Abstract
The sudden outbreak of severe acute respiratory syndrome coronavirus 2 pneumonia posed a significant challenge to medical professionals because treatment of critically ill patients requires the efforts of a multidisciplinary team. To highlight this principle, we examined acute kidney injury (AKI) in IgA-dominant infection-associated glomerulonephritis (GN) and menstrual toxic shock syndrome (mTSS). Both GN and mTSS are rare diseases caused by staphylococcal infection, and renal function is frequently impaired. The resulting AKIs are disparate pathological entities driven by distinct immune mechanisms. We begin by describing the case of a diabetic man with pyopneumothorax following methicillin-resistant Staphylococcus aureus (MRSA). He had endocapillary proliferative GN with in situ IgA-dominant immune-complex formation in the mesangium accompanied by complement C3 deposition in the glomerular capillary wall. By contrast, acute tubular necrosis was observed in a case of mTSS; the patient’s immune response was stimulated differently by MRSA enterotoxin and exotoxin resulting in aberrant IgA deposition, complement activation, and insufficient antibody production. As a multidisciplinary communication covering the fields of nephrology, immunology, and pathology, this report may help clinicians to understand these distinct renal lesions and make optimal therapeutic decisions expeditiously.
Keywords: Acute kidney injury, infection-associated glomerulonephritis, IgA, menstrual toxic shock syndrome, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, renal pathology
Introduction
The sudden outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia in Wuhan caught the majority of the medical community off guard.1 Our previous work reported another de novo zoonotic viral disease recently found to be transmissible in humans and cautioned against potential spread.2 Among critically ill patients who died from coronavirus disease 2019 (COVID-19), 67.0%, 29.0% and 13.5% had acute respiratory distress syndrome, acute kidney injury (AKI) and nosocomial infections, respectively.3 In the face of this grim situation, Chinese medical staff, clinicians and researchers alike, formed a multidisciplinary team (MDT) under the leadership of prominent academician Nan-Shan Zhong.4 In the spirit of the MDT concept, we describe two pathologically disparate cases of AKI in serious pulmonary infection and a rare form of severe sepsis. Both cases were caused by Staphylococcus aureus. We begin by discussing postinfectious glomerulonephritis (GN).
Postinfectious GN is an immune-mediated disorder of the kidney that occurs post hoc following nonrenal infections, especially by streptococci.5 The fundamental pathologic mechanism underlying this entity is believed to be deposition of immune complexes within the glomerular tufts, eliciting various histopathologic changes ranging from mild mesangial proliferation to diffuse exudative proliferation with crescents.6 Accordingly, a wide spectrum of clinical presentations can be observed including subclinical or asymptomatic GN, acute nephritic syndrome and rapidly progressive nephritic syndrome.7 The pathogenic microorganisms, histopathology and clinical presentations associated with postinfectious GN have become increasingly diverse in recent years.8 IgA-dominant postinfectious GN is one such presentation.9
IgA-dominant infection-associated GN is relatively rare and is usually seen in male patients, particularly diabetic patients with staphylococcal infections.10 IgA is the sole or dominant immunoglobulin deposited in the glomeruli and most cases histologically exhibit endocapillary hypercellularity and neutrophil infiltration. In affected patients, these changes may predispose to AKI. Indeed, Haas et al.11 found that only 2% of patients in their cohort had a peak serum creatinine (Scr) within the normal range. Alternatively, S. aureus may also cause a severe infection called menstrual toxic shock syndrome (mTSS) in which AKI is almost unavoidable.12
AKI may be an active participant in the infection rather than a consequence. We consistently found that compromised renal function may expose immunosuppressed patients with kidney disease to higher risk of severe infection.13,14 Hence, clinicians should be familiar with the bidirectional interactions between infection and AKI, a lesson learned in the recent battle against COVID-19.3 The roles of staphylococcal superantigens and immune-mediated injuries in AKI are also discussed.15 Hopefully, our report will promote multidisciplinary collaboration and enable comprehensive treatment by providing a central source of information.
