Abstract
Objective
This study aimed to compare energy and macronutrient intake, birth weight, and anthropometric parameters (mid-upper arm circumference or tricipital skin-fold thickness) between women who had adequate and excessive gestational weight gain (GWG) during pregnancy.
Methods
We studied 115 pregnant women and divided them according to GWG into two groups: adequate GWG (n = 49) and excessive GWG (n = 66). We assessed the medical history, clinical examinations, and dietary habits through a detailed 7-day dietary recall using myfitnesspal software.
Results
Weight, body mass index, mid-upper arm circumference, and tricipital skin-fold thickness were significantly higher at the time of delivery in women with excessive GWG compared with those with adequate GWG. A lipid-based diet was a risk factor for excessive GWG (relative risk: 1.488, 95% confidence interval: 1.112–1.991), whereas a protein-based diet was a protective factor (relative risk: 0.6723, 95% confidence interval: 0.4431–1.020). We found no significant relationship between a carbohydrate-based diet and GWG. The total energy intake was significantly higher in the excessive GWG group than in the adequate GWG group.
Conclusions
Mainly a lipid-based diet in pregnant women might represent a risk factor for excessive GWG. However, a protein-based diet is a protective factor for excessive GWG.
Keywords: Gestational weight gain, macronutrient, energy intake, pregnancy, birth weight, anthropometric parameter
Introduction
In the era of epidemic obesity, healthcare providers and medical interventions should focus on treatment of this worldwide health problem, as well as on its prevention, assuring the wellbeing of future generations.1 Obesity is a disorder with multifactorial determinism involving genetic and environmental factors, such as dietary habits, socio-economical level, and ethnicity.2,3 Birth weight (BW) is an important parameter of further development of obesity during childhood, and even adulthood. BW is mostly affected by maternal factors,4 as well as by different neonatal genetic polymorphisms.5
Among maternal factors, gestational weight gain (GWG) is probably the most important predictor of pregnancy outcomes, and it affects the mother’s and offspring’s wellbeing.6,7 Therefore, inadequate GWG, either insufficient or excessive, might have a negative effect on birth outcomes.8 Complications related to insufficient GWG include preterm delivery, fetal growth restriction, and breastfeeding problems.8 Excessive GWG is associated with a wide-spectrum of short- and long-term pregnancy complications. Short-term complications of excessive GWG include gestational diabetes, gestational arterial hypertension4,9,10 that should be differentiated by other conditions,11 an increased risk of cesarean section delivery,12 and neonatal complications, such previously giving birth to a macrosomic neonate or other life-threatening conditions.13–16 Long-term consequences of excessive GWG include maternal weight retention and excessive neonatal adiposity, which eventually lead to a continuous increase in the obesity rate in the general population.17–19 Excessive GWG is difficult to be reduced after delivery, resulting in maternal adiposity and weight retention. This causes serious medical and economic burdens by increasing the risk of metabolic diseases, such as diabetes mellitus or other obesity-related complications.20
Based on the above-mentioned studies, maternal dietary habits during pregnancy represent one of the most important modifiable variables that influence inappropriate GWG because a positive association has been shown between excessive GWG and high maternal energy intake.21,22 Appropriate dietary interventions provided by a skilled medical team, as well as adequate communication and mother’s weight monitoring, might reduce the prevalence of excessive GWG.23 Moreover, screening programs similar to other severe disorders could be useful in decreasing the incidence of obesity worldwide because it has become a major public health problem.24,25 Nevertheless, studies that aimed to assess maternal dietary habits during pregnancy and their effect on GWG are limited.26 Despite multiple studies having focused on the relationship between dietary patterns and body weight in the general population, these findings may not be generalizable to pregnant women.26 Ethnicity is an important factor that affects dietary habits, and consequently, GWG.27–29 With regard to the macronutrient composition of diet, Lai et al.30 suggested that an optimal GWG could be obtained by increasing the quality of carbohydrate and protein in pregnant women’s diet to prevent excessive GWG during pregnancy.30 Based on these facts, the recommendations for a proper diet during pregnancy consist of a higher quantity of wholegrains, fruits, vegetables, and lean proteins, and a smaller quantity of sweetened food.
