Abstract
Adolescence is an important ontogenetic period that is characterized by behaviors such as enhanced novelty-seeking, impulsivity, and reward preference, which can give rise to an increased risk for substance use. While substance use rates in adolescence are generally on a decline, the current rates combined with emerging trends, such as increases in e-cigarette use, remain a significant public health concern. In this review, we focus on the neurobiological divergences associated with adolescent substance use, derived from a cross-sectional, retrospective, and longitudinal studies, and highlight how the use of these substances during adolescence may relate to behavioral and neuroimaging-based outcomes. Identifying and understanding the associations between adolescent substance use and changes in cognition, mental health, and future substance use risk may assist our understanding of the consequences of drug exposure during this critical window.
Keywords: adolescence, youth, addiction, drug, abuse psychology, special population
Introduction
Adolescence is characterized by a series of developmental changes occurring roughly between 10–19 years, with the timing of onset highly impacted by social, cultural, and nutritional influences (Spear, 2000). During this time, the body experiences increased production of gonadal steroids that contribute to growth and sexual development (Spear, 2000). Additionally, a vast array of neurodevelopmental changes occur during this time, including cortical thinning and gray matter volume (GMV) reductions, increases in white matter volume, synaptic pruning, and reorganization within cortical and limbic regions (Schneider, 2013; Spear, 2014; Jaworska and MacQueen, 2015; Dumontheil, 2016; Thorpe et al., 2020). These neurodevelopmental changes give rise to characteristic behaviors during adolescence, such as improvements in cognition and executive functions; increases in reward sensitivity, novelty-seeking, risk-taking behavior; as well as a tendency to spend more time with peers (Spear, 2000; Choudhury et al., 2006; Romer, 2010). Some of these behavioral characteristics, in turn, contribute to a greater likelihood of initiating substance use (Lisdahl et al., 2018). The temporal overlap between substance use initiation and the vulnerable neurodevelopmental windows makes this an important period to study (Spear, 2000; Thorpe et al., 2020).
Substance use (used broadly to include alcohol and other drugs) by adolescents remains a significant public health concern. According to the most recent National Epidemiologic Survey on Alcohol and Related Conditions, more than 50% of substance use initiation cases occur between the ages 15–19 (Blanco et al., 2018). Moreover, an earlier age of onset of use is significantly associated with the risk of developing a substance use disorder later in life (Taioli and Wynder, 1991; Viner and Taylor, 2007). While the prevalence of substance use has declined in recent years from historical highs, recent surveys show that there have been some specific increases in the past year and that some concerning patterns may be emerging. According to the University of Michigan’s Monitoring the Future Survey in 2019, the prevalence of cannabis use as well as any illicit drug use in students in grades 8–12 have remained consistently high across prior decades (Johnston et al., 2020). Furthermore, nicotine vaping continued to be a concern with over one in three grade 12 students reporting past-year use (with 25.5% of these students indicating past month use), and this prevalence remains substantially higher than other forms of tobacco, including cigarettes, which continues to decline (Johnston et al., 2020). Another emerging trend from the survey suggested that the declining trends in alcohol use and binge drinking may be leveling off (Johnston et al., 2020). Despite the declines from historical highs, by the end of high school, four out of every 10 students reported consuming alcohol in their lifetime. In addition to the increased risk for future substance use, adolescent drug use can also negatively impact ongoing neurodevelopment, which might contribute to the risk for cognitive impairments and psychopathology. A growing body of research predominantly consisting of findings from magnetic resonance imaging (MRI) studies is beginning to unravel the structural and functional changes associated with these clinical outcomes.
This review will outline the cognitive, psychopathological, and future drug use related associations with adolescent substance use, especially related to the emerging trends in this use that have not been addressed in previous reviews. We will also present brain-imaging based neurobiological correlates of these findings when applicable, providing a unique perspective on these associations and potential interactions between behavioral and neural domains. While the specific behaviors under each of the reviewed domains may differ between the drug classes (depending on the availability of research findings), this approach helps to contrast the similarities and differences between the different drugs. We focus on findings from studies of substances most commonly used during adolescence, namely tobacco and e-cigarettes, alcohol, and cannabis (Johnston et al., 2020); while other less prevalent drug classes (e.g., stimulants, ecstasy) are not addressed in this review (for a review see Squeglia et al., 2009a), we chose to include opioids and drug co-use as additional drug classes due to the lack of existing syntheses on these topic. Although brain development continues well into adulthood (Spear, 2014), we limit this review to studies using adolescent sample populations with a mean age of 19-years-old or lower to capture the potential effects of drug use during the most dynamic stages of post-childhood development. This review comes at a time of recreational cannabis legalization and decriminalization by government bodies across the globe despite our somewhat incomplete understanding of its causal impacts on the developing brain alone, or in combination with other drugs commonly used by youth. Importantly, we also summarize the currently available findings surrounding the potential consequences of vaping, which has quickly become one of the most common methods of nicotine and cannabis delivery in youth, one that is still under-represented in the literature to date.
Tobacco and E-Cigarettes
In 2017, it was estimated that 4.9% of adolescents in the United States aged 12–17 were current users of tobacco products, including cigarettes, cigars, smokeless tobacco (i.e., snuff, chew), and pipe tobacco (Substance Abuse and Mental Health Services Administration, 2018). Recent estimates suggest 3.7% of adolescents regularly use cigarettes (Figure 1A; Johnston et al., 2020). These estimates, along with results from the US National Survey on Drug Use and Health, indicate that the prevalence of tobacco use is at its lowest levels since 1991 (Substance Abuse and Mental Health Services Administration, 2019; Johnston et al., 2020). These declining trends in tobacco use, however, contrast with nicotine vaping rates among teens; more adolescents in grades 8, 10, and 12 are estimated to be vaping nicotine than smoking combustible cigarettes (Figure 1B; Johnston et al., 2020), and the rate of use has been steadily increasing since 2011 (US Department of Health and Human Services, 2016). In this age group, nicotine vaping is often perceived as less harmful than traditional smoking (Parker et al., 2018; Jun et al., 2019), likely contributing to the growing proportion of adolescents who experiment with, and regularly use e-cigarettes. Traditional smoking habits are initiated almost exclusively between early adolescence and young adulthood (Substance Abuse and Mental Health Services Administration, 2019), and initiating e-cigarette use in later adulthood is unlikely relative to those under the age of 25 (National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health, 2016).
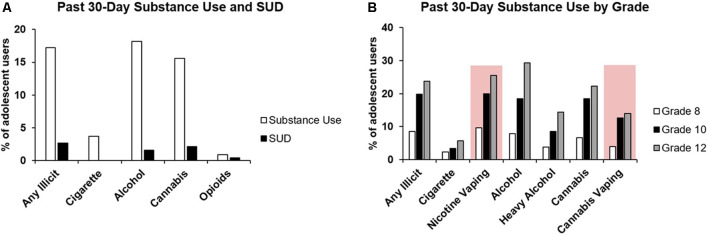
Figure 1.
Prevalence of substance use and substance use disorder in adolescents. (A) Collated data from the 2017 National Survey on Drug Use and Health, 2018 National Survey on Drug Use and Health, and 2019 Monitoring the Future Survey showing the past 30-day substance use by U.S. adolescents, along with the reported percentage of adolescents with specific substance use disorders (Substance Abuse and Mental Health Services Administration, 2018; Substance Abuse and Mental Health Services Administration, 2018; Johnston et al., 2020). (B) Adolescent substance use by school grade (8, 10, 12) as per the 2019 Monitoring the Future report (Johnston et al., 2020). All categories represent self-reported substance use in the past 30 days except for heavy alcohol use (five or more drinks in a row) in the past two weeks. Emerging substance use behaviors (i.e., nicotine and cannabis vaping) are highlighted by a red box.
Nicotine, the primary psychoactive component of cigarette smoke and e-cigarette liquid, is highly addictive and can impact brain development when its use is initiated during adolescence (Thorpe et al., 2020). Nicotine interacts with nicotinic acetylcholine receptors within the body; however, there is a paucity of studies investigating human nicotinic acetylcholine receptors activity and development in the context of adolescent smoking owing to methodological and ethical limitations (e.g., use of radioisotopes in positron emission tomography). As such, most neurobiological studies utilize structural MRI to investigate gross brain morphology; functional MRI (fMRI) to infer brain region activity based on dynamic cerebral blood flow measured through blood oxygenation level-dependent (BOLD) imaging; and diffusion tensor imagining (DTI) to investigate white matter microstructure via water diffusivity across axon bundles (Beres, 2017; Yousaf et al., 2018). Below, we synthesize findings from studies that suggest potential cognitive-, psychopathology-, and future drug use susceptibility-related outcomes associated with nicotine use during the adolescent period, be it through combustible cigarette consumption or e-cigarette use and relate these findings to neural correlates. Summaries of these studies can be found in Supplementary Table S1.
Cognition
Adolescence is a period of attentional development and is characterized by impulsive and risk-taking behaviors (Romer, 2010). Several longitudinal (Treur et al., 2015; Akkermans et al., 2017) and cross-sectional (Tercyak et al., 2002; Jacobsen et al., 2005, 2007b,c) reports implicate a relationship between adolescent smoking and worsened attentional performance relative to non-smoking youth. Though not always significant (Jacobsen et al., 2007b,c), studies consistently report more symptoms of inattention in smokers compared to non-smokers that persist into adulthood (Tercyak et al., 2002; Treur et al., 2015; Akkermans et al., 2017). Performance during selective and divided attention tasks are similarly observed to be poorer in smoking adolescents compared to their non-smoking peers (Jacobsen et al., 2005, 2007b,c; Bi et al., 2017; Li et al., 2017), especially in males (Jacobsen et al., 2005), although divided, but not selective, attentional deficits may be related to nicotine withdrawal (Jacobsen et al., 2005, 2007c). Although performance deficits in some of these attentional tasks may stem from smoking-associated working memory impairments (Jacobsen et al., 2005, 2007c), findings from neural correlate studies conducted in smoking and non-smoking youth suggest that smoking behaviors impact the development and function of attentional brain circuits. Many studies have shown morphological and functional differences between smoking and non-smoking adolescents in the prefrontal cortex (PFC), inferior parietal cortex, and anterior insula that in part comprise the selective and divided attention neural circuits (Elsey et al., 2016). Gray matter loss in the cortex may be exacerbated by smoking; smokers reportedly have lower amounts of gray matter in the frontal cortex (Li et al., 2015; Akkermans et al., 2017; Chaarani et al., 2019), inferior parietal lobe (Li et al., 2015; Akkermans et al., 2017), and insula (Li et al., 2015) than non-smoking controls, and gray matter in the dorsolateral PFC (DLPFC) was negatively correlated with smoking dependency (Li et al., 2015). A recent fMRI study of adolescent smokers found that resting-state functional connectivity (RSFC) was lower between the anterior insula and the DLPFC, amygdala, and striatum of smokers compared to non-smokers (Bi et al., 2017). The activity of the DLPFC appears to be important for divided attention performance, such that greater activation of this brain region is associated with worse performance accuracy when multiple sensory modalities are required (Johnson and Zatorre, 2006), and RSFC between the anterior insula with the DLPFC and inferior parietal cortex may be reduced during acute smoking abstinence (Fedota et al., 2018). Although DLPFC activity has not been monitored during task performance in smoking adolescents, resting-state deficits in the DLPFC during minimal nicotine deprivation conditions found by Bi et al. (2017) suggest smoking-induced functional changes to networks important to divided attention, though the appearance of cognitive impairments such as those found by Jacobsen et al. (2005) may depend on smoking recency. Collectively, these results suggest that the neurotoxic effects of smoking may interfere with the normal developmental trajectory and function of attention-related brain regions and consequently manifest as attentional deficits.
Tobacco use is also suggested to have long-term impacts on inhibitory control, which could prevent future abstinence from smoking through a failure to suppress smoking urges. However, adolescents consistently report fewer withdrawal symptoms relative to adult smokers (McNeill et al., 1986; Rojas et al., 1998) and studies measuring inhibitory control and impulsivity behaviors on adolescent smoking patterns have been conflicting. Counterintuitively, some studies have found that impulsivity (Tercyak et al., 2002) and distractibility (DiFranza et al., 2007) are protective factors against current cigarette use and the loss of control over smoking relative to adolescents without symptoms of impulsivity, whereas others have identified a positive association between impulsivity and cigarette use (Leventhal et al., 2015). These inconsistent findings, as suggested by DiFranza et al. (2007), may be attributed to only some studies controlling for medication status in individuals with co-occurring psychiatric disorders affecting impulsivity such as attention-deficit/hyperactivity disorder (ADHD). Furthermore, there is conflicting evidence surrounding the association between adolescent smoking and inhibitory control performance, with one study finding that smokers commit more errors in the Go/No-Go task (Yin et al., 2016), whereas another study found that adolescent smokers do not show inhibitory control deficits during the Stop-Signal task (Galván et al., 2011) compared to non-smoking peers. However, negative correlations between successful Stop-Signal inhibition trial reaction times and BOLD activation in regions important to inhibitory control have been reported such that greater activation was associated with faster responding (Galván et al., 2011). These correlations indicate that inhibitory control regions (Goldstein and Volkow, 2002; Zhang et al., 2017) are possibly affected by adolescent smoking, supported by findings of smoking-associated abnormalities in the adolescent anterior cingulate (ACC; Lee et al., 2005; Rubinstein et al., 2011b; Bi et al., 2017; Li et al., 2017), insula (Lee et al., 2005; Jacobsen et al., 2007c; Rubinstein et al., 2011a; Li et al., 2015, 2017; Bi et al., 2017), and orbitofrontal cortex (OFC; Dinn et al., 2004; Li et al., 2015; Akkermans et al., 2017). The Go/No-Go and Stop-Signal tasks are thought to rely on unique neural correlates despite sharing a common core network, which may explain the discrepant cognitive results between studies (Zhang et al., 2017; Raud et al., 2020). Taken together, these findings suggest that baseline inhibitory control and impulsive behavior may determine the risk for adolescent smoking, and likewise adolescent smoking may be a detriment to inhibitory control processing.
Aside from potential smoking-induced deficits in attentional and inhibitory processes, there is some evidence that adolescent smoking alters intelligence. A longitudinal study of older adolescent male current smokers, former smokers, and non-smokers found that cognitive abilities related to intelligence quotient (IQ) were negatively correlated with the number of cigarettes smoked per day, that performance deficits were more pronounced in current smokers than non-smokers and former smokers, and that cognitive performance was lower in former smokers than non-smokers (Weiser et al., 2010). Among discordant smoking sibling pairs, smokers were also more likely to have a lower IQ than their non-smoking counterparts. Furthermore, future smoking was more likely in males with lower baseline cognitive scores compared to those who did not initiate smoking, suggesting lower IQ may be predictive of future smoking, which has been supported (Corley et al., 2012; Wraw et al., 2018) and contended (Batty et al., 2007) by other studies comparing childhood IQ with smoking in adulthood.
Adolescent smoking may also impact working memory in a sensory modality-dependent fashion. Auditory working memory accuracy was found to be worse in adolescent smokers compared to non-smokers (Jacobsen et al., 2005, 2007a). These auditory cognitive deficiencies were later recapitulated by the same group, which showed greater smoking-associated deficits in auditory relative to visual cognitive performance (Jacobsen et al., 2007c). These auditory working memory deficits are supported by fMRI findings suggesting that brain regions supporting auditory working memory, such as the inferior frontal gyrus and parietal lobes, show greater activation with worse task performance, suggesting network inefficiency in smokers (Jacobsen et al., 2007a). Likewise, DTI findings suggest that smoking youth have altered white matter integrity compared to non-smokers, as indicated by greater fractional anisotropy (FA), an indirect measure of axonal organization and coherence, in auditory corticothalamic tracts (Jacobsen et al., 2007b). This is in line with findings of a recent meta-analysis of smokers under 30-years-old suggesting brain-wide increases in FA compared to non-smokers, which could represent greater white matter integrity or myelination, or deleterious vasogenic swelling in these tracts (Gogliettino et al., 2016). In addition, smoking-associated differences in hippocampal (Jacobsen et al., 2007c; Rubinstein et al., 2011b) and parahippocampal (Rubinstein et al., 2011b; Li et al., 2015) function and morphology have also been reported in smoking youth, further supporting potential effects of smoking during adolescence on alterations in memory performance.
Age of initiation is an important factor in the trajectory of potential negative outcomes of smoking. Attentional (Treur et al., 2015) and working memory (Jacobsen et al., 2005) performance impairments are less pronounced in those who initiated smoking at a later age. Also, an earlier onset of cigarette use initiation and regular use are both consistently associated with heavier smoking patterns and craving in later adolescence (Stanton, 1995; Everett et al., 1999; Colder et al., 2001; Riggs et al., 2007; Dierker et al., 2012; Buchmann et al., 2013) and adulthood (Taioli and Wynder, 1991; Klein et al., 2013; Lanza and Vasilenko, 2015), as well as greater smoking cue reactivity in adulthood (Mashhoon et al., 2018). Greater cognitive deficits associated with an earlier age of smoking initiation may, therefore, reflect a unique early adolescent vulnerability to the effects of nicotine exposure and/or a cumulative impact of smoking duration on cognition. Although dependence and withdrawal symptoms are reportedly lower in adolescents compared to adults (McNeill et al., 1986; Rojas et al., 1998), dependence in smoking adolescents could lead to loss of smoking autonomy (McNeill et al., 1986; Rojas et al., 1998; DiFranza et al., 2000, 2002). One theory suggests that adolescents are at higher risk for the future negative consequences of smoking because they are less likely to experience negative feelings associated with tobacco use, and thus will continue their habits despite the known health risks, subsequently leading to more damage to the brain through the neurotoxic effects of nicotine (O’Dell et al., 2004). Considering this, early-onset adolescent smoking may cause a greater deviation in the developmental trajectory of attentional-, memory-, inhibitory control-associated brain regions than those who are late-onset users, and consequently worsen the management of withdrawal symptoms during cessation attempts. Together, these studies highlight the importance of early cessation interventions for adolescent smokers, especially for those who initiate their smoking habits at younger ages, to mitigate the potential cognitive impairments that arise from adolescent smoking as well as the known health risks associated with chronic smoking in adulthood.
Psychopathology
Schizophrenia and Psychosis
Heavy nicotine dependence is prevalent in 16–46% of those in the prodromal phase of schizophrenia (Gogos et al., 2019), leading researchers to question if there is a causal relationship between schizophrenia and smoking (i.e., does smoking increase the risk for schizophrenia, or does having schizophrenia promote smoking habits to alleviate disease symptoms?), if the risk for smoking and schizophrenia share common mechanistic underpinnings, or both (Khokhar et al., 2018). The link between adolescence, schizophrenia, and nicotine use has been intensely investigated; almost all schizophrenia diagnoses occur during adolescence and young adulthood, and neurobiological systems that develop during adolescence include those that are implicated in both schizophrenia and smoking (Selemon and Zecevic, 2015). While few studies have found no (Dinn et al., 2004) or a negative association (Zammit et al., 2003) between adolescent smoking and psychosis outcomes, most studies indicate that smoking during adolescence and young adulthood is associated with increased risk for the development of schizophrenia (Weiser et al., 2004; Myles et al., 2012b; McGrath et al., 2016; Mustonen et al., 2018). This increased risk is especially prominent in individuals who engage in heavy smoking behaviors (Weiser et al., 2004; Mustonen et al., 2018) and initiate smoking during early adolescence as compared with older youth (McGrath et al., 2016; Mustonen et al., 2018). Importantly, unaccounted for confounders in these studies may contribute extensively to the observed relationship between smoking onset and future psychotic experiences (Jones et al., 2018). However, the age of smoking onset does not appear to alter the temporal course of psychosis development, as a meta-analysis found that while an earlier age of smoking onset predicted diagnosis, smoking status did not predict an earlier disease onset (Myles et al., 2012a).
Although the etiology of schizophrenia is complex and disrupted the development of many brain regions has been implicated in its emergence, neurobiological abnormalities and cognitive impairments associated with adolescent smoking overlap with those observed in schizophrenia. For instance, patients with schizophrenia present with deficits in cognitive processes such as inhibition, attention, and working memory, and show the aberrant activity of brain regions such as the DLPFC, ACC, and parietal lobes, all of which are implicated in adolescent smoking effects (Selemon and Zecevic, 2015). As previously mentioned, adolescence is a critical period of cortical development, and gray matter loss occurs into adulthood as synapses are pruned. Cortical gray matter reductions undergo steeper declines in those with schizophrenia than healthy individuals, suggesting a link between synapse refinement and development of the disease (Selemon and Zecevic, 2015). Since cortical thickness and GMV is lower in smoking adolescents (Li et al., 2015; Akkermans et al., 2017; Chaarani et al., 2019) and the rate of cortical thinning, though non-significant, is greater in novel smokers compared to non-smokers (Akkermans et al., 2017), it is possible that smoking may exacerbate gray matter declines in youth with a genetic predisposition to develop schizophrenia. Longitudinal MRI studies of adolescent smokers and non-smokers with schizophrenia risk (genetic or environmental) would help to elucidate the potential for additive effects of these factors on gray matter development.
