Abstract
Objectives:
The aims of this secondary analysis were to: (a) characterize medication use following hospital discharge for patients with chronic kidney disease (CKD), and (b) investigate relationships of medication use with the primary composite outcome of acute care utilization 90 days after hospitalization.
Methods:
The CKD-Medication Intervention Trial (CKD-MIT) enrolled acutely ill hospitalized patients with CKD stages 3–5 not dialyzed (CKD 3–5 ND). In this post hoc analysis, data for medication use were characterized, and the relationship of medication use with the primary outcome was evaluated using Cox proportional hazards models.
Results:
Participants were taking a mean of 12.6 (standard deviation=5.1) medications, including medications from a wide variety of medication classes. Nearly half of study participants were taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB). ACE inhibitor/ARB use was associated with decreased risk of the primary outcome (hazard ratio=0.51; 95% confidence interval 0.28–0.95; p=0.03) after adjustment for baseline estimated glomerular filtration rate, age, sex, race, blood pressure, albuminuria, and potential nephrotoxin use.
Conclusions:
A large number, variety, and complexity of medications were used by hospitalized patients with CKD 3–5 ND. ACE inhibitor or ARB use at hospital discharge was associated with a decreased risk of 90-day acute care utilization.
Keywords: Acute illness, hospitalization, medication regimen complexity index, pharmacotherapy, renin–angiotensin system inhibitors
Introduction
Chronic kidney disease (CKD) is a serious chronic condition associated with high rates of morbidity, use of complex medication regimens, and poor overall survival, all of which contribute to high costs of care.1–3 In 2014, expenditures exceeded $50 billion for Medicare beneficiaries with CKD—representing 20% of all Medicare spending in beneficiaries >65 years of age.4 Other chronic conditions such as hypertension, dyslipidemia, diabetes mellitus, and mood disorders are often inadequately managed in this high-risk population.5,6 Reflecting a high comorbidity burden, patients with CKD use more medications than patients without CKD and frequently have complex medication regimens.7 Previous studies have reported that the number of medications taken by patients with CKD ranges widely from 1 to 38 medications.7–9 Two recent reports of medication utilization in patients with CKD reported a mean of eight medications taken per participant. As expected, with increased regimen complexity, the potential for medication-related adverse events and adherence difficulties increases.10,11
Hospitalization is a common occurrence in CKD. Compared to Medicare beneficiaries without CKD, those with CKD stages 3–5 not dialyzed (CKD 3-5 ND) have three- to fivefold higher rates of hospitalization (200 vs. 600–1000 admissions per 1000 patient years, respectively).4 They are also more likely to be re-hospitalized when compared to patients without CKD. Overall readmissions and readmissions resulting in death increase in a stepwise fashion with increased CKD severity.12 Risks of medication-related adverse events are heightened during acute illness and hospital admission, and medication changes made during the hospital stay may increase the risk of adverse events.13,14 Therefore, more complete characterization of medication use in hospitalized patients with CKD is needed to understand usage patterns better and to identify opportunities to improve care following acute illness.
The study aim was to determine relationships between medication use (number of medications taken, medication classes used, and medication regimen complexity) and risk of hospital readmissions and emergency department and urgent care visits for 90 days after hospitalization in patients with CKD 3–5 ND.
Methods
Study design
For patients with CKD 3–5 ND, post hoc analyses were conducted with data on medication use at the hospital-to-home transition from the CKD-Medication Intervention Trial (CKD-MIT; www.clinicaltrials.gov; NCT01459770).15,16 The CKD-MIT was conducted at Providence Sacred Heart Medical Center and Providence Holy Family Hospital in Spokane, Washington, between February 2012 and May 2015. The Providence Institutional Review Board approved the CKD-MIT trial. Conduct of the trial adhered to the principles set forth by the Declaration of Helsinki. All participants provided written informed consent prior to study participation.
