Abstract
Hypothalamic amenorrhoea (HA) accounts for approximately 30% of cases of secondary amenorrhoea in women of reproductive age. It is caused by deficient secretion of hypothalamic gonadotrophin-releasing hormone, which in turn leads to failure of pituitary gonadotrophin and gonadal steroid release. Functional HA (FHA) is defined as HA occurring in the absence of a structural lesion and is predominantly caused by significant weight loss, intense exercise or stress. Treatment of FHA is crucial in avoiding the long-term health consequences on fertility and bone health, in addition to reducing psychological morbidity. This article summarises our understanding of the mechanisms underlying FHA, the evidence base for its clinical management and emerging therapies.
Keywords: amenorrhoea, hypothalamus, infertility, kisspeptin, oestrogen
Introduction
Hypothalamic amenorrhoea (HA) accounts for approximately 30% of cases of secondary amenorrhoea in women of reproductive age.1 It is caused by deficient secretion of hypothalamic gonadotrophin-releasing hormone (GnRH), which in turn leads to failure of pituitary gonadotrophin and gonadal steroid release. Functional HA (FHA) is defined as HA occurring in the absence of a structural lesion, although the terms FHA and HA are often used interchangeably. FHA is predominantly caused by significant weight loss, intense exercise or stress, or a combination of such.1 In addition, there may be a genetic predisposition for the development of FHA, such as heterozygosity for congenital hypogonadotropic hypogonadism.2 Treatment of FHA is crucial to avoid the long-term health consequences on fertility and bone health, in addition to reducing psychological morbidity. Initial management should be focused on resolving the precipitating cause of FHA such as low weight, excessive exercise or stress. In those where FHA persists, treatments are generally limited to hormone replacement with the aim of maintaining oestrogen levels, or ovulation induction or in vitro fertilisation (IVF) in those seeking pregnancy. This article summarises our understanding of the mechanisms underlying FHA, the evidence base for its clinical management and emerging therapies.
Pathophysiology
The normal functioning of the hypothalamic–pituitary–ovarian (HPO) axis is essential for reproductive health. Gonadotropins, i.e. luteinising hormone (LH) and follicle-stimulating hormone (FSH), are secreted by the pituitary gland secondary to pulsatile stimulation by hypothalamic GnRH. HA is defined as the cessation of menstruation due to abnormal signalling between the hypothalamus and the pituitary gland due to deficient pulsatile secretion of GnRH.3 This reduced secretion of GnRH leads to levels of LH and FSH that are insufficient to maintain full folliculogenesis and normal ovulatory ovarian function, with consequent oestrogen deficiency. Rarely, HA may be caused by a structural lesion such as a hypothalamic tumour.3 In the absence of a structural lesion, HA is classified as being functional; FHA is caused by a number of factors including stress and chronic illness.1 However, the majority of cases of FHA result from a relative energy deficit within the body associated with weight loss or exercise.4–6 More rarely, FHA can occur without a precipitating cause, in which case it can be labelled idiopathic HA. The most serious consequences of HA include delayed puberty, amenorrhoea, infertility and the effects of long-standing oestrogen deficiency on bone mineral density (BMD), sexual and genitourinary health and potential effects on cardiovascular health. The link between HA on long-term cardiovascular health is ambiguous, but there is epidemiological data and studies in nonhuman primates that support the hypothesis that even mild ovulatory abnormalities in young women can accelerate development of coronary artery disease.7,8
The link between energy balance and fertility has long been recognised. In the 17th century, Richard Morten observed that amenorrhoea was a cardinal feature of the condition, which was later termed anorexia nervosa (AN). Furthermore in the 1960s, Kennedy and Mitra postulated that a critical body weight is required for reproductive function.9 This permissive action of energy availability on fertility may represent an adaptive response to inhibit the energetic expense of reproduction during prevailing conditions of poor nutrition. A relative calorie deficiency can suppress the HPO axis, such that LH pulsatility is disrupted at a threshold of negative energy availability.10 There is conflicting evidence as to whether low body fat is a cause of amenorrhoea when it falls below a certain threshold.11,12 Menstrual irregularities are common in women who undertake intense exercise (e.g. athletes or ballerinas).13,14 Restrictive eating and strenuous exercise are independent risk factors for HA, but are often seen concurrently, with or without a formal diagnosis of an eating disorder. The ‘female athlete triad’ refers to the interplay between energy availability, menstrual function and bone density; women who exercise frequently tend to have a relative caloric deficiency, menstrual irregularity or HA and reduced BMD, making them more prone to fractures, particularly stress fractures. Importantly, women with the female athlete triad may be of low or normal weight, but those with <85% ideal body weight have been shown to be approximately four times more likely to have menstrual dysfunction and low bone density.13
Stress is another powerful inhibitor of reproductive function. Psychosocial stressors, including externally imposed stress, dysfunctional attitudes and psychiatric morbidity activate the hypothalamic–pituitary–adrenal (HPA) axis, increase corticotrophin-releasing hormone (CRH) and cortisol levels, and sequentially inhibit GnRH secretion.15–17 Administration of CRH has been shown to inhibit gonadotrophin release in healthy female volunteers18 and monkeys.19 Conversely administration of a CRH antagonist stimulates release of GnRH,20 and advances the onset of puberty in rats.21 Furthermore, it has been shown in monkeys that seemingly minor stressors that alone would have minimal impact on reproductive function can interact synergistically, such that combinations of stressors cause a greater impairment of the reproductive axis than any single stressor alone.22 There is also evidence that women’s preconception stress, as measured by salivary alpha-amylase, is associated with a longer time-to-pregnancy and an increased risk of infertility.23
Other less common, but well recognised, causes of FHA include chronic disease, malabsorptive illnesses such as coeliac disease and hypermetabolic states such as severe burns or hyperthyroidism.24
There is considerable inter-person variability in the degree of weight loss, exercise or stress required to result in menstrual disturbance or HA, which is why there are some athletes who are able to conceive despite gruelling training regimes,25 while other women may miss periods secondary to seemingly low levels of nutritional or mental disruption. It is known that a number of genetic mutations can lead to HA in homozygous individuals, such as in KAL1, FGFR1, PROKR2 and GNRHR; in women particularly sensitive to menstrual disturbance many have been shown to be heterozygous for these mutations.2 Similarly, it may be hypothesised that some women may have protective genetic mutations, which enable continuation of fertility despite stressors such as strenuous exercise or weight loss.
