Abstract
Our recent study found that activation of signal transducer and activator of transcription 3 (Stat3) is up-regulated in human brain metastatic cells and contributes to brain metastasis of melanoma. However, the molecular mechanisms underlying this increased Stat3 activation and effect on brain metastasis are unknown. In this report, we showed that the expression of Janus-activated kinase 2 (JAK2), a Stat3 activator, was increased, whereas the expression of a negative regulator of Stat3, suppressor of cytokine signaling-1 (SOCS-1), was reduced in the brain metastatic melanoma cell line A375Br, relative to that in the parental A375P cell line. Consistently, SOCS-1 expression was also lower in the human brain metastatic tissues than in the primary melanoma tissues. Mechanistically, increased JAK2 expression in the A375Br cells was due to, at least in part, its decreased degradation, which was directly correlated with low expression of SOCS-1. Moreover, restoration of SOCS-1 expression resulted in the inhibition of Stat3 activation, whereas depletion of SOCS-1 up-regulated Stat3 activation. These clinical, experimental, and mechanistic findings strongly suggest that increased activation of Stat3 in brain metastatic melanoma cells might be due to decreased SOCS-1 expression. Furthermore, restoration of SOCS-1 expression in brain metastatic A375Br cells significantly inhibited brain metastasis in animal models (P < 0.001). Additionally, alterations of SOCS-1 expression profoundly affected the expression of matrix metalloproteinase-2 (MMP-2), basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF) and the melanoma cell invasion and angiogenesis. Collectively, these data suggest that the loss of SOCS-1 expression is a critical event, leading to elevated Stat3 signaling and overexpression of MMP-2, bFGF, and VEGF, as well as enhanced invasion and angiogenesis of melanoma cells, consequently promoting brain metastasis.
Introduction
Melanoma is one of the few remaining cancers for which the incidence rate is increasing (1). Deaths from malignant melanoma are also increasing (2). Brain metastasis occurs in 40% to 60% of patients with advanced melanoma and is the cause of death in most cases (3–6). However, little is known about the biological and molecular basis of melanoma brain metastases, despite its high incidence and effect on survival.
We previously reported that the signal transducer and activator of transcription 3 (Stat3) activity was higher in human brain metastatic cells than in primary melanoma cells and that high Stat3 activity contributed to brain metastasis of melanoma (7). However, the mechanism(s) responsible for the increased Stat3 activity in brain metastatic cells are unclear. It could be due to elevated activation signals or/and decreased inhibitory signals present in the brain metastatic cells. Studies have shown that numerous cytokines, growth factor, and specific oncogenic proteins can activate Stat3 through receptor-associated Janus-activated kinases (JAK), such as JAK2. Studies have also reported that suppressor of cytokine signaling-1 (SOCS-1), also called JAK-binding protein or Stat-induced Stat inhibitor-1, could be an inhibitory molecule for Stat3 activation (8, 9). Presumably, aberrant expression and disturbed signaling of those molecules could critically affect brain metastasis.
SOCS-1 is a member of the SOCS family of negative regulators of cytokine signal transduction (8–10). SOCS-1 protein was identified as being involved in a negative feedback loop in cytokine signaling. SOCS-1 suppresses cellular responses to various cytokines, including interleukin-6 (IL-6), IL-4, leukemia inhibitory factor, oncostatin M, IFN, and growth hormones (8–14). SOCS-1 turns off cytokine signal transduction, in part, through direct interaction with active JAKs by binding to their activation loop through its SH2 domain, thereby blocking subsequent signaling that requires phosphorylation and activation of the Jak/Stat pathway (15). A second critical role for SOCS-1 in signal down-regulation is through its targeting of associated proteins for proteasomal degradation (16). For example, SOCS-1 has been shown to be an ubiquitin ligase for JAK2 (17, 18) by means of its COOH terminal homology domain SOCS box, which targets JAK2 for ubiquitination and proteasomal degradation.
The roles of SOCS-1 in tumor development and progression seem to be cell type–specific. Several studies indicate SOCS-1 as a tumor suppressor gene. For example, restoration of SOCS-1 suppressed both the growth rate and anchorage-independent growth of hepatocellular carcinoma cells with methylation-silenced SOCS-1 and constitutively activated JAK2 (19). Moreover, ectopic expression of SOCS-1 can block the transforming activity of several oncogenes, including TEL-JAK2 (a constitutively active form of the KIT receptor) and v-ABL (20), and reduce metastasis of BCR-ABL–transformed cells. The tumor suppressor function of SOCS-1 was further shown by a study showing that SOCS-1 had antitumor effects through attenuation of IL-6 signaling (21, 22). Conversely, SOCS-1 might be a marker of progression rather than a tumor suppressor in human melanoma, because the primary melanoma cells express SOCS-1 in vitro and in situ, and SOCS-1 immunoreactivity is closely related to tumor invasion, tumor thickness, and disease stage (23). Another known function of the SOCS-1 protein is its ability to inhibit Stat1 (8, 9, 14), which we found had inhibitory effects on tumor metastasis (24). Nevertheless, the role and regulation of SOCS-1 expression in brain metastasis of melanoma have not been determined.
In this study, we examined SOCS-1 expression levels in melanoma cell cultures and tissues and determined the effects of altered SOCS-1 expression on Stat3 signaling, tumor angiogenesis, invasion, and brain metastasis.
Materials and Methods
Cell lines and culture conditions
Human melanoma cell line A375P and brain metastatic melanoma cell lines A375Br and TXM-18 (7) were used. All cell lines were maintained as adherent monolayers in Eagle’s MEM, supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, L-glutamine, and a vitamin solution (Invitrogen).
Pulse-chase experiments
A375P and A375Br cells (2 × 107 each) were harvested and incubated at 37°C for 15 min in methionine and cysteine-free MEM. Cells were pulsed with 0.5 mCi (18.5 MBq) [35S]methionine (Amersham Biosciences) in 2 mL methionine and cysteine-free MEM for 30 min. Cells were washed thrice in PBS and incubated in chase medium (MEM supplemented with 10% FCS and 10-fold molar excess of Cys and Met) for various times.
Immunoprecipitation and determination of JAK2 degradation
Cell lysates were prepared, as described previously (25). Lysates were then precleared with 100 μL protein A-agarose bead slurry (Amersham Biosciences) for 1 h, and 35S incorporation into total proteins was determined by liquid scintillation counting. Aliquots of 500 μg total protein were incubated with 5 μg anti-JAK2 antibody (Cell Signaling Technology) and 100 μL protein A-agarose bead slurry overnight at 4°C. Agarose beads were collected and washed in ice-cold lysis buffer. Samples were heated and separated on 10% SDS-PAGE by electrophoresis. The gels were fixed and saturated with Amplify solution (Amersham Biosciences), dried, and autoradiographed. For quantification of JAK2 synthesis and degradation, labeled bands were analyzed using the NIH Image software.
