Abstract
Global change influences biogeochemical cycles within and between environmental compartments (i.e., the cryosphere, terrestrial and aquatic ecosystems, and the atmosphere). A major effect of global change on carbon cycling is altered exposure of natural organic matter (NOM) to solar radiation, particularly solar UV radiation. In terrestrial and aquatic ecosystems, NOM is degraded by UV and visible radiation, resulting in the emission of carbon dioxide (CO2) and carbon monoxide, as well as a range of products that can be more easily degraded by microbes (photofacilitation). On land, droughts and land-use change can reduce plant cover causing an increase in exposure of plant litter to solar radiation. The altered transport of soil organic matter from terrestrial to aquatic ecosystems also can enhance exposure of NOM to solar radiation. An increase in emission of CO2 from terrestrial and aquatic ecosystems due to the effects of global warming, such as droughts and thawing of permafrost soils, fuels a positive feedback on global warming. This is also the case for greenhouse gases other than CO2, including methane and nitrous oxide, that are emitted from terrestrial and aquatic ecosystems. These trace gases also have indirect or direct impacts on stratospheric ozone concentrations. The interactive effects of UV radiation and climate change greatly alter the fate of synthetic and biological contaminants. Contaminants are degraded or inactivated by direct and indirect photochemical reactions. The balance between direct and indirect photodegradation or photoinactivation of contaminants is likely to change with future changes in stratospheric ozone, and with changes in runoff of coloured dissolved organic matter due to climate and land-use changes.
1. Introduction
Biogeochemical cycles involve the transformation of materials in the environment and their transport across interfaces between different compartments in the Earth system i.e., land, water, atmosphere and cryosphere (ice, snow and frozen ground), Fig. 1. These cycles govern changes in the concentration and form of carbon, nutrients, and contaminants that affect organisms and ecosystems. Biogeochemical cycles influence the concentration of trace gases in the atmosphere, including carbon dioxide (CO2) and other greenhouse gases, as well as air pollutants. Conversely, biogeochemical cycles in terrestrial and aquatic ecosystems are affected by changes in climate and stratospheric ozone (O3).
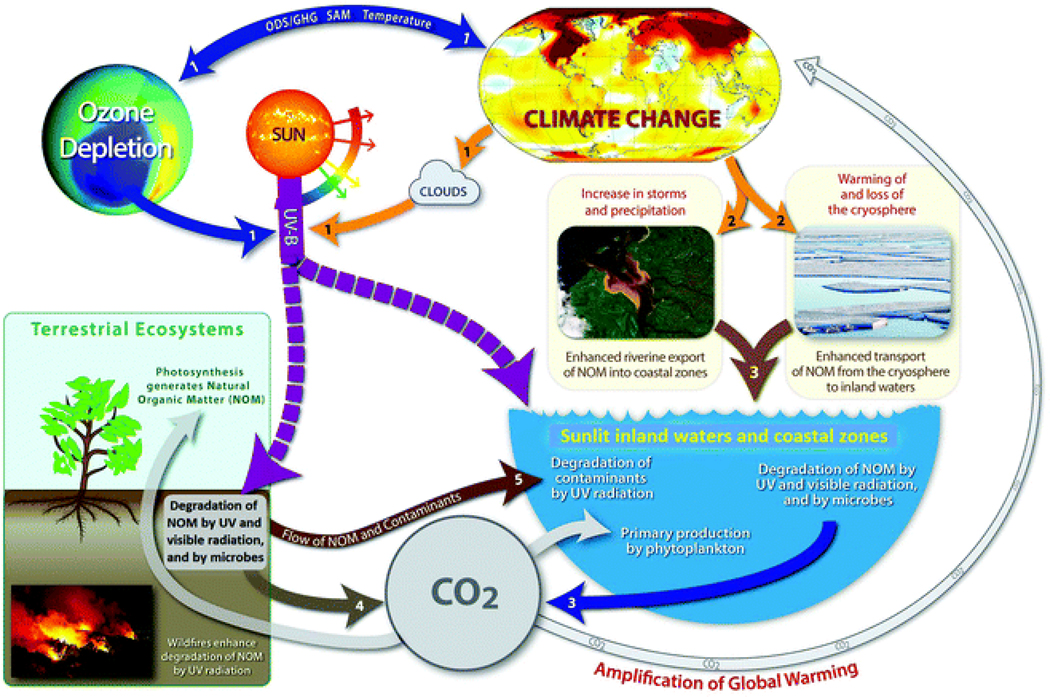
Fig. 1.
Interactive effects of solar radiation (UV and visible) and climate change on biogeochemical cycles. The numbers in the arrows refer to the following effects: (1) stratospheric ozone concentrations are affected by ozone depleting substances (ODSs) and control the intensity of solar UV radiation (see ref. 1). Climate change affects cloud formation and the intensity of UV radiation reaching the Earth’s surface (see ref. 1). Antarctic ozone depletion impacts local climates in the southern hemisphere via changes of the Southern Annular Mode (SAM) (see ref. 1 and 2), while Arctic amplification influences local climates in the northern hemisphere. Interactive effects of solar UV radiation and climate change affect the biogeochemical production and fate of greenhouse gases, particularly CO2, but also methane (CH4) and nitrous oxide (N2O), that impact climate and stratospheric ozone. (2) Important consequences of climate change are warming of and loss of the cryosphere, increasing frequency and intensity of droughts, wildfires, storms, and heavy precipitation events in different regions of the Earth. (3) Loss of the cryosphere and increases in storms and heavy precipitation events result in the enhanced transport of natural organic matter (NOM) from land to water, where it is degraded to CO2, carbon monoxide (CO), and other products by UV and visible radiation, and by microbes. (4) Similarly, degradation of NOM by UV and visible radiation, and by microbes occurs on soil surfaces. (5) Contaminants also undergo UV-induced degradation, either in direct or indirect photochemical reactions. Contaminants include organic contaminants, nanomaterials, microplastics, harmful algal blooms, and viruses.
In this paper, we assess current knowledge on biogeochemical cycles in the context of global change, including changes in stratospheric ozone, climate, land-use, and the interactions between these changes (Fig. 1). We concentrate on the biogeochemical cycling of carbon, precursors of reactive trace gases, and synthetic and biological contaminants. Within that very broad remit, we focus on the effects of solar UV radiation on these cycles. However, rather than the narrow focus on UV-B (280–315 nm) radiation that is appropriate for assessing depletion of stratospheric ozone in isolation, the effects of UV-A (315–400 nm) are now also considered, as well as short-wavelength visible radiation, since both UV and visible radiation are expected to change because of environmental changes (see ref. 1). For example, increases in the frequency and intensity of wildfires and, consequently, enhanced emissions of aerosols affect solar radiation in all the spectral ranges. This paper not only assesses the role of solar UV and visible radiation on the transformation of materials within compartments of the Earth-system but also how global change affects the transport between compartments, for example land–water, cryosphere–water, land–atmosphere, and water–atmosphere.
The transport of materials between land, the cryosphere, and water is strongly influenced by effects of global warming such as thawing of permafrost soils, more frequent and longer lasting heavy precipitation events in some regions of the Earth, and droughts and wildfires in other regions. Stratospheric ozone depletion and warming in polar regions influence weather patterns also in other regions of the southern and northern hemisphere. The major role of ozone depletion in Antarctica on regional climate in the southern hemisphere has been identified (see ref. 1) with concomitant effects on terrestrial and aquatic ecosystems3,4 (see ref. 2 and 5), and biogeochemical cycles.4,6 Similarly, in the northern hemisphere, stratospheric concentrations of ozone in the Arctic have been suggested to affect local climates, with a study showing that years with low Arctic stratospheric ozone in March coincided with colder than normal temperatures over southeastern Europe and southern Asia, but warmer than normal temperatures over northern Asia in March/April.7 Furthermore, changes in the Arctic due to Arctic amplification (i.e., where the Arctic warms faster than elsewhere in response to rising greenhouse-gas concentrations6,8,9) not only affect climate in the Arctic but also at mid-latitudes of the northern hemisphere.9–12 This phenomenon is linked to the jet stream which changes as a consequence of Arctic amplification and loss of sea ice.10,13 As a result, weather patterns have become more persistent and weather extremes more likely.9
Because of heavy precipitation events and loss of the cryosphere, e.g. thawing of permafrost soils, the flow of natural organic matter (NOM) from the land into water bodies is enhanced (Fig. 1 and 2). On land, NOM consists of plant and soil organic matter, whereas, in aquatic ecosystems, debris from algae and bacteria also contribute to NOM. However, in fresh and coastal waters, a large portion of NOM originates from terrestrial ecosystems and consists of terrestrial dissolved organic matter (tDOM), where a large part is coloured dissolved organic matter (CDOM) and particulate organic matter (POM). Therefore, the focus of this assessment regarding carbon cycling is on the effects of solar radiation, particularly solar UV radiation, and climate change on the fate of terrestrial NOM, both on land and in water. The break-down of NOM on land and in water, a process which is influenced by exposure to solar radiation, releases carbon dioxide (CO2) and, to a smaller extent, carbon monoxide (CO) into the atmosphere (Fig. 1 and 2).
Fig. 2.
Plants take up carbon dioxide (CO2) from the atmosphere and convert it to plant organic matter, of which light-absorbing lignin is a major component. When plants die, plant and soil organic matter is decomposed by soil microbes to CO2 and carbon monoxide (CO) that is returned to the atmosphere, and to smaller pools of organic matter (i.e., particulate and terrestrial dissolved organic matter; POM and tDOM, respectively). POM and tDOM are flushed to streams, rivers, and lakes in rain and snow, and to coastal waters via riverine export. On land and in sunlit surface waters, UV and visible radiation help decompose plant organic matter, POM, and tDOM to CO2 and CO. PAR, photosynthetic active radiation (400–700 nm).
Solar UV radiation also plays an important role in the degradation and inactivation of synthetic and biological contaminants in terrestrial and aquatic ecosystems, especially in the case of organic micropollutants that may pass through conventional sewage treatment plants without being degraded.14 Among organic contaminants, antibiotics as well as pesticides are of increasing environmental concern. Pesticides usually enter aquatic ecosystems untreated from non-point sources.15 Furthermore, some organic contaminants are persistent organic pollutants (POPs) with a long lifetime. Semi-volatile POPs can be transported over long distances in the atmosphere and may have environmental effects far from their point of origin, e.g., on penguins of the Southern Ocean.16 In assessing the degradation of contaminants, the effects of climate change also have to be considered, particularly changes in the runoff of CDOM17 (see ref. 5). Models that consider changes in stratospheric ozone and thus UV-B radiation and effects of climate change help to predict rates of photodegradation or photoinactivation of contaminants in environmental systems. Section 5.3 discusses how models can help to quantify degradation of synthetic and biological contaminants in response to global change.
Changes in stratospheric ozone and climate also affect the biogeochemical cycling of trace gases other than CO2, including carbon monoxide (CO), methane (CH4), nitrous oxide (N2O), and halogen compounds. CO is a key player in tropospheric chemistry since it competes with other trace gases for the hydroxyl radical (OH), for example, with CH4, which is an important greenhouse gas. Emissions of CH4 from natural sources including wetlands, permafrost soils, and wildfires contribute approximately 40% of total CH4 emissions.18,19 These natural sources of CH4 are affected by global warming and emissions of CH4 further reinforce global warming. The third most important greenhouse gas is N2O, following CO2 and CH4, which impacts the abundance of stratospheric ozone20 (see ref. 1 and 21). This trace gas is released from terrestrial and aquatic ecosystems and via thawing of permafrost soils. Biogeochemical processes in seawater and on the surface of the cryosphere also play an important role in the formation of halogen compounds other than chlorofluororcarbons (CFCs), which are precursors of reactive halogen species. Among the “natural” halogen compounds, brominated very short-lived substances (BrVSLS) are important trace gases for stratospheric ozone chemistry since they may reach the lowermost stratosphere and participate in the depletion of stratospheric ozone. Reactive halogen species from natural sources also play an important role in tropospheric chemistry, e.g., as oxidants of gaseous elemental mercury.
Here we assess new findings in the realm of biogeochemical cycles under changing stratospheric ozone, solar UV radiation, and climate including the following sections: (2) Stratospheric ozone depletion and biogeochemical cycles: an overview of four decades of research; (3) Roles of interfaces and climate change in carbon cycling mediated by UV and visible radiation; (4) Natural emissions of trace gases that contribute to global warming and affect stratospheric ozone; (5) Effects of stratospheric ozone and climate change on UV-induced transformation of contaminants; (6) Feedbacks on global warming that are mediated by UV and visible radiation; and (7) Major advances and gaps in knowledge (with respect to the interactive effects of solar radiation [UV and visible] and climate change on biogeochemical cycles).
2. Stratospheric ozone depletion and biogeochemical cycles: an overview of four decades of research
Assessing the effects of depletion of stratospheric ozone on biogeochemical cycles has been part of the remit of the Environmental Effects Assessment Panel since 1995. This, our seventh assessment, also follows the 30th anniversary of the Montreal Protocol and the ratification of its Kigali Amendment in 2017. As well as assessing research progress over the last four years, it is timely in this assessment to place that recent research in the context of progress in research and policy over the last four decades.
Part of the success of the Montreal Protocol has been its influence on high quality science, not just the understanding of the mechanisms of stratospheric ozone depletion but also the understanding of the environmental effects of uncontrolled depletion of stratospheric ozone.22 Conversely, the need for high quality science to underpin the Montreal Protocol has been a major stimulus for research across multiple scientific disciplines. As a result, understanding of the environmental effects of ozone depletion, above all, the effects of changes in solar UV-B radiation (280–315 nm), has been transformed over the last three to four decades. That transformation applies to understanding of the effects of UV-B radiation on biogeochemical cycling, which was very poorly developed prior to the 1980s. There was a small foundation of earlier research pertinent to this topic, and since that time much more has been added. For example, by the late 1970s it was well-established that UV radiation could be a significant factor in the degradation of organic pollutants (see e.g., Pinhey and Rigby (1969),23 and Zepp and Cline (1977)24), humic substances (now generally included under the broad heading of dissolved organic matter (DOM)),25,26 and nitrate27 in aquatic systems. By contrast, the role of solar UV radiation in the biogeochemistry of terrestrial ecosystems was effectively unexplored prior to the 1980s.
This research prior to the Montreal Protocol demonstrated that solar UV radiation could play a role in biogeochemical processes and identified fundamental photochemical mechanisms, thus laying the foundation for research stimulated by concerns over ozone depletion. Building on that foundation, research in the early 1980s revealed that the photochemistry of nitrate and coloured dissolved organic matter (CDOM) involved the generation of reactive oxygen species (ROS), such as OH. It became clear that ROS play a major role in the degradation of natural organic compounds as well as contaminants, and also that metals (particularly iron) are involved in the production of OH in sunlit aquatic ecosystems.28
The Montreal Protocol also stimulated the first studies into the effects of UV-B radiation on biogeochemistry in terrestrial ecosystems. The first papers to investigate the effects of UV-B radiation on the decomposition of dead plant material (“litter”) were not published until the mid-1990s. Following initial modelling studies,29 it was confirmed that solar UV radiation could affect the rate of litter degradation30 and enhance emissions of trace gases,31 paving the way for substantial research over the last two decades, e.g., ref. 6 and 32. Some of the trace gases that are emitted from these natural sources, e.g., nitrogen oxides (NOx) and very-short-lived halocarbons, can affect concentrations of ozone in the troposphere and the stratosphere.6
This period of research, stimulated primarily by the need to understand the effects of ozone depletion, lasted from the late 1980s until the beginning of this century. Since then, new advances in both science and policy have led to new perspectives on the role of UV radiation in biogeochemical cycling.
First, we now understand that the effects of solar UV radiation on biogeochemical cycles are not confined to the high irradiances or doses that would occur only with uncontrolled stratospheric ozone depletion. Field manipulations using wavelength-selective filters and laboratory-based studies with environmentally-relevant and well-defined treatments show that a wide range of biogeochemical processes respond significantly to variation in UV radiation within the ambient range. In addition, it is now clear that the results of depletion of stratospheric ozone other than increased UV-B radiation can have major effects on ecosystems that were not anticipated in earlier research. Antarctic ozone depletion has had a strong influence on climate in the southern hemisphere33 (see ref. 1) with concomitant effects on terrestrial and marine ecosystems (see ref. 2 and 5) and biogeochemical cycles.4,6
Second, we now have a much better understanding of the role of factors other than stratospheric ozone in determining surface irradiances of solar UV radiation, both UV-B and longer wavelength UV-A (315–400 nm). The effects of time of day, season and latitude on UV irradiances, all driven by the changes in solar elevation, have been well-defined for many years. However, quantification of the response of UV irradiances to stochastic changes in other factors, including cloud, air pollution and aerosols, continues to improve (see ref. 1). There is also increased awareness that variation in surface irradiances, as typically measured by atmospheric scientists, is not the only factor affecting the exposure of organisms or ecosystems to UV-B radiation. The absorption or reflection of UV radiation by ice or snow may significantly affect the exposure to UV radiation at high latitudes or high altitudes (see ref. 1). In terrestrial ecosystems, plant canopies greatly influence the exposure to UV radiation at the soil surface (see ref. 2), while the exposure to UV radiation in aquatic systems is also substantially influenced by the effects of coloured dissolved organic matter (CDOM) on the penetration of UV radiation through the water column (also see ref. 5).
Third, the successful implementation of the Montreal Protocol and its amendments has prevented uncontrolled, global ozone depletion33 (see ref. 1). While significant seasonal ozone depletion over the Antarctic has occurred annually since the 1980s, changes in total stratospheric ozone in other regions have been small and/or transient (see ref. 1). Except for the effects of ozone ‘holes’ over the Antarctic, and occasionally over the Arctic, measurable effects of changes in stratospheric ozone on levels of surface UV-B radiation have generally been hard to identify against variation due to other factors (see ref. 1). Looking ahead, ozone recovery over the Antarctic is expected to progressively reverse the seasonal increases in surface UV-B radiation measured there (see ref. 1). Beyond the Antarctic, future trends in exposure to surface UV-B radiation will be driven partly by changes in stratospheric ozone and partly by changes in factors other than stratospheric ozone (see ref. 1). These factors include cloud, air pollution, aerosols, ice and snow cover and DOM, which are all expected to be affected by climate change, with marked temporal and geographical variation in the magnitude and in some cases the direction of change (see ref. 1 and 5).
Fourth, it is increasingly evident that knowledge of the effects of solar UV radiation on biogeochemistry is necessary to understand not only responses to environmental change but also the drivers of change. A primary example is the role of solar radiation in determining exchanges of CO2 and other GHGs, for example, the release of CO2 from plant litter or DOM,32,34,35 but also changes in the uptake of CO2 by aquatic organisms (see ref. 5) and, perhaps, terrestrial plants (see ref. 2). Biogeochemical processes driven by sunlight are also pertinent to understanding other current environmental challenges, including the fate of dissolved pollutants such as pesticides or heavy metal compounds, or the increasing problem of plastics contaminating the environment (also see ref. 5 and 36).
