Abstract
Background
Monocyte‐to‐lymphocyte ratio (MLR) and lactate dehydrogenase (LDH) levels are circulating biomarkers that provide information about tumor‐related inflammation and immune suppression. This study aimed to evaluate the prognostic role of MLR and LDH in metastatic colorectal cancer (mCRC).
Material and Methods
This multicentric study analyzed a consecutive cohort of 528 patients with mCRC treated in 2009–2017. The whole population was randomly divided in training and validation cohort. The first was used to identify a threshold for MLR and to create the prognostic model with MLR and MLR‐LDH combined (group 1: MLR‐LDH low; group 2: MLR or LDH high; group 3: MLR‐LDH high). The second cohort was used to validate the model.
Results
At the median follow‐up of 55 months, median overall survival (OS) was 22 months. By multivariate analysis, high MLR >0.49 (hazard ratio [HR], 2.37; 95% confidence interval [C.I.], 1.39–4.04), high LDH (HR, 1.73; 95% C.I., 1.03–2.90) in the first model, group 2 (HR, 2.74; 95% C.I.; 1.62–4.66), and group 3 (HR, 3.73; 95% C.I., 1.94–7.18) in the combined model, had a worse prognosis in terms of OS. These data were confirmed both in the validation set and then in the whole cohort.
Conclusion
MLR and LDH are circulating cost‐effective biomarkers, readily available in clinical practice, that can be useful for predicting the prognosis of patients with mCRC.
Implications for Practice
High monocyte‐to‐lymphocyte ratio (MLR) and lactate dehydrogenase (LDH) levels could be a sign of a tumor's recruitment of suppressive and inflammatory cells worsening prognosis of different types of cancer, including colorectal cancer (CRC). Currently, no data are available for metastatic CRC regarding a cutoff definition for MLR or the prognostic impact of MLR and MLR‐LDH combined. The present study showed in the training cohort and confirmed in the validation and whole cohort that MLR is a reliable and independent laboratory biomarker, which is easy to use, to predict clinical outcomes in patients with mCRC. Moreover, MLR and composite MLR‐LDH could potentially result in an incremental improvement in the prognostic value of these biomarkers, being used as stratification tools for patients with mCRC.
Keywords: Metastatic colorectal cancer, Circulating biomarkers, LDH, MLR, Immune biomarkers
Short abstract
Evidence suggests a pivotal role of the crosstalk between tumor microenvironment and the immune system that may be a hallmark of spreading cancer. This article evaluates the prognostic role of monocyte‐to‐lymphocyte ratio and levels of lactate dehydrogenase in patients with metastatic colorectal cancer.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy worldwide. Despite recent knowledge gained through molecular biology and advances in anticancer treatments, CRC remains the fourth cause of cancer death, with a 5‐year survival rate of 13.8% for metastatic disease 1.
In recent years, mounting evidence has established the pivotal role of the crosstalk between tumor microenvironment (TME) and immune system that has been recognized as an important hallmark of cancer spread 2, 3. Notably, the immune system may either destroy or paradoxically promote and sustain cancer cells growth by modulating regulatory mediators to recruit immunosuppressive and inflammatory cells.
Circulating monocytes are first recruited by tumor and stromal cells through the release of chemokines and growth factors 4, 5. In the tumor site, they are induced to differentiate with tissue resident macrophages into M2 tumor‐associated macrophages (TAMs), acquiring protumor functions, including cancer growth, angiogenesis, dissemination, matrix remodeling, and suppression of adaptive immune response. Recent studies showed that TAMs expressing CD68 and CD163 markers are enriched in many tumor types including CRC and may promote tumorigenesis and cancer progression 6, 7, 8, 9.
Conversely, circulating lymphocytes have an antitumor activity by inducing cytotoxic cell death and inhibiting tumor proliferation, hence several studies showed that the increased lymphocytes infiltration in tumor tissue predicted better survival outcomes in patients with cancer 10, 11, 12.
Therefore, changes in peripheral blood cells composition may reflect TME polarization and its role in cancer growth control 13. Neutrophil‐to‐lymphocyte ratio (NLR), lymphocyte‐to‐monocyte ratio (LMR), and lactate dehydrogenase (LDH) have been proposed as potential prognostic factors in patients with different tumor types 14.
Interestingly, high monocyte‐to‐lymphocyte ratio (MLR), LDH, and NLR could be a sign of a tumor's recruitment of suppressive and inflammatory cells worsening prognosis of different types of cancer, including CRC 10, 11, 12. However, the majority of data in the CRC setting come from studies including mainly Asian patients and early stage tumors 15, 16, 17, 18.
Based on these premises, the present study aimed to evaluate the prognostic impact of circulating immune‐suppressive and inflammatory biomarkers, namely MLR and LDH, in patients with metastatic CRC (mCRC).
Materials and Methods
This was a multicenter, observational, retrospective, cohort study conducted on 528 consecutive patients with mCRC who underwent first‐line chemotherapy and for whom the results of baseline blood tests were known. The study was aimed at evaluating the prognostic impact of baseline MLR and LDH levels in patients with mCRC in terms of overall survival (OS). To define and validate a cutoff for MLR, data were divided into two cohorts, training and validation.
Furthermore, secondary objectives were to identify and validate a threshold of MLR to discriminate patients according to prognosis, evaluate the value of combining MLR and LDH parameters, and evaluate the association between both MLR and the combination of MLR and LDH with other variables (i.e., sidedness, resection of primary tumor, number, and pattern of metastatic sites).
