Abstract
Objective
Studies have demonstrated that tetrandrine reverses multidrug resistance (MDR) in animal models or cell lines derived from multiple cancer types. We examined the potential MDR reversal activity of tetrandrine in a multidrug-resistant variant of a human laryngeal cancer Hep-2 cell line and explored potential mechanisms involved.
Methods
We developed the multidrug-resistant variant cell line (Hep-2/v) by exposing Hep-2 cells to stepwise increasing concentrations of vincristine (VCR). After Hep-2 or Hep-2/v cells were treated with tetrandrine (2.52 µg/mL), MDR was measured by MTT assay, rhodamine 123 retention was measured by flow cytometry, and mRNA and protein expression of multidrug resistance 1 (MDR1), regulator of G-protein signaling 10 (RGS10), high-temperature requirement protein A1 (HTRA1), and nuclear protein 1 (NUPR1) were detected by real-time reverse transcription-PCR and western blotting, respectively.
Results
Tetrandrine significantly lowered the half-maximal inhibitory concentration (IC50) of VCR in Hep-2/v cells, resulting in a 2.22-fold reversal of MDR. Treatment with tetrandrine increased rhodamine 123 retention, downregulated the mRNA and protein expression of MDR1 and RGS10, and upregulated expression of HTRA1 in Hep-2/v cells.
Conclusion
We showed that tetrandrine exerts anti-MDR activity in Hep-2/v cells, possibly by inhibiting MDR1 overexpression-mediated drug efflux and by altering expression of HTRA1 and RGS10.
Keywords: Tetrandrine, multidrug resistance reversal, Hep-2 cells, laryngeal cancer, multidrug resistance 1, high-temperature requirement protein A1, regulator of G-protein signaling 10
Introduction
Laryngeal cancer is one of the most common head and neck cancers. Surgery and radiation therapy are the most frequently used treatments for laryngeal cancer. Chemotherapy is most commonly used with radiation therapy to treat large tumors or tumors that have spread to the lymph nodes or distant areas. Several recent studies have indicated that laryngeal cancer is potentially curable with chemotherapy alone.1 However, multidrug resistance (MDR) is a major obstacle to the success of chemotherapy for laryngeal cancer.
The development of MDR is a multifactorial process that is mediated by multiple mechanisms,2 including reduced drug accumulation due to increased expression of efflux drug transporters, such as multidrug resistance 1 (MDR1)3,4 and multidrug resistance-associated proteins,5 increased drug inactivation resulting from metabolic alterations, increased ability to repair or tolerate DNA lesions due to increased expression of DNA topoisomerases,6 and inhibition of apoptosis by altering the expression of apoptosis-associated genes or proteins, such as p53 and Bcl-2.7,8 Agents directed against the specific targets involved in these mechanisms might reverse MDR.
To date, a wide range of agents have been reported to have MDR reversal activity. Examples include calcium channel blockers,9 calmodulin antagonists,10 and immunomodulators. Although a number of agents that possess potent, long-lasting MDR reversal properties have been identified,11 many have unacceptable side effects when used at effective doses.12 Therefore, great efforts have been made to discover low-toxicity natural herbal substances that have anti-MDR activity.13
Tetrandrine, a compound isolated from Stephania tetandra and other Chinese herbs, is a calcium channel blocker.14 Previous studies demonstrated that tetrandrine and its derivatives could reverse MDR in animal models or cell lines derived from osteosarcoma,15 breast cancer,16 and leukemia.17 However, few studies have investigated whether tetrandrine reverses MDR in laryngeal cancer. Furthermore, the mechanisms underlying tetrandrine-induced MDR reversal in tumor cells are not completely understood. In the present study, we examined the potential MDR reversal activity of tetrandrine in a multidrug-resistant human laryngeal cancer Hep-2 cell variant and explored the potential mechanisms involved.
Material and methods
Ethical approval
Ethical approval was deemed unnecessary because our studies did not involve animal or human experiments.