These new scientific perspectives, which have emerged progressively over several years6,32 are now framed by recent changes in the Montreal Protocol itself. The Protocol has evolved to control not just the original ozone-depleting substances such as chlorofluorocarbons (CFCs), but their replacements, most recently hydrofluorocarbons (HFCs). The control of HFCs under the 2016 Kigali Amendment is notable because HFCs are not ozone-depleting substances but many are greenhouse gases.33 As a result, as well as continuing to be the global mechanism for protecting the stratospheric ozone layer, the Montreal Protocol now includes a specific responsibility for protecting Earth’s climate.
3. Roles of interfaces and climate change in carbon cycling mediated by UV and visible radiation
There are many aspects of climate change that affect biogeochemical carbon cycling by changing the exposure of natural organic matter (NOM) to UV and visible radiation on the surface of the Earth. Important effects are warming of and loss of the cryosphere and more frequent and longer lasting heavy precipitation events. In this section, we assess the interactive effects of solar radiation, particularly solar UV radiation, and climate change on carbon cycling across cryosphere–land–water–air. Solar UV and visible radiation play a role in the degradation of NOM to CO2 and CO, a process that is often enhanced by climate change and can thus feedback to modify climate.
When plants die on land, the organic matter produced by photosynthesis is then decomposed and eventually transformed to inorganic carbon (CO2 and CO). Although most of the carbon turnover is mediated by decomposer organisms, some decomposition of NOM is driven directly by solar UV and visible radiation through photochemical reactions that generate CO2 (Fig. 2).34,37 The degradation of NOM by solar UV and visible radiation occurs on land and in fresh and marine waters after the transfer of NOM from land to water. One of the key controls of photodegradation of NOM on land and in water are compounds that absorb solar radiation. One example is lignin, a compound that provides structural support for plants and is almost exclusively found in plants of terrestrial origin.38 Lignin has been shown to be one of the principal light absorbing compounds in decaying plant material.39–42 In addition to the production of CO2 and CO, photodegradation of NOM on land and in water yields smaller organic compounds that are more bioavailable to microorganisms, a process called photofacilitation12,40,42 (also see ref. 2). In this section, the term “photodegradation” is used to include the integrated effects of solar radiation through photochemical degradation of NOM and photofacilitation of microbial decay.
The fundamental mechanisms of photodegradation of NOM are the same on land and in water. The rate of degradation of NOM via direct photoreactions depends on the quantum yield (efficiency of a photoreaction), and on the rate of light absorption by NOM. The latter is influenced by many factors that are susceptible to changes in stratospheric ozone, climate, and land-use, including the intensity of solar radiation, particularly solar UV radiation at the surface of land or water, and the concentration and absorption properties of NOM. Thus, there are several common ways in which the relative importance of photochemical degradation on land and in water may be affected by climate and change in land-use. Fundamentally, any change in solar radiation (i.e., changes in cloudiness or air pollution events) may contribute to changes in photodegradation of NOM on land and in water. In addition, changes in cover by vegetation can alter the exposure of NOM to solar radiation (i.e., canopy on land or vegetative shading of streams).43,44
In aquatic ecosystems, terrestrially-derived dissolved organic matter (tDOM) can also be degraded via indirect photochemical reactions with the help of photosensitisers that absorb solar (mainly UV) radiation. Important photosensitisers are CDOM, nitrate (NO3−), and iron compounds (section 5.1). Upon absorption of sunlight, photo-excited sensitisers undergo reactions involving dissolved oxygen (O2) to produce reactive oxygen species (ROS), such as OH, superoxide (O2˙−), hydrogen peroxide (H2O2), and singlet oxygen (1O2) (Fig. 3).12,45,46 These ROS can completely degrade tDOM to CO2 47,48 or partially degrade tDOM, resulting in organic matter altered in chemical composition49 to be more or less labile to microbial degradation to CO2 (Fig. 3).50,51 In addition, ROS are harmful to aquatic microbes46 (see ref. 5) and may suppress the degradation of tDOM by microbes.52,53 The relative importance of ROS for increasing the bioavailability of tDOM and for negative effects on microbial communities and their activities remains an open question.
Fig. 3.
Terrestrial dissolved and particulate organic matter (tDOM and POM, respectively) absorb solar UV and visible radiation in fresh and marine waters. This light absorption results in the photodissolution of POM and the photodegradation of tDOM to greenhouse gases (mainly CO2), and to smaller molecules that are readily degraded by microbes to CO2 (i.e., respiration). Reactive oxygen species produced by photo-excited tDOM and POM help to breakdown tDOM and POM to greenhouse gases and smaller organic molecules (modified from Sulzberger and Arey, 2016 12).
Including photodegradation as a pathway for the production of greenhouse gases from terrestrial and aquatic ecosystems improves models of carbon cycling on land54 and improves the understanding of controls on emissions of greenhouse gases from inland and marine waters.35,55–57 For example, in environments where biological decomposition of NOM is relatively slow,35 it is now recognised that photodegradation of NOM on land and in water are important components of the carbon cycle. On land, photodegradation of NOM is important in arid and semi-arid environments where solar UV and visible radiation are high and microbial decomposition is limited by water and availability of carbon.58 In contrast, photodegradation of NOM is important in aquatic ecosystems in Arctic and boreal regions, despite the relatively lower solar UV and visible radiation in these high latitudes compared to temperate and tropical regions. Due in part to slow rates of microbial decomposition in the cold waters at these high latitudes, photodegradation of NOM in aquatic systems is important.35 Given these environmental controls on the relative importance of photodegradation of NOM on land and in water for carbon cycling, e.g., dryness vs. humidity on land, temperature in water, it follows that there are key differences in how these processes may shift in magnitude or location in response to human-caused global changes. These changes may occur through land-use, climate change, and stratospheric ozone depletion in Antarctica (also see ref. 1 and 2), where the latter two result in global warming and altered precipitation patterns. Effects of global changes on the photodegradation of NOM on land and in water are discussed in the next sections.
3.1. Effects of global change on photodegradation of natural organic matter on land
Photodegradation of NOM has been observed in the field,34,37,59,60 and the laboratory.41,61 Several of these studies have demonstrated that, at the ecosystem scale, the loss of carbon via photochemical degradation of NOM in ecosystems with marked seasonality of rainfall could be on a par with microbial respiration. The contribution of photochemical degradation of NOM vs. biotic degradation is often difficult to quantify since these two processes occur simultaneously in most ecosystems, and many of the products of these reactions (e.g., CO2) are identical. Nevertheless, there is increasing confidence that, particularly in semiarid ecosystems, the unexplained high rates of decomposition that occur may be directly related to carbon loss through exposure to solar UV and visible radiation.58,62
In addition to the photochemical degradation of lignin and other light-absorbing organic compounds of plant litter, exposure of plant litter to UV and short-wavelength visible radiation can facilitate biological degradation of NOM in terrestrial ecosystems40,63–66 (also see ref. 2). In dryland ecosystems, modelling of photochemical and photofacilitated degradation of NOM demonstrates substantial contributions to carbon turnover in terrestrial ecosystems.54 The suggested role of photofacilitation is increased microbial access to labile carbohydrates in litter following photochemical degradation of lignin40 (see ref. 2 for a more detailed review). Thus, through the effects of photofacilitation, solar UV and visible radiation play a major role in carbon turnover in a wide range of mesic (moist) terrestrial ecosystems.40
3.2. Land-use change and photodegradation
Changes in land-use continue to be one of the major factors affecting terrestrial ecosystems around the globe. Future conversion of ecosystems for agricultural use, particularly in South America and sub-Saharan Africa,67 extraction of wood and other products, or planting of exotic species for potential carbon mitigation, will all place pressure on soils and carbon reservoirs in terrestrial ecosystems.68 The effects of these changes on photodegradation of NOM have not been considered as of yet, but could have important consequences for carbon cycling at regional and global scales. Afforestation, the planting of woody vegetation in areas that were previously dominated by herbaceous vegetation, can have surprising effects on carbon turnover. In a comparison of paired sites with pine plantations and natural counterparts of grassland and steppes, afforestation caused more than a 60% reduction of the decomposition of litter in arid zones.43 Moreover, the relationship between the decomposition of litter in the paired afforested and natural vegetation was largely explained by differences in the interception of solar radiation before it reached the surface of the soil.43 These results suggest that conversions due to agriculture, deforestation, and afforestation could have large impacts, positive or negative, on carbon cycling via changes in the interception of solar UV and visible radiation.
Climate change due to human activity has been documented worldwide and is of growing concern due to its impacts on the functioning of natural ecosystems. In this context, two important global changes in terrestrial ecosystems, droughts and wildfires, are of relevance to the photodegradation of plant litter. Due to the reduction of plant cover, which enhances exposure of plant litter to solar radiation,43 photochemical degradation of NOM tends to increase under conditions of drought or extreme aridity.69,70 In addition, photofacilitation can play an important role in arid ecosystems since this process stimulates microbial break-down of plant litter.40,64,71 In summary, an important impact of global warming on terrestrial ecosystems is increased exposure to solar UV radiation of previously unexposed NOM. This is due to decreased plant cover and reduced interception of solar radiation. Thawing of permafrost soils and combustion of aboveground vegetation can also result in increased exposure of NOM to solar UV and visible radiation (see the following section).
3.3. Role of global change on photodegradation of tDOM and POM in fresh and coastal waters
In this section, we assess the effects of UV and visible radiation, as well as global change, on the emission of CO2 and CO from fresh and coastal waters via degradation of tDOM. NOM produced by plants moves from land to water in particulate and dissolved form (POM and tDOM, respectively) (see Fig. 2). Most of the terrestrial carbon flushed from land to water is in the form of tDOM72 and, thus, most studies have focused on the photodegradation of tDOM. However, photodegradation of POM is now recognised as important, especially in coastal waters, and we highlight some recent work in this area (section 3.3.2). Loss of the cryosphere is a major global change that enhances the exposure of tDOM to UV and visible radiation (discussed in section 3.3.3).
3.3.1. Photodegradation of tDOM in fresh and coastal waters
To balance terrestrial carbon budgets, the degradation of tDOM in aquatic systems must be accounted for.73–75 Studies published within the last five years have challenged the understanding that microbial decomposition of tDOM was much more important than photodegradation of tDOM. For example, current estimates are that 10–30% of the CO2 released from Arctic and boreal waters comes via photodegradation of tDOM.35,55,57 Given that freshwaters account for 40% of the net exchange of carbon between land and the atmosphere in the Arctic,76 CO2 released from freshwaters via photodegradation of tDOM is important in regional and global carbon budgets. More research related to photodegradation of tDOM in freshwaters is needed since in North American and European freshwaters, concentrations of tDOM have been increasing, a trend called “browning”, which indicates increased inputs of light-absorbing tDOM.77 The causes of browning are currently under debate and may vary by region (also see ref. 5).
tDOM is exported into coastal waters largely by rivers. Flooding of riparian zones due to heavy precipitation events results in increased export of tDOM into marine environments.78 Decades of research have documented the importance of photodegradation of tDOM to CO2 and CO once this organic matter is exported from rivers to the coastal ocean (reviewed in ref. 6 and 42). For example, estimates are that from 3–40% of tDOM exported to coastal waters from rivers can be converted to CO2 and CO within months to a few years.42 Photodegradation of tDOM to CO2 in the ocean has been estimated to offset the net air–sea flux of CO2 by about 8–28%.56
Enhanced runoff also increases the supply of nutrients, for example iron, to phytoplankton. Its availability to phytoplankton is affected by solar UV radiation,79 and by the interactions with other global changes such as acidification of aquatic ecosystems.79–81 While iron is an important micronutrient for phytoplankton (see ref. 5), it can also catalyse the photochemical degradation of tDOM (section 3.3.3). Hence, enhanced export of iron from land to water could enhance uptake of CO2via primary production but also release of CO2via photodegradation of tDOM. The balance between uptake and release of CO2 at coastal interfaces depends on complex interactions.82 Based on air–sea CO2 flux measurements, Laruelle and coauthors83 found that the global coastal ocean is a much smaller sink of CO2 (∼0.2 Pg C per year) than was previously thought, and that many coastal regions are net sources of CO2.
In addition to photodegradation of tDOM to CO2 and CO, ∼70% of tDOM is partially photodegraded (i.e., altered in chemical composition) by sunlight during riverine transit to the Arctic Ocean.35 This partial degradation of tDOM by UV radiation can facilitate or slow microbial respiration of tDOM to CO2.51 It has been known for a long time that photodegradation of tDOM breaks down large biomolecules like lignin into smaller, simpler compounds that microbes use for energy with the production of CO2 (see above and Fig. 3). Hence photofacilitation also plays an important role in aquatic ecosystems and supports aquatic food-webs (also see ref. 5). What is less well-known, and a focus of current research, is how important this photofacilitation process is for the cycling of carbon in freshwaters in a quantitative way. Although currently poorly quantified, the effect of photochemical degradation of tDOM on its conversion to CO2 by microbes is probably substantial given that studies show that microbial respiration of tDOM to CO2 can be increased or decreased by more than two-fold after tDOM has been degraded by UV and visible radiation.84
3.3.2. Photodissolution of POM in fresh and coastal waters
Both tDOM and POM enter aquatic ecosystems when soil organic matter runs off from land to water (Fig. 2). In sunlit surface waters, POM can be altered by photochemical reactions (photodissolution) to yield dissolved organic matter85,86 (i.e., tDOM that can undergo further photodegradation), CO2 and CO87 (Fig. 3). The photodissolution of POM to tDOM can also occur via indirect photoreactions, involving OH.88 In contrast to photodissolution of POM of distinct terrestrial origin, more work has focused on photodissolution of resuspended estuarine sediment and POM in coastal waters.85,89–91 POM from resuspended sediment in coastal waters likely reflects a mixture of sinking particles from different sources of NOM. These sources include terrestrially-derived POM exported from rivers to coastal waters as well as autochthonously-derived POM produced from algal and bacterial detritus.90,92–94 Studies have shown that production of DOM via photodissolution of POM from resuspended sediments resulted in fluxes of DOM that were larger than benthic and riverine fluxes of DOM to coastal waters.85 Others have suggested that 5–15% of POM could undergo photodissolution before settling in coastal waters.95 However, a review highlighted the lack of quantitative information on the contribution of photodissolution of POM to carbon cycling and fluxes of carbon to and from coastal waters.42 Quantifying photodissolution of POM requires an integration of environmental factors including the turbidity of the water column (which controls the UV and visible light exposure of POM) and the apparent quantum yields of photodissolution of POM to products,89 as well as separating out contributions from biological decomposition. These sunlight-induced changes in the rates of photodissolution of POM have consequences for the biogeochemical cycling of POM in aquatic ecosystems including the transfer of POM into bottom sediments.
3.3.3. Warming of and loss of the cryosphere generally increases the likelihood that terrestrial dissolved organic matter will be degraded by UV and visible radiation
It is now recognised that photodegradation of tDOM can account for a substantial fraction of the total CO2 released from Arctic surface waters. Currently, photodegradation may contribute 30% of the CO2 emitted from the water column of Arctic waters,35 which is important because these waters account for 40% of the net atmosphere–land exchange of carbon.76 For example, thawing of permafrost soils in Arctic and boreal regions is predicted to increase export of tDOM to inland and coastal waters.96,97 tDOM of permafrost origin (i.e., previously frozen organic matter) contains less light-absorbing CDOM on a per carbon basis, compared to tDOM draining from the actively thawed soil layers at high latitudes.51,98,99 This implies lower rates of absorption of UV radiation and thus lower rates of photodegradation in the water column.100,101 However, lower CDOM content of permafrost tDOM is offset by higher apparent quantum yields of the photochemical reactions that result in the formation of CO2, CO, and organic compounds that strongly facilitate microbial respiration.57,84,102,103 Thus, increasing export of permafrost tDOM from land to water likely means that photodegradation of tDOM may become an even more important source of CO2 from inland waters of the Arctic.103
Another reason why photodegradation of tDOM will continue to be important in a warming Arctic is because the export of light-absorbing tDOM from land to water and the time of exposure of tDOM to UV radiation in sunlit waters, are dependent on the hydrology of the watershed. Every year during spring in the Arctic (i.e., May–June), snow melt on land flushes tDOM from soils to rivers, ponds, and lakes, resulting in an annual peak in the concentration of light-absorbing tDOM in sunlit surface waters.104–106 During this period of ice and snow melt, and flooding on land, the storage of river water in lakes and on floodplains can be substantial, e.g., up to approximately 50% of the flow of Mackenzie River in the Canadian Arctic can be stored on the floodplain during this time.107 Retention of Mackenzie River water stored on the floodplain during spring snowmelt and floods increases the time that tDOM is exposed to UV radiation. This is because the timing of snowmelt and spring floods coincides with times of peak solar irradiation in the Arctic. The longer the time tDOM is exposed to UV radiation, the more this carbon can be photodegraded to CO2 and CO. In addition, during spring floods, river water is spread in a thin layer (∼2 m thick) across an area more than 11 000 km2 in size,107 which also increases opportunities for tDOM in the water to be photodegraded. This effect of river flooding on annual greenhouse gas production through photodegradation of tDOM in Arctic waters is not currently accounted for in regional carbon budgets.107 This would be important, given that snowmelt and spring flooding may be happening earlier due to warming.
Warming at high latitudes is accelerating melting of ice on rivers, ponds, and lakes and of snow on land; furthermore, it also changes the timing of the melt. For example, melting of ice and snow on water108 and land109 are occurring earlier in the Arctic spring. Earlier retreat of ice and snow has increased the number of days tDOM on inundated land or in water is exposed to UV radiation on average by one day per year from 2000–2013 across the Arctic. Some areas of the Arctic show a faster trend in loss of ice and snow.110 Earlier melt of ice and snow on freshwaters and land in May and June means more overlap with the time of the year when the sun angle is at its highest (and thus, UV radiation reaching the surface is most intense in the Arctic), and when concentrations of light-absorbing tDOM are also at their highest of the year.104,106 Thus, this earlier melt of ice and snow on water and land in the Arctic can substantially increase photodegradation of tDOM to CO2 and CO35 by increasing the time this carbon is exposed to UV radiation.