The study design and main results of the trial have been reported previously.15,16 Briefly, the CKD-MIT was designed to test the impact of a pharmacist-led, in-home medication management intervention compared to usual care on a primary composite outcome of acute care utilization (hospital readmissions and visits to emergency departments or urgent care centers) for 90 days following hospital discharge in adult participants with CKD 3–5 ND. The intervention involved assessment and resolution of medication discrepancies and medication errors during transition from hospital to home. Pharmacists additionally assessed suitability of medication use based on consideration of participants’ kidney function and other comorbidities, with the pharmacists recommending medication changes when appropriate. Participants enrolled in the CKD-MIT were adults (⩾21 years of age) with CKD 3–5 ND identified by at least two measures of estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 for more than 3 months during the year prior to the index hospital admission for acute illness. For study eligibility, eGFR was calculated based on local laboratory reports of creatinine values. A detailed review of prescription and non-prescription medication use was conducted at the baseline assessment within 7 days of hospital discharge.
Study definitions
Medication data were systematically categorized by a pharmacist investigator according to a pre-specified classification system developed for the CKD-MIT by two members of the study team.15,16 For non-prescription medications, over-the-counter (OTC)/herbal medications were operationally defined as any medications that could be obtained without a prescription regardless of whether the participant was instructed to take the product by a health-care provider. Frequently occurring OTC products (e.g., aspirin, vitamin D) were reported individually. OTC/herbal medications encountered less frequently (e.g., cinnamon, vitamin C) were placed into a single “other OTC/herbal” category. While select insulin products can be purchased without a prescription (e.g., regular and isophane (NPH) insulins), all insulin products were classified as “antihyperglycemic agents.” Medication use was analyzed and reported by CKD stage. A single drug category of “ACE inhibitor/ARB” was created for renin–angiotensin system (RAS) inhibitors. All medication classifications were verified by a second pharmacist investigator. Any discordance in classification was discussed among a larger group of study investigators, inclusive of pharmacists and physicians, to reach a consensus. Medication complexity scores were calculated by a study pharmacist and validated by a second study pharmacist using the Medication Regimen Complexity Index (MRCI).10,17–19
Statistical analyses
Descriptive statistics for key demographic variables, number of medications taken, medication class use by CKD 3–5 ND stage, and MRCI scores were calculated (mean±standard deviation (SD) or n/N and %). Associations of the medication use with the primary outcome of 90-day acute care utilization were evaluated using Cox proportional hazards models. The models applied predictors (total number of medications taken, medication classes taken, and MRCI scores) and were adjusted for pre-specified covariates within each model (baseline eGFR, age, sex, race, blood pressure, albuminuria, and potential nephrotoxin use (nonsteroidal anti-inflammatory drugs and/or proton pump inhibitors (PPI)). As some participants experienced more than one event during the study follow-up period, all Cox proportional hazard models represent time to first event. All medication classes outlined were tested for relationships with the primary outcome (Table 2). p-Values <0.05 were considered statistically significant. Data analyses and computations were conducted using IBM SPSS Statistics for Windows v25.0 (IBM Corp., Armonk, NY).
Table 2.
Medication use by class in the CKD-MIT by CKD stage.
