Abstract
Objective
Observational studies have shown that circulating vitamin D (VitD) deficiency is associated with atherogenic lipid patterns among polycystic ovary syndrome (PCOS) patients. However, interventional studies have shown inconsistent results. The aim of this meta-analysis was to investigate how VitD supplementation influences lipid indices in PCOS patients.
Methods
The authors searched four electronic databases through August 2019 to identify randomized controlled trials (RCTs) that assessed the effect of VitD intervention on serum lipids among PCOS patients. Mean differences were generated for statistical evaluation.
Results
We included eight studies and performed nine comparisons across 467 participants. VitD supplementation reduced serum triglyceride levels (−11.88 mg/dL; 95% confidence interval [CI]: −17.03 to −6.73), total cholesterol (−9.09 mg/dL; 95% CI: −14.90 to −3.29), low-density lipoprotein cholesterol (−5.22 mg/dL; 95% CI: −10.32 to −0.13), and very-low-density lipoprotein cholesterol (−2.43 mg/dL; 95% CI: −3.69 to −1.17) compared with no VitD supplementation. However, high-density lipoprotein cholesterol levels showed no differences with or without VitD supplementation (−0.39 mg/dL; 95% CI: −1.39 to 0.61).
Conclusions
VitD supplementation improved serum lipid levels among PCOS patients, but serum high-density lipoprotein cholesterol levels were not changed. VitD intervention might benefit PCOS patients who are at high risk of an atherogenic lipid profile.
Keywords: Vitamin D, polycystic ovary syndrome, lipids, meta-analysis, randomized controlled trial, triglycerides, high-density lipoprotein cholesterol, total cholesterol
Introduction
Polycystic ovary syndrome (PCOS) is a prevalent disease of the endocrine system that affects up to 20% of reproductive-aged women across the globe.1 It is considered to be a complex multisystem syndrome that is associated with both reproductive (ovulatory dysfunction, hyperandrogenism, and infertility) and metabolic abnormalities (i.e., dyslipidemia, insulin resistance, and cardiovascular diseases [CVD]).2,3 Dyslipidemia is probably the most common metabolic abnormality among PCOS patients, with a prevalence of 70%, and it is characterized by elevated levels of a range of serum lipids, but decreased levels of high-density lipoprotein cholesterol (HDL-C).2 These altered lipid levels represent a major determinant of atherosclerosis, which is central to the development of CVD.3 Additionally, women with PCOS have an enhanced risk of stroke and heart disease.4,5 Therefore, lipid-modulating and CVD prevention strategies should be given a high priority in female patients with PCOS.
Vitamin D (VitD) deficiency (VDD) is defined as serum levels of 25-hydroxy VitD (or 25(OH)D) ≤20 ng/mL.6 Women with PCOS are more likely to develop VDD, with a high prevalence of 67% to 85%.7 An increasing amount of evidence shows that VDD is closely associated with unregulated lipid levels and a heightened risk of and CVD.8–10 VitD binding to its receptor regulates the expression of many lipid-associated genes and suggests that it plays a role in the pathogenesis of dyslipidemia in PCOS.11 However, the effectiveness of taking VitD to recover lipid profiles is inconclusive. Some randomized controlled trials (RCTs) have shown favorable changes in the lipid profiles of those supplemented with VitD,12–16 but others have documented non-significant effects of VitD.17–21 Several meta-analyses have been conducted to explore the effect of VitD intervention on lipid levels among PCOS patients, but no clear conclusion has been reached.7,22,23 Since the publication of the studies referenced above, additional RCTs have been conducted and more evidence has been provided about the effect of VitD intervention on comprehensive lipid parameters among PCOS patients, although the results remain inconclusive. Thus, we investigated the current evidence from RCTs to critically evaluate the effectiveness of VitD supplementation on the serum lipid levels in PCOS patients.
Methods
Data sources and search strategy
Two reviewers independently searched PubMed, Web of Science, Embase, and The Cochrane Library up to August 26, 2019 to identify RCTs on the effect of VitD supplementation on the lipid levels in PCOS patients. We combined the Medical Subject Heading terms “Vitamin D” and “polycystic ovary syndrome” as well as the following free-text terms: “25 (OH) D”, “vitamin D”, “cholecalciferol”, “calciferol”, “ergocalciferol”, “25 Hydroxyvitamin D”, “calcitriol”, ”calcifediol”, “PCOS”, “Stein–Leventhal Syndrome”, “Stein Leventhal Syndrome”, “sclerocystic ovarian degeneration”, “sclerocystic ovary syndrome”, “polycystic ovarian syndrome”, “polycystic ovary syndrome”, “sclerocystic ovaries”, and “sclerocystic ovary”. The reference lists from the retrieved citations and relevant review articles were also screened for any eligible studies. Only articles published in English were considered.
Study selection and eligibility criteria
After removing duplicate publications, two independent authors selected the studies in a two-step process. First, the titles and abstracts of eligible references were screened. Second, the relevant articles that were published in full were acquired for a detailed assessment with the aim of identifying eligible studies that met the criteria for this meta-analysis. Disagreements were resolved by a third reviewer.
Inclusion criteria were as follows. (1) Participants: definitively diagnosed PCOS patients based on the Rotterdam criteria,24 the 1990 National Institutes of Health (NIH) criteria,25 or Androgen Excess Society criteria;26 (2) Intervention: VitD was administered orally as VitD3 (cholecalciferol), VitD2 (ergocalciferol), or any other active form of VitD including calcitriol and calcifediol; (3) Comparisons: placebo was applied in the control arm of a study; (4) Outcomes: mean changes from baseline in serum levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), HDL-C, and very-low-density lipoprotein cholesterol (VLDL-C), and the standard deviation (SD) was either reported or was able to be calculated; (5) Study design: RCTs with a parallel design; and (6) when more than one paper with overlapping sample data from the same institute was published, the most recent publication or the publication that contained more detailed information was included.
