Abstract
Degenerative disease of the lumbar spine is often ignored as a potential cause of testicular pain because the exact link between the two remains uncertain. This article reports the case of a 60-year-old man with a 3-year history of low back pain and unexplained right testicular pain for 2 years. Painful symptoms were negatively affecting his social, physical, and sexual functions. After failure to achieve pain relief through multiple types of therapy, the patient sought chiropractic treatment for his condition. Lumbar spine magnetic resonance imaging (MRI) revealed disc protrusion at the L1/L2, L3/L4, and L4/L5 segments causing thecal sac indentations. Due to the absence of direct testicular causes, the medical impression was chronic testicular pain (CTP) complicating lumbar disc disease. The patient experienced regular improvement in his low back and testicular pain with complete resolution of both after 8 weeks of chiropractic treatment. This article describes an overlook of the etiology of this patient’s testicular pain and a successful option in treating the patient. CTP has multifactorial etiology. An excellent treatment outcome depends heavily on recognizing the origin of the pain.
Keywords: Back pain, chiropractic treatment, lumbar disc herniation, testicular pain
Chronic testicular pain (CTP) is a frustrating condition defined as constant or intermittent scrotal pain of at least 3 months duration that significantly interferes with daily activities. About 4.8% of men presenting at a urology clinic for other reasons also had CTP (Ciftci et al., 2010). The pathophysiology of CTP is multifactorial and poorly understood. There is no test to find the cause of testicular pain and the assessment is based on clinical criteria. Direct causes of CTP may include infection, tumor, testicular torsion, varicocele, hydrocele, spermatocele, and trauma. A variety of non-scrotal causes, such as ureteral stone, inguinal hernia, aortic aneurysms, and spinal disorders, can also result in referred pain to the scrotum. Patients complaining of CTP have often been evaluated by multiple providers from different disciplines but often are left with an unexplained etiology for their complaint. The most up-to-date guidelines on the diagnosis and management of CTP are based on expert opinion derived from small cohort studies (Tan & Levine, 2017). This study aims to provide a better picture of an overlooked cause, proposed pathophysiology, and relevant treatment options of testicular pain.
Case Report
A 60-year-old male security guard complained of low back pain for 3 years, along with right testicular pain for 2 years. He had recently experienced severe pain in the right lower back down to the right buttock. Lying supine, right lumbar lateral flexion or heavy lifting could trigger right buttock pain and episodic right testicular pain. The testicular pain was localized to his right hemiscrotum and was described as an aching sharp pain. Back and testicular pain negatively affected his social, physical, and sexual functions. Eventually, he was unable to sit up straight, get a good night’s sleep, or to continue working. The patient had no previous history of localized trauma or chronic systemic diseases. Initial workup by an urologist and an orthopedist found an insignificant right renal cyst, asymptomatic left varicocele, and degenerative lumbar spine disease. A scrotal ultrasound ruled out direct testicular pathologies. For nearly 2 years, he had received pain medication, fluoroscopy-guided lumbar facet joint injection, exercise rehabilitation, and acupuncture, all of which failed to provide substantial, lasting symptom relief. The patient then sought chiropractic care for his condition.
Upon presentation, the size, shape, and texture of his testicles were unremarkable and even bilaterally with unrestricted movement within the scrotal sac. Point tenderness was elicited by palpating at the T12/L1 and L4/L5 intervertebral spaces. A shock-like sensation (Lhermitte’s sign) could be induced by spinal percussion with a reflex hammer at the T12/L1 level. Mobility restrictions were present in the lumbar spine. Sharp pain from the right thoracolumbar area down to the right buttock and testicular pain were triggered with passive lumbar extension and right lateral lumbar flexion. A tight psoas muscle was speculated. Pinch–roll test of the skin revealed local hyperesthesia and tenderness over the right lumbar paraspinal region and right buttock. Lumbar spine magnetic resonance imaging (MRI; Figure 1) revealed disc desiccation (diminished signal intensity) and disc protrusion at the L1/L2, L3/L4, and L4/L5 levels, causing thecal sac indentation. In the absence of significant medical history and direct testicular causes, the subjective findings of this case were consistent with CTP caused by lumbar disc disease.
Figure 1.