Attention-Deficit/Hyperactivity Disorder
Like schizophrenia, problematic nicotine consumption disproportionately affects individuals with ADHD. Multiple theories, such as common mechanistic underpinnings, disease-associated predisposition, and the self-medication hypothesis, have been presented to address why this is the case (Van Amsterdam et al., 2018). While it is clear that ADHD is a risk factor in smoking initiation and dependence (see Glass and Flory, 2010), there is sparse and conflicting evidence surrounding the potential for smoking to impact ADHD trajectory and symptomology in adolescents. Some studies found that ADHD symptoms are more apparent in smokers compared to non-smokers (Dinn et al., 2004; Akkermans et al., 2017) but conflict on which symptoms were associated with regular smoking. Specifically, the longitudinal study by Akkermans et al. (2017) investigating smoking and non-smoking older adolescents with or without an ADHD diagnosis found that symptoms of inattention but not hyperactivity/impulsivity were greater in smokers at baseline, whereas the cross-sectional study by Dinn et al. (2004) of college-aged participants that did not specifically target those with an established ADHD diagnosis found that only hyperactivity/impulsivity symptoms were more likely in smokers compared to non-smokers. Additionally, Akkermans et al. (2017) did not find that the trajectory of inattention symptom count was correlated with smoking status between study time-points. Given the paucity of studies on the topic and conflicting evidence between existing studies, there is little to suggest that smoking during adolescence exacerbates or alleviates ADHD symptoms or alters symptom trajectory. Future longitudinal studies in smoking and non-smoking adolescents with an ADHD diagnosis should be conducted to delineate if this is truly the case considering the significant overlap between smoking and ADHD, and that the self-medication hypothesis is one of the predominant theories in the field to explain why those with ADHD smoke.
Recently, ADHD research has focused on disruptions to cortical thickness and white matter development within the brain that appear to be a characteristic of the disease (van Ewijk et al., 2012; Bouziane et al., 2018; Albajara Sáenz et al., 2019). As with the brains of patients with schizophrenia, imaging studies show that cortical thickness is reduced in the brains of patients with ADHD (Albajara Sáenz et al., 2019), and smoking during adolescence may have additive effects on cortical thickness. Current evidence does not support this theory, but it is important to highlight that only one small cohort longitudinal study to date has investigated cortical thickness in ADHD-affected and non-affected smokers and non-smokers, which may not have had the statistical power needed to observe a relationship (Akkermans et al., 2017). There are also notable overlaps in white matter abnormalities seen in smokers and those with ADHD. Although the direction of differences compared to control subjects conflicted between studies, meta-analysis of white matter integrity in ADHD patients indicated that ADHD individuals have abnormal FA within tracts of the basal ganglia (i.e., caudate nucleus, anterior corona radiata, internal capsule), as well as the cerebellum, corpus callosum, and right forceps minor (van Ewijk et al., 2012). Adolescent smokers show increased FA in the corpus callosum, internal capsule, and inferior longitudinal fasciculus (Jacobsen et al., 2007b; Yu et al., 2016), as well as the corona radiata (Yu et al., 2016) and forceps minor (Jacobsen et al., 2007b) relative to non-smokers. However, the exact relationship between smoking and FA in the corpus callosum remains unclear considering FA in this region has been negatively, rather than positively, correlated with the extent of youth smoking history (Chaarani et al., 2019). These overlapping regional differences in white matter integrity between young smokers and those with ADHD could suggest that the ADHD brain is differentially sensitive to tobacco’s potential effects on white matter compared to those without ADHD. Interestingly, Van Ewijk et al. (2015) found abnormal white matter characteristics in both smoking and ADHD individuals, albeit in opposing directions; that is, lower FA was associated with ADHD, while FA was increased in smokers. Although the significance of these findings is unclear, it has been suggested that pre-morbid differences in white matter integrity in the brains of those with ADHD may contribute to confounding study results and may also be a causal factor in smoking initiation and maintenance as opposed to a consequence of smoking (Groenman et al., 2013; Van Ewijk et al., 2015). Also, a recent study has suggested that the developmental trajectory of white matter during young adolescence (10- to 12-years-old) is only reduced in those taking medications to treat symptoms of ADHD, but not medication-naïve patients (Bouziane et al., 2018). Prior and current treatment of ADHD with medication should, therefore, be included as a variable in investigations of white matter integrity in the ADHD brain of adolescent smokers, as it is unclear whether there is a synergistic effect of smoking and medication history on white matter microstructure across the span of adolescent brain development.
Depression and Anxiety
Studies reliably point to an association between adolescent smoking with depression. Depression and depressive symptoms are consistently observed in smoking adolescents compared to their non-smoking peers (Wu and Anthony, 1999; Goodman and Capitman, 2000; Albers and Biener, 2002; Jacobsen et al., 2007c; Needham, 2007; Ilomäki et al., 2008; Audrain-Mcgovern et al., 2009; Morrell et al., 2010; Slomp et al., 2019). Most studies of this age group found smoking positively predicted the development of depression and depressive symptoms (Brown et al., 1996; Stein et al., 1996; Choi et al., 1997; Goodman and Capitman, 2000; Windle and Windle, 2001; Albers and Biener, 2002; Brook et al., 2002, 2004; Galambos et al., 2004; Duncan and Rees, 2005; Rodriguez et al., 2005; Boden et al., 2010; Moon et al., 2010; Jamal et al., 2011; Beal et al., 2014; Gage et al., 2015), but not all findings have supported this association (Wang et al., 1996; Repetto et al., 2005; Clark et al., 2007; Munafò et al., 2008; Hu et al., 2011; Strong et al., 2014). Importantly, the relationship between smoking and depression in adolescence has been suggested to be bidirectional, such that baseline depression contributes to the risk for future smoking habits just as baseline smoking predicts depression (Brown et al., 1996; Windle and Windle, 2001; Galambos et al., 2004; Needham, 2007; Audrain-Mcgovern et al., 2009; Moon et al., 2010). Some studies also indicate that baseline depression is a considerable factor in the trajectory of depression symptom development in that smoking may mitigate symptom number acceleration, supporting the notion that a subgroup of adolescents smokes to self-medicate (Rodriguez et al., 2005; Needham, 2007; Audrain-Mcgovern et al., 2009). In comparison to depression and depressive symptoms, there is little evidence to suggest adolescent smoking is a predictor of future anxiety (Brown et al., 1996; Gage et al., 2015), but one retrospective, cross-sectional study did observe that an earlier onset of smoking (<15-years-old) was associated with an earlier anxiety diagnosis compared to late-onset smokers (Jamal et al., 2011). However, the cross-sectional and retrospective nature of this study, and that it only included participants that smoked before diagnosis, is a considerable limitation of this finding in concluding the relationship between tobacco use and anxiety disorder. As is the case with the other psychopathologies associated with adolescent smoking, the relationship between smoking, depression, and anxiety is unclear, with evidence supporting that smoking is a causative factor in the development of depression and anxiety, that pre-existing poor mental health facilitates smoking behavior, or that there is an underlying predisposition for smoking, depression, and anxiety to arise independently of each other.
A prevalent theory in the field of smoking, depression, and anxiety is that adolescents smoke to self-medicate, although some argue against this idea (Boden et al., 2010; Beal et al., 2014). Monoamine systems implicated in depression may be indirectly modulated by nicotine’s effects on cholinergic neurotransmission differentially in baseline depressed and non-depressed youth (Dao et al., 2011; Rendu et al., 2011; Pitsillou et al., 2020). This could explain why smoking is generally associated with more depressive symptoms, but deceleration of symptom progression in those with depressive symptomology preceding smoking onset (Rodriguez et al., 2005; Needham, 2007; Audrain-Mcgovern et al., 2009). This is further complicated by the dynamic development of cortical and limbic receptor expression observed during the adolescent critical period in animal models (Thorpe et al., 2020). The introduction of exogenous receptor ligands, such as nicotine may have consequences on neurotransmission that can impact youth behavior and cognition immediately, including the production of positive feelings (e.g., relaxation). However, repetitive insults to these systems by the actions of nicotine may also modulate the expression profile of neurotransmitter receptors, synthesizing enzymes, and metabolizing enzymes, ultimately changing neural activity that could contribute to the risk for depression and anxiety (Thorpe et al., 2020). The possibility of reciprocal feedback between depression and smoking should also be considered in those with smoking onset preceding depression, such that smoking may lead to the emergence of depressive symptoms that are alleviated by maintaining smoking habits.
Future Substance Use
Future Drug and Alcohol Use
Chronic nicotine exposure may lead to an increased risk for neurochemical and pathological changes in the brain, and adolescent smoking is also associated with future substance use. As previously mentioned, adolescent smoking is a strong predictor of future smoking, and this risk is greater with a younger age of use (Taioli and Wynder, 1991; Stanton, 1995; Everett et al., 1999; Colder et al., 2001; Riggs et al., 2007; Dierker et al., 2012; Buchmann et al., 2013; Klein et al., 2013; Lanza and Vasilenko, 2015). Similarly, adolescent and young adult smokers are reported to consume more alcohol (Galván et al., 2011) and cannabis (Caris et al., 2009), and adolescent smoking is a predictor of future substance use (Lewinsohn et al., 1999; Dinn et al., 2004; Ilomäki et al., 2008), especially when smoking is initiated in early adolescence (Brown et al., 1996; Lewinsohn et al., 1999; Ilomäki et al., 2008). The increased risk for future substance use may be a consequence of alterations to the brain’s reward circuit (Rubinstein et al., 2011a,b; Li et al., 2015). For instance, multiple DTI studies have reported higher FA within the basal ganglia of smokers (Jacobsen et al., 2007b; Van Ewijk et al., 2015; Yu et al., 2016), including in fibers of the external capsule that terminate in the ventral striatum (Van Ewijk et al., 2015). The ventral striatum plays an integral role in motivation and reward, and the activity of dopaminergic neurons in this region is thought to be modulated by acute and chronic drug use (Volkow and Morales, 2015). Therefore, disruption of white matter tracts in this region, possibly caused by regular smoking, may leave adolescents susceptible to the rewarding potential of tobacco and other substances. This vulnerability may also extend to a future attention bias toward smoking cues; fMRI studies by Rubinstein et al. (2011a,b) suggest that even adolescent light smokers have blunted neural responses to naturally reinforcing stimuli (i.e., food; Rubinstein et al., 2011a) while simultaneously showing greater neural activation to smoking cues (Rubinstein et al., 2011b) in comparison to non-smokers.
E-Cigarette Use and Transition to Combustible Tobacco Smoking
Although e-cigarettes potentially offer a less harmful alternative to traditional smoking, the use of e-cigarettes may increase the susceptibility for cigarette smoking in youth that would otherwise have not begun smoking (Barrington-Trimis et al., 2016, 2018; Azagba et al., 2017; Miech et al., 2017; Soneji et al., 2017; Spindle et al., 2017; Wills et al., 2017; Parker et al., 2018; Berry et al., 2019; Vogel et al., 2019, 2020), alongside the risk for vaping cannabis (Cassidy et al., 2018; Dai et al., 2018). Adolescents are attracted to e-cigarette flavors, especially those with fruit- and candy-like tastes, and a desire to use e-cigarettes for their taste is frequently cited as a reason for use (Vogel et al., 2019; Jackson et al., 2020). Also, recent findings by Chen et al. (2018) demonstrate that smoking and non-smoking youth report urges to smoke and have greater activation of reward-related brain regions following the presentation of e-cigarette advertisements in comparison to neutral cues. As such, e-cigarette advertisements and the availability of flavored e-cigarette liquids may make use of these devices enticing to youth, encourage initiation, and subsequently lead to the transition to combustibles and other drugs.
The rising popularity of next-generation e-cigarette devices are concerning due to their ability to deliver higher nicotine concentrations in the form of nicotine salts (Boykan et al., 2019). Adolescents who use high nicotine concentration liquids with e-cigarettes are more susceptible to higher frequency and intensity of combustible and e-cigarette use in the future relative to adolescents that do not engage in e-cigarette use or use liquids with lower nicotine concentrations (Goldenson et al., 2017; Boykan et al., 2019). One study suggests that urinary levels of cotinine, a metabolite of nicotine, are higher in adolescent e-cigarette users, relative to levels observed in another study of those who consume combustibles (Benowitz et al., 2018; Goniewicz et al., 2019). However, e-cigarettes can vary widely in the amount of nicotine they deliver per puff (Wagener et al., 2017) and this finding may depend on the model of e-cigarette used by the study population. While the use of newer model e-cigarettes results in almost double the mean urinary cotinine levels compared to traditional smoking, adolescents who use any model of e-cigarettes have lower urinary cotinine levels, than those who smoke combustibles (Boykan et al., 2019). Greater nicotine delivery efficiency apparent in newer generation e-cigarettes (Wagener et al., 2017; Boykan et al., 2019) coupled with the unique vulnerability of adolescents to rewarding substances may result in youth using e-cigarettes consuming higher levels of nicotine when compared to traditional smokers. Despite their potential for harm reduction relative to traditional smoking, the high risk for adolescents to transition from e-cigarettes to combustibles and other drugs, and the possible modulation of neural activity by e-cigarette use, must be considered in future research as well as in the context of marketing and health policies surrounding these devices.
Alcohol
According to the Monitoring the Future Survey conducted in 2019, 18.2% of adolescents in grades 8, 10, and 12 were current alcohol users (Figure 1A; Johnston et al., 2020). Of these, 1.6% reportedly had an alcohol use disorder (AUD; Substance Abuse and Mental Health Services Administration, 2019). Given the overlap between the high degree of neural reorganization and alcohol use initiation during adolescence (Zahr and Pfefferbaum, 2017), it is imperative to consider what impact this interaction may have on neurodevelopment. Alcohol acts primarily via γ-aminobutyric acid type-A and N-methyl-D-aspartate receptors, which regulate inhibitory and excitatory signaling within the brain, respectively (Chandrasekar, 2013; Mallard et al., 2018). An extensive body of evidence suggests that these neurotransmitter systems are affected by alcohol exposure, which may have long-lasting implications on overall neurocircuitry within the brain (Banerjee, 2014). The potential cognitive, psychopathological, and future substance use vulnerability outcomes associated with adolescent alcohol use are summarized in Supplementary Table S2.
Cognition
Several studies have evaluated and identified potential impairments related to adolescent alcohol use on many neurocognitive domains, including attention and inhibitory control. For instance, heavy-drinking adolescents exhibit impulsive choice and attentional bias for alcohol-related cues compared to light-drinkers (Field et al., 2007). Attentional bias for alcohol-related cues was also observed in adolescent social drinkers, suggesting that attentional bias may still emerge with limited alcohol use (Melaugh McAteer et al., 2015). The association between alcohol use and attention has also been demonstrated in a longitudinal study of adolescents who were first assessed before initiation of drinking and followed over three years. In this study, greater hangover symptoms in males predicted worsening of sustained attention (Squeglia et al., 2009b). Similarly, adolescent alcohol drinking altered the developmental trajectory of impulsivity, whereby improvement in impulsivity decelerated following the onset of binge drinking (Ruan et al., 2019). Interestingly, a family history of alcoholism was shown to be protective concerning impulsivity by Jones et al. (2017). Adolescents with a family history of alcoholism who remained alcohol-naïve exhibited a greater decrease in impulsive choice across an eight-year follow-up period compared to those who went on to binge drink. Furthermore, a greater escalation of drinking was associated with greater impulsive choice in this study. The protective effect of a family history of alcoholism is not supported by earlier studies that suggest that youth with a family history of alcoholism exhibit developmental delay in executive functioning, including heightened impulsivity (see review by Cservenka, 2016). Therefore, future studies should focus on the extent to which familial alcoholism interacts with adolescent alcohol use to alter cognition. This may help uncover unique characteristics that may potentially help address some of the discrepant findings related to adolescent alcohol use throughout this section.
Supporting the cognitive differences related to attention and inhibition in adolescent alcohol users, youth who consume alcohol also exhibit neural activity differences. For instance, heavy-drinking adolescents exhibited attenuated activation in the left supplementary motor area, bilateral parietal lobule, right hippocampus, bilateral middle frontal gyrus, left superior temporal gyrus, and the ACC compared to light drinkers during a response inhibition task (Ahmadi et al., 2013). Similarly, Aloi et al. (2018) reported an association between increasing AUD severity and reduced BOLD responses within the ACC and the dorsomedial PFC during the affective Stroop task assessing emotional interference on cognitive functioning. Effects on inhibitory control may be dose-dependent as a longitudinal study of adolescents with low alcohol use did not find any impairments in the development of inhibitory control across adolescence and activation in related networks, such as the dorsal ACC, DLPFC, pre-supplementary motor area, and the posterior parietal cortex (Jurk et al., 2018). However, in a longitudinal assessment of adolescents aged 12–14 with very limited substance use histories at baseline, reduced activation in regions that largely overlapped with the Ahmadi et al. (2013) study during the same inhibitory task predicted transition into heavy alcohol use after approximately four years (Norman et al., 2011). This suggests that activation differences may predate, and possibly contribute to, the initiation of alcohol use. Another study has revealed a bidirectional relationship with reduced activation in frontal, temporal, and parietal regions during inhibitory tasks predicting future heavy drinking, and heavy drinking, in turn, predicting increased activation in frontal, parietal, subcortical, and cerebellar regions over time (Wetherill et al., 2013). Together, these findings suggest that neural vulnerabilities in regions implicated in inhibitory control predict alcohol use, and heavy drinking subsequently may lead to additional alterations. Similarly, Squeglia et al. (2014) have reported a bidirectional relationship with smaller cingulate and rostral ACC volumes at baseline predicting later transition to heavy drinking, and heavy drinking, in turn, predicting greater volume reductions in the left inferior/middle temporal gyrus and left caudate. Another study has demonstrated the reverse relationship between alcohol use and morphological differences, whereby smaller left dorsal and rostral paralimbic ACC volumes predicted later alcohol-related problems (Cheetham et al., 2014). These findings suggest that the relationship between alcohol use and neural differences is complex and on-going prospective studies (like the Adolescent Brain Cognitive Development study of the National Institute on Drug Abuse) that follow adolescents before the initiation of alcohol use and across development may help further clarify directionality.
Adolescent alcohol drinkers appear to exhibit poorer working and verbal memory (Brown et al., 2000; Hanson et al., 2011; Parada et al., 2012), suggesting that alcohol use during this critical window may predispose youth to memory impairments. However, adverse memory-related outcomes may improve after prolonged drinking abstinence. In a longitudinal study, interruption of binge-drinking patterns led to a partial cognitive recovery, with ex-binge drinkers having greater memory consolidation deficits than non-binge drinkers but fewer deficits than continued binge drinkers (Carbia et al., 2017a). In a separate analysis by this group, binge drinkers showed improvements in working memory span but maintained consistent deficits in perseveration errors (Carbia et al., 2017b). However, it is difficult to predict whether these differences in adolescent drinkers compared to their relatively abstinent peers were present before the initiation of alcohol use. In a study of adolescents first assessed at 11-years-old, working memory impairment predicted both baselines and increased frequency of alcohol use over a four-year follow-up period, while there was no evidence supporting the reverse relationship (Khurana et al., 2013). However, in adolescents first assessed before initiation of substance use, extreme-binge drinkers exhibited poorer performance in measures of verbal learning and memory despite equivalent performances at baseline (Nguyen-Louie et al., 2016). The latter study suggests that the effects of alcohol on learning and memory may be mediated by dose. Dose-dependent neurotoxicity of alcohol use is also observed in other neurocognitive domains that were previously discussed, including attention and impulsive choice (Squeglia et al., 2009b; Jones et al., 2017). Therefore, more research is needed to develop strategies to reduce alcohol intake severity that may help temper the neurocognitive consequences related to adolescent alcohol use.
Adolescent alcohol users also differ in the degree of neural recruitment during a memory task performance from non-users. For instance, during verbal recall, non-drinking adolescents show activation of the left hippocampus whereas adolescents who engage in binge drinking do not and recall fewer words. Binge drinking adolescents also show greater activation in the right superior frontal and bilateral parietal cortices, areas implicated in working memory, compared to non-drinkers, suggesting heavier reliance on alternate memory networks (Schweinsburg et al., 2010). Similarly, adequate performance in the spatial working memory task required greater response in prefrontal and temporal regions compensating for diminished activity in the bilateral cerebral areas and the left precentral gyrus in adolescents with AUD (Tapert et al., 2004). The relationship between adolescent drinking and memory may be bidirectional, as the extent of memory-related brain region activation during working memory tasks has been shown to predict future heavy drinking, and heavy drinking, in turn, predicted increased activation over time (Squeglia et al., 2012a). Female adolescents with AUD may be especially vulnerable to abnormal activity patterns, with Caldwell et al. (2005) suggesting greater compensatory activation in the temporal areas for a reduced frontal and cingulate response to the spatial working memory task. In a subsequent study, attenuated frontal, temporal, and cerebellar responses to a spatial working memory task corresponded to deficits in sustained attention and working memory in female binge drinkers. Meanwhile, male binge drinkers’ spatial working memory performance was positively correlated with activation of related brain regions and these individuals showed better spatial working performance compared to controls, suggesting an engagement of compensatory mechanisms (Squeglia et al., 2011). Aberrant neural recruitment during cognitive processes, in turn, may suggest functional compensation for differences in structural connectivity. For instance, adolescent binge drinkers exhibited lower connectivity in major white matter tracts implicated in neurocognitive functioning, whereby FA was reduced in the corpus callosum, corona radiata, superior longitudinal fasciculus, and fronto-occipital fasciculus compared to controls (Jacobus et al., 2009; McQueeny et al., 2009). These results conflict with those by Cardenas et al. (2013), who reported higher FA in the posterior corpus callosum in adolescents with AUD and did not report any regions with lower FA according to drinking status. Since higher FA in the corpus callosum was not related to any measure of alcohol use, it might predict vulnerability to AUD, rather than being a direct consequence of alcohol use. Lower FA reported by the former studies may suggest alcohol’s toxic effects on white matter microstructure as a longitudinal assessment of adolescents aged 14–19 revealed drinking-associated blunted white matter microstructure development, evidenced by decreased FA in the left caudate and inferior frontal occipital fasciculus over than years (Luciana et al., 2013). The participants in this study had no experience with alcohol and did not have any significant premorbid differences at the baseline assessment.