The study was approved by the departmental review board and by the Ethics Committee (Protocol number CRO‐2019–28).
Study Population
All patients had confirmed mCRC diagnosis and provided consent for the use of clinical data, rendered anonymous, for purposes of clinical research, epidemiology, training, and study of diseases.
The series consisted of consecutive patients treated at the Department of Medical Oncology, National Cancer Institute of Aviano and Pordenone (224 patients), at the Oncology Department of Udine (218 patients), and at the Oncology Department of Mauriziano Hospital of Turin (86 patients), Italy, from January 2009 to March 2018. Data concerning age, sidedness (right colon vs. left colon), resection of primary tumor, date of metastatic disease diagnosis, number of metastatic sites, pattern of metastasis, biological profile (RAS and BRAF mutational status), date of first‐line chemotherapy start, type of first‐line treatment, and baseline treatment blood sample values (lymphocytes, monocytes, neutrophils, LDH) have been collected. Data have been retrieved from electronic and paper‐based chart review according to strict privacy standards.
Blood Sample Analysis
MLR was defined as the absolute monocyte count divided by the absolute lymphocyte count; LDH levels were classified as under and over the limits according to a cutoff established by the laboratory of each participating center. Full blood count data were eligible for analysis if performed within 1 month before the start of first‐line chemotherapy.
Statistical Analysis
The data set obtained was randomly divided with a ratio of 50%/50% in a training and validation cohort.
Patients' clinical and pathological characteristics were summarized through descriptive analysis. Categorical variables were described through frequency distribution, whereas continuous variables were reported through median and range. Differences across groups were compared through the chi‐square test for categorical variables. For overall survival analyses, time at risk has been calculated from the date of metastatic disease diagnosis to the date of the event of interest: death or last follow‐up. For univariate survival analysis, OS probabilities were estimated using the Kaplan‐Meier method and compared by log‐rank test. A p value <.05 was considered as statistically significant.
Training Cohort
To identify a threshold of MLR to discriminate patients according to OS, a receiving operator curve (ROC) analysis was performed. Patients subgroups identified by MLR and by MLR and LDH combination were compared using the log‐rank test. A Cox proportional‐hazards regression model, also including potential confounders (e.g., age, biological profile, sidedness) was used to calculate hazard ratios (HRs) of death, with the corresponding 95% confidence intervals (C.I.s), among different subgroups of patients identified by MLR and by the combination of MLR and LDH.
The training cohort was used to create the prognostic model with the cutoff identified for MLR and then with the combination of MLR and LDH levels; we arbitrarily categorized patients into three groups according to MLR and LDH levels (group 1: MLR and LDH low; group 2: MLR or LDH high; group 3: MLR and LDH high).
The performance of the prognostic model was evaluated using the Harrell's discrimination concordance index statistic (which is defined as the probability that predictions and outcomes are concordant: the equivalent of an area under curve (AUC) in the ROC analysis for survival data).
Validation Cohort
The prognostic model was then applied in the validation set. Cox proportional‐hazards regression model, was used to calculate HRs of death, with the corresponding 95% C.I.s, in the different subgroups of patients identified by MLR and by the combination of MLR and LDH.
Pooled Population
The prognostic model was evaluated in the whole cohort. Cox proportional‐hazards regression model was used to calculate HRs of death, with the corresponding 95% C.I.s, among the different subgroups of patients identified by MLR and by the combination of MLR and LDH.
Associations between variables were explored in the whole cohort by using statistical tests (chi‐square, Wilcoxon rank‐sum test, or Kruskal‐Wallis test) as statistically appropriate. Furthermore, subgroup analyses were carried out. Statistical analysis was performed with STATA (Release 14.2.; StataCorp LP, College Station, TX) and with MedCalc.
Sample Size Calculation
The sample size was estimated in order to obtain a good performance of the statistical model for the association between patient and tumor characteristics with outcome measures in the multivariate analysis. The aim of the sampling was the achievement of a good “goodness of fit” from the regression model according to Peduzzi et al. 19, 20. Therefore, considering 20–50 events per variable (EPV) and a final model with approximately 6–7 variables, about 180 and 360 EPV are necessary to obtain an accurate estimation of the statistical model. In the present study, we observed 186 events in the training set, 195 events in the validation set, and 381 in the whole cohort. Therefore, we could define an accurate estimation for the multivariate model.
Results
The MIMIC study included a cohort of 528 patients with a diagnosis of mCRC (clinical, pathological, and treatment characteristics are listed in the Table 1) who underwent first‐line chemotherapy. In the whole cohort, 63% of the patients were younger than 70 years. Of note, 33% of patients had a right tumor location, and 71% received a primary tumor resection. Approximately 35% had more than one metastatic site involved.
Table 1.
