INTRODUCTION
Nutrition assessment and support of critically ill patients is a challenge. The current severe acute respiratory syndrome coronavirus 2 pandemic results in unprecedented rates of acute lung injury (ALI) and acute respiratory distress syndrome. This article reports on our collaborative effort to create and implement a pragmatic nutrition support protocol for critically ill patients.
KNOWLEDGE SCRAMBLE IN CORONAVIRUS DISEASE 2019
Coronavirus disease 2019 (COVID-19) is a rapidly spreading viral infection resulting in prolonged critical illness or death in a subset of patients. With growing case numbers and deaths reported daily, COVID-19 has become one of the most significant public health crises in modern history.
A paucity of literature addresses nutrition support in the setting of COVID-19. In response to this deficiency, the Society of Critical Care Medicine and the American Society for Parenteral and Enteral Nutrition released a joint statement on nutrition therapy in patients requiring intensive care unit (ICU) admission (1) and the European Society for Clinical Nutrition and Metabolism released an expert consensus statement for nutritional support in individuals with COVID-19 (2). We synthesized the existing literature to provide gastroenterologists and nutrition support providers a rational protocol for use in the ICU in the COVID-19 pandemic (Figures 1 and 2).
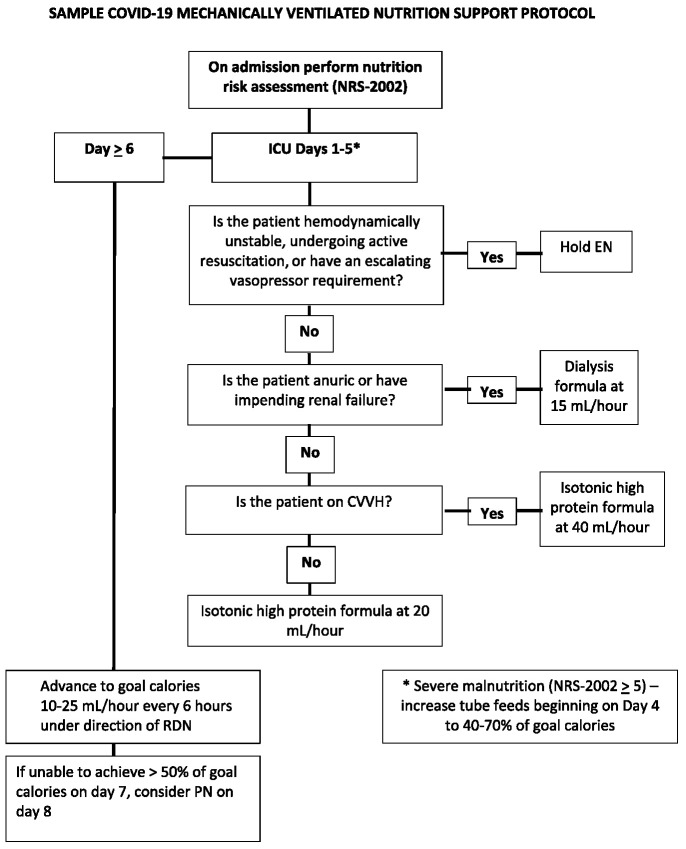
Figure 1.
Sample flowchart for the delivery of nutrition support in a patient with COVID-19 requiring ICU admission. COVID-19, coronavirus disease 2019; CVVH, continuous veno-veno hemofiltration; EN, enteral nutrition; ICU, intensive care unit; PN, parenteral nutrition; RDN, registered dietitian nutritionist.
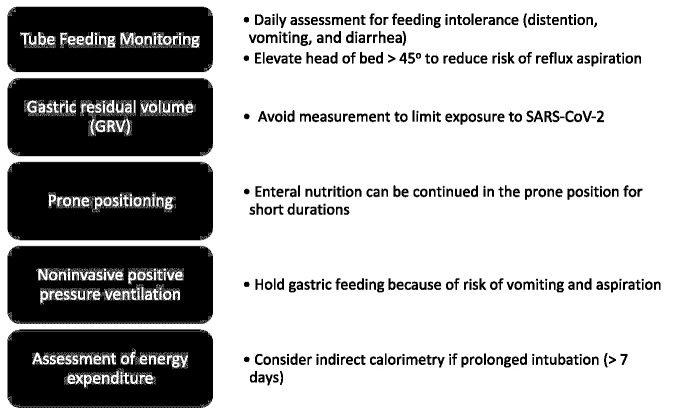
Figure 2.
