Abstract
Introduction
Local treatment of metastases is frequently performed in patients with multiorgan metastatic colorectal carcinoma (mCRC) analogous to selected patients with oligometastatic disease for whom this is standard of care. The ORCHESTRA trial (NCT01792934) was designed to prospectively evaluate overall survival benefit from tumor debulking in addition to chemotherapy in patients with multiorgan mCRC. Here, we report the preplanned safety and feasibility evaluation after inclusion of the first 100 patients.
Methods
Patients were eligible if at least 80% tumor debulking was deemed feasible by resection, radiotherapy and/or thermal ablative therapy. In case of clinical benefit after three or four cycles of respectively 5‐fluorouracil/leucovorin or capecitabine and oxaliplatin ± bevacizumab patients were randomized to tumor debulking followed by chemotherapy in the intervention arm, or standard treatment with chemotherapy.
Results
Twelve patients dropped out prior to randomization for various reasons. Eighty‐eight patients were randomized to the standard (n = 43) or intervention arm (n = 45). No patients withdrew after randomization. Debulking was performed in 82% (n = 37). Two patients had no lesions left to treat, five had progressive disease, and one patient died prior to local treatment. In 15 patients (40%) 21 serious adverse events related to debulking were reported. Postoperative mortality was 2.7% (n = 1). After debulking chemotherapy was resumed in 89% of patients.
Conclusion
Tumor debulking is feasible and does not prohibit administration of palliative chemotherapy in the majority of patients with multiorgan mCRC, despite the occurrence of serious adverse events related to local treatment.
Implications for Practice
This first prospective randomized trial on tumor debulking in addition to chemotherapy shows that local treatment of metastases is feasible in patients with multiorgan metastatic colorectal cancer and does not prohibit administration of palliative systemic therapy, despite the occurrence of serious adverse events related to local treatment. The trial continues accrual, and overall survival (OS) data and quality of life assessment are collected to determine whether the primary aim of >6 months OS benefit with preserved quality of life will be met. This will support evidence‐based decision making in multidisciplinary colorectal cancer care and can be readily implemented in daily practice.
Keywords: Metastatic colorectal cancer, Debulking, Cytoreduction, Stereotactic ablative radiotherapy, Radiofrequency ablation
Short abstract
The ORCHESTRA trial was designed to prospectively evaluate overall survival benefit from tumor debulking in addition to chemotherapy in patients with multi‐organ metastatic colorectal cancer. This article reports the preplanned safety and feasibility evaluation after inclusion of the first 100 patients.
Introduction
In the current multidisciplinary approach of metastatic colorectal cancer (mCRC), local treatment of oligometastases is increasingly performed. Large series of selected patients with liver‐only metastases treated with complete surgical resection suggest that this approach improves 5‐year survival rates to around 30%–60% and offers the only potential for cure [1, 2, 3, 4]. Application of techniques such as radiofrequency ablation (RFA) or microwave ablation or stereotactic ablative radiotherapy potentially increase feasibility of local treatment of metastases [5, 6, 7, 8, 9].
For selected patients with oligometastatic colorectal cancer (CRC), local treatment of metastases is standard of care based on retrospective reports showing long term survival rates. However, reports on the benefit of local treatment for multiorgan metastases of CRC were nonrandomized, single‐center and retrospective and therefore hampered by selection bias [10, 11, 12, 13, 14, 15, 16]. Treatment options with curative intent are generally not available for patients with extensive hepatic and/or extrahepatic mCRC. These patients primarily receive palliative systemic treatment consisting of combination chemotherapy with agents targeting VEGF or EGFR [17, 18]. It is unknown whether patients with extensive disease will benefit from tumor debulking when added to first‐line palliative systemic therapy [19, 20]. The benefit from local treatment of multiorgan metastases for these patients should be evaluated prospectively. Attempted prospective randomized studies were challenged by a lack of clinical equipoise, where both patient and doctors had preferences for either treatment arm based on beliefs of respectively under or overtreatment.
The ORCHESTRA trial (NCT01792934) is a randomized trial, designed to prospectively evaluate overall survival benefit from tumor debulking by resection, radiotherapy and/or thermal ablative therapy in patients with multiorgan mCRC when added to palliative systemic therapy [21]. The current manuscript reports on the preplanned safety and feasibility evaluation of tumor debulking based on the first 100 patients included. This trial examines the interplay of both efficacy and toxicity for the combination of systemic chemotherapy and local therapy. The study design incorporates both systemic and local therapy in the experimental arm and combines local treatment modalities to pursue maximal tumor debulking. The aim is to improve overall survival with at least 6 months of patients with multiorgan mCRC by maximal tumor debulking in addition to palliative chemotherapy.
