Abstract
Objective:
The relationship between microsatellite instability (MSI) and response to neoadjuvant chemoradiation in rectal cancer is not well understood.
Background:
We utilized the National Cancer Database (NCDB) to investigate the association between MSI and pathologic complete response (pCR) in this patient population.
Methods:
We analyzed 5086 patients between 2010 and 2015 with locally advanced rectal cancer who were tested for MSI and treated definitively with chemoradiation followed by surgery. Primary comparison groups were between 4450 MSI-negative(−) and 636 MSI-positive(+) patients. Multivariable regression analysis was conducted to identify demographic, therapeutic, and clinical characteristics predictive of pCR. Cox proportional-hazard ratios were used for survival.
Results:
All patients were treated with definitive chemoradiation (median dose 50.4 Gy) followed by resection within 4 months. MSI(+) patients were associated with earlier year of diagnosis and higher-grade tumors (P < 0.05). The overall pCR rate was 8.6%, including 8.9% for MSI(−) and 5.9% for MSI(+) tumors (P = 0.01). Along with lower T stage, MSI(+) cases were significantly associated with a reduced pCR rate (odds ratio 0.65, 95% confidence interval 0.43–0.96) with multivariable analysis. The 5-year survival for patients with pCR was 93% compared with 73% without it (<0.001).
Conclusion:
Microsatellite instability was independently associated with a reduction in pCR for locally advanced rectal cancer after neoadjuvant chemoradiation in this NCDB-based analysis.
Keywords: chemoradiation, microsatellite instability, National Cancer Database, pathologic complete response, rectal cancer
Of growing interest in the modern management of rectal cancer, the molecular profiling of tumors is an emerging trend in cancer care that aids clinicians both prognostically and therapeutically. Microsatellite instability (MSI) or mismatch repair testing is now recommended for all patients with a history of colorectal cancer, as per the National Comprehensive Cancer Network (NCCN) guidelines.1 An estimated 15% of colorectal patients develop an accumulation of DNA abnormalities (microsatellites) in the genome due to defects in mismatch repair, leaving cells vulnerable to mutations—a phenomenon known as MSI.2–4 A tumor is labeled MSI-high (MSI-H) when at least 40% microsatellite loci are affected by instability, and MSI-low (MSI-L) if less than 40% are.5 Currently, most centers simply report whether a tumor tests positive or negative for MSI via immunohistochemical staining.6
Routine MSI testing in colorectal cancer is not universally performed, but it is increasing as clinicians can use results to help dictate management.7 For instance, early-stage adenocarcinomas of the colon testing positive for MSI carry a favorable prognosis and therefore do not require adjuvant chemotherapy. Some suggest that these tumors are more prone to 5-fluorouracil resistance, although this is controversial.8,9 Conversely, MSI-positive rectal cancers have been linked to a poorer prognosis for reasons not currently understood.10
While there is established evidence regarding the effect of MSI on response to chemotherapy and overall prognosis, there is a paucity of data evaluating the association between MSI, radiosensitivity, and pathologic response to treatment. Because neoadjuvant chemoradiation and resection is the gold standard in locally advanced disease, and because nonoperative management of rectal cancer is gaining popularity, such information may prove to be valuable.11 Furthermore, pathologic complete response (pCR) is a known predictor of improved survival.12 We therefore utilized the National Cancer Database (NCDB) to analyze the association between MSI and pCR rate in locally advanced adenocarcinoma of the rectum treated definitively with chemoradiation and resection.
METHODS
The NCDB is a tumor registry jointly managed by the American Cancer Society and American College of Surgeons, providing the de-identified data used in this institutional board review-exempt study. The database comprises of approximately 70% of cancer cases in the United States from over 1500 hospitals accredited by the Commission on Cancer.13 We queried the database to identify rectal cancers tested for MSI between the years 2004 and 2015, though MSI testing has only been performed since 2010. A complete consolidated standards of reporting trials (CONSORT) diagram depicting the selection process is outlined on Fig. 1.
FIGURE 1.
