Abstract
Feedback indicators can improve chest compression quality during cardiopulmonary resuscitation (CPR). However, the application of feedback indicators in the clinic practice is rare. Pulse oximetry has been widely used and reported to correlate spontaneous circulation restoration during CPR. However, it is unclear if pulse oximetry can monitor the quality of chest compression. We hypothesized that pulse rate monitored by pulse oximetry can be used as a feedback indicator of the chest compression rate during CPR in a porcine model of cardiac arrest. Seven domestic male pigs (30–35 kg) were utilized in this study. Eighteen intermittent chest compression periods of 2 min were performed on each animal. Chest compression and pulse oximetry plethysmographic waveforms were recorded simultaneously. Chest compression and pulse rates were calculated based on both waveforms. Compression interruption and synchronous pulse interruption times were also measured. Agreement was analyzed between pulse rates and synchronous chest compression rates, as well as between compression interruption times and synchronous pulse interruption times. A total of 126 compression periods of 2 min were performed on seven animals. Interclass correlation coefficients and Bland–Altman analysis revealed reliable agreement between pulse rates and synchronous chest compression rates. Similarly, compression interruption and synchronous pulse interruption times obtained also showed high agreement. Pulse rate can be used as an alternative indicator of chest compression rate during CPR in a porcine model of cardiac arrest. Pulse interruption time also can be used to reflect compression interruption time precisely in this model.
Keywords: Cardiac arrest, Cardiopulmonary resuscitation, Pulse rate, Pulse oximetry plethysmographic waveform, Feedback device
Introduction
Cardiac arrest is a major public health issue with high incidence and poor survival rate [1–3]. In order to improve resuscitation outcome and survival rate in patients with cardiac arrest, the 2015 American Heart Association (AHA) guidelines [4] recommended chains of survival, of which cardiopulmonary resuscitation (CPR) was highlighted as the critical lifesaving treatment. During CPR, high-quality chest compression is a key determinant for successful resuscitation and a critical foundation which impacts overall survival [5]. However, maintaining high quality chest compression is challenging, especially during prolonged CPR [6].
Without an accurate measure there can be no improvement [7]. Feedback tools for the metrics of chest compression quality have been developed to improve chest compression quality. Numerous studies have demonstrated that chest compression parameters provided by rescuers are closer to recommendations with feedback devices during resuscitation [8]. Chest compression rate is one key component of high quality CPR, and AHA guidelines recommended chest compression rates of 100–120 compressions/min for cardiac arrest management [9]. However, chest compression rates provided by trained rescuers often fail to attain this standard range [8]. It remains difficult for rescuers to maintain optimal chest compression rate at the scene of cardiac arrest without feedback [10]. Furthermore, the compression interruption times, which negatively correlate with overall survival [9, 11, 12], could also be minimized by feedback indicators [13, 14]. Therefore, feedback indicators can ensure high-quality chest compression during CPR and are associated with an increase in survival [13, 15, 16].
However, feedback indicators are rarely used in clinic practice. It has been reported that the feedback sensor is only used in approximately 50% of all cases in a recent study from Germany [16]. The main reason is that the feedback sensor is not readily available and is inconvenient to carry and use. Importantly, implementation of CPR training program is still challenging in the developing nations, not to mention CPR feedback device [17, 18]. Considering the cost-effectiveness ratio, it is unlikely that the feedback device is affordable, especially in developing nations. Therefore, it is essential to develop a convenient and cost-effective real-time feedback indicator for chest compression quality during CPR.
Pulse oximetry has been widely used around the world. Pulse oximetry plethysmographic waveform monitoring, is not only used for measuring oxygenated hemoglobin, but can also potentially measure peripheral tissue perfusions [19]. In a previous study, we demonstrated that the analysis of pulse oximetry plethysmographic waveform using time and frequency domain methods could be considered feasible, non-invasive markers for spontaneous circulation restoration during CPR [20]. However, it is unknown if pulse oximetry can be used to monitor the quality of chest compression. Therefore, we hypothesized that pulse rate can be used as a feedback indicator of the chest compression rate during CPR in a porcine model of cardiac arrest. In the current study, we also investigated if pulse interruption time can gauge compression interruption time according to real-time pulse oximetry plethysmographic waveform using a porcine model of cardiac arrest.
Materials and methods
All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the Ministry of Science and Technology of the People’s Republic of China. The protocol was approved by the Animal Care and Use Committee of Peking Union Medical College Hospital.