This study aimed to compare energy and macronutrient intake, BW, and anthropometric parameters (mid-upper arm circumference [MUAC] and tricipital skin-fold thickness [TST]) between women who gained adequate GWG and those with excessive GWG during pregnancy.
Material and methods
Study design
We performed a prospective study between July 2017 and July 2019 on pregnant women from Romania. These women were divided into two groups according to GWG as follows: adequate GWG group and excessive GWG group. We defined GWG as the difference between weight at the end of the pregnancy and the preconception body mass index (BMI) as estimated taking into account the initial BMI measured at the time of inclusion in the study (i.e., first trimester weight)31 (Figure 1). According to the Institute of Medicine,32 GWG should be determined by the following criteria while taking into account weight at the beginning of pregnancy: for underweight women (BMI <18.5 kg/m2), the recommended GWG is 12.5 to 18 kg; for normal weight women (BMI = 18.5–24.9 kg/m2), the recommended GWG is 11.5 to 16 kg; for overweight women (BMI = 25–29.9 kg/m2), the recommended GWG is 7.00 to 11.5 kg; and for obese women (BMI >30 kg/m2), the recommended GWG is 5 to 9 kg.32
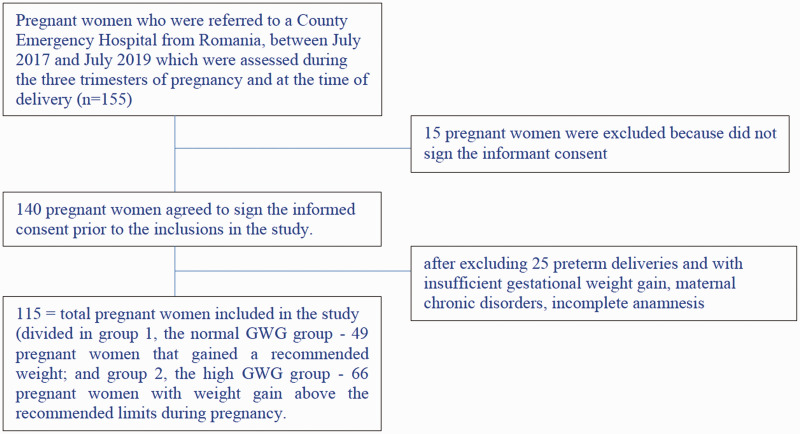
Figure 1.
Flow chart showing selection of the subjects. GWG, gestational weight gain.
Subjects
Among the 155 pregnant women aged between 21 and 39 years who presented for a routine ultrasound during pregnancy, only 140 agreed to sign the informed consent before inclusion in the study. After excluding preterm deliveries and those with insufficient GWG during pregnancy, only 115 pregnant women were eventually included in our study (Figure 1). We decided to exclude preterm deliveries because the women did not have sufficient time for proper weight gain. We excluded those with insufficient GWG because the main focus of our study was assessment of excessive GWG in pregnant women.
We included all pregnant women who presented for a first trimester ultrasound, with a gestational age of approximately 12 to 13 weeks. The exclusion criteria consisted of preterm delivery with insufficient GWG, maternal chronic disorders, incomplete anamnesis, and pregnant women who refused to sign the informed consent form.
Anthropometric measurements and dietary habit assessment
All pregnant women underwent a thorough medical history and clinical exam during each routine consultation, including weight and height. At the time of the delivery (birth time), weight and the height were measured in all pregnant women at the moment of admittance to the hospital. Weight was measured with the Tanita Scale (Tanita Corp., Tokyo, Japan), which was calibrated ( ± 10 g error) daily and height was measured with a stadiometer (PHR Portable Stadiometer, 0.1 cm error; Detecto, Webb City, MO, USA). Moreover, we assessed MUAC and TST at the end of pregnancy. MUAC was assessed at the midpoint between the shoulder and elbow tips using an MUAC measuring tape (in cm) and TST was measured in the same point of the posterior upper arm with a Harpenden Skinfold Caliper (Baty International, West Sussex, UK).