Human tissue specimens and immunohistochemical analyses
Tissue specimens of primary human melanoma and brain metastasis were obtained from a tumor tissue bank maintained at The University of Texas M. D. Anderson Cancer Center. An institutional review board approval was obtained for the study. Sections of paraffin-embedded tissue specimens were stained with an anti–SOCS-1 antibody (1:200 dilution; Zymed Laboratories, Inc.). Tissue sections were immunostained with nonspecific IgG and used as negative controls. Staining was scored by using a three-tier system that incorporated the percentage of positive cells and staining intensity (26): negative (0/+), moderate positive (++), or strong positive (+++). Staining was scored by two investigators, who examined five random fields of each sample while blinded to the clinical data.
Animals
Pathogen-free female athymic BALB/c nude mice were purchased from the Animal Production Area of the National Cancer Institute, Frederick Cancer Research Facility. The animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care in accordance with the current regulations and standards of the U.S. Department of Agriculture, Department of Health and Human Services, and NIH.
Transfection
To generate the pcDNA3.1-SOCS-1 plasmid, SOCS-1 cDNA was released by XbalI digestion of the pEF-FLAG/SOCS-1 plasmid (8) and subcloned into the pcDNA3.1-hygro vector (Invitrogen). A375Br melanoma cells were transfected with either the pcDNA3.1-SOCS-1 or control plasmids using Lipofectamine 2000 (Invitrogen). Stably transfected cell lines were isolated by hygromycin selection (150 μg/mL). TXM-18 cells were also transfected with either the pcDNA3.1-SOCS-1 or the pc-DNA3.1-hygro plasmids. A375P cells were transfected with either SOCS-1 small interfering RNA (siRNA; 5′-CUACCUGAGCUCCUUCCCCUUdTdT-3′; Dharmacon) or control siRNA (100 nmol/L; Ambion).
Analysis of apoptosis by propidium iodide staining
Cells cultured on culture dishes for 48 h were collected and then fixed in 70% alcohol. After fixation, cells were treated with RNase (100 mg/mL) and stained with 40 mg/mL propidium iodide (PI; Sigma). The PI-stained cells were incubated in the dark at room temperature for 30 min and analyzed by flow cytometry with excitation set at 543 nm. The apoptotic rate was calculated from hypodiploid DNA peak of apoptotic cells by Cell Quest software (Becton Dickinson).
In vivo model of brain metastasis
The brain metastasis model used has been described previously (7, 27). Tumor cells (1 × 105 in 0.1 mL of HBSS) were injected into the internal carotid artery. Mice without symptoms were killed 120 d after tumor cell inoculation; other mice were killed when they seemed moribund or developed clinical symptoms, such as immobility, weight loss, or a hunched position. Survival times for each mouse were recorded. The mouse brains were removed, and 10 serial sections every 300 μm through a brain were prepared. The sections were H&E stained for histology analysis to confirm the presence of brain metastases.
Northern blot analysis
Cellular mRNA was then extracted by using a FastTrack mRNA isolation kit (Invitrogen). mRNA (5 μg) was separated electrophoretically on a 1% denaturing formaldehyde agarose gel, transferred to a nylon membrane, and hybrid with [32P]dCTP labeled SOCS-1 cDNA probes. Equal loading of mRNA samples was monitored by hybridizing with a β-actin cDNA probe.
Western blot analysis
Standard Western blotting of whole cell lysates was performed with antibodies to tyrosine-phosphorylated Stat3 (Tyr705), Stat3, phosphorylated p44/42, p44/42 (Cell Signaling Technology), SOCS-1 (Zymed Laboratories, Inc.), or vascular endothelial growth factor (VEGF; Santa Cruz Biotechnology). Equal protein sample loading was monitored by using an anti–β-actin antibody.
Electrophoretic mobility shift assay
The electrophoretic mobility shift assay (EMSA) was performed, as described previously (7, 28). For supershift analyses, the cell extracts were preincubated with specific antibodies against Stat3 (Zymed Laboratories, Inc.). Protein-DNA complexes were resolved on a 6% nondenaturing polyacrylamide gel. The gels were then dried and autoradiographed.
Endothelial cell tube formation assay
The tube formation assay was performed, as described previously (26). Briefly, 250 μL of growth factor–reduced Matrigel (Collaborative Biomedical Products) was pipetted into each well of a 24-well plate and polymerized for 30 min at 37°C. Human umbilical vascular endothelial cells (HUVEC) were harvested after trypsin treatment and suspended in a conditioned medium from 1 × 106 melanoma cells. Next, 2 × 104 HUVECs in 300 μL of conditioned medium were added to each well and incubated at 37°C and 5% CO2 for 20 h. The cultures were photographed under a bright-field microscope.
Real time reverse transcription–PCR
Total RNA was isolated with TRIZOL reagent (Invitrogen). First-strand cDNA was synthesized from 2 μg of total RNA using MMLV reverse transcriptase (Invitrogen). Real-time PCR was carried out with 2 μL of the cDNA and SYBR Green Master Mix (Bio-Rad). Forward and reverse primers for matrix metalloproteinase-2 (MMP-2) and VEGF were as follows: MMP-2 primers (forward 5′-ATGACAGCTGCACCACTGAG-3′, reverse 5′-ATTTGTTGCCCAAGGAAAGTG-3′), VEGF121 primers (forward 5′-GATCAAACCTCACCAAGGCCA-3′, reverse 5′-GCCTCGGCTTGTCACATTTTTC-3′), and VEGF165 primers (forward 5′-CAGATTATGCGGATCAAACCTCA-3′, reverse 5′-CAAGGCCCACAGGGATTTTC-3′). Each sample was run in triplicate for the target gene and the control gene.
Gelatin zymography
MMP-2 activity was measured by a standard assay using serum-free supernatants collected from cell cultures (24 h) and normalized by cell numbers (7). The samples were separated on a 7.5% SDS-PAGE gel containing 1 mg/mL of gelatin. The gel was washed in wash buffer and then incubated for 36 h at 37°C in incubation buffer. Finally, the gel was stained with a 0.1% Coomassie Blue R-250.
Matrigel invasion assay
An in vitro invasion assay was performed, according to the procedure described (7). Briefly, conditioned medium from 1 × 106 HF-U251MG astrocytoma cells was placed in the lower wells to act as a chemoattractant. Melanoma cells (2.5 × 103) in 300 μL of serum-free medium were placed in the upper chambers and incubated at 37°C for 22 h. Next, the cells on the upper surface of the filter were completely removed by using cotton swabs; the cells on the lower surface of the filter were stained with H&E. Cells were counted at a magnification of ×200 (×20 objective and ×10 ocular) in 10 randomly selected fields, and the mean number of cells per field was recorded.
