Abstract
Purpose:
Two modern methods of reirradiation, intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT), are established for patients with recurrent or second primary squamous cell carcinoma of the head and neck (rSCCHN). We performed a retrospective multi-institutional analysis to compare methods.
Methods and Materials:
Data from patients with unresectable rSCCHN previously irradiated to ≥40 Gy who underwent reirradiation with IMRT or SBRT were collected from 8 institutions. First, the prognostic value of our IMRT-based recursive partitioning analysis (RPA) separating those patients with unresectable tumors with an intertreatment interval >2 years or those with ≤2 years and without feeding tube or tracheostomy dependence (class II) from other patients with unresected tumors (class III) was investigated among SBRT patients. Overall survival (OS) and locoregional failure were then compared between IMRT and SBRT by use of 2 methods to control for baseline differences: Cox regression weighted by the inverse probability of treatment and subset analysis by RPA classification.
Results:
The study included 414 patients with unresectable rSCCHN: 217 with IMRT and 197 with SBRT. The unadjusted 2-year OS rate was 35.4% for IMRT and 16.3% for SBRT (P<.01). Among SBRT patients, RPA classification retained an independent association with OS. On Cox regression weighted by the inverse probability of treatment, no significant differences in OS or locoregional failure between IMRT and SBRT were demonstrated. Analysis by RPA class showed similar OS between IMRT and SBRT for class III patients. In all class II patients, IMRT was associated with improved OS (P<.001). Further subset analysis demonstrated comparable OS when ≥35 Gy was delivered with SBRT to small tumor volumes. Acute grade ≥4 toxicity was greater in the IMRT group than in the SBRT group (5.1% vs 0.5%, P<.01), with no significant difference in late toxicity.
Conclusions:
Reirradiation both with SBRT and with IMRT appear relatively safe with favorable toxicity compared with historical studies. Outcomes vary by RPA class, which informs clinical trial design. Survival is poor in class III patients, and alternative strategies are needed.
Summary
In a multi-institutional comparative-effectiveness analysis of definitive reirradiation of locally recurrent or second primary head and neck cancers, stereotactic body radiation therapy and intensity modulated radiation therapy both appear relatively safe with favorable toxicity compared with historical studies. Outcomes vary by recursive partitioning analysis class, which informs clinical trial design. Survival is poor in class III patients, and alternative strategies are needed.
Introduction
Unresectable locoregionally recurrent or second primary squamous cell carcinoma of the head and neck (rSCCHN) that was previously irradiated represents a challenging clinical scenario for which concerns of excessive toxicity and limited survival historically restricted reirradiation application. Pioneering work from Radiation Therapy Oncology Group (RTOG) 9610 and 9911 challenged this paradigm, with conventional hyperfractionated reirradiation plus systemic therapy achieving long-term (2-year) survival in 15% to 26% of rSCCHN cases (1, 2). Despite this, the conventional reirradiation techniques used in RTOG 9610 and 9911 resulted in grade ≥3 acute and late toxicity rates of 63% to 78% and 22% to 37%, respectively, that tempered enthusiasm for reirradiation, especially considering that the median overall survival (OS) in these trials only marginally exceeded that of chemotherapy alone (1-3). Integral to widening the therapeutic ratio of reirradiation for rSCCHN, recent advances in engineering and radiation treatment planning have revolutionized the ability to spare normal tissues and confine radiation dose to the target volume. Numerous reports incorporating modern reirradiation techniques using intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) have been published suggesting that these techniques may improve tumor control, decrease toxicity, and improve quality of life in patients with rSCCHN (4-13).
Patient selection for IMRT versus SBRT is challenging as stark differences exist in the radiobiology, dose per fraction (1.2-2 Gy/fraction vs 6-10 Gy/fraction), overall treatment time (6-8 weeks vs 1-2 weeks), and target definition. Our concurrently reported recursive partitioning analysis (RPA) of patients re-treated with IMRT may help guide treatment selection between the 2 techniques. The RPA is presented in Figure E1 (available online at www.redjournal.org) and consists of 3 distinct and homogeneous classes: class I comprises patients >2 years from the first treatment with resected tumors; class II, those >2 years from the first treatment with unresected tumors or <2 years from the first treatment without organ dysfunction (defined as pretreatment feeding tube or tracheostomy use); and class III, those ≤2 years from the previous course with organ dysfunction (14-16). Whether this system retains prognostic significance in patients treated with SBRT is unclear. Our goals therefore were to perform a multi-institutional analysis to evaluate the RPA’s utility in guiding treatment selection and then to compare survival and toxicity after reirradiation with IMRT or SBRT.
Methods and Materials
Patients, treatment, and follow-up
Following institutional review board approval, a multi-institutional data use agreement was initiated between 9 US academic medical centers, 8 of which contributed to this analysis. The multi-institutional reirradiation consortium included University of Alabama Birmingham, which participated as a full member of the consortium but joined after this initial analysis was completed. A collaborative comprehensive database was assembled to include retrospectively collected data for patients undergoing reirradiation for rSCCHN within a field previously irradiated to ≥40 Gy and then reirradiated with either IMRT to ≥40 Gy or SBRT delivered in 1 to 5 fractions of ≥5 Gy/fraction. The exclusion criteria included patients with nonsquamous histology, concurrent metastatic disease, or unrecorded radiation dose. A CONSORT (Consolidated Standards of Reporting Trials) diagram describing patient selection is shown in Figure E2 (available online at www.redjournal.org). Because this report focuses on definitive reirradiation, patients receiving postoperative reirradiation following definitive salvage surgery were excluded.