Exclusion criteria were as follows: (1) Patients with complications such as other diseases (e.g. diabetes, Cushing’s syndrome, hyperthyroidism, or other hormonal diseases); (2) Studies that co-supplemented patients were excluded if the control group did not receive the exact type of treatment; (3) Studies using cell lines or animals; (4) Data that were reported as the median and interquartile range; or (5) Review articles, conference abstracts, letters, commentaries, case reports, or other articles lacking original data.
Data extraction
Two independent reviewers extracted data independently and in duplicate using a standardized form. Any differences between them were settled by discussion or by a third independent reviewer. Lead author, publication year, the ethnicity of participants, diagnostic criteria of PCOS, sample size, the name of the supplemented VitD, treatment dose (international unit per day), and treatment duration (weeks) were extracted. Baseline and final concentrations (or net changes) of serum-lipids were also assessed. If the changes from baseline were recalculated after adjusting for some baseline characteristics, the adjusted changes were extracted for analysis.
Quality assessment
Two reviewers evaluated the study quality independently using Cochrane’s Collaboration tool.27 Evaluation criteria included the generation of random sequences, participant blinding, outcome evaluation blinding, allocation concealment, incomplete data, and selective reports. The evaluated results were divided into low, unclear, and high bias risk. Any discrepancies were discussed or settled by a third reviewer.
Data synthesis
All parameter measurements were converted to mg/dL for consistency. Specifically, 1 mg/dL = 0.0259 mmol/L for TC, HDL-C, LDL-C and VLDL-C; and 1 mg/dL =0.0113 mmol/L for TG. The effect size was extracted using the mean and SD. Additionally, the standard error (SE) was converted to the SD using the following equation: , where N is the population number that was used to convert the standard error (SE) to SD. Changes in lipid levels from baseline were the main outcome measurement. When not present directly in the original study, we subtracted the lipid levels at initiation from the values that were recorded at the end of the trial. The SD for the changes from baseline was calculated using the following equation: , where SDb is the SD of the baseline, SDe is SD at the end of the trial, and 0.5 was used as an assumed correlation coefficient for end vs. baseline values.28 If two independent intervention groups were designed for comparisons with the same control group, the two groups were combined into a single group based on Dias et al.29 Specifically, the combined sample size was calculated as , the combined mean was calculated as , and the combined SD was calculated as , where N1 and N2 are sample sizes, M1 and M2 are the means, and SD1 and SD2 are the SDs of both groups.
Statistical analysis
Data were analyzed using Stata version 15.1 (StataCorp LLC, College Station, TX, USA). The random-effects model was applied to the generate mean difference (MD) and 95% confidence interval (CI). All calculations accounted for potential clinical heterogeneity. Subgroup analyses were performed based on the VitD doses (low: ≤4000 IU/day, and high: >4000 IU/day) and the intervention duration (short: ≤8 weeks, and long: >8 weeks). The heterogeneity of different studies was evaluated using the Cochrane test and I2.30 Heterogeneity was considered to be low when I2<50%. Studies were sequentially removed to perform a sensitivity analysis. Egger’s assessments31 and Begg’s funnel plots32 were used to assess publication bias. All statistical tests were bilateral. P-values <0.05 were indicative of significant changes in serum lipids.
Results
Study selection
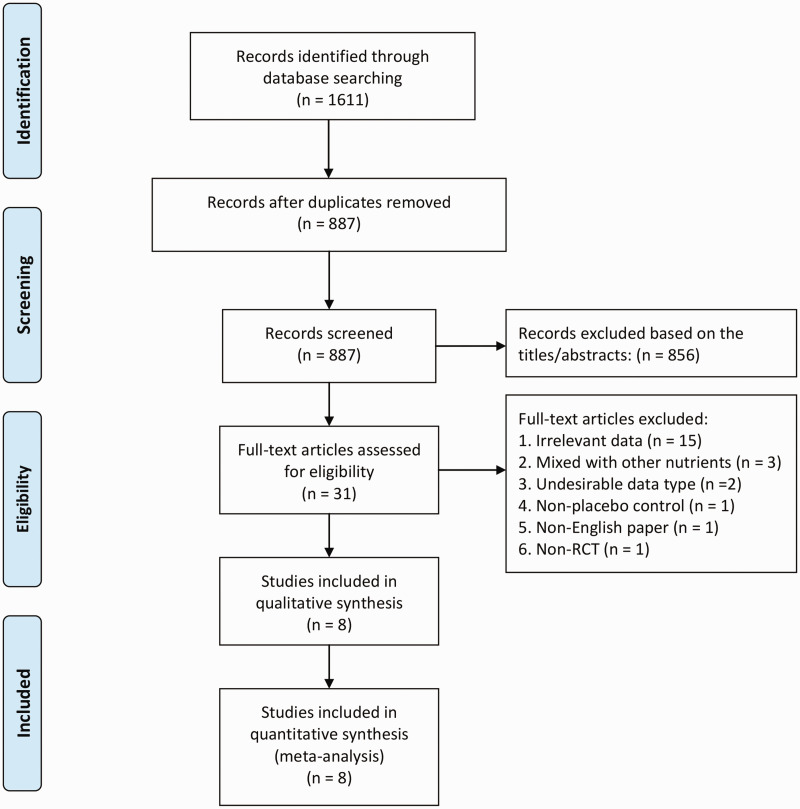
The study selection process is demonstrated in Figure 1. We identified 887 citations after duplications were removed. Following further in-depth screening, 31 complete articles were assessed. After reviewing the full-text of these articles, eight studies met the criteria for this meta-analysis.12–18,21 Among the eight qualified studies, one study involved two pair-wise comparisons with one comparison that used calcium as a co-supplement for both the VitD and the placebo groups.16 This study was enrolled as two separate trials. One study designed two interventional groups (with two different VitD doses) compared with the same control.13 We combined the two interventional groups into a single group using the formula mentioned above. Thus, nine studies from eight RCTs were considered in this meta-analysis.