Cell lines and cell culture
The human laryngeal cancer cell line Hep-2 was provided by the Chinese Academy of Medical Sciences (Beijing, China). Hep-2/v, a drug-resistant human laryngeal cancer Hep-2 cell variant, was developed by exposing Hep-2 cells to stepwise increasing concentrations (from 0.02 to 0.96 µmol/L) of vincristine (VCR, Sigma, St. Louis, MO, USA). Cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (Invitrogen), 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
MTT assay
Hep-2 or Hep-2/v cells were digested with 0.25% trypsin to prepare single cell suspensions. After adjusting the cell density to 5 × 104 cells/mL, the cells were seeded at 100 µL/well in 96-well plates in triplicate and exposed to different concentrations of tetrandrine (0.78, 1.56, 3.13, 6.25, 12.5, 25, or 50 µg/mL, dissolved in 0.1 N HCl and adjusted to pH 6.6–6.8 with 1 N NaOH) or VCR for 72 hours, followed by incubation with MTT solution for 4 hours. RPMI 1640 medium was used as a blank control. At the end of the incubation period, dimethyl sulfoxide was added at 200 µL/well, and the plates were incubated in an air bath shaker at 37°C for 5 minutes. The absorbance at 490 nm (A490) was measured using a microplate reader to assess cell viability. The dose response curve was then plotted to determine the half-maximal (IC50) and 10% (IC10) inhibitory concentrations. The IC10 concentration of tetrandrine was used in subsequent experiments.
Rhodamine 123 retention assay
Hep-2 or Hep-2/v cells (2 × 106), untreated or treated with tetrandrine (2.52 µg/mL) for 48 hours, were harvested to prepare single cell suspensions. Then, 2.5 µL of rhodamine 123 (5 mmol/L; Sigma) was added and the cells were incubated at 37°C for 30 minutes. The cells were then centrifuged at 60 × g to remove the supernatant, washed with fresh medium, and incubated at 37°C for 10 minutes. After washing the cells again with fresh medium, the cells were resuspended in precooled medium and subjected to flow cytometric analysis of rhodamine 123 fluorescence to count the number of rhodamine-positive cells. Rhodamine 123 retention was expressed as the percentage of rhodamine 123-positive cells.
Quantitative real-time reverse transcription-PCR
Total RNA was extracted from Hep-2 or Hep-2/v cells, untreated or treated with tetrandrine (2.52 µg/mL) for 24 hours, and reverse transcribed into cDNA using M-MLV reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Real-time PCR was then performed to determine the expression levels of MDR1, regulator of G-protein signaling 10 (RGS10), high-temperature requirement protein A1 (HTRA1), and nuclear protein 1 (NUPR1); β-actin (ACTB) was used as a control for quantification. The primers used for MDR1, RGS10, HTRA1, NUPR1, and ACTB were as follows: MDR1 (258 bp), 5′-GCACTAAAGTAGGAGACAAAGGAA-3′, 5′-TGACTCTGCCATTCTGAAACAC-3′; RGS10 (304 bp), 5′-GGCCGCCGTCAGACATCCAC-3′, 5′-AGCCGAGACTGCCCCTCCAC-3′; HTRA1 (210 bp), 5′-TGCCTGTCCTGCTGCTTGGC-3′, 5′-ACGGGCCTCCCGAGTTTCCA-3′; NUPR1 (358 bp), 5′-GGCTGGACTCAGGGACCGACT-3′, 5′-TCCGGCCTCCACCTCCGA-3′; and ACTB (250 bp), 5′-CATGTACGTTGCTATCCAGGC-3′, 5′-CTCCTTAATGTCACGCACGAT-3′. The expression level of each mRNA was measured using the 2−ΔΔCt method.
Western blotting
Total cell extracts from Hep-2 or Hep-2/v cells, untreated or treated with tetrandrine (2.52 µg/mL) for 24 hours, were prepared and subjected to spectrophotometric measurement of protein concentration. Forty micrograms of total cell protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked for 1 hour at room temperature in PBS containing 0.3% Tween 20 and 5% skim milk and then incubated overnight at 4°C with an anti-MDR1 antibody (dilution 1:1,000; Chemicon, Temecula, CA, USA), anti-HTRA1 antibody (dilution 1:1,000; Abcam, Cambridge, MA, USA), anti-RGS10 antibody (dilution 1:1,000; Abcam), or anti-actin antibody (dilution 1:1,500; Chemicon). Antibody binding was revealed by incubation with horseradish peroxidase-coupled secondary antibody (dilution 1:5,000; Pierce, Rockford, IL, USA) for 1 hour at room temperature. Chemiluminescence was detected using enhanced chemiluminescence reagents (Pierce). The relative level of MDR1 protein to ACTB was determined by densitometric scanning.