Statistical analysis
Differences in staining scores among the patient specimens were assessed with the χ2 test. Differences in the in vitro data were examined with two-tailed Student’s t tests, and differences between median values of the in vivo data were compared by using the two-tailed Mann-Whitney test.
Results
JAK2 expression is higher in brain metastatic cells than in primary melanoma cells
The highly brain metastatic A375Br melanoma cell line exhibits higher Stat3 activity than that in its parental poorly metastatic A375P primary melanoma cells (7). To study the mechanism that might be responsible for the increased Stat3 activation in brain metastatic cells, we first examined one of the upstream signals of Stat3 activation and JAK2 protein and found a higher JAK2 protein expression in A375Br cells than that in A375P cells (Fig. 1A). Pulse-chase experiments showed that the turnover of [35S]JAK2 in A375Br cells was significantly decreased compared with that in A375P cells (Fig. 1B), whereas no significant difference of 35S incorporation into the total proteins of A375P and A375Br cells was noted (data not shown) at those time points. These results indicated that the increased JAK2 expression in A375Br cells was due, at least in part, to increased stability of JAK2 protein.
Figure 1.
JAK2 expression and turnover in melanoma cells. A, the expression of JAK2 in human melanoma cell lines of different metastatic potential was examined by using immunoprecipitation with anti-JAK2 antibody. Nonspecific IgG was used as a loading control. B, turnover of JAK2 protein. A375P and A375Br cells were pulsed with [35S]methionine for 30 min and chased for the times indicated. Cell protein extracts were used for immunoprecipitation with anti-JAK2 antibodies and subjected to SDS-PAGE and autoradiography. Left, [35S]JAK2 signals were obtained from A375P (top) and A375Br (bottom) protein extracts indicating dynamics of degradation. Right, intensities of the bands were analyzed using the NIH Image software, and the integrated optical densities of the bands at time 0 were set as 100%. Note a time-dependent degradation of JAK2 in A375P and A375Br. C, SOCS-1 protein expression was determined by Western blotting. D, SOCS-1 expression in human primary and brain metastasis melanomas. Immunohistochemical staining with an anti–SOCS-1 antibody was done with 42 specimens of melanoma brain metastases, 40 specimens of primary melanoma, and 8 specimens of normal skin. Percentages of specimens with negatively, moderately, or strongly positive staining for SOCS-1 protein were shown for each group. *, P = 0.001 by χ2 test.
SOCS-1 expression is lower in brain metastatic cells than in primary melanoma cells
Because the degradation of JAK2 is affected by SOCS-1 protein level (17, 18), we then examined the endogenous expression of SOCS-1 in cultures of A375P and A375Br cells. Immunoblotting with an anti–SOCS-1 antibody clearly showed a significantly decreased SOCS-1 expression in A375Br cells compared with that in A375P cells (Fig. 1C).
To determine a possible clinical relevance of our in vitro observation that SOCS-1 expression was decreased in brain metastatic melanoma cells, we analyzed SOCS-1 expression in 42 specimens of melanoma brain metastases, 40 specimens of primary melanoma, and 8 specimens of normal skin by immunohistochemical staining. We found that 9 of the primary melanomas (22.5%) were strongly positive (+++), 17 (42.5.2%) were moderately positive (++), and 14 (35%) were negative for SOCS-1. This is similar to a historical positive percentage (~70%) for primary melanomas regardless of stage (23). In contrast, only 2 of the brain metastases (4.9%) were strongly positive, 16 (38%) were moderately positive, and 24 (57.1%) were negative for SOCS-1. A significantly less SOCS-1 was expressed in melanoma brain metastases than in primary melanoma specimens (P < 0.01, χ2 test; Fig. 1D). We detected SOCS-1 protein expression in only one of the eight normal skin specimens. Representative sections of strong staining (+++), moderate staining (++), and negative or weak staining (0/+) for SOCS-1 were shown in Supplementary Fig. S1. Thus, the human melanoma brain metastases tissues apparently had a substantially lower level of SOCS-1 expression than the primary melanoma tissues.
Restoring SOCS-1 expression inhibits brain metastasis of melanoma cells
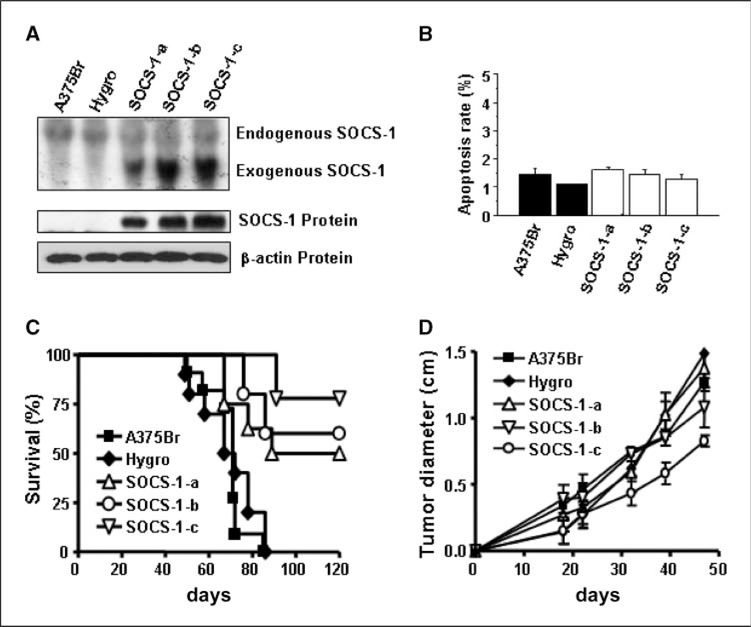
The above studies for the first time provided both experimental and clinical evidence that SOCS-1/JAK2/Stat3 signaling was disturbed, and the disturbance was closely associated with brain metastasis. To determine the causal role of SOCS-1 expression in brain metastasis of melanoma, we genetically modified SOCS-1 expression in brain metastatic A375Br cells by transfecting them with a SOCS-1 expression vector. To avoid clonal selection and variation, we carried out three independent transfections of pcDNA3.1-SOCS-1 in the cell line and pooled G418-resistant colonies to establish stable transfectants, designated as SOCS-1–transfected A375Br cell lines (A375Br-SOCS-1-a, A375Br-SOCS-1-b, and A375Br–SOCS-1-c). A375Br cells were also transfected with pcDNA3.1-hygro vector to generated A375Br-hygro control cells. Northern blotting revealed that A375Br and A375Br-hygro cells expressed very low levels of endogenous SOCS-1 mRNA (Fig. 2A, top). The A375Br-SOCS-1cells expressed exogenous SOCS-1 mRNA of a lower molecular weight than the endogenous form, because the SOCS-1 expression vector contains only the coding region of the SOCS-1 gene. Western blotting also confirmed that SOCS-1 protein expression was increased in the pcDNA3.1-SOCS-1–transfected A375Br cells (Fig. 2A, bottom).