Figure 1.
Flow schematic depicting the literature search and screening process.
Study characteristics
As shown in Table 1, nine studies from eight RCTs that were published between 2013 and 2018 included 467 participants who were enrolled into this study. Eight studies used the Rotterdam diagnostic criteria, and one study used the NIH criteria. The number of participants ranged from 28 to 90. All nine studies used cholecalciferol (VitD3) with a daily dose that ranged from 1000 to 12,000 IU. The intervention duration ranged from 8 to 24 weeks. Mean 25(OH)D levels ranged from 6.9 ± 2.8 ng/mL to 19.9 ± 9.5 ng/mL at baseline, all of which increased to above 20 ng/mL.
Table 1.
Study features in the systematic review.
| Literature | Diagnosis criteria | Subjects (n) | Ethnicity | VitD dose (IU/day) | Intervention | Follow-up time (weeks) | Baseline VitD (ng/mL)a | Final VitD (ng/mL)a |
|---|---|---|---|---|---|---|---|---|
| Rahimi-Ardabili,15 2013 | Rotterdam | 24/26 | Asian: 100% | 2500 | CholecalciferolPlacebo | 8 | 6.9 ± 2.87.3 ± 2.9 | 23.4 ± 6.148.57 ± 3.98 |
| Raja-Khan,18 2014 | NIH | 13/15 | N/A | 12000 | CholecalciferolPlacebo | 12 | 19.9 ± 9.522.2 ± 6.9 | 67.36 ± 28.6222.45 ± 7.02 |
| Asemi-1,16 2015 | Rotterdam | 26/26 | Asian: 100% | 7143 | CholecalciferolPlacebo | 8 | 11.6 ± 4.713.9 ± 2.0 | 23.4 ± 7.114.2 ± 2.2 |
| Asemi-2,16 2015 | Rotterdam | 26/26 | Asian: 100% | 7143 | Cholecalciferol+ Calcium Placebo + Calcium | 8 | 15.1 ± 3.614.0 ± 4.1 | 26.8 ± 7.814.4 ± 4.7 |
| Garg,21 2015 | Rotterdam | 15/17 | Asian: 100% | 4000 | Cholecalciferol + MET Placebo + MET | 24 | 7.7 ± 6.056.8 ± 2.46 | 31.5 ± 13.886.7 ± 2.31 |
| Irani,14 2015 | Rotterdam | 35/18 | Vitamin D group: Hispanic, 69.4%; Asian, 25%; Black, 5.5%; Placebo group: Hispanic, 72.2%; Asian, 22.2%; Black, 5.5% | 7143 | CholecalciferolPlacebo | 8 | 16.3 ± 0.917.0 ± 1.8 | 43.2 ± 2.417.4 ± 1.9 |
| Foroozanfard,13 2017 | Rotterdam | 30/30/30 | Asian: 100% | 40001000 | Cholecalciferol (high-dose) + METCholecalciferol (lose-dose) + METPlacebo + MET | 12 | 13.5 ± 3.114.0 ± 4.614.0 ± 3.5 | 24.3 ± 3.720.7 ± 6.214.1 ± 3.6 |
| Maktabi,17 2017 | Rotterdam | 35/35 | Asian: 100% | 3571 | CholecalciferolPlacebo | 12 | 12.8 ± 4.514.5 ± 5.1 | 27.5 ± 9.814.4 ± 5.2 |
| Dastorani,12 2018 | Rotterdam | 20/20 | Asian: 100% | 3571 | CholecalciferolPlacebo | 8 | 10.5 ± 2.511.0 ± 2.4 | 21.7 ± 5.910.9 ± 2.1 |
aMean ± SD
NIH, the 1990 National Institutes of Health criterion; MET, metformin; VitD, vitamin D; N/A, not applicable; SD, standard deviation.