| Medication class | CKD stage 3A (eGFR 45–59 mL/min/1.73 m2), n/N=49/141 | CKD stage 3B (eGFR 30–44 mL/min/1.73 m2), n/N=52/141 | CKD stage 4/5 (eGFR 15–29 mL/min/1.73 m2), n/N=40/141 | Total combined medication use in CKD-MIT, N=141 | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Antihypertensive agent | 45 | 92 | 46 | 89 | 39 | 98 | 130 | 92 |
| ACE inhibitor/ARB | 29 | 59 | 19 | 37 | 17 | 43 | 65 | 46 |
| Diuretic | 21 | 43 | 31 | 60 | 31 | 78 | 83 | 59 |
| Loop | 19 | 39 | 24 | 46 | 28 | 70 | 71 | 50 |
| Thiazide | 4 | 8 | 6 | 12 | 6 | 15 | 16 | 11 |
| Potassium sparing | 5 | 10 | 7 | 14 | 2 | 5 | 14 | 10 |
| Beta blocker | 21 | 43 | 28 | 54 | 20 | 50 | 69 | 49 |
| Calcium channel blocker | 13 | 27 | 13 | 25 | 18 | 45 | 44 | 31 |
| Dihydropyridine | 7 | 14 | 9 | 17 | 16 | 40 | 32 | 23 |
| Non-dihydropyridine | 6 | 12 | 5 | 10 | 3 | 8 | 14 | 10 |
| Other antihypertensive | 0 | 0 | 7 | 14 | 6 | 15 | 13 | 9 |
| Other OTC/herbal | 32 | 65 | 41 | 79 | 32 | 80 | 105 | 74 |
| Platelet inhibitor | 35 | 71 | 31 | 60 | 23 | 58 | 89 | 63 |
| Aspirin | 33 | 67 | 26 | 50 | 21 | 53 | 80 | 57 |
| Other platelet inhibitor | 6 | 12 | 17 | 33 | 6 | 15 | 29 | 21 |
| Statin | 29 | 59 | 35 | 67 | 22 | 55 | 86 | 61 |
| Gastrointestinal agent | 27 | 55 | 29 | 56 | 21 | 53 | 77 | 55 |
| Proton pump inhibitor (PPI) | 17 | 35 | 22 | 42 | 14 | 35 | 53 | 38 |
| Other gastrointestinal agent | 17 | 35 | 15 | 29 | 10 | 25 | 42 | 30 |
| Antihyperglycemic agent | 18 | 37 | 29 | 56 | 25 | 63 | 72 | 51 |
| Insulin | 13 | 27 | 19 | 37 | 21 | 53 | 53 | 38 |
| Oral antihyperglycemic agent | 10 | 20 | 16 | 31 | 6 | 15 | 32 | 23 |
| Sulfonylurea | 4 | 8 | 7 | 14 | 5 | 13 | 16 | 11 |
| Metformin | 7 | 14 | 6 | 12 | 1 | 3 | 14 | 10 |
| DPP-4 Inhibitor | 2 | 4 | 2 | 4 | 0 | 0 | 4 | 3 |
| Thiazolidinedione | 0 | 0 | 2 | 4 | 0 | 0 | 2 | 1 |
| GLP-1 receptor agonist | 1 | 2 | 1 | 2 | 0 | 0 | 2 | 1 |
| Vitamin D | 20 | 41 | 28 | 54 | 24 | 60 | 72 | 51 |
| Psychoactive agents | 24 | 49 | 28 | 54 | 17 | 43 | 69 | 49 |
| Antidepressant | 18 | 37 | 20 | 39 | 10 | 25 | 48 | 34 |
| Anticonvulsant | 8 | 16 | 9 | 17 | 5 | 13 | 22 | 16 |
| Anxiolytic | 8 | 16 | 4 | 8 | 5 | 13 | 17 | 12 |
| Insomnia medication | 3 | 6 | 4 | 8 | 0 | 0 | 7 | 5 |
| Other psychoactive agent | 1 | 2 | 0 | 0 | 0 | 0 | 1 | <1 |
| Opioid agonist | 16 | 33 | 19 | 37 | 11 | 28 | 46 | 33 |
| Acetaminophen | 13 | 27 | 21 | 40 | 10 | 25 | 44 | 31 |
| Thyroid supplement | 13 | 27 | 13 | 25 | 11 | 28 | 37 | 26 |
| Antibiotic agent | 15 | 31 | 10 | 19 | 11 | 28 | 36 | 26 |
| Anticoagulant | 11 | 22 | 14 | 27 | 11 | 28 | 36 | 26 |
| Warfarin | 9 | 18 | 11 | 21 | 10 | 25 | 30 | 21 |
| Direct oral anticoagulant | 2 | 4 | 3 | 6 | 0 | 0 | 5 | 4 |
| Heparin | 0 | 0 | 0 | 0 | 1 | 3 | 1 | <1 |
| Low molecular weight heparin | 0 | 0 | 0 | 0 | 1 | 3 | 1 | <1 |
| Uric acid lowering agent | 13 | 27 | 9 | 17 | 13 | 33 | 35 | 25 |
| Iron supplements | 10 | 20 | 13 | 25 | 11 | 28 | 34 | 24 |
| Nitrate | 10 | 20 | 13 | 25 | 7 | 18 | 30 | 21 |
| Prescription phosphate binder | 0 | 0 | 2 | 4 | 3 | 8 | 12 | 9 |
| Parkinson’s disease medication | 3 | 6 | 2 | 4 | 1 | 3 | 6 | 4 |
| NSAID | 2 | 4 | 3 | 6 | 0 | 0 | 5 | 4 |
| Erythropoietin stimulating agent | 0 | 0 | 1 | 2 | 1 | 3 | 2 | 1 |
| Potassium binder | 0 | 0 | 1 | 2 | 0 | 0 | 1 | <1 |
| Dementia medication | 0 | 0 | 1 | 2 | 0 | 0 | 1 | <1 |
| Anti-neoplastic | 0 | 0 | 1 | 2 | 0 | 0 | 1 | <1 |
ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; DPP-4: dipeptidyl peptidase-4; GLP-1: glucagon-like peptide-1; NSAID: nonsteroidal anti-inflammatory drug.