Case 1
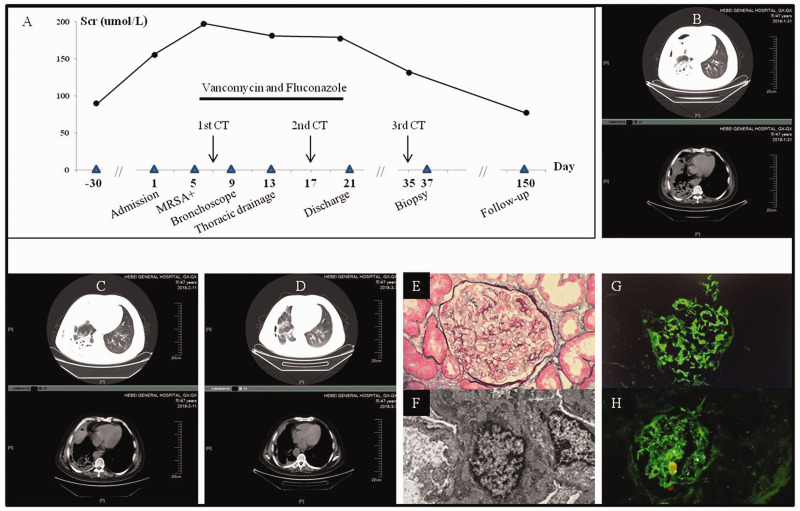
A 47-year-old diabetic man was referred to our facility with fever, pneumonia, pyopneumothorax and AKI (Figure 1). He had been taking oral prednisone for Sjögren syndrome for 6 months; the dose was tapered to 10 mg daily prior to admission. Upon arrival, his temperature was 39.3°C and he was experiencing hemoptysis and dyspnea. Laboratory tests showed white blood cells (WBCs) of 11.3 × 109/L with 94.8% neutrophils, a T-lymphocytes of 320/µL (reference 690–1760/µL), hemoglobin 110 g/L (130–150 g/L), platelets 127 × 109/L (100–300 × 109/L), plasma albumin 32.1 g/L (40–55 g/L), Scr 155.6 µmol/L (88.4–132.6 µmol/L) that was normal 1 month ex ante, fasting blood sugar 8.7 mmol/L (3.9–6.1 mmol/L), total triiodothyronine 1.0 pmol/L (1.3–3.1 pmol/L), IgG 4.1 g/L (7.2–16.5 g/L), IgA 0.97 g/L (0.69–3.28 g/L), complement C3 1.02 g/L (0.85–1.93 g/L) and C4 0.22 g/L (0.12–0.36 g/L), C-reactive protein 470.9 mg/L (<10 mg/L), procalcitonin 1.89 ng/mL (<0.05 ng/mL), and (1,3)-β-D-glucan 23.7 pg/mL (<10 pg/mL). Urinalysis showed 3+ occult blood and 1+ protein. Further diagnostic workup was negative for antistreptolysin O, antinuclear, anti-glomerular basement membrane and antineutrophil cytoplasmic antibodies. Serum immunofixation electrophoresis was also negative. Methicillin-resistant S. aureus (MRSA) was isolated from both blood and purulent thoracic drainage. During his hospital stay, the patient was placed under stringent glycemic control, administered intravenous vancomycin and oral fluconazole, and underwent bronchoscopy and pleural washout. Accordingly, his AKI was mitigated but not fully recovered in tandem with his pulmonary infection. Until then the patient reluctantly accepted the renal biopsy that was previously declined. The result confirmed a diagnosis of IgA-dominant infection-associated GN. The patient’s Scr eventually returned to normal range (77.7 –mol/L).
Figure 1.