Statistical analysis
First, baseline characteristics were tabulated and compared between the IMRT and SBRT cohorts. Demographic characteristics and treatment factors were compared by use of the Wilcoxon rank sum test for continuous variables and the Fisher exact test or Pearson χ2 test for categorical variables where appropriate.
Second, the independent prognostic utility of the concurrently reported RPA classification developed among IMRT patients (Fig. E1; available online at www.redjournal.org) was assessed by use of a mixed-effects multivariate Cox proportional hazards regression model for OS (14). Random effects were modeled within each multivariate Cox regression model to account for potential unobserved baseline differences in patient selection by institution.
Third, OS was compared between the IMRT and SBRT cohorts. Two methods were used to control for baseline cohort differences: (1) Cox proportional hazards regression weighted by the stabilized inverse probability of treatment; and (2) subset analysis by RPA classification. In the RPA class II patients, additional subset analysis by dose and tumor volume was performed given previous literature demonstrating the prognostic utility of these factors in the SBRT population (17). For this subset, small tumors were considered those with a gross tumor volume (GTV) ≤25 cm3 based on the previously described cut point demonstrated in the SBRT literature (17). Because GTV was not available in all patients, when GTV was missing, the recurrent American Joint Committee on Cancer T classification was used as a surrogate to classify tumor bulk, with rT0 to rT2 tumors characterized as “small” and rT3 to rT4 characterized as “large.” The rT classification was used over the rN classification because a statistically significant association between GTV and rT stage was demonstrated in patients with both known factors (Spearman ρ = 0.464 and P<.001 for rT classification and Spearman ρ = 0.174 and P=.057 for rN classification).
A secondary endpoint, the cumulative incidence of locoregional failure (LRF), was defined as any disease recurrence, persistence, or progression documented on physical examination, imaging, or biopsy and was calculated by use of the Gray competing-risk model with death considered a competing risk. Differences for competing-risk models were compared by the Gray test (18).
Acute and late toxicity
Physician-reported toxicity was measured according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Acute toxicity was considered to occur within 90 days of the end of reirradiation. Pre-existing use of a tracheostomy or feeding tube was defined as pre-existing organ dysfunction; continued use was not considered toxicity. Particular attention was given to the following acute events: admission to the hospital for aspiration pneumonia, new tracheostomy use, new feeding tube placement, esophageal stricture dilation, neutropenic fever, and soft tissue necrosis.
Late toxicity was considered an event occurring ≥90 days beyond the end of radiation therapy. CTCAE grade ≥3 events specifically investigated included osteor-adionecrosis, aspiration pneumonia, esophageal stricture, carotid blowout syndrome, and fistula. Feeding tube and tracheostomy dependence beyond 1 year in the absence of disease was also considered late toxicity. Carotid blowout syndrome was defined as arterial bleeding from the head or neck in the absence of uncontrolled locoregional disease.
The crude rate of acute toxicity was compared between the SBRT and IMRT cohorts, and differences were assessed with the Pearson χ2 test. Conversely, a time-to-event analysis was used to calculate the cumulative incidence of late toxicity. This method was used because the issues of censoring (loss to follow-up) and competing risks (death or LRF) become relevant when assessing a time-dependent endpoint (as opposed to acute toxicity, in which all patients are at risk). The cumulative incidence of late toxicity was compared by use of the Gray test. For reference, the cumulative incidence was also calculated via the Kaplan-Meier method with censoring performed at the date of last clinical follow-up, recurrence, or death. Statistical analysis was performed with the R package (version 3.2.3; R Foundation for Statistical Computing, Vienna, Austria). We considered α < .05 significant for all analyses.
Results
Patients, treatment, and follow-up
Baseline patient characteristics by reirradiation technique are outlined in Table 1. The University of Pittsburgh, where SBRT is the favored modality, contributed 80% of the SBRT patients included.
Table 1.