Statistical analysis
Statistical analysis was performed using the SPSS version 11.0 software package (SPSS Inc., Chicago, IL, USA). Numerical data were expressed as mean ± standard deviation (SD). The means between two groups were compared using Student’s t-test. The comparison of multiple means was performed using analysis of variance. Categorical data were compared using the chi-square test.
Results
Tetrandrine partially reverses multidrug resistance of Hep-2/v cells
To determine the maximum noncytotoxic concentration (IC10) of tetrandrine to use in subsequent experiments, we performed the MTT assay to determine the cytotoxicity of tetrandrine by exposing Hep-2 cells to different concentrations of tetrandrine. The results showed that the IC10 for tetrandrine in Hep-2 cells was 2.52 µg/mL.
We next examined the MDR reversal activity of tetrandrine in Hep-2/v cells by exposing the cells to 2.52 µg/mL tetrandrine and a full range of concentrations of VCR. The IC50 values for VCR in Hep-2 and Hep-2/v cells were 0.04 ± 0.01 and 1.8 ± 0.20 µmol/L, respectively; Hep-2/v cells were 45-fold more resistant to VCR than Hep-2 cells. Tetrandrine at 2.52 µg/mL lowered the IC50 for VCR in Hep-2/v cells to 0.81 ± 0.33 µmol/L, indicating a 2.22-fold reversal of MDR.
Tetrandrine increases rhodamine 123 retention in Hep-2/v cells
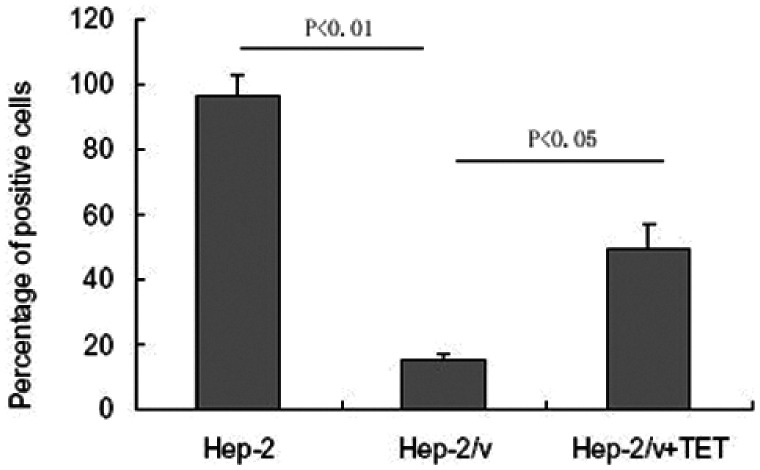
As shown in Figure 1, the percentage of rhodamine 123-positive Hep-2 cells was significantly higher than that of rhodamine 123-positive Hep-2/v cells (96.35 ± 6.56% vs. 15.12 ± 2.23, P < 0.01), suggesting increased efflux of rhodamine 123 in the Hep-2/v cells. Tetrandrine at 2.52 µg/mL significantly increased the percentage of rhodamine 123-positive Hep-2/v cells to 49.26 ± 8.14% (P < 0.05), indicating that tetrandrine could reduce the efflux of rhodamine 123 in Hep-2/v cells.
Figure 1.
Tetrandrine increases rhodamine 123 retention in Hep-2/v cells. Hep-2/v cells were treated with tetrandrine for 48 hours and incubated with rhodamine 123; then, rhodamine 123 fluorescence was detected by flow cytometry to count the number of rhodamine-positive cells. Data shown represent the percentages of rhodamine 123-positive cells expressed as mean ± SD.
TET, tetrandrine; Hep-2, human laryngeal cancer cell line; Hep-2/v, multidrug-resistant human laryngeal cancer Hep-2 cell line variant.