Clinical, imaging, and renal pathologic features of case 1. A. Clinical course of the patient’s severe pulmonary infection and acute kidney injury. Scr: serum creatinine, MRSA: methicillin-resistant Staphylococcus aureus. B. The first computed tomography (CT) scan of the chest on admission showed right pulmonary abscess, pleural effusion and pyopneumothorax on the parenchymal and mediastinal window, respectively. C. The second CT scan during vancomycin treatment showed amelioration of right pulmonary infection. D. The third CT scan showed near absorption of pulmonary infection. E. Light microscopy showed endocapillary proliferative glomerulonephritis. F. Electron microscopy showed electron-dense deposits in the mesangial region. G and H. Immunofluorescence microscopy for IgA and C3, respectively, showed their deposition in the mesangium and capillary wall.
Case 2
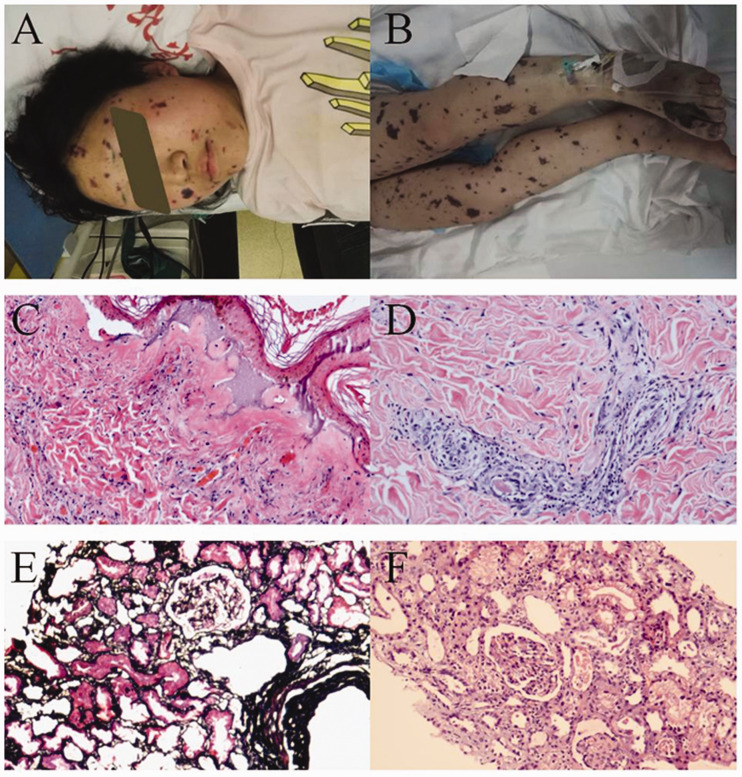
A previously healthy 16-year-old menstruating girl was rushed to our emergency room with fever and hypotension. She had neither vaginal discomfort nor abnormal discharge. Upon admission, her temperature was 37.5°C with a pulse of 137 beats/minute, a respiratory rate of 37 breaths/minute, and a blood pressure of 70/33 mmHg. Extensive skin ecchymosis was visible throughout the body (Figure 2). Subsequent laboratory tests showed WBCs of 90.3 × 109/L with 91.6% neutrophils, hemoglobin 71 g/L, platelets 18 × 109/L, plasma albumin 58.2 g/L, Scr 132.0 µmol/L, complement C3 0.60 g/L, C-reactive protein 195.5 mg/L, and procalcitonin >100 ng/mL. Liver function test results were abnormal with no specific pattern of abnormalities. Bedside chest plain film and blood cultures yielded negative findings. Furthermore, biopsy of the bone marrow and skin lesions revealed grossly normal hyperplasia and leukocytoclastic vasculitis, respectively. Methicillin-sensitive S. aureus was identified from cultures of purulent secretions from buttock skin lesions and palmoplantar desquamations that occurred during her hospital stay. All lines of evidence confirmed a diagnosis of mTSS and treatment included intravenous piperacillin and tazobactam and supportive measures. Her Scr once surged to 800.0 µmol/L with anuria and hypoxemia, requiring renal replacement therapy and mechanical ventilation, respectively. The patient’s condition stabilized after intensive care and her urine volume began to recover. Nonetheless, renal biopsy was performed and expectedly showed acute tubular necrosis with ischemic injury. After 45 days, the patient showed near-complete recovery except for an Scr of 141.2 µmol/L.