Figure 2.
Restoring SOCS-1 expression inhibits brain metastasis of A375Br cells. A, SOCS-1 expression in untransfected, vector-transfected, and SOCS-1–transfected A375Br melanoma cell lines was determined by Northern blotting (top) and Western blotting (bottom). B, apoptosis rate of A375Br, A375Br-hygro and SOCS-1–transfected A375Br cells. Apoptosis analysis was assessed by flow cytometry of PI-stained cells that were cultured on culture dishes for 48 h. Apoptosis rate (%) indicates the cell population in sub-G1 phase of cell cycle. Columns, mean (n = 3);bars, SE. C, survival analyses of nude mice injected with melanoma cells. A brain metastasis assay was performed by injecting 1 × 105 of A375Br, A375Br-hygro, or SOCS-1–transfected A375Br cells into the left internal carotid artery of nude mice. Data were presented as Kaplan-Meier survival curves from the day of injection to day 120. Log-rank tests show significant (P < 0.001) differences in survival between the A375Br-SOCS-1 group and the control group. D, tumor growth in the subcutis of nude mice. Tumor cells (1 × 106 in 0.2 mL of HBSS) were injected over the right scapular region of nude mice (n = 5). The tumor diameters were monitored by weekly examination and measurement of tumors with calipers. The data represent the mean diameter observed in five mice per group. The results shown are for one representative experiment of two.
We evaluated the effect of increased SOCS-1 expression on cell growth and apoptosis in vitro. We found that A375Br, A375Br-hygro, and A375Br-SOCS-1 cells have no difference on growth rates, cell cycling (data not shown), and apoptosis (Fig. 2B). Next, we evaluated the effect of increased SOCS-1 expression on brain metastasis in vivo. The A375Br and A375Br-hygro control cells produced brain metastases in 100% of mice (Table 1). The incidence of brain metastases reduced to 44.4%, 50%, and 22.2% in mice injected with A375Br-SOCS-1-a, A375Br-SOCS-1-b, and A375Br-SOCS-1-c cells, respectively (Table 1). Thus, the average brain metastasis incidence of SOCS-1–transfected A375Br cells is 61.2% lower than that of A375Br and control vector–transfected A375Br cells (P < 0.001). With regard to survival time, all of the mice injected with A375Br or A375Br-hygro cells died before day 87 after injection but 78%, 56%, and 50% of the mice injected with A375Br-SOCS-1 variants were alive at 87 days (and indeed, at 120 days; P < 0.001; Fig. 2C). These results show that up-regulation of SOCS-1 expression significantly inhibited the metastasis of melanoma cells to the brain. However, the growth of A375Br-SOCS-1 cells injected into the subcutis of mice was only slightly inhibited relative to that of control cells (no statistical significance; Fig. 2D). Moreover, the apoptosis rate of A375Br-SOCS-1 subcutaneous tumors was similar to that of A375Br or A375Br-Hygro subcutaneous tumors (Supplementary Fig. S2).
Table 1.
Effects of SOCS-1 expression on brain metastasis in nude mice
| Cell line | Brain metastasis |
|
|---|---|---|
| Incidence* | Survival (d)† | |
| A375Br | 100.0% (11/11) | 50 to 85 |
| A375Br-Hygro | 100.0% (10/10) | 48 to 86 |
| A375Br-SOCS-1-a | 50.0% (4/8) ‡ | 67 to >120 |
| A375Br-SOCS-1-b | 44.4% (4/9) ‡ | 76 to >120 |
| A375 Br-SOCS-1-c | 22.2% (2/9) ‡ | 91 to >120 |
Percentage of mice with brain metastasis (number of positive mice / number of injected mice).
The survival time for brain tumor-free animal was recorded as 120 d.
P < 0.001 compared with A375Br or A375-hygo control cells.
Altering SOCS-1 expression affects Stat3 activation
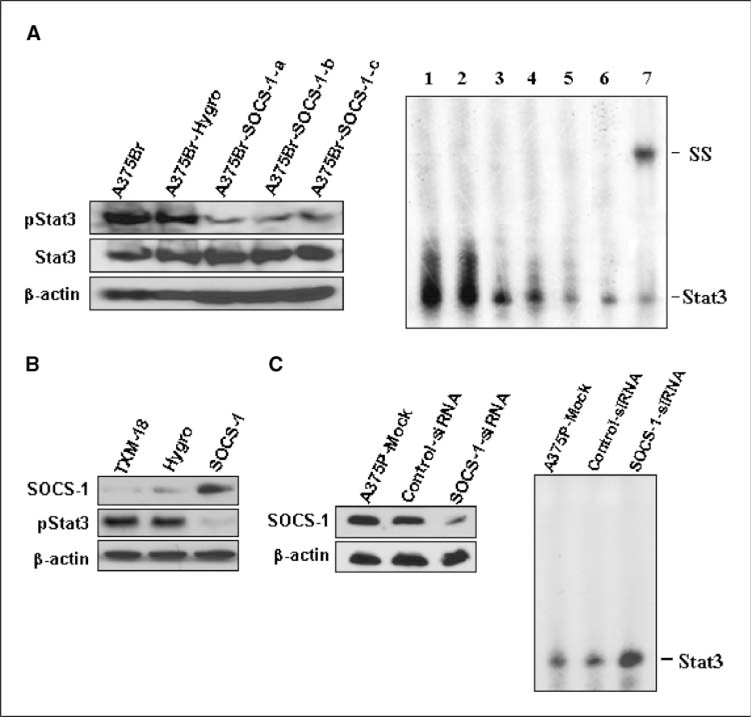
Because SOCS-1 can inhibit the tyrosine phosphorylation of STATs, specifically Stat1 and Stat3, we examined pStat1 and pStat3 protein levels in SOCS-1–transfected A375Br cells by Western blotting. Amounts of tyrosine-phosphorylated Stat3 (pStat3), but not total Stat3, were decreased in all of the A375Br-SOCS-1 cell lines relative to the parental and vector-transfected A375Br cells (Fig. 3A, left). pStat1 was not detected in parental or SOCS-1–transfected A375Br cells, although those cells did express Stat1 protein (data not shown), suggesting that Stat1 is not constitutively activated in these cells. In addition, an EMSA showed significantly lower Stat3-binding activity in the SOCS-1–transfected A375Br cells than in the parental and control vector–transfected cells (Fig. 3A, right). The specificity of Stat3 DNA-binding activity in these cells was confirmed by a competition assay with an unlabeled Stat3 consensus probe and by a supershift assay with a Stat3-specific antibody (Fig. 3A, right). Furthermore, we analyzed the Stat3 activity in metastatic tumors produced by A375Br or SOCS-1–transfected A375Br cells. Although most mice injected with SOCS-1–transfected A375Br cells did not have metastatic tumors in the brain, a few mice developed small lesions, which were confirmed by histology (Supplementary Fig. S3; H&E). We found that the level of tyrosine-phosphorylated Stat3 (pStat3) was drastically decreased in SOCS-1–transfected A375Br tumors compared with A375Br-hygro tumors (Supplementary Fig. S3).