To assess dietary habits, each pregnant woman was asked to describe her nutritional history over the past week using a 7-day food recall (the precise content of each meal for the past 7 days) at the end of every trimester. The information provided by the pregnant women regarding their diet on the last 7 days before the interview was automatically transformed into energy (kcal) and macronutrients (carbohydrates, lipids, and proteins) by software (https://www.myfitnesspal.com). Using the results, we calculated the predominance of macronutrients in their diet. This software is also designed to provide the excess for each macronutrient according to the daily recommended amounts for adults. Using the results from this software, we obtained the energy intake per day and the pattern of the diet, which mainly included a protein-, carbohydrate-, or lipid-based diet. The pregnant woman’s dietary habits were assessed three times during pregnancy at 12 to 13 gestational weeks, at 22 to 23 gestational weeks, and at 35 to 38 gestational weeks. The interview was conducted by an obstetrics specialist with a Master’s degree in nutrition.
Informed consent
All pregnant women signed informed consent for themselves and their newborns before inclusion in the study. The study was approved by the Ethics Committee of the University of Medicine, Pharmacy, Sciences, and Technology from Târgu Mureș (No. 138/05.07.2018), and it was performed according to the principles of the Helsinki Declaration.
Statistical analysis
Statistical analysis comprised descriptive statistics (frequency, mean, median, and standard deviation) and inferential statistics. The Shapiro–Wilk test was applied to assess the distribution of the analyzed series. For comparison of means, we used the Student’s t test for unpaired data in two groups, the Student’s t test with Welch correction for unequal variances, and ANOVA for comparison of three groups. We also used the non-parametric Mann–Whitney and Kruskal–Wallis tests for comparison of median values. The relative risk (RR) was computed as the ratio between the incidence of excessive GWG in the exposed group (number of subjects with excessive GWG with mainly a lipid-based diet divided by the total number of subjects with mainly a lipid-based diet) and the incidence of excessive GWG in the unexposed group (number of subjects with excessive GWG without a mainly lipid-based diet divided by the total number of subjects without a mainly lipid-based diet). We also computed the RR for pregnant women with mainly a carbohydrate-based diet and mainly a protein-based diet. For each calculated RR value, the statistical analysis program also calculated a 95% confidence interval (CI). For p values, the chi-square test for association (contingency) to measure the association between two binary variables was used. The chosen significance threshold for p values was 0.05. Statistical analysis was performed using the program GraphPad Prism, trial variant (San Diego, CA, USA).
Results
Among the 115 pregnant women included in the study, 66 had excessive GWG and 49 had adequate GWG. With regard to sociodemographic characteristics, women in the adequate GWG and excessive GWG groups mostly lived in urban areas. The educational level was similar between the two groups. The majority of pregnant women in the excessive GWG group were primiparas (p = 0.016) (Table 1). The mean age for the adequate GWG group was 29.59 ± 4.28 years, which was similar to that of the excessive GWG group (28.92 ± 4.20 years) (Table 2). The initial BMI was significantly higher in the excessive GWG group compared with the adequate GWG group (p < 0.0001). We found significantly higher values of MUAC and TST at the time of delivery in the excessive GWG group compared with the adequate GWG (both p < 0.0001). We also found that newborns whose mothers were in the excessive GWG had a higher BW compared with those whose mothers were in the adequate GWG group (p = 0.046). With regard to mean energy intake, we found that there was a significantly higher energy intake during all three trimesters in the excessive GWG group compared with the adequate GWG group (all p < 0.001) (Table 2).
Table 1.
Descriptive demographic parameters of the two groups.