Baseline characteristics
| Variables | Frequencies, n (%) (n = 528) |
|---|---|
| Age, yr | |
| ≤70 | 331 (62.69) |
| >70 | 194 (36.74) |
| Missing | 3 (0.57) |
| Sex | |
| Male | 306 (57.95) |
| Female | 222 (42.05) |
| Sidedness | |
| Right | 173 (32.77) |
| Left | 351 (66.48) |
| Missing | 4 (0.76) |
| Primary tumor resection | |
| No | 154 (29.17) |
| Yes | 374 (70.83) |
| Number of metastatic sites | |
| 1 | 290 (54.92) |
| >1 | 187 (35.42) |
| Missing | 51 (9.66) |
| Metastatic sites | |
| Liver | 199 (37.69) |
| Lung | 98 (18.56) |
| Lymphnodes | 71 (13.45) |
| Peritoneum | 18 (22.35) |
| Bone | 13 (2.46) |
| Brain | 3 (0.57) |
| Missing | 26 (4.92) |
| KRAS | |
| Wild type | 244 (46.47) |
| Mutated | 212 (40.15) |
| Missing | 72 (13.64) |
| BRAF | |
| Wild type | 335 (63.44) |
| Mutated | 43 (8.14) |
| Missing | 150 (28.57) |
| NRAS | |
| Wild Type | 347 (65.72) |
| Mutated | 14 (2.65) |
| Missing | 167 (31.63) |
| All RAS | |
| Wild type | 225 (42.61) |
| Mutated | 266 (50.38) |
| Missing | 37 (7.01) |
| LDH level | |
| Under limits | 277 (52.46) |
| Over limits | 113 (21.40) |
| Missing | 138 (26.14) |
| Number of events | |
| Censored | 147 (27.84) |
| Deaths | 381 (72.16) |
Number of treatment lines (411 pts); median value, 2.11. monocyte‐to‐lymphocyte ratio (528 pts); median value, 0.40.
Abbreviation: LDH, lactate dehydrogenase.
The most frequent site of metastatic spread was liver (38%), followed by peritoneum (22%) and lungs (19%). As for the biologic profile, KRAS mutation was found in 40%, NRAS mutation in 3%, and BRAF mutation in 8% of patients. The median follow‐up was 55 months, whereas median OS was 22 months.
No significant differences were observed among clinical and pathological features collected between the two cohorts (supplemental online Table 1).
Training Cohort
The training cohort included 264 patients. The median follow‐up was 53 months, whereas median OS was 22 months.
At univariate analysis (supplemental online Table 2), age ≥ 70 years (HR, 1.53; 95% C.I., 1.14–2.05), metastatic pattern (lymph‐nodes vs. liver: HR, 1.83; 95% C.I., 1.15–2.90; peritoneum vs. liver: HR, 1.48; 95% C.I., 1.01–2.16), BRAF mutation (HR, 1.77; 95% C.I., 1.07–2.92), NLR as continuous variable (HR, 1.01; 95% C.I., 1.01–1.02), MLR as continuous variable (HR, 2.44; 95% C.I., 1.79–3.34), and LDH above limits (HR, 2.10; 95% C.I., 1.47–2.98) were associated with worst prognosis in terms of OS. Conversely, primary tumor resection (HR, 0.37; 95% C.I., 0.27–0.51) and left sidedness (HR, 0.62; 95% C.I., 0.46–0.84) were associated with better survival.
The MLR cutoff identified with ROC analysis was 0.49 (AUC, 0.64; supplemental online Fig. 1). MLR levels higher than 0.49 (called MLR high from here on) predicted worse OS (Fig. 1). By multivariate analysis (Table 2), age ≥ 70 years (HR, 1.83; 95% C.I., 1.13–2.94), BRAF mutation (HR, 2.23; 95% C.I., 1.04–4.77), MLR > 0.49 (HR, 2.37; 95% C.I., 1.39–4.04), and high LDH values (HR, 1.73; 95% C.I., 1.03–2.90) were still associated with shorter OS. Conversely, surgery of primary tumor was associated with longer OS (HR, 0.40; 95% C.I., 0.23–0.68). Similarly, left sidedness showed a trend toward good prognosis (left vs. right: HR, 0.62; 95% C.I., 0.37–1.06). Median OS for MLR high was 13.8 months versus 29.5 months for MLR low.
Figure 1.
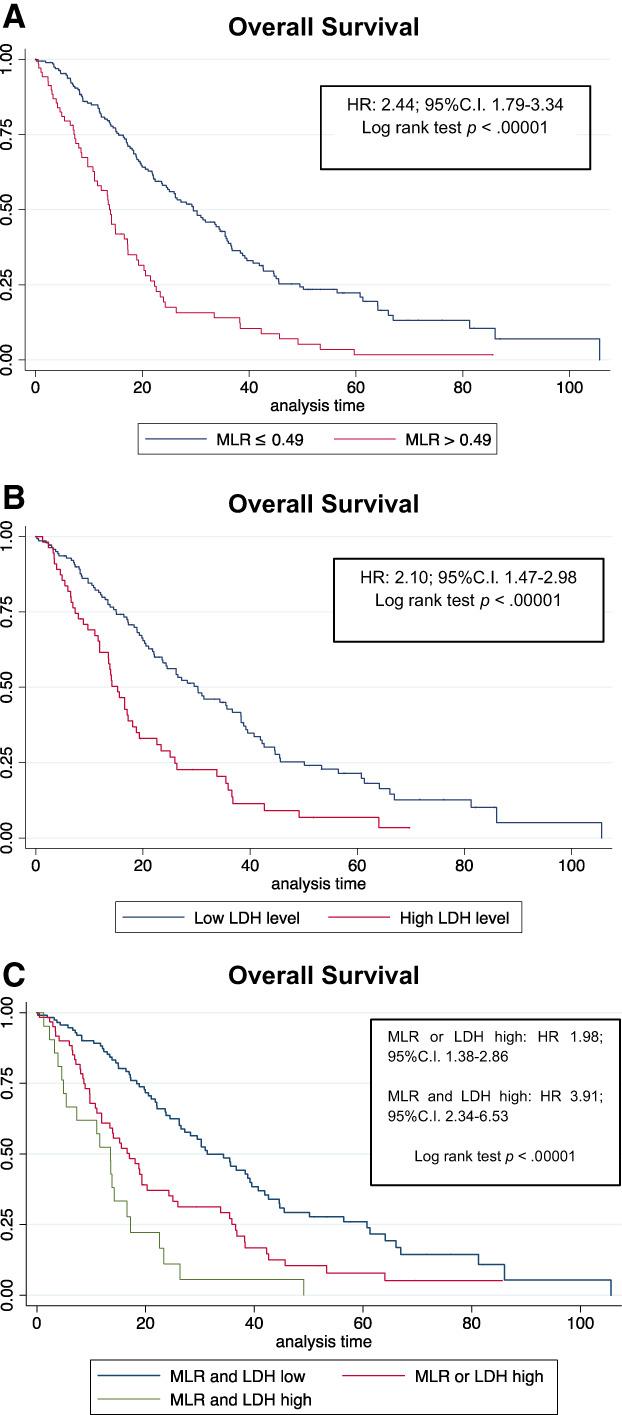
Training cohort: (A): MLR, (B): LDH, and (C): MLR and LDH combination. Abbreviations: C.I., confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase; MLR, monocyte‐to‐lymphocyte ratio.