Figure 3.
Altering SOCS-1 expression affects Stat3 activation. A, left, Stat3 and tyrosine-phosphorylated Stat3 levels in A375Br, A375Br-hygro, and A375Br-SOCS-1 cells were determined by Western blotting. Right, restoring SOCS-1 inhibits the DNA-binding activity of Stat3 in A375Br cells, as determined by EMSA. Nuclear protein extracts (10 μg/sample) of A375Br (lane 1), A375Br-hygro (lane 2), or A375Br-SOCS-1-a, A375Br-SOCS-1-b, and A375Br-SOCS-1-c (lanes 3–5) cells were subjected to EMSA with 32P-labeled oligonucleotide probes containing a consensus-binding motif for Stat3. In some reactions with A375Br protein, unlabeled Stat3 consensus probe (lane 6) or anti-Stat3 antibody (lane 7) was added. The positions of the Stat3 and supershifted (SS) complexes were marked. B, restoring SOCS-1 expression inhibits Stat3 tryrosine phosphorylation in TXM-18 cells. TXM-18 cells were transfected with pcDNA-SOCS-1 or control plasmids twice in 48 h, and then cells were harvested for Western blot analysis of the expression of SOCS-1 and tyrosine-phosphorylated Stat3. C, left, depletion of SOCS-1 expression in zA375P cells by transfection with SOCS-1-siRNA. SOCS-1 expression in mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA–transfected A375P cells was determined by Western blotting. Right, depletion of SOCS-1 increases Stat3 DNA-binding activity in A375P cells. Stat3 DNA-binding activity in mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA–transfected A375P cells was determined by EMSA.
We also used another human melanoma cell line TXM-18, which was isolated from brain metastases of a patient and is highly metastatic to the brain of nude mice (7). In consistent with the results of A375Br, TXM-18 cells have very a low level of SOCS-1 but a high level of tyrosine-phosphorylated Stat3 (pStat3) expression (Fig. 3B). Increased SOCS-1 expression in TXM-18 cells by transfection of SOCS-1 significantly reduced the pStat3 expression (Fig. 3B).
In a parallel set of experiments, we depleted the expression of SOCS-1 in A375P cells (which express high levels of SOCS-1) by transfecting them with SOCS-1–specific siRNA and assessed the Stat3 activity in those cells. SOCS-1 expression was sharply reduced after SOCS-1-siRNA trans fection (Fig. 3C, left), whereas Stat3 activity was increased in the SOCS-1-siRNA–transfected A375P cells compared with that in mock-transfected or control-siRNA-transfected cells, as determined by EMSA (Fig. 3C, right). However, the levels of total and phosphorylated p44/42 mitogen-activated protein kinase in A375P cells did not change upon transfection of SOCS-siRNA (Supplementary Fig. S4A). These findings further confirm that SOCS-1 can specifically suppress Stat3 activation.
Altering SOCS-1 expression affects MMP-2 expression and melanoma cell invasion
Cell growth, invasion, and angiogenesis are critical aspects of melanoma biology. Our initial mechanism study revealed no differences in in vitro growth rates, cell cycling, and apoptosis among the A375Br, A375Br-hygro, and A375Br-SOCS-1 cells (Fig. 2B). However, the A375Br-SOCS-1 cells exhibited significantly decreased invasion through a Matrigel-coated filter compared with control cells (Fig. 4A and B). Increased SOCS-1 expression in TXM-18 cells by transfection of SOCS-1 significantly inhibited invasion of the cells compared with control cells (Fig. 4A and B). In contrast, the SOCS-1-siRNA–transfected A375P cells, in which SOCS-1 level was decreased compared with that in control cells (Supplementary Fig. S4B), exhibited significantly increased invasion (Fig. 4A and B). These results suggested that altered SOCS-1 expression affected the invasiveness of melanoma cells. Moreover, we found that A375Br cells expressed a high level of MMP-2 but a very low level of MMP-9 (data not shown) and that inhibition of MMP-2 expression and activity significantly suppressed the invasiveness of A375 Br cells (Supplementary Fig. S5). Thus, we next assessed the MMP-2 regulation in these cell lines and found that the SOCS-1–transfected A375Br cells exhibited lower MMP-2 RNA and activity levels in vitro than did the parental or vector-transfected A375Br cells (Fig. 4C). Also, the SOCS-1–transfected TXM-18 cells exhibited lower MMP-2 RNA, enzyme activity, and promoter activity levels in vitro than did the parental or vector-transfected TXM-18 cells (Fig. 4C and Supplementary Fig. S4D). Furthermore, MMP-2 expression was consistently decreased in the brain metastatic tumors formed by SOCS-1–transfected A375Br cells in mice (Supplementary Fig. S3). In contrast, SOCS-1-siRNA–transfected A375P cells exhibited higher expression of MMP-2 at both the RNA and protein levels when compared with mock-transfected or control-siRNA-transfected A375P cells (Fig. 4C). Collectively, these results suggest that modifying SOCS-1 levels affects the expression of MMP-2 and the invasiveness of melanoma cells.
Figure 4.
Altering SOCS-1 expression affects MMP-2 expression and melanoma cell invasion. A, the invasive ability of A375Br, A375Br-hygro, A375Br-SOCS-1, TXM-18, TXM-18-hygro, and TXM-18-SOCS-1 cells and that of mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA–transfected A375P cells was determined by Matrigel invasion assay. The numbers of migrated cells that penetrated through Matrigel-coated filter were expressed as the mean number of cells in 10 random fields identified within the lower surface of the filter. *, P < 0.001 compared with A375Br or TXM-18. **, P < 0.01 compared with A375P-mock. Bars, SD for replicates within the assay. Results are from one of two experiments. B, photomicrographs of tumor-cell invasion during the in vitro invasion assay. C, MMP-2 RNA expression was determined in A375Br, A375Br-hygro, A375Br-SOCS-1, TXM-18, TXM-18-hygro, and TXM-18-SOCS-1 cells and in mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA–transfected A375P cells by real-time reverse transcription–PCR (RT-PCR; top); *, P < 0.001 compared with A375Br or A375P-mock. MMP-2 activity was determined in A375Br, A375Br-hygro, A375Br-SOCS-1, TXM-18, TXM-18-hygro, and TXM-18-SOCS-1 cells and in mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA–transfected A375P cells with a gelatinase assay (bottom).
