Abstract
Aim:
To assess the feasibility of a patient-centered complex intervention for multimorbidity (CIM) based on general practice in collaboration with community health-care centers and outpatient clinics.
Methods:
Inclusion criteria were age ≥18 years, diagnoses of two or more of three chronic conditions (diabetes, chronic obstructive pulmonary disease (COPD), and chronic heart conditions), and a hospital contact during the previous year. The CIM included extended consultations and nurse care manager support in general practice and intensified cross-sectorial collaboration. Elements included a structured care plan based on patients’ care goals, coordination of services, and, if appropriate, shifting outpatient clinic visits to general practice, medication review, referral to rehabilitation, and home care. The acceptability dimension of feasibility was assessed with validated questionnaires, observations, and focus groups.
Results:
Forty-eight patients were included (mean age 72.2 (standard deviation (SD) 9.5, range 52–89); 23 (48%) were men. Thirty-seven patients had two diseases; most commonly COPD and cardiovascular disease (46%), followed by diabetes and cardiovascular disease (23%), and COPD and diabetes (15%). Eleven (23%) patients had all three conditions. Focus group interviews with patients with multimorbidity identified three main themes: (1) lack of care coordination existed across health-care sectors before the CIM, (2) extended consultations provided better care coordination, and (3) patients want to be involved in planning their treatment and care. In focus groups, health-care professionals discussed two main themes: (1) patient-centered care and (2) culture and organizational change. Completion rates for questionnaires were 98% (47/48).
Conclusions:
Patients and health-care professionals found the CIM acceptable.
Keywords: Multimorbidity, primary care, feasibility study, extended consultations, cross-sectional collaboration, integrated care, health-care organization, patient involvement, medicine assessment
Introduction
Multimorbidity refers to the coexistence of two or more chronic conditions in the same individual.1 In the Capital Region of Denmark, 22% of individuals aged 16 years and older have multimorbidity.2 The prevalence increases to 25% among adults aged 45–64 years and 60% among those older than 65 years.2 The prevalence of multimorbidity is expected to rise due to increasing life expectancy and improving health-care technologies.3 Multimorbidity is associated with decreased functional capacity, reduced quality of life, and increased mortality.4,5 Also linked to high care utilization and decreased productivity, multimorbidity is costly for health-care systems and society.6,7 A primary challenge in managing multimorbidity is care fragmentation; patients often need services from many providers across health-care sectors.8 For example, patients with multimorbidity in Denmark often receive care from both a general practitioner (GP) and one or more specialists at hospital outpatient clinics and community health-care centers. The Danish Healthcare System is a publicly funded health-care system comparable to health-care systems in other Scandinavian countries and the UK.9 Patients receive care in the primary care sector and might be referred to community health centers for rehabilitation and specialist care in hospital outpatient clinics.10 Patients with chronic conditions routinely receive a yearly consultation at the general practice.11
Patient-centered integrated models of care for patients with multimorbidity have been proposed, including the Sustainable Integrated Care Models For Multi-Morbidity and the World Health Organization integrated patient-centered care model.12–14 Although a recent literature review revealed difficulties in improving outcomes among patients with multimorbidity, interventions in primary care and community settings targeting specific risk factors, such as functional capacity and depression, can improve mortality and depression.12
Based on evidence about improving multimorbidity care management,2,9,14–16 we developed a complex intervention for multimorbidity (CIM) supporting patient-centered integrated care with GPs as primary providers. The CIM focuses on identifying patients with high service needs, extending consultations in general practice, coordinating care, and improving collaboration between general practice, the community health-care center, and the outpatient clinics at the hospital. The study aim is to assess the feasibility of the CIM.