Morphological differences in alcohol-using adolescents relative to abstinent adolescents have also been observed in brain regions implicated in neurocognitive functioning, such as smaller hippocampal, PFC, and cerebellar volumes, as well as thicker frontal cortices (De Bellis et al., 2000, 2005; Nagel et al., 2005; Medina et al., 2008; Squeglia et al., 2012b; Lisdahl et al., 2013), but the directionality of these findings is debated. In one longitudinal study of baseline “alcohol-naive youth” aged 12–21, and another of youth aged 18–23, heavy drinkers exhibited accelerated gray matter loss in the superior frontal gyrus, caudal middle frontal gyrus, and rostral middle frontal gyrus (Pfefferbaum et al., 2018), as well as in the parahippocampus (Meda et al., 2017) compared to no/low drinking controls over two years. A similar observation was made by Squeglia et al. (2015) in lateral frontal and temporal GMV in addition to attenuated white matter growth of the corpus callosum in heavy adolescent drinkers who were followed over eight years. Other studies have demonstrated the reverse relationship between adolescent alcohol use and morphological differences, whereby thinner DLPFC and inferior frontal cortex (Brumback et al., 2016), and higher GMV in the caudate nucleus and the left cerebellum (Kuhn et al., 2019) predicted later increases in alcohol use and alcohol-related problems.
The age of drinking onset may also have important implications for future cognitive and neurobiological abnormalities. An earlier age of first drinking onset predicted worse psychomotor speed and visual attention functioning, but only when the model accounted for drinking duration (Nguyen-Louie et al., 2017). Consistently, participants with an earlier age of weekly drinking onset performed poorer on measures of cognitive inhibition and working memory than those with a later onset age. In light of this evidence, it is suggested that early onset of drinking increases the risk for alcohol-related neurocognitive vulnerabilities and that initiation of alcohol use at younger ages appears to be a risk factor for poorer subsequent neuropsychological functioning. The impact of early adolescent alcohol use upon later working memory was also observed in a larger study of 3,300 participants, with the frequent/binge drinking group displaying impaired working memory at three-year follow-up (Mahedy et al., 2018). While each of the above studies attempted to control for confounding variables, including comorbid substance use, sociodemographic status, and baseline neuropsychological performance, the impact of these confounds was mixed across studies. Nevertheless, even after controlling for these variables, the association between earlier alcohol use and poorer neurocognitive performance remained across both studies. The variability in confounding influences and the different neuropsychological measures taken across studies highlight the need for high-quality, long-term prospective cohort studies with standardized measures to better understand the lasting consequences of adolescent drinking.
Psychopathology
Depression and Anxiety
Several studies have investigated the association between psychiatric illness and alcohol use (see detailed reviews: Fiorentini et al., 2011; Addington et al., 2014). However, whether this association is causal or arises from shared pathophysiology has remained difficult to parse (Khokhar et al., 2018). This notion is further supported by a comprehensive, prospective longitudinal study of participants interviewed from ages 16 through 30 that showed a high prevalence of comorbidity of major depressive disorder (MDD) and AUD. Prospectively, adolescent AUD predicted early adult MDD and early adult MDD predicted adult AUD, suggesting that MDD and AUD are inter-related in a complex manner (Brière et al., 2011). This association has been shown at even sub-clinical levels of alcohol use, with adolescent alcohol use at the age of 13–15 predicting depression at age 17 (Edwards et al., 2014). Interestingly, another study has suggested that the relationship between alcohol use and depression may be mediated by a specific measure of alcohol involvement, whereby problematic use (defined by adverse consequences of alcohol use), but not alcohol intake (defined by the level of alcohol consumption) predicted young adult MDD (Mason et al., 2008). Self-reported alcohol use in adolescence has also been prospectively associated with hypomanic/manic symptoms at age 23 (Fasteau et al., 2017); however, these results solely relied on self-reported alcohol intake and problematic use and will need to be confirmed in future studies with more robust designs. Although the neural basis for the association between adolescent alcohol use and mood disorders has been largely unexplored, AUD symptom severity in adolescents was associated with increased amygdala responses to emotional compared to neutral stimuli (Aloi et al., 2018). However, the directional implications of these findings on the relationship between alcohol use and mood disorders are unclear, highlighting the need for more studies to identify neural markers to help characterize their comorbidity. For instance, activity within neural circuitry that underlie both alcohol use and mood disorders, such as the reward circuit (Russo and Nestler, 2013), in response to paradigms measuring emotional processing should be assessed through neuroimaging techniques.
Socially anxious adolescents have been shown to use alcohol to cope with their symptoms, supporting the self-medication hypothesis (Blumenthal et al., 2010). Furthermore, in a recent large cohort longitudinal study that tracked girls aged 13–17, higher baseline depression severity predicted an increased likelihood of future alcohol use. There was also evidence for an inconsistent, reciprocal relationship with the consumption of one full drink at ages 14 and 16 predicting decreased depression in the next year. However, the latter finding should be interpreted with caution as this association was inconsistent across time and low levels of alcohol drinking are not necessarily pathological and may constitute normative behavior among adolescents (Schleider et al., 2019). A bidirectional relationship has also been reported between alcohol use and internalizing symptoms (e.g., anxiety and depressive symptoms) among adolescents who were prospectively assessed from age 14–16, where alcohol use or internalizing symptoms at age 14 predicted the other at age 16. Another important finding emerged from this study when internalizing symptoms were examined in clusters related to either anxiety or depression. While the Anxious Arousal scale showed a consistent reciprocal relationship with alcohol use, the association between alcohol use and Anhedonic Depression disappeared after controlling for delinquency, highlighting that symptoms of anxiety and depression in the internalizing domain are not interchangeable, which should be considered in future studies. There was also variation within symptoms unique to anxiety as measures from the Anxiety scale was not associated with alcohol use, contrasting what was observed with the Anxious Arousal scale (Parrish et al., 2016). This is consistent with another study showing that while early generalized anxiety symptomology predicted an increased risk for initiation of alcohol use, separation anxiety symptomology predicted decreased risk (Kaplow et al., 2001). It is also important to consider co-occurring externalizing symptoms (e.g., aggression and impulsivity) when assessing the relationship between alcohol use and internalizing symptoms, as externalizing symptoms have been previously shown to mediate this relationship (Colder et al., 2017). Current evidence relating to the association between alcohol and mood disorders is mixed with some supporting the self-medication hypothesis, while others suggesting that adolescent alcohol use may be a risk factor for developing mood disorders.
Future Substance Use
Adolescent alcohol drinking may also contribute to the risk for subsequent alcohol or drug use and dependence in adulthood; adolescent binge drinking predicts an increased risk of adult alcohol dependence, persistent cannabis, and other illicit drug use (Viner and Taylor, 2007; Pampati et al., 2018). The association between early alcohol use and subsequent alcohol-related problems has been further supported by data drawn from two large population studies conducted in two countries with distinct alcohol use policies and cultures. After controlling for a comprehensive number of potential confounders, both early-onset drinking and early onset of excessive drinking in adolescence (aged 14–16) were related to increased risk of alcohol-related problems when assessed at 18- to 25-years-old (Enstad et al., 2019). Impaired decision-making and underlying neural mechanisms in adolescent alcohol users may mediate the relationship between alcohol use and future substance use vulnerability. For instance, adolescent binge drinkers cross-sectionally exhibited poorer performance compared to controls in the Iowa gambling task used to assess effective decision-making, and higher activity in regions implicated in the emotional and incentive-related aspects of decision-making, such as the amygdala and insula. Similarly, connectivity between the OFC and amygdala predicted increases in alcohol use and increased connectivity between these regions has previously been shown to be protective against risk-taking (Peters et al., 2017). Activation differences in response to risky decision-making may both predict and be a consequence of adolescent alcohol drinking. While adolescent binge drinkers showed reduced activation in the dorsal caudate during risky decision-making, reduced frontoparietal activation in binge drinkers was present before they initiated alcohol use (Jones et al., 2016). In another study, an opposite pattern of increased activation in the nucleus accumbens, precuneus, and occipital cortex during risky decision-making predicted earlier initiation of binge drinking (Morales et al., 2018).
Adolescent binge drinking may also alter neural activity during reward processing, with Aloi et al. (2019) showing a cross-sectional association between AUD symptom severity and reduced activity in the posterior cingulate cortex and the striatum. Furthermore, among adolescents who were alcohol-naïve at baseline, those who transitioned into binge drinking after a two-year follow-up period exhibited reduced activity in the left cerebellum compared to controls during reward processing (Cservenka et al., 2015). This cerebellar activity was negatively associated with the average number of drinks consumed/drinking days, suggesting a dose-dependent effect. Differential activation patterns in reward-related regions may also predict increases in alcohol use from age 16–18 in a gender-specific manner; higher ventral striatum activity during reward anticipation was observed in boys, and higher dorsomedial PFC activity and decreased ventral striatum activity during reward anticipation was found in girls (Swartz et al., 2020). Greater activation to alcohol cues in adolescent alcohol users have also been reported, indicating a more intense desire and craving for alcohol, potentially putting them at risk for greater alcohol use in the future (Tapert et al., 2003; Dager et al., 2014; Brumback et al., 2015). Together, these findings suggest that neural markers may both predict alcohol use initiation, and also be a consequence of alcohol’s neurotoxic effects on reward circuitry; these differences may ultimately predispose adolescent alcohol users to excessive drinking in the future. However, research investigating adolescent alcohol use and vulnerability to alcohol and other drugs is scarce and requires considerable attention.
Cannabis
In 2019, approximately 15.6% of U.S. adolescents were current users of cannabis, making it the second most commonly used substance by this age group (Figure 1A; Johnston et al., 2020), and one that requires further attention. Adolescence marks a period in which extensive cortical reorganization and synaptic pruning occur, and mounting evidence points to chronic cannabis use interfering with this process (Renard et al., 2014). Δ9-tetrahydrocannabinol, the primary psychoactive ingredient of cannabis, acts primarily as a partial agonist at the cannabinoid type 1 receptor. Given that cannabinoid type 1 receptors are widely expressed throughout the brain, structural and functional consequences of cannabis exposure are a subject of interest (Pertwee, 1997). Herein, we review the possible consequences of cannabis use during adolescence related to cognition, psychopathology, and future substance use risk, and studies investigating these associations are summarized in Supplementary Table S3.
Cognition
Numerous studies have suggested that adolescent cannabis users are at a heightened risk for adverse cognitive outcomes (see review by Lubman et al., 2015). For instance, cross-sectional studies have reported that adolescent cannabis use is associated with impairments in inhibitory control and attention (Harvey et al., 2007; Lane et al., 2007; Medina et al., 2007a). A longitudinal assessment by Infante et al. (2020) supports this relationship as greater lifetime adolescent cannabis use over 14 years was associated with impairments in inhibitory control and visuospatial functioning. Inhibitory control impairments may be, in turn, related to increased connectivity between the parietal and cerebellar regions, which comprise part of the inhibitory circuit (Behan et al., 2014). Adolescent cannabis users also exhibited hyper-activations in DLPFC and parietal regions during a Go/No-Go task in the absence of group differences in performance, instead suggesting functional compensation (Tapert et al., 2007). The effects of cannabis use on attention in adolescence may be dose-dependent. In a recent large cross-sectional study of adolescents aged 14–21, frequent, but not occasional cannabis users exhibited poorer sustained attention compared to non-users. Interestingly, earlier age of onset of cannabis use appeared to be a risk factor for sustained attention deficits in occasional cannabis users (Scott et al., 2017). This dose-dependency is also apparent in fMRI studies with adolescent chronic cannabis users exhibiting impairments in executive attention and greater activation of the right PFC compared to non-using controls (Abdullaev et al., 2010). Also, cannabis users may be more vulnerable to the adverse effects of cannabis on attention compared to other executive functions as Hanson et al. (2010) showed that working memory and verbal learning deficits improved after three weeks of abstinence in cannabis users, while attention deficits persisted. This study highlights the importance of considering the periods of abstinence from cannabis in cross-sectional studies that differ from one study to another, making it difficult to disentangle acute and lasting effects of adolescent cannabis use on cognition. Therefore, harmonization of protocols relating to the period of abstinence is necessary, in addition to assessing the effect of abstinence from cannabis longitudinally.
Although not specific to inhibitory control or attention, adolescent cannabis users also displayed larger cerebellar vermis volumes compared to controls, which was associated with poorer executive functioning assessed by subsets of the Delis-Kaplan executive function test. This suggests that morphological differences in brain regions may underlie abnormalities related to deficits in higher-level cognitive skills (Medina et al., 2010). Female adolescent cannabis users may be at a greater risk for such differences as Medina et al. (2009) reported larger PFC volumes in female cannabis users, with smaller PFC volumes predicting better executive functioning among cannabis users. Reduced right medial PFC volume (Churchwell et al., 2010) and greater left hippocampal volumes (Ashtari et al., 2011) have also been observed in adolescent heavy cannabis users; however, functional correlates of these morphological differences have yet to be studied in adolescent heavy cannabis users. The hippocampal volume findings conflict with Weiland et al. (2015), who showed that adolescent daily cannabis users did not differ from non-users in hippocampal volumes. Similarly, Scott et al. (2019) reported non-significant differences between frequent and occasional cannabis users, as well as non-users in global or regional brain volumes, cortical thickness, and gray matter density. These findings suggest that adolescent heavy cannabis users may be at a heightened risk for impairments in neurocognitive functioning, and future studies should focus on investigating the functional correlates of the structural differences observed in heavy cannabis users.
Cannabis use during adolescence is also associated with deficits in intelligence. In adolescents first assessed at 13 years of age before the onset of cannabis use and again at 20-years-old, poor short-term and working memory predicted earlier age of onset of cannabis use. Conversely, an earlier age of onset and more frequent use during adolescence was associated with declines in performance in verbal IQ as well as trial and error learning and conditional association learning (Castellanos-Ryan et al., 2017). Similarly, adolescent cannabis use was associated with greater IQ decline and working memory impairments and cessation of cannabis use did not restore neurocognitive functioning (Meier et al., 2012). However, findings regarding adolescent cannabis-associated declines in IQ remain conflicting with one study showing no evidence for IQ decline from ages 12–18, while another indicated that cigarette smoking may be a confounder (Mokrysz et al., 2016; Meier et al., 2018). Familial factors may also contribute to the observed differences in IQ decline between cannabis users and non-users. In a large longitudinal twin-pair study of participants aged 9–11 at baseline, cannabis-using twins did not exhibit greater IQ decline relative to their non-user co-twin when assessed at 18–20 years of age (Jackson et al., 2016). However, a neuroimaging study by Camchong et al. (2017) converges on the former findings with adolescents with cannabis use disorder (CUD) showing decreased caudal ACC RSFC with the left DLPFC and OFC, as well as lower IQ and slower cognitive function across an 18-month follow-up period. Adolescent cannabis use is not consistently associated with deficits in IQ, which may be explained by familial factors and the use of other drugs; therefore, the extent to which these factors interact with the effect of cannabis on the adolescent brain should be considered for other behavioral and neurobiological domains.
Greater amounts of cannabis use have also been prospectively associated with declines in immediate, but not delayed, memory performance (Duperrouzel et al., 2019) and persistent verbal learning impairments (Becker et al., 2015). The latter study also investigated the association between cannabis use and white matter microstructure and found that adolescent cannabis users aged 18–20 at baseline exhibited attenuated FA growth in the superior longitudinal fasciculus, an association fiber that has been largely implicated in cognitive functions (Becker et al., 2015). Also, male adolescent heavy cannabis users exhibited decreased FA in the left temporal lobe, an area implicated in verbal memory. FA reductions were accompanied by complementary increases in radial diffusivity and trace values, all suggestive of decreased myelination (Ashtari et al., 2009). This study reported minimal baseline differences in FA between cannabis users and controls, suggesting that white matter microstructure differences did not predate cannabis use. Interestingly, attenuated loss of cortical thickness across adolescent development (Epstein and Kumra, 2015) and greater GMV (Orr et al., 2019) have been observed in adolescent cannabis users in several regions of the brain bilaterally, both of which have reciprocal relationships with myelination. Cross-sectional studies have reported that adolescent cannabis users also show impairments in working memory, problem-solving, and planning (Harvey et al., 2007; Medina et al., 2007a; Vo et al., 2014). Compensatory hyper-functioning has been reported by fMRI studies in the brains of adolescent cannabis users during task performance, complementing these behavioral findings. For instance, hyper-activation in the DLPFC and the right basal ganglia (Padula et al., 2007; Jager et al., 2010), as well as failure to reduce activation in the right hippocampus (Jacobsen et al., 2004), have been observed in adolescent cannabis users compared to non-users during working memory tasks. In the former studies, activation differences between groups were present despite adequate performance on the task, suggesting that adolescent cannabis users require more neural recruitment to perform the tasks at a comparable level to non-users.
The age of onset also plays a critical role in the effects of adolescent cannabis use on cognition. For instance, adolescent early-onset cannabis use has been associated with poorer sustained attention, impulse control, and verbal IQ compared to a later onset of use in current adult cannabis users (Pope et al., 2003; Fontes et al., 2011). Females may be more susceptible to the effects of earlier initiation of cannabis use on neurocognitive functions, as female adolescents exhibited more spatial working memory deficits compared to males across a five-year follow-up period from a baseline age of 12 (Noorbakhsh et al., 2020). Interestingly, in a longitudinal study that tracked cannabis use across adolescence and into adulthood, earlier onset of cannabis use was associated with longer reaction times during a working memory task, which was mediated by reduced activity in the posterior parietal cortex compared to late-onset use (Tervo-Clemmens et al., 2018). This suggests that early onset of cannabis use may predispose those who continue to use cannabis into adulthood to executive function impairments. Also, Wilson et al. (2000) reported a smaller percentage of cortical gray matter, and a larger percentage of white matter across the whole brain, in adults who initiated cannabis use before age 17 compared to those who initiated their use later. These differences were most prominent in the frontal lobes. However, in a study of adolescent boys followed prospectively into adulthood, no differences were observed in both cortical and subcortical region morphology between non-users and users across different trajectories of cannabis use ranging across infrequent to chronic use (Meier et al., 2019). The mixed findings could be attributed to the differences in study design, whereby some of the aforementioned studies were retrospective, and are therefore susceptible to recall bias. Therefore, the longevity of the effects of adolescent cannabis use on cognitive functions and their neurobiological correlates need to be further elucidated through currently on-going and future prospective longitudinal studies.
Psychopathology
Schizophrenia and Psychosis
Cannabis use is common among first-episode psychosis patients (Katz et al., 2016; Abdel-Baki et al., 2017), and cannabis use has been hypothesized to be a causal factor in these disorders (Toftdahl et al., 2016). More recent data appears to confirm this positive association between adolescent cannabis use and schizophrenia spectrum disorders (Arseneault et al., 2002; Jones et al., 2018), particularly in that cannabis both hastens the onset and amplifies the severity of schizophrenia (Shahzade et al., 2018). However, Hanna et al. (2016) reported better cognitive function in adolescent cannabis users with schizophrenia/schizoaffective disorders, suggesting a potential protective role of cannabis in psychosis-related cognitive dysfunction. Structural MRI studies are not consistent with a neuroprotective effect and have suggested that processes underlying gray matter and cortical maturation may mediate the association between adolescent cannabis use and risk for schizophrenia. Among adolescents aged 10–21, those with CUD and early-onset schizophrenia exhibited decreased GMV in the left superior parietal cortex compared to controls (Kumra et al., 2012). Greater cannabis consumption across an 18-month follow-up period in adolescents with CUD predicted a greater decrease in the left inferior longitudinal fasciculus (Kumra et al., 2012), a white matter tract that was previously shown to be disrupted in adolescents with schizophrenia (Ashtari et al., 2007). Moreover, gender may interact with structural abnormalities mediating the association between cannabis use and schizophrenia. For instance, male adolescent cannabis users, with a high polygenic risk score for schizophrenia across 108 genetic loci exhibited decreased cortical thickness, which was not observed in low-risk male, or high- and low-risk female participants (French et al., 2015). However, gender differences need to be investigated further as current studies report mixed findings. For instance, in a study of Australian adolescents, girls who started using cannabis before the age of 16 displayed higher levels of introvertive anhedonia, a negative schizotypy, than girls who started using cannabis later in adolescence, whereas this association was not present in boys (Albertella et al., 2017). Also, the causal direction of the relationship between adolescent cannabis use and schizophrenia is called into question as Hiemstra et al. (2018) found stronger evidence for a reverse association, showing that schizophrenia genetic risk was predictive of increased cannabis use from age 16 to 20. This study, combined with those outlined above, suggests that the association between adolescent cannabis use with psychosis, while strong, may not be causal, and further study of the functional contributions of the risk of loci identified in these studies might help to unravel this “chicken-or-egg” problem.