Altering SOCS-1 expression affects VEGF and basic fibroblast growth factor expression in and the angiogenic potential of melanoma cells
Because the metastatic process depends on angiogenesis (29), we next sought to determine whether modifying SOCS-1 expression would affect angiogenesis in brain metastatic tumors. We assessed vascularization in brain metastases by staining mouse brain metastasis tissue sections with an anti-CD34 antibody and found that brain metastases produced by A375Br-hygro cells were highly vascularized, whereas brain metastases produced by SOCS-1–transfected cells had considerably lower microvessel density (Supplementary Fig. S3).
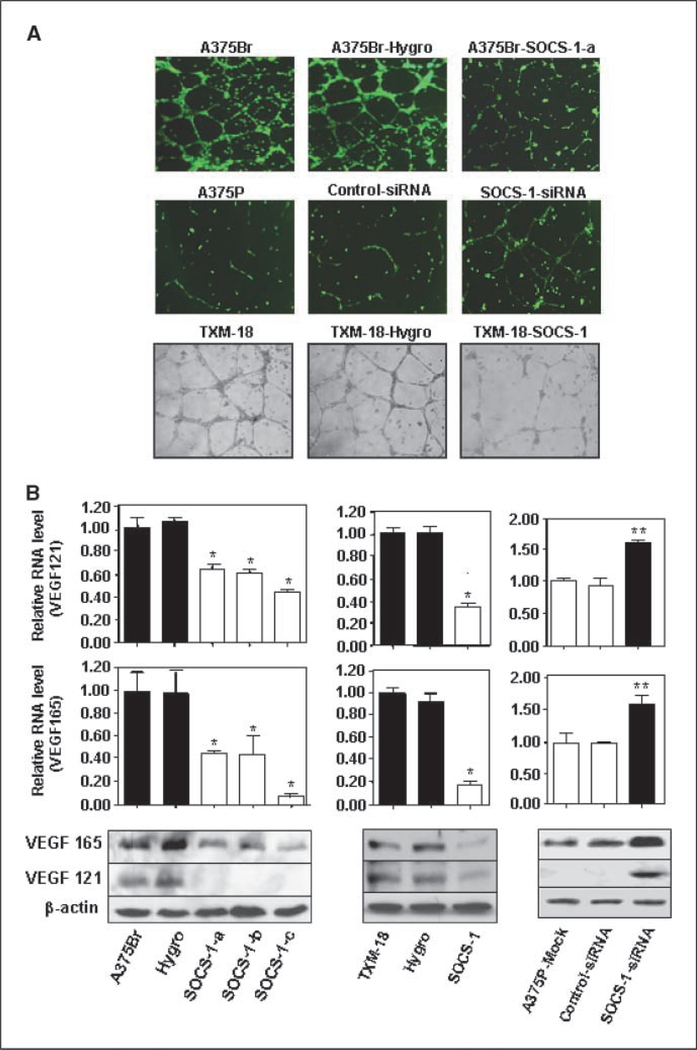
To provide direct evidence that SOCS-1 regulates the angiogenic phenotype of melanoma cells, we conducted an endothelial cell tube formation assay. We found that the conditioned media from A375Br-SOCS-1 cell cultures had a significantly reduced capillary tube formation compared with the conditioned media from parental and vector control-transfected A375Br cells (Fig. 5A). Also, the conditioned media from SOCS-1–transfected TXM-18 cells had a significantly reduced capillary tube formation compared with the conditioned media from parental or vector-transfected TXM-18 cells (Fig. 5A). In contrast, the conditioned medium of A375P cells transfected with SOCS-1-siRNA, but not those transfected with control-siRNA, had greater angiogenic potential than the conditioned medium of A375P cells (Fig. 5A). The down-regulation of SOCS-1 expression in these SOCS-1-siRNA–transfected A375 cells was confirmed by the Western blot analysis of cell lysates (Supplementary Fig. S4C).
Figure 5.
Altering SOCS-1 expression affects VEGF expression and angiogenic potential of melanoma cells. A, the angiogenic potential of A375P and A375Br cells was determined by an endothelial cell tube formation assay. Samples of conditioned medium were prepaared from A375Br, A375Br-hygro, A375Br-SOCS-1, TXM-18, TXM-18-hygro, and TXM-18-SOCS-1 cells and from mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA-transfected A375P cells. Human umbilical cord endothelial cells (2 × 104) in 3 00 μL of conditioned medium were then plated on growth factor–reduced Matrigel to form a capillary tube. Capillary tube formation in each group was photographed. The results shown are representative of two experiments. B, VEGF RNA expression in A375Br, A375Br-hygro, A375Br-SOCS-1, TXM-18, TXM-18-hygro, and TXM-18-SOCS-1 cells and mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA–transfected A375P cells was measured by real-time RT-PCR. *, P < 0.001 compared with A375Br; **, P < 0.01 compared with A375P-mock. C, VEGF protein expression in A375Br, A375Br-hygro, and A375Br-SOCS-1 cells, TXM-18, TXM-18-hygro, and TXM-18-SOCS-1 cells, and in mock-transfected, control-siRNA–transfected, or SOCS-1-siRNA–transfected A375P cells was determined by Western blotting.
Finally, given the established role of VEGF and basic fibroblast growth factor (bFGF) in melanoma angiogenesis (30–32), we evaluated VEGF and bFGF expression in brain metastatic tumors. Consistent with the microvessel density findings, VEGF and bFGF staining was prominent in the brain metastatic lesions, but not in lesions formed by SOCS-1–transfected cells (Supplementary Fig. S3). The expression of VEGF RNA and protein in SOCS-1–transfected A375Br and TXM-18 cells was also significantly decreased compared with control cells (Fig. 5B and C). Moreover, the promoter activity of VEGF in SOCS-1–transfected TXM-18 cells was significantly decreased compared with control cells (Supplementary Fig. S4D). Conversely, expression of VEGF in SOCS-1-siRNA–transfected A375P cells was increased at both RNA and protein levels (Fig. 5B and C). Similar findings were noted for bFGF expression, i.e., increased SOCS-1 expression repressed bFGF expression, whereas knockdown of SOCS-1 expression did the opposite (Supplementary Fig. S6). Collectively, these findings suggest that SOCS-1 inhibits angiogenesis partly through the inhibition of VEGF and bFGF expression.
Discussion
In the present study, we found that SOCS-1 expression was reduced in the brain metastatic melanoma cell line A375Br compared with the original A375P cell line. Likewise, SOCS-1 expression was lower in specimens of human brain metastases than in primary melanoma specimens. The decreased SOCS-1 expression was related to increased JAK2 expression in A375Br cells. Moreover, in an animal model, increased SOCS-1 expression suppressed brain metastasis of human melanoma cells. Increased SOCS-1 expression inhibited the activation of Stat3 in vitro and in vivo, whereas depletion of SOCS-1 increased the activation of Stat3. Furthermore, modifying SOCS-1 expression levels significantly affected the in vitro and in vivo expression of VEGF, bFGF, and MMP-2 and affected melanoma angiogenesis and invasion. To the best of our knowledge, our findings provide the first direct evidence that SOCS-1 has an inhibitory role in the brain metastasis of human melanoma.
A previous report from Li and colleagues indicated that SOCS-1 immunoreactivity was absent or undetectable in the epidermis of normal skin but that primary cutaneous melanomas at different stages expressed variable degrees of SOCS-1 immunoreactivity, with ~30% of primary cutaneous melanomas being negative for SOCS-1 staining regardless of stage (23). These results are consistent with our findings here. However, the previous report also indicated that metastatic melanomas express high levels of SOCS-1, whereas we found that brain metastatic melanomas expressed low levels of SOCS-1. The discrepancy in these results may reflect differences in the sample sizes or the organs assessed. Indeed, the study reported by Li and colleagues included only two cases of distant metastasis (lymph nodes) and nine cases of local metastases (23). Li and colleagues speculated that increased SOCS-1 expression in melanoma cells may either directly promote cell proliferation or render tumor cells insensitive to IFN released by the surrounding tissue, because SOCS-1 can inhibit Stat1. To clarify this issue, we examined the tumor growth and metastatic ability of melanoma cells that overexpressed SOCS-1. We observed that SOCS-1 expression slightly inhibited subcutaneous tumor growth but significantly inhibited brain metastasis of the melanoma cells. These results indicated that SOCS-1 expression in melanoma cells does not promote cell growth in vivo. However, because Stat1 activity was not detected in this experimental system, our results do not indicate that SOCS-1 modulated the in vivo growth by inhibiting Stat1.
We previously showed that activation of Stat3 in human melanoma promotes brain metastasis in nude mice (7). Stat3 is activated by numerous cytokines, growth factors, and oncogenic proteins through activation of JAK2 (33, 34). Conversely, Stat3 activation is negatively regulated by several proteins, including SOCS-1. The findings from our current study showed that less SOCS-1 was expressed in brain metastatic cells than in primary melanoma cells. Moreover, JAK2, which activates Stat3, is targeted for ubiquitination by SOCS-1 and showed increased expression in brain metastatic cells, presumably due to decreased degradation. Furthermore, inhibition of SOCS-1 expression in melanoma cells increased Stat3 activation, whereas enforced SOCS-1 expression in brain metastatic cells inhibited the activation of Stat3. Therefore, increased Stat3 activity in melanoma metastases might be due, at least in part, to decreased SOCS-1 expression.
Increased SOCS-1 expression has been shown to inhibit tumor cell growth induced by receptors for IL-6 and epidermal growth factor by suppressing Stat3 activation (19, 22, 35). However, we found that the growth of SOCS-1–transfected A375Br cells in vitro was no different from that of the A375Br or control vector–transfected cells. This lack of a growth effect could be because the A375Br cells do not require Stat3 activation for growth in vitro. Indeed, our previous study showed that inhibiting Stat3 activation in A375Br cells with a Stat3 dominant-negative construct did not affect growth in vitro (7). Here, we showed that modifying SOCS-1 expression significantly affected expression of VEGF, bFGF, and MMP-2 in vitro and in vivo and affected melanoma angiogenesis and invasion. Therefore, the mechanisms by which SOCS-1 inhibits brain metastases may be attributed, at least in part, to the down-regulation of angiogenesis and invasion. Also, these results are consistent with the previous findings that Stat3 signaling affects the expression of a variety of genes that are critical to invasion and angiogenesis (36–38), including MMPs and VEGF. Because angiogenesis and invasion processes are not unique to brain metastasis, SOCS-1 may not be an organ-specific regulator of metastatic potential. However, that would not downplay the importance of SOCS-1 in brain metastasis, because clinical observations suggest that many patients with melanoma metastatic to the brain also have metastases in the lungs and other organs and that some brain metastases are produced by cells populating lymph node or visceral metastases (i.e., metastasis of metastases; ref. 39).
The mechanisms responsible for the decreased expression of SOCS-1 in brain metastases are not known. SOCS-1 expression has been shown to be down-regulated because of promoter methylation and/or mutation of the SOCS-1 gene in several types of cancer, including hepatocellular carcinoma, multiple myeloma, and gastric, colorectal, ovarian, and breast carcinomas (19, 40–46). However, this does not seem to be the case for melanoma cells, because SOCS-1 transcripts were constitutively present in normal and transformed human melanocytes, whereas SOCS-1 protein was detectable only in melanoma cells (23). Interestingly, SOCS-1 expression may also be controlled by posttranscriptional mechanisms (35, 47). We are currently investigating whether aberrant SOCS-1 expression in melanoma brain metastases results from a change in the stability of the protein. Nonetheless, our present findings strongly suggest that SOCS-1 has an important role in brain metastasis through the regulation of multiple metastasis-associated factors and could be a potential therapeutic target for brain metastasis of melanoma. It would be of great interest to test the efficacy of a novel SOCS-1 mimetic tyrosine kinase inhibitor (22, 48) on brain metastasis of melanoma.
Supplementary Material
Acknowledgments
Grant support: American Cancer Society Research Scholar grant (S. Huang) and National Cancer Institute Cancer Center Support grant CA 16672.