In case this trial meets its primary outcome of 6‐month overall survival benefit, it will provide the evidence that any kind of local ablative therapy in a setting with multiple metastases will be of clinically significant benefit for patients with multiorgan mCRC and could reasonably be extended to patients with oligometastatic mCRC.
This report is focused on feasibility and safety of the local treatment procedures in this patient population. Moreover, we studied the ability to administer adequate palliative systemic treatment in the intervention arm, being the current evidence‐based treatment regimen, compared with patients receiving standard palliative systemic therapy.
Materials and Methods
The ORCHESTRA trial is a randomized multicenter clinical trial for patients with multiorgan mCRC, comparing the combination of chemotherapy and maximal tumor debulking versus chemotherapy alone. All procedures performed involving human participants were in accordance with the ethical standards of the institutional ethical and research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Written informed consent was obtained from all patients included in the ORCHESTRA trial. Patients were 18 years or older and had an indication for first‐line palliative systemic therapy for mCRC. They all had an Eastern Cooperative Oncology Group performance status of 0–2 and adequate bone marrow, liver, and renal function.
Patients with extensive multiorgan mCRC were eligible, as specified in Table 1. Tumor debulking of at least 80% of metastatic lesions by a combination of resection, radiotherapy, or thermal ablative therapy was deemed feasible by a multidisciplinary team, including a specialist in surgical oncology, radiotherapy, radiology, and medical oncology. Metastatic lesions were enumerated on computed tomography (CT) scan. If peritoneal metastases were individual deposits, these were numbered as separate metastatic lesions. In case of diffuse peritoneal carcinomatosis where lesions were difficult to define, this was categorized as “diffuse disease.” If the number of lesions in a single organ exceeded 10, this was also categorized as diffuse disease.
Table 1.
Main eligibility criteria for ORCHESTRA
| Patients with colorectal cancer metastases in at least two different organs if… |
|---|
| More than one extrahepatic metastasis or |
| More than five hepatic metastases not located to one lobe or |
| Either a positive para‐aortal lymph nodes or celiac lymph nodes or adrenal metastases or pleural carcinomatosis or peritoneal carcinomatosis |
| N.B. The primary tumor is excluded as metastatic site |
| Radical tumor debulking is feasible (incomplete tumor debulking is allowed only if at least 80% of metastases can be locally treated) |
Patients who underwent prior local treatment were not excluded. Prior (adjuvant) systemic therapy should have been completed more than 6 months at diagnosis of extrahepatic metastatic disease. Comprehensive inclusion and exclusion criteria are available at clinicaltrials.gov (NCT01792934). All patients received systemic therapy consisting of 5‐fluorouracil/leucovorin or capecitabine with oxaliplatin ± bevacizumab at physician discretion. Systemic therapy consisted of orally administered capecitabine 1,000 mg/m2 twice a day for 2 weeks and oxaliplatin 130 mg/m2 intravenously (CAPOX) on day 1 in a 3‐week cycle or comparable intravenous regimen consisting of oxaliplatin 85 mg/m2 on day 1 and 400 mg/m2 leucovorin followed by 400 mg/m2 5‐fluorouracil bolus and 2,400 mg/m2 continuous infusion over 46 hours (modified FOLFOX6) of each 2‐week cycle. Bevacizumab was added at physician discretion as intravenous infusion over 30–90 minutes on day 1 (in CAPOX regimen 3‐weekly 7.5 mg/kg, referred to as CAPOX(B); in FOLFOX regimen biweekly 5 mg/kg, referred to as FOLFOX(B)). First response evaluation (according to RECIST) [22] on a CT scan of thorax and abdomen was scheduled after three cycles of CAPOX(B) or four cycles of FOLFOX(B) (generally 9 weeks). Follow‐up CT scans were done at least every 3 months.
In case of stable disease or response, patients were randomized to continuation of systemic therapy (standard treatment; arm A), or tumor debulking followed by systemic therapy (intervention; arm B) and were stratified for location of metastases (liver and lung only vs. other), number of metastatic sites (at least two organs), and prior local treatment of metastases (yes/no) as well as gender, baseline lactic acid dehydrogenase (normal or elevated), and response to three cycles of systemic treatment (stable disease vs. [partial] response).
Patients who were randomized in the intervention arm and had stable disease at first evaluation continued systemic therapy (three cycles of CAPOX(B) or four cycles of FOLFOX(B)) followed by debulking if disease remained stable. Bevacizumab was omitted in the treatment cycle prior to tumor debulking. The final local treatment plan was determined by the multidisciplinary team based on metastases present at the latest CT scan.