Animal preparation
A total of seven domestic pigs, weighting 30–35 kg were utilized and fasted overnight, except for access to water. Animals were anesthetized with an intramuscular injection of ketamine (20 mg/kg), then placed in the supine position on a U-shaped fixing frame and received a further intravascular injection with sodium pentobarbital (30 mg/kg).
A pulse oximeter sensor (Mindray Biological Medical Electronic Ltd, Shenzhen, China) was connected to the animal’s tails to monitor pulse oximetry plethysmographic waveform and oxygen saturation. Each pig was intubated with a 6.5 mm endotracheal tube and mechanically ventilated with inspired oxygen of 0.21 and a tidal volume of 10 mL/kg using volume-control mode ventilator (Mindray-E5, Mindray Biological Medical Electronic Ltd, Shenzhen, China). End-Tidal Carbon Dioxide (ETCO2) was monitored using a mainstream CO2 module (Capnostat, Shenzhen, China). Respiratory rates were adjusted to maintain the PetCO2 at 35–40 mmHg.
An electric blanket was used to maintain the animal’s temperature between 36.5 and 37.5 °C throughout, which was monitored rectally. Force and acceleration sensors were fixed on the sternum to collect chest compression waveforms. A 6-F catheter was inserted into the thoracic aorta through the right femoral artery for central aortic blood pressure (ABP) measurements and arterial waveform record.
A 7.5-F Swan-Ganz catheter (Edwards Life Sciences LLC, CA, USA) was advanced from the right femoral vein into the right atrium to measure core blood temperature and right atrial pressure (RAP). Both catheters were flushed intermittently with saline solution containing 5 IU heparin per ml. A 5 F pacing catheter (EP Technologies Inc., Mountain View, CA, USA) was placed from the right external jugular vein into the right ventricle. An electrocardiogram (ECG) was used throughout. A glucose and sodium chloride injection at 5 ml/kg/h was infused prior to the induction of ventricular fibrillation to maintain RAP between 3 and 5 mmHg. ECG, RAP, arterial waveform and pulse oximetry plethysmographic waveform were continuously monitored and collected using a T8 Mindray monitor (Mindray Biological Medical Electronic Ltd, Shenzhen, China). The compressor piston was positioned at the midline level of the fifth interspace.
Experimental procedure
Baseline (BL) measurements were obtained 15 min prior to inducing ventricular fibrillation (VF). VF was induced by 24V/50 Hz AC current with a right ventricular internal pacing electrode and was verified using both ECG waveform and rapid decrease to 20 mmHg in arterial blood pressure. Mechanical ventilation was discontinued after VF was established. The pacing catheter was pulled out before initiating resuscitation.
After 3 min of untreated VF, resuscitation was initiated using a mechanical CPR device (OA O1S-Z1, Shenzhen Light and Precision Automation LTD, Shenzhen, China), which was programmed to provide readings for chest compression depths and related rates. Once chest compression had been initiated, the ventilator was reconnected to the endotracheal tube and parameters (i.e., volume-controlled mode with a constant flow of 30 L/min, zero end-expiratory pressure, FiO2 1.0, RR 10/min, tidal volume of 8 mL/kg, I:E 1:2, and the upper airway pressure limit was set to 60 cmH2O, turning-off the inspiratory triggering sensitivity) were set.
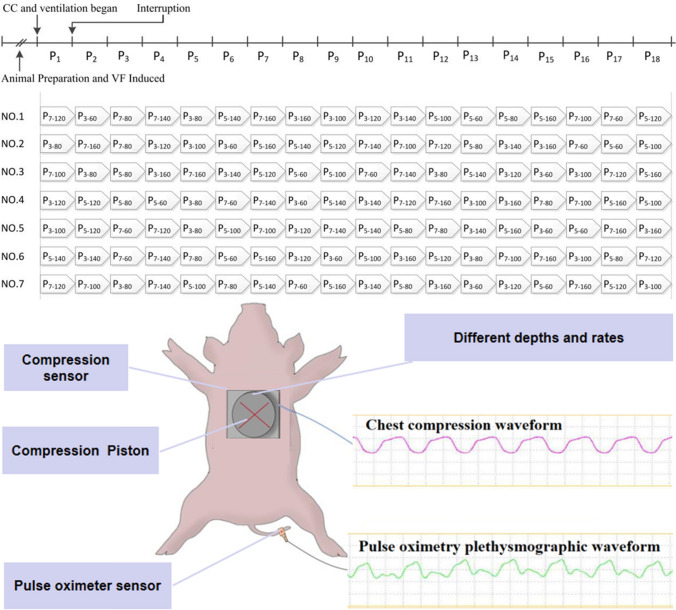
Each pig underwent 18 periods of compression under different settings, including P3–60, P3–80, P3–100, P3–120, P3–140, P3–160, P5–60, P5–80, P5–100, P5–120, P5–140, P5–160, P7–60, P7–80, P7–100, P7–120, P7–140 and P7–160. To clarify, P3–60 refers to a chest compression depth of 3 cm and chest compression rate of 60 times per minute while P5–80 refers to a chest compression depth of 5 cm and chest compression rate repeating at 80 times per minute. Each compression period lasted 2 min, before intentional interruptions, where settings were adjusted and compression was recommenced.