Table 2.
Multivariate analysis
| Variables | Training cohort | Validation cohort | Pooled cohort | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age, ≥70 vs.70 | 1.83 (1.13–2.94) | .013 | 1.36 (0.83–2.23) | .208 | 1.60 (1.16–2.21) | .004 |
| Sidedness left vs. right | 0.63 (0.37–1.07) | .089 | 0.80 (0.50–1.29) | .377 | 0.74 (0.53–1.03) | .078 |
| Surgery of primary tumor, yes vs. no | 0.40 (0.23–0.68) | .001 | 0.95 (0.60–1.51) | .859 | 0.67 (0.48–0.92) | .016 |
| Pattern of metastasis | ||||||
| Lung vs. liver | 1.08 (0.54–2.14) | .826 | 1.41 (0.75–2.66) | .277 | 1.20 (0.77–1.85) | .404 |
| Nodes vs. liver | 1.27 (0.67–2.44) | .456 | 1.07 (0.51–2.22) | .846 | 1.26 (0.79–2.01) | .319 |
| Peritoneum vs. liver | 1.51 (0.86–2.65) | .148 | 3.10 (1.69–5.66) | <.001 | 2.03 (1.36–3.02) | <.001 |
| BRAF, mutated vs. WT | 2.23 (1.04–4.77) | .038 | 1.39 (0.65–2.98) | .389 | 1.79 (1.06–3.01) | .027 |
| MLR, >0.49 vs. <=0.49 | 2.37 (1.38–4.04) | .001 | 2.31 (1.27–4.19) | .006 | 2.15 (1.47–3.14) | <.001 |
| LDH, high vs. low | 1.73 (1.03–2.90) | .037 | 1.85 (1.00–3.41) | .048 | 1.65 (1.15–2.37) | .006 |
| NLR, continuous variable | 1.00 (0.99–1.01) | .347 | 0.97 (0.88–1.06) | .511 | 1.00 (0.99–1.02) | .108 |
Bold values are statistically significant. Italicized text are values of reference.
Abbreviations: CI, confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase; MLR, monocyte‐to‐lymphocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; WT, wild type.
We arbitrarily categorized patients into three groups according to combined MLR and LDH levels (group 1: MLR and LDH low; group 2: MLR or LDH high; group 3: MLR and LDH high).
Patients with MLR or LDH high (HR vs. group 1, 1.98; 95% C.I., 1.38–2.86 in univariate analysis; HR, 2.74; 95% C.I., 1.62–4.66 in multivariate analysis) or both elevated (HR, 3.91 vs. group 1; 95% C.I., 2.34–6.53 in univariate; HR, 3.73; 95% C.I., 1.94–7.18 in multivariate) had a worse prognosis in terms of OS (Table 3; Fig. 1). Median OS for patients with both MLR and LDH low was 31.3 months, for patients with MLR or LDH high was 17.0 months, and for patients with both MLR and LDH high was 13.55 months. The performance established by Harrell's C‐statistic for model with known clinic‐pathological variables was 0.68 and with the addition of MLR was 0.71 or LDH was 0.72. Interestingly, the C‐statistic for the model with known clinical and pathological variables and the combination of MLR and LDH in the three categories was 0.74.
Table 3.
