Abstract
Objective
To assess temporal trends in the association between newly diagnosed atrial fibrillation and death.
Design
Community based cohort study.
Setting
Framingham Heart Study cohort, in 1972-85, 1986-2000, and 2001-15 (periods 1-3, respectively), in Framingham, MA, USA.
Participants
Participants with no atrial fibrillation, aged 45-95 in each time period, and identified with newly diagnosed atrial fibrillation (or atrial flutter) during each time period.
Main outcome measures
The main outcome was all cause mortality. Hazard ratios for the association between time varying atrial fibrillation and all cause mortality were calculated with adjustment for time varying confounding factors. The difference in restricted mean survival times, adjusted for confounders, between participants with atrial fibrillation and matched referents at 10 years after a diagnosis of atrial fibrillation was estimated. Meta-regression was used to test for linear trends in hazard ratios and restricted mean survival times over the different time periods.
Results
5671 participants were selected in time period 1, 6177 in period 2, and 6174 in period 3. Adjusted hazard ratios for all cause mortality between participants with and without atrial fibrillation were 1.9 (95% confidence interval 1.7 to 2.2) in time period 1, 1.4 (1.3 to 1.6) in period 2, and 1.7 (1.5 to 2.0) in period 3 (Ptrend=0.70). Ten years after diagnosis of atrial fibrillation, the adjusted difference in restricted mean survival times between participants with atrial fibrillation and matched referents decreased by 31%, from −2.9 years (95% confidence interval −3.2 to −2.5) in period 1, to −2.1 years (−2.4 to −1.8) in period 2, to −2.0 years (−2.3 to −1.7) in period 3 (Ptrend=0.03).
Conclusions
No evidence of a temporal trend in hazard ratios for the association between atrial fibrillation and all cause mortality was found. The mean number of life years lost to atrial fibrillation at 10 years had improved significantly, but a two year gap compared with individuals without atrial fibrillation still remained.
Introduction
Atrial fibrillation is the most common sustained arrhythmia, with increases in the worldwide incidence and prevalence.1 2 3 4 The lifetime risk of atrial fibrillation is about one in three in men and women aged 55 and older.5 6 Projections show that the prevalence of atrial fibrillation could reach 15.9 million people in the United States by 2050 and 17.9 million people in Europe by 2060.7 8
Results from the Framingham Heart Study published in 1998 showed that newly diagnosed atrial fibrillation was associated with an increased risk of death.9 In a meta-analysis of 66 cohort studies, atrial fibrillation was associated with a 46% relative increase in all cause mortality.10 Raised awareness and regular publication of updated clinical guidelines have facilitated improvements in prognosis after a diagnosis of atrial fibrillation. Many studies have shown trends for improved short term and long term survival in individuals with atrial fibrillation.1 11 12 13 Previous studies did not compare mortality rates between individuals with and without atrial fibrillation, however, and data on temporal trends for the strength of association between atrial fibrillation and death are limited. Moreover, previous studies did not account for time varying confounding affected by previous exposure.14
Evaluating whether the association between atrial fibrillation and death has changed over time is essential to understand the success or failure of current management of atrial fibrillation. We hypothesized that the association between atrial fibrillation and death would decline over time. We examined the temporal trends in the association between newly diagnosed atrial fibrillation and death, measured as a hazard ratio and as a difference in restricted mean survival times,15 in participants in the Framingham Heart Study with nearly 45 years of follow-up.
Methods
Data sources
We analyzed data from the Framingham Heart Study, a community based cohort study designed to investigate risk factors for cardiovascular diseases. The original cohort started in 1948 and enrolled 5209 men and women aged 30-62 from Framingham, Massachusetts. Participants underwent standardized examinations every other year, including medical history, physical examination, laboratory tests, and 12 lead electrocardiograms. Children of the original cohort and their spouses were enrolled in the offspring cohort in 1971 (n=5124), and were examined every four to eight years.16 Adult children from the offspring cohort were enrolled in the third generation cohort in 2002 (n=4095), and were examined every six to eight years.17 The new offspring spouse cohort was started in 2003 (n=103), and the Omni 1 (n=506) and Omni 2 (n=410) cohorts in 1994 and 2003, respectively.
Participants
For practical purposes, we selected three time periods based on calendar years and number of participants: 1972-85, 1986-2000, and 2001-15. In each time period, we selected participants who did not have atrial fibrillation and were aged 45 or older. We excluded participants aged 95 or older or those with prevalent atrial fibrillation at the time of entry. We considered participants at risk from their age at entry in the selected time period until death, age at last follow-up, or age at the end of the time period, whichever came first. Participants were eligible to re-enter the analyses for subsequent time periods, but for different age periods, if they were still alive, aged 45-95, and did not have atrial fibrillation; in such cases, we updated the entry age and the characteristics of each participant at the beginning of the next time period (supplementary fig S1). We considered each index examination with its follow-up period to be a separate person examination.18
We considered participants from examinations 13-28 (1972-2005) for the original cohort; examinations one to nine (1971-2014) for the offspring cohort; examinations one to two (2002-14) for the third generation cohort; examinations one to two (2003-11) for the new offspring spouse cohort; examinations one to four (1994-2014) for the Omni 1 cohort; and examinations one to two (2009-11) for the Omni 2 cohort (supplementary table S1).
Assessment of newly diagnosed atrial fibrillation
The exposure was newly diagnosed atrial fibrillation or atrial flutter as a time varying variable, which was assessed until 31 December 2015. Participants were classified as having newly diagnosed atrial fibrillation if at least two cardiologists from the Framingham Heart Study confirmed that atrial fibrillation or atrial flutter was found on an electrocardiogram from a study examination or from an external clinician, by Holter monitoring, or noted on the hospital records. Staff from the Framingham Heart Study contacted hospital and medical clinics to collect records of outpatient appointments and admissions to hospital for cardiovascular diseases based on their history of health, updated every 24 months. Questions about atrial fibrillation (atrial fibrillation and atrial flutter) were included in the history of health updates for participants outside the clinical visits or admissions to hospital.