| Parameters | Adequate GWG group (n = 49), n (%) | Excessive GWG group (n = 66), n (%) | p value |
|---|---|---|---|
| Environment | |||
| Rural | 14 (28.57) | 14 (21.21) | 0.363 |
| Urban | 35 (71.43) | 52 (78.79) | |
| Education | |||
| Medium school | 17 (34.69) | 34 (51.51) | 0.073 |
| Higher education | 32 (65.30) | 32 (48.49) | |
| Parity | |||
| Primipara | 22 (44.89) | 45 (68.18) | 0.016 |
| Secundipara | 22 (44.89) | 20 (30.30) | |
| Tertipara | 5 (10.20) | 1 (1.51) | |
| Medical history of macrosomia | |||
| Yes | 1 | 2 | 1 |
| No | 48 | 64 | |
| Carbohydrate-based diet | |||
| 1st trimester | 8 (16.33) | 16 (24.24) | 0.329 |
| 2nd trimester | 16 (32.65) | 29 (43.94) | |
| 3rd trimester | 33 (67.34) | 37 (56.06) | |
| Lipid-based diet | |||
| 1st trimester | 2 (4.08) | 18 (27.27) | 0.214 |
| 2nd trimester | 10 (20.41) | 24 (36.36) | |
| 3rd trimester | 8 (16.33) | 19 (28.79) | |
| Protein-based diet | |||
| 1st trimester | 39 (79.59) | 32 (48.49) | 0.383 |
| 2nd trimester | 23 (46.94) | 13 (19.70) | |
| 3rd trimester | 8 (16.33) | 10 (15.15) |
GWG, gestational weight gain.
Table 2.
Descriptive anthropometric parameters at onset and delivery in the two groups.
| Parameters | Adequate GWG group (n = 49) |
Excessive GWG group (n = 66) |
|||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | p value | |
| Age (years) | 29.59 ± 4.28 | 30.00 | 28.92 ± 4.20 | 28.00 | 0.403 |
| H (cm) | 165.50 ± 6.99 | 165.0 | 166.3 ± 6.39 | 166.0 | 0.450 |
| Winitial (kg) | 57.08 ± 8.33 | 56.00 | 66.33 ± 12.41 | 63.50 | *<0.0001 |
| BMIinitial (kg/m2) | 20.79 ± 2.28 | 19.90 | 23.92 ± 3.93 | 23.90 | *<0.0001 |
| Wdelivery (kg) | 71.67 ± 8.95 | 71.00 | 85.30 ± 12.19 | 83.00 | *<0.0001 |
| BMIdelivery (kg/m2) | 26.15 ± 2.54 | 25.50 | 30.80 ± 3.82 | 30.95 | *<0.0001 |
| MUACdelivery (cm) | 28.37 ± 2.41 | 28.00 | 31.24 ± 3.04 | 31.00 | *<0.0001 |
| TSTdelivery (cm) | 25.84 ± 2.64 | 25.00 | 28.53 ± 2.87 | 28.00 | <0.0001 |
| BW (kg) | 3330 ± 276.7 | 3360 | 3451 ± 345.5 | 3435 | 0.046 |
| Energy intake in the 1st trimester | 2041 ± 181.5 | 2036 | 2261 ± 285.8 | 2286 | *<0.0001 |
| Energy intake in the 2nd trimester | 2131 ± 197.1 | 2141 | 2339 ± 294.2 | 2383 | *<0.0001 |
| Energy intake in the 3rd trimester | 2272 ± 224.4 | 2307 | 2470 ± 286.7 | 2517 | 0.0001 |
BMI, body mass index; BW, birth weight; GWG, gestational weight gain; H, height; MUAC, mid-upper arm circumference; SD, standard deviation; TST, tricipital skin-fold thickness; W, weight. *Fisher’s test was used for analysis.
When we assessed the dietary patterns (carbohydrate-based, lipid-based, or protein-based diet) in each trimester of pregnancy, we found that both groups predominantly had a protein-based diet in the first trimester. In the second trimester, the main pattern changed into a predominantly carbohydrate-based diet in both groups. In the last trimester, the dietary pattern changed for both groups, and most of the pregnant women in both groups had a carbohydrate-based diet (Table 1).
With regard to the relationship between dietary habits and GWG, we found that pregnant women with a lipid-based diet had a significantly higher risk for excessive GWG (RR: 1.488, 95% CI: 1.112–1.991, p = 0.025). However, protein-based and carbohydrate-based diets showed no significant risk for excessive GWG in our sample (Table 3).
Table 3.