Other changes in Arctic and boreal hydrology influence the duration of exposure of tDOM to UV radiation. These changes include an increase or decrease in the number and surface areas of small ponds, depending on the regions of the Arctic and boreal zones,111–113 as well as changes in the connectivity of lakes in Greenland.114 In addition, increased turbidity of freshwaters from thawing of permafrost115 could decrease exposure of tDOM to UV radiation. Any change in the exposure of tDOM to UV radiation in lakes and ponds may affect photodegradation of tDOM to greenhouse gases.101,114
The intensity and frequency of droughts and wildfires are increasing worldwide as a result of changes in climate and land-use,9,77,116–119 and in the southern hemisphere, this is being further enhanced by the effect of ozone depletion on climate (see ref. 2). In the Arctic, the current and projected increases in wildfires in boreal forests and Arctic tundra,120,121 could have multiple consequences for boreal and Arctic ecosystems as wildfires are sources of greenhouse gases (including CO2, CO, and CH4), and of volatile hydrocarbons to the atmosphere.122,123 Increasing frequency and extent of fires in the Arctic tundra120 could influence photodegradation of tDOM in several ways. Fires in the Arctic tundra and boreal forest have been shown to transform tDOM into compounds called black carbon124 (also see ref. 5), particularly at high fire temperatures (>600–700 °C).125 Black carbon is a component of tDOM with higher extinction coefficients (where extinction coefficients reflect the absorption properties of components) and thus higher susceptibility to photodegradation.124,126 Wildfires also affect the thawing of permafrost soils; the loss of permafrost with warming after fire has been linked to reduction of the insulating surface organic layer.127 For example, up to 0.5 m of settlement was observed during thaws after recent fires in Alaska, causing impoundment of water and further thawing of permafrost.127 Another effect of wildfires is the decrease in UV and visible radiation reaching the surface of aquatic systems because of the generation of short-lived aerosols that act as cloud-condensation nuclei.77 As a consequence, rates of tDOM photodegradation may decrease. Widespread wildfires in Alaska have resulted in substantially lower UV and visible light in the Alaskan Arctic for the few days in the summer when rivers and lakes are ice-free and otherwise exposed to sunlight. Thus, the fire-related decrease in solar UV and visible radiation reaching the water surface could offset the warming related loss of snow and ice that increase exposure of tDOM to UV radiation. However, at present, no study has investigated the effect of fire on UV radiation reaching Arctic or boreal surface waters.
Finally, there is some indication that thawing permafrost may increase the export of iron to Arctic and boreal inland waters (Fig. 4),128 a trend recently reported for North American and European surface waters.129 Increased concentrations of iron in sunlit surface waters may enhance photodegradation of tDOM via various mechanisms,79 for example, by increasing the rates of light-absorption by tDOM,130 by producing reactive oxygen species (ROS) that degrade tDOM,47 and by catalysing other reactions that photodegrade tDOM.103,131 For example, Page and co-workers47 concluded that degradation of tDOM by ROS, produced in UV-induced iron cycling, could account for 5–10% of the CO2 formed from photodegradation of tDOM in Arctic waters.
Fig. 4.
The red colour in the Saviukviayak River (Alaskan Arctic) is due to high concentrations of iron flushed from land to water. Once in the sunlit river, iron plays a role in photochemical reactions. Photo credit: R. M. Cory.
4. Natural emissions of trace gases that contribute to global warming and affect atmospheric ozone
Several gases released from terrestrial and aquatic ecosystems, or from the surface of the cryosphere, contribute to global warming and changes in stratospheric ozone concentrations. Some of these gases are highly reactive in the troposphere and in the stratosphere. The following sections discuss the formation and environmental significance of CO, CH4, and nitrogen- and halogen-containing trace gases.
4.1. Carbon monoxide
Carbon monoxide is a gas that plays an important role in tropospheric chemistry by contributing to the formation of ozone and by greatly impacting the concentration of OH (also see ref. 21). On a regional scale, CO participates in gas-phase reactions that control local concentrations of ozone and peroxides. On a global scale, CO competes with atmospheric CH4 for OH,132 thus decreasing the atmospheric capacity to oxidise CH4, and indirectly affecting the lifetime of this important greenhouse gas. Although CO itself only weakly absorbs infrared radiation from the Earth, the cumulative indirect radiative forcing of CO, taking into account its effects on the components CO2, CH4, and O3, may be even larger than that of the third most important greenhouse gas, N2O.133
Major sources of CO include direct production from burning of fossil fuels and biomass, e.g. wildfires, and tropospheric oxidation of hydrocarbons.134 Emissions of CO from both terrestrial and aquatic ecosystems are a balance between production, which has light-dependent and light-independent mechanisms, and microbial processes that consume CO. Consumption of CO typically exceeds production at night, while photochemical production becomes dominant during daylight. The resulting significant diurnal variations in CO fluxes have been confirmed in both terrestrial6,135 and aquatic systems.42
Additional studies since our last assessment have confirmed that the production of CO in the ocean and freshwaters occurs primarily via photodegradation of CDOM (section 3). Photodissolution of POM to CDOM also contributes to the formation of CO6,42,56,87,136 (section 3.3.2 and Fig. 3). The measured average ratio of CO2/CO is close to 20 in marine systems and somewhat lower in freshwaters.6 In terrestrial ecosystems, it is well-established that photodegradation of plant litter from a variety of species yields CO, in addition to CO2 (e.g., ref. 6 and 135; and section 3.1). Emissions of CO can be enhanced by fires, not just during the fire itself137 but also in the longer term, since charring transforms organic matter into a broad spectrum of organic constituents (including black carbon), which can emit CO and other gaseous products via photodegradation125,126 (section 3.3.3).
Given these mechanisms, the production of CO is likely to be affected by various elements of environmental change, for example, by increased inputs of CDOM into aquatic systems (see also ref. 5), and increased aridity in terrestrial systems (section 3.1 and ref. 2). In aquatic ecosystems, the concentrations of CDOM and POM may be increased by floods. Photoproduction of CO from POM may be as important as that from CDOM, depending on the season.87 The ratio of the apparent quantum yield of CO photoproduction from POM and CDOM, ΦCO-POM/ΦCO-CDOM, has been shown to increase from UV to visible radiation.87 On the balance of evidence, these environmental changes are likely to increase the production of CO by solar radiation but the magnitude of this increase remains unclear, especially relative to changes in other processes that affect the production and consumption of CO.
4.2. Methane
UV-induced production of methane (CH4) from plant material discussed in previous assessments is no longer considered to be a significant source of CH4.3 Nonetheless, we consider CH4 briefly here because it is not only a potent greenhouse gas but it also indirectly impacts stratospheric ozone. In the Executive Summary of the Scientific Assessment of Ozone Depletion: 2018 33 it is stated: “Outside the Antarctic, CO2, CH4, and N2O will be the main drivers of stratospheric ozone changes in the second half of the 21st century, assuming full compliance with the Montreal Protocol”. In order to predict trends in stratospheric ozone, it is, therefore, important to assess biogeochemical sources of methane, as well as its atmospheric loss-processes.20,138
The contribution of CH4 to radiative forcing is about half of that of CO2 if indirect effects of CH4 emission, such as the production of stratospheric water vapour, also are taken into account.133 The global atmospheric concentrations of CH4 have been increasing in the past three to four decades at various rates. The present net growth rate of CH4 is about 5–10 ppb per year and is higher than it has been for the past 20 years.18,139 Approximately 60% of global emissions of CH4 were anthropogenic in the decade 2003–2012 18,19 through agriculture, waste, and fossil fuel extraction and use. Over the industrial era, concentrations of atmospheric CH4 rose from about 720 ppb before industrialisation to over 1850 ppb in 2017.140 Methane is also formed via biogeochemical processes and sources of CH4 may vary seasonally. For example, in the winter, concentrations of CH4 in the Arctic troposphere are mainly controlled by anthropogenic emissions including sources from Russian fossil fuel industries, whereas, in the summer, emissions from wetland and freshwater sources dominate across the whole region.141 In the atmosphere, OH is the most important sink of CH4 (85% or more18) (also see ref. 21).
The most important natural sources of CH4 are wetlands, particularly tropical wetlands.139,142–145 Emissions of methane from tropical wetlands contribute 60–80% of global emissions from natural wetlands.145 Emissions of CH4 from tropical wetlands are primarily affected by increasing temperatures since the rate of CH4 production by methanogenic bacteria increases with increasing temperature,146,147 which represents a positive feedback on global warming. Zhang and coworkers144 have estimated that, depending on scenarios, feedbacks via emissions of CH4 by wetlands could add an additional radiative forcing of 0.04 W m−2 to 0.19 W m−2 to the global mean by the end of the 21st century. In boreal wetlands, emissions of CH4 are also enhanced during thawing of inundated areas during the cold season (December to May).144
Other natural sources of CH4 include wildfires and biomass burning,122,123 fresh waters (lakes and rivers),141,148 oxygen minimum zones (OMZs) of marine environments,149 and thawing of permafrost soils.148 These sources of CH4 are also affected by climate change, and by depletion of Antarctic ozone (see ref. 2). OMZs are increasing because of increasing sea-surface temperature due to global warming and hence reduced O2 solubility is occurring. In OMZs, methanogenic bacteria are the main source of CH4.149
Global warming is also increasing emissions of CH4 from permafrost soils owing to the increasing thaw of permafrost (section 3.3.3). Release of CH4 from permafrost is moderated by methanotrophs, which oxidise 20–60% of this methane before emission to the atmosphere.150 The area of thermokarst lakes in the Arctic has expanded over the past 60 years.148 The rate of emission of CH4 from these areas was found to be directly proportional to the amount of soil-derived organic carbon entering the lakes because of the erosion of thawing permafrost.148 In summer, emissions of CH4 from freshwater systems have been estimated to represent between 11% and 26% of total emissions from the Arctic.141 Release of CH4 has also been shown to occur from the Arctic seabed where DOM and CH4 are preserved within and beneath the subsea permafrost.151 Hence, emissions of CH4 from thawing permafrost soils might fuel a positive feedback process that further reinforces global warming and thawing of permafrost. Effects of CH4 emissions on future trends in stratospheric ozone depend on regions. Outside the polar regions, the cooling of the stratosphere by water vapor formed via oxidation of CH4 is expected to result in an increase in total column ozone, while inside the polar regions, increases in stratospheric water vapor favours the formation of polar stratospheric clouds, which facilitate ozone depletion in polar spring152 (see ref. 1).
4.3. Nitrogen compounds
The effects of climate change and solar UV radiation on the production of three gases containing nitrogen are assessed here: on nitrous oxide (N2O), and on nitrogen oxides (NOx = NO + NO2).
4.3.1. Nitrous oxide
Nitrous oxide is an important greenhouse gas and its oxidation is the dominant source of stratospheric NOx, which affects the concentrations of stratospheric ozone through the formation of reactive chlorine reservoirs and other stratospheric processes (see ref. 1). The contribution of N2O to radiative forcing of climate between 1750 and 2011 was approximately 10% of that of CO2 (based on estimated changes in concentration).133
In terrestrial ecosystems, N2 and N2O can be formed by UV- and microbially-mediated processes. On soil surfaces that are exposed to solar UV radiation, increased microbial transformations, causing release of N2 and N2O from decomposing litter, have been reported in modelling153 and empirical studies.70,154 It is likely that the increased availability of carbohydrates from photochemical degradation of lignin may facilitate the process of mineralisation of nitrogen-containing natural organic matter by microbes. Emission of N2O via microbial processes increases with temperature and heavy precipitation events.155 The reasons are increased water-filled pore space and faster consumption of O2via respiration and thus a larger anaerobic volume soil fraction.155 In addition, thawing of permafrost soils is an important source of N2O.156–158 Rates of emissions of N2O per unit area from permafrost peatlands in the Arctic were found to be of similar magnitude as those from tropical forest soils, the largest global N2O source from terrestrial ecosystems.156,157 Natural emissions of N2O from land are similar to N2O emissions from anthropogenic activities (mainly agriculture).138
In the ocean, N2O is formed via two pathways, depending on the oxygen concentration.159,160 One pathway of formation of N2O is via nitrification, i.e., microbial oxidation of ammonia (NH4+) to nitrate (NO3−), where N2O is an intermediate product. This biotic formation of N2O involves ammonia-oxidising bacteria and/or Archaea, depending on the salinity of marine environments.161 Nitrification occurs nearly everywhere in the global ocean, where natural organic matter from phytoplankton debris is mineralised, releasing NH4+. Hence, marine production of N2O via nitrification depends on the rates of primary production and re-mineralisation.159,160 In the sunlit zone of the ocean, NH4+ can also be formed via UV-induced transformation of organic nitrogen-containing compounds from phytoplankton debris.42,162
The second pathway of formation of N2O in marine environments is de-nitrification; this process occurs in OMZs where concentrations of dissolved O2 fall below ∼160 μg L−1.160,163 Emissions of N2O via de-nitrification are predicted to increase due to expanding OMZs as a result of global warming and resultant decreased O2 solubility.160 The trend in emission of N2O from the global ocean depends on the balance between nitrification and de-nitrification. Based on a global ocean biogeochemical model, Martinez-Rey and coworkers160 predicted a decrease of 4–12% in emissions of N2O from 2005 to 2100 from the global ocean due to decreasing primary and export production, and reduced transport of N2O from the ocean interior to the ocean surface. On the other hand, emissions of N2O from land are likely to increase, in part due to more frequent and longer lasting heavy precipitation events and enhanced thawing of permafrost in the Arctic.156–158
4.3.2. Nitrogen oxides
The abiotic pathway of NOx formation from soil and snow surfaces is photolysis of NO3− driven by solar UV radiation.164–166 In snow, rates of photolysis of NO3− increased with the concentrations of sea salt (NaCl)166 and, due to the attenuation of the incoming UV radiation, decreased with the depth of snow.165 Hence enhanced snow-melt (section 3.3.3) and thus increased exposure of NO3− to solar UV radiation could result in increased emissions of NOx from snow surfaces. In addition to the UV-induced pathway, NO is produced in soils by microbial nitrification or denitrification, depending on environmental factors such as oxygen- and water-content, temperature, and pH of the soil.167 In the troposphere, NOx controls the formation of ground-level ozone and OH, the latter being critically important to the self-cleaning ability of the atmosphere (also see ref. 21). Furthermore, reactions of NO2 with OH (during the day) and with O3 (during the night) yield nitric acid (HNO3), which undergoes wet deposition and contributes to acid rain.168
4.4. Halogen compounds
Interactive effects of solar UV radiation and climate change play an important role in the production and emission of halogen compounds other than CFCs. These “natural” halogen compounds are formed via UV-induced and microbial processes in seawater and on the surface of the cryosphere and are precursors of reactive halogen species that affect tropospheric and stratospheric chemistry. Among the “natural” halocarbons, very short-lived halogenated substances (VSLSs) are important trace gases for stratospheric ozone chemistry.86,169–172 They may reach the lowermost stratosphere, where they are photochemically transformed into reactive halogen species that act as sinks for ozone. VSLSs may account for ∼25% of stratospheric bromine and a few per cent of stratospheric chlorine.169
In seawater, precursors of reactive halogen species are formed in UV-induced and biological processes. The methyl halides, CH3Br, CH3Cl, and CH3I, are produced through indirect photochemical reactions involving OH.173,174 On the other hand, very short-lived brominated halocarbons (BrVSLS), e.g., bromoform (CHBr3), are formed via biotic processes.86,169–171 CHBr3 and dibromomethane CH2Br2 are the major BrVSLS (with tropospheric lifetimes of <6 months) and account for ∼80% of the very short-lived organic bromine in the marine boundary layer.86 The rate of formation of CHBr3via bromoperoxidase-mediated halogenation of DOM depends on the chemical composition of DOM. Humic acid enhanced the enzyme-mediated production of CHBr3, but amino acids and lignin suppressed production86 (Fig. 5). Ozone depletion potential (ODP)-weighted emissions of CHBr3 (global mean, simulated for 2005) were estimated to account for up to 50% of ODP-weighted anthropogenic emissions of CFC-11.172 Furthermore, results from simulations suggested that, in 2011, depletion of stratospheric ozone from BrVSLS had a radiative effect that was nearly half that from long-lived halocarbons.169 The question arises, whether emissions of BrVSLS from natural sources are one reason for the findings that ozone in the lower stratosphere between 60° S and 60° N has continued to decline since 1998.175
Fig. 5.
Schematic illustration of the formation of halocarbons in seawater that undergo UV-induced reactions in the troposphere yielding reactive halogen species such as bromine monoxide (BrO) that act as oxidants for atmospheric pollutants, e.g., gaseous elementary mercury (Hg0g). Brominated very-short-lived substances (BrVSLS) may reach the lowermost stratosphere and participate in stratospheric ozone depletion.
The extent to which halocarbons reach the lowermost stratosphere depends on their lifetime, and in turn on the concentration of OH in the troposphere, at least for those halocarbons that react with OH, e.g., CH2Br2. Rex and coworkers176 found a coincidence of an “OH minimum zone” over the West Pacific and a relatively long lifetime of CH2Br2. Inside the “OH minimum zone” the lifetime of CH2Br2 was 188 days at 500 hPa, whereas outside this zone, the CH2Br2 lifetime was only 55 days.
In the troposphere, reactive halogen species react with other trace gases such as nitrogen oxides (NOx),177,178 methane (CH4),177 ozone,86,178 and with mercury.178–180 Hence reactive halogen species affect the lifetime of tropospheric pollutants. Reaction of Br with O3 is thought to be responsible for the episodic decline in the concentrations of ozone to near-zero levels in the lower troposphere over the Arctic, following the boreal springtime polar sunrise.181 An important pathway for the formation of Br and Cl in the troposphere is photolysis of molecular chlorine and bromine (Cl2 and Br2, respectively). This process has been shown to occur in the interstitial air of Arctic surface snowpack.178,181
Bromine monoxide (BrO) is the main atmospheric oxidant of gaseous, elemental mercury (GEM, Hg0g), which is emitted by human activities.179 Oxidation of GEM by BrO yields HgII, which is water soluble and, therefore, available for wet deposition.180 In the marine boundary layer of the Southern Ocean, GEM concentrations ranged from 0.4 to 1.9 ng m−3 in austral summer.182 Following deposition to aquatic and terrestrial ecosystems, HgII undergoes methylation yielding methylmercury (MeHg), the form of mercury that enters the food web and is highly toxic (Fig. 5, also see ref. 5).
5. Effects of stratospheric ozone and climate change on UV-induced transformations of contaminants
The previous two sections describe how changes in solar radiation affect carbon cycling and emissions of trace gases, biogeochemical processes that have environmental consequences across large geographical and temporal scales. In this section, we consider effects of solar radiation on biogeochemical processes that, while often more localised, may have more direct consequences for the health of humans and other organisms by altering the environmental fate of toxic chemicals and other contaminants. Many processes that drive carbon cycling, such as reactions involving ROS (Fig. 3), also apply to contaminants. In addition, human activities that occur simultaneously with contaminant release, such as runoff of nitrogen fertilisers, animal or human wastewaters, and oil spills, contribute photosensitising substances that accelerate photoreactions of contaminants. Contaminants are diverse in their origins and may be commercially-produced chemicals intentionally or accidentally released into the environment (section 5.1.1, and ref. 5, 21 and 36). In other cases, such as toxins produced by blue-green algae (cyanobacteria), the toxins are a natural component of ecosystems, but their abundance is increasing because of human activities (section 5.1.2). Contaminants are also highly diverse in their chemistry and responses to solar radiation. UV-B exposure induces direct photoreactions of a wide array of chemical contaminants and initiates free radical processes that oxidise plastics and other commercial products (hence the widespread practice of adding UV-protective substances to prolong lifetimes of plastic (see ref. 36)). Direct photoreactions initiated by the absorption of UV-B radiation by endogenous chromophores may also be the primary mechanism of photodamage in many organisms, for example some pathogenic viruses.183 For other contaminants, photodamage may involve a much greater element of indirect damage induced by photosensitisers such as CDOM and driven by UV-A and visible wavelengths (400–700 nm) as well as UV-B wavelengths.