Outcomes
The primary outcome was all cause mortality. Secondary outcomes included cause specific mortality categorized as cardiovascular deaths (coronary heart disease, cerebrovascular accidents, and other cardiovascular causes) and non-cardiovascular deaths (cancers, other causes, and unknown causes). A panel of three physicians from the Framingham Heart Study conducted death reviews based on: hospital admission and emergency department records; imaging and laboratory reports; physicians' notes; death certificates; autopsy and medical examiners' reports; and a telephone call to family members by a physician in the absence of other records.At least two of the three members of the review panel were required to agree on the cause of death.
Covariates
We considered these clinical characteristics in the analyses: current smoking, body mass index, systolic and diastolic blood pressure, use of drug treatments for hypertension, diabetes mellitus, total cholesterol, use of statins, history of heart failure, history of myocardial infarction, history of stroke or transient ischemic attack, and educational level. Education was defined categorically as no high school degree, high school degree only, some college but no college degree, or a college degree. A panel of three investigators, including a neurologist, reviewed all records from relevant hospital admissions and clinic reported events for a diagnosis of stroke or transient ischemic attack. All covariates, except education, were updated from the repeated examination cycles.
Statistical analyses
To estimate the association between atrial fibrillation and the risk of death, we used Cox proportional hazard models. We defined age as the time scale and we used the counting process style of input, with at risk intervals defined by entry and exit ages. We modeled atrial fibrillation as a time varying covariate to account for the binary exposure status changing once for some participants within a time period from having no atrial fibrillation to having atrial fibrillation. By adding atrial fibrillation as a time varying covariate to the Cox model, the corresponding relative hazard is also time varying and no longer a proportional hazards model. We estimated hazard ratios with 95% confidence intervals. The hazard ratios compared the rate of death between individuals with atrial fibrillation and those who had not yet developed atrial fibrillation at each death time. Model 1 was adjusted for age and sex, model 2 was further adjusted for covariates at entry age, and model 3 further accounted for time varying covariates. Covariates included current smoking, body mass index, systolic and diastolic blood pressure, use of drug treatment for hypertension, diabetes mellitus, total cholesterol, use of statins, history of heart failure, history of myocardial infarction, history of stroke or transient ischemic attack, and educational level. For model 3, we used marginal structural models with inverse probability weighting to adjust for time dependent confounding.19 Because we examined atrial fibrillation as a time varying exposure, a time varying confounder might have been present, affected by previous atrial fibrillation exposure, that acted as an intermediate in the pathway between the atrial fibrillation exposure and the death outcome. For example, participants who developed atrial fibrillation are at higher risk of developing heart failure or myocardial infarction, and heart failure and myocardial infarction are associated with an increased hazard of death. We analyzed the three time periods separately. Trends in hazard ratios across the time periods were tested with meta-regression models of log hazard ratios against a linear term for time period.
To derive absolute measures of the association between atrial fibrillation and death, we also conducted a matched cohort analysis (supplementary fig S2). Within each time period, we matched each participant with atrial fibrillation with up to two referents without atrial fibrillation, according to age at diagnosis of atrial fibrillation, sex, and the Framingham Heart Study cohort. We derived survival curves from age at diagnosis of atrial fibrillation for participants and referents, adjusted for clinical covariates at age of newly diagnosed atrial fibrillation with inverse probability weighting.20 We estimated the adjusted difference in restricted mean survival times between participants with atrial fibrillation and referents at 10 years after a diagnosis of atrial fibrillation.21 22 The restricted mean survival time quantifies the loss in life expectancy over 10 years after a diagnosis of atrial fibrillation when comparing participants with atrial fibrillation with referents. We also tested for temporal trends in the differences in restricted mean survival times across the time periods by a meta-regression model.
Lastly, we examined cause specific mortality. In each time period, we estimated the cumulative incidence of cardiovascular deaths and non-cardiovascular deaths with the Aalen-Johansen estimator to account for competing risks. To assess the association between time varying atrial fibrillation and cause specific deaths, we used an illness-death model and fitted cause specific proportional hazards models with time dependent atrial fibrillation (supplementary fig S3). We computed hazard ratios with 95% confidence intervals. We used the R software 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) with packages survival, etm, and ipw, and the akm_rmst function.21
Subgroup and sensitivity analyses
We performed analyses stratified by sex. We also performed three sensitivity analyses. First, we excluded participants who died within 30 days of newly diagnosed atrial fibrillation, to remove the effect of comorbidities with a high case fatality rate. Second, we categorized newly diagnosed participants with atrial fibrillation into those detected at a study examination and those diagnosed by electrocardiography outside of the Framingham Heart Study examinations. We censored participants with atrial fibrillation detected at a study examination at the time of diagnosis, to account for the fact that these participants are more likely to be asymptomatic compared with atrial fibrillation detected outside of a study examination. Third, we censored participants with newly diagnosed atrial fibrillation associated with an acute reversible precipitant at the time of diagnosis. Secondary precipitants included acute myocardial infarction (within 30 days), acute pericardial disease surgery (within 30 days), thyrotoxicosis, acute alcohol consumption, acute infection, and acute pulmonary pathology.23
Missing data
For all analyses, we used multiple imputations to account for missing values for body mass index, systolic and diastolic blood pressure, smoking status, diabetes, drug treatment for hypertension, total cholesterol, use of statins, and education. We imputed data beginning at examination 11 in the original cohort and examination one in the offspring, third generation, new offspring spouse, Omni 1, and Omni 2 cohorts. We imputed missing covariate values at entry examinations and both missing covariate values and non-attended examinations throughout the follow‐up period. As described previously, we used fully conditional specification methods to impute values sequentially to account for the temporality of covariates across successive examination cycles.24 25 Individuals were removed from the imputation model at death. We used fully conditional specification regression methods for continuous variables, and fully conditional specification discriminant functions for categorical variables. The imputation model included age, sex, current smoking, height, weight, systolic and diastolic blood pressure, drug treatment for hypertension, diabetes mellitus, total cholesterol, use of statins, and educational level. We created 30 imputed datasets and combined estimates with Rubin’s rules. We used PROC MI of the SAS software, version 9.4 (SAS Institute, Cary, NC) to perform the imputation stage.