Methods
CIM development
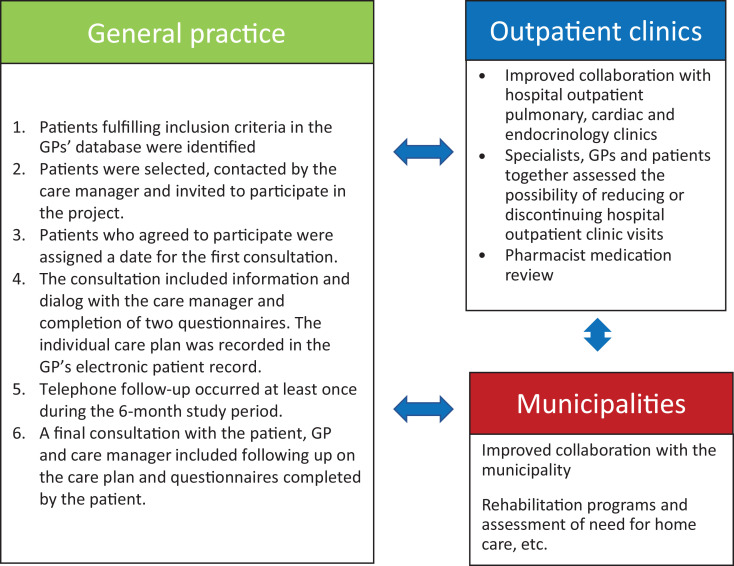
GPs and nurses from general practice, health-care professionals from two community health-care centers (nurses, physiotherapists, occupational therapists, and dieticians), and specialists and nurses from hospital settings participated in developing and testing the CIM (Figure 1). Evidence from literature reviews17,18 and our studies of patients’ experience of care 9,16,19 formed the basis for developing the CIM.
Figure 1.
The patient-centered CIM. CIM: complex intervention for multimorbidity.
It includes an extended GP consultation focusing on patients’ quality of life and goals for care and partnering with them to develop an individualized care plan. GP consultations were extended from a baseline of 10–15 min to a maximum of 60 min. The GP, a nurse care manager (a registered nurse with 3.5 years of education after high school), the patient with multimorbidity and, often, a family member collaboratively developed a care plan using motivational interviewing techniques.20 The care plan included (1) the patient’s chronic conditions, (2) the patient’s care goals, (3) a coordinated care plan with telephone follow-up and future appointments, (4) a plan for medication review in selected patients, (5) potentially shifting hospital outpatient clinic visits to general practice, and (6) referral to community-based rehabilitation and, if needed, home care. The coordinated care plan was shared electronically with the community health center and hospital outpatient clinics. Each extended GP consultation was reimbursed at 150 USD.
The nurse care manager coordinated activities in primary and secondary health-care sectors to support integrated care. All planned activities took place within a 6-month intervention period that ended with a second extended GP consultation focusing on whether the patients’ care goals were fulfilled (Supplementary data S1).
Study setting, participants, and design
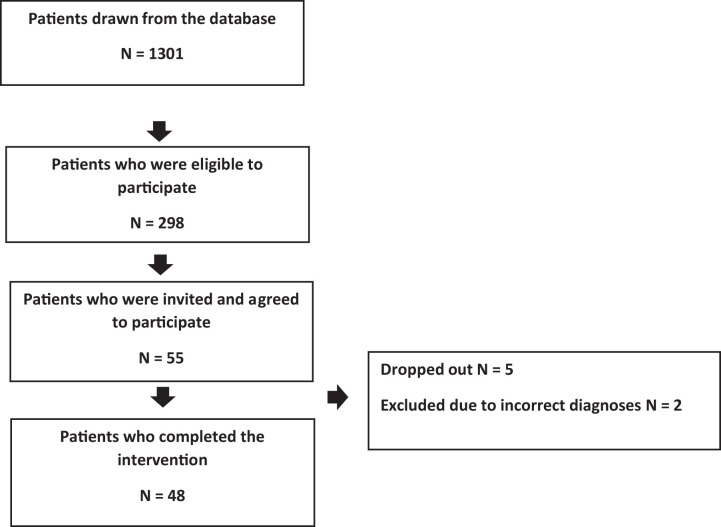
The CIM was implemented in a large general practice in Copenhagen with 9000 registered individuals; it employed five GPs, two registered nurses, and two secretaries. The general practice database was screened for patients meeting inclusion criteria: (1) age ≥18 years; (2) at least two of three conditions: diabetes (International Classification of Primary Care (ICPC) code T90), chronic obstructive pulmonary disease (COPD, ICPC code R95), and cardiovascular disease (ICPC codes K72-K80 and K90-K92); and (3) a hospitalization or visit to an outpatient clinic in the previous year. Patients were consecutively selected until 50 were enrolled (Figure 2). Patients were excluded from the study if they were unable to understand or speak Danish or too ill to complete the questionnaires. All participants received verbal and written information about the study.