Figure 2.
Systemic ecchymosis and skin and renal pathologic findings of case 2. A and B. Extensive skin ecchymosis of the face and legs was observed in the patient with mTSS. C and D. Light microscopy examination of skin lesions showed massive accumulation of neutrophils and karyorrhexis within the dermis of the lesion center with wall necrosis in some of the small vessels and red blood cell overspill. Also visible was vascular endothelial swelling, narrowing of the vascular lumen and infiltration of the vascular wall by neutrophils and lymphocytes within the dermis surrounding the lesion. Subepidermal vesicles were observed as well. These changes were consistent with leukocytoclastic vasculitis. E and F. Light microscopy showed ischemic shrinkage of most glomerular capillary loops and, in the tubular epithelium, shedding of the brush border, lumen expansion, exposure of the basement membrane and epithelial regeneration. Exfoliated cell debris and proteins were observed within the lumen, slight edema of the interstitium and indiscernible changes within the arterioles. These alterations reflected acute tubular necrosis with ischemic injury.
Discussion
The development of modern medical science has been greatly specialized within each discipline, resulting in great depth but narrow focus. This fine division may create invisible barriers among individual medical branches, rendering clinicians less familiar and knowledgeable beyond their field of specialty. Delayed nephrology consultation for patients with acute renal failure was associated with increased mortality.16 We found that a multidisciplinary therapeutic approach was vital in managing critically ill cancer patients with AKI.17 More recently, the survival of patients with severe COVID-19 pneumonia reportedly depended on a similar approach.18 To further highlight the MDT concept, we describe two cases of AKI in patients with GN and mTSS.
MRSA infection is still prevalent in developing countries19 and Staphylococcus is now three times more common than Streptococcus as the causative agent of acute postinfectious GN in the elderly population.20 The situation is more precarious in hemodialysis patients in whom MRSA accounts for the majority of bacterial infections.21 The high prevalence of staphylococcal infection may result in increased incidence of IgA-dominant infection-associated GN. However, this is sometimes overshadowed by the systemic manifestations of infection itself and thus easily overlooked. Therefore, attending clinicians should be more aware of this disease in all aspects, especially its mechanisms, glomerular abnormalities, differential diagnosis and prognosis of renal function.
IgA-dominant infection-associated GN exhibited a male predominance of roughly 4:1, higher than the male-to female ratio of 2:1 reported for poststreptococcal glomerulonephritis.22 In addition, 55% of diagnosed patients had underlying diabetes.19 The principal pathologic mechanisms are now thought to be in situ IgA-dominant immune-complex formation and direct activation of plasmin by bacterial antigens, which may lead to complement activation and inflammation within the glomerulus.23 Interestingly, intense C3 deposition as shown in Figure 1 is a hallmark of this disease and can help distinguish it from IgA nephropathy or IgA vasculitis.24 In MRSA-associated cases, staphylococcal enterotoxin may act as a superantigen, which during inflammatory cascades binds to antigen-presenting cells promoting massive T cell activation, production of proinflammatory cytokines and secretion of IgA.25,26 The advanced glycation end-products in diabetic patients were speculated to be responsible for decreased IgA clearance and/or increased synthesis.27 Because of aberrant IgA deposit and complement activation, the major pathological changes in the glomeruli are endocapillary (63.3%), mesangial (31.8%) and crescent GN (4.9%).9
Based on these pathological changes, the clinical renal presentations of IgA-dominant infection-associated GN may include AKI, hematuria, proteinuria, and hypertension; these overlap considerably with the presentation of general post-infectious GN. In our experience, this disease should be suspected in patients who present with acute GN in the setting of infection, especially in those with known susceptibility to S. aureus and/or diabetes. In this context, the main differential diagnoses consist of poststreptococcal GN, systemic lupus erythematosus, Goodpasture’s syndrome, antineutrophil cytoplasmic antibody-associated vasculitis and monoclonal gammopathy.28 This undertaking requires recognition of subtle risk factors, monitoring for occult clinical symptoms and careful attention to laboratory results.14
The kidney is one of the most affected organs during multiple organ failure associated with sepsis,29 and mTSS places renal function in dire jeopardy.30 In an early report, full-swing acute renal failure was observed in all nine fatal cases of mTSS.31 Unlike IgA-dominant infection-associated GN, S. aureus exotoxin rather than enterotoxin is responsible for mTSS and associated multiple organ failure. Because bacterial invasion of the blood is minimal31 and antibody responses against exotoxin are weak,12 AKI is mainly caused by hemodynamic perturbances, direct injury caused by the exotoxin or both. Thus, staphylococcal superantigens may play a role by enhancing the deleterious actions of the exotoxin and deficiencies in the host’s immune system are implicated.32 Therefore, acute tubular necrosis is predominantly observed in comparison with the glomerular injury in immune-mediated GN (Figure 2). Perplexingly, a number of children with COVID-19 were found to have features in common with TSS according to a recent BMJ report.33 Hence, clinicians need to be aware of any existing and emerging evidence related to this syndrome.
Treatment of IgA-dominant infection-associated GN is primarily focused on achieving control of the precipitating infection and giving supportive measures. Vancomycin has been the cornerstone for treatment of MRSA infections.34 However, this agent may also cause AKI35 and Scr-dependent fine dose adjustment is recommended.36 Glucocorticoids are presumably of little help according to previous reports.37,38 About half of patients may regain initial renal function, whereas end stage renal disease developed in approximately 20% of patients.39 Our report found that recovery of renal function may lag behind recovery from pulmonary infection. Clearance of immune complexes from the glomeruli is a function of time.40 Nonetheless, any protracted recovery of renal function warrants the participation of nephrologists to rule out persistent injury within the glomeruli by renal biopsy. The safety of this procedure was recently questioned in immunocompromised patients or patients with unbridled infection because of the potential risk of sepsis 41 or perinephric abscess.42 We believe that renal biopsy is preferable for cases with uncertain diagnosis or prognosis and should be handled with discretion in cases with bacteremia or septicemia, such as mTSS, in which the etiology of AKI is less ambiguous.
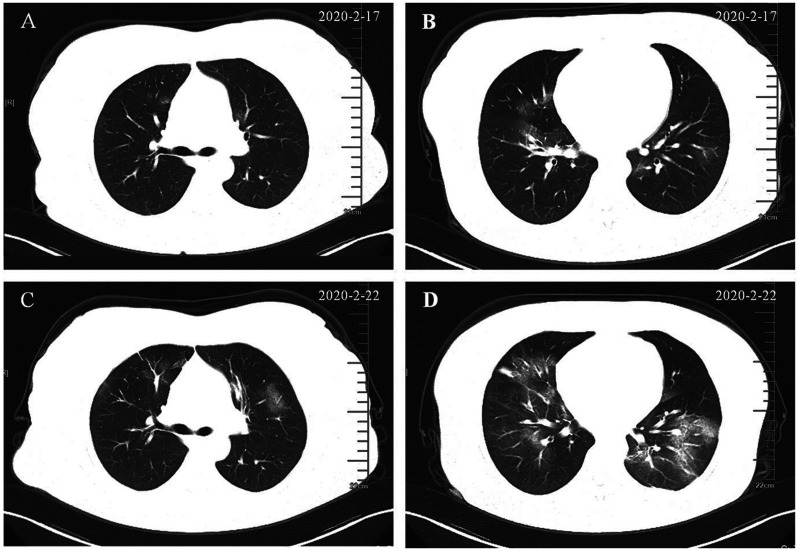