Risk of bias evaluation
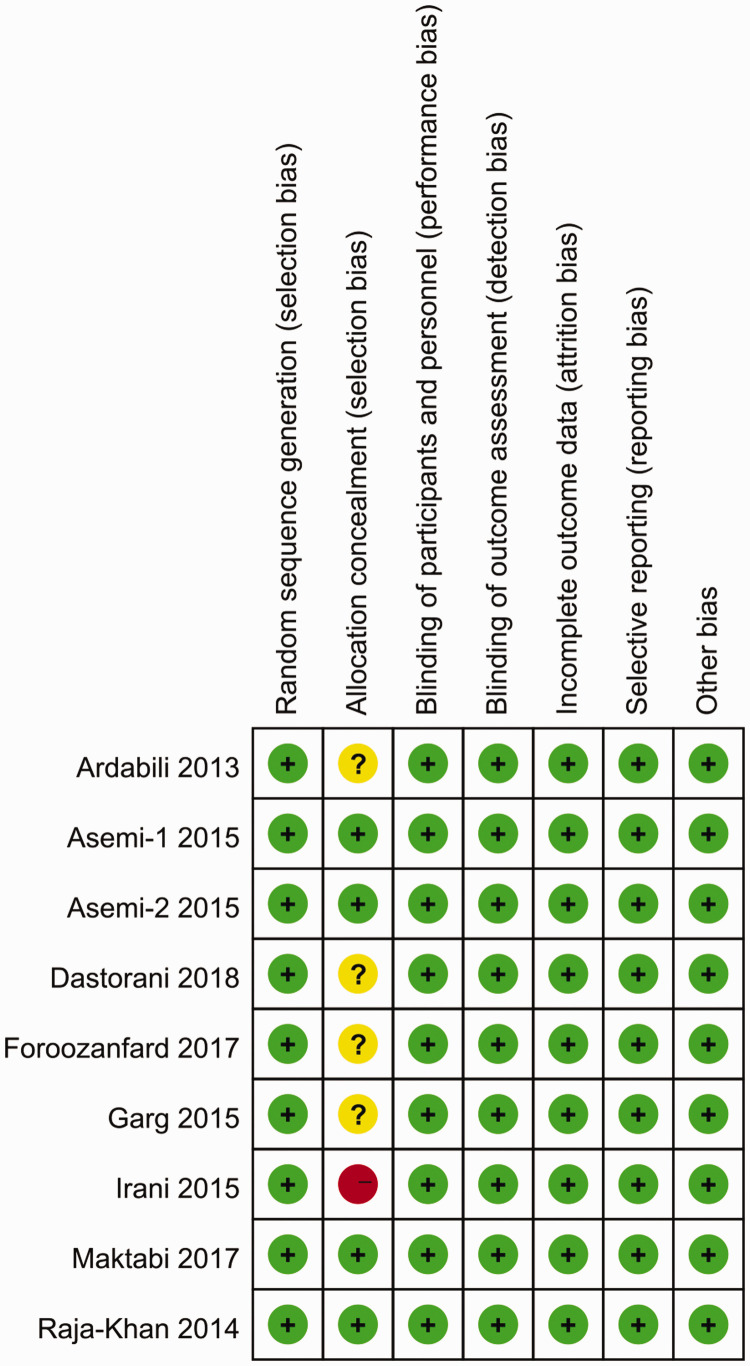
The quality assessment revealed that all possible biases were related to allocation concealment, with one study exhibiting a high risk because of the single-blind study design,14 and four studies had an unclear risk because of insufficient information about the allocation process.12,13,15,21 There was no other bias observed in any of the studies. Quality assessments are outlined in Figure 2.
Figure 2.
Quality assessment of the included studies using the Cochrane Collaboration’s tool. Review authors’ judgments about each risk of bias item for each included study. Green circle: low risk of bias; yellow circle: unclear risk of bias; red circle: high risk of bias.
Meta-analysis
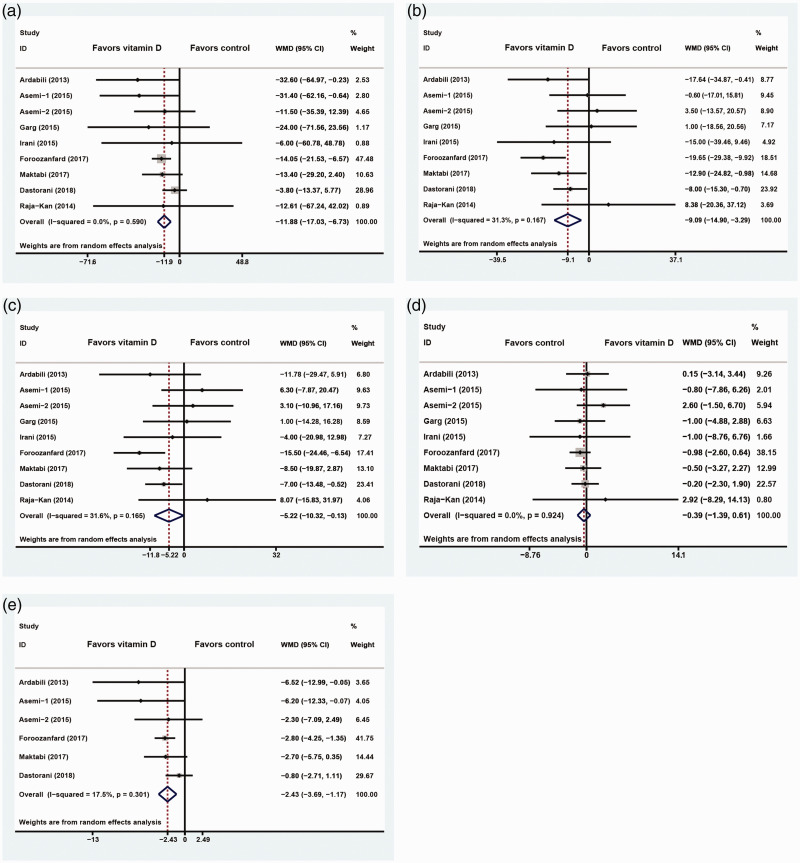
The level of heterogeneity was minimal across the studies (I2<50%). Nine studies with 467 PCOS patients suggested that the addition of exogenous VitD supplementation significantly reduced serum TG (−11.88 mg/dL; 95% CI: −17.03 to −6.73, I2 = 0%), TC (−9.09 mg/dL; 95% CI: −14.90 to −3.29, I2= 31.3%), and LDL-C (−5.22 mg/dL; 95% CI: −10.32 to −0.13, I2 = 31.6%) levels, while the serum HDL-C level remained statistically unchanged (−0.39 mg/dL; 95% CI: −1.39 to 0.61, I2 = 0%; Figure 3a–d). Six studies with 354 PCOS patients showed that VitD markedly improved serum VLDL-C levels (−2.43 mg/dL; 95% CI: −3.69 to −1.17, I2 = 17.5%; Figure 3e).
Figure 3.
Forest plot depicting the effect of vitamin D supplementation on serum TG (a), TC (b), LDL-C (c), HDL-C (d), and VLDL-C (e) levels in PCOS patients. Horizontal lines indicate 95% CIs; boxes show the study-specific weight; diamond represents the combined effect size; dashed line indicates the overall estimate.
TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very-low-density lipoprotein cholesterol; PCOS, polycystic ovary syndrome; CI, confidence interval.
Stratified analyses were performed based on VitD dosing and intervention duration (Table 2). When stratified by the VitD dose, the significant effects for TC, LDL-C, and VLDL remained for the trials at doses of ≤4000 IU/day, but they disappeared for trials with doses >4000 IU/day, while the effects for TG and HDL-C in each subgroup remained consistent with the overall pooled results. When stratified by intervention duration, the effects of VitD on TC were restricted to subgroups with a duration of ≤8 weeks, while the effect for VLDL-C only existed in subgroups with a duration of >8 weeks. Moreover, the intervention duration did not meaningfully influence the effect of VitD on either TG or HDL-C, and the effects of both subgroups was consistent with the overall effect sizes. In addition, VitD did not influence LDL-C regardless of the treatment duration.
Table 2.
Subgroup analysis of factors that influence the effect of VitD on lipid parameters among PCOS patients.
| Variables | Subgroups | Number of MD | Pooled estimates | P value | |
|---|---|---|---|---|---|
| TG | Dosage of VitD (IU/day) | ≤4000 | 4 | −11.40 (−19.03, −3.77) | 0.003 |
| >4000 | 5 | −17.90 (−33.88, −1.93) | 0.028 | ||
| Duration of study (week) | ≤8 | 5 | −12.25 (−24.27, −0.24) | 0.046 | |
| >8 | 4 | −14.11 (−20.75, −7.47) | <0.001 | ||
| TC | Dosage of VitD (IU/day) | ≤4000 | 4 | −13.27 (−19.22, −7.32) | <0.001 |
| >4000 | 5 | −0.20 (−9.09, 8.69) | 0.965 | ||
| Duration of study (week) | ≤8 | 5 | −7.26 (−12.95, −1.58) | 0.012 | |
| >8 | 4 | −10.39 (−21.10, 0.33) | 0.057 | ||
| LDL-C | Dosage of VitD (IU/day) | ≤4000 | 4 | −9.81 (−14.41, −5.21) | <0.001 |
| >4000 | 5 | 2.64 (−4.51, 9.79) | 0.469 | ||
| Duration of study (week) | ≤8 | 5 | −3.71 (−9.43, 2.01) | 0.203 | |
| >8 | 4 | −7.02 (−16.23, 2.18) | 0.135 | ||
| HDL-C | Dosage of VitD (IU/day) | ≤4000 | 4 | −0.57 (−1.66, 0.53) | 0.312 |
| >4000 | 5 | 0.46 (−1.96, 2.88) | 0.708 | ||
| Duration of study (week) | ≤8 | 5 | 0.22 (−1.33, 1.77) | 0.783 | |
| >8 | 4 | −0.82 (−2.13, 0.48) | 0.217 | ||
| VLDL-C | Dosage of VitD (IU/day) | ≤4000 | 4 | −2.30 (−3.77, −0.82) | 0.002 |
| >4000 | 2 | −3.78 (−7.55, −0.00) | 0.050 | ||
| Duration of study (week) | ≤8 | 4 | −2.83 (−5.68, 0.02) | 0.052 | |
| >8 | 2 | −2.78 (−4.09, −1.47) | <0.001 | ||
VitD, vitamin D; PCOS, polycystic ovary syndrome; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very-low-density lipoprotein cholesterol; MD, mean difference.
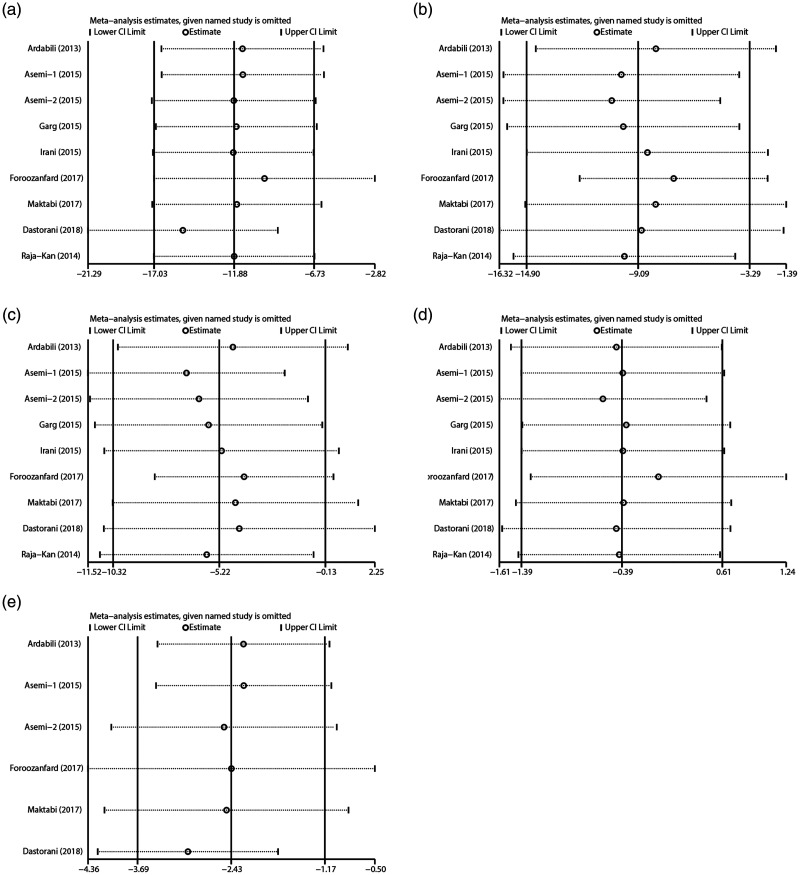
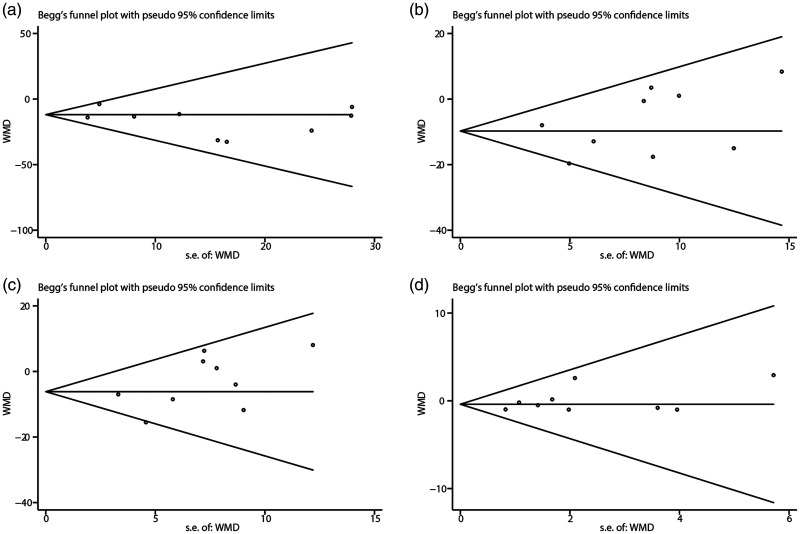
By sequentially removing individual studies, sensitivity showed that no single study excessively influenced the pooled effect sizes of all lipid parameters (Figure 4). In addition, Egger’s assessments and Begg’s funnel plots showed no publication bias based on the lipid indices (Figure 5).