The degradation of contaminants in aquatic ecosystems will be assessed although solar UV and visible radiation also plays an important role in the phototransformation of contaminants in the troposphere (see ref. 21) and on surfaces (e.g., on leaves of plants and surfaces of soils).184,185 Modelling of the fate of contaminants, including both direct and indirect photoreactions, will be evaluated. Contaminant modelling has primarily focused on the aquatic environment, although photoreactions are important reactions that determine the fate of contaminants such as pesticides in terrestrial ecosystems.
5.1. Degradation of contaminants via direct and indirect photoreactions
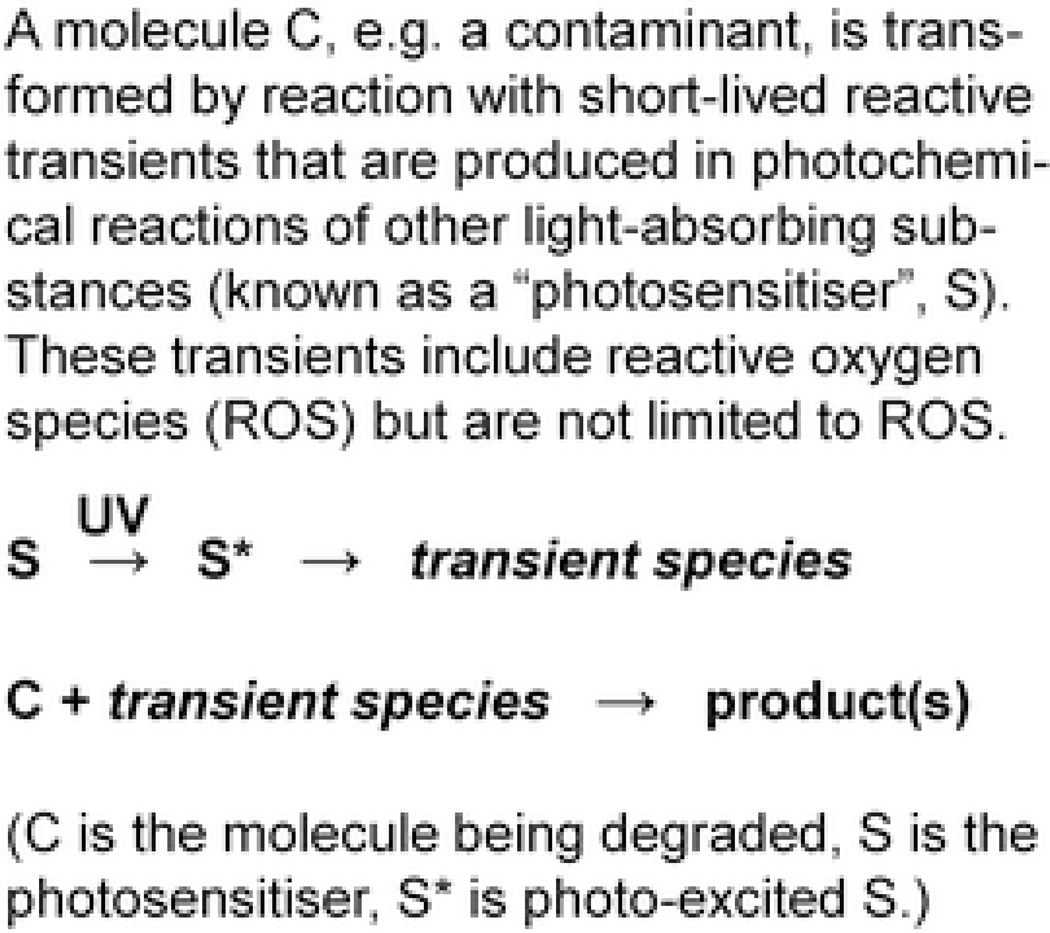
Direct photoreactions (Chart 1) are the simplest mechanisms for photodegradation of contaminants. The rate of photolysis of a contaminant is directly proportional to its concentration; that is, the reaction is described by a first order rate expression. In indirect photoreactions (Chart 2), it is not a contaminant itself that absorbs solar radiation but a photosensitiser. In aquatic ecosystems, important photosensitisers are CDOM, nitrate, and iron compounds (Fig. 6). Absorption of solar radiation by a photosensitiser and subsequent reactions produce reactive transients (i.e., short-lived reactive species). These include triplet CDOM (3CDOM*) as well as reactive oxygen species (ROS) such as the hydroxyl radical (OH), superoxide (O2˙−), hydrogen peroxide (H2O2), and singlet oxygen (1O2) (Fig. 3). These reactive transient species react with a contaminant to form one or more products (Chart 2).
Chart 1.
Direct photoreaction.
Chart 2.
Indirect photoreaction.
Fig. 6.
Schematic illustrating processes that affect photoreactions of contaminants in aquatic environments. The two main pathways, direct and indirect photoreactions, are described in Charts 1 and 2 above.
CDOM plays a double role in the photodegradation of contaminants. On the one hand, CDOM acts as a photosensitiser for contaminants that are degraded or inactivated via indirect photoreactions. On the other hand, CDOM protects contaminants that undergo direct photodegradation from UV-B radiation (Fig. 6). Increasing runoff of CDOM due to thawing of permafrost, and heavy precipitation events, so-called browning of aquatic ecosystems17 (see ref. 5), is likely to enhance indirect photoreactions via several mechanisms. Production of reactive transient species with CDOM acting as the photosensitiser partly offsets the decreases in direct photodegradation rates caused by concurrent protection against UV radiation. Another effect of CDOM acting as the photosensitiser is to shift the photodegradation of contaminants to a greater dependence on UV-A radiation, which penetrates to greater depths in the water column, and hence to a greater dependence on the dynamics of vertical mixing. Droughts would have the opposite effect, i.e., direct photoreactions driven by UV-B radiation would be expected to be predominant because of decreased runoff and thus smaller CDOM concentrations in aquatic ecosystems.
The predicted decline in UV-B radiation in many areas of the Earth due to recovery of stratospheric ozone (see ref. 1) is expected to reduce the rate of direct photodegradation or photoinactivation since light-absorbing contaminants are particularly susceptible to being broken down by UV-B radiation. One key indicator of the dependence of photoprocesses on UV-B radiation is its radiation amplification factor (RAF, see ref. 186). RAFs were originally developed as a measure of the sensitivity of a photoprocess to stratospheric ozone depletion.186 Since they depend on the balance between the effects of shorter (UV-B) and longer wavelengths, with higher RAFs indicating a greater dependence on UV-B radiation, they have much wider application. RAFs for endogenous inactivation of some coliphages187 (viruses used as indicators of biological contamination) are similar to the RAF (1.0–1.1) for the UV index (UVI) used to quantify damaging UV radiation in human skin (see ref. 1). The RAFs for direct photodegradation of chemical contaminants can be of the same order. The RAFs for indirect photoprocesses involving CDOM are typically much smaller (∼0.3) than for the UVI, reflecting the greater role of longer wavelength radiation than that of direct photodamage. However, some indirect processes involving inorganic sensitisers in natural waters can be closer to the UVI RAF, for example the RAF for nitrate-sensitised indirect processes is ∼0.6. These different wavelength responses, reflected in this range of RAFs, mean that the balance between different mechanisms contributing to photochemical changes is expected to vary with multiple elements of global change.
Examples of the extensive work on interactions of UV radiation with chemical and biological contaminants are provided in Table 1, which shows the broad scope of effects that solar UV radiation has on contaminants and that both direct and indirect photoreactions drive the degradation of contaminants. The examples are subdivided according to their susceptibility to direct or indirect photoreactions. However, for many contaminants, both direct and indirect processes are involved, e.g. photoreactions of the lampricides, TFM and niclosamide.188,189 In the last four years there has been a growing realisation of the importance of indirect photoreactions in the environment. This change in perception has partly stemmed from more studies focusing on this photoprocess but also because future projections suggest that the indirect mechanism for degradation of contaminants will increase with environmental change. Triplet state intermediates of CDOM often mediate indirect photoreactions.190 Recent research has shown that chemicals such as “persistent organic pollutants”, thought once to be resistant to biodegradation and photodegradation, can be transformed by indirect pathways.190–192 Indirect photoreactions are often initiated by CDOM, but inorganic substances such as nitrate, hydrogen peroxide, and iron/peroxides (photo-Fenton) can also sensitise photoreactions.193 The pathway of phototransformation of contaminants (via direct and/or indirect photoreactions) may influence the “toxicity” of byproducts formed in these photochemical processes. For example, antibiotics usually undergo indirect photoreactions (see Table 1). In general, indirect photochemical transformations preserve the basic chemical structure or backbone of an antibiotic, which means that many of the by-products of phototransformation have similar antibacterial properties to the original antibiotics. As a consequence, bacterial resistance of antibiotics could be extended from the point where they enter aquatic ecosystems to coastal areas.194
Table 1.
Schematic showing examples of contaminants that are transformed via direct and/or indirect photochemical reactions (photoreactions). Changes in UV radiation, linked to changes in stratospheric ozone, as well as climate, modulate the balance between direct and indirect photoreactions
| Direct photoreactions | Both | Indirect photoreactions |
|---|---|---|
| Photosensitive pesticides. UV-B induced, direct photoreactions dominate. UV-absorbing films can be used to reduce rates of photodegradation.3,207 | Organophosphoro-thionate pesticides. Fenitrothion undergoes direct photodegradation while diazinon is degraded by the indirect, nitrate-sensitised pathway.191 | POPs, PBDEs, and other biorefractory chemicals. Susceptible to indirect photoreactions.78,123,158 |
| Carbonyl compounds (especially aromatic ketones). Absorb in the UV-B region; triplet states participate in H-atom abstraction and electron transfer and also initiate indirect photoreactions.123 | Antibiotics. Direct pathway is dominant for Cipro and indirect pathway for others.111 | Antibiotics. Indirect pathway is usually dominant.111,123 |
| Lampricide. Direct and indirect photoreactions influence fate.119,120 | Nanosilver. CDOM-sensitised photoreactions reduce ionic silver to nanosilver.88 | |
| Graphene oxide. See ref. 88 and 89. | UV filters. Only indirect photoreactions are important.164 | |
| Oil spills. Combination of indirect and direct photodegradation at surfaces, coupled with photofacilitated biodegradation.82,205 | Microplastics. Are produced by indirect photoreactions7 (also see ref. 6). | |
| Pathogenic bacteria, viruses, and protozoans. Undergo direct (endogenous) and indirect (exogenous) photoinactivation133 (see Fig. 8). |
PPPs, persistent organic pollutants; PBDEs, polybrominated diphenyl ethers.
5.1.1. Organic contaminants, nanomaterials, microplastics, and oil spills
Organic contaminants are man-made chemicals including pesticides, pharmaceuticals, household and industrial products, fuels and other petrochemicals, nanomaterials, and microplastics. In some cases, exposure to UV radiation partially transforms contaminants to more toxic substances, e.g., conversion of graphene oxide to polycyclic hydrocarbons.195
Engineered nanomaterials (ENMs) are commonly incorporated as fillers in composites with plastics to improve their mechanical properties, conductivity, thermal stability, flame retardancy and other properties. Some of the resulting plastic nanocomposites are quite stable and likely to be very persistent in the environment. UV-initiated photoreactions play a key role in the release of nanomaterials from polymer composites by inducing weathering of the plastic matrix. The degradation of the polymer matrix is particularly important in this release.196,197 Exposure to UV radiation also plays a role in the weathering of macroplastics to microplastics (1 nm to 5 mm in size), which are widespread contaminants of freshwaters and oceans198 (also see ref. 36). Although plastic nanocomposites have not been shown to similarly form nano-containing microplastics, it is likely that the more persistent nanocomposites may be transported and transformed in a similar fashion. Two papers199,200 have provided overviews of chemical additives present in plastics, including their migration, release, fate and environmental impact during use, disposal, and recycling.
Oil spills result in accumulation of contamination on the water surface where full exposure to solar UV radiation occurs. One example was the Deepwater Horizon oil spill of 2010 where extensive amounts of oil floated on the surface of the Gulf of Mexico for over 100 days. Recent evidence201 has demonstrated that photo-oxidation by sunlight largely accounted for the oxidation of the surface oil (Fig. 7) but it was not possible to fully assess the chemical composition and toxicity of the photo-oxidised residues of the oil spill. However, their study, coupled with another study202 showed that photo-oxidation of the oil and its partially-oxidised residues on beach sands photo-facilitated its microbial degradation by increasing the carbonyl content (Fig. 7), another previously overlooked effect of photo-oxidation on dissipation of oil spills.
Fig. 7.
As oil is increasingly exposed to sunlight, its carbonyl content (Δ) increases, indicative of partial photo-oxidation of hydrocarbons in the oil to form oxidised organic compounds. This change in chemical composition of the oil decreases its chemical dispersion (■) (without much change in its natural dispersion (●)). See ref. 51 for more information (reproduced with permission of the American Chemical Society (ACS) from Fig. 1 of ref. 201
5.1.2. Harmful algal blooms and other biological contaminants
Eutrophication of freshwater and coastal ecosystems causes harmful algal blooms, particularly with cyanobacteria, which are a pervasive global threat to ecosystems and human health. In large temperate lakes, harmful algal blooms are often dominated by Microcystis species that produce potent neurotoxins, threaten drinking water supplies, and stimulate autotrophic carbon production and subsequent hypoxia. In recent years, Lake Erie has experienced two of the largest recorded blooms in its history. In 2011, 2013, 2014, and in 2015 elevated concentrations of the neurotoxin microcystins shut down the drinking water supply to nearly a half million people.203
Key drivers such as temperature and nutrients alone or together do not fully explain patterns of the occurrence or toxicity of harmful algal blooms (e.g., ref. 204). Recently, it has been suggested that photochemical processes driven by CDOM may mitigate the toxicity of harmful algal blooms.205 On the one hand, hydrogen peroxide (H2O2) produced by photochemical reactions of CDOM may be less damaging to toxic strains of Microcystis than non-toxic strains.206,207 Recent work has shown that high concentrations of H2O2 in Lake Erie are associated with toxic strains of Microcystis, while non-toxic strains were dominant when H2O2 concentrations were lower.208,209 On the other hand, photochemical reactions of CDOM may promote the degradation of algal toxins.210 Understanding the balance of these light-mediated effects of CDOM on Microcystis and its toxins is important because concentrations of dissolved organic matter (and thus CDOM) are increasing in North American and European freshwaters17 (see ref. 5).
Harmful algal blooms are not the only health-related microorganisms strongly affected by exposure to solar radiation. Exposure to solar UV radiation in the environment inactivates or kills many viruses, bacteria and other microbes that cause water-borne diseases in humans and other animals183 (also see ref. 5 and 211). As with chemical contaminants (Table 1), pathogenic micro-organisms may be damaged by direct and/or indirect photoreactions, also referred to as endogenous and exogenous inactivation, respectively.183 Recent advances in the understanding and modelling of microbial responses to solar radiation17,183 provide a broad conceptual framework for assessing the responses of contaminants to sunlight, and how those responses may alter given expected changes in stratospheric ozone, climate and other components of global change (section 5.3).
5.2. Modelling of photodegradation or photoinactivation of contaminants
Advances in modelling approaches are allowing improved quantification of the effects of global changes on the fate of synthetic and biological contaminants. Modelling is also used to assist with the design of treatment processes that rely on solar disinfection. Many contaminants are degraded via both direct and indirect photoreactions (Table 1). An important metric for characterizing the fate of contaminants is their half-life (t½, i.e., the time taken for degradation to half of the initial concentration) in a given environmental system. This half-life is calculated from eqn (1),
| (1) |
where ktot is the first-order rate constant of photodegradation or photoinactivation of contaminants and can be modelled including both direct and indirect photoreactions (ktot = kdir + kindir).
5.2.1. Modelling of direct photoreactions
The first-order rate constant of direct photochemical reactions, kdir, depends on the specific rate of light absorption by the contaminant, ka, as well as on the reaction quantum yield (Φ) (eqn (2)).
| (2) |
The quantum yield is the fraction of absorbed light that results in photoreaction. The specific rate of light absorption, ka, is a measure of the spectral overlap of the solar spectral irradiance and the absorption spectrum of the contaminant and is sensitive to changes in the absorption spectra of the contaminants, climate, and stratospheric ozone. The reasons for this sensitivity are that solar spectral irradiance that strikes the surface of a water body, particularly in the UV-B range, depends on concentrations of stratospheric ozone as well as on cloud cover and concentrations and composition of aerosols. Decreased atmospheric ozone in recent decades will have tended to increase rates of UV-B-induced photoreactions of contaminant, primarily direct photoreactions of the type described in the left column of Table 1. As the stratospheric ozone layer recovers (see ref. 1), UV-B radiation will decline in many areas. Assuming this effect would be approximated by estimated changes in UVI, as seems reasonable given the relevant RAFs, this change in ozone would reduce these UV-B photoreactions by up to 5–15%. The effect of stratospheric ozone recovery would be much smaller for indirect photoreactions, given their smaller RAFs.
As discussed above, CDOM is the main light-absorbing compound in many aquatic ecosystems. Therefore, ka depends not only on the extinction coefficient (absorption properties) of a considered contaminant but also on the concentration and absorption properties of CDOM and of all other light-absorbing materials present in a water body, including POM. Hence the specific rate of light absorption by a contaminant, ka, and thus the first-order rate constant of a direct photochemical reaction decreases exponentially with increasing depth of a water body, and with increasing concentration and absorption of solar radiation by CDOM and other light-absorbing components contained in aquatic ecosystems. Simulations of photodegradation of contaminants via direct photoreactions require estimates of the solar spectral irradiance, principally the UV irradiance that strikes the surface of a water body. Mathematical models have been developed to quantify photolysis of contaminants in different aquatic environments and at different solar zenith angles (a function of latitude, time of year and time of day).183,187,212–214
5.2.2. Modelling of indirect photoreactions
The first-order rate constant of indirect photochemical reactions, kindir, depends on the steady-state concentration of a reactive transient species ([RTi]ss) and on the second-order rate constant of reaction of a reactive transient species with a contaminant (ki). The photochemical production of reactive transients involving photosensitisers occurs far into the UV-A and visible region. In aquatic ecosystems, many reactive transients are present and, therefore, indirect photoreactions of contaminants have been quantified by summing individual rate expressions for reaction of a contaminant with each of several reactive transients produced by sunlight in the water (eqn (3)).
| (3) |
The assessment of kindir requires experiments that define the second-order rate constant, ki, for reaction of the contaminant with each transient. An alternative approach is to use action spectra, i.e., weighting functions, in the calculations of second-order rate constants.183,187,214–217 The use of action spectra has been employed mainly to estimate second-order rate constants for biological contaminants. The steady-state concentrations of transients, [RTi]ss, can be directly measured195,215,218–220 or simulated.212,213
5.3. A conceptual framework for assessing changes in the photo-biogeochemistry of contaminants in response to changes in stratospheric ozone and other components of global change
As noted in the Introduction, future changes in exposure to solar UV radiation are likely to be affected not just by changes in stratospheric ozone but by other factors including cloud, aerosols, surface reflectivity coupled with ice/snow loss (see ref. 1), and changes in the concentration of CDOM in water bodies17 (see ref. 5). While changes in stratospheric ozone preferentially affect UV-B radiation, changes in these other factors will also affect UV-A radiation, and even wavelengths greater than 400 nm (see ref. 1). These more heterogeneous changes will affect rates of photochemical processes as well as the balance between direct and indirect photochemistry. The concentration of CDOM in a water body is critical. On the one hand, by attenuating solar radiation, particularly UV radiation, CDOM protects synthetic and biological contaminants that undergo direct photoreactions. On the other hand, CDOM acts as a photosensitiser and, therefore, enables indirect photoreactions. Thus, the balance between different mechanisms by which contaminants are transformed or inactivated depends on their susceptibility to direct or indirect photoreactions (Table 1), on the depth in a water body, and on environmental factors such as the concentration and absorption properties of CDOM. These potentially complex interactions have been considered in a case study.183 This study investigated the role of sunlight in the inactivation of two different viruses at different depths in a water column containing CDOM, which acted as an exogenous photosensitiser in indirect photoinactivation. They modelled the total rate constant, ktot, of photoinactivation as the sum of three mechanisms
Light-independent (dark) mechanisms
Direct light-dependent mechanisms
Indirect light-dependent mechanisms
They compared the effect of depth in the water column on ktot of photoinactivation of two viruses, poliovirus, and the phage MS2, using the model parameters from ref. 221 for a wetland during the month of June in Northern California. Poliovirus undergoes mainly direct photoinactivation, whereas MS2 is mainly inactivated by photosensitised processes (green and brown areas, respectively, in the right panel of Fig. 8).