Baseline characteristics by reirradiation technique
| IMRT (n=217) | SBRT (n=197) | P value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age at reirradiation, median (range), y | 64 (21-93) | 64 (39-90) | <.01 |
| Gender, n (%) | .38 | ||
| Male | 149 (69) | 143 (73) | |
| Female | 68 (31) | 54 (27) | |
| Smoking pack-years | .026 | ||
| Median (range) | 30 (0-250) | 30 (0-150) | |
| Unrecorded, n (%) | 14 (7) | 71 (36) | |
| First diagnosis | |||
| Year of original diagnosis | |||
| Median | 2003 | 2005 | |
| Range | 1972-2013 | 1954-2014 | |
| Original histology, n (%) | |||
| Squamous cell carcinoma | 205 (95) | 194 (98) | |
| Squamous variants* | 3 (1) | 2 (1) | |
| Lymphoma | 2 (1) | 0 (0) | |
| Other | 7 (3) | 1 (1) | |
| Previous surgery to primary, n (%) | |||
| Yes | 96 (44) | 106 (54) | |
| No | 120 (55) | 91 (47) | |
| Unrecorded | 1 (1) | 0 | |
| Previous neck dissection, n (%) | |||
| Yes | 73 (34) | 89 (45) | |
| No | 123 (57) | 104 (53) | |
| Unrecorded | 21 (10) | 4 (2) | |
| Previous systemic therapy, n (%) | <.01 | ||
| Yes | 99 (46) | 126 (64) | |
| No | 117 (54) | 70 (36) | |
| Unrecorded | 1 (1) | 1 (1) | |
| Previous radiation dose, median (range), Gy | 66.4 (41.4-80) | 70 (40-170.7) | <.01 |
| RPA class, n (%) | .78 | ||
| Class II | 184 (85) | 169 (86) | |
| Class III | 33 (15) | 28 (14) | |
| Second diagnosis | |||
| Tumor site, n (%) | <.01 | ||
| Oral cavity | 23 (11) | 35 (18) | |
| Oropharynx | 80 (37) | 53 (27) | |
| Larynx or hypopharynx | 36 (16) | 28 (14) | |
| Sinonasal | 7 (3) | 3 (1) | |
| Neck only | 33 (15) | 41 (21) | |
| Skin or salivary | 1 (1) | 10 (5) | |
| Nasopharynx or base of skull | 37 (17) | 27 (14) | |
| Recurrent or second primary, n (%) | <.01 | ||
| Recurrent | 158 (73) | 176 (89) | |
| Second primary | 59 (27) | 21 (11) | |
| Pre-existing organ dysfunction,† n (%) | 75 (35) | 37 (19) | <.01 |
| Gross tumor volume | .43 | ||
| Median (range), cm3 | 30 (2-515) | 30 (1-427) | |
| Unrecorded, n (%) | 101 (47) | 6 (3) | |
| Second treatment | |||
| Systemic therapy, n (%) | <.01 | ||
| Yes | 183 (84) | 108 (55) | |
| No | 34 (16) | 89 (45) | |
| Radiation dose, median (range), Gy | 60 (40-72) | 40 (16-50) | |
| No. of fractions, median (range) | 33 (12-60) | 5 (1-8) | |
| BED with α/β = 10,‡ median (range), Gy | 72 (48-86) | 72 (28-100) | .27 |
| Time between courses of radiation therapy, median (range), y | 3.1 (0.2-34) | 1.2 (0.1-35) | <.01 |
Abbreviations: BED = biologically equivalent dose; IMRT = intensity modulated radiation therapy; RPA = recursive partitioning analysis; SBRT = stereotactic body radiation therapy.
The category of squamous cell carcinoma variants includes patients with basaloid, adenosquamous, and sarcomatoid differentiation.
Organ dysfunction implies a pre-existing tracheostomy or feeding tube dependence, excluding prophylactic feeding tubes and stomas after a total laryngectomy.
Only those who completed radiation therapy are included.
Patients in the SBRT cohort tended to be older and were more heavily treated, including a higher lifetime dose of previous radiation therapy and more frequent use of chemotherapy. SBRT patients were more likely to be treated for a recurrence rather than a second primary and at a shorter time interval from the previous radiation therapy than IMRT patients. IMRT patients were more likely to have a feeding tube or tracheostomy at the time of reirradiation than SBRT patients. The use of chemotherapy was significantly more common in IMRT patients. Of the IMRT patients, 178 received a concurrent platinum-based regimen, 28 received concurrent cetuximab, 5 received induction chemotherapy alone, and 28 received induction and concurrent chemotherapy. Among SBRT patients, 105 of the 108 receiving systemic therapy received cetuximab, with the others receiving a non-platinum regimen. Acknowledging the inherent caveats of incorporating the biologically effective dose (BED10Gy) in high dose-perfraction techniques such as SBRT, we found no significant differences in the BED10Gy between the IMRT and SBRT groups. IMRT was most commonly delivered by once-daily fractionation (79%) or twice-daily hyperfractionation (20%), while SBRT was exclusively administered by every-other-day fractionation schemas. The elective neck was targeted in 28% of the IMRT patients, whereas in 96% of the SBRT patients, the target volume was gross disease only, with a small elective volume to a single adjacent nodal level added in 4%. In the IMRT cohort, 13 patients (6.0%) did not complete the prescribed dose of radiation therapy, whereas all patients in the SBRT cohort completed therapy (P<.01). The median on-treatment time was 43 days for those who completed IMRT (range, 21-77 days) and 10 days for the SBRT cohort (range, 1-38 days; P<.01).
For survivors, the median follow-up period from the start of radiation therapy was 28 months in the IMRT group (range, 2-130 months) and 24 months (range, 3-120 months) in the SBRT group (P=.50). The median follow-up period for all patients from the start of reirradiation was 8.4 months for IMRT patients (range, 0-130 months) and 7.1 months for SBRT patients (range, 1-120 months; P=.27). There were 162 total deaths in the IMRT group and 173 in the SBRT group, with uncontrolled head and neck cancer representing the most common cause of death (91% of IMRT and 84% of SBRT patients with a known cause of death).