Intensive care unit nutrition supports topics unique to coronavirus disease 2019. SARS-COV-2, severe acute respiratory syndrome coronavirus 2.
NUTRITION SCREENING IN COVID-19
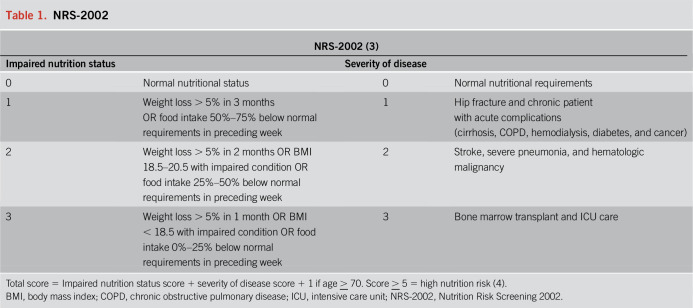
Malnutrition in the ICU is associated with poor outcomes to include prolonged ventilator dependence, increased hospital length of stay, and mortality when compared with well-nourished counterparts. Because gastrointestinal symptoms can be present for a week or longer before hospital admission, patients with COVID-19 are at risk for both malnutrition and refeeding on hospital presentation. Therefore, an ideal screening tool is short, easy to calculate, and reproducible by any healthcare team member that allows for limited patient and provider exposures. The Nutrition Risk Screening 2002 satisfies these criteria (Table 1) (3). This screening measure incorporates age, food intake, weight loss, body mass index (BMI), and illness severity; a score > 5 on admission is associated with ICU mortality (4), highlighting a cohort that may benefit from more aggressive nutritional support.
Table 1.
NRS-2002
ENTERAL NUTRITION SUPPORT
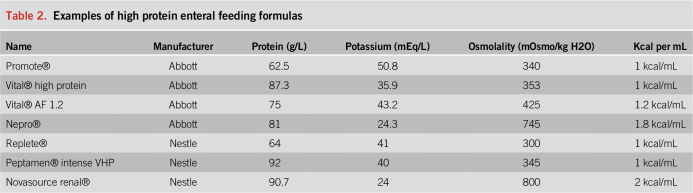
Initiation of early enteral nutrition (EN) within 24–48 hours of admission to the ICU is recommended in ICU-specific nutrition guidelines (5). At the time of intubation, placement of a 10–16 Fr nasogastric or orogastric tube allows for care to be clustered and allows for rapid initiation of EN after patient stabilization. Larger bore tube patency can be maintained with less frequent water flushes, reducing contact time with the patient and minimizing total volume input crucial for the management of patients with acute respiratory distress syndrome. Early initiation of isotonic, low-fiber and high-protein content formulas (Table 2) allows for adequate calorie and protein support while minimizing the risk of clogged feeding tubes and hyperglycemia. As a subset of patients with COVID-19 have acute kidney injury or underlying chronic kidney disease, a dialysis specialty formula is needed in cases of anuria or hyperkalemia. There is insufficient evidence, and the potential to cause harm, in the routine use of immune-modulating enteral formulas or antioxidant additives (6).
Table 2.
Examples of high protein enteral feeding formulas
Provision of calories
Although energy expenditure is best measured by indirect calorimetry in critically ill patients, the prolonged time needed for these measures increases clinician risk for viral exposure and is contrary to the principle of “clustering care,” in which patient care is bundled to limit provider exposures (1). Multivariable equations validated for use in critical care often lack precision in obesity, a problem as early reports in the United States indicate that most critically ill patients with COVID-19 are obese. On validation studies, the Harris-Benedict equation with 50% weight adjustment and the weight-based equations (Figure 3) stratified for degree of obesity are the most reliable for estimation of goal energy needs (7). In individuals receiving propofol for sedation, the additional calories from the lipid emulsion should be accounted for (10% lipid = 1.1 kcal/mL).
Figure 3.