Figure 2.
Flow chart.
A 2-h training program was provided for GPs and their personnel, community health center health-care professionals, and health-care professionals in the outpatient clinics. It included a detailed overview of project activities, characteristics of multimorbidity, health challenges of patients with multimorbidity, and polypharmacy assessment. Roles and clinical responsibilities across the three sectors were discussed.
Assessing feasibility of the CIM
Our study was conducted to establish whether a full trial would be feasible and if improvements to the CIM were needed.21 We focused on acceptability and integration of the CIM with the existing system of care, assessing whether it was accepted by patients and health-care professionals and could be implemented in general practice, community health-care centers, and outpatient clinics at Bispebjerg–Frederiksberg University Hospital. A mixed-methods approach was used.
Focus group interviews
Focus group interviews were conducted separately with participating patients and with health-care professionals, using semi-structured interview guides. AJ conducted two 90-min focus group interviews with three women and three men with multimorbidity aged 56–86 years to explore their experiences of care before and after CIM implementation. JL conducted two 60-min focus group interviews with health-care professionals at the end of the study. One group included four GPs and explored their experience of using the CIM. The other focus group included a GP, three hospital specialists, and one health-care professional from the community health-care center and explored cross-sectoral collaboration. Interviews were audio-recorded and supplemented by field notes from the interviewers and then transcribed verbatim (Supplementary data S2).
Observation of consultations in GP offices
We observed extended consultations in general practice to explore how GPs, care managers, and patients accepted and structured them. Consultations with seven patients (four men and three women aged 58–83 years) and five GPs and two care managers were observed and audio-recorded, transcribed verbatim, and supplemented with field notes taken during consultations.
Qualitative data analysis
Data from focus group interviews were analyzed using content analysis.22 Iterative reading was done to obtain a general impression of the data and to condense them and to identify meaning units sorted into codes and reconceptualized into main themes. Data from observations were analyzed using systematic text condensation to identify key elements and processes of consultations.23 All authors reached consensus on the findings.
Primary outcome measure
The Patient Assessment of Chronic Illness Care (PACIC) is a validated, 20-item questionnaire with 5 subscales assessing patient activation, decision support, goal setting, problem-solving, and coordination and follow-up. Response options range from 1 (never) to 5 (always).24 The total score is calculated as the mean score of all 20 items. The questionnaire has been validated in a Danish context.24 Its acceptability to patients was assessed by completion rates.
Secondary outcome measures
The EuroQol 5-Dimension (EQ-5D-3 L) is a validated 5-item questionnaire assessing generic health status, including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.25 An index score (TTO) is calculated by applying preference weights obtained from the Danish population.26 We also measured overall health with a visual analog and numerical scale,25 with scores ranging from 0 (As bad as I can imagine) to 100 (The best possible). Its acceptability to patients was assessed by completion rates.
Ethical approval
The Danish Data Protection Agency approved the study (j.nr.: 2012-58-0004). Preliminary assessment by the regional ethical committee concluded that it did not require a full formal ethical assessment (protocol nr.: H-17007945).
Results
Population characteristics
Forty-eight patients were included in the study. Their mean age was 72.2 (standard deviation (SD) 9.5, range 52–89) years; 23 (48%) were men (Table 1). Thirty-seven patients had two diseases; the most common disease combinations were COPD and cardiovascular disease (46%), diabetes and cardiovascular disease (23%), and COPD and diabetes (15%). Eleven (23%) patients had all conditions. Fifty-four percent of participants were referred to rehabilitation in the municipality, 29% were referred for a medication review, and 13% discontinued control visits to a hospital outpatient clinic. Seventy-nine percent of participants completed the second consultation.