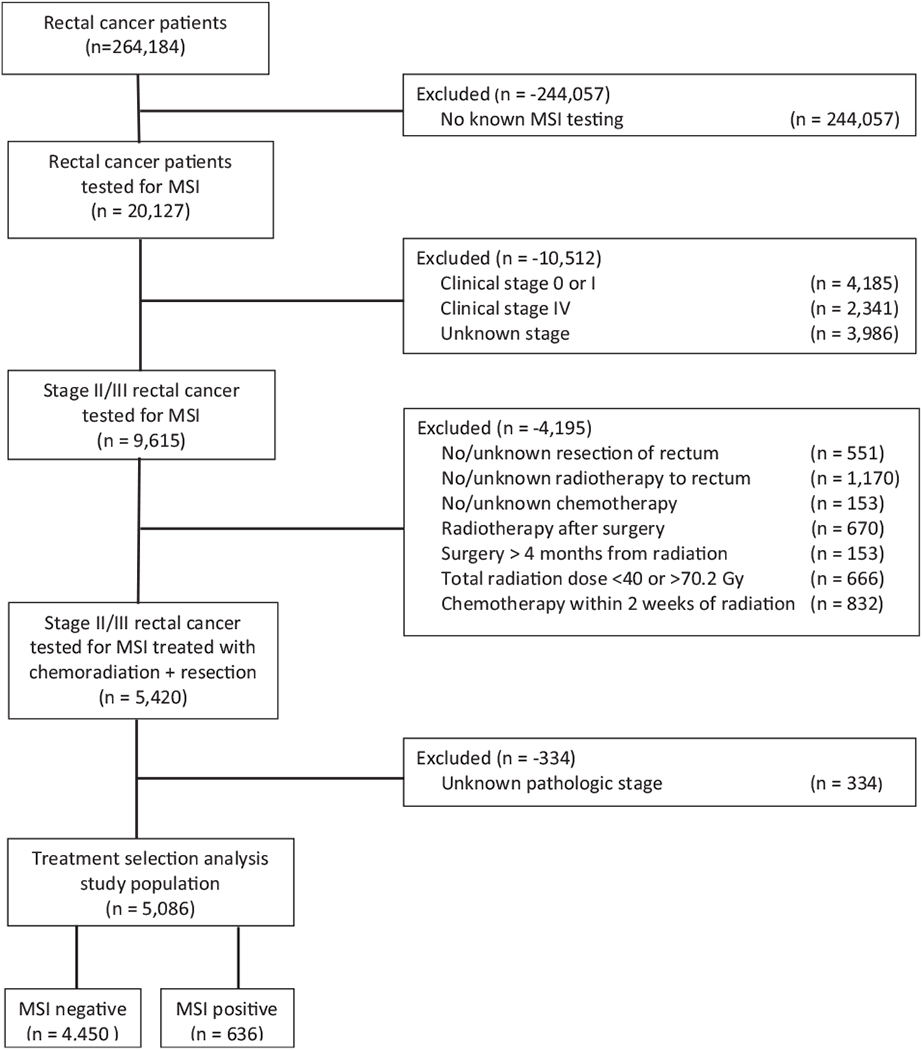
CONSORT diagram.
Ultimately, 5086 patients with locally advanced adenocarcinoma of the rectum, tested for MSI, and treated with neoadjuvant chemoradiation followed by resection with known pathologic staging were eligible for final analysis. Patients were either MSI(−) (stable), or MSI(+), and within the MSI(+) group they were either MSI-H, MSI-L, or MSI-NOS (not otherwise specified). Of note, because almost half of the MSI(+) cases reported no distinction between MSI-H and MSI-L, the 2 main comparison groups were simply MSI(−) and MSI(+), although subset analyses were conducted with MSI-H/MSI-L populations as well.
Stage was defined clinically as per the American Joint Committee on Cancer’s 7th edition staging for rectal cancer. Comorbidity was quantified using the Charlson-Deyo comorbidity index, and socioeconomic data were provided as quartiles of median household income. Locations were assigned in accordance with the US Department of Agriculture Economic Research Service, and facility type was assigned as per Commission on Cancer Accreditation Category.
Statistical analysis was performed via SPSS version 20. Summary statistics were reported for discrete variables, and chi-square tests were used to compare demographic, clinical, and treatment characteristics between the MSI(−) and MSI(+) groups. Bivariate logistic regression models were used to assess the association between independent variables of interest and MSI status. Multivariable logistic regression models were used to detect associations between the aforementioned characteristics and pCR. Factors significant on univariate analysis were entered in a hierarchical fashion using forward selection of the covariates’ likelihood ratios, and for confirmation, the same results were obtained using a stepwise backward elimination procedure. Adjusted odds ratios (ORs) and 95% confidence interval (CIs) are reported, with α = 0.05 used to indicate statistical significance.
Because of the size discrepancy between the MSI(−) and MSI(+) cohorts, a propensity score analysis was utilized to account for a potential lack of balance between the 2 groups.14 Propensity scores were calculated by multivariable logistic regression to provide a score reflecting the conditional probability of testing negative or positive for MSI. The propensity model included observable variables significantly associated with MSI selection on multivariable logistic regression, including race, education, year, and T stage. Cases with missing data were dropped from the propensity score model and only variables with <5% missing data were included. We then created a pseudo population using the conditional probabilities with a distribution of confounding variables in each dose-group arm that was identical to the entire cohort. Subsequently, we constructed a multivariable logistic regression model for pCR, adjusting for propensity score.15 To avoid overcorrection, only factors significant on univariable logistic regression not included in the propensity score were included in the propensity-adjusted model. To strengthen the assumption of balance between groups, the propensity-adjusted score was validated by stratification into propensity score-based quintiles, which demonstrated that standardized difference between the treatment groups was less than 0.10.15 To further validate the propensity-matched analysis, we also performed a case-control matching procedure to randomly match cases and controls based on the aforementioned demographic and disease-related characteristics among the MSI(−) and MSI(+) cohorts. This also allowed us to incorporate grade, which was significantly associated with MSI selection, but had to be excluded from the propensity score model due to a high proportion of missing data (11.6%). The matched cases were then once again entered into a multivariable regression analysis for pCR. Overall survival (OS) was calculated from the date of diagnosis to the date of last contact or death using Kaplan-Meier curves to present the cumulative probability of survival, and log-rank statistics to assess statistical significance between groups. A Cox proportional-hazards model was used for multivariable survival analysis. Note that for survival analysis 1184 patients were excluded for no known followup, though survival was not the major endpoint of this study.