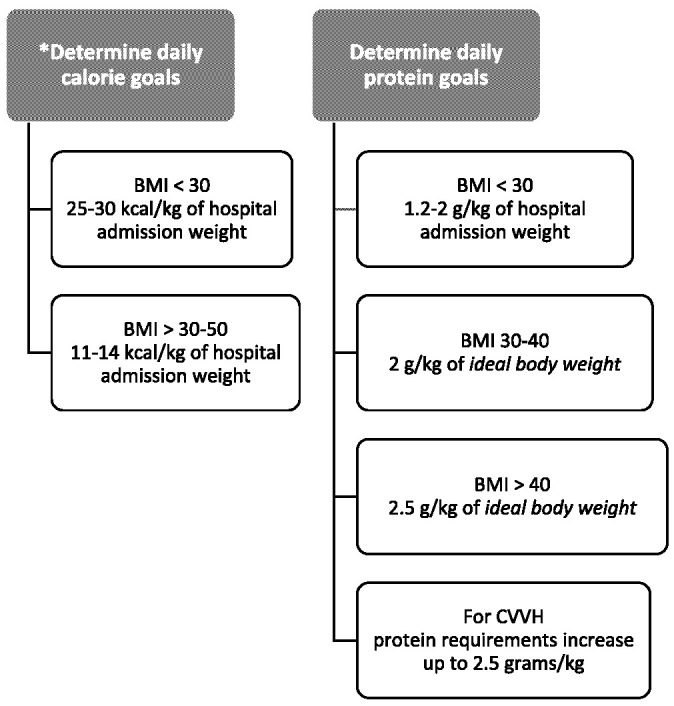
Outline for the calculation of goal calorie and protein needs (5). BMI, body mass index; CVVH, continuous veno-veno hemofiltration.
A critical balance between the timing and amount of calorie provision is required based on the patient's underlying disease state, comorbid conditions, expected prognosis, and the harms of both prolonged underfeeding and early overfeeding. In the Early vs Delayed Enteral Nutrition in the ALI trial (8), 1,000 mechanically ventilated patients were randomized to early trophic feeding (providing 400 kcal/d) or full feeding (providing 1,300 kcal/d) within 48 hours of ALI onset and up to day 6 of the study. No differences in ventilator-free days, 60-day mortality, organ failure-free days, ICU-free days, or infection were found between the 2 study groups.
Although the early vs delayed enteral nutrition trial has been criticized for the degree of underfeeding, a smaller study, INTACT (9), reported that a daily mean intake > 18 kcal/kg over the first 7 days was associated with increased mortality. Based on these studies and the need to limit patient contact in COVID-19, we propose a low calorie, moderate protein feeding protocol over the first 5 ICU days, increasing to goal calories in those surviving to ICU day 6 (Figure 1).
Provision of protein
Central to the issue of hypocaloric feedings is provision of sufficient protein to support immune function and limit lean body mass loss related to the catabolic process of illness (Table 2). Similar to calorie exposures, early excessive protein intake has also been associated with increased mortality in the ICU population (10). A lack of strong data to support optimal protein requirements in critical illness led to the American Society for Parenteral and Enteral Nutrition/Society of Critical Care Medicine expert consensus to use weight-based equations, stratified by BMI, to calculate protein needs (5).
Parenteral nutrition support
Enteral nutrition may prove difficult in patients with gastrointestinal symptoms. In the setting of intolerance to gastric feeding, attempts should be made with promotility agents to support gastric feedings before the use of endoscopically placed jejunal feeding tubes or parenteral nutrition (PN). Initiation of PN should be considered if a patient is unable to achieve goal calorie intake by ICU day 7 (11). In patients with COVID-19, early use of PN has the potential to cause harm secondary to the high volume of fluid needed to provide nutrition. Given the intense inflammatory response associated with severe acute respiratory syndrome coronavirus 2 infection, close monitoring of serum triglycerides should be instituted and lipid formulations should be dose reduced in the setting of serum triglycerides greater than 400 mg/dL (12). Fish oil containing lipid formulations should be considered, if available, based on the limited literature that reports reductions in infectious complications (1).
