, Yaffe K, Ancoli-Israel S, Scheider JL, Cauley JA, Hillier TA, Fink HA, Stone KL, Study of Osteoporotic Fractures Group (2006) Poor sleep is associated with impaired cognitive function in older women: The Study of Osteoporotic Fractures. J Gerontol A Biol Sci Med Sci 61, 405–410. [DOI] [PubMed] [Google Scholar]
- [87].Rasch B, Born J (2013) About sleep’s role in memory. Physiol Rev 93, 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yaffe K, Falvey CM, Hoang T (2014) Connections between sleep and cognition in older adults. Lancet Neurol 13, 1017–1028. [DOI] [PubMed] [Google Scholar]
- [89].Conrad CD (2010) A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsy-chopharmacol Biol Psychiatry 34, 742–755. [DOI] [PubMed] [Google Scholar]
- [90].Rock PL, Roiser JP, Riedel WJ, Blackwell AD (2014) Cognitive impairment in depression: A systematic review and meta-analysis. Psychol Med 44, 2029–2040. [DOI] [PubMed] [Google Scholar]
- [91].Killgore WDS (2010) Effects of sleep deprivation on cognition. Prog Brain Res 185, 105–129. [DOI] [PubMed] [Google Scholar]
- [92].Zhao C, Noble JM, Marder K, Hartman JS, Gu Y, Scarmeas N (2018) Dietary patterns, physical activity, sleep, and risk for dementia and cognitive decline. Curr Nutr Rep 7, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, Shi J, Vitiello MV, Lu L (2018) Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev 40, 4–16. [DOI] [PubMed] [Google Scholar]
- [94].Higgins DM, Martin AM, Baker DG, Vasterling JJ, Ris-brough V (2018) The relationship between chronic pain and neurocognitive function a systematic review. Clin J Pain 34, 262–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Moriarty O, McGuire BE, Finn DP (2011) The effect of pain on cognitive function: A review of clinical and preclinical research. Prog Neurobiol 93, 385–404. [DOI] [PubMed] [Google Scholar]
- [96].Seminowicz DA, Ceko M (2015) Can we exploit cognitive brain networks to treat chronic pain? Pain Manag 5, 399–402. [DOI] [PubMed] [Google Scholar]
- [97].Apkarian AV, Hashmi JA, Baliki MN (2011) Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 152, S49–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ivo R, Nicklas A, Dargel J, Sobottke R, Delank K-S, Eysel P, Weber B (2013) Brain structural and psychometric alterations in chronic low back pain. Eur Spine J 22, 1958–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DM (2014) Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. Neuroimage Clin 4, 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Vachon-Presseau E, Roy M, Martel M-O, Caron E, Marin M-F, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P (2013) The stress model of chronic pain: Evidence from basal cortisol and hippocampal structure and function in humans. Brain 136, 815–827. [DOI] [PubMed] [Google Scholar]
- [101].Innes KE, Selfe TK, Kandati S, Wen S, Huysmans Z(2018) Effects of mantra meditation versus music listening on knee pain, function, and related outcomes in older adults with knee osteoarthritis: An exploratory randomized clinical trial (RCT). Evid Based Complement Alternat Med 2018, 7683897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Sofat N, Ejindu V, Kiely P (2011) What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatology 50, 2157–2165. [DOI] [PubMed] [Google Scholar]
- [103].Lluch E, Torres R, Nijs J, Van Oosterwijck J (2014) Evidence for central sensitization in patients with osteoarthritis pain: A systematic literature review. Eur J Pain 18, 1367–1375. [DOI] [PubMed] [Google Scholar]
- [104].Baker KS, Georgiou-Karistianis N, Gibson SJ, Giummarra MJ (2017) Optimizing cognitive function in persons with chronic pain. Clin J Pain 33, 462–472. [DOI] [PubMed] [Google Scholar]
- [105].Al-Khazraji BK, Appleton CT, Beier F, Birmingham TB, Shoemaker JK (2018) Osteoarthritis, cerebrovascular dysfunction and the common denominator of inflammation: A narrative review. Osteoarthritis Cartilage 26, 462–470. [DOI] [PubMed] [Google Scholar]
- [106].Schaible H-G (2012) Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep 14, 549–556. [DOI] [PubMed] [Google Scholar]