Fig. 8.
Modelled depth dependence of the rate constant ktot of photoinactivation of MS2 coliphage and poliovirus in a water body with the same optical properties and exogenous photosensitiser concentrations as observed in a simulated open water wetland.183 The term “clear water” indicates that ktot was computed assuming water at the surface with no light attenuation or sensitisation by coloured dissolved organic matter (CDOM). kendo and kexo are the first-order rate constants for direct and indirect photoinactivation, respectively. The circles in the right panel represent ktot and include both direct (green) and indirect (brown) photoinactivation (ktot = kendo + kexo). The abbreviations “endo” and “exo” refer to the light-absorbing compounds, i.e., biological molecules inside the viruses and CDOM outside the viruses, respectively (reproduced with permission of the Royal Society of Chemistry from Fig. 10 of ref. 183).
At the surface of clear water, ktot of photoinactivation of poliovirus is 20 times greater than ktot of MS2 photoinactivation (right panel in Fig. 8). By contrast, at the surface of simulated wetland water containing CDOM, ktot of poliovirus photoinactivation is only 2.5 times greater than ktot of MS2 photoinactivation (left panel in Fig. 8). This contrast is due to CDOM acting as a photosensitiser in the simulated wetland water and hence ktot of MS2 photoinactivation is greater than in water without CDOM. At 5 cm depth in water containing CDOM, ktot for MS2 is greater than in clear water since MS2 is mainly susceptible to indirect photoinactivation, whereas ktot for poliovirus is smaller than in clear water, due to the attenuation of UV-B radiation by CDOM. At depths greater than 11 cm, MS2 receives greater photodamage than poliovirus (left panel in Fig. 8) since kendo decreases more strongly with depth than kexo. This phenomenon is due to the fast attenuation of the damaging solar UV-B radiation with depth, while deeper penetrating longer wavelengths are involved in the photoproduction of reactive transients, and hence indirect photoinactivation. This is also shown in the right panel of Fig. 8, where ktot for MS2 is greater than ktot for poliovirus at 20 cm depth. If the water column containing CDOM would be well mixed to about 50 cm deep, ktot averaged over the full depth of the column would be approximately equal for MS2 and poliovirus.
The complex interplay of direct and indirect mechanisms with different wavelength dependencies, absorption properties of any dissolved or suspended material, and the extent of mixing can lead to patterns of photodamage not evident from the responses of organisms measured in clear water, including measurements made under typical laboratory conditions. Since indirect photoreactions mediated by CDOM are affected by wavelengths well into the UV-A and visible region they have much lower sensitivity to changes in ozone and much deeper penetration into water bodies in general. Considering the large effect of CDOM on the inactivation rate constants even with a change of a few cm in depth, we hypothesise that ozone-related changes are much smaller than changes caused by CDOM, even for direct photodegradation. Hence, increases in runoff of CDOM, so-called browning,77 could affect contaminant degradation rates much more substantially in the future than the small changes projected for ozone increases. Conversely, decreases in CDOM related to drought and reduced runoff could result in much larger increases in UV-related contaminant photodegradation than observed or projected ozone changes. Results similar to poliovirus can be demonstrated for direct photoinactivation of coliphage phiX174 by solar UV-B radiation in beach waters of the Great Lakes; like poliovirus, phiX174 photoreacts predominately by the UV-B-induced direct mechanism.187
Given the parallels in the balance of direct and indirect photoprocesses between micro-organisms and chemical contaminants (Table 1), we suggest that the results shown in Fig. 8 can be generalised to include chemical contaminants. For example, photoreaction of the antibiotic ciprofloxin proceeds exclusively by UV-B-induced direct photoreaction, and so its responses may be close to those of poliovirus, whereas other antibiotics photoreact by indirect pathways mediated by CDOM, and so are closer to MS2.222 Likewise, UV-B radiation induces direct photoreaction of the organophosphorus insecticide fenitrothion but diazinon, another organophosphorus insecticide, photoreacts in natural waters by indirect mechanisms that are induced through UV-B radiation absorbed by nitrate ions in the water.193 In this case, the prediction is that fenitrothion will respond to factors such as the concentration of CDOM and the depth in the water column, similar to the response of poliovirus, while diazinon is expected to be more similar to MS2. The latter findings show that not only CDOM but also inorganic compounds such as nitrate can act as photosensitisers, and in many environments the two may occur together. If nitrate as a photosensitiser had been included in the scenario modelled in Fig. 8, then ktot of photoinactivation of MS2 would have been greater at the surface of the simulated water body but would have dropped off more sharply than shown in Fig. 8, i.e., the dependence on depth of ktot for MS2 would be closer to that for poliovirus.
The new modelling techniques as shown in the example above are providing useful insights into the most important mechanisms for contaminant photoreactions and dependence of the rates of these processes on changes in time, location, water depth, and stratospheric ozone concentrations. Other examples apply to chemical contaminants. Photoreactions of contaminants have been modelled in boreal lakes to provide comprehensive maps of indirect and direct photoreactions over a large geographic region of Sweden.219 In the case of indirect photoreactions, recent and projected future changes in “browning” of the lakes are creating unfavourable environments for indirect photoreactions mediated by OH and carbonate radical anions but increasingly favourable environments for indirect photoreactions involving CDOM triplet states and singlet oxygen. Another modelling study of ocean water in Terra Nova Bay, Antarctica, indicated that two sample contaminants, the solar filter, benzophenone-3, and antimicrobial agent, triclosan, would have photochemical half-lives of less than a few days during the Antarctic summer due to indirect photoreactions primarily mediated by CDOM triplet states.215 Other modelling studies in four Alpine lakes of Europe predict that indirect photoreactions in the lakes may be significantly altered by future changes in water alkalinity and dissolved organic carbon caused by climate change.223
6. Feedbacks on global warming that are mediated by UV and visible radiation
The effects of changes in ozone and climate on biogeochemical cycles that are mediated by solar UV and visible radiation have the potential to drive feedbacks to global warming (Fig. 9), although critical gaps in knowledge (section 7) make it difficult to quantify the magnitude of any such feedbacks. Droughts can increase exposure of dead plant material (litter) to solar radiation due to the reduction in plant cover. Hence the photochemical decomposition of litter can be accelerated, yielding increased emissions of CO2 to the atmosphere and organic compounds that can be more easily degraded by microorganisms (photofacilitation), increasing microbial respiration. Wildfires, because of droughts, are direct sources of CO2 and other greenhouse gases, particularly CH4. In addition, wildfires transform NOM into compounds that are more susceptible to photodegradation, which increases the release of CO2. Wildfires also enhance the thawing of permafrost soils. Extreme precipitation events (storms in Fig. 9) and thawing of permafrost soils enhance the flow of soil organic matter from land to fresh and coastal waters and hence the exposure to solar radiation of particulate and dissolved organic matter from soils (POM and tDOM, respectively). In aquatic ecosystems, POM and tDOM undergo photodegradation in similar ways as in terrestrial ecosystems yielding CO2 and other products, where photofacilitation plays an important role. The net results of the interactive effects of UV and visible radiation and climate change on carbon cycling are a reduction of carbon storage in soils, and an increased release of CO2 into the atmosphere, which reinforces climate change (positive feedback).
Fig. 9.
Interactive effects of solar radiation and climate change on the flow of natural organic matter (NOM) from soils into aquatic ecosystems (rivers, lakes, coastal waters), and on the photodegradation of NOM on land and in water. Key to symbols: black arrows indicate interactions between changes in stratospheric ozone, solar UV radiation, and climate and their effects on changes on land (permafrost thawing, storms, droughts, (wild)-fires) that influence the flow of soil organic matter from land to water. Dashed arrows indicate direct effects of solar UV radiation on decomposer organisms (see ref. 2 and 5). Purple arrows indicate the effects of solar UV and visible radiation on the degradation of soil and litter in terrestrial ecosystems and of particulate organic matter (POM) and terrestrial dissolved organic matter (tDOM) in aquatic ecosystems. Green arrows refer to the process of photofacilitation. Brown arrows indicate the flow of carbon from terrestrial to aquatic ecosystems. Red arrows indicate the release of carbon dioxide (CO2) from terrestrial and aquatic ecosystems to the atmosphere and the feedback on climate change. The symbols + and − indicate an enhanced or reduced effect on a change, flow or process.
Climate-change related increases in emissions of greenhouse gases other than CO2 may also cause positive feedbacks on global warming. Photodegradation of tDOM not only yields CO2 but also CO and other products. CO competes with CH4 for OH, where reaction of CH4 with OH is the major sink of CH4 in the troposphere. Thus, CO emission from tDOM photodegradation might fuel a positive feedback on global warming via a longer lifetime of CH4, an important GHG, in the troposphere. Thawing of permafrost soils due to global warming also enhances emissions of N2O, another important GHG. Thus, emissions of N2O from permafrost soils might fuel a positive feedback by reinforcing thawing of permafrost soils.
7. Major advances and gaps in knowledge
As highlighted in the previous sections, there is increasing awareness that a wide range of biogeochemical processes that are mediated by solar radiation, and so liable to be influenced by changes in stratospheric ozone, may interact with effects of climate change such as the loss of the cryosphere, droughts, and extreme precipitation events. These effects enhance the exposure of NOM to solar radiation and thus the likelihood for photodegradation. The processes involved in the photodegradation of NOM are now better understood and include direct and indirect photoreactions, as well as photofacilitation.
Gaps in knowledge relating to carbon cycling include (1) the net effect of land-use change (including conversions due to agriculture, deforestation, and afforestation) on the photodegradation of plant litter, (2) the role of reactive oxygen species (ROS) and iron in the photodegradation of tDOM in aquatic ecosystems, (3) the relative importance of ROS vs. products of tDOM photodegradation on subsequent microbial respiration, (4) the timescales of tDOM photodegradation relative to water residence times and inputs of “fresh” tDOM from land, (5) how rates of tDOM photodegradation will be affected by changes in the ratio of UV and visible radiation reaching surface waters due to changes in ice cover on lakes, cloud cover, wildfire, or changes in stratospheric ozone. Modelling approaches as discussed in section 5.3 for contaminants would greatly help to advance our understanding on these outstanding issues. However, the use of such models is constrained for many biogeochemical processes due to the lack of necessary model inputs. Such inputs might include, for example, a well-defined action spectrum and/or well quantified relationships between the rate of a process and the irradiance or dose of weighted radiation. The progress made in modelling contaminants (see below) provides some encouragement that such gaps could be filled for a wider range of biogeochemical processes.
As for CO2, effects of climate change may also affect rates of CO production via photodegradation of tDOM (section 4.1) and thus tropospheric concentrations of CH4, since CO competes with CH4 for OH. CH4 and N2O are greenhouse gases with indirect or direct impacts on stratospheric ozone. Another group of trace gases that affect stratospheric ozone are the brominated very-short-lived substances (BrVSLS). Gaps in knowledge regarding trace gases are: (1) the ratio of CO2/CO formed via photodegradation of tDOM on a regional and global scale, (2) the reason for the leveling off of tropospheric CH4 concentrations between 1999–2006, (3) the role of UV and visible radiation in facilitating microbial mineralisation of nitrogen-containing NOM in terrestrial and aquatic ecosystems and thus the release of N2O, (4) future N2O emissions from terrestrial and aquatic ecosystems on a global scale, (5) the contribution of BrVSLS emissions from natural sources to ozone depletion in the lower stratosphere.
Since the last Quadrennial Assessment, major advances have been made regarding the biogeochemical cycling of contaminants. Advanced modelling tools as described in section 5.3 have enabled half-lives of contaminants in a given aquatic environment to be predicted for contaminants that are degraded via both direct and indirect photochemical reactions. Gaps in knowledge are: (1) the role of POM in the degradation of contaminants via direct and indirect photoreactions, (2) three-dimensional models that can be used to predict changes in CDOM concentrations and UV radiation, (3) models that can be used to predict UV-induced degradation of contaminants in terrestrial ecosystems, (4) the way in which half-lives of contaminants in a given aquatic ecosystem change with time due to bleaching of CDOM. As noted above, this rapid progress made with contaminants provides an exemplar and stimulus to develop equally powerful modelling approaches to quantify the role of light-driven processes in large-scale biogeochemistry, above all the carbon cycle.