Results
Of 141 participants enrolled in the CKD-MIT, 35% (49/141) had CKD stage 3A, 37% (52/141) had CKD stage 3B, and 28% (40/141) had CKD stage 4/5 ND (Table 1). For all CKD stages, the mean age of participants was ⩾67 years. Most participants were white, although the CKD stage 4/5 ND group was composed of a relatively higher proportion of non-white participants compared to the CKD stage 3A and 3B groups. The majority of participants had diabetes and/or hypertension.
Table 1.
Demographic and clinical characteristics of participants in the CKD-MIT by CKD stage.
| CKD stage 3A (eGFR 45–59 mL/min/1.73 m2), n/N=49/141 | CKD Stage 3B (eGFR 30–44 mL/min/1.73 m2), n/N=52/141 | CKD stage 4/5 (eGFR 15–29 mL/min/1.73 m2), n/N=40/141 | Total combined in CKD-MIT (eGFR 15–59 mL/min/1.73 m2), N=141 | |||||
|---|---|---|---|---|---|---|---|---|
| Age (M±SD) | 68±11 | 72±9 | 67±13 | 69±11 | ||||
| n | % | N | % | n | % | n | % | |
| Sex | ||||||||
| Male | 28 | 57 | 23 | 44 | 23 | 56 | 74 | 52 |
| Female | 21 | 43 | 29 | 56 | 17 | 43 | 67 | 48 |
| Race | ||||||||
| White | 46 | 94 | 43 | 83 | 30 | 75 | 119 | 84 |
| Non-white | 3 | 6 | 9 | 17 | 10 | 25 | 22 | 16 |
| Ethnicity | ||||||||
| Hispanic | 0 | 0 | 3 | 6 | 0 | 0 | 3 | 2 |
| Non-Hispanic | 49 | 100 | 49 | 94 | 40 | 100 | 138 | 98 |
| Diabetes | 22 | 45 | 31 | 60 | 26 | 65 | 79 | 56 |
| Hypertension | 43 | 88 | 43 | 83 | 31 | 78 | 117 | 83 |
| M | SD | M | SD | M | SD | M | SD | |
| Prescription medications | 8.2 | 3.8 | 8.8 | 3.4 | 8.9 | 2.8 | 8.6 | 3.4 |
| OTC/herbal medications | 3.8 | 3.6 | 4.1 | 3.2 | 4.0 | 4.1 | 4.0 | 3.6 |
| Total medications | 12.0 | 5.5 | 12.9 | 4.5 | 12.9 | 5.2 | 12.6 | 5.1 |
| Total MRCI score | 22.8 | 12.6 | 23.9 | 9.8 | 25.1 | 9.5 | 23.9 | 10.7 |
CKD: chronic kidney disease; CKD-MIT: CKD-Medication Intervention Trial; eGFR: estimated glomerular filtration rate; M: mean; MRCI: medication regimen complexity index; OTC: over-the-counter; SD: standard deviation.