Multivariate analysis with MLR‐LDH combination
| Variables | Training cohort | Validation cohort | Pooled cohort | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age, ≥70 vs.70 | 1.79 (1.11–2.86) | .016 | 1.38 (0.85–2.24) | .188 | 1.63 (1.18–2.25) | .003 |
| Sidedness, left vs. right | 0.62 (0.36–1.06) | .089 | 0.81 (0.51–1.29 | .387 | 0.75 (0.54–1.04) | .018 |
| Surgery of primary tumor, yes vs. no | 0.40 (0.24–0.68) | .001 | 0.97 (0.61–1.53) | .904 | 0.67 (0.49–0.94) | .089 |
| Pattern of metastasis | ||||||
| Lung vs. liver | 0.97 (0.47–1.96) | .933 | 1.42 (0.75–2.69) | .273 | 1.17 (0.76–1.82) | .466 |
| Nodes vs. liver | 1.29 (0.68–2.46) | .456 | 1.07 (0.52–2.23) | .839 | 1.29 (0.81–2.05) | .281 |
| Peritoneum vs. liver | 1.39 (0.78–2.44) | .254 | 3.06 (1.68–5.57) | <.001 | 1.97 (1.33–2.93) | .001 |
| BRAF, mutated vs. WT | 2.31 (1.08–4.91) | .029 | 1.37 (0.64–2.93) | .412 | 1.79 (1.06–3.02) | .028 |
| MLR‐LDH combination | ||||||
| MLR or LDH high vs. low | 2.74 (1.62–4.66) | <.001 | 1.86 (1.00–3.47) | .051 | 1.98 (1.38–2.86) | <.001 |
| MLR and LDH high vs. low | 3.73 (1.94–7.18) | <.001 | 4.46 (2.09–9.52) | <.001 | 3.42 (2.16–5.41) | <.001 |
| NLR, continuous variable | 1.00 (0.99–1.01) | .321 | 0.97 (0.88–1.06) | .527 | 1.00 (0.99–1.02) | .081 |
Bold values are statistically significant. Italicized text are values of reference.
Abbreviations: CI, confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase; MLR, monocyte‐to‐lymphocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; WT, wild type.
Validation Cohort
A total of 264 eligible patients were included in the validation cohort. Baseline clinical and pathological characteristics are reported in the Table 2. The median follow‐up was 58 months, the median OS was 22 months. To validate the MLR cutoff and the combination of the two biomarkers found in the training cohort, we carried out a multivariate Cox model including also the variables identified as significant in the training cohort.
In this set, MLR and LDH maintained their unfavorable prognostic value both when analyzed individually (MLR high vs. low: HR, 2.31; 95% C.I., 1.27–4.19; LDH high vs. low: HR, 1.85; 95% C.I., 1.01–3.41) and when combined in the three categories (MLR or LDH high: HR vs. MLR and LDH low, 1.86; 95% C.I., 0.99–3.47; MLR and LDH high: HR vs. MLR and LDH low, 4.46; 95% C.I., 2.09–9.52; supplemental online Fig. 2).
Pooled Population
By multivariate analysis for OS in the whole cohort (n = 528), MLR and LDH confirmed to be independent prognostic factors both individually (MLR high vs. low: HR, 2.15; 95% C.I., 1.47–3.14; LDH high vs. low: HR, 1.65; 95% C.I., 1.15–2.37) and when they were combined (MLR or LDH high: HR vs. MLR and LDH low, 1.98; 95% C.I., 1.38–2.86; MLR and LDH high: HR vs. MLR and LDH low, 3.42; 95% C.I., 2.16–5.41; Fig. 2).
Figure 2.
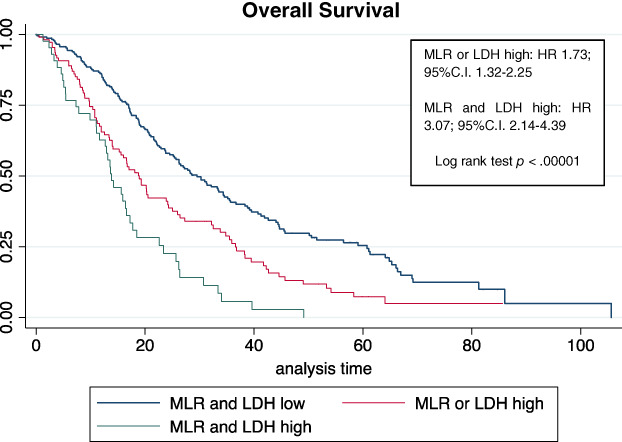
Whole population. Abbreviations: C.I., confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase; MLR, monocyte‐to‐lymphocyte ratio.
Notably, high MLR at first‐line therapy start was associated with one or more metastatic sites (p < .001), pattern of metastasis (p < .001), and primary tumor surgery not executed (p < .001; Fig. 3). Conversely, MLR was not associated with sidedness (p = .202). Interestingly, an exploratory analysis conducted on a subgroup of patients with the availability of the precise information about sidedness (according to right, left, and rectum) showed an association with MLR (Fig. 3).
Figure 3.
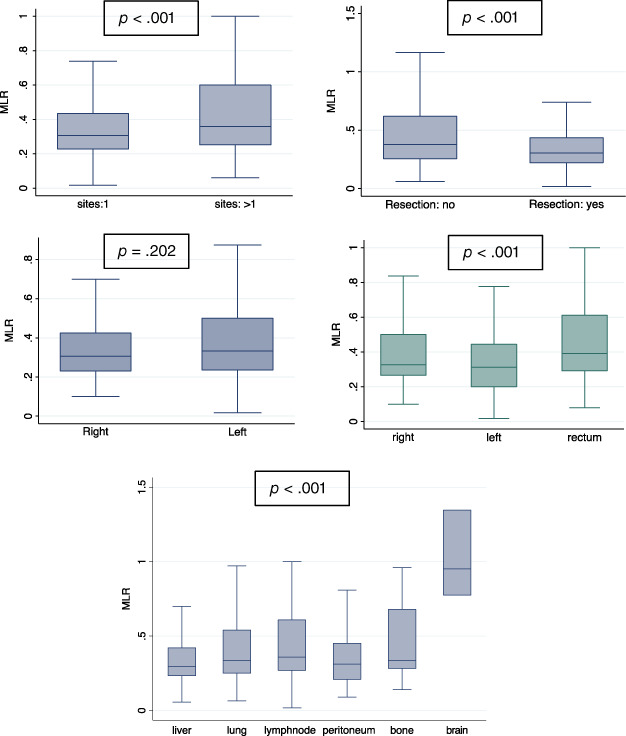
MLR association. Notably, high MLR at first‐line therapy start was associated with one or more metastatic sites (p < .001), pattern of metastasis (p < .001), and primary tumor surgery not executed (p < .001; Fig. 3). Conversely, MLR was not associated with sidedness (p = .202). Interestingly, an exploratory analysis conducted on a subgroup of patients with the availability of the precise information about sidedness (according to right, left, and rectum) showed an association with MLR (Fig. 3).Abbreviation: MLR, monocyte‐to‐lymphocyte ratio.