Figure 4.
Influence analysis of an individual study on the pooled estimate for serum TG (a), TC (b), LDL-C (c), HDL-C (d), and VLDL-C (e) levels among PCOS patients. Open circle indicates the pooled mean difference, and the named study is omitted. Horizontal lines indicate 95% CIs.
TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very-low-density lipoprotein cholesterol; PCOS, polycystic ovary syndrome; CI, confidence interval.
Figure 5.
Begg’s funnel plot for assessing publication bias that measured the effect of VitD supplementation on serum TG (a), TC (b), LDL-C (c), and HDL-C (d) levels among PCOS patients.
TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; PCOS, polycystic ovary syndrome.
Discussion
In this meta-analysis, eight RCTs with nine comparisons among 467 participants supported that VitD intervention improved serum lipid profiles in PCOS patients, which manifested as statistically significant decreases in TG (−11.88 mg/dL), TC (−9.09 mg/dL), LDL-C (−5.22 mg/dL), and VLDL-C (−2.43 mg/dL) levels, while changes in serum HDL-C were negligible (−0.39 mg/dL). Stratified analyses showed that dose and duration influenced the effects of vitamin D supplementation. Administering ≤4000 IU/day of VitD sufficiently reduced TC (−13.27 mg/dL), LDL-C (−9.81 mg/dL), and VLDL-C (−2.30 mg/dL) levels, while a high dose of VitD was not beneficial. For duration, a concrete conclusion cannot be drawn because the optimal result for TC was achieved with a duration ≤8 weeks (−7.26 mg/dL), while the beneficial effect for VLDL-C was observed with a duration >8 weeks (−2.78 mg/dL).
In contrast to the results mentioned above, previous meta-analyses have demonstrated that VitD supplementation had no to minor effects on the lipid profiles of PCOS patients. For example, He et al.7 pooled evidence from two single-arm studies and three RCTs with 130 patients and found no favorable effects of VitD intervention on lipid features, although they confirmed a relationship between VDD and abnormal lipid features. Xue et al.22 analyzed two single-arm studies and four RCTs with 156 patients, and found that serum TG levels decreased after VitD intervention, while LDL-C levels remained unchanged. These studies exhibited great selection, reporting, and confounding bias associated with single-arm studies. Other meta-analyses evaluated this association with evidence from RCTs. For example, Pergialiotis et al.23 confined their analysis to two RCTs with 81 patients and found that there was an effect of VitD on serum TC but not LDL-C. Fang et al.33 concluded from four RCTs and 214 participants that VitD supplementation did not improve any lipid indices. However, these studies were underpowered because of the small sample size. Most recently, Miao et al.34 analyzed six RCTs with 254 participants and summarized that VitD intervention reduced serum TC and LDL-C levels, while TG and HDL-C levels were unchanged. Their results were comparable to our study, except that TG levels were also significantly improved (−11.88 mg/dL; 95% CI: −17.03, to −6.73, I2= 0%) in our study, which is similar to the results of previous RCTs13–16 and meta-analyses.7,22,23 In Miao et al.’s study,34 only post-treatment levels were evaluated when comparing the VitD intervention with placebo. Their results showed a marginally non-significant effect of VitD on TG (−5.59 mg/dL; 95% CI: −12.11 to 0.93, I2= 0%). Given the great variation of individual baseline values, their statistical method may not reflect the authentic effect of VitD intervention. In our study, the main outcome measurements were the changes in lipid levels from baseline, which eliminated the bias caused by different individual baseline values. Our study has higher power and better credibility because of the larger number of included participants. Another advantage of our meta-analysis is that we also included VLDL as a primary outcome, which was strongly associated with CVD events.35 Our results indicated that VLDL is improved after VitD intervention, which further supports the therapeutic effect of VitD among patients with PCOS and dyslipidemia.