RESULTS
Patient Characteristics
Baseline characteristics for this patient population are depicted in Table 1. Briefly, the median age was 59 years with a Caucasian (86%) and male (61%) predominance. The vast majority of patients had T3 tumors (85%) or node-positive disease (59%). There were no missing data for preoperative radiation dose, which exhibited very minor variability—67% receiving 50.4 Gy, 12% receiving 45 Gy, and 7% receiving 54 Gy (interquartile range 50.4–50.4 Gy). All patients had concurrent chemotherapy, 56% with single agent and 41% with 2 agents. All patients received definitive surgical resection, including a total of 228 (5%) total proctocolectomies in the MSI(−) group and 51 (8%) in the MSI(+) group. The number of patients with MSI testing increased from year to year, as the total cohort includes 486 patients diagnosed in 2010 compared with 1185 patients in 2015. Among the 5086 patients tested, 87% (n = 4046) were MSI(−) and 13% (n = 636) were MSI(+), of which 135 cases were MSI-H, 210 cases were MSI-L, and 291 cases were not otherwise specified. Potential demographic and clinical covariates were well-balanced between MSI(−) and MSI(+) patients, with few exceptions: cases diagnosed between 2010 and 2011 were more likely to test MSI(+) relative to 2012 to 2015, and the small portion of high-grade/T4 lesions were also associated with MSI(+) (Table 2).
TABLE 1.
Patient and Treatment Characteristics (N = 5086)
| Characteristic | No. (% or Range) |
|---|---|
| Demographics | |
| Sex | |
| Male | 3100 (61) |
| Female | 1986 (39) |
| Age | |
| Median | 59 (19-90) |
| <60 | 1743 (53.1) |
| ≥60 | 1537 (46.9) |
| Race | |
| White | 4369 (85.9) |
| African American | 412 (8.1) |
| Other/unknown | 305 (6.0) |
| Comorbidity score | |
| 0 | 4029 (59.8) |
| 1 | 829 (16.3) |
| 2+ | 228 (4.5) |
| Insurance | |
| Not insured | 226 (4.4) |
| Private | 2849 (56.0) |
| Government | 1953 (38.4) |
| Treatment facility type | |
| Community cancer program | 295 (5.8) |
| Academic/research program | 1917 (37.7) |
| Comprehensive cancer program/other | 2874 (56.5) |
| Treatment facility location | |
| Metro counties | 4098 (82.3) |
| Urban counties | 794 (15.9) |
| Rural counties | 88 (1.8) |
| Income, US dollars | |
| <48,000 | 2012 (39.7) |
| ≥48,000 | 3059 (60.3) |
| Distance to treatment facility, miles | |
| ≤10 | 2391 (47.0) |
| >10 | 2679 (52.7) |
| Location | |
| East | 1944 (38.2) |
| Central | 1913 (37.6) |
| West | 870 (17.1) |
| Year of diagnosis | |
| 2010–2011 | 1105 (21.7) |
| 2012–2013 | 1702 (33.5) |
| 2014–2015 | 2279 (44.8) |
| Disease characteristics | |
| Clinical T stage | |
| T1/T2 | 317 (6.2) |
| T3 | 4312 (84.8) |
| T4 | 428 (8.4) |
| Clinical T size | |
| <2 cm | 724 (14.2) |
| 2–5 cm | 2517 (49.5) |
| >5 cm | 1237 (24.3) |
| Clinical N stage | |
| N0 | 2026 (39.8) |
| N1 | 2408 (47.3) |
| N2 | 607 (11.9) |
| Grade | |
| Well differentiated | 403 (7.9) |
| Moderately differentiated | 3533 (69.5) |
| Poorly differentiated | 537 (10.6) |
| Microsatellite instability | |
| Negative (stable) | 4450 (87.5) |
| Positive (instable) | 636 (12.5) |
| Treatment characteristics | |
| Radiation dose, Gy | |
| Median (Gy) | 50.4 (40.4–70.2) |
| <50.4 | 769 (15.1) |
| ≥50.4 and <54 | 3754 (73.8) |
| ≥54 | 563 (11.1) |
| Time from XRT to surgery | |
| ≤2 mos | 2679 (52.7) |
| 2–4mos | 2407 (47.3) |
| Preoperative chemotherapy | |
| Single agent | 2850 (56.0) |
| Double agent | 2088 (41.1) |
| Unknown | 148 (2.9) |
Note: If sum of percentages is <100, then the remaining data are missing/unreported. Gy, gray.
TABLE 2.
