Abstract
Background
We aimed to validate a simple Comprehensive Geriatric Assessment (CGA) in older adults with diffuse large B‐cell lymphoma (DLBCL) in China and to evaluate the tolerability and efficacy of CGA‐driven therapy.
Materials and Methods
In total, 78 patients with DLBCL aged ≥60 years were evaluated using CGA with the following parameters: age ≥ 80 years, activities of daily living (ADL), instrumental ADL, and modified cumulative illness rating score for geriatrics. Patients were grouped as fit, unfit, or frail. Patients classified as fit received standard‐dose rituximab plus CHOP, whereas patients in the latter two groups received reduced‐dose or reduced‐agent therapy. The overall response rate (ORR), overall survival (OS), progression‐free survival (PFS), and toxicities in the three groups were evaluated.
Results
According to the CGA, 45 (57.5%) patients were classified as fit, 5 (6.4%) as unfit, and 28 (35.9%) as frail. The ORR was 82.1% (64/78) among all the patients, including 55 patients (70.6%) who achieved complete response and 9 patients (11.5%) who achieved partial response. In the fit and unfit + frail groups, it achieved 97.8% and 60.6%, respectively. In total, 26 (33.3%) patients (10/45 [22.2%] fit and 16/33 [48.5%] unfit + frail) showed disease progression or recurrence. The median follow‐up time was 18 months (range, 5–62). The 3‐year OS and PFS rates were 82% and 58%, respectively. There were no treatment‐related deaths.
Conclusion
A simple CGA in older adults with DLBCL may be an effective tool for guiding therapeutic strategies in China.
Implications for Practice
Diffuse large B‐cell lymphoma (DLBCL) is the most common malignant lymphoma in older adults. The simple tool, Comprehensive Geriatric Assessment (CGA), is proved to be an effective method to identify older adults with DLBCL who are suitable for standard‐dose R‐CHOP regimen therapy. This is the first prospective trial in China to evaluate the tolerability and efficacy of CGA‐driven therapy for older adults with DLBCL, and the result showed that this simple CGA may be an effective tool for guiding therapeutic strategies.
Keywords: Older adult, Diffuse large B‐cell lymphoma, Comprehensive geriatric assessment
Short abstract
Clinicians should take into account geriatric assessment results when recommending chemotherapy. This article evaluates the tolerability and efficacy of Comprehensive Geriatric Assessment‐driven therapy in China.
Introduction
Diffuse large B‐cell lymphoma (DLBCL) is the most common malignant lymphoma, accounting for nearly 40% of all non‐Hodgkin's lymphoma cases. The median age of onset is about 65 years, and the incidence increases with age 1. The current standard, first‐line therapy for DLBCL is rituximab plus CHOP (R‐CHOP), which improves the survival outcome of young patients and some older patients 2. However, about 40% of older patients cannot tolerate a standard dose of R‐CHOP because of their older age, poor performance status, or the presence of comorbidities 3. Recent studies indicate that the Comprehensive Geriatric Assessment (CGA) may help identify older adults with DLBCL who are suitable for a therapy regimen of standard‐dose R‐CHOP 4, 5, 6, 7, 8, 9, 10, 11. American Society of Clinical Oncology expert panel suggests that clinicians should take into account geriatric assessment results when recommending chemotherapy 12. International Society of Geriatric Oncology (SIOG) expert panel suggests that pretreatment evaluation should consider comorbidities, functional, social, and psychological constraints in older adults with DLBCL, which will facilitate identifying those patients’ intolerance to R‐CHOP 13, 14. Tucci et al. used a simple geriatric score to define different categories of older adults with DLBCL. The specific criteria for each group are shown in Table 1; however, the treatment decision was made by clinicians 5. In the fit group, when patients received standard‐dose R‐CHOP, the 2‐year overall survival (OS) rate was 88%, compared with that of patients who received reduced‐dose therapy, which was only 25% (p = .0001). In the frail group, there was no significant difference in OS between patients receiving standard‐dose R‐CHOP and those receiving reduced‐dose therapy; however, the incidence of nonhematological toxicity was significantly higher in patients receiving standard‐dose R‐CHOP than in those receiving reduced‐dose therapy 5. The results showed that the CGA was an essential tool used to support treatment decisions, whereas the application of the CGA in China has not been widely studied. In a noninterventional study in our center, we validated the simple geriatric score in older adults with DLBCL (>70 years), as Tucci et al. had proposed, and it showed that the CGA is feasible 11. However, there is no report on CGA‐driven therapy for older adults with DLBCL in China. Therefore, this prospective study was performed to evaluate the tolerability and efficacy of CGA‐driven therapy in China.