Utility of RPA in patients treated with SBRT
To investigate the prognostic significance of the RPA classes defined by our concurrently reported manuscript among SBRT patients (14), a multivariate Cox regression was performed within the SBRT group only (Table E1; available online at www.redjournal.org). Because this manuscript investigates only patients treated nonoperatively, patients grouped into RPA class I were not included. After adjustment for potential confounders including random effects by treating institution, the RPA class remained independently associated with OS (hazard ratio [HR] for class III vs class II, 1.707; 95% confidence interval [CI], 1.099-2.653; P=.017). In addition to RPA class, we found that younger age, second primary tumor, and smaller tumor volume were independently associated with improved OS for the SBRT group. The time between radiation therapy courses and organ dysfunction was not entered into the SBRT-only model because these variables are components of the RPA classification. The variance of the random-effect term accounting for unobserved institutional bias was small (4.0 × 10−4), implying that institutional selection differences were well specified by the observed fixed effects.
Comparison of IMRT and SBRT by Cox regression weighted by inverse probability of treatment
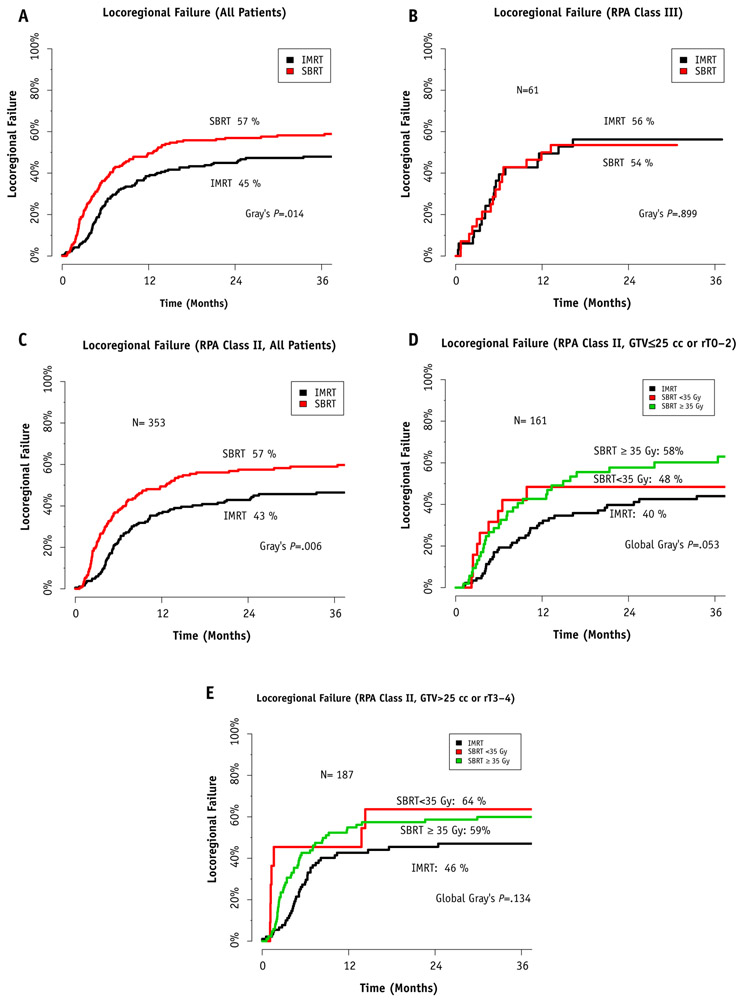
Overall, the unadjusted 2-year Kaplan-Meier estimate of OS was 35.4% (95% CI, 29.3%-42.8%) in the IMRT group and 16.3% (95% CI, 11.7%-22.7%) in the SBRT group (P<.001, log-rank test) (Fig. 1A). The corresponding median survival periods were 13.3 months (95% CI, 10.5-16.9 months) for IMRT patients and 7.8 months (95% CI, 6.8-9.8 months) for SBRT patients. The cumulative incidence of LRF when accounting for the competing risk of death in the IMRT group compared with the SBRT group was 45.4% (95% CI, 38.5%-52.3%) versus 57.0% (95% CI, 54.8%-68.6%; P=.014, Gray test) (Fig. 2A). On univariate Cox regression among all patients, the RPA demonstrated prognostic significance for OS (HR for class III vs class II, 1.914; 95% CI, 1.426-2.527; P<.0001) (Table E2; available online at www.redjournal.org). Multivariate Cox regression among all patients identified factors associated with OS and is presented in Table 2. This model also accounted for random effects by institution, and this variance was also small (1.6 × 10−5), again implying that potential institutional selection bias was well specified by the model’s fixed effects.