Comparative Baseline Characteristics for Microsatellite Status
| Characteristics | MSI(−), n = 4450 | MSI(+), n = 636 | OR | 95% CI | P |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 2713 (61.0) | 387 (60.8) | 1 | Reference | |
| Female | 1737 (39.0) | 249 (39.2) | 1.05 | 0.85–1.19 | 0.96 |
| Age, yrs | |||||
| <60 | 2470 (55.5) | 366 (57.5) | 1 | Reference | |
| ≥60 | 1980 (44.5) | 270 (42.5) | 0.92 | 0.78–1.09 | 0.33 |
| Race | |||||
| White | 3811 (85.6) | 558 (87.7) | 1 | Reference | |
| Black | 386 (8.3) | 44 (6.9) | 0.82 | 0.59–1.13 | 0.22 |
| Other | 271 (6.1) | 34 (5.3) | 0.86 | 0.59–1.24 | 0.41 |
| Year | |||||
| 2010–2011 | 940 (21.1) | 165 (25.9) | 1 | Reference | |
| 2012–2013 | 1506 (33.8) | 196 (30.8) | 0.74 | 0.59–0.93 | 0.01 |
| 2014–2015 | 2004 (45.0) | 275 (43.2) | 0.78 | 0.64–0.96 | 0.02 |
| Comorbid (Charlson-Deyo) | |||||
| 0 | 3508 (78.8) | 521 (81.9) | 1 | Reference | |
| 1 | 740 (16.6) | 89 (14.0) | 0.81 | 0.64–1.03 | 0.08 |
| ≥2 | 202 (4.5) | 26 (4.1) | 0.87 | 0.57–1.32 | 0.50 |
| Clinical T stage | |||||
| T1/T2 | 281 (6.4) | 36 (5.7) | 1 | Reference | |
| T3 | 3786 (85.6) | 526 (83.2) | 1.08 | 0.76–1.55 | 0.66 |
| T4 | 358 (8.1) | 70 (11.1) | 1.53 | 0.99–2.35 | 0.06 |
| Primary tumor size, cm | |||||
| <2 | 632 (16.1) | 92 (16.5) | 1 | Reference | |
| 2–5 | 2200 (56.1) | 317 (57.0) | 0.99 | 0.77–1.27 | 0.94 |
| >5 | 1090 (27.8) | 147 (26.4) | 0.93 | 0.70–1.22 | 0.59 |
| Differentiation | |||||
| Well | 352 (9.0) | 51 (9.0) | 1 | Reference | |
| Moderately | 3116 (79.7) | 417 (73.9) | 0.92 | 0.68–1.26 | 0.62 |
| Poorly | 441 (11.3) | 96 (17.0) | 1.50 | 1.04–2.17 | 0.03 |
| Radiotherapy dose, Gy | |||||
| <50.4 | 667 (15.0) | 102 (16.0) | 1 | Reference | |
| ≥50.4 and <54 | 3292 (74.0) | 462 (72.6) | 0.92 | 0.73–1.16 | 0.46 |
| ≥54 | 491 (11.0) | 72 (11.3) | 0.96 | 0.69–1.33 | 0.80 |
| Time from XRT to surgery | |||||
| ≤2 mos | 2347 (52.7%) | 332 (52.2) | 1 | Reference | |
| >2 mos | 2103 (47.3) | 304 (47.8) | 1.02 | 0.87–1.21 | 0.80 |
| Initial chemotherapy | |||||
| Single agent | 2495 (56.1) | 355 (55.8) | 1 | ||
| Double agent | 1833 (41.2) | 255 (40.1) | 0.98 | 0.82–1.16 | 0.80 |
Response to Neoadjuvant Therapy
After neoadjuvant chemoradiation, the proportion of pathologic T1/T2, T3, and T4 lesions were 24%, 47%, and 5% compared with 6%, 85%, and 9% clinically staged T1/T2, T3, and T4 lesions before treatment. Node positivity decreased from 59% to 35% after chemoradiation. Tumor down-staging and nodal sterilization occurred in 50.2% and 57% of cases, respectively. pCR was achieved in 437 cases (8.6%), with an 8.9% rate among MSI(−) patients and 5.9% rate for MSI(+) patients (OR 0.65, P = 0.01).With propensity score-matched univariable and multivariable regression analysis, MSI(+) patients remained an independent predictor of reduced pathologic complete response (OR 0.65, 95% CI 0.43–0.96). Higher income, more recent year of diagnosis, and lower T stage were also all independently associated with increased pCR (Table 3). Secondary analysis with case-control matching further corroborated our findings, with MSI(+) tumors proving to be the lone predictor of reduced pCR (OR 0.57, 95% CI 0.35–0.91), although year of diagnosis in 2014 to 2015 trended with increased pCR (OR 1.86, 95% CI 0.98–3.56). None of the following variables demonstrated an association with pCR with either univariable or multivariable analysis: sex, age, race, comorbidity score, insurance, facility, education, distance from facility, environment (urban/metropolitan/rural), N stage, grade, dose, use of doublet (instead of singlet chemotherapy), or timing from chemoradiation to surgery.
TABLE 3.