Patient and public involvement
Funding was not available to train or support patients or members of the public to work with us on this study. The Framingham Heart Study is ongoing and the internal question arose from clinical need; no patients were present in this setting. We then appraised these secondary data and analyzed them without public or patient involvement, as we did not have patients or members of the public available to us with the level of methodological and statistical experience to analyze or interpret these results.
Results
Characteristics of participants
We included 10 816 participants without atrial fibrillation at entry, of whom 5660 contributed to more than one time period (supplementary table S2). Supplementary figure S4 shows flow diagrams for the selection of participants: 5671 contributed to time period 1 (1972-85), 6177 to period 2 (1986-2000), and 6174 to period 3 (2001-15). The proportions of participants who were non-smokers, had diabetes, or received drug treatment for hypertension increased over time (table 1).
Table 1.
Characteristics of participants at entry, and of participants with atrial fibrillation at diagnosis, in each time period
| All participants | Participants with atrial fibrillation | ||||||
|---|---|---|---|---|---|---|---|
| Time period 1 (1972-85; n=5671) | Time period 2 (1986-2000; n=6177) | Time period 3 (2001-15; n=6174) | Time period 1 (1972-85; n=305) | Time period 2 (1986-2000; n=596) |
Time period 3 (2001-15; n=468) | ||
| Age (years) | 59.0 (10.5) | 59.9 (12.2) | 61.0 (12.5) | 74.4 (9.5) | 76.6 (10.5) | 77.8 (10.7) | |
| Sex (% women) | 54.6 (n=3094) | 56.2 (n=3474) | 56.7 (n=3501) | 48.2 (n=147) | 45.8 (n=273) | 45.7 (n=214) | |
| Education (%) | |||||||
| No high school degree | 26.6 (n=1415) | 15.3 (n=867) | 4.9 (n=278) | 42.5 (n=122) | 28.6 (n=160) | 10.5 (n=47) | |
| High school degree only | 34.1 (n=1810) | 31.2 (n=1776) | 23.7 (n=1353) | 28.9 (n=83) | 33.1 (n=185) | 32.7 (n=147) | |
| Some college | 21.3 (n=1130) | 25.2 (n=1435) | 29.6 (n=1685) | 16.7 (n=48) | 30.6 (n=115) | 22.5 (n=101) | |
| College graduate | 18.0 (n=956) | 28.3 (n=1610) | 41.8 (n=2380) | 11.9 (n=34) | 17.7 (n=99) | 34.3 (n=154) | |
| Systolic blood pressure (mm Hg) | 134 (21) | 132 (21) | 126 (18) | 145 (23) | 142 (22) | 135 (20) | |
| Diastolic blood pressure (mm Hg) | 80 (11) | 78 (10) | 75 (10) | 77 (12) | 74 (11) | 71 (11) | |
| Hypertension treatment (%) | 18.9 (n=1070) | 26.3 (n=1616) | 36.3 (n=2227) | 44.4 (n=134) | 56.5 (n=331) | 65.3 (n=303) | |
| Body mass index | 26.4 (4.3) | 27.0 (4.9) | 28.1 (5.6) | 26.3 (4.5) | 27.7 (4.9) | 28.8 (5.6) | |
| Current smoker (%) | 32.1 (n=1789) | 20.0 (n=1230) | 10.3 (n=635) | 20.1 (n=59) | 12.5 (n=74) | 6.6 (n=31) | |
| Diabetes mellitus (%) | 5.7 (n=304) | 6.8 (n=402) | 10.8 (n=628) | 12.3 (n=35) | 17.7 (n=83) | 22.6 (n=92) | |
| Total cholesterol (mmol/L) | 5.8 (1.1) | 5.6 (1.1) | 4.9 (0.9) | 5.7 (1.3) | 5.2 (1.1) | 4.6 (0.9) | |
| Use of statins (%) | 1.8 (n=99) | 3.2 (n=198) | 28.7 (n=1771) | 1.7 (n=5) | 8.1 (n=48) | 48.5 (n=227) | |
| Previous heart failure (%) | 1.1 (n=62) | 1.2 (n=75) | 1.0 (n=64) | 8.5 (n=26) | 6.7 (n=40) | 4.5 (n=21) | |
| Previous myocardial infarction (%) | 4.7 (n=265) | 4.7 (n=287) | 3.5 (n=213) | 15.1 (n=46) | 15.1 (n=90) | 8.8 (n=41) | |
| Previous stroke or transient ischemic attack (%) | 2.4 (n=133) | 3.1 (n=194) | 3.0 (n=184) | 12.5 (n=38) | 10.6 (n=63) | 8.8 (n=41) | |
Data are mean (standard deviation) or percentage (frequency). Corresponding percentages of missing values for time periods 1, 2, and 3, respectively: body mass index=1.0%, 3.7%, and 6.1%; systolic blood pressure=0.1%, 0.1%, and 0.2%; diastolic blood pressure=0.1%, 0.1%, and 0.2%; current smoker=1.7%, 0.2%, and 0.1%; diabetes mellitus=6.1%, 4.5%, and 6.2%; hypertension treatment=0.3%, 0.4%, and 0.6%; total cholesterol=3.8%, 5.7%, and 6.1%; use of statins=0.1%, 0.2%, and 0.2%; education=6.3%, 7.9%, and 7.7%.