Tetrandrine decreases mRNA and protein expression of MDR1 in Hep-2/v cells
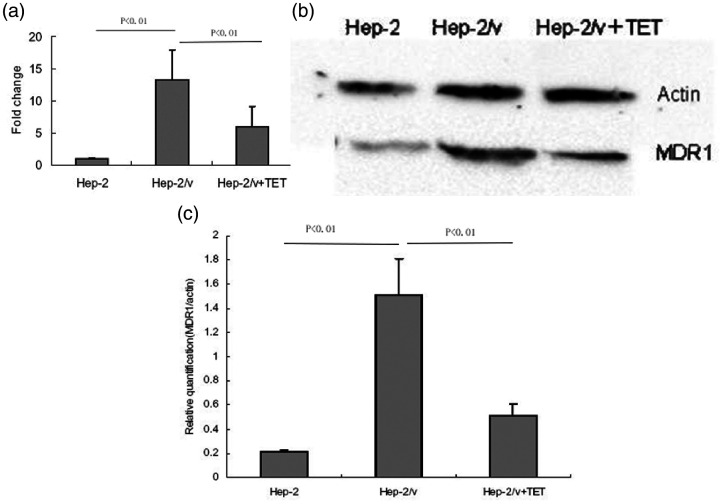
To examine the effect of tetrandrine on the mRNA expression of MDR1 in Hep-2/v cells, real-time RT-PCR was performed. As shown in Figure 2a, the expression level of MDR1 mRNA was significantly higher in Hep-2/v cells than in Hep-2 cells (P < 0.01). Treatment with tetrandrine resulted in an approximately 50% decline in the expression level of MDR1 mRNA in Hep-2/v cells (P < 0.01).
Figure 2.
Tetrandrine downregulates MDR1 mRNA and protein expression in Hep-2/v cells. (a) Relative expression of MDR1 mRNA in Hep-2 cells, untreated Hep-2/v cells, and Hep-2/v cells treated with tetrandrine. The relative levels of MDR1 mRNA in different types of cells were determined by real-time RT-PCR. Data shown are expressed as mean ± SD. (b) Representative images of western blots used to quantify the expression of MDR1 protein (relative to β-actin) in Hep-2 cells, untreated Hep-2/v cells, and Hep-2/v cells treated with tetrandrine. (c) Relative expression of MDR1 protein in Hep-2 cells, untreated Hep-2/v cells, and Hep-2/v cells treated with tetrandrine. Western blot data in Figure 2 and Figure 3 were derived from samples in the same group. Data shown are expressed as mean ± SD.
TET, tetrandrine; Hep-2, human laryngeal cancer cell line; Hep-2/v, multidrug-resistant human laryngeal cancer Hep-2 cell line variant; MDR1, multidrug resistance 1.
We next examined the effect of tetrandrine on the protein expression of MDR1 in Hep-2/v cells using western blot analysis (Figure 2b and 2c). The expression level of MDR1 protein was significantly higher in Hep-2/v cells than in Hep-2 cells (P < 0.01). Treatment with tetrandrine significantly decreased the expression of MDR1 protein in Hep-2/v cells (P < 0.01).
Tetrandrine alters mRNA and protein expression of HTRA1 and RGS10 in Hep-2/v cells
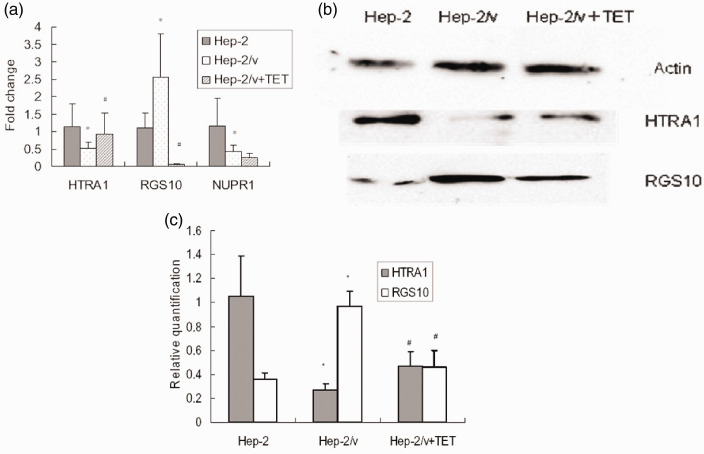
To examine the effect of tetrandrine on the mRNA expression of HTRA1, RGS10, and NUPR1 in Hep-2/v cells, real-time RT-PCR was performed. As shown in Figure 3a, the mRNA expression of HTRA1 and NUPR1 was significantly lower and that of RGS10 significantly higher in Hep-2/v cells than in Hep-2 cells (all P < 0.01). Treatment with tetrandrine significantly altered the expression of HTRA1 and RGS10 but did not affect expression of NUPR1 in Hep-2/v cells (P < 0.01).
Figure 3.