MLR‐LDH combination high was associated with number of metastatic sites ≥1 (p < .001), pattern of metastasis (p = .047), and primary tumor surgery not executed (p < .001).
In the subgroup analysis, no interaction was observed among subgroups stratified for MLR status (high vs. low; supplemental online Fig. 3) and for MLR and LDH status (supplemental online Fig. 4).
Discussion
Increasing evidence shows that cancer‐related inflammation and immune suppression favor tumor initiation and progression in CRC, becoming a critical player in the oncogenic process. Inflammatory and immune‐suppressive indexes, namely NLR, LMR, and LDH, are gaining momentum as prognostic indicators.
However, published studies evaluating the role of inflammatory indexes are heterogeneous, mostly with variable cutoff values and were conducted in patients with resectable CRC 21, 22, 23, 24, 25, 26.
The present study, conducted on 528 patients, aimed to evaluate the prognostic role of such circulating biomarkers in mCRC. Moreover, although data for NLR and LDH about the optimal cutoff are defined, for MLR there are still few and conflicting data. Therefore, the data set was randomly divided in two balanced cohorts to establish and validate the statistically optimal MLR cutoff value.
Interestingly, MLR high (cutoff point identified as >0.49) at the start of first‐line therapy negatively impacted on survival. To assess the prognostic role of the MLR cutoff value established in the training cohort, the multivariate model was tested in a validation set: MLR >0.49 and high LDH levels were confirmed to be associated with worse prognosis.
Of note, MLR indirectly reflects the complex interplay between TME, with its degree of progression, and the host immune system, with its ability to govern clinical behavior of CRC to determine survival outcome. Chemokine‐ligand (CCL)2, CCL21, and vascular endothelial growth factor (VEGF) are responsible for the recruitment of circulating monocytes into primary tumors and the metastatic niche, where local chemokines (including interleukin [IL]‐10 and TGF‐β) promote their differentiation into M2 phenotype TAMs. Hence, they induce tumor growth promotion, tissue remodeling, angiogenesis, adaptive immunity suppression, and invasion 27, 28, 29. Conversely, lymphocytes are crucial for the adaptive immune response that inhibits tumor cell proliferation and migration and determines cytotoxic cell death. A decrease in lymphocytes absolute count potentially reflects an insufficient response of the host immune system to the tumor, consequently enhancing tumor progression and metastatic processes 22, 30, 31.
Moreover, evidence is emerging about the role of inflammation in the tumorigenesis and progression of many malignancies, which negatively influences survival outcomes. In particular, recent studies show that baseline LDH and its dynamic change have an established independent prognostic impact 32, 33, 34, 35. Lactate produced by cancer cells (a product of LDH‐A gene) has a pivotal role in the TME, by activating the recruitment of circulating monocytes and resident macrophages to tumor site and then influencing the polarization of TAMs into M2‐like subsets 36, 37, 38.
Remarkably, LDH plays a key role in cancer maintenance and progression inducing several key features in TME through several proinflammatory cytokines such as TNF‐α, IL‐1, IL‐6, reactive oxygen and nitrogen species, prostaglandins, nuclear factor‐κB, and microRNAs. Namely, it modulates the activity of arginase I and inhibits the innate and adaptive immune response by promoting immune‐suppressive cytokines.
Therefore, a combined composite stratification that included MLR and LDH levels was developed (group 1: MLR and LDH low; group 2: MLR or LDH high; group 3: MLR and LDH high). An association with worse prognosis in terms of OS was observed with both MLR or LDH over the limits and both elevated. Data were confirmed also in the validation set.
Of note, LDH is a product of aerobic and anaerobic glycolysis. Therefore, as a byproduct of the first one, it can provide information about cell proliferation, whereas as a byproduct of the second one, it is strongly linked to hypoxia and angiogenesis with overexpression of VEGF‐A and VEGFR‐1 and macrophage polarization 39, 40, 41. Preclinical and clinical studies has already shown that serum LDH levels could be a circulating biomarker of angiogenesis and could predict benefit to anti‐VEGF in mCRC 42.
Because both a high monocyte count and a low lymphocyte count reflect a suppressed immune activity, a high MLR may be a sign of elevated tumor burden 43. In fact, in the present study, MLR and MLR‐LDH combined were associated with the number of metastatic sites: high levels were observed in patients with more than one site involved. Moreover, MLR and MLR‐LDH combined were associated with pattern of metastasis and primary tumor surgery. Conversely, they were not associated with sidedness (right vs. left). Interestingly, an exploratory analysis according to sidedness as right, left, and rectum showed an association with MLR: right and rectum tumors have high MLR levels.