Relationships between dietary habits and GWG in pregnant women.
| Parameters | Excessive GWG group (n = 66, 57.40% from the total group) | Normal GWG group (n = 49, 42.60% from the total group) | p value | |
|---|---|---|---|---|
| Mainly carbohydrate-based diet | ||||
| Yes | 30 (45.45%) | 19 (38.77%) | RR: 1.12295% CI: 0.8205–1.536p = 0.474 | |
| No | 36 (54.55%) | 30 (61.23%) | ||
| Total | 66 | 49 | ||
| Mainly lipid-based diet | ||||
| Yes | 20 (30.30%) | 6 (12.24%) | RR: 1.48895% CI: 1.112–1.991p = 0.025 | |
| No | 46 (69.70%) | 43 (87.76%) | ||
| Total | 66 | 49 | ||
| Mainly protein-based diet | ||||
| Yes | 15 (22.72%) | 20 (40.81%) | RR: 0.672395% CI: 0.4431–1.020p = 0.060 | |
| No | 51 (77.18%) | 29 (59.19%) | ||
| Total | 66 | 49 | ||
GWG, gestational weight gain; RR, relative risk; CI, confidence interval.
During all three trimesters of pregnancy, pregnant women from both groups with a lipid-based diet had higher energy intakes than women with protein/carbohydrate-based diets. This finding was expected because lipids have a higher caloric density than proteins and carbohydrates.
Discussion
Our study showed a higher number of pregnant women with GWG above the recommended limits compared with those with adequate GWG. Currently, there is a proven trend of weight gain and obesity in the general population, and consequently, the prevalence of women with excessive GWG has reached alarming rates.32 A study performed on Chinese pregnant women showed that in 2011, 38.2% of women presented with excessive GWG.8 Previous studies by our team showed that, in Romanian women, the prevalence of pregnant women with excessive GWG has also increased during recent years.14,33,34
In our study, pre-pregnancy BMI was significantly higher in women with excessive GWG compared with those with adequate GWG in pregnancy. A previous study showed that American women whose pre-pregnancy BMI was high were more predisposed to gain weight above the recommended limits in pregnancy.35–37 Therefore, pre-pregnancy BMI is an important predictor for excessive GWG. The association between high pre-pregnancy BMI and excessive GWG has become an even more pressing issue since the Pregnancy Risk Assessment Monitoring System showed that obesity had increased in women of childbearing age from 13% to 22% from 1993 to 2002.38
Our study also showed that pregnant women with excessive GWG had a significant higher energy intake during pregnancy compared with those with adequate GWG. Weight gain during pregnancy not only differs during the three trimesters, but these differences are related to certain complications. GWG during the first two trimesters is an important indicator of maternal obesity.39 The effect of diet on inappropriate weight gain is not the same in pregnant and non-pregnant women because GWG is affected by maternal factors, such as fat accretion or increased blood volume, and fetal-related factors, such as amniotic fluid or fetal weight.40 Multiple studies have reported that increased energy intake in pregnancy is correlated with a higher risk for excessive GWG, and consequently, with an increased absolute weight gain.41–43 This finding is in accordance with results from our study.
Multiple studies have focused on assessing the relationships between fat, carbohydrate, and protein in GWG, but the findings remain controversial.43–46 Our study showed that a dietary pattern mainly based on lipids during pregnancy significantly increased the risk for excessive GWG. Nevertheless, we did not individually assess the role of saturated and unsaturated fats, or that of animal or vegetal fat. Other studies failed to show any association between total fat or saturated fat intake during pregnancy and GWG.44–46 However, a prospective study performed on 1388 American pregnant women showed that replacement of carbohydrates by monounsaturated fat was associated with a 37% lower risk for excessive GWG.44 Another study performed on the same population showed that animal fat intake was significantly related to GWG at 27 gestational weeks, but it failed to show the same association for vegetable fat.43
With regard to protein intake during pregnancy, our study showed that pregnant women whose diet was mainly based on protein had a lower risk for gaining excessive weight during pregnancy. Therefore, a protein-based diet during pregnancy might be considered as a protective factor for excessive GWG. However, findings of this dietary choice in the literature remain contradictory. Several studies showed no association between total protein energy intake and GWG.44,46 However, a cross-sectional analysis by Lagiou et al.43 showed that weight gain during pregnancy increased by 3.11 kg for every standard deviation increase in protein intake.