Based on operating reports and radiotherapy treatment delivery, the number of treated metastases was documented and classified as tumor debulking of >80% of metastatic lesions or not.
Adverse events (AEs) were documented according to Common Terminology Criteria for Adverse Events version 4.03 and documented to be related to systemic therapy (only grade > 2), related to local therapy, or not related. AEs related to local treatment were graded according to the Clavien Dindo classification of surgical complications as well [23]. Serious adverse events (SAEs) were reported to the competent authority for adverse events that resulted in death, were life‐threatening, required inpatient hospitalization or caused prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, or required intervention to prevent permanent impairment or damage. Safety reports were drawn up and evaluated by an independent Data Safety Monitoring Board after inclusion of 25, 50, and 100 patients. Study continuation was based on the interim report on safety and feasibility after inclusion of 100 (of 478) patients.
A 20% dropout rate prior to randomization because of progression on first‐line systemic therapy or other reasons was taken into account in the power analysis. A total of 478 patients are anticipated to be included to randomize 382 patients and meet the primary endpoint of an overall survival benefit of >6 months (power 80%, type I error rate 5%). The study was deemed feasible if less than 10% of patients withdrew from the study after randomization of 20% of the total number of patients (n = 76). Secondary endpoints include progression‐free survival and quality of life, as well as evaluation of potential biomarkers such as carcinoembryonic antigen, microRNA, circulating endothelial cells, and platelet‐derived RNA.
Results
Between May 2013 and May 2015, the first 100 patients were included in 16 secondary and tertiary hospitals in The Netherlands that are part of the Dutch Colorectal Cancer Group. Patients had a median age of 65 years (range, 30–78), and 67% were male. Of these 100 patients, 71% had a left‐sided primary tumor, and 63% presented with synchronous metastatic disease. In 72% the primary tumor was resected, and 34 patients had prior local treatment of metastases. In 35% more than two organs were involved in metastatic disease (up to five organs). Patients had a median of six metastatic lesions (interquartile range [IQR], five). Twenty‐six percent had fewer than five lesions, 43% had five to ten lesions, and 31% had more than ten lesions or diffuse (peritoneal) disease. There were no significant differences in clinical parameters between both treatment arms prior to start of chemotherapy (Table 2). Liver metastases were present in 81%, 50% had lung metastasis, and 57% had distant lymph node metastases [12, 24]. Peritoneal disease was present in 33%, and, respectively, 7%, 5%, and 3% had bone, adrenal gland, or skin/subcutaneous metastases. The majority of patients were treated with CAPOX; one patient was treated with FOLFOX. Bevacizumab was added in 62%. Seventy percent of patients in arm A and 64% of patients in arm B completed eight cycles of CAP(OX).
Table 2.
Patient demographics and clinical characteristics
| Baseline characteristics | Total (n = 100), n (%) | Standard treatment arm (n = 43), n (%) | Intervention arm B (n = 45), n (%) | p value |
|---|---|---|---|---|
| Gender: Male | 67 (67) | 29 (67) | 31 (69) | .88 |
| Age <65 | 51 (51) | 25 (58) | 21 (47) | .28 |
| Synchronous | 63 (63) | 30 (70) | 26 (58) | .24 |
| Left‐sided primary tumor | 71 (71) | 26 (60) | 35 (78) | .08 |
| Primary in situ | 28 (28) | 14 (41) | 12 (27) | .55 |
| Number of metastases | .89 | |||
| <5 | 26 (26) | 11 (26) | 10 (22) | |
| 5–10 | 43 (43) | 18 (42) | 21 (47) | |
| >10 or diffuse | 31 (31) | 14 (33) | 14 (31) | |
| Number of organs involved | .35 | |||
| 2 | 65 (65) | 29 (67) | 26 (58) | |
| >2 | 35 (35) | 14 (33) | 19 (42) | |
| CEA >5 μg/L | 78 (78) | 31 (79) | 37 (84) | .18 |
| LDH normal | 77 (77) | 37 (86) | 34 (65) | .28 |
| Prior tumor treatments | ||||
| Prior (neo) adjuvant chemotherapy | 19 | 9 (21) | 10 (22) | .88 |
| Prior chemoradiation | 14 | 4 (9) | 8 (17) | .25 |
| Previous local treatment | 34 | 17 (40) | 12 (27) | .20 |
Abbreviations: CEA, carcinoembryonic antigen; LDH, lactic acid dehydrogenase.