Table 1.
Participant characteristics at baseline, mean (SD).
| Total, N = 48 | Men, N = 23 | Women, N = 25 | COPD + CVD, N = 22 | Diabetes + CVD, N = 11 | Diabetes + COPD, N = 4 | Diabetes + COPD + CVD, N = 11 | |
|---|---|---|---|---|---|---|---|
| Age, years | 72.2 (9.5) | 71.0 (9.2) | 73.3 (9.8) | 72.1 (8.9) | 77.6 (10.3) | 67.0 (10.9) | 68.9 (7.6) |
| Total PACIC score | 2.7 (0.7) | 2.6 (0.8) | 2.7 (0.7) | 2.6 (0.7) | 2.4 (0.8) | 3.3 (0.9) | 2.9 (0.6) |
| EQ-5D TTO | 0.6 (0.4) | 0.6 (0.3) | 0.7 (0.2) | 0.7 (0.2) | 0.5 (0.4) | 0.6 (0.3) | 0.6 (0.3) |
| EQ-VAS | 0.5 (0.2) | 0.6 (0.2) | 0.5 (0.2) | 0.6 (0.2) | 0.4 (0.2) | 0.5 (0.2) | 0.5 (0.2) |
SD: standard deviation; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; EQ-5D TTO, EuroQol 5-Dimension index score; EQ-VAS, EuroQol visual analog scale; PACIC, patient assessment of chronic illness care survey.
Focus group interviews with patients with multimorbidity
We identified three main themes in focus group interviews with patients: (1) lack of care coordination across health-care sectors existed before the CIM, (2) extended GP consultations provided better care coordination, and (3) patients want to be involved in planning their care.
Many patients dealt with a lack of care coordination across settings before the CIM. Due to the complexity of multimorbidity care, poor coordination can place them at risk for poor treatment outcomes and is exemplified by a comment from the 61-year-old wife of a patient with multimorbidity: “I think that it should be stated somewhere that here is a patient with several chronic conditions, that the health-care organisations should share information for.”
Patients also felt forced to take charge of care plans for their diseases: “I generally lack coordination of…my care. So, I feel it necessary to keep the overview myself.”. (JIL, 64 years old, with heart disease, post-polio syndrome, and diabetes).
Another negative effect of poor care coordination is a devaluation of patients’ priorities, needs, and wants. One patient explained: “Often, I hear ‘no, this is more important to you or you have to do this’. The worst case is when you are just met with ‘try and talk to another department about this’.” All informants agreed that extended consultations were beneficial, and most felt they improved care coordination. A daughter helped her 81-year-old mother recall the consultations: “You had the chance to discuss more issues, and you had been suggested to take this diabetes course at the healthcare center.” Patients perceived extra consultation time as positive when it was used to provide individualized care planning. As a 63-year-old man stated, “Then the GP has this piece of paper which she acts according to; she calls me and has this whole thing coordinated.” Individualized patient-centered consultations resonated with what patients requested.
The third theme reflects the finding that the most important issue for patients with multimorbidity was involvement in planning their care. All participants felt their unique knowledge about their diseases enabled them to contribute substantially to planning treatments and medications but described their individual preferences and values as not being systematically solicited. Unfortunately, participants did not provide information on how the CIM could be improved.
Focus group interviews with health-care professionals
We identified two themes in focus group interviews with health-care professionals: (1) patient-centered care and (2) culture and organizational change. The structure of the PACIC and EQ-5D-3 L consistently helped shift the focus of consultations from GPs to patients with multimorbidity, which enhanced patients’ awareness of the need for and motivation for lifestyle changes.
GPs experienced extended consultations as making it possible to learn more about patients’ lives and goals, supporting increased patient involvement. Health-care professionals learned that suffering from multimorbidity worried patients surprisingly little, compared to concerns about being able to perform activities of daily living.
The second theme of cultural and organizational change reflects our finding that extended consultations were necessary for GPs to better understand patients’ situations and prepare for subsequent consultations.
Before adopting the CIM, they lacked time to collaborate with outpatient clinics. Communication between sectors was also suboptimal. Extended consultations posed a logistical challenge for GPs, but, as one GP explained, the clear benefits were a motivating factor.
Something else that’s very central and that is the time. That it is extremely liberating to have a consultation where we may have a lot to catch up upon, but there is one hour allocated to this patient, we have the time to get into things, we have time to read the journal properly before the patient entering the door and we have time with the patient.
They had often experienced cross-sectoral and hospital collaboration as difficult for patients, and cross-sectoral meetings were mentioned as an opportunity to ensure better care plans and align health services. A GP described his concerns,
In the project, we could elucidate that it is possible to find ways to make things a bit smarter for the patients and for each other. In that way, we can work more efficiently without wasting a lot of time with repetitions and patients who do not understand the fragmented cross-sectoral health care system. I’m very concerned about that.
In addition, there was a lack of clarity about how to communicate; GPs perceived that patients expected information to be shared across sectors but often needed to repeat their stories due to poor data sharing, “I want to say that from our point of view, the more information we get from them about the patients, the better we can also start because the patients have the expectation that we are actually talking together.”
Health-care professionals viewed the use of an individual care plan as supporting cross-sectoral communication and data sharing.
Observation of consultations
We observed seven consistent elements of consultations based both on the points needed for the written action plan, and elements of traditional GP consultations. Four elements were required to complete the written care plan: completing questionnaires, care planning and treatments, proposing changes to care in other sectors, such as attending rehabilitation and reducing visits to hospital outpatient clinics, and reviewing medications. These elements served to structure the consultation. The written care plan helped the GP and the patient to comprehend the extended consultation as different than the traditional consultation. Besides helping to maintain an organized structure, the completed questionnaire initiated important topics such as changes in lifestyle. Also, the duration of the consultation was important when it came to initiate the talk about lifestyle changes. Finally, it was important for the GP to keep the new consultation and its purpose separated from other types of consultations to keep focus.
Three elements were like usual consultations: an introduction to the consultation, which differed from traditional consultations where the patient is encouraged to present health problems, collecting data from patients, and referring patients’ disease-specific questions to the yearly checkups. Despite the GPs explanation about the specific purpose and framing of the present consultation, patients asked for test results and other information regarding specific illnesses usually provided in the traditional yearly checkups of chronic disease.
Acceptability of questionnaires
Both questionnaires had completion rates of 98% (47/48). Mean (SD) total PACIC score was 2.7 (0.7) (Table 1). Mean (SD) EQ-5D-3 L index and health status scale scores were 0.63 (0.28) and 59.4 (19.5), respectively, for all participants.
Discussion
Our feasibility study in a large general practice revealed that longer consultations, use of a care manager, and development of individual care plans were valuable elements of the CIM. The use of the questionnaires was not intended to be a part of the intervention; however, the use turned out to be beneficial for both patients and health professionals regarding providing a structure of the consultations and talking about lifestyle changes in the consultations.
Feasibility of the CIM
Varying definitions of multimorbidity across studies make direct comparisons with our findings difficult, but previous reports provide context for our findings. Key themes from patient focus group interviews are consistent with findings from other studies.8,14,27 An overview of systematic reviews found that patients with multimorbidity seldom participate in clinical decision-making.28 A qualitative study investigating the involvement of patients with multimorbidity in service planning concluded that a need exists to reorganize care delivery to support care coordination, putting patient preferences at the center.16
Health-care professionals experienced the CIM as increasing patient-centeredness of care. Extended consultations allowed them to give patients more guidance, support, and information and to spend more time listening to them, which led to a better understanding of patients’ individual situations. In other studies, taking additional time, involving patients, and improving information sharing were also important factors in care for patients with multimorbidity.8,29,30
A complex intervention for multimorbidity
The development of the CIM is consistent with the literature suggesting the use of a systematic process,31 a framework when designing a model of care or planning a randomized controlled trial,32–34 the template for intervention description and replication checklist to provide transparency in the intervention process,35 and a guideline when reporting a feasibility study.36
Our findings support the importance of care managers in goal setting and care planning for patients with multimorbidity. They also reduced GP workloads. Communication of patient information across health-care sectors was insufficient, and health-care professionals were unsure about what information to communicate. Information technology systems varied across health-care sectors, reducing options for sharing patient information.
It is unclear how to reduce inappropriate use of polypharmacy and its consequences among patients with multimorbidity. A German study found no effect of medication review in general practice on quality of life or functional status of patients with multimorbidity.37 A systematic review concluded that the effectiveness of interventions targeting polypharmacy was uncertain.38 In our study, a pharmacist performed medication reviews for 25% of participants. This may indicate that many participants’ medications were appropriate but may also arise from our small sample size.
Few patients in our study wanted to transfer their hospital outpatient visits to general practice. Notably, 54% of study participants were referred to municipal rehabilitation. The CIM may increase awareness of the need to refer patients to rehabilitation. However, data on rehabilitation rates in the primary care sector are sparse.39 Factors influencing GP referrals include previous successful referral of other patients and awareness and accessibility of rehabilitation referral programs and procedures.40
Health-care professionals perceived insufficient time and economic incentives as barriers to making organizational changes. They experienced CIM as more patient-centered and supportive of integrated care than usual care, and it could be more profitable over the long run. Other barriers include low levels of organizational readiness for change.
Feasibility of questionnaires
The purpose of assessing the PACIC and EQ-5D-3 L questionnaires as primary and secondary outcome measures, respectively, was to measure the feasibility of using the two questionnaires in patients with multimorbidity and to compare the scoring with other similar studies. Our observed baseline mean total PACIC score was higher than the overall score in a study among slightly older patients with multimorbidity.41 Variations in PACIC scores across studies are difficult to assess due to discrepancies in patient characteristics, health-care systems, and sample sizes.
Mean (SD) EQ-5D-3 L TTO and health status scale scores were consistent with findings from a randomized controlled trial among patients with both heart disease and diabetes.42 Unfortunately, the data collection at 6 months did not occur as scheduled. Consequently, we had only baseline measurements. The feasibility of the questionnaires will be further tested in a pilot study before performing a cluster randomized controlled trial.
Strengths and limitations
Strengths of our study include the development of an evidence-based CIM. Focus group interviews support a dynamic and creative dialogue that was ideal for discussing patients’ and health-care professionals’ experiences. Observations allowed us to understand the structure of extended consultations. Our study population had disease patterns similar to those identified in the Capital Region,16 indicating that our sample was suitable for testing the feasibility of CIM. Despite careful planning, questionnaire completion rates at follow-up visits were inadequate, precluding comparison of baseline and follow-up PACIC, and health status data. Similarly, an administrative error at the general practice precluded tracking participant flow.
Conclusions
The CIM was feasible and could be integrated with existing care systems. It should be refined and tested in a pilot study before performing a cluster randomized controlled trial. Patient identification should be improved to ensure that the right patients receive the time-consuming and costly services. Collaboration between sectors could be more effective and may decrease the number of hospital outpatient visits. Developing feasible organizational innovations in existing health-care systems requires improved collaboration and sharing of patient information across sectors.
Supplemental material
Supplement_1_and_2_9_April_2020jg for A complex intervention for multimorbidity in primary care: A feasibility study by Hanne Birke, Ramune Jacobsen, Alexandra BR Jønsson, Ann Dorrit Kristiane Guassora, Marie Walther, Thomas Saxild, Jannie T Laursen, Maria Helena Dominquez Vall-Lamora and Anne Frølich in Journal of Comorbidity
Footnotes
Implications: The CIM offers a feasible new approach for optimizing the management of patients with multimorbidity but further study is needed.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Hanne Birke  https://orcid.org/0000-0001-5923-2170
https://orcid.org/0000-0001-5923-2170
Maria Helena Dominquez Vall-Lamora  https://orcid.org/0000-0002-7089-2636
https://orcid.org/0000-0002-7089-2636
Supplemental material: Supplemental material for this article is available online.