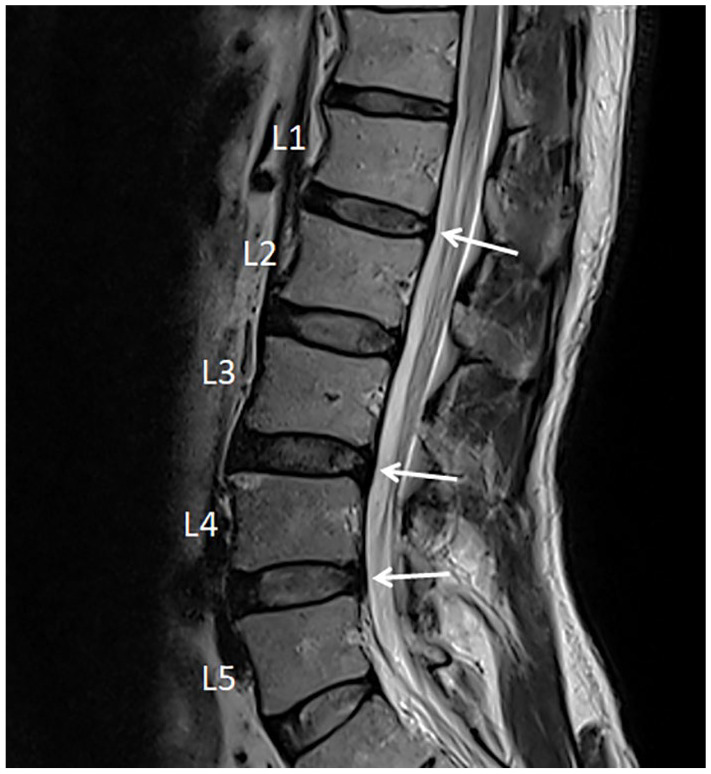
Sagittal T2 weighted magnetic resonance (MR) image revealed marginal osteophyte formation of the lumbar vertebrae and lumbar disc desiccation (low water content/diminished signal intensity) with posterior protrusion at the L1/L2, L3/L4, and L4/L5 levels causing thecal indentation.
The patient underwent chiropractic treatment consisting of therapeutic ultrasound and spinal manipulation with a high-velocity, low-amplitude force applied at the thoracolumbar junction daily for 6 days. Low back pain and subsequent sleep disturbance were reduced following 1 week of treatment. His pain score declined from 9/10 to 7/10 on the numeric rating scale (NRS-11). Subsequently, treatment sessions consisted of intermittent motorized traction (Spine Decompression Device, MID Series, WIZ Medical, Korea) focused on the T12/L1 segment, therapeutic ultrasound, and spinal manipulation. Frequency of treatments was reduced to twice weekly for the following 7 weeks. Both back and testicular pain diminished every week and were fully resolved near the end of treatment. Oswestry Disability Index (ODI) score decreased significantly (78% at intake, 6% at 8 weeks). He recovered and was able to resume normal daily activities with a sense of heightened well-being. A 12-month follow-up phone call confirmed that the patient did not have any delayed complications, nor did he experience a recurrence of his symptoms.
Discussion
The testicles develop embryologically in the upper abdomen and descend into the scrotum shortly before birth. On their descent, the testes bring their sympathetic nerve supply with them from T10 to L1 segments and parasympathetic nerve supply from the S2 to S4 segments (Patel, 2017; Quallich & Arslanian-Engoren, 2013). The somatic supply to the testicles and scrotum originates from the L1–L2 and S2–S4 nerve roots through the iliohypogastric, ilioinguinal, genitofemoral, and pudendal nerves (Patel, 2017). The genitofemoral nerve, formed by a union of branches from the L1 and L2, splits into the genital and femoral branches after passing through the psoas muscle (Figure 2). The genital branch provides sensation to the cremaster muscle as well as the hemiscrotum in men and labia in women. The psoas major inserts on the lumbar discs, on the vertebral bodies, and on the transverse processes of the T12 to L5. As the psoas adheres to the lateral aspect of the fibrous annulus of the intervertebral discs, compression or inflammatory irritation derived from the lumbar discs can agitate the psoas muscle.
Figure 2.