Compression interruption times between two compression periods were randomly generated with times ranging from 10 to 100 s. The sequence of 18 compression periods for each animal was also generated randomly. Necropsy was regularly performed to detect any experimental insults. The entire experimental procedure has been provided in Fig. 1.
Fig. 1.
Experimental procedures. VF ventricular fibrillation, CC chest compression, P period, NO animal number
Measurements
Chest compression waveform, arterial waveform, ECG and pulse oximetry plethysmographic waveform during each period were recorded simultaneously through a computer data acquisition system (Heart Lung Recovery Platform 3.0, Mindray Biological Medical Electronic Ltd, Shenzhen, China). Chest compression and pulse rates per two seconds during each chest compression period were calculated using this software according to the chest compression waveform and pulse oximetry plethysmographic waveform.
Compression interruption time is defined as the initial time of chest compression waveform minus the end time of the last chest compression waveform. Synchronous pulse interruption time is defined as the initial time of pulse oximetry plethysmographic waveform minus the end time of the last pulse oximetry plethysmographic waveform. Then, consistency between pulse rates and the synchronous chest compression rates during chest compression were statistically analyzed. Compression interruption times and the synchronous pulse interruption times were analyzed for agreement.
Statistical analysis
Statistical analysis was performed with SPSS 19.0 for Windows (SPSS, Chicago, IL, USA) and GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). Baseline measurements were reported as means with corresponding standard deviations (SD). Agreement between various measures was performed using intraclass correlation coefficients (ICCs) and Bland–Altman analysis. A value of 2-tailed p < 0.05 was considered statistically significant.
Results
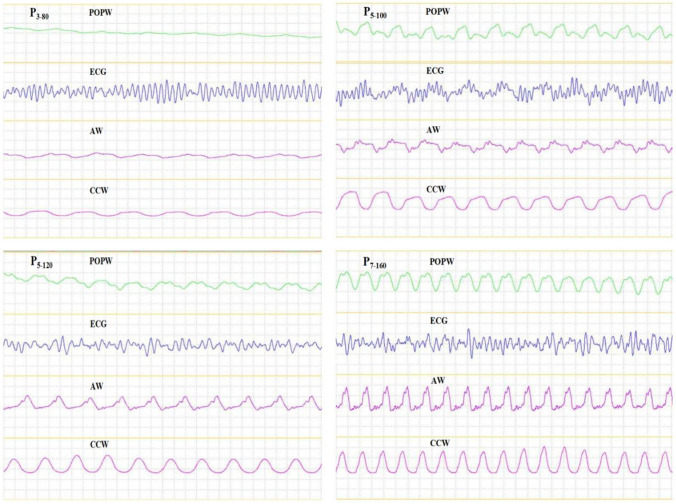
Baseline characteristics, including body weights, heart rates, respiratory rates, mean arterial pressure, ETCO2 and SpO2 measures are provided in Table 1. Ventricular fibrillation was induced successfully in seven animals. In total 126 compression periods of 2 min were performed. Chest compression waveform, arterial waveform and pulse oximetry plethysmographic waveform had synchronous frequency variations during chest compression, although, this was not so when compared to ECG readings. Pulse oximetry plethysmographic waveform, ECG, arterial waveform and chest compression waveform during chest compression periods of P3–80, P5–100, P5–120 and P7–160 are provided in Fig. 2.
Table 1.
Baseline characteristics
| Body weight, kg | 33.7 ± 0.7 |
| Heart rate, bpm | 87.4 ± 1.7 |
| RR, bpm | 13.4 ± 1.0 |
| MAP, mmHg | 125.1 ± 16.9 |
| ETCO2, mmHg | 39.4 ± 1.6 |
| SpO2, % | 96.5 ± 1.1 |
MAP mean aortic pressure, RR respiratory rate, ETCO2 end-tidal carbon dioxide, SpO2 pulse oxygen saturation
Values are presented as mean ± SD
Fig. 2.
Pulse oximetry plethysmographic waveform, ECG, arterial waveform and Chest compression waveform during chest compression periods of P3–80, P5–100, P5–120 and P7–160 POPW, pulse oximetry plethysmographic waveform; ECG, electrocardiogram; AW, arterial waveform; CCW, chest compression waveform; P3–80, a chest compression depth of 3 cm and chest compression rate of 80 times per minute
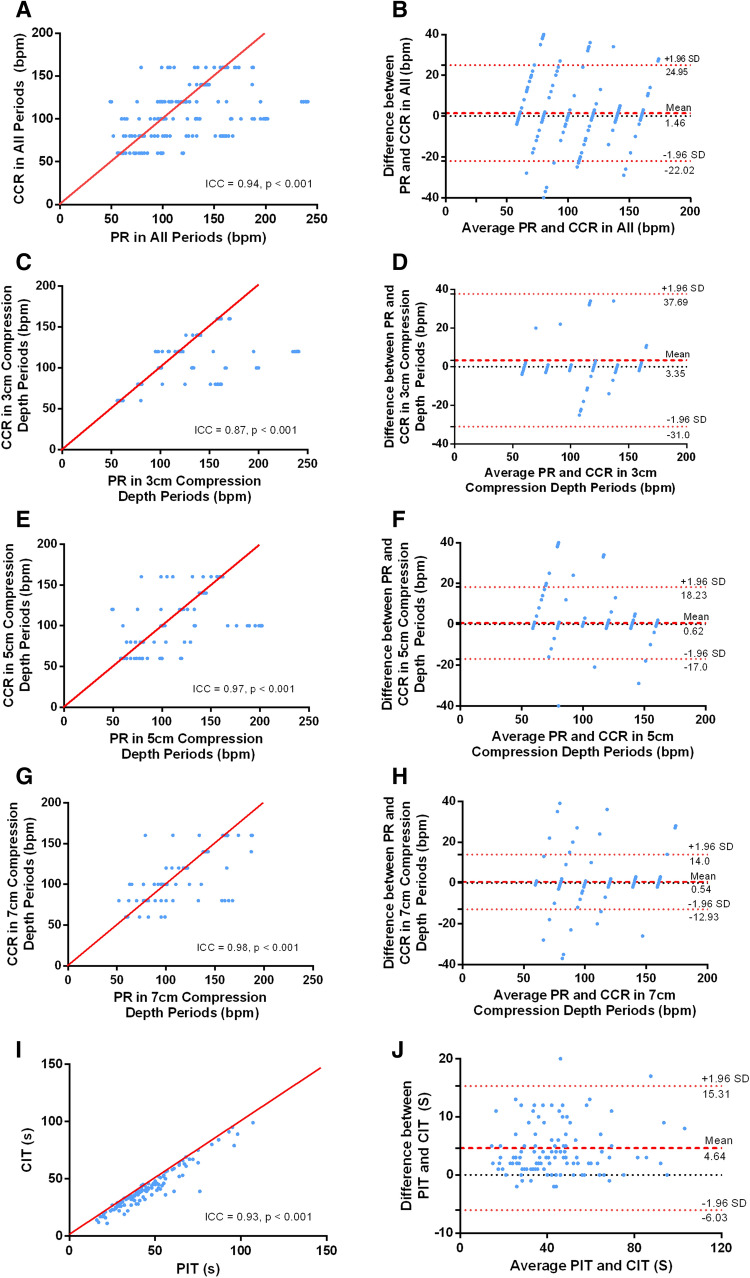
Using ICC, high levels of agreement were observed between chest compression and synchronous pulse rates across all periods (ICC = 0.94, p < 0.001). Chest compression depths of 3 cm (ICC = 0.87, p < 0.001), 5 cm (ICC = 0.97, p < 0.001) and 7 cm (ICC = 0.98, p < 0.001) were also consistent. Similarly, there was high agreement between pulse interruption and synchronous compression interruption times (ICC = 0.93, p < 0.001).
Using Bland–Altman analysis, low bias and limits of agreement were shown when chest compression rates were compared with synchronous pulse rates across all periods (Mean bias 1.46 bpm; 95% confidence interval [CI] 1.18–1.74 bpm), as well as in chest compression depths of 3 cm (mean bias 3.35 bpm; 95% CI 2.62 bpm to 4.07 bpm), 5 cm (mean bias 0.62 bpm; 95% CI 0.26 bpm to 0.98 bpm) and 7 cm (mean bias 0.54 bpm; 95% CI 0.26 bpm to 0.81 bpm). Similarly, when pulse interruption times were compared with synchronous compression interruption times, the relatively low levels of bias and limited agreement were observed (mean bias 4.64 s; 95% CI: 3.64 to 5.64 s). Please see Table 2; Fig. 3 for the details.
Table 2.
Agreement and Bland–Altman bias of rates and interruption times
| Agreement: ICC (95% CI) |
Bland–Altman: bias (95% CI) |
|
|---|---|---|
| Pulse rates and chest compression rates | ||
| All periods | 0.940 (0.936 to 0.943) | 1.46 bpm (1.18 bpm to 1.74 bpm) |
| 3 cm chest compression depth periods | 0.874 (0.858 to 0.887) | 3.35 bpm (2.62 bpm to 4.07 bpm) |
| 5 cm chest compression depth periods | 0.966 (0.963 to 0.969) | 0.62 bpm (0.26 bpm to 0.98 bpm) |
| 7 cm chest compression depth periods | 0.980 (0.978 to 0.981) | 0.54 bpm (0.26 bpm to 0.81 bpm) |
| Pulse interruption times and compression interruption times | 0.927 (0.693 to 0.971) | 4.64 s (3.64 to 5.64 s) |
ICC intraclass correlation coefficients, CI confidence intervals, bpm beats per minute
Fig. 3.
Concordance of rates and interruption times. PR pulse rate, CCR chest compression rate, PIT pulse interruption time, CIT compression interruption time
Discussion
This experiment confirms that there are two determining factors (i.e., chest compression rate and compression interruption time) which affect the quality of CPR. However, these can be effectively monitored using pulse oximetry plethysmographic waveform technology. Based on our analysis of pulse oximetry plethysmographic waveform in this porcine cardiac arrest model, pulse rate can accurately represent chest compression rate in real-time. Pulse interruption time can also be used to represent synchronous compression interruption time. Therefore, pulse oximetry plethysmographic waveform may be used as a real-time feedback mechanism which would simplify procedures and improve CPR quality and outcomes.
In the present study, high agreement and synchronicity between pulse rate and chest compression rate were demonstrated. According to chest compression data from a previous study [21], depths were set to 3 cm, 5 and 7 cm, with unmatched compression rates set to 60, 80, 100, 120, 140 and 160 times per minute. High agreement between pulse and simultaneous chest compression rates were observed even though complete resuscitation times for each pig exceeded 30 min. Initial time points for most periods were however, greater than 10 min after CPR commencement. Chest compression waveform and pulse oximetry plethysmographic waveform appeared synchronous once chest compression commenced and when chest compression stopped. Chest compression waveform and pulse oximetry plethysmographic waveform also disappeared simultaneously at that stage. Even though comparing pulse rate and simultaneous chest compression rate in chest compression 3 cm, 5 and 7 cm depths separately, high agreement was also evident across all three subgroups. High levels of agreement and synchronicity indicate that pulse rate could represent chest compression rate accurately, providing instantaneous feedback.
Some CPR instruments, even mobile device applications have emerged and could provide real-time CPR feedback parameters [22, 23]. However, these feedback tools are limited because they may only represent CPR performance metrics while neglecting genuine physiological responses. Being different from these feedback tools, pulse oximetry plethysmographic waveform could provide more physiological information and is therefore a viable alternative, although further research is necessary. The principal of pulse oximetry plethysmographic waveform is that pulse oximetry may distinguish the pulsatile components of arterial blood from non-pulsatile components of other tissues. The added advantage, is that this approach is sensitive to capture real-time changes in peripheral blood flow which is superior to non-invasive feedback indicators [24].