Acknowledgements
This paper has been reviewed in accordance with the U.S. Environmental Protection Agency’s (U.S. EPA) peer and administrative review policies and approved for publication. Mention of trade names or commercial products does not constitute an endorsement or recommendation for use by the U.S. EPA. We thank Andrew Netherwood for his assistance with drafting and improving Fig. 1.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Bais F, Bernhard G, McKenzie RL, Aucamp PJ, Young PJ, Ilyas M, Jöckel P. and Deushi M, Ozone–climate interactions and effects on solar ultraviolet radiation, Photochem. Photobiol. Sci, 2019, 10.1039/C8PP90059K, 18 [DOI] [PubMed] [Google Scholar]
- 2.Bornman JF, Barnes PW, Robson TM, Robinson SA, Jansen MAK, Ballaré CL and Flint SD, Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems, Photochem. Photobiol. Sci, 2019, 10.1039/C8PP90061B, 18 [DOI] [PubMed] [Google Scholar]
- 3.Bornman JF, Barnes PW, Robinson SA, Ballaré CL, Flint SD and Caldwell MM, Solar ultraviolet radiation and ozone depletion-driven climate change: effects on terrestrial ecosystems, Photochem. Photobiol. Sci, 2015, 14, 88–107 [DOI] [PubMed] [Google Scholar]
- 4.Robinson SA and Erickson DJ, Not just about sunburn - the ozone hole’s profound effect on climate has significant implications for Southern Hemisphere ecosystems, Glob. Change Biol, 2015, 21, 515–527 [DOI] [PubMed] [Google Scholar]
- 5.Williamson E, Neale PJ, Hylander S, Rose KC, Figueroa FL, Robinson S, Häder D-P, Wängberg S-Å and Worrest RC, The interactive effects of stratospheric ozone depletion, UV radiation, and climate change on aquatic ecosystems, Photochem. Photobiol. Sci, 2019, 10.1039/C8PP90062K, 18 [DOI] [PubMed] [Google Scholar]
- 6.Erickson J, Sulzberger B, Zepp RG and Austin AT, Effects of stratospheric ozone depletion, solar UV radiation, and climate change on biogeochemical cycling: interactions and feedbacks, Photochem. Photobiol. Sci, 2015, 14, 127–148 [DOI] [PubMed] [Google Scholar]
- 7.Ivy J, Solomon S, Calvo N. and Thompson DWJ, Observed connections of Arctic stratospheric ozone extremes to Northern Hemisphere surface climate, Environ. Res. Lett, 2017, 12, 024004 [Google Scholar]
- 8.Francis JA and Vavrus SJ, Evidence for a wavier jet stream in response to rapid Arctic warming, Environ. Res. Lett, 2015, 10, 014005 [Google Scholar]
- 9.Overland JE, Dethloff K, Francis JA, Hall RJ, Hanna E, Kim SJ, Screen JA, Shepherd TG and Vihma T, Nonlinear response of mid-latitude weather to the changing Arctic, Nat. Clim. Change, 2016, 6, 992–999 [Google Scholar]
- 10.Francis JA and Vavrus SJ, Evidence linking Arctic amplification to extreme weather in mid-latitudes, Geophys. Res. Lett, 2012, 39, 051000 [Google Scholar]
- 11.Kretschmer M, Coumou D, Donges JF and Runge J, Using causal effect networks to analyze different Arctic drivers of midlatitude winter circulation, J. Clim, 2016, 29, 4069–4081 [Google Scholar]
- 12.Sulzberger B. and Arey JS, Impacts of polar changes on the UV-induced mineralization of terrigenous dissolved organic matter, Environ. Sci. Technol, 2016, 50, 6621–6631 [DOI] [PubMed] [Google Scholar]
- 13.Ronalds B, Barnes E. and Hassanzadeh P, A barotropic mechanism for the response of jet stream variability to Arctic amplification and sea ice loss, J. Clim, 2018, 31, 7069–7085 [Google Scholar]
- 14.von U. Gunten Oxidation processes in water treatment: Are we on track?, Environ. Sci. Technol, 2018, 52, 5062–5075 [DOI] [PubMed] [Google Scholar]
- 15.Topaz T, Egozi R, Eshel G. and Chefetz B, Pesticide load dynamics during stormwater flow events in Mediterranean coastal streams: Alexander stream case study, Sci. Total Environ, 2018, 625, 168–177 [DOI] [PubMed] [Google Scholar]
- 16.Ellis S, Cipro CVZ, Ogletree CA, Smith KE and Aronson RB, A 50-year retrospective of persistent organic pollutants in the fat and eggs of penguins of the Southern Ocean, Environ. Pollut, 2018, 241, 155–163 [DOI] [PubMed] [Google Scholar]
- 17.Williamson CE, Madronich S, Lal A, Zepp RG, Lucas RM, Overholt EP, Rose KC, Schladow SG and Lee-Taylor J, Climate change-induced increases in precipitation are reducing the potential for solar ultraviolet radiation to inactivate pathogens in surface waters, Sci. Rep, 2017, 7, 13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crill PM and Thornton BF, Whither methane in the IPCC process?, Nat. Clim. Change, 2017, 7, 678–680 [Google Scholar]
- 19.Saunois M, Bousquet P, Poulter B, Peregon A, Ciais P, Canadell JG, Dlugokencky EJ, Etiope G, Bastviken D, Houweling S, Janssens-Maenhout G, Tubiello FN, Castaldi S, Jackson RB, Alexe M, Arora VK, Beerling DJ, Bergamaschi P, Blake DR, Brailsford G, Brovkin V, Bruhwiler L, Crevoisier C, Crill P, Covey K, Curry C, Frankenberg C, Gedney N, Hoglund-Isaksson L, Ishizawa M, Ito A, Joos F, Kim HS, Kleinen T, Krummel P, Lamarque JF, Langenfelds R, Locatelli R, Machida T, Maksyutov S, McDonald KC, Marshall J, Melton JR, Morino I, Naik V, O’Doherty S, Parmentier FJW, Patra PK, Peng CH, Peng SS, Peters GP, Pison I, Prigent C, Prinn R, Ramonet M, Riley WJ, Saito M, Santini M, Schroeder R, Simpson IJ, Spahni R, Steele P, Takizawa A, Thornton BF, Tian HQ, Tohjima Y, Viovy N, Voulgarakis A, van Weele M, van der Werf GR, Weiss R, Wiedinmyer C, Wilton DJ, Wiltshire A, Worthy D, Wunch D, Xu XY, Yoshida Y, Zhang B, Zhang Z. and Zhu Q, The global methane budget 2000–2012, Earth Syst. Sci. Data, 2016, 8, 697–751 [Google Scholar]
- 20.Fleming L, George C, Heard DE, Jackman CH, Kurylo MJ, Mellouki W, Orkin VL, Swartz WH, Wallington TJ, Wine PH and Burkholder JB, The impact of current CH4 and N2O atmospheric loss process uncertainties on calculated ozone abundances and trends, J. Geophys. Res.: Atmos, 2015, 120, 5267–5293 [Google Scholar]
- 21.Wilson SR, Madronich S, Longstreth JD and Solomon KR, Interactive effects of changing stratospheric ozone and climate on tropospheric composition and air quality, and the consequences for human and ecosystem health, Photochem. Photobiol. Sci, 2019, 10.1039/C8PP90064G, 18 [DOI] [PubMed] [Google Scholar]
- 22.Andrady L, Aucamp PJ, Austin A, Bais AF, Ballaré CL, Barnes PW, Bernhard GH, Bornman JF, Caldwell MM, De Gruijl FR, Erickson DJ, Flint SD, Gao K, Gies P, Häder D-P, Ilyas M, Longstreth J, Lucas RM, Madronich S, McKenzie RL, Neale R, Norval M, Pandy KK, Paul ND, Rautio M, Redhwi HH, Robinson SA, Rose K, Shao M, Sinha RP, Solomon KR, Sulzberger B, Takizawa Y, Tang X, Torikai A, Tourpali K, van der Leun JC, Wängberg S-Å, Williamson CE, Wilson SR, Worrest RC, Young AR and Zepp RG, Environmental effects of ozone depletion and its interactions with climate change: 2014 assessment Executive summary, Photochem. Photobiol. Sci, 2015, 14, 14–18 [DOI] [PubMed] [Google Scholar]
- 23.Pinhey JT and Rigby RDG, Photo-reduction of chloro- and bromo-aromatic compounds, Tetrahedron Lett., 1969, 1267–1270 [Google Scholar]
- 24.Zepp RG and Cline DM, Rates of direct photolysis in aquatic environment, Environ. Sci. Technol, 1977, 11, 359–366 [Google Scholar]
- 25.Chen Y, Khan SU and Schnitzer M, Ultraviolet irradiation of dilute fulvic acid solutions, Soil Sci. Soc. Am. J, 1978, 42, 292–296 [Google Scholar]
- 26.Kramer CJM Degradation by sunlight of dissolved fluorescing substances in the upper layers of the eastern Atlantic Ocean, Neth. J. Sea Res, 1979, 13, 325–329 [Google Scholar]
- 27.Zafiriou OC and True MB, Nitrite photolysis in seawater by sunlight, Mar. Chem, 1979, 8, 9–32 [Google Scholar]
- 28.Zepp RG, Schlotzhauer PF and Sink RM, Photosensitized transformations involving electronic-energy transfer in natural-waters - role of humic substances, Environ. Sci. Technol, 1985, 19, 74–81 [Google Scholar]
- 29.Moorhead DL and Callaghan T, Effects of increasing ultraviolet-B radiation on decomposition and soil organic-matter dynamics - A synthesis and modeling study, Biol. Fertil. Soils, 1994, 18, 19–26 [Google Scholar]
- 30.Gehrke C, Johanson U, Callaghan TV, Chadwick D. and Robinson CH, The impact of enhanced ultraviolet-B radiation on litter quality and decomposition processes in Vaccinium leaves from the sub-Arctic, Oikos, 1995, 72, 213–222 [Google Scholar]
- 31.Tarr MA, Miller WL and Zepp RG, Direct carbon-monoxide photoproduction from plant matter, J. Geophys. Res.: Atmos., 1995, 100, 11403–11413 [Google Scholar]
- 32.Zepp RG, Erickson DJ, Paul ND and Sulzberger B, Effects of solar UV radiation and climate change on biogeochemical cycling: interactions and feedbacks, Photochem. Photobiol. Sci, 2011, 10, 261–279 [DOI] [PubMed] [Google Scholar]
- 33.WMO, Executive Summary: Scientific Assessment of Ozone Depletion: World Meteorological Organization, Global Ozone Research and Monitoring Project, World Meteorological Organization Report No. 58, Geneva Switzerland, 2018, p. 67 [Google Scholar]
- 34.Austin AT and Vivanco L, Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation, Nature, 2006, 442, 555–558 [DOI] [PubMed] [Google Scholar]
- 35.Cory RM, Ward CP, Crump BC and Kling GW, Sunlight controls water column processing of carbon in arctic fresh waters, Science, 2014, 345, 925–928 [DOI] [PubMed] [Google Scholar]
- 36.Andrady L, Pandey KK and Heikkilä AM, Interactive effects of solar UV radiation and climate change on material damage, Photochem. Photobiol. Sci, 2019, 10.1039/C8PP90065E, 18 [DOI] [PubMed] [Google Scholar]
- 37.Brandt LA, Bohnet C. and King JY, Photochemically induced carbon dioxide production as a mechanism for carbon loss from plant litter in arid ecosystems, J. Geophys. Res.: Biogeosci, 2009, 114, G02004 [Google Scholar]
- 38.Weng JK and Chapple C, The origin and evolution of lignin biosynthesis, New Phytol., 2010, 187, 273–285 [DOI] [PubMed] [Google Scholar]
- 39.Austin AT and Ballaré CL, Dual role of lignin in plant litter decomposition in terrestrial ecosystems, Proc. Natl. Acad. Sci. U. S. A, 2010, 107, 4618–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin AT, Mendez MS and Ballaré CL, Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 4392–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Y, Scarlett RD and King JY, Effects of UV photodegradation on subsequent microbial decomposition of Bromus diandrus litter, Plant Soil, 2015, 395, 263–271 [Google Scholar]
- 42.Mopper K, Kieber DJ, Stubbins A, Hansell D. and Carlson C, Marine Photochemistry of Organic Matter: Processes and Impacts, Biogeochemistry of Marine Dissolved Organic Matter, Academic Press, 2015, 389–450 [Google Scholar]
- 43.Araujo PI and Austin AT, A shady business: pine afforestation alters the primary controls on litter decomposition along a precipitation gradient in Patagonia, Argentina, J. Ecol, 2015, 103, 1408–1420 [Google Scholar]
- 44.Ballaré CL and Pierik R, The shade-avoidance syndrome: multiple signals and ecological consequences, Plant, Cell Environ., 2017, 40, 2530–2543 [DOI] [PubMed] [Google Scholar]
- 45.Cory RM, Cotner JB and McNeill K, Quantifying interactions between singlet oxygen and aquatic fulvic acids, Environ. Sci. Technol, 2009, 43, 718–723 [DOI] [PubMed] [Google Scholar]
- 46.Wolf R, Thrane J-E, Hessen DO and Andersen T, Modelling ROS formation in boreal lakes from interactions between dissolved organic matter and absorbed solar photon flux, Water Res., 2018, 132, 331–339 [DOI] [PubMed] [Google Scholar]
- 47.Page SE, Logan JR, Cory RM and McNeill K, Evidence for dissolved organic matter as the primary source and sink of photochemically produced hydroxyl radical in Arctic surface waters, Environ. Sci.: Processes Impacts, 2014, 16, 807–822 [DOI] [PubMed] [Google Scholar]
- 48.Trusiak, Treibergs LA, Kling GW and Cory RM, The role of iron and reactive oxygen species in the production of CO2 in Arctic soil waters, Geochim. Cosmochim. Acta, 2018, 224, 80–95 [Google Scholar]
- 49.Waggoner C, Wozniak AS, Cory RM and Hatcher PG, The role of reactive oxygen species in the degradation of lignin derived dissolved organic matter, Geochim. Cosmochim. Acta, 2017, 208, 171–184 [Google Scholar]
- 50.Cory RM, McNeill K, Cotner JP, Amado A, Purcell JM and Marshall AG, Singlet oxygen in the coupled photochemical and biochemical oxidation of dissolved organic matter, Environ. Sci. Technol, 2010, 44, 3683–3689 [DOI] [PubMed] [Google Scholar]
- 51.Ward CP, Nalven SG, Crump BC, Kling GW and Cory RM, Photochemical alteration of organic carbon draining permafrost soils shifts microbial metabolic pathways and stimulates respiration, Nat. Commun, 2017, 8, 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anesio M, Graneli W, Aiken GR, Kieber DJ and Mopper K, Effect of humic substance photodegradation on bacterial growth and respiration in lake water, Appl. Environ. Microbiol, 2005, 71, 6267–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Judd KE, Crump BC and Kling GW, Bacterial responses in activity and community composition to photo-oxidation of dissolved organic matter from soil and surface waters, Aquat. Sci, 2007, 69, 96–107 [Google Scholar]
- 54.Adair C, Parton WJ, King JY, Brandt LA and Lin Y, Accounting for photodegradation dramatically improves prediction of carbon losses in dryland systems, Ecosphere, 2017, 8, 1–1629552374 [Google Scholar]
- 55.Koehler B, Landelius T, Weyhenmeyer GA, Machida N. and Tranvik LJ, Sunlight-induced carbon dioxide emissions from inland waters, Global Biogeochem. Cycles, 2014, 28, 696–711 [Google Scholar]
- 56.Powers LC and Miller WL, Photochemical production of CO and CO2 in the Northern Gulf of Mexico: Estimates and challenges for quantifying the impact of photochemistry on carbon cycles, Mar. Chem, 2015, 171, 21–35 [Google Scholar]
- 57.Vachon D, Lapierre JF and del Giorgio PA, Seasonality of photochemical dissolved organic carbon mineralization and its relative contribution to pelagic CO2 production in northern lakes, J. Geophys. Res.: Biogeosci, 2016, 121, 864–878 [Google Scholar]
- 58.Austin AT Has water limited our imagination for aridland biogeochemistry?, Trends Ecol. Evol, 2011, 26, 229–235 [DOI] [PubMed] [Google Scholar]
- 59.Gliksman D, Rey A, Seligmann R, Dumbur R, Sperling O, Navon Y, Haenel S, De Angelis P, Arnone JA and Grunzweig JM, Biotic degradation at night, abiotic degradation at day: positive feedbacks on litter decomposition in drylands, Glob. Change Biol, 2017, 23, 1564–1574 [DOI] [PubMed] [Google Scholar]
- 60.Rutledge S, Campbell DI, Baldocchi D. and Schipper LA, Photodegradation leads to increased CO2 losses from terrestrial organic matter, Glob. Change Biol, 2010, 16, 3065–3074 [Google Scholar]
- 61.Lee H, Rahn T. and Throop H, An accounting of C-based trace gas release during abiotic plant litter degradation, Glob. Change Biol, 2012, 18, 1185–1195 [Google Scholar]
- 62.Ballaré CL and Austin AT, UV radiation and terrestrial ecosystems: Emerging perspectives, Cabi Publishing-C a B Int, Wallingford, 2017 [Google Scholar]
- 63.Baker NR and Allison SD, Ultraviolet photodegradation facilitates microbial litter decomposition in a Mediterranean climate, Ecology, 2015, 96, 1994–2003 [DOI] [PubMed] [Google Scholar]
- 64.Gaxiola and JJ Armesto, Understanding litter decomposition in semiarid ecosystems: linking leaf traits, UV exposure and rainfall variability, Front. Plant Sci, 2015, 6, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gliksman D, Haenel S. and Grunzweig JM, Biotic and abiotic modifications of leaf litter during dry periods affect litter mass loss and nitrogen loss during wet periods, Funct. Ecol, 2018, 32, 831–839 [Google Scholar]
- 66.Wang J, Liu LL, Wang X, Yang S, Zhang BB, Li P, Qiao CL, Deng MF and Liu WX, High night-time humidity and dissolved organic carbon content support rapid decomposition of standing litter in a semi-arid landscape, Funct. Ecol, 2017, 31, 1659–1668 [Google Scholar]
- 67.Schmitz C, van Meijl H, Kyle P, Nelson GC, Fujimori S, Gurgel A, Havlik P, Heyhoe E, d’Croz DM and Popp A, Land-use change trajectories up to 2050: Insights from a global agro-economic model comparison, Agric. Econ, 2014, 45, 69–84 [Google Scholar]
- 68.Smith P, Davis SJ, Creutzig F, Fuss S, Minx J, Gabrielle B, Kato E, Jackson RB, Cowie A. and Kriegler E, Biophysical and economic limits to negative CO2 emissions, Nat. Clim. Change, 2016, 6, 42–50 [Google Scholar]
- 69.Almagro M, Maestre FT, Martinez-Lopez J, Valencia E. and Rey A, Climate change may reduce litter decomposition while enhancing the contribution of photodegradation in dry perennial Mediterranean grasslands, Soil Biol. Biochem, 2015, 90, 214–223 [Google Scholar]
- 70.Huang, Zhao HM and Li Y, Litter decomposition in hyper-arid deserts: Photodegradation is still important, Sci. Total Environ, 2017, 601, 784–792 [DOI] [PubMed] [Google Scholar]
- 71.Day TA, Guenon R. and Ruhland CT, Photodegradation of plant litter in the Sonoran Desert varies by litter type and age, Soil Biol. Biochem, 2015, 89, 109–122 [Google Scholar]
- 72.Adame MF, Wright SF, Grinham A, Lobb K, Reymond CE and Lovelock CE, Terrestrial-marine connectivity: Patterns of terrestrial soil carbon deposition in coastal sediments determined by analysis of glomalin related soil protein, Limnol. Oceanogr, 2012, 57, 1492–1502 [Google Scholar]
- 73.Biddanda B. Global significance of the changing freshwater carbon cycle, EOS, 2017, 15–17 [Google Scholar]
- 74.Cole JJ, Prairie YT, Caraco NF, McDowell WH, Tranvik LJ, Striegl RG, Duarte CM, Kortelainen P, Downing JA, Middelburg JJ and Melack J, Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget, Ecosystems, 2007, 10, 171–184 [Google Scholar]
- 75.Raymond PA, Hartmann J, Lauerwald R, Sobek S, McDonald C, Hoover M, Butman D, Striegl R, Mayorga E, Humborg C, Kortelainen P, Durr H, Meybeck M, Ciais P. and Guth P, Global carbon dioxide emissions from inland waters, Nature, 2013, 503, 355–359 [DOI] [PubMed] [Google Scholar]
- 76.McGuire D, Anderson LG, Christensen TR, Dallimore S, Guo LD, Hayes DJ, Heimann M, Lorenson TD, Macdonald RW and Roulet N, Sensitivity of the carbon cycle in the Arctic to climate change, Ecol. Monogr, 2009, 79, 523–555 [Google Scholar]