During follow-up, 305 (5.4%) participants developed atrial fibrillation in time period 1, 596 (9.6%) in period 2, and 468 (7.6%) in period 3. For participants with atrial fibrillation, mean age at diagnosis, and the proportions of participants with diabetes and receiving drug treatment for hypertension, increased over time, whereas relatively fewer participants had prevalent heart failure or myocardial infarction over time (table 1). In participants with atrial fibrillation, 66 (21.6%) were detected during a study examination in time period 1, 43 (7.8%) in period 2, and seven (1.5%) in period 3 (supplementary table S3). Also, in participants with atrial fibrillation, 105 (34.4%) were secondary to a precipitant in time period 1, 265 (44.4%) in period 2, and 201 (42.9%) in period 3 (supplementary table S4). The characteristics of participants with atrial fibrillation at diagnosis and of their matched referents are reported in supplementary table S5.
Temporal trend in the association between newly diagnosed atrial fibrillation and all cause mortality
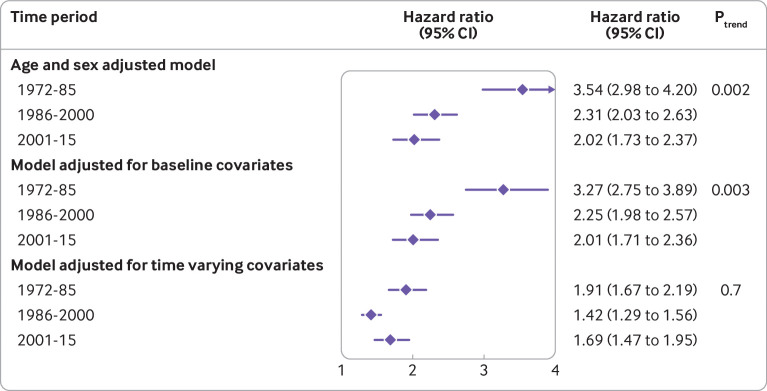
Newly diagnosed atrial fibrillation, compared with individuals without atrial fibrillation, was associated with an increased hazard of death in all time periods and all models (fig 1). In the model adjusted for time varying covariates, we found no evidence of a temporal trend in hazard ratios (Ptrend=0.70), with a hazard ratio of 1.91 (95% confidence interval 1.67 to 2.19) in time period 1, 1.42 (1.29 to 1.56) in period 2, and 1.69 (1.47 to 1.95) in period 3. Results from the matched cohort design were also consistent (supplementary table S6).
Fig 1.
Temporal trends for the association between newly diagnosed atrial fibrillation and all cause mortality. Data are hazard ratios (95% confidence intervals) for the association between time varying atrial fibrillation and death (in participants with v those without atrial fibrillation). Multivariable models were adjusted for the clinical covariates listed in table 1. Linear trends across time periods were tested by meta-regression models of log hazard ratios
To assess the loss in lifetime over 10 years after a diagnosis of atrial fibrillation, we analyzed 305 participants with atrial fibrillation and 610 matched referents in time period 1, 589 participants and 1170 referents in period 2, and 453 participants and 892 referents in period 3. Figure 2 shows the adjusted Kaplan-Meier survival curves by time period. The adjusted difference in the restricted mean survival time at 10 years between participants with atrial fibrillation and referents was −2.85 years (95% confidence interval −3.21 to −2.50) in time period 1, −2.10 years (−2.35 to −1.84) in period 2, and −1.99 years (95% CI −2.26 to −1.72) in period 3 (Ptrend=0.03).
Fig 2.
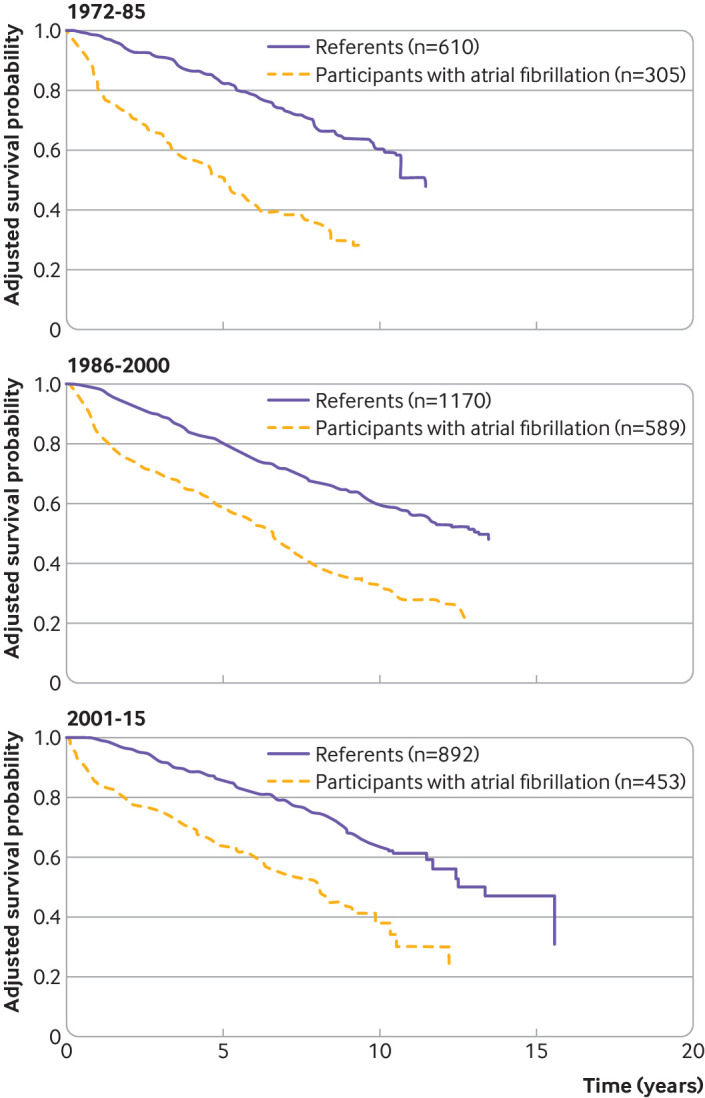
Adjusted survival curves for participants with atrial fibrillation and matched referents. Kaplan-Meier curves since time of diagnosis of atrial fibrillation for participants with atrial fibrillation and referents, matched on age at diagnosis of atrial fibrillation, sex, and Framingham Heart Study cohort, adjusted for clinical covariates at age of diagnosis of atrial fibrillation. The adjusted difference in the restricted mean survival time at 10 years between participants with atrial fibrillation and referents is the area between the survival curves: −2.85 years (95% confidence interval −3.21 to −2.50) in time period 1, −2.10 years (−2.35 to −1.84) in period 2, and −1.99 years (95% CI −2.26 to −1.72) in period 3 (Ptrend=0.03)
Subgroup analysis and sensitivity analyses
In the sex stratified analyses, we found an increased hazard of death associated with newly diagnosed atrial fibrillation in women and men (table 2). After adjusting for time varying covariates, the hazard ratio in time period 2 was larger in women than in men (1.73 v 1.23, P=0.005) but no evidence of a difference between the sexes in time periods 1 and 3 was found (P=0.06 and P=0.07, supplementary table S7). We found evidence of a decrease in hazard ratios over time in women, but not in men. The temporal trends in the differences in restricted mean survival times in men and women were consistent with the main analysis. In men, the difference in restricted mean survival time was −2.89 years in time period 1 versus −2.23 years in period 3 (Ptrend=0.59); in women, the difference in restricted mean survival time was −3.01 years in time period 1 versus −1.78 years in period 3 (Ptrend=0.04; supplementary fig S5 and fig S6).
Table 2.
Association between newly diagnosed atrial fibrillation and all cause mortality, by sex
| Time period | Hazard ratio (95% CI) for all cause death between participants with v without atrial fibrillation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | ||||||||||
| No of deaths/all participants |
No of deaths/participants with atrial fibrillation | Model 1 adjusted for age and sex | Model 2 adjusted for covariates at entry | Model 3 adjusted for time varying covariates | No of deaths/all participants | No of deaths/participants with atrial fibrillation | Model 1 adjusted for age and sex | Model 2 adjusted for covariates at entry | Model 3 adjusted for time varying covariates | ||
| 1972-85 | 485/3094 | 82/147 | 4.05 (3.17 to 5.18) | 3.63 (2.82 to 4.65) | 2.20 (1.81 to 2.67) | 568/2577 | 79/158 | 3.10 (2.44 to 3.95) | 2.92 (2.28 to 3.74) | 1.69 (1.39 to 2.06) | |
| 1986-2000 | 725/3474 | 151/273 | 2.87 (2.39 to 3.44) | 2.83 (2.36 to 3.40) | 1.73 (1.52 to 1.98) | 632/2703 | 147/323 | 1.92 (1.59 to 2.31) | 1.84 (1.53 to 2.22) | 1.23 (1.07 to 1.41) | |
| 2001-15 | 522/3501 | 96/214 | 1.85 (1.48 to 2.32) | 1.96 (1.56 to 2.47) | 1.49 (1.21 to 1.83) | 400/2673 | 107/254 | 2.22 (1.77 to 2.78) | 2.11 (1.68 to 2.66) | 1.95 (1.59 to 2.39) | |
| Ptrend | — | — | <0.001 | <0.001 | 0.007 | — | — | 0.37 | 0.37 | 0.76 | |
Data are hazard ratios (95% confidence interval) for the association between time varying atrial fibrillation and death. Models 2 and 3 were adjusted for clinical covariates listed in table 1. Linear trends across time periods were tested with meta-regression models of log hazard ratios.
In a sensitivity analysis, we removed deaths within 30 days of a diagnosis of atrial fibrillation and the results were consistent (supplementary table S8). Results after censoring participants with atrial fibrillation detected at a Framingham Heart Study examination are reported in supplementary table S9 and figure S7. Results after censoring secondary atrial fibrillation are reported in supplementary table S10 and figure S8. Both sensitivity analyses were consistent with the main findings for hazard ratios and for differences in restricted mean survival times.
Temporal trend in the association between newly diagnosed atrial fibrillation and cause specific mortality
We found a decrease in cardiovascular mortality over time in all participants, with a lifetime risk of cardiovascular death of 35% (95% confidence interval 32% to 39%) in time period 1 and 16% (13% to 18%) in period 3 (supplementary fig S9). In contrast, non-cardiovascular mortality was similar across the three time periods (supplementary fig S10). Across all time periods, the association between atrial fibrillation and cardiovascular death was larger than for non-cardiovascular death (table 3). No evidence of change over time was found for the association between atrial fibrillation and cardiovascular death, whereas the strength of association between atrial fibrillation and non-cardiovascular death decreased over time (hazard ratio 3.2 in time period 1 v 2.0 in time period 3, table 3). We also used the matched cohort approach to estimate the association between atrial fibrillation and cause specific mortality (supplementary table S11). The results were consistent with the main analyses.
Table 3.
Association between newly diagnosed atrial fibrillation and cause specific mortality
| Time period | Hazard ratio (95% CI) for cause specific death between participants with v without atrial fibrillation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular death | Non-cardiovascular death | ||||||||
| No of deaths | Model 1 adjusted for age and sex | Model 2 adjusted for covariates at entry | Model 3 adjusted for time varying covariates | No of deaths |
Model 1 adjusted for age and sex | Model 2 adjusted for covariates at entry | Model 3 adjusted for time varying covariates | ||
| 1972-85 (n=5671) | 425 | 6.11 (4.76 to 7.85) | 5.54 (4.30 to 7.14) | 5.00 (3.84 to 6.51) | 628 | 3.16 (2.47 to 4.05) | 3.14 (2.45 to 4.03) | 3.16 (2.47 to 4.03) | |
| 1986-2000 (n=6177) | 351 | 4.18 (3.28 to 5.32) | 3.82 (3.00 to 4.87) | 2.99 (2.30 to 3.88) | 1006 | 2.65 (2.25 to 3.12) | 2.68 (2.28 to 3.16) | 2.36 (1.99 to 2.79) | |
| 2001-15 (n=6174) | 175 | 6.74 (4.90 to 9.26) | 6.63 (4.78 to 9.18) | 6.69 (4.80 to 9.31) | 747 | 1.95 (1.61 to 2.38) | 2.03 (1.66 to 2.47) | 2.04 (1.67 to 2.50) | |
| Ptrend | — | 0.86 | 0.76 | 0.71 | — | 0.002 | 0.005 | 0.008 | |
Data are hazard ratios (95% confidence interval) from an illness death model; cause specific proportional hazards models were fitted with time dependent atrial fibrillation. Models 2 and 3 were adjusted for clinical covariates listed in table 1. Linear trends across time periods were tested by meta-regression models of log hazard ratios.
Discussion
Principal findings
Over nearly 45 years of observation in a community based cohort, we found that newly diagnosed atrial fibrillation was associated with an increased hazard of death over three time periods. No evidence of a temporal trend in hazard ratios between newly diagnosed atrial fibrillation and all cause mortality was found. The differences in restricted mean survival times 10 years after newly diagnosed atrial fibrillation, however, decreased over time, from 2.9 to 2.0 years of life expectancy after atrial fibrillation, between time periods 1 and 3. In the analyses of cause specific mortality, we found a decreasing trend in the association between atrial fibrillation and non-cardiovascular death, but not in relation to cardiovascular death.
Possible explanations for our findings
Several reasons could explain a decrease in the association between atrial fibrillation and death over time. The first clinical guideline recommendations for the treatment of atrial fibrillation were published in the US in 2001,26 and since then, new evidence has regularly been incorporated into updates to the guideline. Primary and secondary prevention of thromboembolism are important to improve life expectancy in individuals with atrial fibrillation.27 28 In 1954, the US Food and Drug Administration approved the use of warfarin, which was the only long term anticoagulant drug until the introduction in 2010 of non-vitamin K antagonist oral anticoagulants. Vitamin K antagonists, such as warfarin, reduced the risk of all cause mortality by 26% compared with placebo,29 whereas non-vitamin K antagonist oral anticoagulants reduced all cause mortality by 10% compared with warfarin.30 The use of anticoagulation might be inadequate as physicians’ adherence to clinical recommendations varies, but temporal trends in the prescription of anticoagulation suggest improvements over time.31 32 Another reason for an expected reduction in mortality associated with newly diagnosed atrial fibrillation is the potential early identification of individuals at low risk because of increased awareness, and use of routine electrocardiographs and extended electrocardiographic monitoring devices. Individuals at low risk might have a more favorable prognosis because of starting treatments early for modifiable risk factors and prevention of stroke.
As life expectancy of the general population is increasing, in part because of reduced mortality rates at older ages,33 our findings could indicate a similar reduction in mortality rate in individuals with atrial fibrillation over time. The reasons for the lack of greater improvement in the prognosis in individuals with atrial fibrillation are unknown. Individuals with atrial fibrillation have a high prevalence of comorbidities and the level of multimorbidity reflects the prognosis.34 To our knowledge, however, no studies have examined the temporal trends in the burden of comorbidities and related mortality in participants with atrial fibrillation compared with individuals without atrial fibrillation. That the burden of comorbidities has grown over time because mortality rates in the general population have declined is likely,33 and the burden of multimorbidity could be related to a prognosis that is difficult to improve.
In the analyses of temporal trends, we measured the association between atrial fibrillation and death with the conventional hazard ratio and the difference in restricted mean survival times. Absolute measures of association, such as years of life lost, might be particularly important in public health decision making.35 36 We found that the hazard ratios decreased over time when adjusted for age, sex, and baseline covariates, but when adjusting for time varying confounders, the trend was reduced. Evidence of a decreasing trend in the differences in restricted mean survival time, however, was found. Interaction analysis depends on the analysis scale, and interaction on the absolute scale (difference in restricted mean survival time) without interaction on the multiplicative scale (hazard ratio) is mathematically possible.37 Also, detecting interactions with multiplicative models, such as the Cox regression model, is uncommon.38 Results from additive models, however, such as the trends seen in years of life lost, might not apply to populations other than those with similar risk factor distributions as the participants in the Framingham Heart Study.38 Another potential explanation for the discrepancy is that, in the Cox model, we considered participants with no atrial fibrillation; we modeled atrial fibrillation as a time varying variable, and we adjusted for time varying variables to account for differences between participants over the course of their trajectories. In contrast, when analyzing restricted mean survival times, we considered participants with atrial fibrillation and matched referents, and we adjusted for fixed covariates at the age of diagnosis of atrial fibrillation.
We found evidence of higher all cause mortality after a diagnosis of atrial fibrillation in women than in men in time period 2 (1986-2000), but no difference in hazard ratios between women and men for time periods 1 (1972-85) or 3 (2001-15) was found. A larger increase in mortality rate after atrial fibrillation in women is similar to previous results.9 39 In a recent meta-analysis of 30 studies, the ratio of hazard ratios for women compared with men was 1.12 (95% confidence 1.07 to 1.17).39
Comparison with other studies
Several studies have examined temporal trends in survival after newly diagnosed atrial fibrillation.1 11 12 40 These studies, however, compared participants with atrial fibrillation across different years, and most studies did not assess the rate of death in participants with atrial fibrillation compared with those without atrial fibrillation. Hence these temporal trends from previous studies do not account for the underlying potential improvements in survival of the general population. In a study comparing mortality in individuals with atrial fibrillation among Medicare beneficiaries, older than 65, from 1993 to 2007, with the US general population,41 no evidence of improvement in relative mortality was found, consistent with the results of our study.41
Limitations of the study
Our study had important limitations. We did not have enough information on treatments related to atrial fibrillation, such as anticoagulation or drug treatments for underlying cardiovascular diseases. Previous studies showed increased use of anticoagulation in patients with atrial fibrillation over time in the US.31 42 43 The increasing use of anticoagulation in patients with atrial fibrillation partly explains the improvement in survival in this group. We combined the diagnoses of atrial fibrillation and atrial flutter, and included no information on the pattern of atrial fibrillation, such as paroxysmal or chronic atrial fibrillation, and therefore we could not report on temporal trends in specific subtypes.
The recent establishment of the newest Framingham cohorts, including the third generation cohort and the Omni 2 cohort, limited the length of follow-up. Also, we could not definitively establish a causal relation between atrial fibrillation and death, and we cannot rule out residual confounding from severity of disease or unmeasured factors, such as genetics or variables related to lifestyle. Although the Framingham Heart Study included families, we did not account for shared environmental factors that could affect the development of atrial fibrillation and subsequent hazard of death. Previous studies have shown familial aggregation of atrial fibrillation.44 In patients with atrial fibrillation, however, those with and those without a first degree relative affected by atrial fibrillation had a similar risk of stroke and coronary events.45
Atrial fibrillation is frequently unrecognized, and we acknowledge that temporal trends in earlier detection of atrial fibrillation over time might be present. The mean age at diagnosis of atrial fibrillation has increased over time, however, and could be because of improved treatment of cardiac risk factors (eg, hypertension, smoking cessation) and better management of cardiac conditions (eg, heart failure and myocardial infarction), leading to a later diagnosis of atrial fibrillation. Moreover, participants and referents were matched according to age at diagnosis of atrial fibrillation, and our analysis compared life expectancy from the same index age in each set of participants with atrial fibrillation and referents, across all time periods. This finding suggests that lead time bias is unlikely to explain our findings.
Also, our multiple imputation analyses assumed that covariates were missing at random. We did not assess the sensitivity of analyses under the missing-at-random assumption to the not-missing-at-random assumption. Another limitation is that our statistical test for temporal trends was based on meta-regression across only three time periods and might have limited the statistical power, particularly in subgroups of men and women. We performed multiple trend tests without adjustment for multiplicity and we cannot exclude the possibility of spurious findings. Finally, our results might not apply to other racial or ethnic groups because the Framingham Heart Study has mainly European participants.
Conclusions
We found no evidence of a temporal trend in hazard ratios between newly diagnosed atrial fibrillation and all cause mortality. The hazard ratios for non-cardiovascular death declined over time but no evidence of a temporal trend for cardiovascular death was found. Mortality associated with newly diagnosed atrial fibrillation remained high compared with individuals without atrial fibrillation, despite showing some improvements over the past 45 years. More than 10 years after a diagnosis of atrial fibrillation, individuals with atrial fibrillation lose about two years of life compared with matched referents.
What is already known on this topic
Newly diagnosed atrial fibrillation is associated with an increased hazard of death
Short term and long term survival probabilities in individuals with atrial fibrillation have improved over time
What this study adds
Comparing participants with and without atrial fibrillation in the community based Framingham Heart Study, we did not find evidence of temporal trends in the relative rate of dying from all causes
The mean number of life years lost to atrial fibrillation at 10 years improved significantly over 45 years, but a two year gap compared with individuals without atrial fibrillation was still present
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: NV, LF, and LT developed the hypothesis and study design. QH and LT did the statistical analysis. NV and LT wrote the first and successive drafts of the manuscript. All authors contributed to the study concept and design, interpretation of the data, and drafting or critical revision of the manuscript for important intellectual content or additionally to data acquisition. QH and LT had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. LT is the guarantor. We thank the participants and staff of the Framingham Heart Study for their valuable contributions.
Funding: This work was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI), Framingham Heart Study (NHLBI/NIH contract No HHSN268201500001I), and the Boston University School of Medicine. This work was also supported by the American Heart Association (18SFRN34110082, 18SFRN34150007, and 1RC1HL101056) and the NIH/NHLBI (R01 HL128914, R01 HL092577, and R01 HL126136). The Framingham Heart Study is supported by 75N92019D00031. The funding sources had no role in the design, analysis, and reporting of the study.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI), Framingham Heart Study, and the Boston University School of Medicine for the submitted work; LF reports grants from the Health Research Foundation of Central Denmark Region, personal fees from Bristol-Myers Squibb, personal fees from Pfizer, personal fees from Bayer, and personal fees from Merck Sharp and Dohme, outside of the submitted work. EJB serves as an uncompensated member of the MyHeartLab Steering Committee. The MyHeartLab Study is a principal investigator initiated study from the University of California San Francisco (UCSF): principal investigator Jeffrey Olgin, through a research grant to UCSF from Samsung. NV, QH, MF-G, and LT have no competing interests.
Ethical approval: The Framingham Heart Study was approved by the Boston Medical Center and Boston University Medical Campus institutional review board (protocol No H-32132). Participants signed informed consent at study examinations.
Data sharing: Participant level data are available at the database of Genotypes and Phenotypes (https://www.ncbi.nlm.nih.gov/gap/) and BioLINCC (https://biolincc.nhlbi.nih.gov/home/).
LT affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Results from the Framingham Heart Study are routinely disseminated to participants by the study website and newsletters as well as social media outlets. Findings will be disseminated by the media departments of the authors’ institutes. Results of the study will also be linked in the Framingham Heart Study website and shared by social media outlets for participants and relevant patient and public communities.
References
- 1. Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154-62. 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lane DA, Skjøth F, Lip GYH, Larsen TB, Kotecha D. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 2017;6:e005155. 10.1161/JAHA.116.005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martinez C, Katholing A, Wallenhorst C, Granziera S, Cohen AT, Freedman SB. Increasing incidence of non-valvular atrial fibrillation in the UK from 2001 to 2013. Heart 2015;101:1748-54. 10.1136/heartjnl-2015-307808. [DOI] [PubMed] [Google Scholar]
- 4. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837-47. 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Magnussen C, Niiranen TJ, Ojeda FM, et al. BiomarCaRE Consortium Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017;136:1588-97. 10.1161/CIRCULATIONAHA.117.028981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Staerk L, Wang B, Preis SR, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018;361:k1453. 10.1136/bmj.k1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119-25. 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 8. Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746-51. 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-52. 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- 10. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ 2016;354:i4482. 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 11. Miyasaka Y, Barnes ME, Bailey KR, et al. Mortality trends in patients diagnosed with first atrial fibrillation. A 21-year community-based study. J Am Coll Cardiol 2007;49:986-92. 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt M, Ulrichsen SP, Pedersen L, Bøtker HE, Nielsen JC, Sørensen HT. 30-year nationwide trends in incidence of atrial fibrillation in Denmark and associated 5-year risk of heart failure, stroke, and death. Int J Cardiol 2016;225:30-6. 10.1016/j.ijcard.2016.09.071. [DOI] [PubMed] [Google Scholar]
- 13. Freeman JV, Wang Y, Akar J, Desai N, Krumholz H. National trends in atrial fibrillation hospitalization, readmission, and mortality for Medicare beneficiaries, 1999-2013. Circulation 2017;135:1227-39. 10.1161/CIRCULATIONAHA.116.022388. [DOI] [PubMed] [Google Scholar]
- 14. Mansournia MA, Etminan M, Danaei G, Kaufman JS, Collins G. Handling time varying confounding in observational research. BMJ 2017;359:j4587. 10.1136/bmj.j4587. [DOI] [PubMed] [Google Scholar]
- 15. Staerk L, Preis SR, Lin H, et al. Novel risk modeling approach of atrial fibrillation with restricted mean survival times: application in the Framingham Heart Study community-based cohort. Circ Cardiovasc Qual Outcomes 2020;13:e005918. 10.1161/CIRCOUTCOMES.119.005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med 1975;4:518-25. 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 17. Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328-35. 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 18. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840-4. 10.1001/jama.1994.03510350050036 [DOI] [PubMed] [Google Scholar]
- 19. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550-60. 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 20. Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 2005;24:3089-110. 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 21. Conner SC, Sullivan LM, Benjamin EJ, LaValley MP, Galea S, Trinquart L. Adjusted restricted mean survival times in observational studies. Stat Med 2019;38:3832-60. 10.1002/sim.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearce N. Analysis of matched case-control studies. BMJ 2016;352:i969. 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lubitz SA, Yin X, Rienstra M, et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation 2015;131:1648-55. 10.1161/CIRCULATIONAHA.114.014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nevalainen J, Kenward MG, Virtanen SM. Missing values in longitudinal dietary data: a multiple imputation approach based on a fully conditional specification. Stat Med 2009;28:3657-69. 10.1002/sim.3731. [DOI] [PubMed] [Google Scholar]
- 25. Conner SC, Lodi S, Lunetta KL, et al. Refining the association between body mass index and atrial fibrillation: G-formula and restricted mean survival times. J Am Heart Assoc 2019;8:e013011. 10.1161/JAHA.119.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fuster V, Rydén LE, Asinger RW, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) North American Society of Pacing and Electrophysiology ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology. Circulation 2001;104:2118-50. 10.1161/circ.104.17.2118. [DOI] [PubMed] [Google Scholar]
- 27. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [correction in: J Am Coll Cardiol 2019;74:599]. J Am Coll Cardiol 2019;74:104-32. 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 28. Kirchhof P, Benussi S, Kotecha D, et al. ESC Scientific Document Group 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 29. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 30. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62. 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 31. Katz DF, Maddox TM, Turakhia M, et al. Contemporary trends in oral anticoagulant prescription in atrial fibrillation patients at low to moderate risk of stroke after guideline-recommended change in use of the CHADS2 to the CHA2DS2-VASc score for thromboembolic risk assessment: analysis from the National Cardiovascular Data Registry’s outpatient practice innovation and clinical excellence atrial fibrillation registry. Circ Cardiovasc Qual Outcomes 2017;10:e003476. 10.1161/CIRCOUTCOMES.116.003476. [DOI] [PubMed] [Google Scholar]
- 32. Gadsbøll K, Staerk L, Fosbøl EL, et al. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J 2017;38:899-906. 10.1093/eurheartj/ehw658. [DOI] [PubMed] [Google Scholar]
- 33. Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743-800. 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chamberlain AM, Alonso A, Gersh BJ, et al. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: a population-based study. Am Heart J 2017;185:74-84. 10.1016/j.ahj.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poole C. Commentary: some thoughts on consequential epidemiology and causal architecture. Epidemiology 2017;28:6-11. 10.1097/EDE.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 36. Poole C. On the origin of risk relativism. Epidemiology 2010;21:3-9. 10.1097/EDE.0b013e3181c30eba. [DOI] [PubMed] [Google Scholar]
- 37. Greenland S, Rothman K. Concepts of interaction. In: Rothman K, Greenland S, Lash T, eds. Modern epidemiology. Lippincott Williams & Wilkins, 2008: 71-83. [Google Scholar]
- 38. Spiegelman D, VanderWeele TJ. Evaluating public health interventions: 6. Modeling ratios or differences? Let the data tell us. Am J Public Health 2017;107:1087-91. 10.2105/AJPH.2017.303810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016;532:h7013. 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frost L, Vestergaard P, Mosekilde L, Mortensen LS. Trends in incidence and mortality in the hospital diagnosis of atrial fibrillation or flutter in Denmark, 1980-1999. Int J Cardiol 2005;103:78-84. 10.1016/j.ijcard.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 41. Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes 2012;5:85-93. 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes 2012;5:615-21. 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rowan SB, Bailey DN, Bublitz CE, Anderson RJ. Trends in anticoagulation for atrial fibrillation in the U.S.: an analysis of the national ambulatory medical care survey database. J Am Coll Cardiol 2007;49:1561-5. 10.1016/j.jacc.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 44. Lubitz SA, Yin X, Fontes JD, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA 2010;304:2263-9. 10.1001/jama.2010.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang SH, Kuo CF, Chou IJ, et al. Association of a family history of atrial fibrillation with incidence and outcomes of atrial fibrillation: a population-based family cohort study. JAMA Cardiol 2017;2:863-70. 10.1001/jamacardio.2017.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material