Today we are engaged in containing COVID-19, which requires that we are clinicians first and specialist physicians second.43 Though conceived in the view of nephrology, we tried to convey an important message that latent diseases associated with a prima facie renal injury should be carefully considered in light of the MDT concept. Thus, we prepared this report as a review instead of a case series. As such, inflammatory cascades such as those induced by staphylococcal superantigens may evolve into cytokine storms. In this regard, the mechanism of cardiac, pulmonary and renal injury in COVID-19 is believed to be a systemic cytokine storm syndrome and/or direct cellular injury caused by the virus.44,45 A patient with SARS-CoV-2 fulminant myocarditis in moribund condition was successfully saved following treatment with glucocorticoids and human immunoglobulin.46 In February we successfully identified an asymptomatic patient with pneumonia (Figure 3) who initially presented with incident proteinuria. He received four nucleic acid tests for SARS-COV-2 within 7 days while in preemptive isolation, with only the last being positive. Supporting our rationale, a more recent report found that 34% of patients with COVID-19 developed massive albuminuria on the first day of admission.45 We aim to share this frontline knowledge with fellow medical staff fighting the current pandemic.
Figure 3.
Chest CT evolution of a patient with COVID-19, who initially presented with incident proteinuria and showed no signs of respiratory disease. A and B. The first CT scan showed patchy ground-glass opacity and a negative nucleic acid test conducted on the same day. C and D. Corresponding slices of the second CT scan 5 days later showed progressive pulmonary lesions. Two more nucleic acid tests between the two scans remained negative and the fourth one, conducted 2 days after the second scan was positive. We speculate that proteinuria was likely a renal involvement of COVID-19.
Using evidence from a variety fields of expertise, we re-examined two cases of AKI in patients with IgA-dominant infection-associated GN and mTSS from the perspective of the underlying immune responses, mechanisms of damage, structural changes of the nephron and timing of biopsy. Our experience may be of help to clinicians in general and infectious disease specialists in particular when combating intractable and sometimes inexorably lethal disease such as COVID-19.
Acknowledgements
We thank Dr. Lin Kang of the Department of Pathology, Hebei Provincial General Hospital, for preparing pathological materials and Mr. Eric J. Gayetsky (Dublin, OH, USA) for proofreading the manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of Hebei General Hospital and written informed consent was obtained from patient (case 1) or the guardians (case 2).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Hui-zhi Zhao https://orcid.org/0000-0002-6967-5028
References
- 1.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet 2020; 395: 470–473. DOI: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Hu M, Wei D, et al. From severe herpes zoster to rare suid herpesvirus encephalitis: a new twist of the Varicellovirus genus infection in patients with kidney diseases. Int J Med Sci 2020; 17: 745–750. DOI: 10.7150/ijms.41952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang XB, Yu Y, Xu JQ, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. DOI: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005; 5: 685–694. [DOI] [PubMed] [Google Scholar]
- 6.Nasr SH, Markowitz GS, Stokes MB, et al. Acute postinfectious glomerulonephritis in the modern era: experience with 86 adults and review of the literature. Medicine (Baltimore) 2008; 87: 21–32. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Iturbe B, Batsford S. Pathogenesis of poststreptococcal glomerulonephritis a century after Clemens von Pirquet. Kidney Int 2007; 71: 1094–1104. [DOI] [PubMed] [Google Scholar]
- 8.Kanjanabuch T, Kittikowit W, Eiam-Ong S. An update on acute postinfectious glomerulonephritis worldwide. Nat Rev Nephrol 2009; 5: 259–269. [DOI] [PubMed] [Google Scholar]
- 9.Nasr SH, D’Agati VD. IgA-dominant postinfectious glomerulonephritis: a new twist on an old disease. Nephron Clin Pract 2011; 119: c18–c25. [DOI] [PubMed] [Google Scholar]