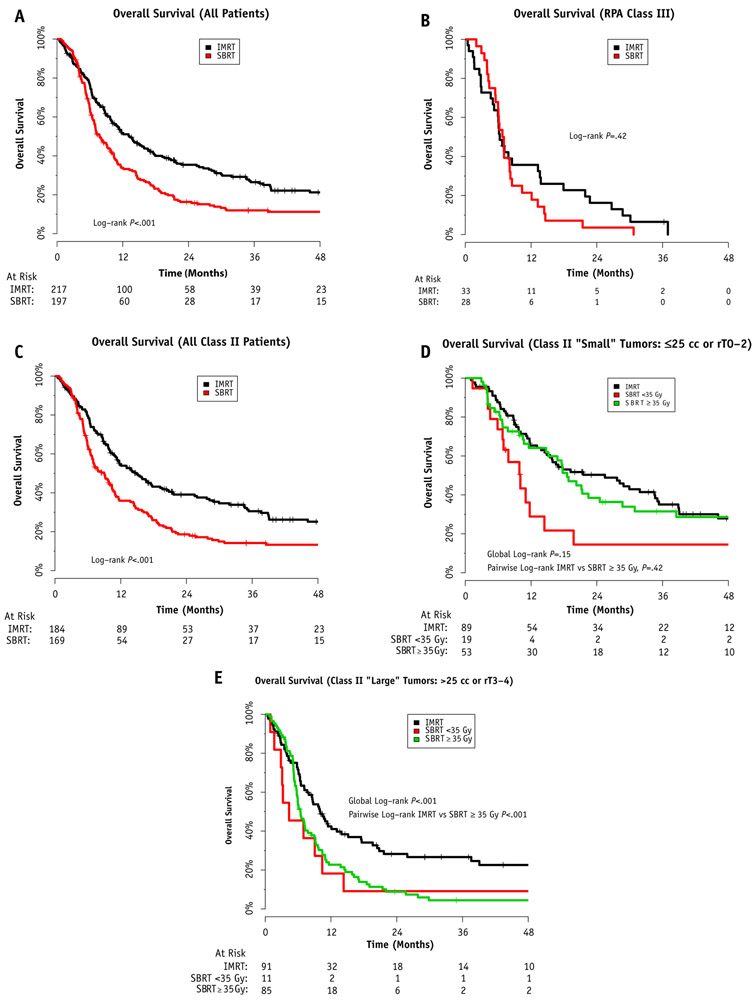
Fig. 1.
Comparative overall survival between intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) for recurrent or second primary squamous cell carcinoma of the head and neck across recursive partitioning analysis (RPA) class. (A) Overall survival for all patients. (B) RPA class III—unresectable disease with pre-existing severe airway or swallowing dysfunction. (C) All RPA class II patients. (D) RPA class II with small tumors—unresectable disease with no pre-existing severe airway or swallowing dysfunction and gross tumor volume ≤25 cm3 or rT0 to rT2. (E) RPA class II with large tumors—unresectable disease with no pre-existing severe airway or swallowing dysfunction and gross tumor volume >25 cm3 or rT3 to rT4. Results are stratified by dose.
Fig. 2.
Comparative locoregional control between intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) for recurrent or second primary squamous cell carcinoma of the head and neck across recursive partitioning analysis (RPA) class. (A) Overall locoregional control for all patients. (B) RPA class III—unresectable disease with pre-existing severe airway or swallowing dysfunction. (C) All RPA class II patients. (D) RPA class II with small tumors—unresectable disease with no pre-existing severe airway or swallowing dysfunction and gross tumor volume (GTV) ≤25 cm3 or rT0 to rT2. (E) RPA class II with large tumors—unresectable disease with no pre-existing severe airway or swallowing dysfunction and GTV >25 cm3 or rT3 to rT4. Results are stratified by dose.
Table 2.
Multivariate Cox proportional hazards regression for overall survival in all patients
| Variable | Multivariate (11 df, 329 deaths) |
|||
|---|---|---|---|---|
| HR | P value | 95% CI | ||
| Age | Continuous, per year | 1.025 | <.0001* | 1.014-1.0361 |
| Gender | Male vs female | 0.707 | .005* | 0.556-0.898 |
| Tumor site | Nasopharynx, base of skull, sinonasal, or other | Ref | Ref | Ref |
| Oral cavity, oropharynx, larynx, hypopharynx, or neck | 1.413 | .020* | 1.056-1.890 | |
| Recurrence or second primary | Recurrent vs second primary | 1.477 | .022* | 1.059-2.061 |
| Organ dysfunction | Yes vs no | 1.357 | .017* | 1.055-1.745 |
| Time between RT | >2 y vs ≤2 y | 0.682 | .003* | 0.529-0.878 |
| Previous use of systemic therapy | Yes vs no | 1.362 | .013* | 1.066-1.740 |
| Systemic therapy during re-RT | Yes vs no | 1.025 | .856 | 0.783-1.342 |
| Previous dose of RT | >70 Gy vs ≤70 Gy | 1.290 | .065 | 0.984-1.691 |
| Tumor size | Large (GTV >25 cm3 or rT3-rT4) vs small (GTV ≤25 cm3 or rT0-rT2) | 1.839 | <.0001* | 1.461-2.315 |
| Re-RT type | SBRT vs IMRT | 1.141 | .338 | 0.871-1.493 |
Abbreviations: CI = confidence interval; GTV = gross tumor volume; HR = hazard ratio; IMRT = intensity modulated radiation therapy; Ref = reference category; RT = radiation therapy; SBRT = stereotactic body radiation therapy.
Statistically significant.