CRC is a heterogeneous disease whose complexity results from multiple genetic and epigenetic alterations that interfere with tumor biology and disease characteristics 44. Interestingly, in the present study, no interaction was observed between MLR and RAS or BRAF molecular mutations. These results are consistent with scientific evidence available, suggesting that a high MLR denote an aggressive biology in a way that is independent of common molecular alterations 21. Nevertheless, they may be influenced by the small number of patients with molecular mutations detected. Larger cohorts are needed to eventually undertake association with molecular characteristics that associate with worse clinical outcomes.
The results of this study confirm and highlight the clinical utility of MLR and LDH as biomarkers of systemic immune and inflammatory response and independent predictors of survival in patients receiving a first‐line treatment for mCRC. Notably, the study evaluated a heterogeneous cohort of unselected, real‐world patients, hence these results might hold a considerable clinical utility to identify mCRC patients with worst survival. Indeed, they are consistent with the evidence available and support the prospective use of the cutoff identified in larger cohorts of patients with mCRC candidate to receive a first‐line therapy.
A strength of this study is the large sample size of the whole cohort and the randomly division in training and validation cohort. Furthermore, the results of this study can be considered consistent with the role of monocyte‐derived TAMs in promoting tumor cell migration, invasion, metastatic potential, and malignant cells tropism 45, 46. Although there is evidence that inflammation and immune suppression have a driving role in promoting cancer progression, TME is surrounded by a halo of unanswered questions. Therefore, the precise role of the different actors involved in the suppressive TME and progression need to be further investigated.
Conclusion
MLR is a reliable and independent laboratory biomarker, which is easy to use, to predict clinical outcomes in patients with mCRC. Moreover, MLR and composite MLR‐LDH could potentially result in an incremental improvement in the prognostic value of these biomarkers, being used as stratification tools for patients with mCRC. Their evaluation is accessible and inexpensive, with a considerable extent of reproducibility. Future studies with a multicenter and prospective design in larger cohorts of patients are crucial to validate our results.
Author Contributions
Conception/design: Debora Basile, Marcella Montico, Giuseppe Aprile, Fabio Puglisi
Provision of study material or patients: Luisa Foltran, Nicoletta Pella, Angela Buonadonna, Massimo Di Maio, Gianpiero Fasola, Fabio Puglisi
Collection and/or assembly of data: Debora Basile, Silvio Ken Garattini, Carla Corvaja, Francesco Cortiula, Giacomo Pelizzari, Marco Audisio, Camilla Lisanti
Data analysis and interpretation: Debora Basile, Marcella Montico, Lorenzo Gerratana, Elena Ongaro, Donatella Iacono, Giovanni Gerardo Cardellino, Giuseppe Aprile, Fabio Puglisi
Manuscript writing: Debora Basile, Silvio Ken Garattini, Carla Corvaja, Francesco Cortiula, Valentina Fanotto
Final approval of manuscript: Debora Basile, Silvio Ken Garattini, Carla Corvaja, Marcella Montico, Francesco Cortiula, Giacomo Pelizzari, Lorenzo Gerratana, Marco Audisio, Camilla Lisanti, Valentina Fanotto, Elena Ongaro, Donatella Iacono, Giovanni Gerardo Cardellino, Luisa Foltran, Nicoletta Pella, Angela Buonadonna, Giuseppe Aprile, Massimo Di Maio, Gianpiero Fasola, Fabio Puglisi
Disclosures
Massimo Di Maio: Bristol‐Myers Squibb, Merck Sharp & Dohme, Pfizer, Eisai, Takeda, AstraZeneca, Millennium Pharmceuticals (C/A), Tesaro (RF); Fabio Puglisi: AstraZeneca, Roche (RF), Amgen, Eli Lilly & Co, Novartis, Pfizer, Roche (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Siegel RL, Miller KD, Fedewa SA et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 3. Cammarota R, Bertolini V, Pennesi G et al. The tumor microenvironment of colorectal cancer: Stromal TLR‐4 expression as a potential prognostic marker. J Transl Med 2010;8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sawa‐Wejksza K, Dudek A, Lemieszek M et al. Colon cancer‐derived conditioned medium induces differentiation of THP‐1 monocytes into a mixed population of M1/M2 cells. Tumour Biol 2018;40:1010428318797880. [DOI] [PubMed] [Google Scholar]
- 5. Pollard JW. Tumour‐educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71–78. [DOI] [PubMed] [Google Scholar]
- 6. Szebeni GJ, Vizler C, Kitajka K, et al. Inflammation and cancer: Extra‐ and intracellular determinants of tumor‐associated macrophages as tumor promoters. Mediators Inflamm 2017;2017:9294018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pander J, Heusinkveld M, van der Straaten T et al. Activation of tumor‐promoting type 2 macrophages by EGFR‐targeting antibody cetuximab. Clin Cancer Res 2011;17:5668–5673. [DOI] [PubMed] [Google Scholar]
- 8. Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011;9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res 2016;39:1588–1596. [DOI] [PubMed] [Google Scholar]
- 10. Marín Hernández C, Piñero Madrona A, Gil Vázquez PJ, et al. Usefulness of lymphocyte‐to‐monocyte, neutrophil‐to‐monocyte and neutrophil‐to‐lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol 2018;20:476–483. [DOI] [PubMed] [Google Scholar]