Depression and Anxiety
Adolescent cannabis users, particularly females, maybe at a heightened risk for mood disorders. Among Norwegian adolescents aged 13–17, cannabis users reported more anxiety and depressive symptoms compared to non-users. Girls reported slightly more symptoms compared to boys despite the lower prevalence of cannabis use among girls (Kaasbøll et al., 2018). Similarly, more internalizing symptoms in female adolescent cannabis users were associated with larger amygdalar volumes (McQueeny et al., 2011); this association was not observed in male participants. Conversely, other studies have found no association between adolescent cannabis use and differences in amygdala volumes between adolescent cannabis users and non-using controls (Ashtari et al., 2011; Weiland et al., 2015). It is important to note that the number of female participants in the McQueeny et al. (2011) study was small and future studies with more female participants would be needed to confirm these results. Despite limited evidence for differences in amygdalar morphology between adolescent cannabis users and non-users, amygdalar hypersensitivity in response to angry faces has been reported in adolescent cannabis users, which could predispose individuals to future mood disorders (Spechler et al., 2015). However, these results are conflicted by a more recent study that showed no differences in amygdalar responsivity to emotional stimuli in adolescents with CUD (Aloi et al., 2018). Psychiatric comorbidity may have masked any association between CUD symptomology and amygdala responsiveness in the latter study. Furthermore, in adolescent cannabis users, depressive symptoms were positively associated with increased connectivity between the left OFC and left parietal regions, while anxiety symptoms were negatively associated with increased connectivity between bilateral OFC with right occipital and temporal regions (Subramaniam et al., 2018). Similarly, decreased FA and increased radial diffusivity and trace in the thalamic radiation were observed in older adolescents with a history of heavy cannabis use (Ashtari et al., 2009); decreased FA in the thalamic radiation has also been previously shown in young adult patients with depression (Lai and Wu, 2014). Also, smaller global white matter volumes were associated with more depressive symptoms in adolescent cannabis users (Medina et al., 2007b), suggesting that white matter abnormalities may extend beyond what is observed at a microstructure level.
A recent meta-analysis of longitudinal studies indicated that adolescent cannabis use is associated with a modest risk of developing depression in young adulthood (Gobbi et al., 2019). A recent population-based cohort of young adults who were retrospectively assessed for adolescent cannabis use and followed over 30 years has also captured this. Adolescent cannabis use, particularly an earlier onset of use, as well as more frequent use was associated with adult depression, independent of adult cannabis and other substance use (Hengartner et al., 2020). Adolescent cannabis use may further exacerbate depressive symptoms in males with mild depression at baseline, with limited evidence to support the self-medication hypothesis, whereby depressive symptoms predicted only slight increases in later cannabis use (Womack et al., 2016). Similarly, anxiety symptoms do not appear to predate adolescent cannabis use and may instead depend on the frequency of use. In a recent longitudinal study, adolescents with higher levels of cannabis use reported more persisting anxiety over the next year compared to less frequent users; anxiety levels at baseline did not predict differences in cannabis use between the groups (Duperrouzel et al., 2018). In longitudinal studies, gender differences in the relationship between adolescent cannabis use and anxiety/depressive symptoms have shown an opposite trend to those reported by cross-sectional studies discussed above. In a large adolescent sample balanced for gender, baseline cannabis use at age 16 predicted increases in depressive symptoms in over three years among male, but not, female African American adolescents (Assari et al., 2018). Another study found an association between escalating cannabis use and decreased connectivity between nucleus accumbens and medial PFC that predicted higher levels of depressive symptoms (Lichenstein et al., 2017). Decreased growth in FA in the right anterior thalamic radiation was also observed over three years in adolescent cannabis users (Becker et al., 2015), suggesting possible shared pathophysiology with young adult patients with depression (Lai and Wu, 2014). Overall, both imaging and behavioral findings support a strong relationship between adolescent cannabis use and mood disorders that appear to uniquely interact with gender; neural markers that may give rise to these differences between males and females should be investigated in future studies.
Future Substance Use
In addition to the relationships between adolescent cannabis use and the risk for schizophrenia and mood disorders, longitudinal studies have revealed that occasional and early-onset cannabis use in adolescence predicts nicotine use and dependence, harmful alcohol consumption, and other illicit drug use in adulthood (Degenhardt et al., 2010; Swift et al., 2012; Scholes-Balog et al., 2016; Jin et al., 2017; Taylor et al., 2017; Pampati et al., 2018). When examining the risk of future drug dependence as a consequence of adolescent cannabis use, it may also be important to consider different cannabis use behaviors, such as using cannabis in social settings vs. solitary use. Solitary cannabis use may present as a risk factor for future cannabis dependence as a recent study showed that compared to social-only use, solitary use is associated with greater cannabis use, as well as CUD symptoms in young adulthood (Creswell et al., 2015). However, these results should be interpreted with caution as the association between solitary cannabis use and future CUD symptoms disappeared after controlling for adolescent CUD symptoms. Interestingly, early-onset cannabis use has previously been associated with anti-social behavior, which may, in turn, promote solitary use (Scholes-Balog et al., 2016). Similar findings have been observed for cannabis vaping, which has been relatively under-studied compared to combustible cannabis use and nicotine vaping despite its prevalence. Cassidy et al. (2018) recently observed that youth entering college are more likely to initiate cannabis vaping if they have a prior history of any cannabis or e-cigarette use, and the risk for vaping cannabis scales with the number of peers also engaging in use. However, the frequency and intensity of use among those who initiate cannabis vaping in social settings and the risk for the development of CUD in these populations are not defined, nor is the use of cannabis vaping in younger adolescent populations.
Earlier onset and greater duration of cannabis use were also associated with risky and impulsive decision-making in adolescent users (Solowij et al., 2012), and impaired decision-making, in turn, may promote substance use. Neuroimaging studies suggest functional compensation as De Bellis et al. (2013) reported that adolescents with CUD exhibit higher activity in the left superior parietal lobule, left lateral occipital cortex, and bilateral precuneus during risky decision-making despite no group differences in performance. Despite cross-sectional associations between cannabis use and poor decision-making in youth aged 14–17 at baseline, cannabis use was not associated with changes in decision-making over a one-year follow-up period (Duperrouzel et al., 2019). This suggests that impaired decision-making may predate cannabis use initiation in adolescence. This is in line with a structural MRI study showing that smaller OFC volume, implicated in decision-making, predicted the initiation of cannabis use by the age of 16 (Cheetham et al., 2012). Adolescent cannabis users also exhibited diminished ability to disengage motivational circuitry during non-rewarding events in the monetary incentive delay task despite normal performance as evidenced by heightened striatal activity in cannabis users compared to non-users, which could drive risk-seeking behavior even in the face of negative outcomes (Jager et al., 2013). Additionally, adolescents with CUD exhibited greater accuracy across trials in the monetary delay task, and greater functional global connectivity across networks that included mesocorticolimbic nodes during monetary reward anticipation (Nestor et al., 2019). The group also showed enhanced integration, defined as higher information exchange between regions and a greater number of connections to the nearest nodes, alluding to neural refinement deficiencies. Superior performance may be mediated by higher motivation as there were no group differences in performance and global connectivity within different trials; however, an earlier study showed reduced motivation in adolescent heavy cannabis users, which may instead indicate lack of motivation at greater consumption levels, potentially failing to seek treatment (Lane et al., 2005). In another study, adolescent cannabis users exhibited an enhanced neural response to both wins and losses, the latter suggesting greater sensitivity during negative feedback (Acheson et al., 2015). In contrast to results from Nestor et al. (2019), Acheson et al. (2015) showed that despite seeing no differences in global connectivity, analyses of the individual paths revealed that adolescent cannabis users differed in connectivity from controls in one-third of the total paths analyzed in response to losses, but no individual path differences were observed during wins. Although these results confirm differences in sensitivity to negative feedback observed in previous studies, it differentially highlights the importance of assessing connectivity within individual networks when investigating alterations in reward circuitry. Both behavioral and neural findings indicate that adolescent cannabis use may increase the risk for future substance use and associated behaviors; however, further research is needed to assess the effects of vaping and different cannabis use behaviors (e.g., solitary vs. social) on this relationship.
Opioids
In 2017, an estimated 3.1% of adolescents aged 12–17 had misused opioids in the past year (Figure 1A; Substance Use and Mental Health Services Administration, 2018). While these numbers are lower than the prevalence for other substances, the alarming trends of problematic opioid use in North America, the high mortality associated with opioid use, and their exclusion from previous reviews on this topic necessitate further attention. Opioids produce their effects by modulating the excitability of neurons via mu, kappa, and delta-opioid receptors as well as nociception receptors. Little is known about opioid receptor development and the consequences of opioid use during adolescence; however, the endogenous opioid system has been observed to change throughout adolescent development, highlighting the necessity for future research during this vulnerable window (Thorpe et al., 2020). Due to fewer findings, this section is considerably narrower in its scope compared to the previously reviewed substances (Supplementary Table S4).
Cognition
Very little clinical work has been conducted on the long-term effects of opioids on memory and cognition. Given that adolescent opioid use is rarely unaccompanied by other substance use, it is difficult to attribute any effects to opioids on their own. One study found that opioid-dependent adolescents had significantly impaired working memory, but was unable to determine if these deficits were substance-induced or pre-existing before use (Vo et al., 2014). However, the opioid-using group had similar levels of cannabis use as a cannabis-only using group in the same study and the working memory deficits seen were comparable to those of cannabis-only users. Future studies looking into the effects of long-term prescription opioid use in adolescence on cognition are warranted. This would allow for the study of opioids in populations that do not use other substances and give insight into the neurocognitive effects of illicit opioids without the confound of other drugs.
Psychopathology
Studies conducted on the effects of opioids relating to cognition and psychopathology have shown higher rates of comorbid psychiatric disorders such as MDD, substance use disorder, ADHD, antisocial personality disorder, borderline personality disorder, and post-traumatic stress disorder compared to non-users (Mills et al., 2004; Subramaniam and Stitzer, 2009; Subramaniam et al., 2009; Edlund et al., 2015). Though retrospective, adolescents with MDD and non-medical prescription opioid use often reported MDD to predate opioid use, suggesting MDD to be a risk factor for future opioid abuse (Edlund et al., 2015). In a cross-sectional study of 14- to 18-year-olds, Subramaniam and Stitzer (2009) found that 83% of adolescents with opioid use disorders had a co-occurring psychiatric disorder. Thus, opioid use and several psychopathologies appear to be related but, unfortunately, the directional relationship between opioids and their comorbidities is not known, highlighting the need for future longitudinal studies.
Future Substance Use
As discussed in previous sections, many substances are associated with an increased risk of future substance use. Opioids are likely not an exception to this trend, and it is thus alarming that they are both regularly prescribed to adolescents and often available in lower doses in over-the-counter products such as acetaminophen and cough syrups (Van Hout and Norman, 2016). Indeed, one study showed that students in grade 12 who had ever used prescription opioids were 33% more likely to misuse opioids by the age of 23, independent of their cannabis, cigarette, and alcohol use (Miech et al., 2015). Additionally, adolescents that misuse prescription opioids were more likely to initiate heroin use, with a younger age of initiation of non-medical prescription opioid use being strongly associated with the subsequent development of opioid use disorder (Cerdá et al., 2015; Schepis and Hakes, 2017). Given the potential for prescription opioid use to increase susceptibility to opioid misuse, it is important that health professionals carefully weigh the benefits and potential detriments that opioids might have on adolescent neurodevelopment when deciding on treatment options.
Co-Use
The co-use of substances is common among adolescents. The National Longitudinal Study of Adolescent to Adult Health found that nearly one in five adolescents report using cigarettes, alcohol, and cannabis, either individually or in combination before the age of 16 (Moss et al., 2014). For clarity, we define co-use as either concurrent, in which multiple substances are used on different occasions, or simultaneous, in which substances are used on the same occasion. During adolescence, it was more common to have used cigarettes, alcohol, and cannabis concurrently than it was to have only used one of the substances individually. The survey also reported the rates of using alcohol and nicotine as 22%; cannabis and nicotine as 21.6%; and alcohol and cannabis as 34.1%. Nearly all research on adolescent substance use (as well as most reviews on the topic) has focused on individual use, but using multiple substances is more common than individual use. This underscores the need for research into the combined effects of substances on adolescent neurodevelopment. Furthermore, the neural correlates of co-use are especially understudied, highlighting the need for future research in this area. The studies to date investigating the effects of co-use are summarized in Supplementary Table S5.
Alcohol and Nicotine
Currently, human studies on the neurobiological changes associated with combined alcohol and nicotine use in adolescence do not exist and the same is true of the effects on cognition. However, significantly increased risk for psychopathology and future substance use has been observed.
Psychopathology
A study looking at substance use and psychiatric comorbidity in subjects aged 13–15 found that regular alcohol and nicotine use had an additive risk for psychiatric disorders, with especially high risk for depressive disorder (Boys et al., 2003). A 2016 study found that alcohol and cigarette consumption increased physical aggression in adolescents aged 14–16 (Matuszka et al., 2016). This increase was significantly greater than that observed in non-concurrent users, showing greater effects in combination than those of the individual substances.
Future Substance Use
Nicotine and alcohol also have additive effects on the risk for future substance use in that concurrent use predicts a greater risk of future substance abuse. A U.S. national survey on alcohol users aged 12–20 found that subjects with a past-year smoking status drank more alcohol on average and had a higher risk for AUD than those that drank equal amounts without smoking (Grucza and Bierut, 2006). These results were the strongest in younger participants. In line with these findings, a longitudinal study found similar results, showing that by age 15, alcohol users that smoked tobacco consumed more alcohol and cannabis (Schmid et al., 2007).
Cannabis and Nicotine
As with combined alcohol and nicotine use, no studies addressing the effects of combined cannabis and nicotine during adolescence on cognition exist. However, some evidence points to increased risk of psychiatric disorders and increased substance use following combined cannabis and nicotine consumption.
Psychopathology
A cross-sectional study looking at combined substance use and psychiatric morbidity in adolescents aged 13–15 found that regular cannabis and nicotine use had an additive risk for psychiatric disorders (Boys et al., 2003). This risk was especially high for the development of depressive disorders and was increased further with the addition of regular alcohol consumption. Longitudinal studies on the effects of combined substance use on psychiatric morbidity are warranted to understand the directionality of this relationship.
Future Substance Use
The combined use of cannabis and nicotine has also been associated with increased substance use. In a cross-sectional study of cigarette smoking 13–17-year-olds, the frequency of cannabis use was associated with increased measures of nicotine addiction (Rubinstein et al., 2014). A cross-sectional fMRI study by Karoly et al. (2015) found that adolescents that used tobacco alone had decreased BOLD response in the nucleus accumbens during a monetary incentive delay task compared to non-using peers. However, these differences were not seen in those using both tobacco and cannabis. Cannabis may be counteracting the effects of tobacco on the nucleus accumbens, possibly explaining why the frequency of cannabis use is associated with increased measures of nicotine addiction in these populations; however, results from longitudinal studies investigating this relationship are required before any hypotheses can be made with confidence.
Cannabis and Alcohol
Cognition
The effect of cannabis and alcohol co-use on cognition seems to largely depend on the cognitive behavior being measured. In a longitudinal population-based analysis of grade 7 students, Morin et al. (2019) found that among co-users, cannabis, but not alcohol, was associated with short-term neurotoxic effects on working memory and inhibitory control as well as long-term effects on perceptual reasoning and delayed memory recall. In another study, hangover symptoms among adolescent heavy drinkers were associated with worse verbal learning and memory but these deficits were not seen in adolescents with similar alcohol consumption and heavy cannabis use (Mahmood et al., 2010). The finding that cannabis may provide some neuroprotective effects against heavy alcohol use is also supported by some imaging studies. Alcohol and cannabis appear to have opposing effects on cortical thickness; among co-users, lifetime cannabis use is associated with decreased cortical thickness, while lifetime alcohol use is associated with increased cortical thickness (Jacobus et al., 2014, 2015). Co-users have also shown differential white matter changes associated with cognition, suggesting a possible neuroadaptation resulting in additive and subtractive responses to substance use (Bava et al., 2010). Other studies have also found these subtractive effects, with alcohol alone affecting white matter integrity, but not both alcohol and cannabis; this further suggests possible neuroprotective effects of cannabis when combined with alcohol (Jacobus et al., 2009; Bava et al., 2013; Infante et al., 2018). However, a longitudinal study that compared users at baseline to their three-year follow-up found similar decreases in white matter integrity for both alcohol and co-users (Jacobus et al., 2013a).
In some psychosocial and cognitive domains, co-use appears to have additive deficits. Co-users are more likely to drive intoxicated (Shillington and Clapp, 2001; Terry-McElrath et al., 2014) and have legal problems (Shillington and Clapp, 2001; Green et al., 2016) than those that use each substance individually, suggesting that co-use may play a role in processes, such as inhibitory control (Galambos et al., 2005). A study by Winward et al. (2014) also found that adolescent users of both substances performed worse on a working memory task. Some neuroimaging studies support these results. A study that compared white matter integrity pre- and post-substance use initiation found that initiation of combined alcohol and cannabis use was associated with decreased white matter integrity in most tracts, including the corpus callosum, corticospinal tract, occipital fasciculus, forceps major, internal capsule, and corona radiata, while the initiation of alcohol-only was not linked to changes in white matter integrity (Jacobus et al., 2013b). Interestingly, in most regions at the baseline time point, youth who would later initiate both alcohol and cannabis use demonstrated FA greater than or equal to youth that initiated alcohol use only. This pre-existing increased white matter integrity could explain the supposed neuroprotective effects of cannabis suggested in other studies (Jacobus et al., 2009; Bava et al., 2013). A later study by the same group also found that alcohol-only initiators and controls have greater cortical thickness before initiation compared to those that initiated both cannabis and alcohol, further suggesting that some neurophysiological differences in these groups precede substance use (Jacobus et al., 2016). Functional MRI studies in co-users have shown dysfunction in frontal and temporal regions, and a decoupled association between hippocampal symmetry and verbal learning (Schweinsburg et al., 2005, 2011; Medina et al., 2007b). An fMRI study found decreased BOLD response in the thalamus, insula, and striatum versus non-users when taking risks (Claus et al., 2018). A cross-sectional DTI study by Bava et al. (2009) showed altered frontoparietal networks and fiber projections within circuits responsible for the modulation of complex sensory, motor, and cognitive processing, namely in fibers of the postcentral gyrus, splenium of the corpus callosum, inferior frontal region, and left superior longitudinal fasciculus. With some studies suggesting cannabis to be neuroprotective, some findings appearing to be the result of an individual substance, and others suggesting co-use to have additive deficits, it is difficult to make any clear conclusions. These conflicting findings are likely the result of significant methodological differences and the potential for different use cases to result in distinct findings (e.g., binge drinking vs. heavy drinking vs. AUD or simultaneous use vs. concurrent use). Thus, further studies are required to make sense of the complicated relationship between alcohol and cannabis co-use during adolescence.
Psychopathology
Compared to alcohol or cannabis use alone, adolescent use of both substances is associated with an increased likelihood of a depressive disorder (Boys et al., 2003). This relationship is also supported by DTI studies; adolescent co-use was associated with decreased FA in the inferior frontal region and left superior longitudinal fasciculus, regions that are similarly altered in adolescent depression (Bava et al., 2009; Cullen et al., 2010).
Future Substance Use
Co-use of cannabis and alcohol appears to potentiate future substance use. Co-users consumed more illicit drugs (Magill et al., 2009; Green et al., 2016; Hayaki et al., 2016; Patrick et al., 2018) than those that used alcohol only. There is also evidence that the simultaneous use of alcohol and cannabis together have greater effects on risk for future substance use-related problems than concurrent use (Brière et al., 2011). Similarly, simultaneous users show increased use of illicit drugs compared with those who concurrently use both substances (Patrick et al., 2018). Unfortunately, without longitudinal studies following adolescents before substance use initiation, it is difficult to infer the directionality of these relationships; therefore, interpretations of these results should be cautiously done.
Limitations
Studies investigating drug-associated alterations to adolescent neurodevelopment have several limitations. Foremost, it is necessary to highlight the difficulty of recruiting younger participants due to issues surrounding parental consent, which may hinder researchers’ ability to match key variables between users and non-user controls, such as age, use of other substances, and underlying psychiatric comorbidities. Emerging evidence also underscores the importance of matching study participants based on genetic variation, as genetic variation in a variety of genes have been associated with increased risk for substance use and associated behaviors, and mediate the effects of adolescent substance use (Hines et al., 2015; Patriquin et al., 2015). Controlling for the use of other substances is also of importance as the contribution of multiple drugs to the observed neurobiological and behavioral differences are difficult to disentangle. Also, to control for the acute effects of substance use, a criterion of abstinence is put in place in many studies; however, withdrawal symptoms may confound the results of studies that employ such a design. Abstinence may also be self-reported by participants in place of objective measures, such as urine analysis, making it difficult to isolate acute drug effects from those that are long-lasting. Furthermore, this review is limited in its scope to the potential effects of nicotine, alcohol, and cannabis given their use prevalence during adolescence and, in the case of opioids, the emerging nature of the opioid epidemic. However, adolescents are known to consume a wide variety of other, albeit relatively under-investigated, drugs such as cocaine, ecstasy, and inhalants (Johnston et al., 2020).