- 77.Williamson E, Overholt EP, Brentrup JA, Pilla RM, Leach TH, Schladow SG, Warren JD, Urmy SS, Sadro S, Chandra S. and Neale PJ, Sentinel responses to droughts, wildfires, and floods: effects of UV radiation on lakes and their ecosystem services, Front. Ecol. Environ, 2016, 14, 102–109 [Google Scholar]
- 78.Majidzadeh H, Uzun H, Ruecker A, Miller D, Vernon J, Zhang HY, Bao SW, Tsui MTK, Karanfil T. and Chow AT, Extreme flooding mobilized dissolved organic matter from coastal forested wetlands, Biogeochemistry, 2017, 136, 293–309 [Google Scholar]
- 79.Sulzberger B. Light-induced redox cycling of iron: roles for CO2 uptake and release by aquatic ecosystems, Aquat. Geochem, 2015, 21, 65–80 [Google Scholar]
- 80.Hutchins DA and Boyd PW, Marine phytoplankton and the changing ocean iron cycle, Nat. Clim. Change, 2016, 6, 1072–1079 [Google Scholar]
- 81.Shi DL, Kranz SA, Kim JM and Morel FMM, Ocean acidification slows nitrogen fixation and growth in the dominant diazotroph Trichodesmium under low-iron conditions, Proc. Natl. Acad. Sci. U. S. A, 2012, 109, E3094–E3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.N. Gruber Ocean biogeochemistry: Carbon at the coastal interface, Nature, 2015, 517, 148–149 [DOI] [PubMed] [Google Scholar]
- 83.Laruelle G, Lauerwald R, Pfeil B. and Regnier P, Regionalized global budget of the CO2 exchange at the air-water interface in continental shelf seas, Global Biogeochem. Cycles, 2014, 28, 1199–1214 [Google Scholar]
- 84.Cory RM, Crump BC, Dobkowski JA and Kling GW, Surface exposure to sunlight stimulates CO2 release from permafrost soil carbon in the Arctic, Proc. Natl. Acad. Sci. U. S. A, 2013, 110, 3429–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kieber RJ, Whitehead RF and Skrabal SA, Photochemical production of dissolved organic carbon from resuspended sediments, Limnol. Oceanogr, 2006, 51, 2187–2195 [Google Scholar]
- 86.Liu YN, Thornton DCO, Bianchi TS, Arnold WA, Shields MR, Chen J. and Yvon-Lewis SA, Dissolved organic matter composition drives the marine production of brominated very short-lived substances, Environ. Sci. Technol, 2015, 49, 3366–3374 [DOI] [PubMed] [Google Scholar]
- 87.Song GS, Richardson JD, Werner JP, Xie HX and Kieber DJ, Carbon monoxide photoproduction from particles and solutes in the Delaware estuary under contrasting hydrological conditions, Environ. Sci. Technol, 2015, 49, 14048–14056 [DOI] [PubMed] [Google Scholar]
- 88.Appiani E. and McNeill K, Photochemical production of singlet oxygen from particulate organic matter, Environ. Sci. Technol, 2015, 49, 3514–3522 [DOI] [PubMed] [Google Scholar]
- 89.Estapa ML, Mayer LM and Boss E, Rate and apparent quantum yield of photodissolution of sedimentary organic matter, Limnol. Oceanogr, 2012, 57, 1743–1756 [Google Scholar]
- 90.Helms R, Glinski DA, Mead RN, Southwell MW, Avery GB, Kieber RJ and Skrabal SA, Photochemical dissolution of organic matter from resuspended sediments: Impact of source and diagenetic state on photorelease, Org. Geochem, 2014, 73, 83–89 [Google Scholar]
- 91.Mayer M, Thornton KH and Schick LL, Bioavailability of organic matter photodissolved from coastal sediments, Aquat. Microb. Ecol, 2011, 64, 275–284 [Google Scholar]
- 92.Estapa L. and Mayer LM, Photooxidation of particulate organic matter, carbon/oxygen stoichiometry, and related photoreactions, Mar. Chem, 2010, 122, 138–147 [Google Scholar]
- 93.Mayer M, Schick LL, Hardy KR and Estapa ML, Photodissolution and other photochemical changes upon irradiation of algal detritus, Limnol. Oceanogr, 2009, 54, 1688–1698 [Google Scholar]
- 94.Pisani, Yamashita Y. and Jaffe R, Photo-dissolution of flocculent, detrital material in aquatic environments: Contributions to the dissolved organic matter pool, Water Res., 2011, 45, 3836–3844 [DOI] [PubMed] [Google Scholar]
- 95.Mayer LM, Schick LL, Bianchi TS and Wysocki LA, Photochemical changes in chemical markers of sedimentary organic matter source and age, Mar. Chem, 2009, 113, 123–128 [Google Scholar]
- 96.Dean JF, van der Velde Y, Garnett MH, Dinsmore KJ, Baxter R, Lessels JS, Smith P, Street LE, Subke JA, Tetzlaff D, Washbourne I, Wookey PA and Billett MF, Abundant pre-industrial carbon detected in Canadian Arctic headwaters: implications for the permafrost carbon feedback, Environ. Res. Lett, 2018, 13, 034024 [Google Scholar]
- 97.Toohey RC, Herman-Mercer NM, Schuster PF, Mutter EA and Koch JC, Multidecadal increases in the Yukon River Basin of chemical fluxes as indicators of changing flowpaths, groundwater, and permafrost, Geophys. Res. Lett, 2016, 43, 12120–12130 [Google Scholar]
- 98.Stubbins, Mann PJ, Powers L, Bittar TB, Dittmar T, McIntyre CP, Eglinton TI, Zimov N. and Spencer RGM, Low photolability of yedoma permafrost dissolved organic carbon, J. Geophys. Res.: Biogeosci., 2017, 122, 200–211. [Google Scholar]
- 99.Wang JJ, Lafrenière MJ, Lamoureux SF, Simpson AJ, Gélinas Y. and Simpson MJ, Differences in riverine and pond water dissolved organic matter composition and sources in Canadian high Arctic watersheds affected by active layer detachments, Environ. Sci. Technol, 2018, 52, 1062–1071 [DOI] [PubMed] [Google Scholar]
- 100.Cory RM, Harrold KH, Neilson BT and Kling GW, Controls of dissolved organic matter (DOM) degradation in a headwater stream: the influence of photochemical and hydrological conditions in determining light-limitation or substrate-limitation of photo-degradation, Biogeosci. Discuss, 2015, 12, 9793–9838 [Google Scholar]
- 101.Cory RM and Kling GW, Interactions between sunlight and microorganisms influence dissolved organic matter degradation along the aquatic continuum, Limnol. Oceanogr. Lett, 2018, 3, 102–116 [Google Scholar]
- 102.Hong J, Xie HX, Guo LD and Song GS, Carbon monoxide photoproduction: Implications for photoreactivity of Arctic permafrost-derived soil dissolved organic matter, Environ. Sci. Technol, 2014, 48, 9113–9121 [DOI] [PubMed] [Google Scholar]
- 103.Ward CP and Cory RM, Complete and partial photo-oxidation of dissolved organic matter draining permafrost soils, Environ. Sci. Technol, 2016, 50, 3545–3553 [DOI] [PubMed] [Google Scholar]
- 104.Fouché J, Lafreniere MJ, Rutherford K. and Lamoureux S, Seasonal hydrology and permafrost disturbance impacts on dissolved organic matter composition in High Arctic headwater catchments, Arctic. Sci, 2017, 3, 378–405 [Google Scholar]
- 105.Gareis JAL and Lesack LFW, Fluxes of particulates and nutrients during hydrologically defined seasonal periods in an ice-affected great Arctic river, the Mackenzie, Water Resour. Res, 2017, 53, 6109–6132 [Google Scholar]
- 106.Mann PJ, Spencer RGM, Hernes PJ, Six J, Aiken GR, Tank SE, McClelland JW, Butler KD, Dyda RY and Holmes RM, Pan-Arctic trends in terrestrial dissolved organic matter from optical measurements, Front. Earth Sci, 2016, 4, 1–18 [Google Scholar]
- 107.Gareis JAL and Lesack LFW, Photodegraded dissolved organic matter from peak freshet river discharge as a substrate for bacterial production in a lake-rich great Arctic delta, Arctic. Sci, 2018, 4, 557–583 [Google Scholar]
- 108.Cox CJ, Stone RS, Douglas DC, Stanitski DM, Divoky GJ, Dutton GS, Sweeney C, George JC and Longenecker DU, Drivers and environmental responses to the changing annual snow cycle of Northern Alaska, Bull. Am. Meteorol. Soc, 2017, 98, 2559–2577 [Google Scholar]
- 109.Smejkalova T, Edwards ME and Dash J, Arctic lakes show strong decadal trend in earlier spring ice-out, Sci. Rep, 2016, 6, 38449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bring A, Fedorova I, Dibike Y, Hinzman L, Mard J, Mernild SH, Prowse T, Semenova O, Stuefer SL and Woo MK, Arctic terrestrial hydrology: A synthesis of processes, regional effects, and research challenges, J. Geophys. Res.: Biogeosci, 2016, 121, 621–649 [Google Scholar]
- 111.Andresen G. and Lougheed VL, Disappearing Arctic tundra ponds: Fine-scale analysis of surface hydrology in drained thaw lake basins over a 65year period (1948–2013), J. Geophys. Res.: Biogeosci, 2015, 120, 466–479 [Google Scholar]
- 112.Carroll ML, Townshend JRG, DiMiceli CM, Loboda T. and Sohlberg RA, Shrinking lakes of the Arctic: Spatial relationships and trajectory of change, Geophys. Res. Lett, 2011, 38, 049427 [Google Scholar]
- 113.Nitze, Grosse G, Jones BM, Arp CD, Ulrich M, Fedorov A. and Veremeeva A, Landsat-based trend analysis of lake dynamics across northern permafrost regions, Remote Sens., 2017, 9, 640 [Google Scholar]
- 114.Osburn CL, Anderson NJ, Stedmon CA, Giles ME, Whiteford EJ, McGenity TJ, Dumbrell AJ and Underwood GJC, Shifts in the source and composition of dissolved organic matter in southwest Greenland lakes along a regional hydro-climatic gradient, J. Geophys. Res.: Biogeosci, 2017, 122, 3431–3445 [Google Scholar]
- 115.Jolivel M. and Allard M, Impact of permafrost thaw on the turbidity regime of a subarctic river: the Sheldrake River, Nunavik, Quebec, Arctic. Sci, 2017, 3, 451–474 [Google Scholar]
- 116.Berg, Findell K, Lintner B, Giannini A, Seneviratne SI, van den Hurk B, Lorenz R, Pitman A, Hagemann S, Meier A, Cheruy F, Ducharne A, Malyshev S. and Milly PCD, Land-atmosphere feedbacks amplify aridity increase over land under global warming, Nat. Clim. Change, 2016, 6, 869–874 [Google Scholar]
- 117.Heffernan The mystery of the expanding tropics, Nature, 2016, 530, 21–23 [DOI] [PubMed] [Google Scholar]
- 118.Jolly WM, Cochrane MA, Freeborn PH, Holden ZA, Brown TJ, Williamson GJ and Bowman DM, Climate-induced variations in global wildfire danger from 1979 to 2013, Nat. Commun, 2015, 6, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Santin C. and Doerr SH, Fire effects on soils: the human dimension, Philos. Trans. R. Soc. London, Ser. B, 2016, 371, 20150171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu FS, Higuera PE, Duffy P, Chipman ML, Rocha AV, Young AM, Kelly R. and Dietze MC, Arctic tundra fires: Natural variability and responses to climate change, Front. Ecol. Environ, 2015, 13, 369–377 [Google Scholar]
- 121.Veraverbeke S, Rogers BM, Goulden ML, Jandt RR, Miller CE, Wiggins EB and Randerson JT, Lightning as a major driver of recent large fire years in North American boreal forests, Nat. Clim. Change, 2017, 7, 529–534 [Google Scholar]
- 122.Kim B. and Sarkar S, Impact of wildfires on some greenhouse gases over continental USA: A study based on satellite data, Remote Sens. Environ, 2017, 188, 118–126 [Google Scholar]
- 123.Parker J, Boesch H, Wooster MJ, Moore DP, Webb AJ, Gaveau D. and Murdiyarso D, Atmospheric CH4 and CO2 enhancements and biomass burning emission ratios derived from satellite observations of the 2015 Indonesian fire plumes, Atmos. Chem. Phys, 2016, 16, 10111–10131 [Google Scholar]
- 124.Ward CP, Sleighter RL, Hatcher PG and Cory RM, Insights into the complete and partial photooxidation of black carbon in surface waters, Environ. Sci.: Processess Impacts, 2014, 16, 721–731 [DOI] [PubMed] [Google Scholar]
- 125.Santin C, Doerr SH, Merino A, Bryant R. and Loader NJ, Forest floor chemical transformations in a boreal forest fire and their correlations with temperature and heating duration, Geoderma, 2016, 264, 71–80 [Google Scholar]
- 126.Wagner and Jaffé R, Effect of photodegradation on molecular size distribution and quality of dissolved black carbon, Org. Geochem, 2015, 86, 1–4 [Google Scholar]
- 127.Brown DRN, Jorgenson MT, Douglas TA, Romanovsky VE, Kielland K, Hiemstra C, Euskirchen ES and Ruess RW, Interactive effects of wildfire and climate on permafrost degradation in Alaskan lowland forests, J. Geophys. Res.: Biogeosci, 2015, 120, 1619–1637 [Google Scholar]
- 128.Colombo N, Salerno F, Gruber S, Freppaz M, Williams M, Fratianni S. and Giardino M, Review: Impacts of permafrost degradation on inorganic chemistry of surface fresh water, Glob. Planet. Change, 2018, 162, 69–83 [Google Scholar]
- 129.Bjorneras C, Weyhenmeyer GA, Evans CD, Gessner MO, Grossart HP, Kangur K, Kokorite I, Kortelainen P, Laudon H, Lehtoranta J, Lottig N, Monteith DT, Noges P, Noges T, Oulehle F, Riise G, Rusak JA, Raike A, Sire J, Sterling S. and Kritzberg ES, Widespread increases in iron concentration in european and North American freshwaters, Global Biogeochem. Cycles, 2017, 31, 1488–1500 [Google Scholar]
- 130.Xiao YH, Sara-Aho T, Hartikainen H. and Vähätalo AV, Contribution of ferric iron to light absorption by chromophoric dissolved organic matter, Limnol. Oceanogr, 2013, 58, 653–662 [Google Scholar]
- 131.Gu YF, Lensu A, Peramaki S, Ojala A. and Vähätalo AV, Iron and pH regulating the photochemical mineralization of dissolved organic carbon, ACS Omega, 2017, 2, 1905–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Strode A, Duncan BN, Yegorova EA, Kouatchou J, Ziemke JR and Douglass AR, Implications of carbon monoxide bias for methane lifetime and atmospheric composition in chemistry climate models, Atmos. Chem. Phys, 2015, 15, 11789–11805 [Google Scholar]
- 133.Myhre G, Shindell D, Bréon FM, Collins W, Fuglestvedt J, Huang J, Koch D, Lamarque JF, Lee D, Mendoza B, Nakajima T, Robock A, Stephens G, Takemura T. and Zhang H, Anthropogenic and Natural Radiative Forcing, Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Stocker TF, Qin D., Plattner G-K, Tignor M., Allen SK, Boschung J., Nauels A., Xia Y., Bex V. and Midgley PM, Cambridge Univeristy Press, Cambridge, United Kingdom and New York, USA, 2013, 659–740 [Google Scholar]
- 134.Yin Y, Chevallier F, Ciais P, Broquet G, Fortems-Cheiney A, Pison I. and Saunois M, Decadal trends in global CO emissions as seen by MOPITT, Atmos. Chem. Phys, 2015, 15, 13433–13451 [Google Scholar]
- 135.Pihlatie M, Rannik Ü, Sami Haapanala S, Peltola O, Shurpali N, Martikainen P, Lind S, Hyvönenn N, Virkajärvi P, Zahniser M. and Mammarella I, Seasonal and diurnal variation in CO fluxes from an agrcultural bioenergy crop, Biogeosciences, 2016, 13, 5471–5485 [Google Scholar]
- 136.Song GS and Xie HX, Spectral efficiencies of carbon monoxide photoproduction from particulate and dissolved organic matter in laboratory cultures of Arctic sea ice algae, Mar. Chem, 2017, 190, 51–65 [Google Scholar]
- 137.Carvalho J Jr., Amaral S, Costa M, Neto TS, Veras C, Costa F, Van Leeuwen T, Krieger Filho G, Tourigny E. and Forti M, CO2 and CO emission rates from three forest fire controlled experiments in Western Amazonia, Atmos. Environ, 2016, 135, 73–83 [Google Scholar]
- 138.Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Piao S. and Thomton P, Carbon and Other Biogeochemical Cycles, Climate Chang 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Stocker TF, Qin D., Plattner G-K, Tignor M., Allen SK, Boschung J., Nauels A., Xia Y., Bex V. and Midgley PM, Cambridge University Press, Cambridge, UK and New York, NY, USA, 2013 [Google Scholar]
- 139.Brownlow R, Lowry D, Fisher RE, France JL, Lanoiselle M, White B, Wooster MJ, Zhang T. and Nisbet EG, Isotopic ratios of tropical methane emissions by atmospheric measurement, Global Biogeochem. Cycles, 2017, 31, 1408–1419 [Google Scholar]
- 140.Prather MJ and Holmes CD, Overexplaining or underexplaining methane’s role in climate change, Proc. Natl. Acad. Sci. U. S. A, 2017, 114, 5324–5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thonat, Saunois M, Bousquet P, Pison I, Tan ZL, Zhuang QL, Crill PM, Thornton BF, Bastviken D, Dlugokencky EJ, Zimov N, Laurila T, Hatakka J, Hermansen O. and Worthy DEJ, Detectability of Arctic methane sources at six sites performing continuous atmospheric measurements, Atmos. Chem. Phys, 2017, 17, 8371–8394 [Google Scholar]
- 142.Miller SM, Worthy DEJ, Michalak AM, Wofsy SC, Kort EA, Havice TC, Andrews AE, Dlugokencky EJ, Kaplan JO, Levi PJ, Tian HQ and Zhang BW, Observational constraints on the distribution, seasonality, and environmental predictors of North American boreal methane emissions, Global Biogeochem. Cycles, 2014, 28, 146–160 [Google Scholar]
- 143.Pandey S, Houweling S, Krol M, Aben I, Monteil G, Nechita-Banda N, Dlugokencky EJ, Detmers R, Hasekamp O, Xu XY, Riley WJ, Poulter B, Zhang Z, McDonald KC, White JWC, Bousquet P. and Rockmann T, Enhanced methane emissions from tropical wetlands during the 2011 La Niña, Sci. Rep, 2017, 7, 45759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Z, Zimmermann NE, Stenke A, Li X, Hodson EL, Zhu GF, Huang CL and Poulter B, Emerging role of wetland methane emissions in driving 21st century climate change, Proc. Natl. Acad. Sci. U. S. A, 2017, 114, 9647–9652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu Q, Peng CH, Ciais P, Jiang H, Liu JX, Bousquet P, Li SQ, Chang J, Fang XQ, Zhou XL, Chen H, Liu SR, Lin GH, Gong P, Wang M, Wang H, Xiang WH and Chen J, Interannual variation in methane emissions from tropical wetlands triggered by repeated El Nino Southern Oscillation, Glob. Change Biol, 2017, 23, 4706–4716 [DOI] [PubMed] [Google Scholar]
- 146.Pugh CA, Reed DE, Desai AR and Sulman BN, Wetland flux controls: how does interacting water table levels and temperature influence carbon dioxide and methane fluxes in northern Wisconsin?, Biogeochemistry, 2018, 137, 15–25 [Google Scholar]
- 147.Sihi D, Inglett PW, Gerber S. and Inglett KS, Rate of warming affects temperature sensitivity of anaerobic peat decomposition and greenhouse gas production, Glob. Change Biol, 2018, 24, E259–E274 [DOI] [PubMed] [Google Scholar]
- 148.Anthony W, Daanen R, Anthony P, von Deimling TS, Ping CL, Chanton JP and Grosse G, Methane emissions proportional to permafrost carbon thawed in Arctic lakes since the 1950s, Nat. Geosci, 2016, 9, 679–682 [Google Scholar]