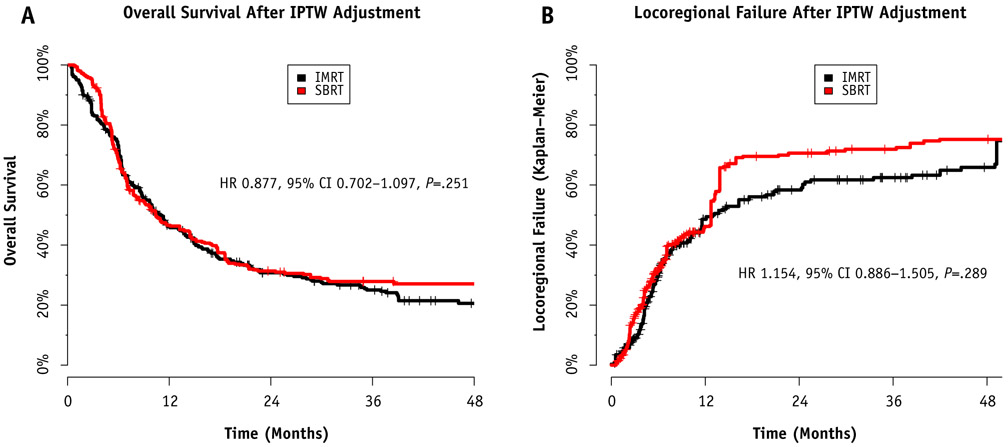
The first of 2 methods used to control for baseline differences when comparing IMRT and SBRT was Cox regression weighted by the inverse probability of treatment. After we used the inverse probability of treatment–weighted model to control for age, gender, smoking pack-years, previous systemic therapy use, previous radiation dose, tumor location, second primary rather than recurrence, time interval between courses of radiation therapy, use of systemic therapy during reirradiation, and tumor size, there were no statistically significant differences in OS between IMRT and SBRT (HR, 0.877; 95% CI, 0.702-1.097; P=.251). Similarly, there was no statistically significant difference in the cumulative incidence of LRF (HR, 1.154; 95% CI, 0.886-1.505; P=.289). The Kaplan-Meier curves weighted by the inverse probability of treatment are presented in Figure 3.
Fig. 3.
Comparison between intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) after weighting by inverse probability of treatment (IPTW). (A) Comparison regarding overall survival. (B) Comparison regarding locoregional failure. No significant differences were noted in either comparison after adjustment. Abbreviations: CI = confidence interval; HR = hazard ratio.
Subset analysis by RPA class
The second method used to compare OS and LRF between the IMRT and SBRT groups was subset analysis by RPA class. Among RPA class III patients (n=61), no significant difference in OS was detected between IMRT and SBRT (2-year OS rate, 16.2% for IMRT vs 3.6% for SBRT; P=.42) (Fig. 1B). Among all RPA class II patients (n=353), treatment with IMRT was associated with improved OS (2-year OS rate, 39.1% for IMRT vs 18.6% for SBRT; P<.001) (Fig. 1C).
To control further for the known prognostic factors of tumor bulk and SBRT dose (17), additional subset analyses of RPA class II patients were performed. The results of these subsets are presented in Figure 1D and E. For small tumors (Fig. 1D), there was no significant difference in OS between those treated with IMRT and those treated with SBRT to doses of ≥35 Gy (2-year OS rate, 50.3% for IMRT vs 14.4% for SBRT to <35 Gy vs 38.5% for SBRT to ≥35 Gy; global log-rank test, P=.15; pair-wise log-rank test for IMRT vs SBRT to ≥35 Gy, P=.42). For large tumors (Fig. 1E), treatment with IMRT was associated with improved OS compared with treatment with SBRT to either dose (28.2% for IMRT vs 9.1% for SBRT to <35 Gy vs 8.8% for SBRT to ≥35 Gy; global log-rank test, P<.001; pair-wise log-rank test for IMRT vs SBRT to ≥35 Gy, P<.001). The cumulative incidence of LRF for the same subsets is presented in Figure 2, with findings similar to those from the OS analysis.
Acute and late toxicity
Before treatment, 35% of IMRT patients and 19% of SBRT patients were dependent on a feeding tube or tracheostomy, and subsequent feeding tube or tracheostomy use was not counted as acute or late toxicity (except when patients remained disease free for ≥1 year). In addition to these rates of baseline dysfunction, new grade ≥3 acute toxicity occurred in 16.6% of IMRT patients and 11.7% of SBRT patients. This difference did not demonstrate statistical significance (P=.15, χ2 test). Grade ≥4 acute toxicity (fistula development, intensive care unit admission, or life-threatening bleeding) was more common in the IMRT cohort (5.1% vs 0.5%, P<.01). Grade 5 acute deaths including bleeding considered unrelated to tumor progression occurred in 1.8% of IMRT patients and 0.5% of SBRT patients (P=.42). Details regarding the nature of events observed in each group are presented in Table E3 (available online at www.redjournal.org).