Furthermore, the cross-sectional design of many studies reviewed here limits conclusions on causal directions as there is a possibility that observed neuroimaging and behavioral differences predate the onset of substance use. While we addressed studies that highlighted neurobiological or cognitive factors antedate to substance use here, studies that did not account for underlying between-subject differences may contribute to discrepant findings. In the absence of controlled trials, longitudinal studies are more useful in inferring directional relationships between drug use and neurobiological consequences, especially when baseline measurements are carried out before the onset of substance use. Therefore, more longitudinal analyses, especially studies that are concerned with structural and functional differences within the brain, are needed.
Future Directions
Future prospective longitudinal studies (e.g., the on-going Adolescent Brain Cognitive Development Study of the National Institute on Drug Abuse) looking at the markers of neurobiological function (e.g., brain imaging) before the appearance of substance use could help uncover the mechanistic underpinnings of the long-term consequences of substance use that have been reviewed here. Importantly, as studies indicate compounding detrimental effects of adolescent and prenatal drug exposure on neurological and cognitive outcomes (Jacobsen et al., 2007b,c), not all studies outlined here control for prenatal drug exposure. Future studies would benefit from investigating the impacts of drug exposure at multiple developmental points and how this compares with adolescent-exclusive use.
The neurobiological and cognitive consequences of vaping should also be the target of future studies, as the persisting effects of adolescents using electronic drug delivery devices relative to traditional (i.e., combustible) delivery methods are largely unknown outside of future drug use susceptibility. To date, only one neuroimaging study investigating young adult e-cigarette experimenters and those at risk to try e-cigarettes exists in the literature (Garrison et al., 2018), though the mean age of participants in this study was above our 19-year-old cutoff age. Another study (Chen et al., 2018) using a group of participants with a mean age within our cutoff also investigated neural activity, however, it was in in response to e-cigarette advertisements, and the participants in this study were selected based on combustible smoking status rather than e-cigarette use. Even fewer studies have been conducted on the outcomes of cannabis vaping during adolescence despite increasing trends of vaping cannabis, as well as edible use, both of which have been associated with heavier cannabis use (Patrick et al., 2020). Neurobiological investigations of cannabis and nicotine vaping susceptibility and potential for harm, especially surrounding the transition to combustibles, in this at-risk population, must, therefore, become a priority for future studies.
Moreover, through the reverse translation of findings from clinical populations, the causal underpinnings of the consequences of adolescent substance use can be uncovered. Related to the emerging trends such as the increases in vaping, the availability of animal models of self-administration using electronic devices, combined with pre-clinical neuroimaging methods, will help establish the direct causal consequences of adolescent vaping (Hines et al., 2015; Freels et al., 2020). Lastly, while our review did not address specific therapeutic attempts to reverse the effects of adolescent drug use, future studies can begin to target these changes toward the development of strategies that help to reduce or prevent some of the deleterious effects of adolescent substance exposure, especially if these interventions can be targeted for use in adolescence.
Conclusion
Despite the overall recent downward trends in adolescent substance use the prevalence of adolescent substance use remains a significant public health concern, largely due to the consequences of this use and the especially vulnerable window of neurodevelopment during this period. In this review, we highlighted the neurobiological and behavioral changes that arise from adolescent nicotine, alcohol, cannabis, and opioid use or their combination. Specifically, adolescent drug exposure may contribute to increased risk for the development of cognitive deficits, psychopathology, or subsequent substance use disorders that may be related to the structural and functional changes in the brain. Investigating mechanisms underlying these alterations may provide novel avenues for the development of therapeutics that target these mechanisms to prevent and reduce the harm associated with substance use in adolescence.
Author Contributions
SH drafted the cannabis and alcohol literature review and the “Limitations” section. Besides, SH was responsible for consolidating the review, formatting Supplementary Tables S1–S5, and the formatting of the review. HT drafted the nicotine literature review, the “Future Directions” section, and Figure 1, as well as helped format Supplementary Tables S1–S5. JF drafted the opioid and the co-use literature review and the “Introduction” section. RM contributed to the literature review and Figure 1. JK formulated the idea for the review and guided the research and writing process. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- ACC
Anterior Cingulate Cortex
- ADHD
Attention Deficit Hyperactivity Disorder
- AUD
Alcohol Use Dependence
- BOLD
Blood Oxygenation Level-Dependent
- CUD
Cannabis Use Dependence
- DLPFC
Dorsolateral Prefrontal Cortex
- DTI
Diffusion Tensor Imaging
- EEG
Electroencephalography
- FA
Fractional Anisotropy
- fMRI
Functional Magnetic Resonance Imaging
- GMV
Gray Matter Volume
- IQ
Intelligence Quotient
- MDD
Major Depressive Disorder
- MRI
Magnetic Resonance Imaging
- OFC
Orbitofrontal Cortex
- PFC
Prefrontal Cortex
- RSFC
Resting-State Functional Connectivity.
Footnotes
Funding. This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council award (RGPIN-2019-05121) to JK.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2020.00298/full#supplementary-material.
References
- Abdel-Baki A., Ouellet-Plamondon C., Salvat E., Grar K., Potvin S. (2017). Symptomatic and functional outcomes of substance use disorder persistence 2 years after admission to a first-episode psychosis program. Psychiatry Res. 247, 113–119. 10.1016/j.psychres.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Abdullaev Y., Posner M. I., Nunnally R., Dishion T. J. (2010). Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav. Brain Res. 215, 45–57. 10.1016/j.bbr.2010.06.023 [DOI] [PubMed] [Google Scholar]
- Acheson A., Ray K. L., Hines C. S., Li K., Dawes M. A., Mathias C. W., et al. (2015). Functional activation and effective connectivity differences in adolescent marijuana users performing a simulated gambling task. J. Addict. 2015:783106. 10.1155/2015/783106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J., Case N., Saleem M. M., Auther A. M., Cornblatt B. A., Cadenhead K. S. (2014). Substance use in clinical high risk for psychosis: a review of the literature. Early Interv. Psychiatry 8, 104–112. 10.1111/eip.12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi A., Pearlson G. D., Meda S. A., Dager A., Potenza M. N., Rosen R., et al. (2013). Influence of alcohol use on neural response to Go/No-Go task in college drinkers. Neuropsychopharmacology 38, 2197–2208. 10.1038/npp.2013.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkermans S. E. A., Van Rooij D., Rommelse N., Hartman C. A., Hoekstra P. J., Franke B., et al. (2017). Effect of tobacco smoking on frontal cortical thickness development: a longitudinal study in a mixed cohort of adhd-affected and -unaffected youth. Eur. Neuropsychopharmacol. 27, 1022–1031. 10.1016/j.euroneuro.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albajara Sáenz A., Villemonteix T., Massat I. (2019). Structural and functional neuroimaging in attention-deficit/hyperactivity disorder. Dev. Med. Child Neurol. 61, 399–405. 10.1111/dmcn.14050 [DOI] [PubMed] [Google Scholar]
- Albers A. B., Biener L. (2002). The role of smoking and rebelliousness in the development of depressive symptoms among a cohort of Massachusetts adolescents. Prev. Med. 34, 625–631. 10.1006/pmed.2002.1029 [DOI] [PubMed] [Google Scholar]
- Albertella L., Le Pelley M. E., Copeland J. (2017). Cannabis use in early adolescence is associated with higher negative schizotypy in females. Eur. Psychiatry 45, 235–241. 10.1016/j.eurpsy.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Aloi J., Blair K. S., Crum K. I., Meffert H., White S. F., Tyler P. M., et al. (2018). Adolescents show differential dysfunctions related to Alcohol and Cannabis Use Disorder severity in emotion and executive attention neuro-circuitries. Neuroimage Clin. 19, 782–792. 10.1016/j.nicl.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloi J., Meffert H., White S. F., Blair K. S., Hwang S., Tyler P. M., et al. (2019). Differential dysfunctions related to alcohol and cannabis use disorder symptoms in reward and error-processing neuro-circuitries in adolescents. Dev. Cogn. Neurosci. 36:100618. 10.1016/j.dcn.2019.100618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L., Cannon M., Poulton R., Murray R., Caspi A., Moffitt T. E. (2002). Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 325, 1212–1213. 10.1136/bmj.325.7374.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M., Avants B., Cyckowski L., Cervellione K. L., Roofeh D., Cook P., et al. (2011). Medial temporal structures and memory functions in adolescents with heavy cannabis use. J. Psychiatr. Res. 45, 1055–1066. 10.1016/j.jpsychires.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M., Cervellione K., Cottone J., Ardekani B. A., Sevy S., Kumra S. (2009). Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J. Psychiatr. Res. 43, 189–204. 10.1016/j.jpsychires.2009.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M., Cottone J., Ardekani B. A., Cervellione K., Szeszko P. R., Wu J., et al. (2007). Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch. Gen. Psychiatry 64, 1270–1280. 10.1001/archpsyc.64.11.1270 [DOI] [PubMed] [Google Scholar]
- Assari S., Mistry R., Caldwell C. H., Zimmerman M. A. (2018). Marijuana use and depressive symptoms; gender differences in African American adolescents. Front. Psychol. 9:2135. 10.3389/fpsyg.2018.02135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-Mcgovern J., Rodriguez D., Kassel J. D. (2009). Adolescent smoking and depression: evidence for self-medication and peer smoking mediation. Addiction 104, 1743–1756. 10.1111/j.1360-0443.2009.02617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azagba S., Baskerville N. B., Foley K. (2017). Susceptibility to cigarette smoking among middle and high school e-cigarette users in Canada. Prev. Med. 103, 14–19. 10.1016/j.ypmed.2017.07.017 [DOI] [PubMed] [Google Scholar]
- Banerjee N. (2014). Neurotransmitters in alcoholism: a review of neurobiological and genetic studies. Indian J. Hum. Genet. 20, 20–31. 10.4103/0971-6866.132750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis J. L., Kong G., Leventhal A. M., Liu F., Mayer M., Boley Cruz T., et al. (2018). E-cigarette use and subsequent smoking frequency among adolescents. Pediatrics 142:e20180486. 10.1542/peds.2018-0486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis J. L., Urman R., Berhane K., Unger J. B., Cruz T. B., Pentz M. A., et al. (2016). E-cigarettes and future cigarette use. Pediatrics 138:e20160379. 10.1542/peds.2016-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty G. D., Deary I. J., Macintyre S. (2007). Childhood IQ in relation to risk factors for premature mortality in middle-aged persons: the aberdeen children of the 1950s study. J. Epidemiol. Community Health 61, 241–247. 10.1136/jech.2006.048215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S., Frank L. R., Mcqueeny T., Schweinsburg B. C., Schweinsburg A. D., Tapert S. F. (2009). Altered white matter microstructure in adolescent substance users. Psychiatry Res. 173, 228–237. 10.1016/j.pscychresns.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S., Jacobus J., Mahmood O., Yang T. T., Tapert S. F. (2010). Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 72, 347–354. 10.1016/j.bandc.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S., Jacobus J., Thayer R. E., Tapert S. F. (2013). Longitudinal changes in white matter integrity among adolescent substance users. Alcohol. Clin. Exp. Res. 37, E181–E189. 10.1111/j.1530-0277.2012.01920.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal S. J., Negriff S., Dorn L. D., Pabst S., Schulenberg J. (2014). Longitudinal associations between smoking and depressive symptoms among adolescent girls. Prev. Sci. 15, 506–515. 10.1007/s11121-013-0402-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. P., Collins P. F., Lim K. O., Muetzel R. L., Luciana M. (2015). Longitudinal changes in white matter microstructure after heavy cannabis use. Dev. Cogn. Neurosci. 16, 23–35. 10.1016/j.dcn.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan B., Connolly C. G., Datwani S., Doucet M., Ivanovic J., Morioka R., et al. (2014). Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology 84, 131–137. 10.1016/j.neuropharm.2013.05.027 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Nardone N., Jain S., Dempsey D. A., Addo N., St Helen G., et al. (2018). Comparison of urine 4-(methylnitrosamino)-1–(3)Pyridyl-1-butanol and cotinine for assessment of active and passive smoke exposure in urban adolescents. Cancer Epidemiol. Biomarkers Prev. 27, 254–261. 10.1158/1055-9965.EPI-17-0671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres A. M. (2017). Time is of the essence: a review of electroencephalography (EEG) and event-related brain potentials (ERPs) in language research. Appl. Psychophysiol. Biofeedback 42, 247–255. 10.1007/s10484-017-9371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry K. M., Fetterman J. L., Benjamin E. J., Bhatnagar A., Barrington-Trimis J. L., Leventhal A. M., et al. (2019). Association of electronic cigarette use with subsequent initiation of tobacco cigarettes in US youths. JAMA Netw. Open 2:e187794. 10.1001/jamanetworkopen.2018.7794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y., Yuan K., Guan Y., Cheng J., Zhang Y., Li Y., et al. (2017). Altered resting state functional connectivity of anterior insula in young smokers. Brain Imaging Behav. 11, 155–165. 10.1007/s11682-016-9511-z [DOI] [PubMed] [Google Scholar]
- Blanco C., Florez-Salamanca L., Secades-Villa R., Wang S., Hasin D. S. (2018). Predictors of initiation of nicotine, alcohol, cannabis and cocaine use: results of the national epidemiologic survey on alcohol and related conditions (NESARC). Am. J. Addict. 27, 477–484. 10.1111/ajad.12764 [DOI] [PubMed] [Google Scholar]
- Blumenthal H., Leen-Feldner E. W., Frala J. L., Badour C. L., Ham L. S. (2010). Social anxiety and motives for alcohol use among adolescents. Psychol. Addict. Behav. 24, 529–534. 10.1037/a0019794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden J. M., Fergusson D. M., Horwood L. J. (2010). Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. Br. J. Psychiatry 196, 440–446. 10.1192/bjp.bp.109.065912 [DOI] [PubMed] [Google Scholar]
- Bouziane C., Caan M. W. A., Tamminga H. G. H., Schrantee A., Bottelier M. A., De Ruiter M. B., et al. (2018). ADHD and maturation of brain white matter: a DTI study in medication naive children and adults. Neuroimage Clin. 17, 53–59. 10.1016/j.nicl.2017.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykan R., Messina C. R., Chateau G., Eliscu A., Tolentino J., Goniewicz M. L. (2019). Self-reported use of Tobacco, E-cigarettes and marijuana versus urinary biomarkers. Pediatrics 143:e20183531. 10.1542/peds.2018-3531 [DOI] [PubMed] [Google Scholar]
- Boys A., Farrell M., Taylor C., Marsden J., Goodman R., Brugha T., et al. (2003). Psychiatric morbidity and substance use in young people aged 13–15 years: results from the Child and Adolescent Survey of Mental Health. Br. J. Psychiatry 182, 509–517. 10.1192/bjp.182.6.509 [DOI] [PubMed] [Google Scholar]
- Brière F. N., Fallu J. S., Descheneaux A., Janosz M. (2011). Predictors and consequences of simultaneous alcohol and cannabis use in adolescents. Addict. Behav. 36, 785–788. 10.1016/j.addbeh.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Brook D. W., Brook J. S., Zhang C., Cohen P., Whiteman M. (2002). Drug use and the risk of major depressive disorder, alcohol dependence and substance use disorders. Arch. Gen. Psychiatry 59, 1039–1044. 10.1001/archpsyc.59.11.1039 [DOI] [PubMed] [Google Scholar]
- Brook J. S., Schuster E., Zhang C. (2004). Cigarette smoking and depressive symptoms: a longitudinal study of adolescents and young adults. Psychol. Rep. 95, 159–166. 10.2466/pr0.95.5.159-166 [DOI] [PubMed] [Google Scholar]
- Brown R. A., Lewinsohn P. M., Seeley J. R., Wagner E. F. (1996). Cigarette smoking, major depression and other psychiatric disorders among adolescents. J. Am. Acad. Child Adolesc. Psychiatry 35, 1602–1610. 10.1097/00004583-199612000-00011 [DOI] [PubMed] [Google Scholar]
- Brown S. A., Tapert S. F., Granholm E., Delis D. C. (2000). Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol. Clin. Exp. Res. 24, 164–171. 10.1111/j.1530-0277.2000.tb04586.x [DOI] [PubMed] [Google Scholar]
- Brumback T., Squeglia L. M., Jacobus J., Pulido C., Tapert S. F., Brown S. A. (2015). Adolescent heavy drinkers’ amplified brain responses to alcohol cues decrease over one month of abstinence. Addict. Behav. 46, 45–52. 10.1016/j.addbeh.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback T. Y., Worley M., Nguyen-Louie T. T., Squeglia L. M., Jacobus J., Tapert S. F. (2016). Neural predictors of alcohol use and psychopathology symptoms in adolescents. Dev. Psychopathol. 28, 1209–1216. 10.1017/s0954579416000766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A. F., Blomeyer D., Jennen-Steinmetz C., Schmidt M. H., Esser G., Banaschewski T., et al. (2013). Early smoking onset may promise initial pleasurable sensations and later addiction. Addict. Biol. 18, 947–954. 10.1111/j.1369-1600.2011.00377.x [DOI] [PubMed] [Google Scholar]
- Caldwell L. C., Schweinsburg A. D., Nagel B. J., Barlett V. C., Brown S. A., Tapert S. F. (2005). Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 40, 194–200. 10.1093/alcalc/agh134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J., Lim K. O., Kumra S. (2017). Adverse effects of cannabis on adolescent brain development: a longitudinal study. Cereb. Cortex 27, 1922–1930. 10.1093/cercor/bhw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia C., Cadaveira F., Caamano-Isorna F., Rodriguez-Holguin S., Corral M. (2017a). Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PLoS One 12:e0171393. 10.1371/journal.pone.0171393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia C., Cadaveira F., Lopez-Caneda E., Caamano-Isorna F., Rodriguez Holguin S., Corral M. (2017b). Working memory over a six-year period in young binge drinkers. Alcohol 61, 17–23. 10.1016/j.alcohol.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Cardenas V. A., Greenstein D., Fouche J. P., Ferrett H., Cuzen N., Stein D. J., et al. (2013). Not lesser but Greater fractional anisotropy in adolescents with alcohol use disorders. Neuroimage Clin. 2, 804–809. 10.1016/j.nicl.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caris L., Wagner F. A., Rios-Bedoya C. F., Anthony J. C. (2009). Opportunities to use drugs and stages of drug involvement outside the United States: evidence from the Republic of Chile. Drug Alcohol Depend. 102, 30–34. 10.1016/j.drugalcdep.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy R. N., Meisel M. K., Diguiseppi G., Balestrieri S., Barnett N. P. (2018). Initiation of vaporizing cannabis: individual and social network predictors in a longitudinal study of young adults. Drug Alcohol Depend. 188, 334–340. 10.1016/j.drugalcdep.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N., Pingault J. B., Parent S., Vitaro F., Tremblay R. E., Seguin J. R. (2017). Adolescent cannabis use, change in neurocognitive function and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev. Psychopathol. 29, 1253–1266. 10.1017/s0954579416001280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá M., Santaella J., Marshall B. D., Kim J. H., Martins S. S. (2015). Nonmedical prescription opioid use in childhood and early adolescence predicts transitions to heroin use in young adulthood: a national study. J. Pediatr. 167, 605–612; e601–e602. 10.1016/j.jpeds.2015.04.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaarani B., Kan K. J., Mackey S., Spechler P. A., Potter A., Orr C., et al. (2019). Low smoking exposure, the adolescent brain and the modulating role of CHRNA5 polymorphisms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 672–679. 10.1016/j.bpsc.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar R. (2013). Alcohol and NMDA receptor: current research and future direction. Front. Mol. Neurosci. 6:14. 10.3389/fnmol.2013.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A., Allen N. B., Whittle S., Simmons J. G., Yucel M., Lubman D. I. (2012). Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol. Psychiatry 71, 684–692. 10.1016/j.biopsych.2011.10.029 [DOI] [PubMed] [Google Scholar]
- Cheetham A., Allen N. B., Whittle S., Simmons J., Yucel M., Lubman D. I. (2014). Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology 231, 1731–1742. 10.1007/s00213-014-3483-8 [DOI] [PubMed] [Google Scholar]
- Chen Y., Fowler C. H., Papa V. B., Lepping R. J., Brucks M. G., Fox A. T., et al. (2018). Adolescents’ behavioral and neural responses to e-cigarette advertising. Addict. Biol. 23, 761–771. 10.1111/adb.12510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W. S., Patten C. A., Gillin J. C., Kaplan R. M., Pierce J. P. (1997). Cigarette smoking predicts development of depressive symptoms among U.S. adolescents. Ann. Behav. Med. 19, 42–50. 10.1007/BF02883426 [DOI] [PubMed] [Google Scholar]
- Choudhury S., Blakemore S. J., Charman T. (2006). Social cognitive development during adolescence. Soc. Cogn. Affect. Neurosci. 1, 165–174. 10.1093/scan/nsl024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell J. C., Lopez-Larson M., Yurgelun-Todd D. A. (2010). Altered frontal cortical volume and decision making in adolescent cannabis users. Front. Psychol. 1:225. 10.3389/fpsyg.2010.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Haines M. M., Head J., Klineberg E., Arephin M., Viner R., et al. (2007). Psychological symptoms and physical health and health behaviours in adolescents: a prospective 2-year study in East London. Addiction 102, 126–135. 10.1111/j.1360-0443.2006.01621.x [DOI] [PubMed] [Google Scholar]
- Claus E. D., Feldstein Ewing S. W., Magnan R. E., Montanaro E., Hutchison K. E., Bryan A. D. (2018). Neural mechanisms of risky decision making in adolescents reporting frequent alcohol and/or marijuana use. Brain Imaging Behav. 12, 564–576. 10.1007/s11682-017-9723-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder C. R., Mehta P., Balanda K., Campbell R. T., Mayhew K. P., Stanton W. R., et al. (2001). Identifying trajectories of adolescent smoking: an application of latent growth mixture modeling. Health Psychol. 20, 127–135. 10.1037/0278-6133.20.2.127 [DOI] [PubMed] [Google Scholar]
- Colder C. R., Shyhalla K., Frndak S., Read J. P., Lengua L. J., Hawk L. W., Jr., et al. (2017). The prospective association between internalizing symptoms and adolescent alcohol involvement and the moderating role of age and externalizing symptoms. Alcohol. Clin. Exp. Res. 41, 2185–2196. 10.1111/acer.13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley J., Gow A. J., Starr J. M., Deary I. J. (2012). Smoking, childhood IQ and cognitive function in old age. J. Psychosom. Res. 73, 132–138. 10.1016/j.jpsychores.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Creswell K. G., Chung T., Clark D. B., Martin C. S. (2015). Solitary cannabis use in adolescence as a correlate and predictor of cannabis problems. Drug Alcohol Depend. 156, 120–125. 10.1016/j.drugalcdep.2015.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A. (2016). Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 158, 8–21. 10.1016/j.drugalcdep.2015.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A., Gillespie A. J., Michael P. G., Nagel B. J. (2015). Family history density of alcoholism relates to left nucleus accumbens volume in adolescent girls. J. Stud. Alcohol Drugs 76, 47–56. 10.15288/jsad.2015.76.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K. R., Klimes-Dougan B., Muetzel R., Mueller B. A., Camchongg J., Houri A., et al. (2010). Altered white matter microstructure in adolescencts with major depression: a preliminary study. J. Am. Acad. Child Adolesc. Psychiatry. 49. 10.1097/00004583-201002000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager A. D., Anderson B. M., Rosen R., Khadka S., Sawyer B., Jiantonio-Kelly R. E., et al. (2014). Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction 109, 585–595. 10.1111/add.12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Catley D., Richter K. P., Goggin K., Ellerbeck E. F. (2018). Electronic cigarettes and future marijuana use: a longitudinal study. Pediatrics 141:e20173787. 10.1542/peds.2017-3787 [DOI] [PubMed] [Google Scholar]
- Dao J. M., McQuown S. C., Loughlin S. E., Belluzzi J. D., Leslie F. M. (2011). Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology 36, 1319–1331. 10.1038/npp.2011.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M. D., Clark D. B., Beers S. R., Soloff P. H., Boring A. M., Hall J., et al. (2000). Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry 157, 737–744. 10.1176/appi.ajp.157.5.737 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Narasimhan A., Thatcher D. L., Keshavan M. S., Soloff P., Clark D. B. (2005). Prefrontal cortex, thalamus and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol. Clin. Exp. Res. 29, 1590–1600. 10.1097/01.alc.0000179368.87886.76 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Wang L., Bergman S. R., Yaxley R. H., Hooper S. R., Huettel S. A. (2013). Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend. 133, 134–145. 10.1016/j.drugalcdep.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L., Coffey C., Carlin J. B., Swift W., Moore E., Patton G. C. (2010). Outcomes of occasional cannabis use in adolescence: 10-year follow-up study in Victoria, Australia. Br. J. Psychiatry 196, 290–295. 10.1192/bjp.bp.108.056952 [DOI] [PubMed] [Google Scholar]
- Dierker L., Swendsen J., Rose J., He J., Merikangas K., Tobacco Etiology Research Network (Tern) (2012). Transitions to regular smoking and nicotine dependence in the Adolescent National Comorbidity Survey (NCS-A). Ann. Behav. Med. 43, 394–401. 10.1007/s12160-011-9330-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza J. R., Rigotti N. A., Mcneill A. D., Ockene J. K., Savageau J. A., St Cyr D., et al. (2000). Initial symptoms of nicotine dependence in adolescents. Tob. Control 9, 313–319. 10.1136/tc.9.3.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza J. R., Savageau J. A., Fletcher K., Pbert L., O’Loughlin J., Mcneill A. D., et al. (2007). Susceptibility to nicotine dependence: the development and assessment of nicotine dependence in youth 2 study. Pediatrics 120, e974–e983. 10.1542/peds.2007-0027 [DOI] [PubMed] [Google Scholar]
- DiFranza J. R., Savageau J. A., Rigotti N. A., Fletcher K., Ockene J. K., Mcneill A. D., et al. (2002). Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob. Control 11, 228–235. 10.1136/tc.11.3.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinn W. M., Aycicegi A., Harris C. L. (2004). Cigarette smoking in a student sample: neurocognitive and clinical correlates. Addict. Behav. 29, 107–126. 10.1016/j.addbeh.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Dumontheil I. (2016). Adolescent brain development. Curr. Opin. Behav. Sci. 10, 39–44. Available online at: 10.1016/j.cobeha.2016.04.012 [DOI] [Google Scholar]
- Duncan B., Rees D. I. (2005). Effect of smoking on depressive symptomatology: a reexamination of data from the National Longitudinal Study of Adolescent Health. Am. J. Epidemiol. 162, 461–470. 10.1093/aje/kwi219 [DOI] [PubMed] [Google Scholar]
- Duperrouzel J., Hawes S. W., Lopez-Quintero C., Pacheco-Colon I., Comer J., Gonzalez R. (2018). The association between adolescent cannabis use and anxiety: a parallel process analysis. Addict. Behav. 78, 107–113. 10.1016/j.addbeh.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duperrouzel J. C., Hawes S. W., Lopez-Quintero C., Pacheco-Colon I., Coxe S., Hayes T., et al. (2019). Adolescent cannabis use and its associations with decision-making and episodic memory: preliminary results from a longitudinal study. Neuropsychology 33, 701–710. 10.1037/neu0000538 [DOI] [PubMed] [Google Scholar]
- Edlund M. J., Forman-Hoffman V. L., Winder C. R., Heller D. C., Kroutil L. A., Lipari R. N., et al. (2015). Opioid abuse and depression in adolescents: results from the national survey on drug use and health. Drug Alcohol Depend. 152, 131–138. 10.1016/j.drugalcdep.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Edwards A. C., Heron J., Dick D. M., Hickman M., Lewis G., Macleod J., et al. (2014). Adolescent alcohol use is positively associated with later depression in a population-based U.K. cohort. J. Stud. Alcohol Drugs 75, 758–765. 10.15288/jsad.2014.75.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsey J. W., Crowley M. J., Mencl W. E., Lacadie C. M., Mayes L. C., Potenza M. N. (2016). Relationships between impulsivity, anxiety and risk-taking and the neural correlates of attention in adolescents. Dev. Neuropsychol. 41, 38–58. 10.1080/87565641.2016.1167212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstad F., Evans-Whipp T., Kjeldsen A., Toumbourou J. W., Von Soest T. (2019). Predicting hazardous drinking in late adolescence/young adulthood from early and excessive adolescent drinking-a longitudinal cross-national study of Norwegian and Australian adolescents. BMC Public Health 19:790. 10.1186/s12889-019-7099-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein K. A., Kumra S. (2015). Altered cortical maturation in adolescent cannabis users with and without schizophrenia. Schizophr. Res. 162, 143–152. 10.1016/j.schres.2014.11.029 [DOI] [PubMed] [Google Scholar]
- Everett S. A., Warren C. W., Sharp D., Kann L., Husten C. G., Crossett L. S. (1999). Initiation of cigarette smoking and subsequent smoking behavior among U.S. high school students. Prev. Med. 29, 327–333. 10.1006/pmed.1999.0560 [DOI] [PubMed] [Google Scholar]
- Fasteau M., Mackay D., Smith D. J., Meyer T. D. (2017). Is adolescent alcohol use associated with self-reported hypomanic symptoms in adulthood? Findings from a prospective birth cohort. Psychiatry Res. 255, 232–237. 10.1016/j.psychres.2017.05.034 [DOI] [PubMed] [Google Scholar]
- Fedota J. R., Ding X., Matous A. L., Salmeron B. J., Mckenna M. R., Gu H., et al. (2018). Nicotine abstinence influences the calculation of salience in discrete insular circuits. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 150–159. 10.1016/j.bpsc.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Christiansen P., Cole J., Goudie A. (2007). Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction 102, 579–586. 10.1111/j.1360-0443.2007.01743.x [DOI] [PubMed] [Google Scholar]
- Fiorentini A., Volonteri L. S., Dragogna F., Rovera C., Maffini M., Mauri M. C., et al. (2011). Substance-induced psychoses: a critical review of the literature. Curr. Drug. Abuse Rev. 4, 228–240. 10.2174/1874473711104040228 [DOI] [PubMed] [Google Scholar]
- Fontes M. A., Bolla K. I., Cunha P. J., Almeida P. P., Jungerman F., Laranjeira R. R., et al. (2011). Cannabis use before age 15 and subsequent executive functioning. Br. J. Psychiatry 198, 442–447. 10.1192/bjp.bp.110.077479 [DOI] [PubMed] [Google Scholar]
- Freels T. G., Baxter-Potter L. N., Lugo J. M., Glodosky N. C., Wright H. R., Baglot S. L., et al. (2020). Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. J. Neurosci. 40, 1897–1908. 10.1523/JNEUROSCI.2416-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French L., Gray C., Leonard G., Perron M., Pike G. B., Richer L., et al. (2015). Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. JAMA Psychiatry 72, 1002–1011. 10.1001/jamapsychiatry.2015.1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage S. H., Hickman M., Heron J., Munafo M. R., Lewis G., Macleod J., et al. (2015). Associations of cannabis and cigarette use with depression and anxiety at age 18: findings from the avon longitudinal study of parents and children. PLoS One 10:e0122896. 10.1371/journal.pone.0122896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos N., Leadbeater B., Barker E. (2004). Gender differences in and risk factors for depression in adolescence: a 4-year longitudinal study. Int. J. Behav. Dev. 28, 16–25. 10.1080/01650250344000235 [DOI] [Google Scholar]
- Galambos N. L., Macdonald S. W., Naphtali C., Cohen A. L., De Frias C. M. (2005). Cognitive performance differentiates selected aspects of psychosocial maturity in adolescence. Dev. Neuropsychol. 28, 473–492. 10.1207/s15326942dn2801_2 [DOI] [PubMed] [Google Scholar]
- Galván A., Poldrack R. A., Baker C. M., Mcglennen K. M., London E. D. (2011). Neural correlates of response inhibition and cigarette smoking in late adolescence. Neuropsychopharmacology 36, 970–978. 10.1038/npp.2010.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison K. A., O’Malley S. S., Gueorguieva R., Krishnan-Sarin S. (2018). A fMRI study on the impact of advertising for flavored e-cigarettes on susceptible young adults. Drug Alcohol Depend. 186, 233–241. 10.1016/j.drugalcdep.2018.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass K., Flory K. (2010). Why does ADHD confer risk for cigarette smoking? A review of psychosocial mechanisms. Clin. Child Fam. Psychol. Rev. 13, 291–313. 10.1007/s10567-010-0070-3 [DOI] [PubMed] [Google Scholar]
- Gobbi G., Atkin T., Zytynski T., Wang S., Askari S., Boruff J., et al. (2019). Association of cannabis use in adolescence and risk of depression, anxiety and suicidality in young adulthood: a systematic review and meta-analysis. JAMA Psychiatry 76, 426–434. 10.1001/jamapsychiatry.2018.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliettino A. R., Potenza M. N., Yip S. W. (2016). White matter development and tobacco smoking in young adults: a systematic review with recommendations for future research. Drug Alcohol Depend. 162, 26–33. 10.1016/j.drugalcdep.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos A., Skokou M., Ferentinou E., Gourzis P. (2019). Nicotine consumption during the prodromal phase of schizophrenia-a review of the literature. Neuropsychiatr. Dis. Treat. 15, 2943–2958. 10.2147/NDT.s210199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenson N. I., Leventhal A. M., Stone M. D., Mcconnell R. S., Barrington-Trimis J. L. (2017). Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 171, 1192–1199. 10.1001/jamapediatrics.2017.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. Z., Volkow N. D. (2002). Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159, 1642–1652. 10.1176/appi.ajp.159.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz M. L., Boykan R., Messina C. R., Eliscu A., Tolentino J. (2019). High exposure to nicotine among adolescents who use Juul and other vape pod systems (‘Pods’). Tob. Control 28, 676–677. 10.1136/tobaccocontrol-2018-054565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman E., Capitman J. (2000). Depressive symptoms and cigarette smoking among teens. Pediatrics 106, 748–755. 10.1542/peds.106.4.748 [DOI] [PubMed] [Google Scholar]
- Green K. M., Musci R. J., Johnson R. M., Matson P. A., Reboussin B. A., Ialongo N. S. (2016). Outcomes associated with adolescent marijuana and alcohol use among urban young adults: a prospective study. Addict. Behav. 53, 155–160. 10.1016/j.addbeh.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenman A. P., Oosterlaan J., Rommelse N., Franke B., Roeyers H., Oades R. D., et al. (2013). Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction 108, 1503–1511. 10.1111/add.12188 [DOI] [PubMed] [Google Scholar]
- Grucza R. A., Bierut L. J. (2006). Cigarette smoking and the risk for alcohol use disorders among adolescent drinkers. Alcohol. Clin. Exp. Res. 30, 2046–2054. 10.1111/j.1530-0277.2006.00255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna R. C., Shalvoy A., Cullum C. M., Ivleva E. I., Keshavan M., Pearlson G., et al. (2016). Cognitive function in individuals with psychosis: moderation by adolescent cannabis use. Schizophr. Bull. 42, 1496–1503. 10.1093/schbul/sbw030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. L., Cummins K., Tapert S. F., Brown S. A. (2011). Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol. Addict. Behav. 25, 127–142. 10.1037/a0022350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. L., Winward J. L., Schweinsburg A. D., Medina K. L., Brown S. A., Tapert S. F. (2010). Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict. Behav. 35, 970–976. 10.1016/j.addbeh.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M. A., Sellman J. D., Porter R. J., Frampton C. M. (2007). The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 26, 309–319. 10.1080/09595230701247772 [DOI] [PubMed] [Google Scholar]
- Hayaki J., Anderson B. J., Stein M. D. (2016). Dual cannabis and alcohol use disorders in young adults: problems magnified. Subst. Abus. 37, 579–583. 10.1080/08897077.2016.1176613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner M. P., Angst J., Ajdacic-Gross V., Rössler W. (2020). Cannabis use during adolescence and the occurrence of depression, suicidality and anxiety disorder across adulthood: findings from a longitudinal cohort study over 30 years. J. Affect. Disord. 272, 98–103. 10.1016/j.jad.2020.03.126 [DOI] [PubMed] [Google Scholar]
- Hiemstra M., Nelemans S. A., Branje S., Van Eijk K. R., Hottenga J. J., Vinkers C. H., et al. (2018). Genetic vulnerability to schizophrenia is associated with cannabis use patterns during adolescence. Drug Alcohol Depend. 190, 143–150. 10.1016/j.drugalcdep.2018.05.024 [DOI] [PubMed] [Google Scholar]
- Hines L. A., Morley K. I., Mackie C., Lynskey M. (2015). Genetic and environmental interplay in adolescent substance use disorders. Curr. Addict. Rep. 2, 122–129. 10.1007/s40429-015-0049-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. C., Griesler P., Schaffran C., Kandel D. (2011). Risk and protective factors for nicotine dependence in adolescence. J. Child Psychol. Psychiatry 52, 1063–1072. 10.1111/j.1469-7610.2010.02362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilomäki R., Riala K., Hakko H., Lappalainen J., Ollinen T., Rasanen P., et al. (2008). Temporal association of onset of daily smoking with adolescent substance use and psychiatric morbidity. Eur. Psychiatry 23, 85–91. 10.1016/j.eurpsy.2007.10.009 [DOI] [PubMed] [Google Scholar]
- Infante M. A., Courtney K. E., Castro N., Squeglia L. M., Jacobus J. (2018). Adolescent Brain surface area Pre- and post-cannabis and alcohol initiation. J. Stud. Alcohol Drugs 79, 835–843. 10.15288/jsad.2018.79.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante M. A., Nguyen-Louie T. T., Worley M., Courtney K. E., Coronado C., Jacobus J. (2020). Neuropsychological trajectories associated with adolescent alcohol and cannabis use: a prospective 14-year study. J. Int. Neuropsychol. Soc. 26, 480–491. 10.1017/S1355617719001395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A., Green B., Erythropel H. C., Kong G., Cavallo D. A., Eid T., et al. (2020). Influence of menthol and green apple e-liquids containing different nicotine concentrations among youth e-cigarette users. Exp. Clin. Psychopharmacol. 10.1037/pha0000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson N. J., Isen J. D., Khoddam R., Irons D., Tuvblad C., Iacono W. G., et al. (2016). Impact of adolescent marijuana use on intelligence: results from two longitudinal twin studies. Proc. Natl. Acad. Sci. U S A 113, E500–E508. 10.1073/pnas.1516648113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L. K., Krystal J. H., Mencl W. E., Westerveld M., Frost S. J., Pugh K. R. (2005). Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol. Psychiatry 57, 56–66. 10.1016/j.biopsych.2004.10.022 [DOI] [PubMed] [Google Scholar]
- Jacobsen L. K., Mencl W. E., Constable R. T., Westerveld M., Pugh K. R. (2007a). Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology 193, 557–566. 10.1007/s00213-007-0797-9 [DOI] [PubMed] [Google Scholar]
- Jacobsen L. K., Picciotto M. R., Heath C. J., Frost S. J., Tsou K. A., Dwan R. A., et al. (2007b). Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J. Neurosci. 27, 13491–13498. 10.1523/JNEUROSCI.2402-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L. K., Slotkin T. A., Mencl W. E., Frost S. J., Pugh K. R. (2007c). Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology 32, 2453–2464. 10.1038/sj.npp.1301398 [DOI] [PubMed] [Google Scholar]
- Jacobsen L. K., Mencl W. E., Westerveld M., Pugh K. R. (2004). Impact of cannabis use on brain function in adolescents. Ann. N Y Acad. Sci. 1021, 384–390. 10.1196/annals.1308.053 [DOI] [PubMed] [Google Scholar]
- Jacobus J., Castro N., Squeglia L. M., Meloy M. J., Brumback T., Huestis M. A., et al. (2016). Adolescent cortical thickness pre- and post marijuana and alcohol initiation. Neurotoxicol. Teratol. 57, 20–29. 10.1016/j.ntt.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Mcqueeny T., Bava S., Schweinsburg B. C., Frank L. R., Yang T. T., et al. (2009). White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol. Teratol. 31, 349–355. 10.1016/j.ntt.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Squeglia L. M., Bava S., Tapert S. F. (2013a). White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. 214, 374–381. 10.1016/j.pscychresns.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Squeglia L. M., Infante M. A., Bava S., Tapert S. F. (2013b). White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 3, 396–414. 10.3390/brainsci3010396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Squeglia L. M., Meruelo A. D., Castro N., Brumback T., Giedd J. N., et al. (2015). Cortical thickness in adolescent marijuana and alcohol users: a three-year prospective study from adolescence to young adulthood. Dev. Cogn. Neurosci. 16, 101–109. 10.1016/j.dcn.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Squeglia L. M., Sorg S. F., Nguyen-Louie T. T., Tapert S. F. (2014). Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J. Stud. Alcohol Drugs 75, 729–743. 10.15288/jsad.2014.75.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G., Block R. I., Luijten M., Ramsey N. F. (2010). Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry 49, 561–572, 572.e1–572.e3. 10.1016/j.jaac.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G., Block R. I., Luijten M., Ramsey N. F. (2013). Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: a cross-sectional multicenter fMRI study. J. Psychoactive Drugs 45, 156–167. 10.1080/02791072.2013.785837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M., Does A. J., Penninx B. W., Cuijpers P. (2011). Age at smoking onset and the onset of depression and anxiety disorders. Nicotine Tob. Res. 13, 809–819. 10.1093/ntr/ntr077 [DOI] [PubMed] [Google Scholar]
- Jaworska N., MacQueen G. (2015). Adolescence as a unique developmental period. J. Psychiatry Neurosci. 40, 291–293. 10.1503/jpn.150268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L. Z., Rangan A., Mehlsen J., Andersen L. B., Larsen S. C., Heitmann B. L. (2017). Association between use of cannabis in adolescence and weight change into midlife. PLoS One 12:e0168897. 10.1371/journal.pone.0168897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. A., Zatorre R. J. (2006). Neural substrates for dividing and focusing attention between simultaneous auditory and visual events. NeuroImage 31, 1673–1681. 10.1016/j.neuroimage.2006.02.026 [DOI] [PubMed] [Google Scholar]
- Johnston L. D., Miech R. A., O’Malley P. M., Bachman J. G., Shulenberg J. E., Patrick M. E. (2020). Monitoring the Future National Survey Results on Drug Use 1975–2019: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research. [Google Scholar]
- Jones S. A., Cservenka A., Nagel B. J. (2016). Binge drinking impacts dorsal striatal response during decision making in adolescents. NeuroImage 129, 378–388. 10.1016/j.neuroimage.2016.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. J., Gage S. H., Heron J., Hickman M., Lewis G., Munafo M. R., et al. (2018). Association of combined patterns of tobacco and cannabis use in adolescence with psychotic experiences. JAMA Psychiatry 75, 240–246. 10.1001/jamapsychiatry.2017.4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. A., Steele J. S., Nagel B. J. (2017). Binge drinking and family history of alcoholism are associated with an altered developmental trajectory of impulsive choice across adolescence. Addiction 112, 1184–1192. 10.1111/add.13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J., Kim S. H., Wu L. (2019). Tobacco risk information and comparative risk assessment of E-cigarettes Vs. cigarettes: application of the reinforcing spirals model. J. Health Commun. 24, 422–431. 10.1080/10810730.2019.1630526 [DOI] [PubMed] [Google Scholar]
- Jurk S., Mennigen E., Goschke T., Smolka M. N. (2018). Low-level alcohol consumption during adolescence and its impact on cognitive control development. Addict. Biol. 23, 313–326. 10.1111/adb.12467 [DOI] [PubMed] [Google Scholar]
- Kaasbøll C., Hagen R., Gråwe R. W. (2018). Population-based associations among cannabis use, anxiety and depression in norwegian adolescents. J. Child Adol. Subst. Abus. 27, 238–243. 10.1080/1067828x.2018.1462281 [DOI] [Google Scholar]
- Kaplow J. B., Curran P. J., Angold A., Costello E. J. (2001). The prospective relation between dimensions of anxiety and the initiation of adolescent alcohol use. J. Clin. Child Psychol. 30, 316–326. 10.1207/s15374424jccp3003_4 [DOI] [PubMed] [Google Scholar]
- Karoly H. C., Bryan A. D., Weiland B. J., Mayer A., Dodd A., Feldstein Ewing S. W. (2015). Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev. Cogn. Neurosci. 16, 5–15. 10.1016/j.dcn.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz G., Kunyvsky Y., Hornik-Lurie T., Raskin S., Abramowitz M. Z. (2016). Cannabis and alcohol abuse among first psychotic episode inpatients. Isr. J. Psychiatry Relat. Sci. 53, 10–15. [PubMed] [Google Scholar]
- Khokhar J. Y., Dwiel L. L., Henricks A. M., Doucette W. T., Green A. I. (2018). The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr. Res. 194, 78–85. 10.1016/j.schres.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A., Romer D., Betancourt L. M., Brodsky N. L., Giannetta J. M., Hurt H. (2013). Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addiction 108, 506–515. 10.1111/add.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H., Sterk C. E., Elifson K. W. (2013). Initial smoking sxperiences and current smoking behaviors and perceptions among current smokers. J. Addict. 2013:491797. 10.1155/2013/491797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Mascharek A., Banaschewski T., Bodke A., Bromberg U., Buchel C., et al. (2019). Predicting development of adolescent drinking behaviour from whole brain structure at 14 years of age. Elife 8:e44056. 10.7554/eLife.44056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S., Robinson P., Tambyraja R., Jensen D., Schimunek C., Houri A., et al. (2012). Parietal lobe volume deficits in adolescents with schizophrenia and adolescents with cannabis use disorders. J. Am. Acad. Child Adolesc. Psychiatry 51, 171–180. 10.1016/j.jaac.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Lai C. H., Wu Y. T. (2014). Alterations in white matter micro-integrity of the superior longitudinal fasciculus and anterior thalamic radiation of young adult patients with depression. Psychol. Med. 44, 2825–2832. 10.1017/s0033291714000440 [DOI] [PubMed] [Google Scholar]
- Lane S. D., Cherek D. R., Pietras C. J., Steinberg J. L. (2005). Performance of heavy marijuana-smoking adolescents on a laboratory measure of motivation. Addict. Behav. 30, 815–828. 10.1016/j.addbeh.2004.08.026 [DOI] [PubMed] [Google Scholar]
- Lane S. D., Cherek D. R., Tcheremissine O. V., Steinberg J. L., Sharon J. L. (2007). Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addict. Behav. 32, 977–990. 10.1016/j.addbeh.2006.07.007 [DOI] [PubMed] [Google Scholar]
- Lanza S. T., Vasilenko S. A. (2015). New methods shed light on age of onset as a risk factor for nicotine dependence. Addict. Behav. 50, 161–164. 10.1016/j.addbeh.2015.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Lim Y., Wiederhold B. K., Graham S. J. (2005). A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl. Psychophysiol. Biofeedback 30, 195–204. 10.1007/s10484-005-6377-z [DOI] [PubMed] [Google Scholar]
- Leventhal A. M., Strong D. R., Kirkpatrick M. G., Unger J. B., Sussman S., Riggs N. R., et al. (2015). Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 314, 700–707. 10.1001/jama.2015.8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P. M., Rohde P., Brown R. A. (1999). Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction 94, 913–921. 10.1046/j.1360-0443.1999.94691313.x [DOI] [PubMed] [Google Scholar]
- Li Y., Yuan K., Cai C., Feng D., Yin J., Bi Y., et al. (2015). Reduced frontal cortical thickness and increased caudate volume within fronto-striatal circuits in young adult smokers. Drug Alcohol Depend. 151, 211–219. 10.1016/j.drugalcdep.2015.03.023 [DOI] [PubMed] [Google Scholar]
- Li Y., Yuan K., Guan Y., Cheng J., Bi Y., Shi S., et al. (2017). The implication of salience network abnormalities in young male adult smokers. Brain Imaging Behav. 11, 943–953. 10.1007/s11682-016-9568-8 [DOI] [PubMed] [Google Scholar]
- Lichenstein S. D., Musselman S., Shaw D. S., Sitnick S., Forbes E. E. (2017). Nucleus accumbens functional connectivity at age 20 is associated with trajectory of adolescent cannabis use and predicts psychosocial functioning in young adulthood. Addiction 112, 1961–1970. 10.1111/add.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K. M., Sher K. J., Conway K. P., Gonzalez R., Feldstein Ewing S. W., Nixon S. J., et al. (2018). Adolescent brain cognitive development (ABCD) study: overview of substance use assessment methods. Dev. Cogn. Neurosci. 32, 80–96. 10.1016/j.dcn.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K. M., Thayer R., Squeglia L. M., Mcqueeny T. M., Tapert S. F. (2013). Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 211, 17–23. 10.1016/j.pscychresns.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman D. I., Cheetham A., Yücel M. (2015). Cannabis and adolescent brain development. Pharmacol. Ther. 148, 1–16. 10.1016/j.pharmthera.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Luciana M., Collins P. F., Muetzel R. L., Lim K. O. (2013). Effects of alcohol use initiation on brain structure in typically developing adolescents. Am. J. Drug Alcohol Abuse 39, 345–355. 10.3109/00952990.2013.837057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill M., Barnett N. P., Apodaca T. R., Rohsenow D. J., Monti P. M. (2009). The role of marijuana use in brief motivational intervention with young adult drinkers treated in an emergency department. J. Stud. Alcohol Drugs 70, 409–413. 10.15288/jsad.2009.70.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahedy L., Field M., Gage S., Hammerton G., Heron J., Hickman M., et al. (2018). Alcohol use in adolescence and later working memory: findings from a large population-based birth cohort. Alcohol Alcohol. 53:501. 10.1093/alcalc/agy005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood O. M., Jacobus J., Bava S., Scarlett A., Tapert S. F. (2010). Learning and memory performances in adolescent users of alcohol and marijuana: interactive effects. J. Stud. Alcohol Drugs 71, 885–894. 10.15288/jsad.2010.71.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard T. T., Ashenhurst J. R., Harden K. P., Fromme K. (2018). GABRA2, alcohol and illicit drug use: an event-level model of genetic risk for polysubstance use. J. Abnorm. Psychol. 127, 190–201. 10.1037/abn0000333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y., Betts J., Farmer S. L., Lukas S. E. (2018). Early onset cigarette smokers exhibit greater P300 reactivity to smoking-related stimuli and report greater craving. Brain Res. 1687, 173–184. 10.1016/j.brainres.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. A., Kosterman R., Haggerty K. P., Hawkins J. D., Redmond C., Spoth R. L., et al. (2008). Dimensions of adolescent alcohol involvement as predictors of young-adult major depression. J. Stud. Alcohol Drugs 69, 275–285. 10.15288/jsad.2008.69.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszka B., Bácskai E., Czobor P., Gerevich J. (2016). Physical aggression and concurrent alcohol and tobacco use among adolescents. Int. J. Ment. Health Addict. 15, 90–99. 10.1007/s11469-015-9630-6 [DOI] [Google Scholar]
- McGrath J. J., Alati R., Clavarino A., Williams G. M., Bor W., Najman J. M., et al. (2016). Age at first tobacco use and risk of subsequent psychosis-related outcomes: a birth cohort study. Aust. N Z J. Psychiatry 50, 577–583. 10.1177/0004867415587341 [DOI] [PubMed] [Google Scholar]
- McNeill A. D., West R. J., Jarvis M., Jackson P., Bryant A. (1986). Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology 90, 533–536. 10.1007/bf00174074 [DOI] [PubMed] [Google Scholar]
- McQueeny T., Padula C. B., Price J., Medina K. L., Logan P., Tapert S. F. (2011). Gender effects on amygdala morphometry in adolescent marijuana users. Behav. Brain Res. 224, 128–134. 10.1016/j.bbr.2011.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T., Schweinsburg B. C., Schweinsburg A. D., Jacobus J., Bava S., Frank L. R., et al. (2009). Altered white matter integrity in adolescent binge drinkers. Alcohol. Clin. Exp. Res. 33, 1278–1285. 10.1111/j.1530-0277.2009.00953.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda S. A., Dager A. D., Hawkins K. A., Tennen H., Raskin S., Wood R. M., et al. (2017). Heavy drinking in college students is associated with accelerated gray matter volumetric decline over a 2 year period. Front. Behav. Neurosci. 11:176. 10.3389/fnbeh.2017.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K. L., Hanson K. L., Schweinsburg A. D., Cohen-Zion M., Nagel B. J., Tapert S. F. (2007a). Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J. Int. Neuropsychol. Soc. 13, 807–820. 10.1017/s1355617707071032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K. L., Schweinsburg A. D., Cohen-Zion M., Nagel B. J., Tapert S. F. (2007b). Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol. Teratol. 29, 141–152. 10.1016/j.ntt.2006.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K. L., McQueeny T., Nagel B. J., Hanson K. L., Schweinsburg A. D., Tapert S. F. (2008). Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol. Clin. Exp. Res. 32, 386–394. 10.1111/j.1530-0277.2007.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K. L., McQueeny T., Nagel B. J., Hanson K. L., Yang T. T., Tapert S. F. (2009). Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict. Biol. 14, 457–468. 10.1111/j.1369-1600.2009.00166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K. L., Nagel B. J., Tapert S. F. (2010). Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 182, 152–159. 10.1016/j.pscychresns.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M. H., Caspi A., Ambler A., Harrington H., Houts R., Keefe R. S., et al. (2012). Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. U S A 109, E2657–E2664. 10.1073/pnas.1206820109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M. H., Caspi A., Danese A., Fisher H. L., Houts R., Arseneault L., et al. (2018). Associations between adolescent cannabis use and neuropsychological decline: a longitudinal co-twin control study. Addiction 113, 257–265. 10.1111/add.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M. H., Schriber R. A., Beardslee J., Hanson J., Pardini D. (2019). Associations between adolescent cannabis use frequency and adult brain structure: a prospective study of boys followed to adulthood. Drug Alcohol Depend. 202, 191–199. 10.1016/j.drugalcdep.2019.05.012 [DOI] [PubMed] [Google Scholar]
- Melaugh McAteer A., Curran D., Hanna D. (2015). Alcohol attention bias in adolescent social drinkers: an eye tracking study. Psychopharmacology 232, 3183–3191. 10.1007/s00213-015-3969-z [DOI] [PubMed] [Google Scholar]
- Miech R., Johnston L., O’Malley P. M., Keyes K. M., Heard K. (2015). Prescription opioids in adolescence and future opioid misuse. Pediatrics 136, e1169–e1177. 10.1542/peds.2015-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R. A., Patrick M. E., O’Malley P. M., Johnson L. D. (2017). E-cigarette use as a predictor of cigarette smoking: results from a 1-year follow-up of a national sample of 12th grade students. Tob. Control 26, e106–e111. 10.1136/tobaccocontrol-2016-053291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K. L., Teesson M., Darke S., Ross J., Lynskey M. (2004). Young people with heroin dependence: findings from the Australian Treatment Outcome Study (ATOS). J. Subst. Abuse Treat. 27, 67–73. 10.1016/j.jsat.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Mokrysz C., Landy R., Gage S. H., Munafo M. R., Roiser J. P., Curran H. V. (2016). Are IQ and educational outcomes in teenagers related to their cannabis use? A prospective cohort study. J. Psychopharmacol. 30, 159–168. 10.1177/0269881115622241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S. S., Mo B. C., Basham R. (2010). Adolescent depression and future smoking behavior: a prospective study. Child Adolesc. Soc. Work J. 27, 405–422. 10.1007/s10560-010-0212-y [DOI] [Google Scholar]
- Morales A. M., Jones S. A., Ehlers A., Lavine J. B., Nagel B. J. (2018). Ventral striatal response during decision making involving risk and reward is associated with future binge drinking in adolescents. Neuropsychopharmacology 43, 1884–1890. 10.1038/s41386-018-0087-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin J.-F. G., Afzali M. H., Bourque J., Stewart S. H., Séguin J. R., O’Leary-Barrett M., et al. (2019). A population-based analysis of the relationship between substance use and adolescent cognitive development. Am. J. Psychiatry 176, 98–106. 10.1176/appi.ajp.2018.18020202 [DOI] [PubMed] [Google Scholar]
- Morrell H. E., Cohen L. M., McChargue D. E. (2010). Depression vulnerability predicts cigarette smoking among college students: gender and negative reinforcement expectancies as contributing factors. Addict. Behav. 35, 607–611. 10.1016/j.addbeh.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss H. B., Chen C. M., Yi H. Y. (2014). Early adolescent patterns of alcohol, cigarettes and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 136, 51–62. 10.1016/j.drugalcdep.2013.12.011 [DOI] [PubMed] [Google Scholar]
- Munafò M. R., Hitsman B., Rende R., Metcalfe C., Niaura R. (2008). Effects of progression to cigarette smoking on depressed mood in adolescents: evidence from the national longitudinal study of adolescent health. Addiction 103, 162–171. 10.1111/j.1360-0443.2007.02052.x [DOI] [PubMed] [Google Scholar]
- Mustonen A., Ahokas T., Nordström T., Murray G. K., Mäki P., Jääskeläinen E., et al. (2018). Smokin’ hot: adolescent smoking and the risk of psychosis. Acta Psychiatr. Scand. 138, 5–14. 10.1111/acps.12863 [DOI] [PubMed] [Google Scholar]
- Myles N., Newall H., Compton M. T., Curtis J., Nielssen O., Large M. (2012a). The age at onset of psychosis and tobacco use: a systematic meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 47, 1243–1250. 10.1007/s00127-011-0431-3 [DOI] [PubMed] [Google Scholar]
- Myles N., Newall H. D., Curtis J., Nielssen O., Shiers D., Large M. (2012b). Tobacco use before, at, and after first-episode psychosis: a systematic meta-analysis. J. Clin. Psychiatry 73, 468–475. 10.4088/jcp.11r07222 [DOI] [PubMed] [Google Scholar]
- Nagel B. J., Schweinsburg A. D., Phan V., Tapert S. F. (2005). Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 139, 181–190. 10.1016/j.pscychresns.2005.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health (2016). E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion (Us) Office on Smoking and Health. [PubMed] [Google Scholar]
- Needham B. L. (2007). Gender differences in trajectories of depressive symptomatology and substance use during the transition from adolescence to young adulthood. Soc. Sci. Med. 65, 1166–1179. 10.1016/j.socscimed.2007.04.037 [DOI] [PubMed] [Google Scholar]
- Nestor L. J., Behan B., Suckling J., Garavan H. (2019). Cannabis-dependent adolescents show differences in global reward-associated network topology: a functional connectomics approach. Addict. Biol. 25:e12752. 10.1111/adb.12752 [DOI] [PubMed] [Google Scholar]
- Nguyen-Louie T. T., Matt G. E., Jacobus J., Li I., Cota C., Castro N., et al. (2017). Earlier alcohol use onset predicts poorer neuropsychological functioning in young adults. Alcohol. Clin. Exp. Res. 41, 2082–2092. 10.1111/acer.13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie T. T., Tracas A., Squeglia L. M., Matt G. E., Eberson-Shumate S., Tapert S. F. (2016). Learning and memory in adolescent moderate, binge, and extreme-binge drinkers. Alcohol. Clin. Exp. Res. 40, 1895–1904. 10.1111/acer.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh S., Afzali M. H., Boers E., Conrod P. J. (2020). Cognitive function impairments linked to alcohol and cannabis use during adolescence: a study of gender differences. Front. Hum. Neurosci. 14:95. 10.3389/fnhum.2020.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A. L., Pulido C., Squeglia L. M., Spadoni A. D., Paulus M. P., Tapert S. F. (2011). Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 119, 216–223. 10.1016/j.drugalcdep.2011.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell L. E., Bruijnzeel A. W., Ghozland S., Markou A., Koob G. F. (2004). Nicotine withdrawal in adolescent and adult rats. Ann. N Y Acad. Sci. 1021, 167–174. 10.1196/annals.1308.022 [DOI] [PubMed] [Google Scholar]
- Orr C., Spechler P., Cao Z., Albaugh M., Chaarani B., Mackey S., et al. (2019). Grey matter volume differences associated with extremely low levels of cannabis use in adolescence. J. Neurosci. 39, 1817–1827. 10.1523/JNEUROSCI.3375-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula C. B., Schweinsburg A. D., Tapert S. F. (2007). Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol. Addict. Behav. 21, 478–487. 10.1037/0893-164x.21.4.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampati S., Buu A., Hu Y. H., Mendes De Leon C. F., Lin H. C. (2018). Effects of alcohol and cigarette use on the initiation, reinitiation and persistence of cannabis use from adolescence to emerging adulthood. Addict. Behav. 79, 144–150. 10.1016/j.addbeh.2017.12.019 [DOI] [PubMed] [Google Scholar]
- Parada M., Corral M., Mota N., Crego A., Rodriguez Holguin S., Cadaveira F. (2012). Executive functioning and alcohol binge drinking in university students. Addict. Behav. 37, 167–172. 10.1016/j.addbeh.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Parker M. A., Villanti A. C., Quisenberry A. J., Stanton C. A., Doogan N. J., Redner R., et al. (2018). Tobacco product harm perceptions and new use. Pediatrics 142:e20181505. 10.1542/peds.2018-1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish K. H., Atherton O. E., Quintana A., Conger R. D., Robins R. W. (2016). Reciprocal relations between internalizing symptoms and frequency of alcohol use: findings from a longitudinal study of Mexican-origin youth. Psychol. Addict. Behav. 30, 203–208. 10.1037/adb0000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick M. E., Kloska D. D., Terry-Mcelrath Y. M., Lee C. M., O’Malley P. M., Johnston L. D. (2018). Patterns of simultaneous and concurrent alcohol and marijuana use among adolescents. Am. J. Drug Alcohol Abuse 44, 441–451. 10.1080/00952990.2017.1402335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick M. E., Miech R. A., Kloska D. D., Wagner A. C., Johnston L. D. (2020). Trends in marijuana vaping and edible consumption From 2015 to 2018 among adolescents in the US. JAMA Pediatr. 6:e200175. 10.1001/jamapediatrics.2020.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin M. A., Bauer I. E., Soares J. C., Graham D. P., Nielsen D. A. (2015). Addiction pharmacogenetics: a systematic review of the genetic variation of the dopaminergic system. Psychiatr. Genet. 25, 181–193. 10.1097/ypg.0000000000000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R. G. (1997). Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 74, 129–180. 10.1016/s0163-7258(97)82001-3 [DOI] [PubMed] [Google Scholar]
- Peters S., Peper J. S., Van Duijvenvoorde A. C. K., Braams B. R., Crone E. A. (2017). Amygdala-orbitofrontal connectivity predicts alcohol use two years later: a longitudinal neuroimaging study on alcohol use in adolescence. Dev. Sci. 20:e12448. 10.1111/desc.12448 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Kwon D., Brumback T., Thompson W. K., Cummins K., Tapert S. F., et al. (2018). Altered brain developmental trajectories in adolescents after initiating drinking. Am. J. Psychiatry 175, 370–380. 10.1176/appi.ajp.2017.17040469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsillou E., Bresnehan S. M., Kagarakis E. A., Wijoyo S. J., Liang J., Hung A., et al. (2020). The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Mol. Biol. Rep. 47, 753–770. 10.1007/s11033-019-05129-3 [DOI] [PubMed] [Google Scholar]
- Pope H. G., Jr., Gruber A. J., Hudson J. I., Cohane G., Huestis M. A., Yurgelun-Todd D. (2003). Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 69, 303–310. 10.1016/s0376-8716(02)00334-4 [DOI] [PubMed] [Google Scholar]