Participants in the CKD-MIT were taking a mean of 8.6 (n=141, SD=3.4) prescription medications when assessed in the home within 7 days after hospital discharge. They were also taking a mean of 4.0 (SD=3.6) OTC/herbal medications, giving a mean of 12.6 (SD=5.1) total medications (Table 2). The mean number of prescription, OTC/herbal, and total medications utilized by patients with CKD-ND stage 3A, stage 3B, and stage 4/5 was found to be similar. When exploring the complexity of medication regimens, mean MRCI scores were similar among groups when examined by CKD stage (means of 23–25).
The most common medication class utilized in the combined cohort was antihypertensive agents (92% of participants; 130/141). Nearly half (46%; 65/141) of all participants received angiotensin-converting enzyme (ACE) inhibitor/angiotensin II receptor blocker (ARB), with the highest use in earlier CKD stages (59%; 29/141). No participants were receiving both an ACE inhibitor and an ARB in combination. Other commonly used classes were OTC/herbal medications (74% of participants; 105/141), platelet inhibitors (63% of participants; 89/141), statins (61% of participants, 86/141), gastrointestinal agents (55% of participants; 77/141), and antihyperglycemic agents (51% of participants; 72/141). PPIs were taken by 38% of participants (53/141) in the combined CKD-MIT, and 33% of participants (46/141) were receiving opioid agonists.
The primary outcome (acute care use within 90 days of hospital discharge) occurred in 43% (60/141) of participants in the CKD-MIT. In total, 37 hospitalizations and 30 visits to the emergency department or urgent care occurred in the 90-day CKD-MIT follow-up period. The three most common diagnoses for hospital readmission were: cardiovascular events (30%; 11/37 re-hospitalizations); gastrointestinal diseases (30%; 11/37), and infections (27%; 8/37). The most frequent diagnostic categories for emergency department urgent care visits were: infections (27%; 8/30 visits), gastrointestinal diseases (17%; 5/30 visits), and cardiovascular events (17%; 5/30 visits).16 Neither MRCI scores nor total number of medications taken were related to the primary outcome. Of all medication classes, ACE inhibitor or ARB use was associated with a decreased risk of experiencing the primary outcome (hazard ratio=0.51; 95% confidence interval 0.28–0.95; p=0.03) in the fully adjusted model (Table 3 and Figure 1). ACE inhibitor or ARB dose did not influence the fully adjusted model.
Table 3.
Cox proportional hazards analyses for ACE inhibitor/ARB use with model covariate adjustments for the primary outcome (acute care utilization: hospital readmissions and emergency department and urgent care visits within 90 days after hospitalization).
| HR (95% CI) | |
|---|---|
| Model 1 | |
| ACE inhibitor/ARB use | 0.52 (0.29–0.94) |
| Model 2 | |
| ACE inhibitor/ARB use | 0.53 (0.29–095) |
| Baseline eGFR | 0.99 (0.97–1.01) |
| Model 3 | |
| ACE inhibitor/ARB use | 0.47 (0.25–0.86) |
| Baseline eGFR | 0.99 (0.97–1.02) |
| Age | 0.98 (0.97–1.01) |
| Sex | 0.96 (0.54–1.70) |
| Race | 0.94 (0.41–2.13) |
| Model 4* | |
| ACE inhibitor/ARB use | 0.51 (0.28–0.95) |
| Baseline eGFR | 1.00 (0.97–1.02) |
| Age | 0.98 (0.96–1.01) |
| Sex | 1.02 (0.57–1.82) |
| Race | 1.03 (0.44–2.40) |
| Mean SBP | 1.01 (0.99–1.02) |
| Use of potential nephrotoxic agent† | 0.76 (0.42–1.40) |
| Albuminuria (urine albumin–creatine ratio) | |
| 30–300 mg/g | 0.67 (0.28–1.61) |
| >300 mg/g | 1.31 (0.58–2.97) |
*Fully adjusted model.
