To the editor,
Coronavirus disease 2019 (COVID-19) can lead to systemic coagulation activation and thrombotic complications. Therefore, heparin treatment has been recommended for COVID-19 [1]. One study showed that heparin treatment appeared to be associated with better prognosis in patients with severe COVID-19 with markedly elevated D-dimer levels [2]. However, the incidence of venous thromboembolism (VTE) is high with routine thromboprophylaxis [3].
Heparin-induced thrombocytopenia (HIT) is an immune complication of heparin therapy caused by antibodies that bind to complexes of platelet factor 4 and heparin (PF4/heparin antibody complex). It is recognized that anti-PF 4/heparin antibodies commonly develop after heparin exposure [4]. We present a case of acute pulmonary thrombosis due to COVID-19 for which veno-arterial extracorporeal membrane oxygenation (VA-ECMO) therapy was administered, and thrombocytopenia that developed with PF4/heparin antibody formation. The patient was successfully treated with low dosage introduction of argatroban.
A 37-year-old man was admitted to our hospital with dyspnea. Eight days before admission, he developed a persistent fever and a loss of taste. Three days before admission, a polymerase chain reaction (PCR) test result for SARS-CoV-2 at a nearby hospital was positive. Soon after entering a quarantine accommodation facility, he experienced dyspnea and was transferred to our hospital.
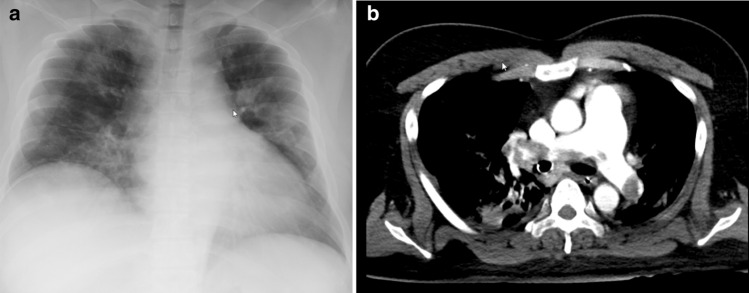
At admission, he was 175 cm in height and weighed 110 kg (body mass index: 35.9). He had no reported medical history. His body temperature was 39.2 °C. He required 3 L/minute of oxygen therapy. Oral favipiravir and ciclesonide (inhalation route) were introduced to treat COVID-19. Blood examination revealed elevated levels of AST (63 IU/L), ALT (43 IU/L), LDH (700 IU/L), and CRP (3.28 mg/dL). The D-dimer level was 1.62 µg/mL. Chest X-ray showed diffuse bilateral ground glass opacities (Fig. 1a).
Fig. 1.
a Chest X ray at admission. Bilateral ground glass opacities were observed on the chest X ray obtained at admission. b Chest CT on day 8. Massive bilateral pulmonary thromboembolism was observed
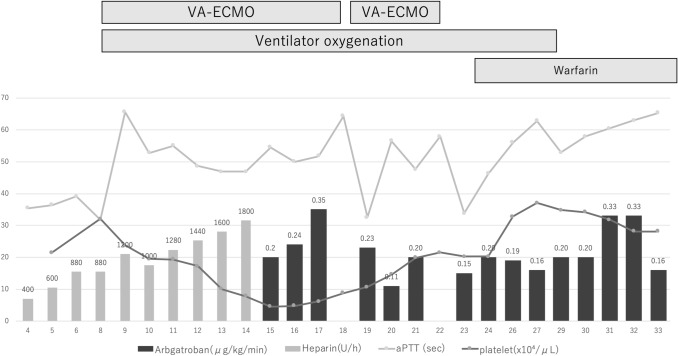
On day 3 after admission, blood examination revealed acutely elevated D-dimer (13.721 µg/mL), without findings suggestive of VTE, such as Homan’s sign or lower limb grasp pain. His dyspnea had improved since admission. Thus, we introduced prophylactic intravenous unfractionated heparin therapy (5000 IU bolus and 300 IU/h initially), and the dosage was adjusted according to the activated partial thromboplastin time (aPTT). However, on day 8, following minimal physical mobility, his consciousness deteriorated, and he had a seizure. Although, these symptoms immediately improved, obvious deterioration of oxygenation was observed. Chest computed tomography revealed a massive pulmonary thromboembolism (Fig. 1b), and the patient was intubated and transferred to the intensive care unit. Ventilation alone did not provide sufficient oxygenation, and therefore, VA-ECMO therapy was also introduced. On day 13, blood examination revealed thrombocytopenia, and the patient exhibited shivering. Culture tests, including four sets of blood culture, were negative. Simultaneously, a white thrombus began to adhere increasingly to the exchange membrane. On day 15, the blood examination showed a continuous decrease in the platelet count. Thus, the 4 Ts score for heparin-induced thrombocytopenia was 6, which fell into the ‘high’ category [5]. Immunoassay using the latex agglutination method was positive for PF4/heparin antibody complex as well (3.1U/mL). Tests for anti-beta-2 glycoprotein I antibody and lupus anticoagulant were both negative. The patient was clinically diagnosed with heparin-induced thrombocytopenia; heparin treatment was stopped, and intravenous argatroban therapy (0.2 µg/kg/min) was initiated. A gradual increase in the platelet count was observed (Fig. 2). The argatroban dosage was adjusted through aPTT monitoring, four times per day (target aPTT: 60 s). On day 18, because of bleeding during the removal of ECMO, VA-ECMO was relocated to the other side of his femoral vasculature with a hemostasis operation by emergency laparotomy. On day 22, ECMO therapy was terminated. On day 29, the ventilator was removed. On day 35, argatroban therapy was terminated. The patient was moved to a general ward, and rehabilitation treatment was performed with warfarinization.