Tetrandrine alters mRNA and protein expression of HTRA1 and RGS10 in Hep-2/v cells. (a) Relative expression of HTRA1, RGS10, and NUPR1 mRNAs in Hep-2 cells, untreated Hep-2/v cells, and Hep-2/v cells treated with tetrandrine. The relative levels of HTRA1, RGS10, and NUPR1 mRNA in different types of cells were determined by real-time reverse transcription-PCR. Data shown are expressed as mean ± SD. (b) Representative images of western blots used to quantify the expression of HTRA1 and RGS10 proteins (relative to β-actin) in Hep-2 cells, untreated Hep-2/v cells, and Hep-2/v cells treated with tetrandrine. (c) Relative expression of HTRA1 and RGS10 proteins in Hep-2 cells, untreated Hep-2/v cells, and Hep-2/v cells treated with tetrandrine. Western blot data in Figure 2 and Figure 3 were derived from samples in the same group. Data shown are expressed as mean ± SD. *P < 0.01 vs. Hep-2 group, #P < 0.01 vs. Hep-2/v group
TET, tetrandrine; HTRA1, high-temperature requirement protein A1; RGS10, regulator of G-protein signaling 10; and NUPR1, nuclear protein 1; Hep-2, human laryngeal cancer cell line; Hep-2/v, multidrug-resistant human laryngeal cancer Hep-2 cell line variant.
We next examined the effect of tetrandrine on the protein expression of HTRA1 and RGS10 in Hep-2/v cells using western blot analysis (Figures 3b and 3c). The expression of HTRA1 protein was significantly lower and that of RGS10 protein significantly higher in Hep-2/v cells than in Hep-2 cells (both P < 0.01). Treatment with tetrandrine significantly increased the expression of HTRA1 protein and decreased that of MDR1 protein in Hep-2/v cells (both P < 0.01).
Discussion
Previous studies have shown that tetrandrine can reverse MDR in several tumor cell lines. Tetrandrine prevented the introduction of paclitaxel-induced MDR in osteosarcoma cells by inhibiting Pgp overexpression through a mechanism involving the inhibition of nuclear factor (NF)-κB signaling.15 Tetrandrine downregulated GCS gene expression by acting on Pglycoprotein to enhance the cytotoxic effect of daunorubicin (DNR) on human leukemia cell line K562/A02 and to reverse DNR resistance of K562/A02 cells.17 In the present study, the IC50 values in Hep-2/v cells treated with VCR alone were significantly increased. In contrast, the IC50 values in Hep-2/v cells treated with the VCR-tetrandrine combination were reduced, indicating that tetrandrine allows the retention of chemotherapy drugs during VCR treatment. We showed that tetrandrine at the IC10 concentration resulted in a 2.22-fold reversal of MDR in Hep-2/v cells. We also found that tetrandrine significantly increased rhodamine 123 retention and decreased the mRNA and protein expression of MDR1 in Hep-2/v cells. Tetrandrine upregulated mRNA and protein expression of HTRA1 and downregulated that of RGS10 in Hep-2/v cells. These findings suggest that tetrandrine can reverse MDR of Hep-2 cells, possibly via multiple mechanisms. In our previous studies, HTRA1, NUPR1, and RGS10 were identified as putative target genes by comparing the expression profiles of microRNAs and mRNAs of Hep-2 cells and Hep-2/v cells using microarray assays.18 Upregulation of HTRA1 expression attenuates DDP (cisplatin) resistance, and paclitaxel-induced cytotoxicity has been reported.19 Studies have demonstrated that NUPR1 can protect some cancer cells from apoptosis and confer resistance to some chemotherapeutic drugs.20,21 RGS10 was upregulated in Hep-2/v cells, and it may represent a novel MDR-associated protein; however, to date, no reports have shown a link between RGS10 expression and chemoresistance of cancer cells.
MDR1 overexpression-mediated drug efflux is an important mechanism that allows tumor cells to acquire MDR.22 Our results showed that retention of rhodamine 123 was significantly decreased and expression of MDR1 mRNA and protein were significantly upregulated in Hep-2/v cells. These findings suggest that MDR1 overexpression-mediated drug efflux is an important mechanism underlying MDR in Hep-2/v cells. Treatment with tetrandrine significantly increased retention of rhodamine 123 and decreased the mRNA and protein expression of MDR1 in Hep-2/v cells. This result is consistent with previous studies demonstrating that tetrandrine exerts anti-MDR activity by inhibiting MDR1 overexpression and increasing intracellular drug accumulation.23,24 However, MDR1 has a crucial role in healthy cells and MDR1 inhibitors fail because of their toxicity. Several studies have reported on the expression of MDR1 in immune cells, where it plays a protective role against xenobiotics and toxins.25 The inhibition of MDR1 expression in healthy cells may reduce its protective effects.