Once cardiac arrest occurs, the purpose of chest compression is to restore blood flow, especially to provide blood to circulate the brain and heart. High quality chest compression can generate essential blood flow to prevent functional complications of vital organs. Therefore, chest compression guided by monitoring blood perfusion might be the ideal choice. A recent review recommended supporting a precision based CPR strategy using individual patient’s physiology to guide resuscitation, such as coronary perfusion pressure, arterial diastolic blood pressure and ETCO2 [25]. However, because of the invasive nature of these interventions, complex operations and time lost due to CPR interruptions, these additional indicators are difficult to implement in every scenario. Compared with these alternative indicators, and due to the non-invasive nature, pulse oximetry plethysmographic waveform could be more easily obtainable. Every pulse captured by pulse oximetry plethysmographic waveform represents a chest compression which generates forward blood flow.
This study also demonstrated that pulse interruption times correspond precisely with compression interruption times in real-time. According to the principles of pulse oximetry and pulsatile blood, these could also easily and quickly be detected using pulse oximetry plethysmographic waveform. Therefore, pulse interruption times based on pulse oximetry plethysmographic waveform could be used to reflect the onset and end of interruptions, accurately and simultaneously. A rapid and accurate reminder of interruptions in real-time is of course, crucial for improving CPR quality and outcomes.
In this study, high synchronicity between pulse oximetry plethysmographic waveform and arterial waveform were showed. Besides, our previous study demonstrated that both the area under the curve (AUC) and amplitude (Amp) of pulse oximetry plethysmographic waveform correlated with CPP and ETCO2 in animal models [26]. These findings combined suggest that pulse oximetry plethysmographic waveform has the potential to provide information around the quality of chest compression, which considerably simplifies procedures.
Furthermore, we observed when comparing to 5 and 7 cm chest compression depths, the pulse oximetry plethysmographic waveform at a depth of 3 cm displayed relative flatness. Therefore, the agreement is not equally high compared to 5 and 7 cm compressions, although pulse rate is still generated by analyzing pulse oximetry plethysmographic waveform using the software at chest compression depths of 3 cm. From another perspective, this could be used to prompt rescuers, if the pulse oximetry plethysmographic waveform appears or becomes platykurtic, this shows blood flow to peripheral regions of the body has decreased. One of the possible reasons for this is insufficient depth of chest compression. These findings combined with our previous study [26] reveal that pulse oximetry plethysmographic waveform may also represent genuine blood flow produced by chest compression; however, further research is required to confirm this assertion.
In clinical practice, pulse oximetry plethysmographic waveform monitoring is extensively utilized because it is non-invasive, easily obtainable, low-cost and a convenient approach. Consequently, it is easy to achieve using pulse rate as an alternative, real-time feedback indicator of chest compression rate and pulse interruption time for reminding compression interruption time based on analysis of pulse oximetry plethysmographic waveform. A previous study having similar technology showed cerebral oximetry allows real-time, non-invasive cerebral oxygenation monitoring during CPR [27]. Comparatively, unlike monitoring blood oxygenation, detecting peripheral pulsatile blood flow caused by chest compression is the physiological monitoring principle for real-time feedback in the analysis of pulse oximetry plethysmographic waveforms. Therefore, the promising value of pulse oximetry plethysmographic waveform might not only monitor chest compression quality, but also provide guidance for optimization of vital organ perfusion, even have the potential to predict prognosis during CPR. Further research is necessary to explore these promising values across a human sample in the future.
Study limitations
We acknowledge that there were several limitations in this study. First, animals were utilized in this study which means findings may not translate to a human population or to a relatively unhealthy human population. Further research is necessary to confirm these findings before generalizing to a human population. Secondly, a healthy porcine model of cardiac arrest was established by induced fibrillation in this study, while many of those whom encounter cardiac arrest are either suffering comorbid disorders or with multiple morbidities. Therefore, the effectiveness of pulse oximetry plethysmographic waveform in cardiac arrest caused by other etiologies requires further evaluation. Finally, pulse oximetry plethysmographic waveform appears to be affected by motion. As a result, selecting an appropriate monitoring site to avoid potential signal interference, which requires a further research.
Conclusions
We conclude that pulse rate can be an alternative indicator of chest compression rate and pulse interruption time can also be used to indicate compression interruption time precisely in this porcine model of cardiac arrest.
Author contributions
Conceptualization: JX, XY, YF, LY; Supervision: JX, XY, HZ; Funding acquisition: XY; Methodology: YF, LY; Experiment: YF, KJ, JD, SY, LZ; Writing - Original Draft: LY; Writing - Review & Editing: JX, LY, SS; Analysis: LY, YL.
Funding
This study received funding from the Capital Health Research and Development Fund (2011-4001-04), the National Health and Family Planning Commission of the People’s Republic of China Special Research Fund (201502019).