Prior to randomization, two patients went off study because of toxicity of systemic treatment, two patients died, and five patients withdrew consent before starting or during the first cycles of systemic therapy (no reason specified). Three patients had progressive disease and were not randomized per protocol. Eighty‐eight patients were randomized to the standard (n = 43) or intervention arm (n = 45). No patients withdrew after randomization (Fig. 1).
Figure 1.
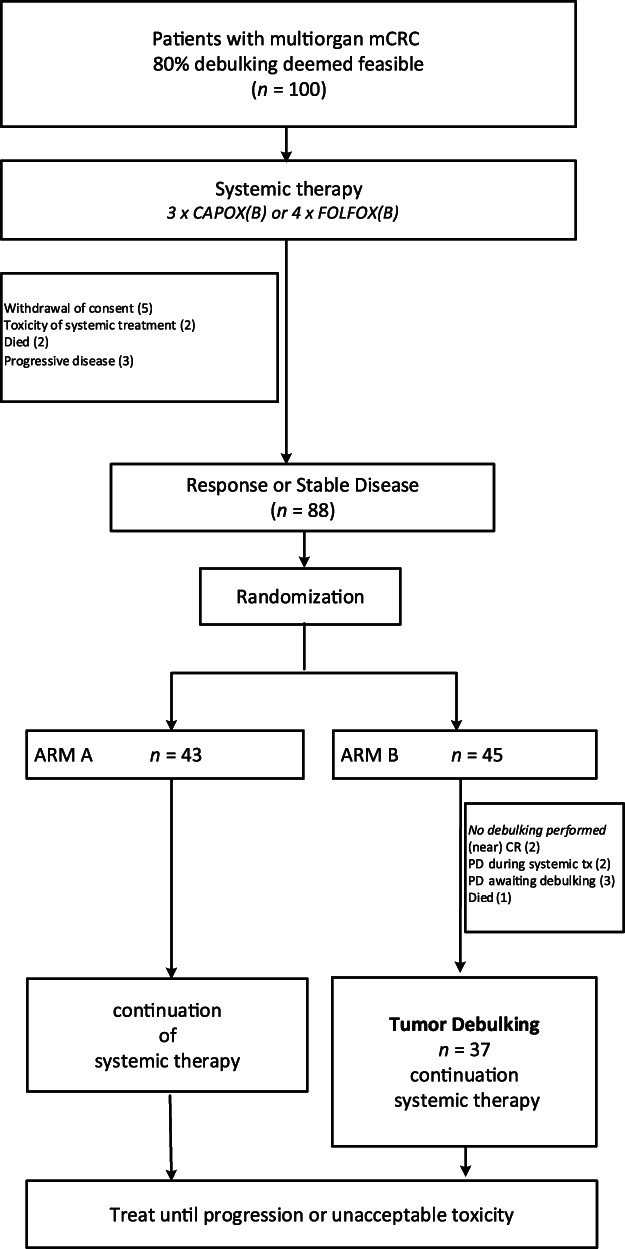
Consort diagram.
Abbreviations: CAPOX(B), capecitabine and oxaliplatin (bevacizumab); CR, complete response; FOLFOX(B), 5‐fluorouracil/leucovorin and oxaliplatin (bevacizumab); mCRC, metastatic colorectal cancer; PD, progressive disease; tx, therapy.
Debulking
In Table 3, local treatment details of patients in arm B are summarized. Protocol debulking was performed in 37 (82%) patients. In 14 patients debulking was performed with one single modality; the other patients required combined modalities. Four patients (11%) were treated by three modalities (surgery, RFA, and radiotherapy). In 31 patients (69%), debulking of ≥80% of metastatic lesions was achieved. The total duration of hospital admission in days was median 9 (IQR, 15). This included elective hospital admissions for surgery and (percutaneous) RFA and unplanned readmissions (seven patients; 16%). In seven patients (16%) a colostomy was created as part of the debulking procedure. In five patients with liver metastases hemihepatectomy was needed as part of debulking; 14 patients had wedge resections or segmentectomy, and in 12 (43%) patients RFA was used preoperatively. Radiotherapy was administered in an outpatient setting. Patients had a median of six radiotherapy sessions (IQR, 10). The median total local treatment days including hospital admission and radiotherapy visits was 14 days (IQR, 19).
Table 3.
Local treatment characteristics of patients in intervention arm B
| Local treatment characteristics | n (%) |
|---|---|
| Intervention arm | 45 (51) |
| Debulking performed | 37 (82) |
| At least 80% treated | 31 (69) |
| Number of modalities, median (range) | 2 (1–3) |
| One modality | |
| Surgery only | 8 (21.6) |
| Radiotherapy only | 4 (10.8) |
| RFA only | 2 (5.4) |
| Two modalities | |
| Surgery and RFA | 6 (16) |
| Surgery and radiotherapy | 11 (29.7) |
| RFA and radiotherapy | 2 (5.4) |
| Three modalities: Surgery and RFA and radiotherapy | 4 (10.8) |
| Total hospital local treatment days (surgical admission, [unplanned] readmissions, RT sessions, and/or percutaneous RFA treatment), median (IQR) | 14 (19) |
Abbreviations: IQR, interquartile range; RFA, radiofrequency ablation; RT, radiotherapy.
In 13% of patients in arm B, debulking was not performed because of progressive disease (n = 5) or death (n = 1) prior to local treatment. Two patients who had stable disease at randomization progressed during the following courses of systemic therapy, and debulking was not performed as per protocol. Two patients showed progressive disease awaiting local treatment (one patient with a newly diagnosed brain metastasis, one patient with progressive and unresectable liver metastases). One patient's disease was unresectable because of unexpected finding of extensive peritoneal carcinomatosis at laparotomy. One patient died in a motor vehicle accident prior to local treatment. On imaging prior to debulking, two patients had near complete response, with lesions too small to treat after systemic therapy (Fig. 1).
Adverse Events
A total of 77 SAEs were reported in 50 patients of the cohort of 100 patients. Thirty‐two events occurred prior to randomization in 25 patients. In arm A, 17 events occurred (in 11 patients) and 28 in arm B (in 21 patients). In arm A, 6 SAEs were related to systemic therapy, and 11 were not related. Of the SAEs in arm B, 1 was related to systemic therapy, 6 were unrelated, and 21 were related to local treatment (in 15 patients; Table 4). In arm B, all AEs in surgical debulking were documented (Table 5) and graded according to the Clavien Dindo classification of surgical complications (Table 6). Thirty‐two complications were reported in 19 patients (51%), from which 11 (in 9 patients; 24%) were grade ≥ 3 according to Clavien Dindo. Postoperative 90‐day mortality was 2.7% (n = 1; hepatic failure). One other patient deceased from respiratory insufficiency caused by pneumonitis, which was possibly related to the stereotactic radiotherapy treatment that the patient underwent 11 months before.
Table 4.
Systemic therapy
| Parameter | Arm A (n = 43), n (%) | Arm B (n = 45), n (%) |
|---|---|---|
| Systemic therapy | ||
| Chemotherapy | ||
| CAPOX | 42 (98) | 45 (100) |
| FOLFOX | 1 (2) | 0 |
| Bevacizumab | 25 (58) | 30 (67) |
| Completed equivalent of eight cycles CAP(OX) | 30 (70) | 29 (64) |
| Response at first evaluation | ||
| Complete remission | 0 (0) | 1 (2) |
| Partial remission | 21 (48) | 20 (44) |
| Stable disease | 22 (51) | 24 (53) |
| Progressive disease | n/a | n/a |
Abbreviations: CAPOX, capecitabine and oxaliplatin; FOLFOX, 5‐fluorouracil/leucovorin and oxaliplatin; n/a, not applicable.
Table 5.
Serious adverse events according to Common Terminology Criteria for Adverse Events version 4.03
| Serious adverse events | Arm A (n = 43), n (%) | Arm B (n = 45), n (%) |
|---|---|---|
| All serious adverse events | 11(26) a | 21(47) b |
| Not related | 7 (16) | 6 (13) |
| Related to chemotherapy | 5 (12) | 1 (2) |
| Related to local treatment | n/a | 15 (33) |
| 90‐day mortality | n/a | 1 (2.7) |
In arm A, one event grade > 3.
In arm B, four events grade > 3 according to Common Terminology Criteria for Adverse Events version 4.03 and two events grade 5.
Abbreviation: n/a, not applicable.
Table 6.
Adverse events of debulking procedures grade ≥ 3 according to Clavien Dindo classification of surgical complications
| Clavien Dindo grade | Complication |
|---|---|
| 3 | Presacral abscess |
| Urinary anastomotic leak | |
| Wound abscess | |
| Pleural effusion | |
| Colonic perforation | |
| Abdominal sepsis | |
| Biliary anastomotic leak/duct leakage (3×) | |
| 4 | Ileus |
| 5 | Hepatic failure |
Chemotherapy was resumed in 89% of patients. Four patients who did not resume chemotherapy all had stable disease at randomization and therefore completed seven (of eight) cycles of CAPOX prior to debulking. One patient could not restart because of complications of debulking; one did not restart the first‐line systemic treatment because of progressive disease. In the other two patients, the treating physician did not restart because the patients had no evaluable disease left and no symptoms to palliate after debulking had taken place. Altogether, 83% of patients who underwent debulking completed (the equivalent of) eight cycles CAP(OX). In general, 70% of patients in arm A and 64% of patients in arm B (p = .65) completed the equivalent of eight cycles of CAP(OX) (Table 4).
The median time to restart systemic treatment was 12.5 weeks (IQR, 6.75) after completion of the last preoperative cycle of systemic therapy. The median interval between the last debulking event and restarting systemic therapy was 5 weeks (IQR, 6).
Discussion
The current report shows that tumor debulking is safe and does not prohibit administration of palliative chemotherapy in the majority of patients with multiorgan mCRC. Completing tumor debulking had substantial impact for the patients involved. Inevitably, serious adverse events occurred. The morbidity and 90‐day mortality are comparable to those of previous studies on surgical resection of CRC liver metastases [25, 26, 27, 28, 29]. Patients randomized in arm B who underwent debulking after seven cycles of systemic therapy because of stable disease at first evaluation did not have more SAEs related to the procedures despite having received more cycles of chemotherapy [30].
This initial evaluation also demonstrates that it is feasible to prospectively include and randomize patients with mCRC between palliative systemic treatment and tumor debulking combined with palliative systemic treatment. It was challenging to get consensus on feasibility of tumor debulking, which may potentially be hampered by a lack of clinical equipoise of members in the multidisciplinary team. Commitment and close collaboration grew in time in the participating centers that include patients.
To our knowledge, only one prospective study on patients with resection for extrahepatic disease has been published by Wei et al. This phase II study of metastasectomy for both intrahepatic and extrahepatic disease enrolled 26 patients with generally less extensive disease (median one extrahepatic organ involved with a median of two extrahepatic lesions) and reported 19% major morbidity and 4% mortality [29].
There is heterogeneity in the different local treatment techniques used for tumor debulking, which may influence outcome. However, limited randomized data on direct comparison of the different local treatment techniques are available, and the individual techniques show acceptable local control rates [20, 31]. In the study protocol we defined surgical resection of metastatic lesions as the preferred local treatment. Depending on the location and size of the metastasis, thermal ablation or radiotherapy was considered.
No patients withdrew from ORCHESTRA study participation after randomization. Three patients developed disease progression in the interval between chemotherapy and resection (6.7%) and were excluded from local treatment. This is less than described by Vigano et al. for patients with resectable colorectal liver metastases awaiting resection [32]. The reported early disease progression (<8 weeks) occurred in approximately 15% of patients who underwent liver resection and had extremely poor survival after liver resection (0% at 2 years). To prevent tumor progression and poor oncological outcome we aimed for a short chemotherapy‐free interval [28]. Systemic therapy was resumed within a median of 12.5 weeks after finishing preoperative chemotherapy. There was a median of 5 weeks’ interval between last local treatment and start of postoperative chemotherapy, which could be considered a “morbidity‐associated chemotherapy interval.” In previous studies of patients undergoing two‐stage hepatectomy for resectable CRC liver metastases, the interval between pre‐ and postoperative chemotherapy was median 18.7 weeks and median 9.8 weeks from the stage 2 resection [26]. In a series of patients who underwent major hepatectomy (at least three segments) for mCRC, with 4% having extrahepatic disease, postoperative chemotherapy was given in 87%, starting median 6 weeks postoperatively [28].
There was no significant difference in the amount of cycles of systemic therapy given between the study arms, and a comparable proportion of patients completed at least eight cycles of CAPOX(±B) (or the equivalent in FOLFOX) in both treatment arms.
Conclusion
The preplanned safety and feasibility report of the ORCHESTRA trial demonstrates that it is feasible to perform tumor debulking in patients with multiorgan mCRC without prohibiting administration of palliative systemic therapy, despite the occurrence of SAEs related to local treatment. This study addresses a topical issue in everyday practice of multidisciplinary CRC care with a study design compatible with current treatment, enabling the results to be readily implemented in daily practice. The ORCHESTRA trial will continue accrual to determine whether the primary aim of >6 months overall survival benefit of additional tumor debulking will be met.
Author Contributions
Conception/design: Elske C. Gootjes, Eric P. van der Stok, Tineke E. Buffart, Cornelis Verhoef, Henk M.W. Verheul
Provision of study material or patients: Mariette Labots, Jurriaan B. Tuynman, Martijn R. Meijerink, Albert J. ten Tije, Jan‐Willem B. de Groot, Mathijs Hendriks, Esther van Meerten, Joost Nuyttens
Collection and/or assembly of data: Lotte Bakkerus
Data analysis and interpretation: Barbara M. Zonderhuis, Peter M. van de Ven, Cornelis J.A. Haasbeek, Dirk J. Grunhagen
Manuscript writing: Elske C. Gootjes, Eric P. van der Stok, Tineke E. Buffart, Lotte Bakkerus, Mariette Labots, Barbara M. Zonderhuis, Jurriaan B. Tuynman, Martijn R. Meijerink, Peter M. van de Ven, Cornelis J.A. Haasbeek, Albert J. ten Tije, Jan‐Willem B. de Groot, Mathijs P. Hendriks, Esther van Meerten, Joost J.M.E. Nuyttens, Dirk J. Grunhagen, Cornelis Verhoef, Henk M.W. Verheul
Final approval of manuscript: Elske C. Gootjes, Eric P. van der Stok, Tineke E. Buffart, Lotte Bakkerus, Mariette Labots, Barbara M. Zonderhuis, Jurriaan B. Tuynman, Martijn R. Meijerink, Peter M. van de Ven, Cornelis J.A. Haasbeek, Albert J. ten Tije, Jan‐Willem B. de Groot, Mathijs P. Hendriks, Esther van Meerten, Joost J.M.E. Nuyttens, Dirk J. Grunhagen, Cornelis Verhoef, Henk M.W. Verheul
Disclosures
Martijn R. Meijerink: Angiodynamics (C/A), Medtronics‐Covidien, Angiodynamics (RF, H); Jan‐Willem de Groot: Bristol‐Myers Squibb, Pierre Fabre, Servier Merck Sharp & Dohme, Novartis (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The publication is on behalf of the ORCHESTRA study group, which further includes Grootscholten C, Tanis PJ, Torrenga H, van Zweeden AA, Helgason HH, Hamberg P, Los M, Hoekstra R, Vreugdenhil G, van Halteren H, de Wilt JHW, Bloemendal HJ, Beeker A, Beerepoot LV, Pruijt, JFM, ten Bokkel Huinink D, Haberkorn B, Hospers GAP, Jansen RLH, Meerum Terwogt, Polee M, Troost M, Vermaas M, Trajkovic M, Meulenbeld H. The ORCHESTRA trial was supported by the not‐for‐profit Foundation “Blokker‐Verwer,” the Dutch Cancer Foundations (KWF: Koninging Wilhelmina Fonds voor de Nederlandse Kankerbestrijding), and an unrestricted grant from Roche.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Primrose JN. Surgery for colorectal liver metastases. Br J Cancer 2010;102:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rees M, Tekkis PP, Welsh FK et al. Evaluation of long‐term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg 2008;247:125–135. [DOI] [PubMed] [Google Scholar]
- 3. van der Pool AE, de Wilt JH, Lalmahomed ZS et al. Optimizing the outcome of surgery in patients with rectal cancer and synchronous liver metastases. Br J Surg 2010;97:383–390. [DOI] [PubMed] [Google Scholar]
- 4. Tomlinson JS, Jarnagin WR, DeMatteo RP et al. Actual 10‐year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575–4580. [DOI] [PubMed] [Google Scholar]
- 5. van der Pool AE, Mendez RA, Wunderink W et al. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg 2010;97:377–382. [DOI] [PubMed] [Google Scholar]
- 6. Bae SH, Kim MS, Cho CK et al. High dose stereotactic body radiotherapy using three fractions for colorectal oligometastases. J Surg Oncol 2012;106:138–143. [DOI] [PubMed] [Google Scholar]
- 7. Baschnagel AM, Mangona VS, Robertson JM et al. Lung metastases treated with image‐guided stereotactic body radiation therapy. Clin Oncol (R Coll Radiol) 2013;25:236–241. [DOI] [PubMed] [Google Scholar]
- 8. Ruers T, Van Coevorden F, Punt CJ et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase II trial. J Natl Cancer Inst 2017;109:djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cirocchi R, Trastulli S, Boselli C et al. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev 2012;(6):CD006317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hadden WJ, de Reuver PR, Brown K et al. Resection of colorectal liver metastases and extra‐hepatic disease: A systematic review and proportional meta‐analysis of survival outcomes. HPB (Oxford) 2016;18:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adam R, de Haas RJ, Wicherts DA et al. Concomitant extrahepatic disease in patients with colorectal liver metastases: When is there a place for surgery? Ann Surg 2011;253:349–359. [DOI] [PubMed] [Google Scholar]
- 12. de Haas RJ, Wicherts DA, Adam R. Resection of colorectal liver metastases with extrahepatic disease. Dig Surg 2008;25:461–466. [DOI] [PubMed] [Google Scholar]
- 13. Chua TC, Saxena A, Liauw W et al. Hepatectomy and resection of concomitant extrahepatic disease for colorectal liver metastases–a systematic review. Eur J Cancer 2012;48:1757–1765. [DOI] [PubMed] [Google Scholar]
- 14. Elias D, Liberale G, Vernerey D et al. Hepatic and extrahepatic colorectal metastases: When resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol 2005;12:900–909. [DOI] [PubMed] [Google Scholar]
- 15. Hwang M, Jayakrishnan TT, Green DE et al. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease. Eur J Cancer 2014;50:1747–1757. [DOI] [PubMed] [Google Scholar]
- 16. Leung U, Gonen M, Allen PJ et al. Colorectal cancer liver metastases and concurrent extrahepatic disease treated with resection. Ann Surg 2017;265:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Venook AP, Niedzwiecki D, Lenz HJ et al. Effect of first‐line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with kras wild‐type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA 2017;317:2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heinemann V, von Weikersthal LF, Decker T et al. Folfiri plus cetuximab versus folfiri plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (FIRE‐3): A randomised, open‐label, phase 3 trial. Lancet Oncol 2014;15:1065–1075. [DOI] [PubMed] [Google Scholar]
- 19. Park JH, Kim TY, Lee KH et al. The beneficial effect of palliative resection in metastatic colorectal cancer. Br J Cancer 2013;108:1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gootjes EC, Bakkerus L, Ten Tije AJ et al. The value of tumour debulking for patients with extensive multi‐organ metastatic colorectal cancer. Eur J Cancer 2018;103:160–164. [DOI] [PubMed] [Google Scholar]
- 21. Elske CG, Tineke EB, Tol MP et al. The ORCHESTRA trial: A phase III trial of adding tumor debulking to systemic therapy versus systemic therapy alone in multi‐organ metastatic colorectal cancer (mCRC). J Clin Oncol 2016;34(suppl 4):TPS788s. [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 23. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adam R, Wicherts DA, de Haas RJ et al. Patients with initially unresectable colorectal liver metastases: Is there a possibility of cure? J Clin Oncol 2009;27:1829–1835. [DOI] [PubMed] [Google Scholar]
- 25. Ubink I, Jongen JMJ, Nijkamp MW et al. Surgical and oncologic outcomes after major liver surgery and extended hemihepatectomy for colorectal liver metastases. Clin Colorectal Cancer 2016;15:e193–e198. [DOI] [PubMed] [Google Scholar]
- 26. Kambakamba P, Linecker M, Alvarez FA et al. Short chemotherapy‐free interval improves oncological outcome in patients undergoing two‐stage hepatectomy for colorectal liver metastases. Ann Surg Oncol 2016;23:3915–3923. [DOI] [PubMed] [Google Scholar]
- 27. Ruers T, Punt C, Van CF et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non‐resectable colorectal liver metastases: A randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012;23:2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Correa‐Gallego C, Gonen M, Fischer M et al. Perioperative complications influence recurrence and survival after resection of hepatic colorectal metastases. Ann Surg Oncol 2013;20:2477–2484. [DOI] [PubMed] [Google Scholar]
- 29. Wei AC, Coburn NG, Devitt KS et al. Survival following resection of intra‐ and extra‐hepatic metastases from colorectal cancer: A phase II trial. Ann Surg Oncol 2016;23:2644–2651. [DOI] [PubMed] [Google Scholar]
- 30. Zorzi D, Laurent A, Pawlik TM et al. Chemotherapy‐associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg 2007;94:274–286. [DOI] [PubMed] [Google Scholar]
- 31. Di Martino M, Rompianesi G, Mora‐Guzman I et al. Systematic review and meta‐analysis of local ablative therapies for resectable colorectal liver metastases. Eur J Surg Oncol 2020;46:772–781. [DOI] [PubMed] [Google Scholar]
- 32. Vigano L, Darwish SS, Rimassa L et al. Progression of colorectal liver metastases from the end of chemotherapy to resection: A new contraindication to surgery? Ann Surg Oncol 2018;25:1676–1685. [DOI] [PubMed] [Google Scholar]