References
- 1. Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet 2012; 380: 7–9. [DOI] [PubMed] [Google Scholar]
- 2. Schiøtz ML, Stockmarr A, Høst D, et al. Social disparities in the prevalence of multimorbidity—a register-based population study. BMC Public Health 2017; 17: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bähler C, Huber CA, Brüngger B, et al. Multimorbidity, health care utilization and costs in an elderly community-dwelling population: a claims data based observational study. BMC Health Serv Res 2015; 15: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA—J Am Med Assoc 2015; 314: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palladino R, Lee JT, Ashworth M, et al. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing 2016; 45: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagl A, Witte J, Hodek JM, et al. Relationship between multimorbidity and direct healthcare costs in an advanced elderly population. Results of the PRISCUS trial. Z Gerontol Geriatr 2012; 45: 146–154. [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Cocker F, Kilpatrick M, et al. The associations of multimorbidity with health-related productivity loss in a large and diverse public sector setting. J Occup Environ Med 2018; 60: 528–535. [DOI] [PubMed] [Google Scholar]
- 8. Salisbury C, Man MS, Bower P, et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet 2018; 392: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schiøtz ML, Høst D, Christensen MB, et al. Quality of care for people with multimorbidity—a case series. BMC Health Serv Res 2017; 17: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vrangbaek K. The Danish health care system. Int Heal Profiles Commonw Fund, https://international.commonwealthfund.org/countries/denmark/ (2017, accessed 17 May 2017).
- 11. Frølich A, Jacobsen R, Knai C. Assessing chronic disease management in European health systems: country reports In: Nolte E, Knai C. (eds) European observatory on health systems and Policies. Copenhagen: World Health Organization, 2015, pp. 1–15. [PubMed] [Google Scholar]
- 12. Smith SM, Wallace E, Tom OD, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016; 3: 1–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leijten FRM, Struckmann V, van Ginneken E, et al. The SELFIE framework for integrated care for multi-morbidity: development and description. Health Policy 2018; 122: 12–22. [DOI] [PubMed] [Google Scholar]
- 14. Mercer S, Furler J, Moffat K, et al. Multimorbidity: technical series on safer primary care. Geneva, https://apps.who.int/iris/bitstream/handle/10665/252275/9789241511650-eng.pdf;jsessionid=0E421BBAD6C4DEB6C7246817311CB219?sequence=1 (2016, accessed 11 May 2016). [Google Scholar]
- 15. Smith SM, Bayliss EA, Mercer SW, et al. How to design and evaluate interventions to improve outcomes for patients with multimorbidity. J Comorbidity 2013; 3: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schiøtz M, Høst D, Frølich A. Involving patients with multimorbidity in service planning: perspectives on continuity and care coordination. J Comorbidity 2016; 6: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith SM, Wallace E, O’Dowd T, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016; 3: CD006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith SM, Soubhi H, Fortin M, et al. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ 2012; 345: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiøtz ML, Frølich A, Jensen AK, et al. Polypharmacy and medication deprescribing: a survey among multimorbid older adults in Denmark. Pharmacol Res Perspect 2018; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Resnicow K, McMaster F. Motivational interviewing: moving from why to how with autonomy support. Int J Behav Nutr Phys Act 2012; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abbott JH. The distinction between randomized clinical trials (RCTS) and preliminary feasibility and pilot studies: what they are and are not. J Orthop Sports Phys Ther 2014; 44: 555–558. [DOI] [PubMed] [Google Scholar]
- 22. Graneheim UH, Lundman B. Qualitative content analysis in nursing research : concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today 2004; 24: 105–112. [DOI] [PubMed] [Google Scholar]