The 2-year cumulative incidence of grade ≥3 late toxicity that was not pre-existing and that occurred beyond 90 days after treatment controlling for the competing risk of recurrence or death was 12.4% (95% CI, 7.7%-17.1%) in the IMRT cohort compared with 11.6% (95% CI, 7.0%-16.2%) in the SBRT cohort (P=.69, Gray test) (Fig. 4, Table E3 [available online at www.redjournal.org]). The 2-year cumulative incidence of competing risks (progression or death) was 73.1% in the IMRT cohort and 79.2% in the SBRT cohort, implying that fewer than 1 in 5 patients is alive and disease free without having had late effects at 2 years after reirradiation (Fig. 4). This result was no different when calculated by the Kaplan-Meier method censoring for death or recurrence (2-year incidence, 32.5% for IMRT vs 33.3% for SBRT; P=.61, log-rank test). A bleeding event occurred beyond 90 days in the absence of disease progression in 4 patients (carotid blowout syndrome), 2 in the IMRT cohort and 2 in the SBRT cohort, for an overall crude rate of 1.0%.
Fig. 4.
Cumulative incidence of grade ≥3 late toxicity by treatment technique. Abbreviations: IMRT = intensity modulated radiation therapy; SBRT = stereotactic body radiation therapy.
Discussion
In this report we present the largest comparison to date of 2 established modern reirradiation schedules. Our data suggest that both reirradiation approaches are relatively safe and carry acute and late toxicity rates that compare favorably with historical reirradiation studies. Both will likely play a role in the treatment of rSCCHN moving forward. The concurrently reported RPA classification developed among IMRT patients retained an independent association with OS, providing external validation of its usefulness in the prognostic classification of these patients. Taken together, the findings of this report provide a step toward clarifying the historically challenging process of treatment selection for these patients faced with multiple reasonable options including palliative care, systemic therapy alone, or reirradiation with conventional IMRT or SBRT.
Both IMRT and SBRT techniques have radiobiological and logistic considerations that complicate treatment selection (19). Conventionally fractionated (or hyper-fractionated) IMRT is a protracted regimen that allows for tumor reoxygenation and normal tissue repair during therapy (20). On the other hand, SBRT is designed as an ablative regimen that may incorporate alternative radiobiological mechanisms such as direct vascular endothelial damage (21). The choice between these treatment options has thus far been informed by institutional practice patterns, physician comfort with the techniques, and patient preferences. This study is the first to provide comparative data between SBRT and IMRT in these patients, which can help guide these choices. Despite inherent differences in both underlying biology and logistics, these data would seem to support relative equipoise in terms of toxicity and survival. Potential advantages of SBRT >35 Gy being lower life-threatening acute effects potentially favoring continued addition of novel systemic therapies especially for smaller tumor volumes and poor prognosis RPA class III patients. IMRT, by allowing for a wider treatment volume, may offer advantage in larger tumors at higher risk of microscopic extension at the expense of additional acute toxicity.
The role of systemic therapy in future directions for this patient population underscores the need to further refine these initial observations in future studies. While the early experience with SBRT was performed without systemic therapy, recent studies have clarified the safety of the addition of concurrent cetuximab with this approach (10-12), and a randomized study investigating the addition of concurrent docetaxel to a cetuximab-SBRT backbone is under way at the University of Pittsburgh. The recent approval of programmed death inhibitors in second-line recurrent-metastatic head and neck cancer has spawned optimism that these agents will have an important role to play in the reirradiation setting as well. The RTOG is soon to open the 3507 KEYSTROKE study, a randomized study investigating the addition of pembrolizumab to SBRT in patients with unresectable recurrent or second primary head and neck cancer to interrogate this question.
Our study also importantly demonstrates that both IMRT reirradiation and SBRT reirradiation are safe and carry acute and late toxicity rates that compare favorably with historical reirradiation studies in which 3-dimensional conformal radiation therapy was used. The method of toxicity collection in this study is important to note, as pretreatment baseline deficits in feeding tube or tracheostomy use were not considered toxicity. Therefore, the toxicities reported are objective physician-reported events qualifying as grade ≥3 as defined by CTCAE version 4.0 and likely related directly to the delivery of reirradiation with or without systemic therapy. This report also applies actuarial analysis of late toxicity adjusted for the significant competing risk of subsequent failure or death. When we account for these factors particularly relevant to this population, the probability of a patient having additional late toxicity clearly due to reirradiation appears reasonable.
Although this is the largest comparative report of modern reirradiation strategies to date, limitations exist. The retrospective study design, including underlying selection and information biases, creates uncertainty about our findings. In addition, the baseline differences between the SBRT and IMRT groups were significant and limit the ability to draw definitive conclusions. For example, while GTV is a known prognostic factor for SBRT patients (17), these data were not generally collected for IMRT patients. We partially accounted for this by using T classification as a surrogate for tumor volume, but this method lacks precision. Follow-up of these patients was per institutional routine rather than on a formal clinical trial. As such, this report may underestimate rates of either LRF or toxicity (or both) because of underreporting. Finally, human papillomavirus status was available on only a minority of recurrent or second primary oropharynx cancers, and additional subset analysis of this distinct disease is warranted (22, 23). Despite the aforementioned limitations, these data provide the most robust comparative analysis to date of outcomes with modern techniques, which can help inform multidisciplinary discussion and treatment recommendations. As clinical trials are being developed to better understand how to incorporate systemic therapy, specifically immunotherapy, with these reirradiation modalities, these data can also provide a useful baseline for clinical trial design (24-28).
Conclusions
Reirradiation with IMRT and SBRT is better tolerated than historical controls. The concurrently proposed RPA classification is an externally valid prognostic tool that can be used to guide treatment recommendations and clinical trial design. Survival for class III patients is poor, and SBRT, systemic therapy alone, and supportive care are all reasonable strategies. Future study is needed to further optimize outcomes.