The Endocrine Society guidelines recommended that those with VitD deficiency are administered 50,000 IU of VitD3 per week for an 8-week period, which is followed a low dose of 1500 to 2000 IU/day of VitD3 that should be taken as maintenance treatment.6 The delivery method or formula has, however, not been standardized, particularly in populations with different coexisting diseases. Our results revealed that the desired effects of VitD in terms of TC, LDL-C, and VLDL-C were only observed in the low dose (≤4000 IU/day) subgroup, while the high dose subgroup exhibited no obvious benefits. Similarly, Xue et al.22 reported in their meta-analysis that only low VitD doses (<50,000 IU) improved the serum levels of TG in patients with PCOS.22 As indicated by Sanders et al.,36 a high-dose VitD intervention, such as bolus doses of VitD >500,000 IU, is associated with some adverse effects such as fracture, gastrointestinal upset, and altered biochemical index. Therefore, we concluded that low-dose VitD (≤4000 IU/day) is enough to benefit lipid profiles without inducing unfavorable effects. For duration, the desired effect of VitD for TC was only observed in subgroups with a short duration (≤8 weeks), while the desired effect for VLDL-C only existed in subgroups that had a long duration (>8 weeks). Taking into account these results along with the recommendation from the Endocrine Society Guidelines, we believe that administration of VitD for a period of 8 weeks is sufficient to improve TC levels in PCOS patients. The null results seen with a longer duration may result from poor participant compliance, while inconsistent results regarding VLDL-C may be explained by the small number of trials.
The mechanisms through which VitD improves lipid profiles have been intensely studied. VitD reduces cholesterol synthesis by activating the transcription of insulin-induced gene-2 (Insig-2), which downregulates SREBP-2 and inhibits HMGR activity, which is the rate-limiting step in cholesterol synthesis.37 VitD also reduces TG accumulation in differentiated 3T3-L1 adipocytes and facilitates fatty acid β-oxidation, thereby protecting against excessive fat mass deposition and an associated metabolic disturbance.38 The link between VitD and HDL-C is not as clear. Studies in VDR knockout mice demonstrated higher HDL-C and hepatic apoA-1 (the main component of HDL-C) mRNA expression relative to their wild-type counterparts.39 However, human studies have yielded conflicting results, and the serum 25(OH)D levels were positively associated with plasma apoA-1 and HDL, while an inverse association was observed in the small intestine and hepatocytes.40
As mentioned above, basic research has elucidated promising pathophysiological pathways that seem to associate VitD with lipid metabolism. Cross-sectional studies have also suggested a positive correlation between VDD and an unfavorable lipid profile in patients with PCOS.41,42 Although several interventional studies have explored the causality of this linkage, a consistent conclusion has still not been reached. Recently, Menichini et al.43 summarized that serum TG was significantly decreased after VitD intervention in four RCTs with PCOS patients, while no changes were found in LDL-C, HDL-C, and TC. However, this conclusion was based on a systematic review without statistical support. A meta-analysis is supposed to provide more robust evidence when lacking a large-scale controlled clinical trial. However, previous meta-analyses demonstrated that VitD had no to minor effects on the lipid profile of PCOS patients. Therefore, strong evidence that links VDD to the development of dyslipidemia in patients with PCOS was lacking. This is the largest meta-analysis to date that focused on the comprehensive parameters of the lipid profile, and our results pointed to a cause–effect association between VDD and dyslipidemia in women presenting with PCOS. Thus, VDD may not just be a complication or manifestation of PCOS, but rather, it may be partially involved in the pathogenesis of PCOS. Our results support the general use of VitD among PCOS patients as a therapeutic agent for dyslipidemia. Further basic research and larger high-quality prospective studies are still needed to test this hypothesis.
This is the largest meta-analysis to date that focused on the parameters of a lipid profile in women presenting with PCOS, with evidence obtained and analyzed from RCTs. The randomization of participants allows more objective findings to be synthesized. Some limitations should be considered in this meta-analysis. We only included studies that showed dose–response associations between VitD administration and changes in lipid levels. Because of our limited sample size, statistical errors occurred. This meta-analysis was largely limited to Asian populations, and our conclusions may not be easily generalizable to other global populations.
Conclusions
This meta-analysis of RCTs demonstrated that VitD supplementation could improve serum lipid indices, with no influence on HDL-C levels in PCOS patients. Dose and duration of VitD administration influenced its effects on lipid profiles. VitD intervention might benefit PCOS patients who are at high risk of VitD deficiency and an atherogenic lipid profile. Because of potential drawbacks of this current analysis, future well-designed RCTs with a larger sample size are required with the intention of further confirming our key conclusions.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (Grant number LQ19H040005) and the Medical and Health Science and Technology Research Program of Zhejiang Province (Grant number 2018KY220; 2019RC114).