- 23. Malterud K. Kvalitative metoder i medicinsk forskning: en innføring In Norwegian. 3rd ed Oslo: Universitetsforlaget, 2011. [Google Scholar]
- 24. Maindal HT, Sokolowski I, Vedsted P. Adaptation, data quality and confirmatory factor analysis of the Danish version of the PACIC questionnaire. Eur J Public Health 2012; 22: 31–36. [DOI] [PubMed] [Google Scholar]
- 25. Van Reenen M, Janssen B. EQ-5D-3L User Guide, EuroQol Research Foundation. https://euroqol.org/publications/user-guides (accessed December 2018). [Google Scholar]
- 26. Sørensen J, Davidsen M, Gudex C, et al. Danish EQ-5D population norms. Scand J Public Health 2009; 37: 467–474. [DOI] [PubMed] [Google Scholar]
- 27. Mercer SW, Fitzpatrick B, Guthrie B, et al. The CARE Plus study—a whole-system intervention to improve quality of life of primary care patients with multimorbidity in areas of high socioeconomic deprivation: exploratory cluster randomised controlled trial and cost-utility analysis. BMC Med 2016; 14: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu X, Mishra GD, Jones M. Evidence on multimorbidity from definition to intervention: an overview of systematic reviews. Ageing Res Rev 2017; 37: 53–68. [DOI] [PubMed] [Google Scholar]
- 29. Fried TR, Tinetti ME, Iannone L. Primary care clinicians’ experiences with treatment decision-making for older persons with multiple conditions. Arch Intern Med 2011; 171: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bayliss EA, Edwards AE, Steiner JF, et al. Processes of care desired by elderly patients with multimorbidities. Fam Pract 2008; 25: 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davidson P, Halcomb E, Hickman L, et al. Beyond the rhetoric : what do we mean by a ‘Model of Care’?. Aust J Adv Nurs 2006; 23: 47–55. [PubMed] [Google Scholar]
- 32. Stokes J, Man MS, Guthrie B, et al. The foundations framework for developing and reporting new models of care for multimorbidity. Ann Fam Med 2017; 15: 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical research council guidance. BMJ 2008; 337: 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eldridge SM, Lancaster GA, Campbell MJ, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One 2016; 11: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: 1–12. [DOI] [PubMed] [Google Scholar]
- 36. Lancaster GA, Thabane L. Guidelines for reporting non-randomised pilot and feasibility studies. Pilot Feasibility Stud 2019; 5: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muth C, Uhlmann L, Haefeli WE, et al. Effectiveness of a complex intervention on prioritising multimedication in multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open 2018; 8: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rankin A, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. Epub ahead of print 2018. DOI: 10.1002/14651858.CD008165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kommunernes landsforening. Almen praksis henvisning til kommunale forebyggelsestilbud (2014–2017). In Danish, https://www.kl.dk/media/14640/a2fnivq8zzeoqjc2ygbc.pdf (accessed 21 August 2018).
- 40. Cox NS, Oliveira CC, Lahham A, et al. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the theoretical domains framework. J Physiother 2017; 63: 84–93. [DOI] [PubMed] [Google Scholar]
- 41. Petersen JJ, Paulitsch MA, Mergenthal K, et al. Implementation of chronic illness care in German primary care practices—how do multimorbid older patients view routine care? A cross-sectional study using multilevel hierarchical modeling. BMC Health Serv Res 2014; 14: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dunbar SB, Reilly CM, Gary R, et al. Randomized clinical trial of an integrated self-care intervention for persons with heart failure and diabetes: quality of life and physical functioning outcomes. J Card Fail 2015; 21: 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement_1_and_2_9_April_2020jg for A complex intervention for multimorbidity in primary care: A feasibility study by Hanne Birke, Ramune Jacobsen, Alexandra BR Jønsson, Ann Dorrit Kristiane Guassora, Marie Walther, Thomas Saxild, Jannie T Laursen, Maria Helena Dominquez Vall-Lamora and Anne Frølich in Journal of Comorbidity