- 10.Caetano J, Pereira F, Oliveira S, et al. IgA-dominant postinfectious glomerulonephritis induced by methicillin-sensitive Staphylococcus aureus. BMJ Case Rep. 2015; 2015: bcr2014208513. DOI: 10.1136/bcr-2014-208513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas M, Racusen LC, Bagnasco SM. IgA-dominant postinfectious glomerulonephritis: a report of 13 cases with common ultrastructural features. Hum Pathol 2008; 39: 1309–1316. [DOI] [PubMed] [Google Scholar]
- 12.Berger S, Kunerl A, Wasmuth S, et al. Menstrual toxic shock syndrome: case report and systematic review of the literature. Lancet Infect Dis 2019; 19: e313–e321. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Jia Y, Chu B, et al. Nocardiosis in kidney disease patients under immunosuppressive therapy: case report and literature review. Int J Med Sci 2019; 16: 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Zhang Y, Ping F, et al. Predicting risk of pulmonary infection in patients with primary membranous nephropathy on immunosuppressive therapy: the AIM-7C score. Nephrology 2019; 24: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 15.Koyama A, Kobayashi M, Yamaguchi N, et al. Glomerulonephritis associated with MRSA infection: a possible role of bacterial superantigen. Kidney Int 1995; 47: 207–216. [DOI] [PubMed] [Google Scholar]
- 16.Mehta RL, McDonald B, Gabbai F, et al. Nephrology consultation in acute renal failure: does timing matter? Am J Med 2002; 113: 456–461. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Zhang Y, Li QX, et al. Acute kidney injury in cancer patients and impedance cardiography-assisted renal replacement therapy: Experience from the onconephrology unit in a Chinese tertiary hospital. Exp Ther Med 2017; 14: 5671–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Wu C, Zheng X, et al. Clinical features and multidisciplinary treatment outcome of COVID-19 pneumonia: A report of three cases. J Formos Med Assoc 2020; S0929-6646(20): 30144–30153. DOI: 10.1016/j.jfma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebremedhn G, Gebremariam TT, Wasihun AG, et al. Prevalence and risk factors of methicillin-resistant Staphylococcus aureus colonization among HIV patients in Mekelle, Northern Ethiopia. Springerplus 2016; 5: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasr SH, Fidler ME, Valeri AM, et al. Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol 2011; 22: 187–195. [DOI] [PubMed] [Google Scholar]
- 21.Kan LP, Lin JC, Chiu SK, et al. Methicillin-resistant Staphylococcus aureus bacteremia in hemodialysis and nondialysis patients. J Microbiol Immunol Infect 2014; 47: 15–22. [DOI] [PubMed] [Google Scholar]
- 22.Muscatello DJ, O’Grady KA, Neville K, et al. Acute poststreptococcal glomerulonephritis: public health implications of recent clusters in New South Wales and epidemiology of hospital admissions. Epidemiol Infect 2001; 126: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasr SH, Radhakrishnan J, D’Agati VD. Bacterial infection-related glomerulonephritis in adults. Kidney Int 2013; 83: 792–803. [DOI] [PubMed] [Google Scholar]
- 24.Satoskar AA, Suleiman S, Ayoub I, et al. Staphylococcus infection-associated GN-spectrum of IgA staining and prevalence of ANCA in a single-center cohort. Clin J Am Soc Nephrol 2017; 12: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satoskar AA, Nadasdy G, Plaza JA, et al. Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol 2006; 1: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 26.Yoh K, Kobayashi M, Yamaguchi N, et al. Cytokines and T-cell responses in superantigen-related glomerulonephritis following methicillin-resistant Staphylococcus aureus infection. Nephrol Dial Transplant 2000; 15: 1170–1174. [DOI] [PubMed] [Google Scholar]