Subjects, Materials, and Methods
From February 2014 to February 2019, patients with newly diagnosed DLBCL aged ≥60 years were enrolled in this study at our hospital; all patients signed informed consent forms. Patients with HIV infection, primary central nervous system lymphoma, transformed DLBCL, or a history of hematological neoplasms were excluded. The end time for follow‐ups was April 1, 2019. This study was approved by the Ethics Committee of Beijing Hospital, National Center of Gerontology, Beijing, China. It was registered on http://www.chictr.org.cn/index.aspx and was assigned the registration number ChiCTR‐OOC‐16006794.
All patients were evaluated using a medical history, a physical examination, imaging and organ function, and a CGA. CGA stratification was performed using the following parameters: (a) age, (b) activities of daily living (ADL), (c) instrumental activities of daily living (IADL), and (d) modified cumulative illness rating score for geriatrics (MCIRS‐G). According to the result of the CGA, the patients were exclusively divided into the fit, unfit, or frail group. The specific criteria for each group are the same as Tucci et al. had proposed (Table 1).
Therapeutic strategies were decided based on the CGA group. It was suggested that patients in the fit group should receive standard‐dose R‐CHOP therapy (rituximab 375 mg/m2 intravenous [i.v.] on day 1; cyclophosphamide 750 mg/m2 i.v. on day 2; doxorubicin 50 mg/m2 i.v. on day 2; vincristine 1.4 mg/m2, with the total dose not exceeding 2 mg/day i.v. on day 2; and prednisone 100 mg per day orally on days 2–6). Treatment was repeated every 3 weeks, and patients received between six and eight cycles of treatment. In contrast, it was recommended that those patients in the unfit or frail groups should receive a regimen of reduced‐dose of anthracycline of R‐CHOP (doxorubicin 25 mg/m2 i.v. on day 2; other drug dosage was the same as standard‐dose R‐CHOP) or R‐COP (rituximab 375 mg/m2 i.v. on day 1; cyclophosphamide 750 mg/m2 i.v. on day 2; vincristine 1.4 mg/m2, with a total dose not exceeding 2 mg/day i.v. on day 2; prednisone 100 mg/day orally on days 2–6) or R‐miniCHOP (rituximab 375 mg/m2 i.v. on day 1; cyclophosphamide 400 mg/m2 i.v. on day 2; doxorubicin 25 mg/m2 i.v. on day 2; vincristine 1 mg on day 2; and prednisone 40 mg/m2 orally on days 2–6). Treatment was repeated every 3 weeks, and patients received between six and eight cycles.
Therapeutic Evaluation
After the fourth courses and at the end of the treatment, patients were evaluated by positron‐emission tomography–computed tomography or enhanced computed tomography. Clinical evaluations were obtained from patients every 3 months for the first 2 years and then every 6 months. Treatment response in this study was evaluated using overall response rate (ORR), OS, and progression‐free survival (PFS). The ORR includes complete response (CR) and partial response (PR). Disease progression (PD) or recurrence was evaluated according to the literature 15. OS was defined as the time from diagnosis to death for any reason, and PFS was defined as the time from diagnosis to disease progression or death. Follow‐up information was collected through outpatient and inpatient medical records or telephone calls. Adverse events were assessed according to World Health Organization criteria 16.
Statistical Analysis
SPSS 20.0 statistical software (SPSS Inc., Chicago, IL) was used for statistical analysis. For qualitative data, cases and percentages were used; the differences between qualitative data were determined using the chi‐square and Fisher's exact tests. The OS and PFS rates were evaluated by the Kaplan‐Meier method, and the differences were compared by using a log‐rank test. In all cases, p values <.05 were considered as statistically significant (two sided).