- [107].Weber A, Mak SH, Berenbaum F, Sellam J, Zheng Y-P, Han Y, Wen C (2019) Association between osteoarthritis and increased risk of dementia: A systemic review and meta-analysis. Medicine (Baltimore) 98, e14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J (2018) A comprehensive review of nonsteroidal anti-inflammatory drug use in the elderly. Aging Dis 9, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhang CX, Wang Y, Wang DY, Zhang JD,Zhang FF (2018) NSAID exposure and risk of Alzheimer’s disease: An updated meta-analysis from cohort studies. Front Aging Neurosci 10, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Fink HA, Jutkowitz E, McCarten JR, Hemmy LS, Butler M, Davila H, Ratner E, Calvert C, Barclay TR, Brasure M, Nelson VA, Kane RL (2018) Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: A systematic review. Ann Intern Med 168, 39–51. [DOI] [PubMed] [Google Scholar]
- [111].Kuzma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ (2018) Stroke and dementia risk: A systematic review and meta-analysis. Alzheimers Dement 14, 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11, 718–726. [DOI] [PubMed] [Google Scholar]
- [113].Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, Tian G (2017) Head injury as a risk factor for dementia and Alzheimer’s disease: A systematic review and meta-analysis of 32 observational studies. PLoS One 12, e0169650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Cherbuin N, Kim S, Anstey KJ (2015) Dementia risk estimates associated with measures of depression: A systematic review and meta-analysis. BMJ Open 5, e008853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gimson A, Schlosser M, Huntley JD, Marchant NL (2018) Support for midlife anxiety diagnosis as an independent risk factor for dementia: A systematic review. BMJ Open 8, e019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gulpers B, Ramakers I, Hamel R, Kohler S, Oude Voshaar RC, Verhey F (2016) Anxiety as a predictor for cognitive decline and dementia: A systematic review and meta-analysis. Am J Geriatr Psychiatry 24, 823–842. [DOI] [PubMed] [Google Scholar]
- [117].Albanese E, Launer LJ, Egger M, Prince MJ, Giannakopoulos P, Wolters FJ, Egan K (2017) Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst) 8, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Emmerzaal TL, Kiliaan AJ, Gustafson DR (2015) 2003–2013: A decade of body mass index, Alzheimer’s disease, and dementia. J Alzheimers Dis 43, 739–755. [DOI] [PubMed] [Google Scholar]
- [119].Ghaderpanahi M, Sharifi F, Mirarefin M, Badamchizade Z, Larijani B, Fakhrzadeh H, Fakhrzadeh H, Sharifi F (2012) Association between late-life body mass index, waist circumference, and dementia: Kahrizak Elderly Study. J Am GeriatrSoc 60, 173–174. [DOI] [PubMed] [Google Scholar]
- [120].Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB (2009) Association between late-life body mass index and dementia The Kame Project. Neurology 72, 1741–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kivimaki M, Luukkonen R, Batty GD, Ferrie JE, Pentti J, Nyberg ST, Shipley MJ, Alfredsson L, Fransson EI, Goldberg M, Knutsson A, Koskenvuo M, Kuosma E, Nordin M, Suominen SB, Theorell T, Vuoksimaa E, Westerholm P, Westerlund H, Zins M, Kivipelto M, Vahtera J, Kaprio J, Singh-Manoux A, Jokela M (2018) Body mass index and risk of dementia: Analysis of individual-level data from 1.3 million individuals. Alzheimers Dement 14, 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Verlinden VJ, van der Geest JN, de Bruijn RF, Hofman A, Koudstaal PJ, Ikram MA (2015) Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimers Dement 12, 144–153. [DOI] [PubMed] [Google Scholar]
- [123].Lang LD, Clifford A, Wei L, Zhang DM, Leung D, Augustine G, Danat IM, Zhou WJ, Copeland JR, Anstey KJ, Chen RL (2017) Prevalence and determinants of undetected dementia in the community: A systematic literature review and a meta-analysis. BMJ Open 7, e011146.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Koller D, Bynum JPW (2015)DementiaintheUSA: State variation in prevalence. J Public Health 37, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Macfarlane GJ, Barnish MS, Jones GT (2017) Persons with chronic widespread pain experience excess mortality: Longitudinal results from UK Biobank and meta-analysis. Ann Rheum Dis 76, 1815–1822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