Use of NSAID and/or PPI.
CI: confidence interval; HR: hazard ratio; SBP: systolic blood pressure.
Figure 1.
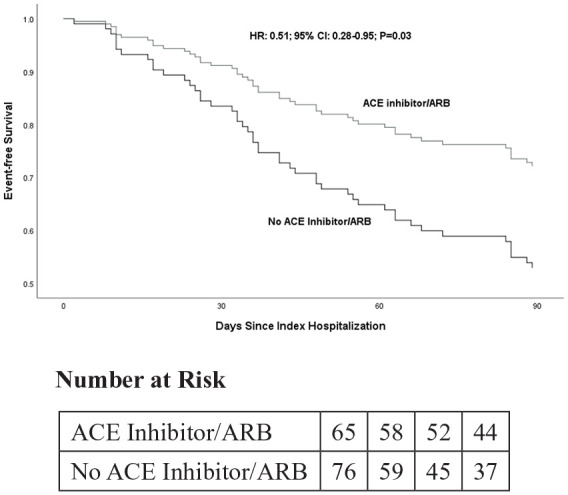
Kaplan–Meier event-free survival for the primary outcome (acute care utilization: hospital readmissions and emergency department and urgent care visits within 90 days after hospitalization) by ACE inhibitor/ARB use in the fully adjusted Cox proportional hazards model (N=141). ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker.
Discussion
This analysis of CKD-MIT data provides a comprehensive account of medication use during the hospital-to-home transition in people with CKD 3–5 ND, a population at high risk for adverse drug events and hospital readmissions.12 Information about medication use was obtained from study participants, their family members, and/or caregivers and categorized by a CKD-MIT classification algorithm to provide an accurate account of medications taken. Following discharge, participants took more than a dozen medications on average, with the most frequently used classes being antihypertensive agents, followed by OTC/herbal medications, antiplatelet agents, and statins. ACE inhibitor or ARB use following discharge from hospital was associated with a decreased risk of experiencing the primary outcome of acute care utilization within 90 days following hospitalization. However, the total number of medications and MRCI scores did not predict the primary outcome.
Acute care utilization in the first 90 days after hospitalization occurred in 43% (60/141) of participants enrolled in the CKD-MIT.16 ACE inhibitors or ARBs were prescribed to 46% (65/141) of study participants at hospital discharge. This relatively low rate of ACE inhibitor or ARB use could be accounted for, at least in part, by the common clinical practice of discontinuing RAS inhibitors during acute illness (“sick days”) because of concerns of acute kidney injury (AKI), hyperkalemia, or hypotension.20 Conversely, it is possible that bias by indication for ACE inhibitor or ARB use may contribute to the observed favorable effects, which should be evaluated in future studies. Nevertheless, the risk of 90-day acute care utilization was reduced by nearly half in ACE inhibitor or ARB users. A recent study reported that medication appropriateness and regimen complexity were not associated with 30- or 90-day hospital readmissions, but use of RAS inhibitors was associated with reduced occurrence of 30- or 90-day readmissions by about half.21 Another study reported that ACE inhibitor or ARB use within 6 months following hospital discharge for AKI was associated with lower mortality after 2 years.22
Patients re-hospitalized for an adverse drug event were reported to take more medications after hospitalization compared to those who were not readmitted (11.2 vs. 7.8 medications, respectively).10 We found that patients with CKD were taking more than a dozen medications on average following hospitalization. However, a relationship between the number of medications and risk of acute care visits following hospitalization was not detected. A systematic review reported that outcomes such as hospital readmission and medication adherence were significantly impacted by higher MRCI scores.23 Patients who were readmitted had a mean MRCI of 30 at the index hospitalization discharge compared to an MRCI of 20 for those who were not readmitted. Mean calculated MRCI scores in this analysis of the CKD-MIT were similar to scores seen in those studies. However, one study demonstrated that mean MRCI scores for adults with multiple chronic conditions that were calculated based on the hospital discharge list were significantly higher than mean MRCI scores that were calculated based on participant-reported home medication use.11 In that study, MRCI scores calculated using the hospital discharge list were predictive of hospital readmission within 30 days of discharge, whereas MRCI scores calculated based on participant-reported medication use were not predictive of hospital readmission.11 Similarly, in the current analysis, MRCI scores calculated in participants with CKD based on participant-reported medication use did not relate to post-hospitalization use of acute care. It is possible the high severity of illness in CKD may have subsumed most of the risk.
Another area of heightened interest is the treatment of pain and use of psychotropic agents in the CKD population. The high rates of opioid (~30% across all groups) and psychotropic medication (~50% across all groups) use was notable in the CKD-MIT. Pain is a common symptom in patients with CKD. Yet, use of pain medication in this population has risks.24–26 Previous reports have suggested psychotropic medication use and co-occurring serious mental illness as a potential mediator of increased re-hospitalization risk in patients with CKD.27
The findings of this study should be considered within its limitations. First, medication use was recorded by interviewing patients and caregivers during a home visit or during a visit to the research center within 7 days following hospital discharge. Thus, medication use reported in the present analysis included short-term medication use after hospitalization and did not account for medications under temporary discontinuation. Second, interest in study participation was likely greater in CKD-aware patients, which may introduce selection bias for adherence and other health-related behaviors. Third, the main diagnoses for which medications were being utilized were not always known, and therefore could not be consistently used to determine medication indication. For example, if a medication such as duloxetine was part of a patient’s regimen, it may not be apparent whether it was being used to treat depression or neuropathy. To avoid inaccurate assumptions concerning indications, medications were categorized generally by class. Due to the limited sample size, there was insufficient power to determine associations of ACE inhibitors or ARBs separately. Fourth, confounding variables such as changes in laboratory values, types of care (e.g., specialists, in-home caregivers), socioeconomic status, and health literacy were not available. Overall, these limitations speak to the difficulties of assessing and managing medications in this complex, severely ill population. Yet, despite its limitations, the present study provides valuable new knowledge about overall medication use, as well as observations about possible benefits of RAS inhibitor use in the early post-discharge period for hospitalized patients with CKD.
Conclusions
In conclusion, this post hoc analysis of the CKD-MIT highlights the large number and variety of medication classes utilized and the complexity of regimens consumed by patients in the CKD 3–5 ND population. Notably, ACE inhibitor or ARB use at hospital discharge in patients with CKD 3–5 ND was associated with a decreased risk of the primary outcome of 90-day acute care utilization. This finding warrants further investigation to evaluate for improved clinical outcomes by early reinstitution of ACE inhibitor or ARB therapy following hospitalization for acute illness.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.J.N., K.B.D., R.Z.A., R.A.S., L.G., H.M.M., B.J.G., and C.F.C. report no conflicts of interest. S.M.M. received research funding from the Bristol-Myers Squibb Foundation, Ringful Health, LLC, and consults for Consistent Care Company. K.R.T. received consulting fees from Eli Lilly and Company, Boehringer Ingelheim, Astra Zeneca, Gilead, Goldfinch Bio, and Novo Nordisk.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; grant number R34DK094016).
ORCID iD: Joshua J Neumiller  https://orcid.org/0000-0002-4734-7402
https://orcid.org/0000-0002-4734-7402
References
- 1. Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–1917. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 3. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018. [Google Scholar]
- 4. Fraser SD, Roderick PJ, May CR, et al. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol 2015; 16: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox CS, Muntner P, Chen AY, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network Registry. Circulation 2010; 121: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McPherson S, Barbosa-Leiker C, Daratha K, et al. Association of co-occurring serious mental illness with emergency hospitalization in people with chronic kidney disease. Am J Nephrol 2014; 39: 260–267. [DOI] [PubMed] [Google Scholar]
- 7. Bailie GR, Eisele G, Liu L, et al. Patterns of medication use in the RRI-CKD study: focus on medications with cardiovascular effects. Nephrol Dial Transplant 2005; 20: 1110–1115. [DOI] [PubMed] [Google Scholar]
- 8. Manley HJ, Garvin CG, Drayer DK, et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant 2004; 19: 1842–1848. [DOI] [PubMed] [Google Scholar]
- 9. Al-Ramahi R. Medication prescribing patterns among chronic kidney disease patients in a hospital in Malaysia. Saudi J Kidney Dis Transpl 2012; 23: 403–408. [PubMed] [Google Scholar]
- 10. Willson MN, Greer CL, Weeks DL. Medication regimen complexity and hospital readmission for an adverse drug event. Ann Pharmacother 2014; 48: 26–32. [DOI] [PubMed] [Google Scholar]
- 11. Schoonover H, Corbett CF, Weeks DL, et al. Predicting potential postdischarge adverse drug events and 30-day unplanned hospital readmissions from medication regimen complexity. J Patient Saf 2014; 10: 186–191. [DOI] [PubMed] [Google Scholar]
- 12. Daratha KB, Short RA, Corbett CF, et al. Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol 2012; 7: 409–416. [DOI] [PubMed] [Google Scholar]
- 13. Doody HK, Peterson GM, Watson D, et al. Retrospective evaluation of potentially inappropriate prescribing in hospitalized patients with renal impairment. Curr Med Res Opin 2015; 31: 525–535. [DOI] [PubMed] [Google Scholar]
- 14. Hassan Y, Al-Ramahi RJ, Aziz NA, et al. Adverse drug events in patients with chronic kidney disease. Int J Pharmacol Ther 2010; 48: 571–576. [DOI] [PubMed] [Google Scholar]
- 15. Alicic RZ, Short RA, Corbett CL, et al. Medication intervention for chronic kidney disease patients transitioning from hospital to home: study design and baseline characteristics. Am J Nephrol 2016; 44: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuttle KR, Alicic RZ, Short RA, et al. Medication therapy management after hospitalization in CKD: a randomized clinical trial. Clin J Am Soc Nephrol 2018; 13: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George J, Phun YT, Bailey MJ, et al. Development and validation of the medication regimen complexity index. Ann Pharmacother 2004; 38: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 18. Patel CH, Zimmerman KM, Fonda JR, et al. Medication complexity, medication number, and their relationships to medication discrepancies. Ann Pharmacother 2016; 50: 534–540. [DOI] [PubMed] [Google Scholar]
- 19. Colavecchia AC, Putney DR, Johnson ML, et al. Discharge medication complexity and 30-day heart failure readmissions. Res Social Adm Pharm 2017; 13: 857–863. [DOI] [PubMed] [Google Scholar]
- 20. Schoolwerth AC, Sica DA, Ballermann BJ, et al. Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation 2001; 104: 1985–1991. [DOI] [PubMed] [Google Scholar]
- 21. Tesfaye WH, Peterson GM, Castelino RL, et al. Medication-related factors and hospital readmission in older adults with chronic kidney disease. J Clin Med 2019; 8: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brar S, Ye F, James MT, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med 2018; 178: 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alves-Conceicão V, Rocha KSS, Silva FVN, et al. Medication regimen complexity measured by MRCI: a systematic review to identify health outcomes. Ann Pharmacother 2018; 52: 1117–1134. [DOI] [PubMed] [Google Scholar]
- 24. Barbosa-Leiker C, McPherson S, Darath K, et al. Association between prescription opioid use and biomarkers of kidney disease in US adults. Kidney Blood Press Res 2016; 41: 365–373. [DOI] [PubMed] [Google Scholar]
- 25. White DM. Appropriate use of opioids in patients with kidney diseases. Clin J Am Soc Nephrol 2018; 13: 675–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagar VR, Birthi P. Chronic opioid pain management for chronic kidney disease. J Pain Palliat Care Pharmacother 2015; 29: 48–50. [DOI] [PubMed] [Google Scholar]
- 27. McPherson S, Barbosa-Leiker C, Daratha K, et al. Association of co-occurring serious mental illness with emergency hospitalization in people with chronic kidney disease. Am J Nephrol 2014; 39: 260–267. [DOI] [PubMed] [Google Scholar]