Results
Patient Characteristics
Among 78 patients with newly diagnosed DLBCL, 45 were male (57.7%) and 33 were female (42.3%). The median age was 69.5 years (range, 60–90). There were 25 patients (33%) with double expressor lymphoma and no patients with double hit lymphoma. Using the CGA, 45 (57.5%) patients were classified as fit, 5 (6.4%) as unfit, and 28 (35.9%) as frail. In the fit group, 71.1% (32/45) patients had grade 2 comorbidities. The most common comorbidities were hypertension and diabetes, respectively accounting for 40.6% (13/32) and 25.0% (8/32). The mean of total CIRS‐G scores in the fit group was 2.3 (range, 0–8). All patients in the unfit group had grade 2 comorbidities. The most common comorbidity was hypertension. All of the patients in frail group had grade 3–4 comorbidities. The most common comorbidities were hypertension, cardiovascular disease, and diabetes, respectively observed in 39.3% (11/28), 21.4% (6/28), and 14.3% (4/28) of the patients (Table 2). The mean of total CIRS‐G scores in the unfit + frail group was 7.4 (range, 2–16).
Table 2.
Allocation of patients into three groups according to CGA
| Factor and group | Fit, n (%) | Unfit, n (%) | Frail, n (%) |
|---|---|---|---|
| CGA | 45 (57.7) | 5 (6.4) | 28 (35.9) |
| ADL | |||
| 6 | 45 (100.0) | 5 (100.0) | 26 (92.9) |
| ≤5 | 0 (0.0) | 0 (0.0) | 2 (7.1) |
| IADL | |||
| 8 | 45 (100.0) | 4 (80.0) | 22 (78.6) |
| ≤7 | 0 (0.0) | 1 (20.0) | 6 (21.4) |
| MCIRS‐G | |||
| 2 | 32 (71.1) | 5 (100.0) | 0 (0) |
| 3–4 | 0 (0.0) | 0 (0.0) | 28 (100) |
| Age ≥80 yr | 0 (0.0) | 4 (80.0) | 8 (28.6) |
Abbreviations: ADL, activities of daily living; CGA, Comprehensive Geriatric Assessment; IADL, instrumental ADL; MCIRS‐G, modified cumulative illness rating score for geriatrics.
Table 1.
The classified criteria of patients according to comprehensive geriatric assessment
| CGA category | |||
|---|---|---|---|
| Factor | Fit | Unfit | Frail |
| ADL | 6 | 5 | <5 |
| IADL | And 8 | Or 6–7 | Or <6 |
| MCIRS‐G | And no comorbidity score 3–4 (and <5 comorbidity score 2) | Or no comorbidity score 3–4 (and 5–8 comorbidity score 2) | Or ≥1 comorbidity score 3–4 (or >8 comorbidity score 2) |
| Age | And <80 yr | Or ≥80 yr fit | Or ≥80 yr unfit |
Abbreviations: ADL, activities of daily living; CGA, Comprehensive Geriatric Assessment; IADL, instrumental ADL; MCIRS‐G, modified cumulative illness rating score for geriatrics.
In the fit group, 51.1% of patients (23/45) had an advanced disease (Ann Arbor stage III–IV), 88.9% (40/45) had albumin (ALB) ≥35 g/L, whereas in the unfit + frail group, 78.8% of patients (26/33) had an advanced disease, and 60.6% (20/33) had ALB ≥35 g/L. This difference in the number of Ann Arbor stage and the ALB values is statistically significant (p < .05). No significant difference was observed in other clinical features, such as B symptoms, bone marrow involvement, extranodal site involvement (>2 sites), lactate dehydrogenase level at initial treatment, and pathological subtype, between the two groups (p > .05; Table 3).
Table 3.
Characteristics and outcome of patients classified as fit or as unfit + frail according to Comprehensive Geriatric Assessment
| Factor and group | Fit, n (%) | Unfit + frail, n (%) | X2 | p value |
|---|---|---|---|---|
| Sex | ||||
| Male | 26 (57.8) | 19 (57.6) | 0.000 | <.999 |
| Female | 19 (42.2) | 14 (42.4) | ||
| Ann Arbor stage | ||||
| I–II | 22 (48.9) | 7 (21.2) | 6.244 | .018 |
| III–IV | 23 (51.1) | 26 (78.8) | ||
| B symptoms | ||||
| Yes | 30 (66.7) | 16 (48.5) | 2.601 | .162 |
| No | 15 (33.3) | 17 (51.5) | ||
| Bone marrow involvement | ||||
| No | 41 (91.1) | 28 (84.8) | 0.247 | .619 |
| Yes | 4 (8.9) | 5 (15.2) | ||
| Extranodal disease | ||||
| 0–1 | 24 (53.3) | 13 (39.4) | 1.484 | .257 |
| ≥2 | 21 (46.7) | 20 (60.6) | ||
| LDH | ||||
| <245 U/L | 29 (64.4) | 21 (63.6) | 0.005 | <.999 |
| ≥245 U/L | 16 (35.6) | 12 (36.4) | ||
| ALB | ||||
| ≥35 g/L | 40 (88.9) | 20 (60.6) | 8.579 | .003 |
| <35 g/L | 5 (11.1) | 13 (39.4) | ||
| Pathological subtypes | ||||
| GCB | 13 (28.9) | 11 (33.3) | 0.557 | .611 |
| ABC | 31 (68.9) | 18 (54.6) | ||
| Unidentified | 1 (2.2) | 4 (12.1) | ||
| Double expression | ||||
| Yes | 14 (31.1) | 11 (33.3) | 0.043 | <.999 |
| No | 31 (68.9) | 22 (66.7) |
Abbreviations: ABC, activated B‐cell; ALB, albumin; GCB, germinal center B‐cell; LDH, lactate dehydrogenase.