- 27.Kanauchi M, Kawano T, Dohi K. Serum IgA levels in patients with diabetic nephropathy and IgA nephropathy superimposed on diabetes mellitus. Diabetes Res Clin Pract 2000; 48: 113–118. [DOI] [PubMed] [Google Scholar]
- 28.Downing NS, McMullan CJ, Rennke HG, et al. Complements from the lung. N Engl J Med 2018; 379: 1767–1773. [DOI] [PubMed] [Google Scholar]
- 29.Fan H, Zhao Y, Chen GD, et al. Health insurance status and risk factors of mortality in patients with septic acute kidney injury in Ningbo, China. J Int Med Res 2019; 47: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sada R, Fukuda S, Ishimaru H. Toxic shock syndrome due to community-acquired methicillin-resistant Staphylococcus aureus infection: two case reports and a literature review in Japan. IDCases 2017; 8: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larkin SM, Williams DN, Osterholm MT, et al. Toxic shock syndrome: clinical, laboratory, and pathologic findings in nine fatal cases. Ann Intern Med 1982; 96: 858–864. [DOI] [PubMed] [Google Scholar]
- 32.Kulhankova K, King J, Salgado-Pabón W. Staphylococcal toxic shock syndrome: superantigen-mediated enhancement of endotoxin shock and adaptive immune suppression. Immunol Res 2014; 59: 182–187. [DOI] [PubMed] [Google Scholar]
- 33.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020; 369: m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandell LA. Infections in the compromised host. J Int Med Res 1990; 18: 177–190. [DOI] [PubMed] [Google Scholar]
- 35.Luan Y, Sun Y, Duan S, et al. Pathogenic bacterial profile and drug resistance analysis of community-acquired pneumonia in older outpatients with fever. J Int Med Res 2018; 46: 4596–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navalkele B, Pogue JM, Karino S, et al. Risk of acute kidney injury in patients on concomitant vancomycin and piperacillin-tazobactam compared to those on vancomycin and cefepime. Clin Infect Dis 2017; 64: 116–123. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava RN, Moudgil A, Bagga A, et al. Crescentic glomerulonephritis in children: a review of 43 cases. Am J Nephrol 1992; 12: 155–161. [DOI] [PubMed] [Google Scholar]
- 38.Orfila C, Lepert JC, Modesto A, et al. Rapidly progressive glomerulonephritis associated with bacterial endocarditis: efficacy of antibiotic therapy alone. Am J Nephrol 1993; 13: 218–222. [DOI] [PubMed] [Google Scholar]
- 39.Bu R, Li Q, Duan ZY, et al. Clinicopathologic features of IgA-dominant infection associated glomerulonephritis: a pooled analysis of 78 cases. Am J Nephrol 2015; 41: 98–106. [DOI] [PubMed] [Google Scholar]
- 40.Hebert LA. Disposition of IgA-containing circulating immune complexes. Am J Kidney Dis 1988; 12: 388–392. [DOI] [PubMed] [Google Scholar]
- 41.Luciano RL, Moeckel GW. Update on the native kidney biopsy: Core curriculum 2019. Am J Kidney Dis 2019; 73: 404–415. [DOI] [PubMed] [Google Scholar]
- 42.Illeperuma PB, Dissanayake HA, Wijewickrama ES. Retroperitoneal abscess with subcutaneous extension: case report of a rare complication of percutaneous renal biopsy. BMC Nephrol 2018; 19: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Rio C, Malani PN. 2019 Novel coronavirus-important information for clinicians. JAMA 2020; 323: 1039–1040. DOI: 10.1001/jama.2020.1490. [DOI] [PubMed] [Google Scholar]
- 44.Rizzo P, Vieceli Dalla Sega F, Fortini F, et al. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol 2020; 115: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naicker S, Yang CW, Hwang SJ, et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int 2020; 97: 824–828. DOI: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu H, Ma F, Wei X, et al. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J 2020; ehaa190. DOI: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]