Fig. 2.
Clinical course and the transition of platelet level and aPTT. aPTT activated partial thromboplastin time, VA-ECMO veno-arterial extracorporeal membrane oxygenation
COVID-19 is commonly complicated by coagulopathy, and a markedly increased D-dimer level is associated with high mortality [6]. Thus, the International Society on Thrombosis and Haemostasis recommends the introduction of low molecular weight heparin [1]. In our hospital, unfractionated heparin therapy was administered prophylactically while monitoring D-dimer or fibrinogen levels, especially in patients at high risk for VTE. In contrast, one study indicated that a high level of P4/heparin antibody complex was observed in most patients with COVID-19 in the ICU [7], and that HIT occurred not only in patients with heparin exposure, but also in heparin-naive patients. The researchers of that study hypothesized that spontaneous HIT may result from the virus itself, due to a secondary bacterial infection, such as PF4-conjugated Staphylococcus aureus or Escherichia coli, or from severe tissue damage [8]. In our case, the apparent bacterial infection was absent, and we also used heparin intravenously. Thus, heparin usage may trigger the PF4/heparin antibody complex formation.
Argatroban, a direct thrombin inhibitor, has been approved for HIT treatment. The usual initial dose is 2 µg/kg/min via continuous intravenous infusion, adjusted to the steady-state aPTT, at 1.5-3 times the initial baseline value. However, we initiated therapy at a dosage lower than the recommended dosage, and the aPTT value remained at 1.5–2.0 times the initial baseline value successfully. Saegel et al. [9] found that in the critical care setting or in patients at high risk for bleeding, initial dose reduction of argatroban may be necessary. In fact, Patell et al. described three of five patients diagnosed as HIT with COVID-19 developed major hemorrhagic events after introducing direct thrombin inhibitors [10].
In conclusion, a COVID-19 patient who developed pulmonary thromboembolism was administered heparin therapy and ECMO; the patient was diagnosed with HIT by thrombocytopenia and ECMO membrane thrombosis. He was successfully treated by replacing heparin with low dosage of argatroban. When thrombocytopenia is observed in COVID-19 patients, HIT should be listed as a differential diagnosis, and clinicians should consider using low dosage of argatroban while monitoring the coagulation function status carefully.
Author contributions
All authors meet the ICMJE authorship criteria. YO was responsible for organization and coordination of this case report. YO, TN, TA, KN, JK, MK, HK, and IG treated with the patient and made diagnosis. All authors contributed to the writing of the final manuscript. All authors have read and approved the manuscript.
Funding
None.
Compliance with ethical standards
Conflict of interest
All the authors decalred that tehy have no conflict of interest.
Informed consent
The patient provided informed consent for the publication of this report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth/14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MC, Bouman CC, Beenen LF, Kootte RS, Heijmans J, Smits LP. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arepally GM. Heparin-induced thrombocytopenia. Blood. 2017;129:2864–2872. doi: 10.1182/blood-2016-11-709873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 6.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Zhang X, Xiao Y, Gao T, Wang G, Wang Z, Zhang Z, Hu Y, Dong Q, Zhao S, Yu L. Heparin-induced thrombocytopenia is associated with a high risk of mortality in critical COVID-19 patients receiving heparin-involved treatment. medRxiv. 2020 doi: 10.1101/2020.04.23.20076851. [DOI] [Google Scholar]
- 8.Krauel K, Pötschke C, Weber C, Kessler W, Fürll B, Ittermann T, Maier S, Hammerschmidt S, Bröker BM, Greinacher A. Platelet factor 4 binds to bacteria, [corrected] inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood. 2011;117:1370–1378. doi: 10.1182/blood-2010-08-301424. [DOI] [PubMed] [Google Scholar]
- 9.Saugel B, Phillip V, Moessmer G, Schmid RM, Huber W. Argatroban therapy for heparin induced thrombocytopenia in ICU patients with multiple organ dysfunction syndrome: a retrospective study. Crit Care. 2010;14:R90. doi: 10.1186/cc9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patell R, Khan A, Bogue T, Merrill M, Koshy A, Bindal P, Joyce R, Aird WC, Neuberg D, Bauer KA, Zwicker JI. Heparin inducet thrombocytopenia antibodies in COVID-19. Am J Hematol. 2020 doi: 10.1002/ajh.25935. [DOI] [PMC free article] [PubMed] [Google Scholar]