All 45 patients in the fit group received standard‐dose R‐CHOP. In the unfit + frail group, 33 patients received reduced‐dose or reduced‐agent therapy. Among them, 10 patients were treated with R‐COP, 11 were treated with R‐mini‐CHOP, and 12 were treated with a reduced‐dose of anthracycline of R‐CHOP. A total of 71 (91.0%) patients completed between six and eight cycles of chemotherapy, 5 (6.4%) patients completed 5 cycles of chemotherapy, and 2 patients completed four cycles of chemotherapy.
Therapeutic Response
The ORR for all patients was 82.1% (64/78), including 55 patients (70.6%) who achieved CR and 9 patients (11.5%) who achieved PR. The ORR was 97.8% (44/45) in the fit group and 60.6% (20/33) in the unfit + frail group. Therefore, there was a significant difference in ORR between the two groups (x2 = 17.862, p < .0001). The CR rates for the fit and unfit + frail groups were 84.4% and 51.5%, respectively, and the difference between these values was statistically significant (x2 = 9.927, p = .002). A total of 26 (33.3%) patients had a PD or disease recurrence, of which 22.2% (10/45) were in the fit group, and 48.5% (16/33) were in the unfit + frail group, and there was a significant difference between the two groups (x2 = 5.909, p = .028).
Survival
Lymphoma progression was the main cause of death in patients. A total of nine patients died, including two patients from the fit group and seven patients from the unfit + frail group. The median follow‐up time was 18 months (range, 5–62). The 2‐year OS rate was 86% for all patients, which was 98% for the fit group and 69% for the unfit + frail group. The 3‐year OS rate was 82% for all patients, which was 91% for the fit group and 69% for the unfit + frail group. Of all 78 patients evaluated, 26 (33.3%) patients showed PD or disease recurrence. The 2‐year PFS was 62%, 72%, and 47% for all patients, the fit group, and the unfit + frail group, respectively. The 3‐year PFS was 58%, 72%, and 35% for all patients, the fit group, and the unfit + frail group, respectively (Figs. 1, 2).
Figure 1.
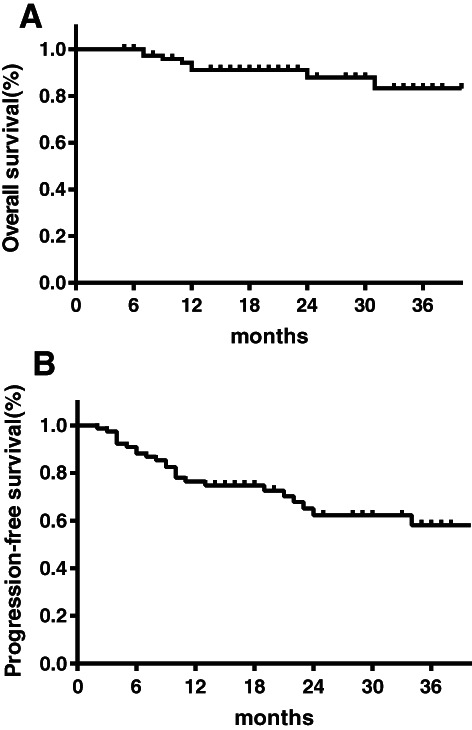
Survival outcomes for all the older adults with DLBCL according to the CGA‐driven therapy. (A): Overall survival. (B): Progression‐free survival.
Figure 2.