- 149.Chronopoulou PM, Shelley F, Pritchard WJ, Maanoja ST and Trimmer M, Origin and fate of methane in the Eastern Tropical North Pacific oxygen minimum zone, ISME J., 2017, 11, 1386–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singleton CM, McCalley CK, Woodcroft BJ, Boyd JA, Evans PN, Hodgkins SB, Chanton JP, Frolking S, Crill PM, Saleska SR, Rich VI and Tyson GW, Methanotrophy across a natural permafrost thaw environment, ISME J., 2018, 12, 2544–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shakhova N, Semiletov I. and Sergienko V, et al. , The East Siberian Arctic Shelf: towards further assessment of permafrost-related methane fluxes and role of sea-ice, Philos. Trans. R. Soc., A, 2015, 373, 214051 —214064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Revell E, Stenke A, Rozanov E, Ball W, Lossow S. and Peter T, The role of methane in projections of 21st century stratospheric water vapour, Atmos. Chem. Phys, 2016, 16, 13067–13080 [Google Scholar]
- 153.Chen, Parton WJ, Adair EC, Asao S, Hartman MD and Gao W, Simulation of the effects of photodecay on long-term litter decay using DayCent, Ecosphere, 2016, 7, 22 [Google Scholar]
- 154.Wang X, Throop HL and Gill T, A novel method to continuously monitor litter moisture - A microcosm-based experiment, J. Arid Environ, 2015, 115, 10–13 [Google Scholar]
- 155.Smith KA, Ball T, Conen F, Dobbie KE, Massheder J. and Rey A, Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes, Eur. J. Soil Sci, 2018, 69, 10–20 [Google Scholar]
- 156.Voigt C, Lamprecht RE, Marushchak ME, Lind SE, Novakovskiy A, Aurela M, Martikainen PJ and Biasi C, Warming of subarctic tundra increases emissions of all three important greenhouse gases - carbon dioxide, methane, and nitrous oxide, Glob. Change Biol, 2017, 23, 3121–3138 [DOI] [PubMed] [Google Scholar]
- 157.Voigt C, Marushchak ME, Lamprecht RE, Jackowicz-Korczynski M, Lindgren A, Mastepanov M, Granlund L, Christensen TR, Tahvanainen T, Martikainen PJ and Biasi C, Increased nitrous oxide emissions from Arctic peatlands after permafrost thaw, Proc. Natl. Acad. Sci. U. S. A, 2017, 114, 6238–6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang G, Peng Y, Marushchak M, Chen Y, Wang G, Li F, Zhang D, Wang J, Yu J, Liu L, Qin S, Kou D. and Yang Y, Magnitude and pathways of increased nitrous oxide emissions from uplands following permafrost thaw, Environ. Sci. Technol, 2018, 52, 9162–9169 [DOI] [PubMed] [Google Scholar]
- 159.Battaglia G. and Joos F, Marine N2O emissions from nitrification and denitrification constrained by modern observations and projected in multimillennial global warming simulations, Global Biogeochem. Cycles, 2018, 32, 92–121 [Google Scholar]
- 160.Martinez-Rey J, Bopp L, Gehlen M, Tagliabue A. and Gruber N, Projections of oceanic N2O emissions in the 21st century using the IPSL Earth system model, Biogeosciences, 2015, 12, 4133–4148 [Google Scholar]
- 161.Monteiro, Séneca J, Torgo L, Cleary DFR, Gomes NCM, Santoro AE and Magalhäes C, Environmental controls on esturarine nitrifying communities along a salinity gradient, Aquat. Microb. Ecol, 2017, 80, 167–180 [Google Scholar]
- 162.Paulot F, Jacob DJ, Johnson MT, Bell TG, Baker AR, Keene WC, Lima ID, Doney SC and Stock CA, Global oceanic emission of ammonia: Constraints from seawater and atmospheric observations, Global Biogeochem. Cycles, 2015, 29, 1165–1178 [Google Scholar]
- 163.Trimmer M, Chronopoulou PM, Maanoja ST, Upstill-Goddard RC, Kitidis V. and Purdy KJ, Nitrous oxide as a function of oxygen and archaeal gene abundance in the North Pacific, Nat. Commun, 2016, 7, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Benedict KB and Anastasio C, Quantum yields of nitrite (NO2−) from the potolysis of nitrate (NO3−) in ice at 313 nm, J. Phys. Chem. A, 2017, 121, 8474–8483 [DOI] [PubMed] [Google Scholar]
- 165.Berhanu A, Savarino J, Erbland J, Vicars WC, Preunkert S, Martins JF and Johnson MS, Isotopic effects of nitrate photochemistry in snow: a field study at Dome C, Antarctica, Atmos. Chem. Phys, 2015, 15, 11243–11256 [Google Scholar]
- 166.Morenz KJ, Shi QW, Murphy JG and Donaldson DJ, Nitrate photolysis in salty snow, J. Phys. Chem. A, 2016, 120, 7902–7908 [DOI] [PubMed] [Google Scholar]
- 167.Pilegard K. Processes regulating nitric oxide emissions from soils, Philos. Trans. R. Soc. London, Ser. B, 2014, 368, 20130126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Newland MJ, Martinerie P, Witrant E, Helmig D, Worton DR, Hogan C, Sturges WT and Reeves CE, Changes to the chemical state of the Northern Hemisphere atmosphere during the second half of the twentieth century, Atmos. Chem. Phys, 2017, 17, 8269–8283 [Google Scholar]
- 169.Hossaini R, Chipperfield MP, Montzka SA, Rap A, Dhomse S. and Feng W, Efficiency of short-lived halogens at influencing climate through depletion of stratospheric ozone, Nat. Geosci, 2015, 8, 186–190 [Google Scholar]
- 170.Sinnhuber BM and Meul S, Simulating the impact of emissions of brominated very short lived substances on past stratospheric ozone trends, Geophys. Res. Lett, 2015, 42, 2449–2456 [Google Scholar]
- 171.Stemmler, Hense I. and Quack B, Marine sources of bromoform in the global open ocean - global patterns and emissions, Biogeosciences, 2015, 12, 1967–1981 [Google Scholar]
- 172.Tegtmeier S, Ziska F, Pisso I, Quack B, Velders GJM, Yang X. and Kruger K, Oceanic bromoform emissions weighted by their ozone depletion potential, Atmos. Chem. Phys, 2015, 15, 13647–13663 [Google Scholar]
- 173.Carpenter LJ and Nightingale PD, Chemistry and release of gases from the surface ocean, Chem. Rev, 2015, 115, 4015–4034 [DOI] [PubMed] [Google Scholar]
- 174.Parker M. and Mitch WA, Halogen radicals contribute to photooxidation in coastal and estuarine waters, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 5868–5873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ball T, Alsing J, Mortlock DJ, Staehelin J, Haigh JD, Peter T, Tummon F, Stubi R, Stenke A, Anderson J, Bourassa A, Davis SM, Degenstein D, Frith S, Froidevaux L, Roth C, Sofieva V, Wang R, Wild J, Yu PF, Ziemke JR and Rozanov EV, Evidence for a continuous decline in lower stratospheric ozone offsetting ozone layer recovery, Atmos. Chem. Phys, 2018, 18, 1379–1394 [Google Scholar]
- 176.Rex M, Wohltmann I, Ridder T, Lehmann R, Rosenlof K, Wennberg P, Weisenstein D, Notholt J, Kruger K, Mohr V. and Tegtmeier S, A tropical West Pacific OH minimum and implications for stratospheric composition, Atmos. Chem. Phys, 2014, 14, 4827–4841 [Google Scholar]
- 177.Custard D, Pratt KA, Wang SY and Shepson PB, Constraints on Arctic atmospheric chlorine production through measurements and simulations of Cl2 and ClO, Environ. Sci. Technol, 2016, 50, 12394–12400 [DOI] [PubMed] [Google Scholar]
- 178.Custard D, Raso ARW, Shepson PB, Staebler RM and Pratt KA, Production and release of molecular bromine and chlorine from the Arctic coastal snowpack, ACS Earth Space Chem., 2017, 1, 142–151 [Google Scholar]
- 179.Coburn S, Dix B, Edgerton E, Holmes CD, Kinnison D, Liang Q, ter Schure A, Wang SY and Volkamer R, Mercury oxidation from bromine chemistry in the free troposphere over the southeastern US, Atmos. Chem. Phys, 2016, 16, 3743–3760 [Google Scholar]
- 180.Kaulfus S, Nair U, Holmes CD and Landing WM, Mercury wet scavenging and deposition differences by precipitation type, Environ. Sci. Technol, 2017, 51, 2628–2634 [DOI] [PubMed] [Google Scholar]
- 181.Pratt KA, Custard KD, Shepson PB, Douglas TA, Pohler D, General S, Zielcke J, Simpson WR, Platt U, Tanner DJ, Huey LG, Carlsen M. and Stirm BH, Photochemical production of molecular bromine in Arctic surface snowpacks, Nat. Geosci, 2013, 6, 351–356 [Google Scholar]
- 182.Wang JC, Xie ZQ, Wang FY and Kang H, Gaseous elemental mercury in the marine boundary layer and air-sea flux in the Southern Ocean in austral summer, Sci. Total Environ, 2017, 603, 510–518 [DOI] [PubMed] [Google Scholar]
- 183.Nelson KL, Boehm AB, Davies-Colley RJ, Dodd MC, Kohn T, Linden KG, Liu Y, Maraccini PA, McNeill K, Mitch WA, Nguyen TH, Parker KM, Rodriguez RA, Sassoubre LM, Silverman AI, Wigginton KR and Zepp RG, Sunlight-mediated inactivation of microorganisms in water: A review of mechanisms and modeling approaches, Environ. Sci. Processes Impacts, 2018, 20, 1089–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Eyheraguibel B, ter Halle A. and Richard C, Photodegradation of bentazon, clopyralid, and triclopyr on model leaves: importance of a systematic evaluation of pesticide photostability on crops, J. Agric. Food Chem, 2009, 57, 1960–1966 [DOI] [PubMed] [Google Scholar]
- 185.Marques, Mari M, Audi-Miro C, Sierra J, Soler A, Nadal M. and Domingo JL, Climate change impact on the PAH photodegradation in soils: Characterization and metabolites identification, Environ. Int, 2016, 89–90, 155–165 [DOI] [PubMed] [Google Scholar]
- 186.McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M. and Madronich S, Ozone depletion and climate change: Impacts on UV radiation, Photochem. Photobiol. Sci, 2011, 10, 182–198 [DOI] [PubMed] [Google Scholar]
- 187.Zepp RG, Cyterski M, Wong K, Molina M, Georgacopoulos O, Acrey B, Whelan G. and Parmar R, Biological weighting functions for evaluating the role of sunlight-induced inactivation of coliphages at selected beaches and nearby tributaries, Environ. Sci. Technol, 2018, 52, 13068–13076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McConville B, Hubert TD and Remucal CK, Direct photolysis rates and transformation pathways of the lampricides TFM and niclosamide in simulated sunlight, Environ. Sci. Technol, 2016, 50, 9998–10006 [DOI] [PubMed] [Google Scholar]
- 189.McConville MB, Mezyk SP and Remucal CK, Indirect photodegradation of the lampricides TFM and niclosamide, Environ. Sci.: Processes Impacts, 2017, 19, 1028–1039 [DOI] [PubMed] [Google Scholar]
- 190.McNeill K. and Canonica S, Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties, Environ. Sci.: Processes Impacts, 2016, 18, 1381–1399 [DOI] [PubMed] [Google Scholar]
- 191.Grannas M. Photochemistry of Organic Pollutants in/on Snow and Ice, Implications and Consequences of Anthropogenic Pollution in Polar Environments, R. KallenbornSpringer, Berlin, Heidelberg, 2016, 41–58 [Google Scholar]
- 192.Rosario-Ortiz FL and Canonica S, Probe compounds to assess the photochemical activity of dissolved organic matter, Environ. Sci. Technol, 2016, 50, 12532–12547 [DOI] [PubMed] [Google Scholar]
- 193.Ukpebor JE and Halsall CJ, Effects of dissolved water constituents on the photodegradation of fenitrothion and diazinon, Water, Air, Soil Pollut., 2012, 223, 655–666 [Google Scholar]
- 194.Ge LK, Halsall C, Chen CE, Zhang P, Dong QQ and Yao ZW, Exploring the aquatic photodegradation of two ionisable fluoroquinolone antibiotics - Gatifloxacin and balofloxacin: Degradation kinetics, photobyproducts and risk to the aquatic environment, Sci. Total Environ, 2018, 633, 1192–1197 [DOI] [PubMed] [Google Scholar]
- 195.Hou W-C, Henderson WM, Chowdhury I, Goodwin DG, Chang XJ, Martin S, Fairbrother DH, Bouchard D. and Zepp RG, The contribution of indirect photolysis to the degradation of graphene oxide in sunlight, Carbon, 2016, 110, 426–437 [Google Scholar]
- 196.Wohlleben, Kingston C, Carter J, Sahle-Demessie E, Vázquez-Campos S, Acrey B, Chen C-Y, Walton E, Egenolf H, Müller P. and Zepp R, NanoRelease: Pilot interlaboratory comparison of a weathering protocol applied to resilient and labile polymers with and without embedded carbon nanotubes, Carbon, 2017, 113, 346–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wohlleben and N. Neubauer, Quantitative rates of release from weathered nanocomposites are determined across 5 orders of magnitude by the matrix, modulated by the embedded nanomaterial, NanoImpact, 2016, 1, 39–45 [Google Scholar]
- 198.Andrady L, Torikai A, Redhwi HH, Pandey KK and Gies P, Consequences of stratospheric ozone depletion and climate change on the use of materials, Photochem. Photobiol. Sci, 2015, 14, 170–184 [DOI] [PubMed] [Google Scholar]
- 199.Hahladakis JN, Velis CA, Weber R, Iacovidou E. and Purnell P, An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling, J. Hazard. Matter, 2018, 344, 179–199 [DOI] [PubMed] [Google Scholar]
- 200.Hermabessiere L, Dehaut A, Paul-Pont I, Lacroix C, Jezequel R, Soudant P. and Duflos G, Occurrence and effects of plastic additives on marine environments and organisms: A review, Chemosphere, 2017, 182, 781–793 [DOI] [PubMed] [Google Scholar]
- 201.Ward CP, Sharpless CM, Valentine DL, French-McCay DP, Aeppli C, White HK, Rodgers RP, Gosselin KM, Nelson RK and Reddy CM, Partial photochemical oxidation was a dominant fate of Deepwater Horizon surface oil, Environ. Sci. Technol, 2018, 52, 1797–1805 [DOI] [PubMed] [Google Scholar]
- 202.Harriman BH, Zito P, Podgorsld DC, Tarr MA and Suflita JM, Impact of photooxidation and biodegradation on the fate of oil spilled during the Deepwater Horizon Incident: Advanced stages of weathering, Environ. Sci. Technol, 2017, 51, 7412–7421 [DOI] [PubMed] [Google Scholar]
- 203.Michalak M, Anderson EJ, Beletsky D, Boland S, Bosch NS, Bridgeman TB, Chaffin JD, Cho K, Confesor R. and Daloğlu I, Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions, Proc. Natl. Acad. Sci. U. S. A, 2013, 110, 6448–6452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Steffen MM, Belisle BS, Watson SB, Boyer GL and Wilhelm SW, Status, causes and controls of cyanobacterial blooms in Lake Erie, J. Great Lakes Res, 2014, 40, 215–225 [Google Scholar]
- 205.Paerl HW and Otten TG, Blooms bite the hand that feeds them, Science, 2013, 342, 433–434 [DOI] [PubMed] [Google Scholar]
- 206.Dziallas C. and Grossart H-P, Increasing oxygen radicals and water temperature select for toxic Microcystis sp., PLoS One, 2011, 6, e25569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zilliges, Kehr J-C, Meissner S, Ishida K, Mikkat S, Hagemann M, Kaplan A, Börner T. and Dittmann E, The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions, PLoS One, 2011, 6, e17615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Berry MA, Davis TW, Cory RM, Duhaime MB, Johengen TH, Kling GW, Marino JA, Den Uyl PA, Gossiaux D. and Dick GJ, Cyanobacterial harmful algal blooms are a biological disturbance to western Lake Erie bacterial communities, Environ. Microbiol, 2017, 19, 1149–1162 [DOI] [PubMed] [Google Scholar]
- 209.Cory R, Davis T, Dick G, Johengen T, Denef V, Berry M, Page S, Watson S, Yuhas K. and Kling G, Seasonal dynamics in dissolved organic matter, hydrogen peroxide, and cyanobacterial blooms in Lake Erie, Front. Mar. Sci, 2016, 3, 54 [Google Scholar]
- 210.Bittar TB, Vieira AAH, Stubbins A. and Mopper K, Competition between photochemical and biological degradation of dissolved organic matter from the cyanobacteria Microcystis aeruginosa, Limnol. Oceanogr, 2015, 60, 1172–1194 [Google Scholar]
- 211.Lucas RM, Yazar S, Young AR, Norval M, de Gruijl FR, Takizawa Y, Rhodes LE, Sinclair CA and Neale RE, Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate, Photochem. Photobiol. Sci, 2019, 10.1039/C8PP90060D [DOI] [PubMed] [Google Scholar]
- 212.Bodrato M. and Vione D, APEX (Aqueous Photochemistry of Environmentally occurring Xenobiotics): a free software tool to predict the kinetics of photochemical processes in surface waters, Environ. Sci.: Processes Impacts, 2014, 16, 732–740 [DOI] [PubMed] [Google Scholar]
- 213.Kohn T, Mattle MJ, Minella M. and Vione D, A modeling approach to estimate the solar disinfection of viral indicator organisms in waste stabilization ponds and surface waters, Water Res., 2016, 88, 912–922 [DOI] [PubMed] [Google Scholar]
- 214.Nguyen MT, Silverman AI and Nelson KL, Sunlight inactivation of MS2 coliphage in the absence of photosensitizers: Modeling the endogenous inactivation rate using a photoaction spectrum, Environ. Sci. Technol, 2014, 48, 3891–3898 [DOI] [PubMed] [Google Scholar]
- 215.Minella M, Maurino V, Minero C. and Vione D, A model assessment of the ability of lake water in Terra Nova Bay, Antarctica, to induce the photochemical degradation of emerging contaminants, Chemosphere, 2016, 162, 91–98 [DOI] [PubMed] [Google Scholar]
- 216.Silverman I, Nguyen MT, Schilling IE, Wenk J. and Nelson KL, Sunlight inactivation of viruses in open-water unit process treatment wetlands: modeling endogenous and exogenous inactivation rates, Environ. Sci. Technol, 2015, 49, 2757–2766 [DOI] [PubMed] [Google Scholar]
- 217.Silverman I. and Nelson KL, Modeling the endogenous sunlight inactivation rates of laboratory strain and wastewater E. coli and Enterococci using biological weighting functions, Environ. Sci. Technol, 2016, 50, 12292–12301 [DOI] [PubMed] [Google Scholar]
- 218.Avetta, Fabbri D, Minella M, Brigante M, Maurino V, Minero C., Pazzi M.and Vione D., Assessing the phototransformation of diclofenac, clofibric acid and naproxen in surface waters: model predictions and comparison with field data, Water Res., 2016, 105, 383–394 [DOI] [PubMed] [Google Scholar]
- 219.Koehler, Barsotti F, Minella M, Landelius T, Minero C, Tranvik LJ and Vione D, Simulation of photoreactive transients and of photochemical transformation of organic pollutants in sunlit boreal lakes across 14 degrees of latitude: A photochemical mapping of Sweden, Water Res., 2018, 129, 94–104 [DOI] [PubMed] [Google Scholar]
- 220.Mattle MJ, Vione D. and Kohn T, Conceptual model and experimental framework to determine the contributions of direct and indirect photoreactions to the solar disinfection of MS2, phiX174, and adenovirus, Environ. Sci. Technol, 2015, 49, 334–342 [DOI] [PubMed] [Google Scholar]
- 221.Silverman I, Peterson BM, Boehm AB, McNeill K. and Nelson KL, Sunlight inactivation of human viruses and bacteriophages in coastal waters containing natural photosensitizers, Environ. Sci. Technol, 2013, 47, 1870–1878 [DOI] [PubMed] [Google Scholar]
- 222.Mangalgiri KP and Blaney L, Elucidating the stimulatory and inhibitory effects of dissolved organic matter from poultry litter on photodegradation of antibiotics, Environ. Sci. Technol, 2017, 51, 12310–12320 [DOI] [PubMed] [Google Scholar]
- 223.Minella M, Leoni B, Salmaso N, Savoye L, Sommaruga R. and Vione D, Long-term trends of chemical and modelled photochemical parameters in four Alpine lakes, Sci. Total Environ, 2016, 541, 247–256 [DOI] [PubMed] [Google Scholar]