- Raud L., Westerhausen R., Dooley N., Huster R. J. (2020). Differences in unity: the go/no-go and stop signal tasks rely on different mechanisms. NeuroImage 210:116582. 10.1016/j.neuroimage.2020.116582 [DOI] [PubMed] [Google Scholar]
- Renard J., Krebs M. O., Le Pen G., Jay T. M. (2014). Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci. 8:361. 10.3389/fnins.2014.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendu F., Peoc’h K., Berlin I., Thomas D., Launay J. M. (2011). Smoking related diseases: the central role of monoamine oxidase. Int. J. Environ. Res. Public Health 8, 136–147. 10.3390/ijerph8010136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto P. B., Caldwell C. H., Zimmerman M. A. (2005). A longitudinal study of the relationship between depressive symptoms and cigarette use among African American adolescents. Health Psychol. 24, 209–219. 10.1037/0278-6133.24.2.209 [DOI] [PubMed] [Google Scholar]
- Riggs N. R., Chou C. P., Li C., Pentz M. A. (2007). Adolescent to emerging adulthood smoking trajectories: when do smoking trajectories diverge and do they predict early adulthood nicotine dependence? Nicotine Tob. Res. 9, 1147–1154. 10.1080/14622200701648359 [DOI] [PubMed] [Google Scholar]
- Rodriguez D., Moss H. B., Audrain-Mcgovern J. (2005). Developmental heterogeneity in adolescent depressive symptoms: associations with smoking behavior. Psychosom. Med. 67, 200–210. 10.1097/01.psy.0000156929.83810.01 [DOI] [PubMed] [Google Scholar]
- Rojas N. L., Killen J. D., Haydel K. F., Robinson T. N. (1998). Nicotine dependence among adolescent smokers. Arch. Pediatr. Adolesc. Med. 152, 151–156. 10.1001/archpedi.152.2.151 [DOI] [PubMed] [Google Scholar]
- Romer D. (2010). Adolescent risk taking, impulsivity, and brain development: implications for prevention. Dev. Psychobiol. 52, 263–276. 10.1002/dev.20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H., Zhou Y., Luo Q., Robert G. H., Desrivieres S., Quinlan E. B., et al. (2019). Adolescent binge drinking disrupts normal trajectories of brain functional organization and personality maturation. Neuroimage Clin. 22:101804. 10.1016/j.nicl.2019.101804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M. L., Luks T. L., Dryden W. Y., Rait M. A., Simpson G. V. (2011a). Adolescent smokers show decreased brain responses to pleasurable food images compared with nonsmokers. Nicotine Tob. Res. 13, 751–755. 10.1093/ntr/ntr046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M. L., Luks T. L., Moscicki A. B., Dryden W., Rait M. A., Simpson G. V. (2011b). Smoking-related cue-induced brain activation in adolescent light smokers. J. Adolesc Health 48, 7–12. 10.1016/j.jadohealth.2010.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M. L., Rait M. A., Prochaska J. J. (2014). Frequent marijuana use is associated with greater nicotine addiction in adolescent smokers. Drug Alcohol Depend. 141, 159–162. 10.1016/j.drugalcdep.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S. J., Nestler E. J. (2013). The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–625. 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis T. S., Hakes J. K. (2017). Age of initiation, psychopathology, and other substance use are associated with time to use disorder diagnosis in persons using opioids nonmedically. Subst. Abus. 38, 407–413. 10.1080/08897077.2017.1356791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleider J. L., Ye F., Wang F., Hipwell A. E., Chung T., Sartor C. E. (2019). Longitudinal reciprocal associations between anxiety, depression, and alcohol use in adolescent girls. Alcohol. Clin. Exp. Res. 43, 98–107. 10.1111/acer.13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B., Hohm E., Blomeyer D., Zimmermann U. S., Schmidt M. H., Esser G., et al. (2007). Concurrent alcohol and tobacco use during early adolescence characterizes a group at risk. Alcohol Alcohol. 42, 219–225. 10.1093/alcalc/agm024 [DOI] [PubMed] [Google Scholar]
- Schneider M. (2013). Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 354, 99–106. 10.1007/s00441-013-1581-2 [DOI] [PubMed] [Google Scholar]
- Scholes-Balog K. E., Hemphill S. A., Evans-Whipp T. J., Toumbourou J. W., Patton G. C. (2016). Developmental trajectories of adolescent cannabis use and their relationship to young adult social and behavioural adjustment: a longitudinal study of Australian youth. Addict. Behav. 53, 11–18. 10.1016/j.addbeh.2015.09.008 [DOI] [PubMed] [Google Scholar]
- Schweinsburg A. D., McQueeny T., Nagel B. J., Eyler L. T., Tapert S. F. (2010). A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol 44, 111–117. 10.1016/j.alcohol.2009.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg A. D., Schweinsburg B. C., Cheung E. H., Brown G. G., Brown S. A., Tapert S. F. (2005). fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 79, 201–210. 10.1016/j.drugalcdep.2005.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg A. D., Schweinsburg B. C., Nagel B. J., Eyler L. T., Tapert S. F. (2011). Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction 106, 564–573. 10.1111/j.1360-0443.2010.03197.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. C., Rosen A. F. G., Moore T. M., Roalf D. R., Satterthwaite T. D., Calkins M. E., et al. (2019). Cannabis use in youth is associated with limited alterations in brain structure. Neuropsychopharmacology 44, 1362–1369. 10.1038/s41386-019-0347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. C., Wolf D. H., Calkins M. E., Bach E. C., Weidner J., Ruparel K., et al. (2017). Cognitive functioning of adolescent and young adult cannabis users in the philadelphia neurodevelopmental cohort. Psychol. Addict. Behav. 31, 423–434. 10.1037/adb0000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon L. D., Zecevic N. (2015). Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl. Psychiatry 5:e623. 10.1038/tp.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzade C., Chun J., Delisi L. E., Manschreck T. C. (2018). Patterns in adolescent cannabis use predict the onset and symptom structure of schizophrenia-spectrum disorder. Schizophr. Res. 197, 539–543. 10.1016/j.schres.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Shillington A. M., Clapp J. D. (2001). Substance use problems reported by college students: combined marijuana and alcohol use versus alcohol-only use. Subst. Use Misuse 36, 663–672. 10.1081/ja-100103566 [DOI] [PubMed] [Google Scholar]
- Slomp F. M., Bara T. S., Picharski G. L., Cordeiro M. L. (2019). Association of cigarette smoking with anxiety, depression, and suicidal ideation among brazilian adolescents. Neuropsychiatr. Dis. Treat. 15, 2799–2808. 10.2147/ndt.s217069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N., Jones K. A., Rozman M. E., Davis S. M., Ciarrochi J., Heaven P. C., et al. (2012). Reflection impulsivity in adolescent cannabis users: a comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology 219, 575–586. 10.1007/s00213-011-2486-y [DOI] [PubMed] [Google Scholar]
- Soneji S., Barrington-Trimis J. L., Wills T. A., Leventhal A. M., Unger J. B., Gibson L. A., et al. (2017). Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 171, 788–797. 10.1001/jamapediatrics.2017.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463. 10.1016/s0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Spear L. P. (2014). Adolescents and alcohol: acute sensitivities, enhanced intake and later consequences. Neurotoxicol. Teratol. 41, 51–59. 10.1016/j.ntt.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spechler P. A., Orr C. A., Chaarani B., Kan K. J., Mackey S., Morton A., et al. (2015). Cannabis use in early adolescence: evidence of amygdala hypersensitivity to signals of threat. Dev. Cogn. Neurosci. 16, 63–70. 10.1016/j.dcn.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle T. R., Hiler M. M., Cooke M. E., Eissenberg T., Kendler K. S., Dick D. M. (2017). Electronic cigarette use and uptake of cigarette smoking: a longitudinal examination of U.S. college students. Addict. Behav. 67, 66–72. 10.1016/j.addbeh.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Jacobus J., Tapert S. F. (2009a). The influence of substance use on adolescent brain development. Clin. EEG Neurosci. 40, 31–38. 10.1177/155005940904000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Spadoni A. D., Infante M. A., Myers M. G., Tapert S. F. (2009b). Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol. Addict. Behav. 23, 715–722. 10.1037/a0016516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Pulido C., Wetherill R. R., Jacobus J., Brown G. G., Tapert S. F. (2012a). Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J. Stud. Alcohol Drugs 73, 749–760. 10.15288/jsad.2012.73.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Sorg S. F., Schweinsburg A. D., Wetherill R. R., Pulido C., Tapert S. F. (2012b). Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology 220, 529–539. 10.1007/s00213-011-2500-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Rinker D. A., Bartsch H., Castro N., Chung Y., Dale A. M., et al. (2014). Brain volume reductions in adolescent heavy drinkers. Dev. Cogn. Neurosci. 9, 117–125. 10.1016/j.dcn.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Schweinsburg A. D., Pulido C., Tapert S. F. (2011). Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol. Clin. Exp. Res. 35, 1831–1841. 10.1111/j.1530-0277.2011.01527.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Tapert S. F., Sullivan E. V., Jacobus J., Meloy M. J., Rohlfing T., et al. (2015). Brain development in heavy-drinking adolescents. Am. J. Psychiatry 172, 531–542. 10.1176/appi.ajp.2015.14101249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton W. R. (1995). DSM-III-R tobacco dependence and quitting during late adolescence. Addict. Behav. 20, 595–603. 10.1016/0306-4603(95)00019-9 [DOI] [PubMed] [Google Scholar]
- Stein J. A., Newcomb M. D., Bentler P. M. (1996). Initiation and maintenance of tobacco smoking: changing personality correlates in adolescence and young adulthood. J. Appl. Soc. Psychol. 26, 160–187. 10.1111/j.1559-1816.1996.tb01844.x [DOI] [Google Scholar]
- Strong C., Juon H. S., Ensminger M. E. (2014). Long-term effects of adolescent smoking on depression and socioeconomic status in adulthood in an urban African American cohort. J. Urban Health 91, 526–540. 10.1007/s11524-013-9849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam G. A., Stitzer M. A. (2009). Clinical characteristics of treatment-seeking prescription opioid vs. heroin-using adolescents with opioid use disorder. Drug Alcohol Depend. 101, 13–19. 10.1016/j.drugalcdep.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam G. A., Stitzer M. L., Woody G., Fishman M. J., Kolodner K. (2009). Clinical characteristics of treatment-seeking adolescents with opioid versus cannabis/alcohol use disorders. Drug Alcohol Depend. 99, 141–149. 10.1016/j.drugalcdep.2008.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam P., Rogowska J., Dimuzio J., Lopez-Larson M., Mcglade E., Yurgelun-Todd D. (2018). Orbitofrontal connectivity is associated with depression and anxiety in marijuana-using adolescents. J. Affect. Disord. 239, 234–241. 10.1016/j.jad.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2018). Key Substance Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health [Online]. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Available online at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.htm. Accessed 2019. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2019). First-Episode Psychosis and Co-Occurring Substance Use Disorders. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Swartz J. R., Weissman D. G., Ferrer E., Beard S. J., Fassbender C., Robins R. W., et al. (2020). Reward-related brain activity prospectively predicts increases in alcohol use in adolescents. J. Am. Acad. Child Adolesc. Psychiatry 59, 391–400. 10.1016/j.jaac.2019.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift W., Coffey C., Degenhardt L., Carlin J. B., Romaniuk H., Patton G. C. (2012). Cannabis and progression to other substance use in young adults: findings from a 13-year prospective population-based study. J. Epidemiol. Community Health 66:e26. 10.1136/jech.2010.129056 [DOI] [PubMed] [Google Scholar]
- Taioli E., Wynder E. L. (1991). Effect of the age at which smoking begins on frequency of smoking in adulthood. N. Engl. J. Med. 325, 968–969. 10.1056/nejm199109263251318 [DOI] [PubMed] [Google Scholar]
- Tapert S. F., Cheung E. H., Brown G. G., Frank L. R., Paulus M. P., Schweinsburg A. D., et al. (2003). Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch. Gen. Psychiatry 60, 727–735. 10.1001/archpsyc.60.7.727 [DOI] [PubMed] [Google Scholar]
- Tapert S. F., Schweinsburg A. D., Barlett V. C., Brown S. A., Frank L. R., Brown G. G., et al. (2004). Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol. Clin. Exp. Res. 28, 1577–1586. 10.1097/01.alc.0000141812.81234.a6 [DOI] [PubMed] [Google Scholar]
- Tapert S. F., Schweinsburg A. D., Drummond S. P., Paulus M. P., Brown S. A., Yang T. T., et al. (2007). Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology 194, 173–183. 10.1007/s00213-007-0823-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M., Collin S. M., Munafo M. R., Macleod J., Hickman M., Heron J. (2017). Patterns of cannabis use during adolescence and their association with harmful substance use behaviour: findings from a UK birth cohort. J. Epidemiol. Community Health 71, 764–770. 10.1136/jech-2016-208503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercyak K. P., Lerman C., Audrain J. (2002). Association of attention-deficit/hyperactivity disorder symptoms with levels of cigarette smoking in a community sample of adolescents. J. Am. Acad. Child Adolesc. Psychiatry 41, 799–805. 10.1097/00004583-200207000-00011 [DOI] [PubMed] [Google Scholar]
- Terry-McElrath Y. M., O’Malley P. M., Johnston L. D. (2014). Alcohol and marijuana use patterns associated with unsafe driving among U.S. high school seniors: high use frequency, concurrent use, and simultaneous use. J. Stud. Alcohol Drugs 75, 378–389. 10.15288/jsad.2014.75.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo-Clemmens B., Simmonds D., Calabro F. J., Day N. L., Richardson G. A., Luna B. (2018). Adolescent cannabis use and brain systems supporting adult working memory encoding, maintenance, and retrieval. NeuroImage 169, 496–509. 10.1016/j.neuroimage.2017.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe H. H. A., Hamidullah S., Jenkins B. W., Khokhar J. Y. (2020). Adolescent neurodevelopment and substance use: receptor expression and behavioral consequences. Pharmacol. Ther. 206:107431. 10.1016/j.pharmthera.2019.107431 [DOI] [PubMed] [Google Scholar]
- Toftdahl N. G., Nordentoft M., Hjorthoj C. (2016). Prevalence of substance use disorders in psychiatric patients: a nationwide Danish population-based study. Soc. Psychiatry Psychiatr. Epidemiol. 51, 129–140. 10.1007/s00127-015-1104-4 [DOI] [PubMed] [Google Scholar]
- Treur J. L., Willemsen G., Bartels M., Geels L. M., Van Beek J. H., Huppertz C., et al. (2015). Smoking during adolescence as a risk factor for attention problems. Biol. Psychiatry 78, 656–663. 10.1016/j.biopsych.2014.06.019 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (2016). E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services. [Google Scholar]
- Van Amsterdam J., Van Der Velde B., Schulte M., Van Den Brink W. (2018). Causal factors of increased smoking in ADHD: a systematic review. Subst. Use Misuse 53, 432–445. 10.1080/10826084.2017.1334066 [DOI] [PubMed] [Google Scholar]
- Van Ewijk H., Groenman A. P., Zwiers M. P., Heslenfeld D. J., Faraone S. V., Hartman C. A., et al. (2015). Smoking and the developing brain: altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls. Hum. Brain Mapp. 36, 1180–1189. 10.1002/hbm.22695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk H., Heslenfeld D. J., Zwiers M. P., Buitelaar J. K., Oosterlaan J. (2012). Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 36, 1093–1106. 10.1016/j.neubiorev.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Van Hout M. C., Norman I. (2016). Misuse of non-prescription codeine containing products: recommendations for detection and reduction of risk in community pharmacies. Int. J. Drug Policy 27, 17–22. 10.1016/j.drugpo.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Viner R. M., Taylor B. (2007). Adult outcomes of binge drinking in adolescence: findings from a UK national birth cohort. J. Epidemiol. Community Health 61, 902–907. 10.1136/jech.2005.038117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo H. T., Schacht R., Mintzer M., Fishman M. (2014). Working memory impairment in cannabis- and opioid-dependent adolescents. Subst. Abus. 35, 387–390. 10.1080/08897077.2014.954027 [DOI] [PubMed] [Google Scholar]
- Vogel E. A., Cho J., McConnell R. S., Barrington-Trimis J. L., Leventhal A. M. (2020). Prevalence of electronic cigarette dependence among youth and its association with future use. JAMA Netw. Open 3:e1921513. 10.1001/jamanetworkopen.2019.21513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel E. A., Prochaska J. J., Ramo D. E., Andres J., Rubinstein M. L. (2019). Adolescents’ E-cigarette use: increases in frequency, dependence, and nicotine exposure over 12 months. J. Adolesc Health 64, 770–775. 10.1016/j.jadohealth.2019.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Morales M. (2015). The brain on drugs: from reward to addiction. Cell 162, 712–725. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Wagener T. L., Floyd E. L., Stepanov I., Driskill L. M., Frank S. G., Meier E., et al. (2017). Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob. Control 26, e23–e28. 10.1136/tobaccocontrol-2016-053041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. Q., Fitzhugh E. C., Turner L., Fu Q., Westerfield R. C. (1996). Association of depressive symptoms and school adolescents’ smoking: a cross-lagged analysis. Psychol. Rep. 79, 127–130. 10.2466/pr0.1996.79.1.127 [DOI] [PubMed] [Google Scholar]
- Weiland B. J., Thayer R. E., Depue B. E., Sabbineni A., Bryan A. D., Hutchison K. E. (2015). Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J. Neurosci. 35, 1505–1512. 10.1523/JNEUROSCI.2946-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M., Reichenberg A., Grotto I., Yasvitzky R., Rabinowitz J., Lubin G., et al. (2004). Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am. J. Psychiatry 161, 1219–1223. 10.1176/appi.ajp.161.7.1219 [DOI] [PubMed] [Google Scholar]
- Weiser M., Zarka S., Werbeloff N., Kravitz E., Lubin G. (2010). Cognitive test scores in male adolescent cigarette smokers compared to non-smokers: a population-based study. Addiction 105, 358–363. 10.1111/j.1360-0443.2009.02740.x [DOI] [PubMed] [Google Scholar]
- Wetherill R. R., Squeglia L. M., Yang T. T., Tapert S. F. (2013). A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology 230, 663–671. 10.1007/s00213-013-3198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills T. A., Knight R., Sargent J. D., Gibbons F. X., Pagano I., Williams R. J. (2017). Longitudinal study of E-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob. Control 26, 34–39. 10.1136/tobaccocontrol-2015-052705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W., Mathew R., Turkington T., Hawk T., Coleman R. E., Provenzale J. (2000). Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J. Addict. Dis. 19, 1–22. 10.1300/j069v19n01_01 [DOI] [PubMed] [Google Scholar]
- Windle M., Windle R. C. (2001). Depressive symptoms and cigarette smoking among middle adolescents: prospective associations and intrapersonal and interpersonal influences. J. Consult. Clin. Psychol. 69, 215–226. 10.1037/0022-006x.69.2.215 [DOI] [PubMed] [Google Scholar]
- Winward J. L., Hanson K. L., Tapert S. F., Brown S. A. (2014). Heavy alcohol use, marijuana use and concomitant use by adolescents are associated with unique and shared cognitive decrements. J. Int. Neuropsychol. Soc. 20, 784–795. 10.1017/s1355617714000666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack S. R., Shaw D. S., Weaver C. M., Forbes E. E. (2016). Bidirectional associations between cannabis use and depressive symptoms from adolescence through early adulthood among at-risk young men. J. Stud. Alcohol Drugs 77, 287–297. 10.15288/jsad.2016.77.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraw C., Der G., Gale C. R., Deary I. J. (2018). Intelligence in youth and health behaviours in middle age. Intelligence 69, 71–86. 10.1016/j.intell.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. T., Anthony J. C. (1999). Tobacco smoking and depressed mood in late childhood and early adolescence. Am. J. Public Health 89, 1837–1840. 10.2105/ajph.89.12.1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Yuan K., Feng D., Cheng J., Li Y., Cai C., et al. (2016). Inhibition control impairments in adolescent smokers: electrophysiological evidence from a Go/NoGo study. Brain Imaging Behav. 10, 497–505. 10.1007/s11682-015-9418-0 [DOI] [PubMed] [Google Scholar]
- Yousaf T., Dervenoulas G., Politis M. (2018). Advances in MRI methodology. Int. Rev. Neurobiol. 141, 31–76. 10.1016/bs.irn.2018.08.008 [DOI] [PubMed] [Google Scholar]
- Yu D., Yuan K., Zhang B., Liu J., Dong M., Jin C., et al. (2016). White matter integrity in young smokers: a tract-based spatial statistics study. Addict. Biol. 21, 679–687. 10.1111/adb.12237 [DOI] [PubMed] [Google Scholar]
- Zahr N. M., Pfefferbaum A. (2017). Alcohol’s effects on the brain: neuroimaging results in humans and animal models. Alcohol Res. 38, 183–206. [PMC free article] [PubMed] [Google Scholar]
- Zammit S., Allebeck P., Dalman C., Lundberg I., Hemmingsson T., Lewis G. (2003). Investigating the association between cigarette smoking and schizophrenia in a cohort study. Am. J. Psychiatry 160, 2216–2221. 10.1176/appi.ajp.160.12.2216 [DOI] [PubMed] [Google Scholar]
- Zhang R., Geng X., Lee T. M. C. (2017). Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Struct. Funct. 222, 3973–3990. 10.1007/s00429-017-1443-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.