ORCID iD
References
- 1.Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016; 2: 16057. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic ovarian syndrome (PCOS): long-term metabolic consequences. Metabolism 2018; 86: 33–43. [DOI] [PubMed] [Google Scholar]
- 3.Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med 2019; pii: S1050-1738(19)30128-8. [DOI] [PubMed] [Google Scholar]
- 4.Glintborg D, Rubin KH, Nybo M, et al. Cardiovascular disease in a nationwide population of Danish women with polycystic ovary syndrome. Cardiovasc Diabetol 2018; 17: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Groot PC, Dekkers OM, Romijn JA, et al. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update 2011; 17: 495–500. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- 7.He C, Lin Z, Robb SW, et al. Serum vitamin D levels and polycystic ovary syndrome: a systematic review and meta-analysis. Nutrients 2015; 7: 4555–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TJ. Vitamin D and cardiovascular disease. Annul Rev Med 2016; 67: 261–272. [DOI] [PubMed] [Google Scholar]
- 9.Alyami AM, Lam V, Soares MJ, et al. The association of vitamin D status with dyslipidaemia and biomarkers of endothelial cell activation in older Australians. Nutrients 2016; 8: pii: E457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Peng M, Chen S, et al. Vitamin D deficiency is associated with dyslipidemia: a cross-sectional study in 3788 subjects. Curr Med Res Opin 2019; 35: 1059–1063. [DOI] [PubMed] [Google Scholar]
- 11.Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 2010; 20: 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dastorani M, Aghadavod E, Mirhosseini N, et al. The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod Biol Endocrinol 2018; 16: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foroozanfard F, Talebi M, Samimi M, et al. Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res 2017; 49: 612–617. [DOI] [PubMed] [Google Scholar]
- 14.Irani M, Seifer DB, Grazi RV, et al. Vitamin D supplementation decreases TGF-beta1 bioavailability in PCOS: a randomized placebo-controlled trial. J Clin Endocrinol Metab 2015; 100: 4307–4314. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi-Ardabili H, Pourghassem Gargari B, Farzadi L. . Effects of vitamin D on cardiovascular disease risk factors in polycystic ovary syndrome women with vitamin D deficiency. J Endocrinol Invest 2013; 36: 28–32. [DOI] [PubMed] [Google Scholar]
- 16.Asemi Z, Foroozanfard F, Hashemi T, et al. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr 2015; 34: 586–592. [DOI] [PubMed] [Google Scholar]
- 17.Maktabi M, Chamani M, Asemi Z. The effects of vitamin D supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res 2017; 49: 493–498. [DOI] [PubMed] [Google Scholar]
- 18.Raja-Khan N, Shah J, Stetter CM, et al. High-dose vitamin D supplementation and measures of insulin sensitivity in polycystic ovary syndrome: a randomized, controlled pilot trial. Fertil Steril 2014; 101: 1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeeshan J, Papageorgiou M, Harshal D, et al. A randomized, controlled trial of vitamin D supplementation on cardiovascular risk factors, hormones, and liver markers in women with polycystic ovary syndrome. Nutrients 2019; 11: pii: E188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trummer C, Schwetz V, Kollmann M, et al. Effects of vitamin D supplementation on metabolic and endocrine parameters in PCOS: a randomized-controlled trial. Eur J Nutr 2019; 58: 2019–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg G, Kachhawa G, Ramot R, et al. Effect of vitamin D supplementation on insulin kinetics and cardiovascular risk factors in polycystic ovarian syndrome: a pilot study. Endocr Connect 2015; 4: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue Y, Xu P, Xue K, et al. Effect of vitamin D on biochemical parameters in polycystic ovary syndrome women: a meta-analysis. Arch Gynecol Obstet 2017; 295: 487–496. [DOI] [PubMed] [Google Scholar]
- 23.Pergialiotis V, Karampetsou N, Panagopoulos P, et al. The effect of vitamin D supplementation on hormonal and glycaemic profile of patients with PCOS: a meta-analysis of randomised trials. Int J Clin Pract 2017; 71: e12957. [DOI] [PubMed] [Google Scholar]
- 24.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19: 41–47. [DOI] [PubMed] [Google Scholar]
- 25.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach In: A Dunaif, JR Givens, FP Haseltine, GR Merriam. (eds) Polycystic ovary syndrome. Boston: Blackwell Scientific, 1992, pp.377–384. [Google Scholar]
- 26.Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006; 91: 4237–4245. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Follmann D, Elliott P, Suh I, et al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992; 45: 769–773. [DOI] [PubMed] [Google Scholar]
- 29.Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013; 33: 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 33.Fang F, Ni K, Cai Y, et al. Effect of vitamin D supplementation on polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin 2017; 26: 53–60. [DOI] [PubMed] [Google Scholar]
- 34.Miao CY, Fang XJ, Chen Y, et al. Effect of vitamin D supplementation on polycystic ovary syndrome: a meta-analysis. Exp Ther Med 2020; 19: 2641–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pechlaner R, Tsimikas S, Yin X, et al. Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol 2017; 69: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders KM, Nicholson GC, Ebeling PR. Is high dose vitamin D harmful? Calcif Tissue Int 2013; 92: 191–206. [DOI] [PubMed] [Google Scholar]
- 37.Li S, He Y, Lin S, et al. Increase of circulating cholesterol in vitamin D deficiency is linked to reduced vitamin D receptor activity via the Insig-2/SREBP-2 pathway. Mol Nutr Food Res 2016; 60: 798–809. [DOI] [PubMed] [Google Scholar]
- 38.Larrick BM, Kim KH, Donkin SS, et al. 1,25-Dihydroxyvitamin D regulates lipid metabolism and glucose utilization in differentiated 3T3-L1 adipocytes. Nutr Res 2018; 58: 72–83. [DOI] [PubMed] [Google Scholar]
- 39.Wang JH, Keisala T, Solakivi T, et al. Serum cholesterol and expression of ApoAI, LXRbeta and SREBP2 in vitamin D receptor knock-out mice. J Steroid Biochem Mol Biol 2009; 113: 222–226. [DOI] [PubMed] [Google Scholar]
- 40.Jaimungal S, Wehmeier K, Mooradian AD, et al. The emerging evidence for vitamin D-mediated regulation of apolipoprotein A-I synthesis. Nutr Res 2011; 31: 805–812. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Lv S, Li F, et al. Vitamin D deficiency is associated with metabolic risk factors in women with polycystic ovary syndrome: a cross-sectional study in Shaanxi, China. Front Endocrinol (Lausanne) 2020; 11: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krul-Poel YHM, Koenders PP, Steegers-Theunissen RP, et al. Vitamin D and metabolic disturbances in polycystic ovary syndrome (PCOS): a cross-sectional study. PLoS One 2018; 13: e0204748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menichini D, Facchinetti F. Effects of vitamin D supplementation in women with polycystic ovary syndrome: a review. Gynecol Endocrinol 2020; 36: 1–5. [DOI] [PubMed] [Google Scholar]