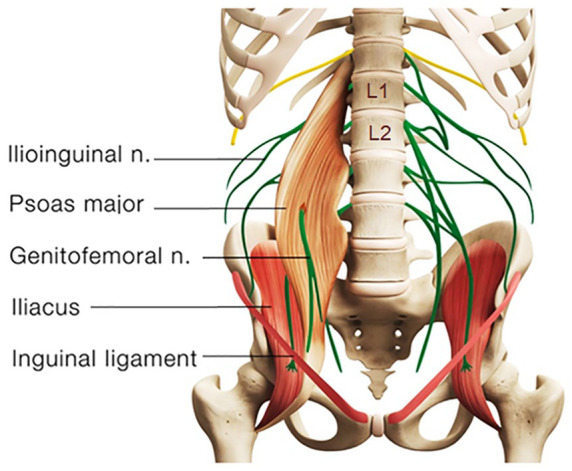
Schematic diagram showing anatomical relations of the lumbar spine, psoas muscle, and genitofemoral nerve. The genitofemoral nerve is formed from the L1 and L2 nerve roots and splits into the genital and femoral branches after passing through the psoas muscle. Nerve root impingement due to discitis and facet arthrosis could radiate pain into the ipsilateral testicle. A genitofemoral entrapment could also cause chronic testicular pain.
As shown in the present case, disc protrusion at the L1/L2, L3/L4, and L4/L5 levels was observed along with right psoas spasticity and right testicular pain. This patient had three potential pain sources. First, lumbar radiculopathy from encroachment or irritation of the L2 nerve root could radiate pain into the ipsilateral testicle (Patel, 2017). Second, discitis and/or facet arthrosis at other levels of the patient’s lumbar spine might also be capable of referring pain, transmitted nonsegmentally via the paravertebral sympathetic chain to the L1–L2 nerve roots and in the genitofemoral nerve to the testicle (Doubleday et al., 2003; Peng et al., 2014). Third, the genitofemoral nerve penetrates the psoas muscle before splitting into the genital and femoral branches (Figure 2). Genitofemoral entrapment at the spastic psoas might also be involved in the advent of testicular pain (Masarani & Cox, 2003). Many of these hypotheses, as in this case, remain unconfirmed.
The pathophysiology of testicular pain is multifactorial and poorly understood. Diagnostic and treatment recommendations are based on expert opinion derived from small cohort studies (Patel, 2017). The appropriate approach to a patient presenting with CTP is to first rule out the possibility of serious pathology or red flag symptoms. When imaging is deemed necessary, a high-resolution scrotal ultrasound with color-flow Doppler is the primary modality used to detect and diagnose abnormalities within the scrotum. There can often be straightforward explanations for the CTP such as varicocele, infection, tumor, or referred pain from non-scrotal sources, but more often the etiology remains unexplained (Quallich & Arslanian-Engoren, 2013). Because of the overlapping sensory innervations and anatomic variability, it is difficult to differentiate the particular nerve involvement in cases of testicular pain.
The first step in the management of CTP with no red flag signs remains conservative in nature. Consideration should include the use of antibiotics, nonsteroidal anti-inflammatory medications, pelvic floor physical therapy, or spermatic cord block. When conservative measures have failed, microsurgical testicular denervation and orchiectomy have been described as procedures of last resort. Unsuccessful recognition of the causative factors for CTP often leads to surgical failure, poor outcomes, and years of psychological distress (Doubleday et al., 2003). There is a scarcity of data on the association of CTP with degenerative lumbar spine disease. Case reports (Doubleday et al., 2003; Rowell & Rylander, 2012) revealed significant efficacy of manipulative therapies for alleviating testicular pain of spinal origin. According to the Clinical Guidelines for Diagnosis and Treatment of Lumbar Disc Herniation With Radiculopathy (Kreiner et al., 2014), spinal manipulation is an option for radiculopathy caused by discogenic disease. Remission of CTP observed in the present case appears to support that spinal manipulation could be a treatment regimen for testicular pain from this cause.
The exact mechanism underlying the action of chiropractic for the relief of discogenic pain remains unclear. Chiropractic trial in this case consisted of therapeutic ultrasound and spinal manipulation, along with intermittent motorized lumbar traction. Reported thermal effects of ultrasound upon tissue include raised tissue temperature, enhanced circulation, reduced muscle spasm, and increased extensibility of collagen fibers and a proinflammatory response (Speed, 2001). Biomechanical effects of chiropractic manipulation on symptom relief include relaxation of hypertonic musculature, release of entrapped nerves, disruption of periarticular adhesions, and restoration of spinal alignment (Onel et al., 1989). Lumbar traction is a form of decompression therapy, creating increased space and negative pressure of the intervertebral space. Intermittent mechanical traction can facilitate diffusion of nutrients for the disc and initiate the healing process. The main limitation of the present study is that the cause of testicular pain and mechanism of symptom relief are still uncertain without a control group. As a study retrospective in nature, the items regarding diagnosis, management, and outcome monitoring were not necessarily comprehensive. Larger scale studies regarding the efficacy of nonsurgical regimens for CTP are needed to provide corroboration.
Conclusion
Testicular pain comes from muscle, joint, or nerve issues that cannot easily be identified. Recognition of pain referral patterns and appropriate intervention are essential for the management of testicular pain.
Acknowledgments
None.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Consent: A copy of the written consent is available for review by the editor-in-chief of this journal.
ORCID iD: Eric Chun Pu Chu  https://orcid.org/0000-0002-0893-556X
https://orcid.org/0000-0002-0893-556X
References
- Ciftci H., Savas M., Yeni E., Verit A., Topal U. (2010). Chronic orchialgia and associated diseases. Current Urology, 46(2), 67–70. 10.1159/000253415 [DOI] [Google Scholar]
- Doubleday K. L., Kulig K., Landel R. (2003). Treatment of testicular pain using conservative management of the thoracolumbar spine: A case report. Archives of Physical Medicine Rehabilitation, 84(12), 1903–1905. 10.1016/S0003-9993(03)00283-1 [DOI] [PubMed] [Google Scholar]
- Kreiner D. S., Hwang S. W., Easa J. E., Resnick D. K., Baisden J. L., Bess S., Cho C. H., DePalma M. J., Dougherty P., Fernand R., Ghiselli G., Hanna A. S., Lamer T., Lisi A. J., Mazanec D., Meagher R. J., Nucci R. C., Patel R. D., Sembrano J. N. . . . North American Spine Society. (2014). An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine Journal, 14(1), 180–191. 10.1016/j.spinee.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Masarani M., Cox R. (2003). The aetiology, pathophysiology and management of chronic orchialgia. BJU International, 91(5), 435–437. 10.1046/j.1464-410X.2003.04094.x [DOI] [PubMed] [Google Scholar]
- Onel D., Tuzlaci M., Sari H., Demir K. (1989). Computed tomographic investigation of the effect of traction on lumbar disc herniations. Spine, 14(1), 82–90. 10.1097/00007632-198901000-00017 [DOI] [PubMed] [Google Scholar]
- Patel A. P. (2017). Anatomy and physiology of chronic scrotal pain. Translational Andrology and Urology, 6(Suppl 1), S51–S56. 10.21037/tau.2017.05.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B., Li D., Pang X. (2014). Degenerative lumbar spondylolisthesis with testicular pain. Pain Medicine, 15(1), 169–170. 10.1111/pme.12246 [DOI] [PubMed] [Google Scholar]
- Quallich S. Q., Arslanian-Engoren C. (2013). Chronic testicular pain in adult men: An integrative literature review. American Journal of Men’s Health, 7(5), 402–413. 10.1177/1557988313476732 [DOI] [PubMed] [Google Scholar]
- Rowell R., Rylander S. (2012). Low-back pain, leg pain, and chronic idiopathic testicular pain treated with chiropractic care. Journal of Alternative and Complementary Medicine, 18(4), 420–422. 10.1089/acm.2010.0698 [DOI] [PubMed] [Google Scholar]
- Speed C. A. (2001). Therapeutic ultrasound in soft tissue lesions, Rheumatology, 40(12), 1331–1336. 10.1093/rheumatology/40.12.1331 [DOI] [PubMed] [Google Scholar]
- Tan W. P., Levine L. A. (2017). What can we do for chronic scrotal content pain? World J Men’s Health, 35(3), 146–155. 10.5534/wjmh.17047 [DOI] [PMC free article] [PubMed] [Google Scholar]