Our study showed no significant risk for women with a carbohydrate-based diet to develop excessive GWG. Similar to our results, carbohydrate intake has been previously shown to have either a negative effect or no effect on GWG.43,46 The cross-sectional NHANES analysis failed to show any relationship between GWG and energy from carbohydrates in the studied participants’ diet.46 However, Lagiou et al.43 found that for every standard deviation increase in total carbohydrate intake, GWG decreased by 5.22 kg. Based on the above-mentioned findings, we consider that there is a complex relationship between a pregnant woman’s diet, GWG, gestational complications, postpartum weight retention, and BW.
The main limitation of our study is the relatively small number of pregnant women included in the study. Additionally, these women were assessed only at the time of the first routine pregnancy consultation, and not before pregnancy. Furthermore, our research involved only macronutrient analysis in the diet of pregnant women, without taking into account different types of diets. Moreover, we did not assess the distinction between the sources of protein and fat, as well as between simple and complex carbohydrates. Another limitation is that our study included pregnant women from a single geographic area and we did not follow our participants longitudinally. Because excessive GWG is associated with higher postpartum weight retention,17–19 an inadequate diet during pregnancy could affect postpartum weight retention. However, this was not assessed in the present study, which is another limitation of our study. Strengths of our study include the accuracy of the statistical analysis, assessment of different anthropometric parameters, such as MAUC and TST, neonatal outcome represented by BW, and all measurements were performed by a single trained person. Moreover, to the best of our knowledge, this is the first study to be performed in Romania that assessed the effect of macronutrient and energy intake on GWG. Our study needs to be expanded to a greater geographic area of Romania, and even to Europe. We also need to take into account other dietary details for precise identification of each dietary habit during pregnancy and its effect on GWG.
Conclusions
Our study shows that a predominantly lipid-based diet during pregnancy might represent a risk factor for excessive GWG. A protein-based diet appears to be a protective factor for excessive GWG. Moreover, energy intake during all three trimesters has a significant negative effect on GWG, resulting in weight gain above the recommended limits. Pre-pregnancy BMI is higher in pregnant women with excessive GWG than in those with adequate GWG. Certain dietary habits might increase the risk for excessive GWG, leading to increased BW with adverse neonatal outcomes. Therefore, health professionals should be aware of the types of diets involved in excessive GWG to decrease the incidence of maternal obesity and even neonatal obesity. Additionally, dietary strategies implemented in women of childbearing age could also contribute to proper weight gain during pregnancy. Despite all of these findings, further studies are necessary on larger samples, including dietary patterns, and not only assessment of macronutrients, to determine the precise role of diet in development of obesity in the mother and offspring.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-4-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-5-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-6-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Contributors’ statement
Dr Rugină Cosmin, Prof Cristina Oana Mărginean, Dr Lorena Elena Meliț, Dr Viviana Modi, and Dr Claudiu Mărginean conceptualized and designed the study, drafted the initial manuscript, and revised the manuscript. Dr Dana Valentina Giga performed statistical analysis. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Cristina Oana Mărginean https://orcid.org/0000-0003-2119-1726
References
- 1.WHO | Overweight and obesity, https://www.who.int/gho/ncd/risk_factors/overweight/en/ (accessed 25 January 2019).