Supplementary Material
Footnotes
Presented in abstract form at the 58th Annual Meeting of the American Society for Radiation Oncology (ASTRO); September 25 to 28, 2016; Boston, MA; and selected for presentation at the Best of ASTRO 2016 meeting; November 11 to 12, 2016; Fort Lauderdale, FL.
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of re-irradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 2008;30:281–288. [DOI] [PubMed] [Google Scholar]
- 2.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily re-irradiation in recurrent squamous cell carcinoma of the head and neck: Results of Radiation Therapy Oncology Group protocol 9911. J Clin Oncol 2007;25:4800–4805. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 4.Sulman EP, Schwartz DL, Le TT, et al. IMRT re-irradiation of head and neck cancer-disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys 2009;73:399–409. [DOI] [PubMed] [Google Scholar]
- 5.Lee N, Chan K, Bekelman JE, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 2007;68: 731–740. [DOI] [PubMed] [Google Scholar]
- 6.Biagioli MC, Harvey M, Roman E, et al. Intensity-modulated radiotherapy with concurrent chemotherapy for previously irradiated, recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 69:1067–1073. [DOI] [PubMed] [Google Scholar]
- 7.Duprez F, Madani I, Bonte K, et al. Intensity-modulated radiotherapy for recurrent and second primary head and neck cancer in previously irradiated territory. Radiother Oncol 2009;93:563–569. [DOI] [PubMed] [Google Scholar]
- 8.Chen AM, Vazquez E, Michaud AL, et al. Functional and quality-of-life outcomes after reirradiation for head and neck cancer. Laryngoscope 2014;124:1807–1812. [DOI] [PubMed] [Google Scholar]
- 9.Heron DE, Ferris RL, Karmouzis M, et al. Stereotactic body radiotherapy for recurrent squamous cell carcinoma of the head and neck: Results of a phase I dose-escalation trial. Int J Radiat Oncol Biol Phys 2009;75:1493–1500. [DOI] [PubMed] [Google Scholar]
- 10.Vargo JA, Ferris RL, Ohr J, et al. A prospective phase II trial of reirradiation with stereotactic body radiotherapy plus cetuximab in patients with recurrent previously-irradiated squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2015;91:480–488. [DOI] [PubMed] [Google Scholar]
- 11.Comet B, Kramar A, Faivre-Pierret M, et al. Salvage stereotactic reirradiation with or without cetuximab for locally recurrent head-and-neck cancer: A feasibility study. Int J Radiat Oncol Biol Phys 2012;84: 203–209. [DOI] [PubMed] [Google Scholar]
- 12.Lartigau EF, Tresch E, Thariat J, et al. Multi institutional phase II study of concomitant stereotactic re-irradiation and cetuximab for recurrent head and neck cancer. Radiother Oncol 2013;109:281–285. [DOI] [PubMed] [Google Scholar]
- 13.Vargo JA, Heron DE, Ferris RL, et al. Prospective evaluation of patient-reported quality-of-life outcomes following SBRT ± cetuximab for locally-recurrent, previously-irradiated head and neck cancer. Radiother Oncol 2012;104:91–95. [DOI] [PubMed] [Google Scholar]
- 14.Ward MC, Riaz N, Caudell JJ, et al. Refining patient selection for reirradiation of head and neck squamous cell carcinoma in the IMRT era: A multi-insitution cohort study by MIRI collaborative. Int J Radiation Oncol Biol Phys 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanvetyanon T, Padhya T, McCaffrey J, et al. Prognostic factors for survival after salvage reirradiation of head and neck cancer. J Clin Oncol 2009;27:1983–1991. [DOI] [PubMed] [Google Scholar]
- 16.Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict locoregional control after re-irradiation for head and neck cancer. Radiother Oncol 2014;111:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rewigema JC, Heron DE, Ferris RL, et al. The impact of tumor volume and radiotherapy dose on outcome in previously irradiated recurrence squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. Am J Clin Oncol 2011;34: 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–1154. [Google Scholar]
- 19.Practical considerations in the re-irradiation of recurrence and second primary head-and-neck cancer: Who, why, how, and how much? Int J Radiat Oncol Biol Phys 2011;81:1211–1219. [DOI] [PubMed] [Google Scholar]
- 20.Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: A randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014;89:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 2014;88:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol 2014;32:3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis KS, Vargo JA, Ferris RL, et al. Stereotactic body radiotherapy for recurrent oropharyngeal cancer—Influence of HPV status and smoking history. Oral Oncol 2014;50:1104–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiwert TY, Burtness B, Weiss J, et al. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV–associated head and neck (H/N) cancer. J Clin Oncol 2014; 32:5s. [Google Scholar]
- 25.Seiwert T, Haddad R, Gupta S. Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN) (2015) preliminary results from KEYNOTE-012 expansion cohort. J Clin Oncol 2015;33s:LBA6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong SJ, Heron DE, Stenson K, et al. Locoregional recurrent or second primary head and neck cancer: Management strategies and challenges. Am Soc Clin Oncol Educ Book 2016;35:e284–e292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.