- 11. Zhu J‐Y, Liu CC, Wang L, et al. Peripheral blood lymphocyte‐to‐monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: A multicenter retrospective study. J Cancer 2017;8:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Q, Hu T, Zheng E et al. Prognostic role of the lymphocyte‐to‐monocyte ratio in colorectal cancer. Medicine (Baltimore) 2017;96:e7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koh YW, Kang HJ, Park C et al. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin's lymphoma: Correlation with tumor‐associated macrophages. The Oncologist 2012;17:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pelizzari G, Basile D, Zago S et al. Lactate dehydrogenase (LDH) response to first‐line treatment predicts survival in metastatic breast cancer: First clues for a cost‐effective and dynamic biomarker. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao WW, Zhang LN, You KY et al. A low lymphocyte‐to‐monocyte ratio predicts unfavorable prognosis in pathological T3N0 rectal cancer patients following total mesorectal excision. J Cancer 2015;6:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kozak M, von Eyben R, Pai J et al. The prognostic significance of pretreatment hematologic parameters in patients undergoing resection for colorectal cancer. Am J Clin Oncol 2015;40:405–412. [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Jia H, Yu W et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer 2016;139:220–231. [DOI] [PubMed] [Google Scholar]
- 18. Chan JCY, Chan DL, Diakos CI et al. The lymphocyte‐to‐monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg 2017;265:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peduzzi P, Concato J, Kemper E et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–1379. [DOI] [PubMed] [Google Scholar]
- 20. Steyerberg EW, Eijkemans MJ, Harrell FE Jr et al. Prognostic modeling with logistic regression analysis: In search of a sensible strategy in small data sets. Med Decis Making 2001;21:45–56. [DOI] [PubMed] [Google Scholar]
- 21. Chen ZY, Raghav K, Lieu CH et al. Cytokine profile and prognostic significance of high neutrophil‐lymphocyte ratio in colorectal cancer. Br J Cancer 2015;112:1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stotz M, Pichler M, Absenger G et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer 2014;110:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guthrie GJK, Charles KA, Roxburgh CSD et al. The systemic inflammation‐based neutrophil‐lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–230. [DOI] [PubMed] [Google Scholar]
- 24. Li MX, Liu XM, Zhang XF et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in colorectal cancer: A systematic review and meta‐analysis. Int J Cancer 2014;134:2403–2413. [DOI] [PubMed] [Google Scholar]
- 25. Kaneko M, Nozawa H, Sasaki K et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in advanced colorectal cancer patients receiving oxaliplatin‐based chemotherapy. Oncology 2012;82:261–268. [DOI] [PubMed] [Google Scholar]
- 26. Wu Y, Li C, Zhao J et al. Neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J Surg Oncol 2016;14:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mantovani A, Allavena P, Sica A et al. Cancer‐related inflammation. Nature 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- 28. Mantovani A, Sozzani S, Locati M et al. Macrophage polarization: Tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–555. [DOI] [PubMed] [Google Scholar]
- 29. Evani SJ, Prabhu RG, Gnanaruban V et al. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. FASEB J 2013;27:3017–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Terzić J, Grivennikov S, Karin E et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101–2114.e5. [DOI] [PubMed] [Google Scholar]
- 31. Cézé N, Thibault G, Goujon G et al. Pre‐treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol 2011;68:1305–1313. [DOI] [PubMed] [Google Scholar]
- 32. Eton O, Legha SS, Moon TE et al. Prognostic factors for survival of patients treated systemically for disseminated melanoma. J Clin Oncol 1998;16:1103–1111. [DOI] [PubMed] [Google Scholar]
- 33. Manola J, Atkins M, Ibrahim J et al. Prognostic factors in metastatic melanoma: A pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 2000;18:3782–3793. [DOI] [PubMed] [Google Scholar]
- 34. Agarwala SS, Keilholz U, Gilles E et al. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951). Eur J Cancer 2009;45:1807–1814. [DOI] [PubMed] [Google Scholar]
- 35. Kelderman S, Heemskerk B, van Tinteren H et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 2014;63:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Colegio OR, Chu N‐Q, Szabo AL et al. Functional polarization of tumour‐associated macrophages by tumour‐derived lactic acid. Nature 2014;513:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mlecnik B, Tosolini M, Kirilovsky A et al. Histopathologic‐based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011;29:610–618. [DOI] [PubMed] [Google Scholar]
- 38. Becht E, de Reyniès A, Giraldo NA et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res 2016;22:4057–4066. [DOI] [PubMed] [Google Scholar]
- 39. Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 2008;134:703–707. [DOI] [PubMed] [Google Scholar]
- 40. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark 2017;19:353–363. [DOI] [PubMed] [Google Scholar]
- 41. Feng Y, Xiong Y, Qiao T et al. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med 2018;7:6124–6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marmorino F, Salvatore L, Barbara C et al. Serum LDH predicts benefit from bevacizumab beyond progression in metastatic colorectal cancer. Br J Cancer 2017;116:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hu P, Shen H, Wang G et al. Prognostic significance of systemic inflammation‐based lymphocyte‐ monocyte ratio in patients with lung cancer: Based on a large cohort study. PloS One 2014;9:e108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cancer Genome Atlas Network . Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263–266. [DOI] [PubMed] [Google Scholar]
- 46. Wyckoff JB, Wang Y, Lin EY et al. Direct visualization of macrophage‐assisted tumor cell intravasation in mammary tumors. Cancer Res 2007;67:2649–2656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables