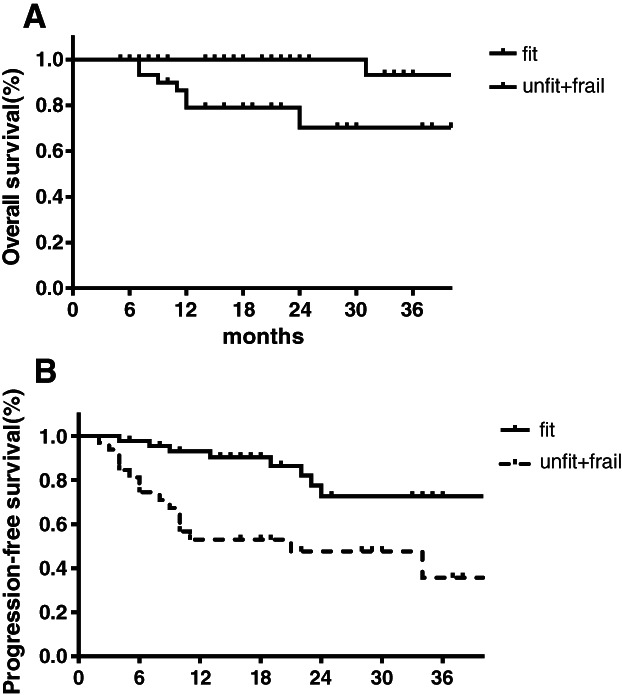
Survival outcomes in older adults with DLBCL classified as fit or as “unfit + frail” according to CGA. (A): Overall survival. (B): Progression‐free survival.
Toxicity Response
There was no treatment‐related mortality in 78 patients. A total of 52.6% (41/78) of patients had hematological grade 3–4 toxicity, of which 51.1% (23/45) were in the fit group, and 54.5% (18/33) were in the unfit + frail group. Additionally, 20.0% (9/45) of patients in the fit group and 15.2% (5/33) of patients in the unfit + frail group had gastrointestinal adverse reactions, and febrile neutropenia was observed in 8.9% (4/45) and 27.3% (9/33) of patients in the fit and unfit + frail groups, respectively. The incidence of drug‐induced lung injury was 6.7% (3/45) in the fit group and 12.1% (4/33) in the unfit + frail group, and four patients had cardiovascular adverse reactions, including two in the fit group and two in the unfit + frail group. A total of 33.3% (15/45) of patients in fit group and 42.4% (14/33) of patients in the unfit + frail group had treatment delays during the course of treatment because of treatment‐related toxicity. According to the CGA grouping, there was no significant difference in treatment‐related toxicity between the fit group and the unfit + frail group (p > .05).
Discussion
Nowadays, the decision to treat an older patient with DLBCL is based on the clinical experience of physicians. However, clinical experience is subjective, and the individual judgments of different doctors could diverge. Thus, it is time to explore how to accurately stratify and rationally treat older patients with DLBCL. The National Comprehensive Cancer Network and SIOG guidelines recommend routine CGA in older adults with cancer (aged over 65 years) 17, 18. In this clinical study, the CGA scales were used, and it takes only 15–30 minutes to finish the whole evaluation 7. Therefore, the CGA is a feasible tool.
Older adults are generally defined as adults aged 65 and above in most developed countries, whereas in developing countries, it is defined as adults aged 60 years and above. Question remains whether patients aged between 60 and 65 should be included in the study. In a cross‐sectional study in our center in China, we assessed the CGA in older adults with non‐Hodgkin's lymphoma; the result showed that among the relatively younger patients aged 60–64 years, 25% (3/12) were considered as frail 19. Therefore, we think it is better to include older adults aged 60–64 years in this study. In addition, in the unfit + frail group in this study, 18.18% of patients (6/33) were aged between 60 and 64. The reasons for the relatively younger patients in the unfit + frail group are completely due to grade 3–4 comorbidities such as cardiovascular disease, hypertension, and diabetes. Therefore, in developing countries, routine CGA in adults aged 60–64 with DLBCL could be considered.
The CGA stratification scales include ADL, IADL, and MCIRS‐G. The ADL scale is a six‐item scale assessing basic self‐care activities, including feeding, dressing, bathing, toileting, transfer, and continence. The IADL scale was used to assess a patient's basic abilities to maintain an independent life, such as preparing food, laundry, using the phone, shopping, the ability to travel, taking drugs, housekeeping, and handling money. Complications were assessed according to the MCIRS‐G scale, which includes cardiovascular, respiratory, digestive, urinary, musculoskeletal, skin, and endocrine system complications. In an Italian Lymphoma Italian Foundation (FIL) Research Group study 20 of 792 patients aged >65 years with newly diagnosed DLBCL, functional status (ADL, IADL), complications (MCIRS‐G), and age were assessed, and patients were divided into fit, unfit, and frail groups. The results showed that the fit, unfit, and frail groups accounted for 41.4% (328), 26.1% (207), and 32.5% (257) of patients, respectively. In this study, we refer to the CGA stratification scales of the Italian FIL research. Our results show that 57.7%, 6.4%, and 35.9% of patients were classed as fit, unfit, or frail. In our research, the frail patients accounted for about one‐third of all patients, which was similar to that in the Italian FIL group.
All of the patients in our frail group had grade 3–4 comorbidities, the most common of which were hypertension, cardiovascular disease, and diabetes. Janssen‐Heijnen et al. evaluated 234 patients with non‐Hodgkin's lymphoma (NHL) aged >60 years to analyze the prevalence of comorbidities 21. Their results showed that 75% of patients had comorbidities, including cardiovascular diseases, hypertension, and diabetes mellitus. In a study of 73 patients with NHL aged >75 years, 79.4% patients had comorbidities, including 52% with cardiovascular diseases 6. The previously discussed Italian FIL study showed that the most common comorbidities in the frail group were cardiovascular disease and muscular system disease 5. This study and previous literature have shown that the most common comorbidities are cardiovascular disease and hypertension. The presence of comorbidities reduced a patient's tolerance to standard‐dose R‐CHOP, resulting in them receiving reduced‐dose chemotherapy.
In this study, our results show that the ORR was 82.1%, including 70.6% of patients who achieved CR and 11.5% of patients who achieved PR. For all patients, the 2‐ and 3‐year OS rates were 86% and 82%, respectively. The 2‐year PFS of all patients was 62%, and the 3‐year PFS was 58%. The GELA LNH‐98.5 study enrolled 60–80‐year‐old patients who fulfilled the following criteria: no previous therapy or history of hematological neoplasms, a performance status score of 0–2, Ann Arbor stage II–IV, and no severe comorbidities 22. When patients received R‐CHOP, the ORR was 83%, including 75% of patients who achieved CR, and the 2‐year OS was 70%. In our study, we also included patients who were >80 years and those who had impaired physical function and severe comorbidities. Our study included more unfit + frail patients than those from the GELA LNH‐98.5 study; however, our OS rate is significantly higher. In the RICOVER‐60 study, the patients were aged <80 years and had no previous history of lymphoma or severe comorbidities 23. They had an ORR of 81%, including 78% of patients who achieved CR and a 3‐year OS of 78.1%. The ORR and CR rates from our study as are similar to those of the RICOVER‐60 study; however, our OS was slightly higher.
Olivieri et al. enrolled 91 older adults with DLBCL in their study, all of whom were aged >65 years. In their study, the therapeutic strategies were decided based on the CGA; the fit group received standard‐dose R‐CHOP, the unfit group received R‐CDOP, and the frail group received R‐mini‐CHOP. Their results showed that the ORR of all patients was 79%, the CR rate was 73.6%, and the 2‐year OS was 62% 7. This suggested that CGA‐driven therapy is effective.
In a historical study at our center, 37 older adults with DLBCL aged >70 years were enrolled, and treatment decisions were made by clinicians 11. The ORR of these patients was 64.9%, the CR rate was 43.3%, and the 2‐year OS was 51.4% 11. Therefore, the ORR and OS of patients in this study were higher than those in our previous study. It is expected that we should further validate the effectiveness of CGA‐driven therapy in the future through the development of a randomized controlled study.
In this study, our results show that the incidence of PD or recurrence in the unfit and frail group is higher than that in the fit group (48% vs. 22%). Because the reductions in chemotherapy dose intensity are associated with the poorer prognosis, a question could be raised if the assessment results of older adult patients with DLBCL were negatively impacted by lymphoma, leading to insufficient dose in the treatment. To examine the correlation, it is noteworthy to review the process of CGA and discern if the reason that makes patients fall into the unfit and frail group is because of the disease itself or because of patients’ baseline ability. In this study, CGA stratification was performed based on four parameters, namely age, ADL, IADL, and MCIRS‐G. Among the four parameters, ADL and IADL may be affected by the disease itself, whereas the other two are irrelevant. In frail group of the study, all the 28 patients had grade 3–4 comorbidities. According to the standard of classification, whether their ADL and/or IADL is impaired or not, they should be grouped into frail group, and thus the disease had no impact on their assessment result. In the unfit group, among five patients, four were included because they were aged ≥80 years, which is irrelevant with the disease, and the other one was there because of impaired IADL (6 scores). For the patient with impaired IADL, his IADL score was the same when we assessed his IADL 3 months before the diagnosis of DLBCL. Besides, the patient did not manifest any symptoms 3 months ago. The result indicates that his impaired IADL was due to baseline abilities instead of lymphoma. Thus, through the above analysis, we could reach the conclusion that the classification of the unfit and frail group in the study should be mainly due to baseline abilities and not affected by the disease itself. Considering the possibility of the affection of lymphoma to ADL and IADL, we suggest that if the patients are classified into the unfit and frail group only because of impaired ADL and IADL, the ADL and IADL results before the diagnosis should be used to minimize the possibility that the assessment result is linked with the disease. If the score is different, the impaired ADL and IADL may be caused by lymphoma itself. In the future, a repeated CGA is recommended before each course of chemotherapy, and the therapy could be more precise based on the result of reassessment. There's no clinical research in this field, and more clinical practice is needed to prove the effectiveness.
There was no treatment‐related mortality among the 78 patients in this study, whereas in GELA98.5, the treatment‐related mortality rate was 11% in the CHOP group and 13% in R‐CHOP group 22. In the RICOVER‐60 study, the treatment‐related mortality rates were 8% and 7% in the eight and six treatment cycle groups, respectively 23. This suggests that CGA‐driven therapy is safe and controllable.
This study has several limitations. First, this study is in the absence of a control group, which may influence the accuracy of our findings. Second, the sample size was small, so we cannot reach a robust conclusions. To improve, more prospective randomized trials of CGA as strata criterion should be planned in the future. A prospective randomized controlled study is ongoing in our center (ChiCTR1800016732).
This study is the first exploration of CGA‐driven stratified therapy in China. The results show that CGA‐driven therapy is feasible and effective.
Conclusion
We preliminarily explored the effectiveness and safety of CGA‐driven therapy in older adults with DLBCL in China. Patients who underwent stratified therapy had a better treatment response rate, OS, and PFS, and the toxicity and side effects were controllable.
Author Contributions
Conception/Design: Hui Liu; Jie‐fei Bai, Hui‐Xiu Han,
Provision of study material or patients: Ru Feng, Jiang‐Tao Li, Ting Wang, Chun‐Li Zhang,
Collection and/or assembly of data: Hui‐Xiu Han, Ru Feng, Jiang‐Tao Li,
Data analysis and interpretation: Jie‐fei Bai, Hui‐Xiu Han,
Manuscript writing: Jie‐fei Bai, and Hui Liu
Final approval of manuscript: Jie‐fei Bai, Hui‐Xiu Han, Ru Feng, Jiang‐Tao Li, Ting Wang, Chun‐Li Zhang, Hui Liu
Disclosures
The authors indicated no financial relationships.
Acknowledgments
Supported by Application of Clinical Features of Science and Technology Commission, grant no Z171100001017200, Z181100001718162. Jie‐fei Bai and Hui Liu were involved in the editing of the manuscript.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Sun J, Yang Q, Lu Z et al. Distribution of lymphoid neoplasms in China: Analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol 2012;138:429–434. [DOI] [PubMed] [Google Scholar]
- 2. Chinese Society of Hematology; Chinese Medical Association; Chinese Society of Lymphoma et al. Chinese guidelines for diagnosis and treatment of diffuse large B cell lymphoma(2013) [in Chinese]. Zhonghua Xue Ye Xue Za Zhi 2013;34:816–819. [DOI] [PubMed] [Google Scholar]
- 3. Pfreundschuh M. How I treat elderly patients with diffuse large B‐cell lymphoma. Blood 2010;116:5103–5110. [DOI] [PubMed] [Google Scholar]
- 4. Tucci A, Ferrari S, Bottelli C et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer 2009;115:4547–4553. [DOI] [PubMed] [Google Scholar]
- 5. Tucci A, Martelli M, Rigacci L et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B‐cell lymphoma: A prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 2015;56:921–926. [DOI] [PubMed] [Google Scholar]
- 6. Marchesi F, Cenfra N, Altomare L et al. A retrospective study on 73 elderly patients (≥75years) with aggressive B‐cell non Hodgkin lymphoma: Clinical significance of treatment intensity and comprehensive geriatric assessment. J Geriatr Oncol 2013;4:242–248. [DOI] [PubMed] [Google Scholar]
- 7. Olivieri A, Gini G, Bocci C et al. Tailored therapy in an unselected population of 91 elderly patients with DLBCL prospectively evaluated using a simplified CGA. The Oncologist 2012;17:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spina M, Balzarotti M, Uziel L et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B‐cell lymphoma. The Oncologist 2012;17:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merli F, Luminari S, Rossi G et al. Outcome of frail elderly patients with diffuse large B‐cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: Results from a study of the Fondazione Italiana Linfomi. Leuk Lymphoma 2014;55:38–43. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida M, Nakao T, Horiuchi M et al. Analysis of elderly patients with diffuse large B‐cell lymphoma: Aggressive therapy is a reasonable approach for ‘unfit’ patients classified by comprehensive geriatric assessment. Eur J Haematol 2016;96:409–416. [DOI] [PubMed] [Google Scholar]
- 11. Jiangtao Li, Hui Liu, Jiefei Bai et al. The effect of comprehensive geriatric assessment on the therapeutic decision‐making in elderly patients with diffuse large B‐cell lymphoma. Chin J Geriatr 2017;36:269–273. [Google Scholar]
- 12. Mohile SG, Dale W, Somerfield MR et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Oncol Pract,2018;36:2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morrison VA, Hamlin P, Soubeyran P et al. Diffuse large B‐cell lymphoma in the elderly: impact of prognosis, comorbidities, geriatric assessment, and supportive care on clinical practice. An International Society of Geriatric Oncology (SIOG) expert position paper. J Geriatr Oncol 2015;6:141–152. [DOI] [PubMed] [Google Scholar]
- 14. Morrison VA, Hamlin P, Soubeyran P et al; International Society of Geriatric Oncology . Approach to therapy of diffuse large B‐cell lymphoma in the elderly: The International Society of Geriatric Oncology (SIOG) expert position commentary. Ann Oncol 2015;26:1058–1068. [DOI] [PubMed] [Google Scholar]
- 15. Cheson BD, Pfistner B, Juweid ME et al; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 16. Miller AB, Hoogstraten B, Staquet M et al. Reporting results of cancer treatment. Cancer 1981;47:207–214. [DOI] [PubMed] [Google Scholar]
- 17. Horwitz SM, Zelenetz AD, Gordon LI et al. NCCN Guidelines Insights: Non‐Hodgkin's Lymphomas, Version 3.2016. J Natl Compr Canc Netw 2016;14:1067–1079. [DOI] [PubMed] [Google Scholar]
- 18. Wildiers H, Heeren P, Puts M et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H, Gao M, Mei D et al. A comparative study of comprehensive geriatric assessment in elder patients with non‐Hodgkin's lymphoma [in Chinese]. Zhonghua Nei Ke Za Zhi 2018;57:330–334. [DOI] [PubMed] [Google Scholar]
- 20. Merli F, Luminari S, Tucci A et al. The elderly project by the Fondazione Italiana Linfomi (FIL): A Prospective Multidimensional Assessment of Elderly Patients with Diffuse Large B‐Cell Lymphoma. Blood 2016;128:3049. [Google Scholar]
- 21. Janssen‐Heijnen ML, van Spronsen DJ, Lemmens VE et al. A population‐based study of severity of comorbidity among patients with non‐Hodgkin's lymphoma: Prognostic impact independent of International Prognostic Index. Br J Haematol 2005;129:597–606. [DOI] [PubMed] [Google Scholar]
- 22. Coiffier B, Thieblemont C, Van Den Neste E et al. Long‐term outcome of patients in the LNH‐98.5 trial, the first randomized study comparing rituximab‐CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d'Etudes des Lymphomas de l'Adulte. Blood 2010;116:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfreundschuh M, Schubert J, Ziepert M et al. Six versus eight cycles of bi‐weekly CHOP‐14 with or without rituximab in elderly patients with aggressive CD20+ B‐cell lymphomas: A randomised controlled trial (RICOVER‐60). Lancet Oncol 2008;9:105–116. [DOI] [PubMed] [Google Scholar]