GASTROSTOMY TUBES
The placement of gastrostomy tubes in the United States in the setting of critical illness has more than doubled over the past 20 years, accounting for roughly 50% of all gastrostomy tubes placed and occurring in 2.4% of critically ill adults (13). Placement is particularly common in individuals with ICU-acquired weakness that results in discharge to long-term care facilities. Although generally recommended when the provision of EN is expected to exceed 4 weeks (14), percutaneous endoscopic gastrostomy (PEG) tube placement in patients with COVID-19 should be deferred unless complications of nasoenteral tubes develop (i.e., sinusitis and esophageal ulceration). When needed for longer term care, PEG tubes should be placed once disposition is arranged. Standard recommendations from the recent joint societal statements should be followed for PPE in endoscopy at the time of PEG placement (15).
CONCLUSION
The worldwide pandemic of COVID-19 has resulted in unprecedented stressors on the healthcare system, balancing an ability to care for patients in critical illness while providing safety to healthcare workers. These factors mandate implementation of an organized pathway for nutrition support with the potential to improve patient outcomes while limiting healthcare worker risk. Implementation of a simple and pragmatic protocol at the institutional or regional level allows uniformity of nutrition support in an otherwise unpredictable environment.
CONFLICTS OF INTEREST
Guarantor of the article: Dejan Micic, MD.
Specific author contributions: D.M. contributed to the conception and design and completed the first draft of the manuscript. E.W. contributed to the conception and design and drafting of the manuscript. C.S. contributed to the conception and design and drafting of the manuscript.
Financial support: None to report.
Potential competing interests: None to report.
REFERENCES
- 1.Martindale R, Patel JJ, Taylor B, et al. Nutrition therapy in the patient with COVID-19 disease requiring ICU Care2020 (https://www.nutritioncare.org/).
- 2.Barazzoni R, Bischoff SC, Breda J, et al. ; endorsed by the ESPEN Council. Espen expert statements and practical guidance for nutritional management of individuals with sars-cov-2 infection. Clin Nutr 2020;39(6):1631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin Nut. 2003;22(3):321–36. [DOI] [PubMed] [Google Scholar]
- 4.Maciel L, Franzosi OS, Nunes DSL, et al. Nutritional risk screening 2002 cut-off to identify high-risk is a good predictor of ICU mortality in critically ill patients. Nutr Clin Pract 2019;34(1):137–41. [DOI] [PubMed] [Google Scholar]
- 5.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40(2):159–211. [DOI] [PubMed] [Google Scholar]
- 6.Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 2011;306(14):1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogensen KM, Andrew BY, Corona JC, et al. Validation of the society of critical care medicine and American society for parenteral and enteral nutrition recommendations for caloric provision to critically ill obese patients: A pilot study. JPEN J Parenter Enteral Nutr 2016;40(5):713–21. [DOI] [PubMed] [Google Scholar]
- 8.Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA 2012;307(8):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braunschweig CA, Sheean PM, Peterson SJ, et al. Intensive nutrition in acute lung injury: A clinical trial (INTACT). JPEN J Parenter Enteral Nutr 2015;39(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Koning MLY, Koekkoek W, Kars J, et al. Association of PROtein and CAloric intake and clinical outcomes in adult SEPTic and non-septic ICU patients on prolonged mechanical ventilation: The PROCASEPT retrospective study. JPEN J Parenter Enteral Nutr 2020;44(3):434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365(6):506–17. [DOI] [PubMed] [Google Scholar]
- 12.Adolph M, Heller AR, Koch T, et al. Lipid emulsions—guidelines on parenteral nutrition, chapter 6. Ger Med Sci 2009;7:Doc22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law AC, Stevens JP, Walkey AJ. Gastrostomy tube use in the critically ill, 1994-2014. Ann Am Thorac Soc 2019;16(6):724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClave SA, DiBaise JK, Mullin GE, et al. ACG clinical guideline: Nutrition therapy in the adult hospitalized patient. Am J Gastroenterol 2016;111(3):315–34; quiz 35. [DOI] [PubMed] [Google Scholar]
- 15.AASLD A, AGA, ASGE. COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers 2020. (https://gi.org/2020/03/15/joint-gi-society-message-on-covid-19/). Accessed April 1, 2020. [Google Scholar]