The emergence of MDR in tumor cells is multifactorial, involving multiple mechanisms.2–5 Accordingly, the mechanisms of action of MDR reversal agents are diverse. In addition to inhibiting MDR1 overexpression-mediated drug efflux, tetrandrine may exert anti-MDR activity in tumors by promoting chemotherapeutic drug-induced apoptosis.
In Hep-2/v cells, mRNA expression of HTRA1 and NUPR1 was significantly downregulated and that of RGS10 upregulated compared with expression in Hep-2 cells. Treatment with tetrandrine upregulated HTRA1 expression and downregulated RGS10 expression in Hep-2/v cells. Interestingly, a previous study demonstrated that upregulation of HTRA1 expression attenuates chemotherapeutic drug-induced cytotoxicity, whereas forced expression of HTRA1 enhances such a cytotoxic effect.26 These observations suggest that tetrandrine antagonizes MDR, possibly via mechanisms associated with upregulation of HTRA1 expression and downregulation of RGS10 expression.
Although significant numbers of MDR modulators have been identified, dose-limiting toxic effects and adverse pharmacokinetic interactions with chemotherapeutic agents have precluded their clinical application.27 In contrast, many natural products are less toxic, do not affect the pharmacokinetics of chemotherapeutic drugs, and may possess similar effectiveness to MDR modulators in reversing MDR.28 Previous studies have indicated that tetrandrine has no apparent toxic or adverse effect on the pharmacokinetics of chemotherapeutic drugs in mice when administered at plasma concentrations capable of reversing MDR.13,29 These findings, together with the observation that tetrandrine may have MDR reversal activity in different types of tumor cells,15–17 suggest that tetrandrine might have clinical potential as an MDR reversal agent in laryngeal cancer.
Conclusions
MDR was partially reversed in Hep-2/v cells after treatment with tetrandrine. Inhibition of MDR1 overexpression-mediated drug efflux might be a major mechanism responsible for tetrandrine-induced MDR reversal in Hep-2/v cells. Additionally, the anti-MDR activity exerted by tetrandrine in Hep-2/v cells may occur by mechanisms associated with altered HTRA1 and RGS10 expression. These findings have implications for the potential clinical use of tetrandrine as an MDR reversal agent in laryngeal cancer.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Science and Technology Development Plan of Jilin Province of China (20200801025GH) and Development and Reform Commission of Jilin Provincial (2019C049-8).
ORCID iD
Yachun Li https://orcid.org/0000-0002-4681-1007
References
- 1.Haigentz M, Jr, Silver CE, Rinaldo A, et al. Definitive chemotherapy: a new frontier in the fight against laryngeal cancer. Eur Arch Otorhinolaryngol 2010; 267: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stavrovskaya AA. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) 2000; 65: 95–106. [PubMed] [Google Scholar]
- 3.Zhigang H, Qi Z, Jugao F, et al. Reverse multidrug resistance in laryngeal cancer cells by knockdown MDR1 gene expression. J Otolaryngol Head Neck Surg 2009; 38: 440–448. [PubMed] [Google Scholar]
- 4.Li D, Zhou L, Huang J, et al. Effect of multidrug resistance 1/P-glycoprotein on the hypoxia-induced multidrug resistance of human laryngeal cancer cells. Oncol Lett 2016; 12: 1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature 2007; 446: 749–757. [DOI] [PubMed] [Google Scholar]
- 6.Delgado JL, Hsieh CM, Chan NL, et al. Topoisomerases as anticancer targets. Biochem J 2018; 475: 373–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye S, Shen J, Choy E, et al. p53 overexpression increases chemosensitivity in multidrug-resistant osteosarcoma cell lines. Cancer Chemother Pharmacol 2016; 77: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao C, Cao X, Fu Z, et al. Boschniakia Rossica polysaccharide triggers laryngeal carcinoma cell apoptosis by regulating expression of Bcl-2, caspase-3, and P53. Med Sci Monit 2017; 23: 2059–2064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Williams JB, Buchanan CM, Pitt WG. Codelivery of doxorubicin and verapamil for treating multidrug resistant cancer cells. Pharm Nanotechnol 2018; 6: 116–123. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Zhang Y, Chen Y, et al. A novel calmodulin antagonist O-(4-ethoxyl-butyl)-berbamine overcomes multidrug resistance in drug-resistant MCF-7/ADR breast carcinoma cells. J Pharm Sci 2010; 99: 3266–3275. [DOI] [PubMed] [Google Scholar]
- 11.Anreddy N, Gupta P, Kathawala RJ, et al. Tyrosine kinase inhibitors as reversal agents for ABC transporter mediated drug resistance. Molecules 2014; 19: 13848–13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Wei D, Han X, et al. The combinational effect of vincristine and berberine on growth inhibition and apoptosis induction in hepatoma cells. J Cell Biochem 2014; 115: 721–730. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Liu X, Li W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget 2016; 7: 40800–40815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhagya N, Chandrashekar KR. Tetrandrine and cancer - An overview on the molecular approach. Biomed Pharmacother 2018; 97: 624–632. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, Li F, Xu T, et al. Tetrandrine prevents multidrug resistance in the osteosarcoma cell line, U-2OS, by preventing Pgp overexpression through the inhibition of NF-kappaB signaling. Int J Mol Med 2017; 39: 993–1000. [DOI] [PubMed] [Google Scholar]
- 16.Jiang M, Zhang R, Wang Y, et al. Reduction-sensitive paclitaxel prodrug self-assembled nanoparticles with tetrandrine effectively promote synergistic therapy against drug-sensitive and multidrug-resistant breast cancer. Mol Pharm 2017; 14: 3628–3635. [DOI] [PubMed] [Google Scholar]
- 17.Chen B, Yin L, Cheng J, et al. Effect of D,L-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol and tetrandrine on the reversion of multidrug resistance in K562/A02 cells. Hematology 2011; 16: 24–30. [DOI] [PubMed] [Google Scholar]
- 18.Yin W, Wang P, Wang X, et al. Identification of microRNAs and mRNAs associated with multidrug resistance of human laryngeal cancer Hep-2 cells. Braz J Med Biol Res 2013; 46: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien J, Aletti G, Baldi A, et al. Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J Clin Invest 2006; 116: 1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark DW, Mitra A, Fillmore RA, et al. NUPR1 interacts with p53, transcriptionally regulates p21 and rescues breast epithelial cells from doxorubicin-induced genotoxic stress. Curr Cancer Drug Targets 2008; 8: 421–430. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury UR, Samant RS, Fodstad O, et al. Emerging role of nuclear protein 1 (NUPR1) in cancer biology. Cancer Metastasis Rev 2009; 28: 225–232. [DOI] [PubMed] [Google Scholar]
- 22.Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol 2010; 46: 308–316. [DOI] [PubMed] [Google Scholar]
- 23.Sun YF, Wink M. Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from Stephania tetrandra can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Phytomedicine 2014; 21: 1110–1119. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Liu XD, Liu Q, et al. Reversal of P-glycoprotein-mediated multidrug resistance by the novel tetrandrine derivative W6. J Asian Nat Prod Res 2015; 17: 638–648. [DOI] [PubMed] [Google Scholar]
- 25.Bossennec M, Di Roio A, Caux C, et al. MDR1 in immunity: friend or foe? Oncoimmunology 2018; 7: e1499388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao W, Zhu F, Duan Y, et al. HtrA1 resensitizes multidrug-resistant hepatocellular carcinoma cells by targeting XIAP. Biomed Pharmacother 2015; 70: 97–102. [DOI] [PubMed] [Google Scholar]
- 27.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci 2000; 11: 265–283. [DOI] [PubMed] [Google Scholar]
- 28.Karthikeyan S, Hoti SL. Development of fourth generation ABC inhibitors from natural products: a novel approach to overcome cancer multidrug resistance. Anticancer Agents Med Chem 2015; 15: 605–615. [DOI] [PubMed] [Google Scholar]
- 29.Bhagya N, Chandrashekar KR. Tetrandrine–A molecule of wide bioactivity. Phytochemistry 2016; 125: 5–13. [DOI] [PubMed] [Google Scholar]