Multivariable Binary Logistic Models for Pathologic Complete Response
| Characteristics | Without Propensity Score |
Propensity Score-adjusted |
||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Hazard of Death (95% CI) | P | |
| Microsatellite Instability | ||||
| Negative | Reference | Reference | ||
| Positive | 0.65 (0.43–0.96) | 0.03 | 0.65 (0.43–0.97) | 0.03 |
| Income, dollars | ||||
| <48,000 | Reference | Reference | ||
| ≥48,000 | 1.28 (1.01–1.62) | 0.04 | 1.24 (0.97–1.58) | 0.09 |
| Year | ||||
| 2010–2011 | Reference | — | ||
| 2012–2013 | 1.20 (0.86–1.69) | 0.28 | ||
| 2014–2015 | 1.59 (1.17–2.18) | 0.003 | ||
| T stage | ||||
| T1/T2 | Reference | — | ||
| T3 | 0.60 (0.41–0.87) | 0.01 | ||
| T4 | 0.25 (0.13–0.49) | <0.001 | ||
Survival
The median follow-up for all patients was 31 months, with 3 and 5-year survival of 87% and 74%, respectively. The 3 and 5-year survival for patients achieving pCR was 96% and 93% compared with 86% and 73% for patients with residual disease after chemoradiation (P < 0.001) (Fig. 2). Variables associated with an increased risk of death upon multivariable analysis include single (instead of double) agent chemotherapy, treatment at a nonacademic facility, older age, male sex, nonprivate insurance, lower income, higher comorbidity score, higher grade, and radiation dose less than 50.4 Gy. MSI status did not correlate with survival.
FIGURE 2.
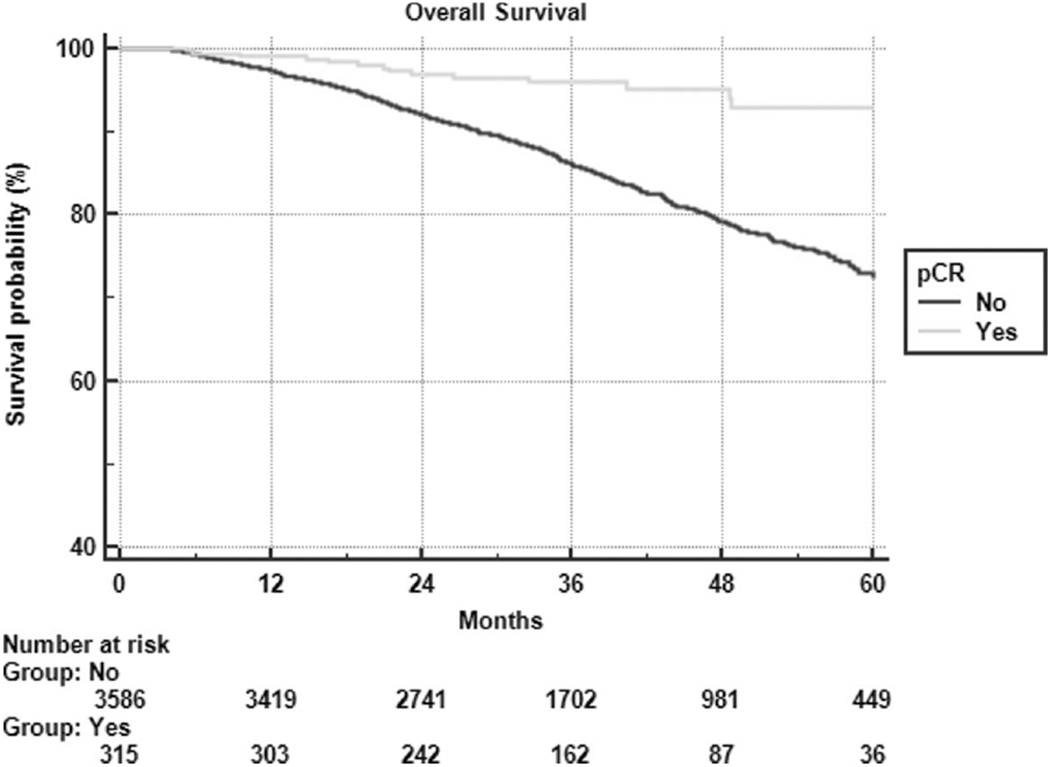
Kaplan-Meier overall survival partitioned by pathologic complete response.