- 2.Mǎrginean CO, Mǎrginean C, Meliţ LE. New insights regarding genetic aspects of Childhood obesity: a minireview. Front Pediatr 2018; 6: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mărginean C, Meliț L, Ghiga D, et al. Early inflammatory status related to pediatric obesity (STROBE compliant article). Front Pediatr 2019; 7: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mărginean C, Mărginean CO, Bănescu C, et al. Impact of demographic, genetic, and bioimpedance factors on gestational weight gain and birth weight in a Romanian population: A cross-sectional study in mothers and their newborns: the Monebo study (STROBE-compliant article). Medicine (Baltimore) 2016; 95: e4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mărginean CO, Bănescu C, Duicu C, et al. Angiotensin-converting enzyme gene insertion/deletion polymorphism in nutritional disorders in children. Eur J Nutr 2015; 54: 1245–1254. [DOI] [PubMed] [Google Scholar]
- 6.Mărginean C, Mărginean CO, Iancu M, et al. The role of TGF-β1 869 T > C and PPAR γ2 34 C > G polymorphisms, fat mass, and anthropometric characteristics in predicting childhood obesity at birth: A cross-sectional study according the parental characteristics and newborn’s risk for child obesity (the newborns obesity’s risk) NOR study. Medicine (Baltimore) 2016; 95: e4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Liu Y, Zhang W. Joint and independent associations of gestational weight gain and pre-pregnancy body mass index with outcomes of pregnancy in Chinese women: a retrospective cohort study. PLoS One 2015; 10: e0136850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep) 2008; 168: 1–223. [PMC free article] [PubMed] [Google Scholar]
- 9.Park JE, Park S, Daily JW, et al. Low gestational weight gain improves infant and maternal pregnancy outcomes in overweight and obese Korean women with gestational diabetes mellitus. Gynecol Endocrinol 2011; 27: 775–781. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Ao D, Yang H, et al. Gestational weight gain and risk of gestational diabetes mellitus among Chinese women. Chin Med J 2014; 127: 1255–1260. [PubMed] [Google Scholar]
- 11.Mărginean CO, Meliţ LE, Moldovan H, et al. Lead poisoning in a 16-year-old girl: a case report and a review of the literature (CARE compliant). Medicine (Baltimore) 2016; 95: e4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham LE, Brunner Huber LR, Thompson ME, et al. Does amount of weight gain during pregnancy modify the association between obesity and cesarean section delivery? Birth 2014; 41: 93–99. [DOI] [PubMed] [Google Scholar]
- 13.Mărginean C, Mărginean CO, Iancu M, et al. The FTO rs9939609 and LEPR rs1137101 mothers-newborns gene polymorphisms and maternal fat mass index effects on anthropometric characteristics in newborns: A cross-sectional study on mothers-newborns gene polymorphisms-The FTO-LEPR Study (STROBE-compliant article). Medicine (Baltimore) 2016; 95: e5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mărginean CO, Mărginean C, Bănescu C, et al. The relationship between MMP9 and ADRA2A gene polymorphisms and mothers-newborns’ nutritional status: an exploratory path model (STROBE compliant article). Pediatr Res. Epub ahead of print 21 February 2019. DOI: 10.1038/s41390-019-0347-2. [DOI] [PMC free article] [PubMed]
- 15.Kim SY, Sharma AJ, Sappenfield W, et al. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol 2014; 123: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meliț LE, Mărginean CO, Georgescu A, et al. Complications of sepsis in infant. A case report. J Crit Care Med (Targu Mures) 2016; 2: 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser A, Tilling K, Macdonald-Wallis C, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr 2011; 93: 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sridhar SB, Darbinian J, Ehrlich SF, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol 2014; 211: 259.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ensenauer R, Chmitorz A, Riedel C, et al. Effects of suboptimal or excessive gestational weight gain on childhood overweight and abdominal adiposity: results from a retrospective cohort study. Int J Obes (Lond) 2013; 37: 505–512. [DOI] [PubMed] [Google Scholar]
- 20.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyes NR, Klotz AA, Herring SJ. A qualitative study of motivators and barriers to healthy eating in pregnancy for low-income, overweight, African-American mothers. J Acad Nutr Diet 2013; 113: 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tielemans MJ, Garcia AH, Peralta Santos A, et al. Macronutrient composition and gestational weight gain: a systematic review. Am J Clin Nutr 2016; 103: 83–99. [DOI] [PubMed] [Google Scholar]
- 23.Mărginean CO, Meliţ LE, Chinceşan M, et al. Communication skills in pediatrics - the relationship between pediatrician and child. Medicine (Baltimore) 2017; 96: e8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zerjav Tansek M, Groselj U, Angelkova N, et al. Phenylketonuria screening and management in southeastern Europe - survey results from 11 countries. Orphanet J Rare Dis 2015; 10: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groselj U, Tansek MZ, Smon A, et al. Newborn screening in southeastern Europe. Mol Genet Metab 2014; 113: 42–45. [DOI] [PubMed] [Google Scholar]
- 26.Tobias D, Bac W. Diet during pregnancy and gestational weight gain. Curr Nutr Rep 2014; 3: 289–297. [Google Scholar]
- 27.Uusitalo U, Arkkola T, Ovaskainen ML, et al. Unhealthy dietary patterns are associated with weight gain during pregnancy among Finnish women. Public Health Nutr 2009; 12: 2392–2399. [DOI] [PubMed] [Google Scholar]
- 28.Tielemans MJ, Erler NS, Leermakers ETM, et al. A priori and a posteriori dietary patterns during pregnancy and gestational weight gain: the Generation R Study. Nutrients 2015; 7: 9383–9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin D, Lee KW, Song WO. Dietary patterns during pregnancy are associated with gestational weight gain. Matern Child Health J 2016; 20: 2527–2538. [DOI] [PubMed] [Google Scholar]
- 30.Lai JS, Soh SE, Loy SL, et al. Macronutrient composition and food groups associated with gestational weight gain: the GUSTO study. Eur J Nutr 2019; 58: 1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krukowski RA, West DS, DiCarlo M, et al. Are early first trimester weights valid proxies for preconception weight? BMC Pregnancy Childbirth 2016; 16: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press (US), http://www.ncbi.nlm.nih.gov/books/NBK32813/ (2009, accessed 30 March 2019). [Google Scholar]
- 33.Mărginean C, Bănescu CV, Mărginean CO, et al. Glutathione S-transferase (GSTM1, GSTT1) gene polymorphisms, maternal gestational weight gain, bioimpedance factors and their relationship with birth weight: a cross-sectional study in Romanian mothers and their newborns. Rom J Morphol Embryol 2017; 58: 1285–1293. [PubMed] [Google Scholar]
- 34.Marginean C, Marginean C, Iancu M, et al. MC4R and ENPP1 gene polymorphisms and their implication in maternal and neonatal risk for obesity. Sci Rep 2019; 9: 10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA 2004; 291: 2847–2850. [DOI] [PubMed] [Google Scholar]
- 36.Helms E, Coulson CC, Galvin SL. Trends in weight gain during pregnancy: a population study across 16 years in North Carolina. Am J Obstet Gynecol 2006; 194: e32–e34. [DOI] [PubMed] [Google Scholar]
- 37.Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J Am Diet Assoc 2003; 103: 48–54. [DOI] [PubMed] [Google Scholar]
- 38.Kim SY, Dietz PM, England L, et al. Trends in pre-pregnancy obesity in nine states, 1993-2003. Obesity (Silver Spring) 2007; 15: 986–993. [DOI] [PubMed] [Google Scholar]
- 39.Wei X, He JR, Lin Y, et al. The influence of maternal dietary patterns on gestational weight gain: a large prospective cohort study in China. Nutrition 2019; 59: 90–95. [DOI] [PubMed] [Google Scholar]
- 40.Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol 1976; 19: 489–513. [DOI] [PubMed] [Google Scholar]
- 41.Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. Am J Obstet Gynecol 2004; 191: 530–536. [DOI] [PubMed] [Google Scholar]
- 42.Olafsdottir AS, Skuladottir GV, Thorsdottir I, et al. Maternal diet in early and late pregnancy in relation to weight gain. Int J Obes (Lond) 2006; 30: 492–499. [DOI] [PubMed] [Google Scholar]
- 43.Lagiou P, Tamimi RM, Mucci LA, et al. Diet during pregnancy in relation to maternal weight gain and birth size. Eur J Clin Nutr 2004; 58: 231–237. [DOI] [PubMed] [Google Scholar]
- 44.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol 2009; 201: 58.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Althuizen E, Van Poppel MNM, Seidell JC, et al. Correlates of absolute and excessive weight gain during pregnancy. J Womens Health (Larchmt) 2009; 18: 1559–1566. [DOI] [PubMed] [Google Scholar]
- 46.Shin D, Bianchi L, Chung H, et al. Is gestational weight gain associated with diet quality during pregnancy? Matern Child Health J 2014; 18: 1433–1443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-4-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-5-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research
Supplemental material, sj-pdf-6-imr-10.1177_0300060520933808 for Relationships between excessive gestational weight gain and energy and macronutrient intake in pregnant women by Cosmin Rugină, Cristina Oana Mărginean, Lorena Elena Meliţ, Dana Valentina Giga, Viviana Modi and Claudiu Mărginean in Journal of International Medical Research