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the Ministry of Science and Technology of the People’s Republic of China. The protocol was approved by the Animal Care and Use Committee of Peking Union Medical College Hospital.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yangyang Fu and Lu Yin are co-first authors
Contributor Information
Jun Xu, Email: xujunfree@126.com.
Xuezhong Yu, Email: yxzpumch@126.com.
References
- 1.Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation. 2010;81(11):1479–87. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on E. Prevention Statistics C. Stroke Statistics S Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Luc G, Baert V, Escutnaire J, Genin M, Vilhelm C, Di Pompeo C, Khoury CE, Segal N, Wiel E, Adnet F, Tazarourte K, Gueugniaud PY, Hubert H, On behalf GRR Epidemiology of out-of-hospital cardiac arrest: a French national incidence and mid-term survival rate study. Anaesth Crit Care Pain Med. 2019;38(2):131–5. doi: 10.1016/j.accpm.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Kronick SL, Kurz MC, Lin S, Edelson DP, Berg RA, Billi JE, Cabanas JG, Cone DC, Diercks DB, Foster JJ, Meeks RA, Travers AH, Welsford M. Part 4: systems of care and continuous quality improvement: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):397–413. doi: 10.1161/CIR.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 5.Neumar RW, Eigel B, Callaway CW, Estes III NA, Jollis JG, Kleinman ME, Morrison LJ, Peberdy MA, Rabinstein A, Rea TD, Sendelbach S, American Heart A. American Heart Association response to the 2015 institute of medicine report on strategies to improve cardiac arrest survival. Circulation. 2015;132(11):1049–70. doi: 10.1161/CIR.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 6.Wik L, Kramer-Johansen J, Myklebust H, Sørebø H, Svensson L, Fellows B, Steen PA. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293(3):299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 7.Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, Abella BS, Kleinman ME, Edelson DP, Berg RA, Aufderheide TP, Menon V, Leary M, Cpr Quality Summit Investigators tAHAECCC, the Council on Cardiopulmonary CCP, Resuscitation Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128(4):417–35. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 8.Kirkbright S, Finn J, Tohira H, Bremner A, Jacobs I, Celenza A. Audiovisual feedback device use by health care professionals during CPR: a systematic review and meta-analysis of randomised and non-randomised trials. Resuscitation. 2014;85(4):460–71. doi: 10.1016/j.resuscitation.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Kleinman ME, Brennan EE, Goldberger ZD, Swor RA, Terry M, Bobrow BJ, Gazmuri RJ, Travers AH, Rea T. part 5: adult basic life support and cardiopulmonary resuscitation quality: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):414–35. doi: 10.1161/CIR.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 10.Deakin CD, Sidebottom DB, Potter R. Can rescuers accurately deliver subtle changes to chest compression depth if recommended by future guidelines? Resuscitation. 2018;124:58–62. doi: 10.1016/j.resuscitation.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Cheskes S, Schmicker RH, Verbeek PR, Salcido DD, Brown SP, Brooks S, Menegazzi JJ, Vaillancourt C, Powell J, May S, Berg RA, Sell R, Idris A, Kampp M, Schmidt T, Christenson J, Resuscitation Outcomes Consortium i The impact of peri-shock pause on survival from out-of-hospital shockable cardiac arrest during the Resuscitation Outcomes Consortium PRIMED trial. Resuscitation. 2014;85(3):336–42. doi: 10.1016/j.resuscitation.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christenson J, Andrusiek D, Everson-Stewart S, Kudenchuk P, Hostler D, Powell J, Callaway CW, Bishop D, Vaillancourt C, Davis D, Aufderheide TP, Idris A, Stouffer JA, Stiell I, Berg R, Resuscitation Outcomes Consortium I Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009;120(13):1241–1247. doi: 10.1161/CIRCULATIONAHA.109.852202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobrow BJ, Vadeboncoeur TF, Stolz U, Silver AE, Tobin JM, Crawford SA, Mason TK, Schirmer J, Smith GA, Spaite DW. The influence of scenario-based training and real-time audiovisual feedback on out-of-hospital cardiopulmonary resuscitation quality and survival from out-of-hospital cardiac arrest. Ann Emerg Med. 2013;62(1):47–56.e41. doi: 10.1016/j.annemergmed.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 14.Nassar BS, Kerber R. Improving CPR performance. Chest. 2017;152(5):1061–9. doi: 10.1016/j.chest.2017.04.178. [DOI] [PubMed] [Google Scholar]
- 15.Kramer-Johansen J, Myklebust H, Wik L, Fellows B, Svensson L, Sørebø H, Steen PA. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation. 2006;71(3):283–92. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Lakomek F, Lukas RP, Brinkrolf P, Mennewisch A, Steinsiek N, Gutendorf P, Sudowe H, Heller M, Kwiecien R, Zarbock A, Bohn A. Real-time feedback improves chest compression quality in out-of-hospital cardiac arrest: a prospective cohort study. PLoS ONE. 2020;15(2):e0229431. doi: 10.1371/journal.pone.0229431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anto-Ocrah M, Maxwell N, Cushman J, Acheampong E, Kodam RS, Homan C, Li T. Public knowledge and attitudes towards bystander cardiopulmonary resuscitation (CPR) in Ghana, West Africa. Int J Emerg Med. 2020;13(1):29. doi: 10.1186/s12245-020-00286-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veronese JP, Wallis L, Allgaier R, Botha R. Cardiopulmonary resuscitation by Emergency Medical Services in South Africa: barriers to achieving high quality performance. Afr J Emerg Med. 2018;8(1):6–11. doi: 10.1016/j.afjem.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severinghaus JW. History, status and future of pulse oximetry. Adv Exp Med Biol. 1987;220:3–8. doi: 10.1007/978-1-4613-1927-6_1. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Xu J, Han F, Walline J, Zheng L, Fu Y, Zhu H, Chai Y, Yu X. Identification of return of spontaneous circulation during cardiopulmonary resuscitation via pulse oximetry in a porcine animal cardiac arrest model. J Clin Monit Comput. 2019;33(5):843–51. doi: 10.1007/s10877-018-0230-4. [DOI] [PubMed] [Google Scholar]
- 21.Idris AH, Guffey D, Pepe PE, Brown SP, Brooks SC, Callaway CW, Christenson J, Davis DP, Daya MR, Gray R, Kudenchuk PJ, Larsen J, Lin S, Menegazzi JJ, Sheehan K, Sopko G, Stiell I, Nichol G, Aufderheide TP, Resuscitation Outcomes Consortium I Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med. 2015;43(4):840–8. doi: 10.1097/CCM.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 22.Brown LL, Lin Y, Tofil NM, Overly F, Duff JP, Bhanji F, Nadkarni VM, Hunt EA, Bragg A, Kessler D, Bank I, Cheng A, International Network for Simulation-based Pediatric Innovation RECPRI Impact of a CPR feedback device on healthcare provider workload during simulated cardiac arrest. Resuscitation. 2018;130:111–7. doi: 10.1016/j.resuscitation.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Sarma S, Bucuti H, Chitnis A, Klacman A, Dantu R. Real-time mobile device-assisted chest compression during cardiopulmonary resuscitation. Am J Cardiol. 2017;120(2):196–200. doi: 10.1016/j.amjcard.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med. 2005;31(10):1316–26. doi: 10.1007/s00134-005-2790-2. [DOI] [PubMed] [Google Scholar]
- 25.Marquez AM, Morgan RW, Ross CE, Berg RA, Sutton RM. Physiology-directed cardiopulmonary resuscitation: advances in precision monitoring during cardiac arrest. Curr Opin Crit Care. 2018;24(3):143–50. doi: 10.1097/MCC.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Li C, Zheng L, Han F, Li Y, Walline J, Fu Y, Yao D, Zhang X, Zhang H, Zhu H, Guo S, Wang Z, Yu X. Pulse oximetry: a non-invasive, novel marker for the quality of chest compressions in porcine models of cardiac arrest. PLoS ONE. 2015;10(10):e0139707. doi: 10.1371/journal.pone.0139707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parnia S, Yang J, Nguyen R, Ahn A, Zhu J, Inigo-Santiago L, Nasir A, Golder K, Ravishankar S, Bartlett P, Xu J, Pogson D, Cooke S, Walker C, Spearpoint K, Kitson D, Melody T, Chilwan M, Schoenfeld E, Richman P, Mills B, Wichtendahl N, Nolan J, Singer A, Brett S, Perkins GD, Deakin CD. Cerebral oximetry during cardiac arrest: a multicenter study of neurologic outcomes and survival. Crit Care Med. 2016;44(9):1663–74. doi: 10.1097/CCM.0000000000001723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.