DISCUSSION
TNM staging remains the gold standard for the prognostic classification of rectal cancer; however, outcome variability within stages may be at least, in part, attributable to heterogeneity in molecular profile.16 MSI is strongly, though not exclusively, associated with deficient mismatch repair enzymes, which is most often seen in hereditary nonpolyposis colon cancer—a disease primarily involving the proximal colon.17 For a myriad of reasons, radiotherapy has a limited role in the primary treatment of proximal colon cancer, and therefore the association, if any, between MSI and radioresponsiveness has not been well studied. The few studies that have explored this concept failed to establish such a relationship, but were limited by small (n < 100) cohorts.18,19 Shin et al20 proposed that MSI should promote radiosensitivity because MSI tumors are deficient in the mechanisms necessary to repair radiation-induced DNA double-strand breaks, and also effector proteins that promote tumor growth. Such radiosensitivity has been demonstrated in mouse models with MSI cell lines.21,22
The aforementioned data suggest a role for routine MSI testing in rectal malignancies, as has been conducted with increasing frequency per our analysis. Naturally, more patients were being tested from 1 year to the next. Unexpectedly, however, was the proportionately higher percentage of MSI(+) patients in 2010 to 2011 relative to 2012 to 2015. Perhaps, testing methods changed with time, although most studies indicate that both immunohistochemical staining and PCR assay are almost equally effective at detecting MSI.6 In addition to year of diagnosis, higher grade and T4 tumors were also more likely to test positive for MSI, suggesting that there may be a link between microsatellite instability and known unfavorable prognosticators in rectal cancer.23
Interestingly, MSI has been consistently associated with both improved survival in colon cancer and decreased survival in rectal cancer.10,24 While the prognostic implication of MSI-H varies by site, MSI-L seemingly carries a poorer prognosis regardless.25–27 Reasons for this dichotomous relationship are not well understood, though some suggest that the rectum is less likely to harbor co-mutations such as gene that encodes for the protein b-raf (B-RAF)—a correlate of improved survival.28 Although no association with survival and MSI status was noted in this study (with relatively short follow-up), there was a nonstatistically significant higher risk of death in MSI-L patients [hazard ratio (HR) 1.33, 95% CI 0.92–1.91). Response to treatment, however, did demonstrate a correlation with survival.
Pathologic complete response after neoadjuvant therapy is a strong indicator of outcome in rectal cancer, almost doubling the survival rate in a recent study.29 Similarly, patients with pCR after chemoradiation in our patient population were far less likely to encounter death than those harboring residual disease (HR 0.28, P < 0.001). In the landmark trial that established neoadjuvant radiotherapy with concurrent 5-fluorouracil as the standard of care in locally advanced rectal cancer, Sauer et al30 reported a pCR rate of 8%, mirroring the overall pCR rate in our study. It should be noted that a recent NCDB analysis of neoadjuvant chemoradiation in rectal cancer reported a pCR rate of 23%,31 although they included a greater proportion of clinical T1 and T2 tumors relative to this study. Subsequent attempts to improve pCR rate since the Sauer trial included the addition of oxaliplatin, extended capecitabine, and dose-escalated radiotherapy, with better but varying results, between 15% and 27%.32–34 Perhaps, tumor biology plays as significant a role in response to therapy as the therapy itself, as suggested by the clinical and molecular correlates of treatment response demonstrated in this analysis.
Unsurprisingly, lower T stage was the strongest predictor of pCR with multivariable analysis, as was a higher income and more recent diagnosis (Fig. 3). Of interest, dose-escalated radiation (over 54 Gy) and double agent chemotherapy were not associated with increased pCR rate, although both correlated with longer survival. Completing surgery within 2 months or after 2 months of finishing radiotherapy also showed no association with pCR or survival, suggesting that our study sample had adequate time for tumor down-staging to pCR to develop.
FIGURE 3.
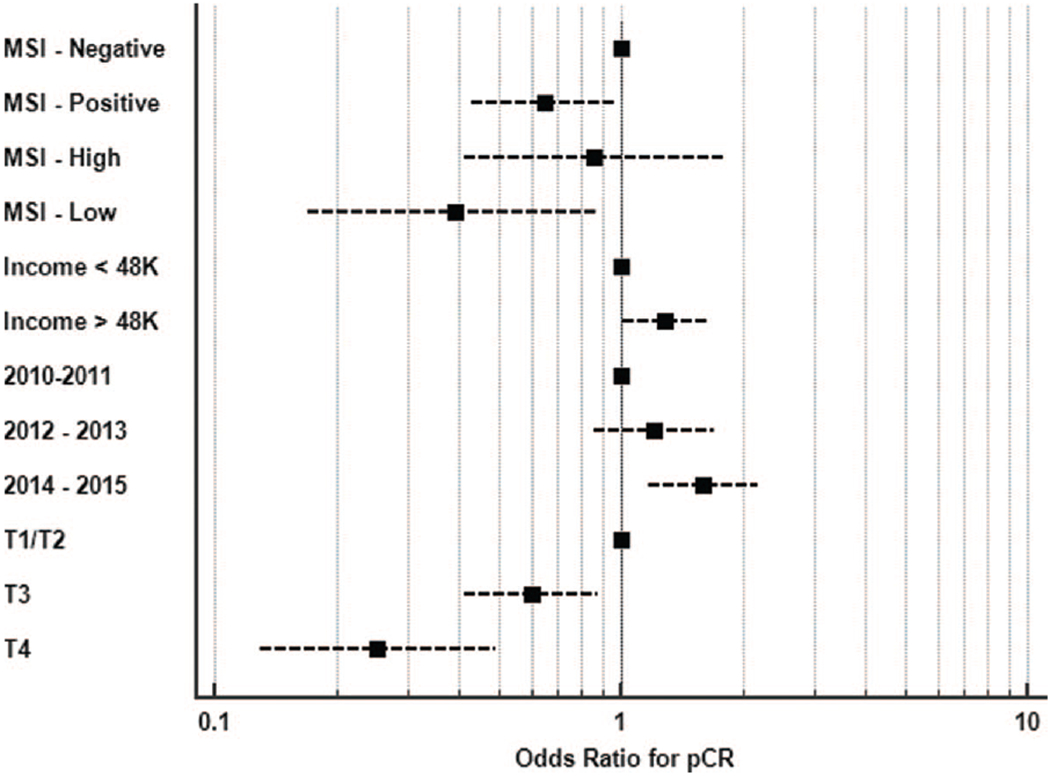
Forest plot with odds ratios for pCR based on multivariable regression analysis. Note: MSI-high/MSI-low testing conducted independently of MSI-negative/MSI-positive to avoid confounding covariability.