We thank Dr. Douglas J. Hilton (The Walter and Eliza Hall Institute for Medical Research) for the SOCS-1 cDNA expression vector and Theresa Willis and Christine F. Wogan for the editorial comments.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst 1999;91:675–90. [DOI] [PubMed] [Google Scholar]
- 2.Rigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin 2000;50:215–36. [DOI] [PubMed] [Google Scholar]
- 3.Amer MH, Al-Sarraf M, Vaitkevicius VK. Clinical presentation, natural history and prognostic factors in advanced malignant melanoma. Surg Gynecol Obstet 1979;149:687–92. [PubMed] [Google Scholar]
- 4.Sampson JH, Carter JH Jr., Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 1998;88:11–20. [DOI] [PubMed] [Google Scholar]
- 5.Wen PY, Loeffler JS. Management of brain metastases. Oncology (Huntingt) 1999;13:941–54. [PubMed] [Google Scholar]
- 6.Davey P Brain metastases. Curr Probl Cancer 1999;23: 59–98. [DOI] [PubMed] [Google Scholar]
- 7.Xie TX, Huang FJ, Aldape KD, et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res 2006;66:3188–96. [DOI] [PubMed] [Google Scholar]
- 8.Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature 1997; 387:917–21. [DOI] [PubMed] [Google Scholar]
- 9.Endo TA, Masuhara M, Yokouchi M, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 1997;387:921–4. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson SE, Willson TA, Farley A, et al. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J 1999;18:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander WS, Starr R, Fenner JE, et al. SOCS1 is a critical inhibitor of interferon γ signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 1999;98:597–608. [DOI] [PubMed] [Google Scholar]
- 12.Morita Y, Naka T, Kawazoe Y, et al. Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor α-induced cell death in fibroblasts. Proc Natl Acad Sci U S A 2000; 97:5405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sporri B, Kovanen PE, Sasaki A, Yoshimura A, Leonard WJ. JAB/SOCS1/SSI-1 is an interleukin-2-induced inhibitor of IL-2 signaling. Blood 2001;97:221–6. [DOI] [PubMed] [Google Scholar]
- 14.Song MM, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem 1998;273:35056–62. [DOI] [PubMed] [Google Scholar]
- 15.Yasukawa H, Misawa H, Sakamoto H, et al. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J 1999;18:1309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JG, Farley A, Nicholson SE, et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci U S A 1999;96:2071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frantsve J, Schwaller J, Sternberg DW, Kutok J, Gilliland DG. Socs-1 inhibits TEL-JAK2-mediated transformation of hematopoietic cells through inhibition of JAK2 kinase activity and induction of proteasome-mediated degradation. Mol Cell Biol 2001;21:3547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamizono S, Hanada T, Yasukawa H, et al. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem 2001;276:12530–8. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa H, Matsubara K, Qian GS, et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity.[see comment]. Nat Genet 2001;28:29–35. [DOI] [PubMed] [Google Scholar]
- 20.Rottapel R, Ilangumaran S, Neale C, et al. The tumor suppressor activity of SOCS-1. Oncogene 2002;21:4351–62. [DOI] [PubMed] [Google Scholar]
- 21.Lee TL, Yeh J, Van Waes C, Chen Z. Epigenetic modification of SOCS-1 differentially regulates STAT3 activation in response to interleukin-6 receptor and epidermal growth factor receptor signaling through JAK and/or MEK in head and neck squamous cell carcinomas. Mol Cancer Ther 2006;5:8–19. [DOI] [PubMed] [Google Scholar]
- 22.Flowers LO, Subramaniam PS, Johnson HM. A SOCS-1 peptide mimetic inhibits both constitutive and IL-6 induced activation of STAT3 in prostate cancer cells. Oncogene 2005;24:2114–20. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Metze D, Nashan D, et al. Expression of SOCS-1, suppressor of cytokine signalling-1, in human melanoma. J Invest Dermatol 2004;123:737–45. [DOI] [PubMed] [Google Scholar]
- 24.Huang S, Bucana CD, Van Arsdall M, Fidler IJ. Stat1 negatively regulates angiogenesis, tumorigenicity and metastasis of tumor cells. Oncogene 2002;21:2504–12. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-κB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res 2000;6:2573–81. [PubMed] [Google Scholar]
- 26.Gong W, Wang L, Yao JC, et al. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res 2005;11:1386–93. [DOI] [PubMed] [Google Scholar]
- 27.Schackert G, Fidler IJ. Development of in vivo models for studies of brain metastasis. Int J Cancer 1988;41:589–94. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, Jean D, Luca M, Tainsky MA, Bar-Eli M. Loss of AP-2 results in down-regulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. EMBO J 1998;17:4358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumour cells and endothelial cells: the “angiogenesis progression” hypothesis. Eur J Cancer 1996;32A:2438–50. [DOI] [PubMed] [Google Scholar]
- 30.Singh RK, Gutman M, Radinsky R. Heterogeneity of cytokine and growth factor gene expression in human melanoma cells with different metastatic potentials. J Interferon Cytokine Res 1995;15:81–7. [DOI] [PubMed] [Google Scholar]
- 31.Amino N, Ideyama Y, Yamano M, et al. YM-201627: an orally active antitumor agent with selective inhibition of vascular endothelial cell proliferation. Cancer Lett 2006; 238:119–27. [DOI] [PubMed] [Google Scholar]
- 32.Sola F, Farao M, Ciomei M, Pastori A, Mongelli N, Grandi M. FCE 27266, a sulfonic distamycin derivative, inhibits experimental and spontaneous lung and liver metastasis. Invasion Metastasis 1995;15:222–31. [PubMed] [Google Scholar]
- 33.Bromberg J Stat proteins and oncogenesis. J Clin Invest 2002;109:1139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H, Jove R. The STATs of cancer-new molecular targets come of age. Nat Rev Cancer 2004;4:97–105. [DOI] [PubMed] [Google Scholar]
- 35.Narazaki M, Fujimoto M, Matsumoto T, et al. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc Natl Acad Sci U S A 1998;95:13130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dechow TN, Pedranzini L, Leitch A, et al. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci U S A 2004;101:10602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002;21:2000–8. [DOI] [PubMed] [Google Scholar]
- 38.Xie TX, Wei D, Liu M, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 2004;23:3550–60. [DOI] [PubMed] [Google Scholar]
- 39.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg 1978;135:807–10. [DOI] [PubMed] [Google Scholar]
- 40.Fukushima N, Sato N, Sahin F, Su GH, Hruban RH, Goggins M. Aberrant methylation of suppressor of cytokine signalling-1 (SOCS-1) gene in pancreatic ductal neoplasms. Br J Cancer 2003;89:338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagai H, Naka T, Terada Y, et al. Hypermethylation associated with inactivation of the SOCS-1 gene, a JAK/STAT inhibitor, in human hepatoblastomas. J Hum Genet 2003;48:65–9. [DOI] [PubMed] [Google Scholar]
- 42.Fujitake S, Hibi K, Okochi O, et al. Aberrant methylation of SOCS-1 was observed in younger colorectal cancer patients. J Gastroenterol 2004;39: 120–4. [DOI] [PubMed] [Google Scholar]
- 43.Oshimo Y, Kuraoka K, Nakayama H, et al. Epigenetic inactivation of SOCS-1 by CpG island hypermethylation in human gastric carcinoma. Int J Cancer 2004;112: 1003–9. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland KD, Lindeman GJ, Choong DY, et al. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene 2004;23:7726–33. [DOI] [PubMed] [Google Scholar]
- 45.Galm O, Yoshikawa H, Esteller M, Osieka R, Herman JG. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood 2003;101:2784–8. [DOI] [PubMed] [Google Scholar]
- 46.Weniger MA, Melzner I, Menz CK, et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene 2006;25:2679–84. [DOI] [PubMed] [Google Scholar]
- 47.Kamura T, Sato S, Haque D, et al. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev 1998;12:3872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flowers LO, Johnson HM, Mujtaba MG, Ellis MR, Haider SM, Subramaniam PS. Characterization of a peptide inhibitor of Janus kinase 2 that mimics suppressor of cytokine signaling 1 function. J Immunol 2004;172:7510–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.