Tumors testing positive for MSI independently correlated with a reduced pCR rate on multivariable analysis, both with and without propensity matching, and also with case-control matching. These findings are consistent with the known poorer prognosis of MSI(+) patients with rectal cancer, and also corroborate the notion of chemo/radio-resistance for MSI(+) tumors in the neoadjuvant setting. Such information may be valuable to gastrointestinal oncologists who are currently exploring different avenues of neoadjuvant therapy, such as the omission of radiotherapy in the preoperative radiation or selective preoperative radiation and evaluation before chemotherapy and total mesorectal excision (PROSPECT) trial or more aggressive upfront chemotherapy with total neoadjuvant therapy.35,36 Furthermore, with the growing trend of nonoperative management of patients requiring abdominoperineal resection, it may benefit clinicians to test for MSI and exercise organ-preserving management with caution for those with MSI(+) tumors, which may be relatively chemo/radio-resistant for reasons not yet understood.37
Presuming that such a resistance to conventional therapies exist in MSI(+) rectal cancers, immunotherapy may provide a pathway to help navigate it. In a small but prospective trial of progressive metastatic carcinoma, 40% of mismatch repair-deficient colorectal cancers responded to the Programmed Death-1 (PD-1) blocker pembrolizumab compared with 0% of patients mismatch repair proficient colorectal cancers.38 This concept is being further explored in the ongoing NRG GI004 phase III trial, designed to evaluate the addition of atezolizumab to conventional chemotherapy in metastatic colorectal cancer with deficient mismatch repair genes.39 If immunotherapy proves to be effective in this setting, perhaps it can play a role in the neoadjuvant setting for MSI(+) rectal cancers in future clinical trials.
Limitations
While our findings are thought-provoking, they must be considered with the selection bias inherent to any large retrospective study. The numbers supplied by the NCDB allow investigators to explore associations that are otherwise difficult to unveil due to a limited sample size. Nevertheless, unobserved confounding variables limit the interpretation of observational data, regardless of attempts to mitigate bias with multivariable analysis and propensity and crosscontrol matching. Additionally, clinical treatment response, salvage therapies, specific chemotherapeutic agents, and number of cycles administered are not included in the data, which may have otherwise affected the interpretation of results. It should be, however, noted that survival was not a primary endpoint in this study. It is also difficult to draw conclusions from the survival analysis that was conducted, as locally advanced rectal cancer has a good overall prognosis, and therefore longer follow-up is needed to truly determine associations with survival and covariates such as MSI, which has only been collected in more recent years.
The NCDB contained MSI data on only 8% of rectal cancer patients, creating the most pertinent source of selection bias for this study. Reasons for MSI testing were not provided, so perhaps testing was reserved for cases that did not respond well to conventional therapy (which may also help explain the low overall pCR rate), or perhaps it was only routinely being conducted at academic centers. Therefore, caution should be used when extrapolating these results to all cases of microsatellite instability. Nevertheless, these findings provide enough data to warrant further investigation and strengthens the case for routine MSI testing for locally advanced (if not all stages of) rectal cancer. Lastly, we could not evaluate MSI-H against MSI-L as precisely as we would like to, given the limitations of the MSI “not otherwise specified” category. The absence of specificity is likely because most centers perform MSI testing with immunohistochemical staining, which cannot distinguish between MSI-H and MSI-L among MSI(+) tumors, unlike with PCR testing. We did conduct an independent subset multivariable analysis and found that the pCR rate was 4.3% for MSI-L (OR 0.46, P = 0.02) and 8.1% for MSI-H (OR 0.90, P = 0.74). Correlations with complete response were statistically validated in a multivariable setting for both MSI(+) in overall analysis and MSI-L in subset analysis. Based on the trends suggested in this subset analysis, perhaps PCR testing for MSI-H/ MSI-L should be recommended after immunohistochemical staining for MSI(+) tumors.
CONCLUSIONS
To our knowledge this is the first comprehensive study to analyze the association between MSI and pathologic response to chemoradiation. Herein, we revealed a significant correlation between MSI(+) tumors and a reduction in pCR. The NCCN suggests MSI testing for all patients with colorectal cancers, a recommendation supported by the results of our analysis. MSI is a known prognosticator, but it might also help select appropriate patients for neoadjuvant and definitive management. Perhaps, future trials may investigate the role of dose-escalated radiation and/or immunotherapy in MSI(+) disease that is resistant to conventional treatment. Clearly, we have only scratched the surface of molecular profiling in rectal cancer, which may harbor mutations in KRAS, BRAF, PD-L1, and 18q, among many others. A greater understanding of these mutations and their mechanisms will hopefully lead to appropriate selection for optimal therapies.
ACKNOWLEDGMENTS
The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines () Rectal Cancer NCCN.org.; 2018. Available at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed May 14, 2018.
- 2.Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara T, Stolker JM, Watanabe T, et al. Accumulated clonal genetic alterations in familial and sporadic colorectal carcinomas with widespread instability in microsatellite sequences. Am J Pathol. 1998;153:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frame-shift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–997. [DOI] [PubMed] [Google Scholar]
- 5.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 6.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–1048. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Li J. Era of universal testing of microsatellite instability in colorectal cancer. World J Gastrointest Oncol. 2013;5:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li LS, Morales JC, Veigl M, et al. DNA mismatch repair (MMR)-dependent 5-fluorouracil cytotoxicity and the potential for new therapeutic targets. Br J Pharmacol. 2009;158:679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samowitz WS, Curtin K, Wolff RK, et al. Microsatellite instability and survival in rectal cancer. Cancer Causes Control. 2009;20:1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sammour T, Price BA, Krause KJ, et al. Nonoperative management or ‘watch and wait’ for rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy: a critical appraisal. Ann Surg Oncol. 2017;24:1904–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dossa F, Acuna SA, Rickles AS, et al. Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol. 2018;4:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winchester DP, Stewart AK, Bura C, et al. The National Cancer Database: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004;85: 1–3. [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami H, Zaanan A, Sinicrope FA. MSI testing and its role in the management of colorectal cancer Opinion Statement. Curr Treat Options Oncol. 2015;16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Soler M, Pérez-Carbonell L, Guarinos C, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013;144:926–932. [DOI] [PubMed] [Google Scholar]
- 18.Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol. 2009;74: 673–688. [DOI] [PubMed] [Google Scholar]
- 19.Smith FM, Reynolds JV, Miller N, et al. Pathological and molecular predictors of the response of rectal cancer to neoadjuvant radiochemotherapy. Eur J Surg Oncol. 2006;32:55–64. [DOI] [PubMed] [Google Scholar]
- 20.Shin J-S, Tut TG, Yang T, et al. Radiotherapy response in microsatellite instability related rectal cancer. Korean J Pathol. 2013;47:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franchitto A, Pichierri P, Piergentili R, et al. The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene. 2003;22:2110–2120. [DOI] [PubMed] [Google Scholar]
- 22.Barwell J, Pangon L, Hodgson S, et al. Biallelic mutation of MSH2 in primary human cells is associated with sensitivity to irradiation and altered RAD51 foci kinetics. J Med Genet. 2007;44:516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valentini V, van Stiphout RGPM, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163–3172. [DOI] [PubMed] [Google Scholar]
- 24.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917–923. [PubMed] [Google Scholar]
- 25.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. [DOI] [PubMed] [Google Scholar]
- 26.Nazemalhosseini Mojarad E, Kashfi SMH, Mirtalebi H, et al. Low level of microsatellite instability correlates with poor clinical prognosis in stage II colorectal cancer patients. J Oncol. 2016;2016:2196703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright CM, Dent OF, Newland RC, et al. Low level microsatellite instability may be associated with reduced cancer specific survival in sporadic stage C colorectal carcinoma. Gut. 2005;54:103–108. doi: 10.1136/gut.2003.034579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. [DOI] [PubMed] [Google Scholar]
- 29.Sinukumar S, Patil P, Engineer R, et al. Clinical Outcome of patients with complete pathological response to neoadjuvant chemoradiotherapy for locally advanced rectal cancers: the Indian scenario. Gastroenterol Res Pract. 2014;2014:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 31.Al-Sukhni E, Attwood K, Mattson DM, et al. Predictors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2016;23:1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habr-Gama A, Rodrigo Perez BO, Sabbaga J, et al. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52:1927–1934. [DOI] [PubMed] [Google Scholar]
- 34.Van Wickle JD, Paulson ES, Landry JC, et al. Adaptive radiation dose escalation in rectal adenocarcinoma: a review. J Gastrointest Oncol. 2017;8:902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bossé D, Mercer J, Raissouni S, et al. PROSPECT eligibility and clinical outcomes: results from the Pan-Canadian Rectal Cancer Consortium. Clin Colorectal Cancer. 2016;15:243–249. [DOI] [PubMed] [Google Scholar]
- 36.George TJ, Yothers G, Hong TS, et al. Utilizing total neoadjuvant therapy (TNT) in rectal cancer: NRG-GI002, a phase II clinical trial platform. J Clin Oncol. 2017;35(4_suppl):TS814–TS1814. [Google Scholar]
- 37.Ferrari L, Fichera A. Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterol Rep. 2015;3:277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Combination Chemotherapy, Bevacizumab, and/or Atezolizumab in Treating Patients With Deficient DNA Mismatch Repair Metastatic Colorectal Cancer: Full Text View: ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT02997228. Accessed May 17, 2018