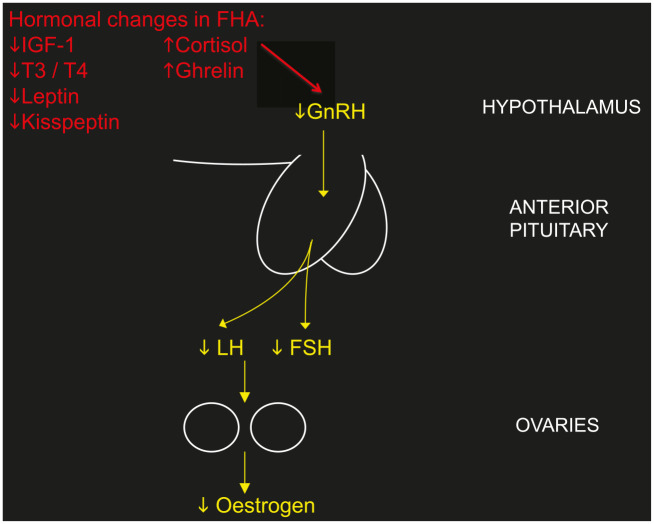
The processes by which GnRH is suppressed by causative agents such as weight loss, exercise or stress are multifactorial, as there are many neuromodulatory signals that alter hypothalamic GnRH function, both inhibitory and stimulatory. The HPO axis and the HPA axis are tightly linked, with the HPA axis activated by nutritional or other stress-reducing GnRH secretion and subsequent LH pulsatility from the pituitary gland.26 GnRH may be suppressed by the common hormonal abnormalities that are associated with FHA including decreased insulin-like growth factor 1 (IGF-1),27 increased cortisol,28,29 increased ghrelin,30,31 decreased thyroid hormone levels, especially triiodothyronine, and reduced leptin32 (Figure 1). Kisspeptin signalling has been implicated as the common intermediate signalling factor, acting downstream of leptin and other neuromodulatory signalling systems to modulate activity of GnRH.33
Figure 1.
The effect of hormonal abnormalities associated with FHA on suppressing the hypothalamic–pituitary–ovarian axis.
FHA, functional hypothalamic amenorrhoea; FSH, follicle-stimulating hormone; GnRH, gonadotrophin-releasing hormone; IGF-1, insulin-like growth factor 1; LH, luteinising hormone; T3, triiodothyronine; T4, thyroxine.
Clinical assessment
Diagnosis of HA is based on symptoms of amenorrhoea, biochemical findings of low oestradiol (<50 ng/ml) with normal/low gonadotrophins (LH and FSH both <10 IU/L with a ratio ~1),32 and, usually, evidence of a causative factor. HA is a diagnosis of exclusion, and other important causes of amenorrhoea must be ruled out.34 These include polycystic ovarian syndrome (PCOS), premature ovarian insufficiency (which may have a genetic cause, such as Turner’s syndrome), uterine abnormalities (congenital, Asherman’s syndrome), endocrine disorders (thyroid dysfunction, Cushing’s syndrome), hyperprolactinaemia, Sheehan’s syndrome (hypopituitarism secondary to major obstetric haemorrhage) and pituitary tumours. The input of a multidisciplinary team including gynaecologists, fertility specialists and endocrinologists may be required to reach a diagnosis. Women with FHA usually present with menstrual disturbance, infertility, symptoms of the cause of FHA such as stress, anxiety, weight loss or an eating disorder or, more rarely, with a consequence of FHA such as fractures or sexual dysfunction. Some cases of FHA are masked by use of hormonal contraception, for example, the oral contraceptive pill (OCP), Depo-Provera, implant or Mirena intrauterine system, and only become clinically apparent once contraception is ceased. A detailed history is arguably the most powerful diagnostic tool in determining the underlying cause of amenorrhoea. Sensitive, but thorough questioning regarding eating patterns and exercise is crucial. It is also important to explore potential stressors, such as work- or study-related stress or personal stress, in addition to psychiatric disorders such as anxiety and depression. Use of a validated questionnaire, such as the Perceived Stress Scale,35 may be helpful in facilitating these conversations, although there is a lack of validated questionnaires specifically designed to elucidate eating and exercise patterns in FHA. Thorough clinical examination is useful. It may help point to a diagnosis of HA, with signs of AN, for example, or provide diagnostic information for other causes of menstrual disturbance (e.g. hirsutism, acne or physical characteristics of Turner’s syndrome). Height and weight should be used to calculate body mass index (BMI) and, if available, it is helpful to measure body fat percentage, using the bioelectrical impedance method, as some women who exercise strenuously or maintain a restrictive diet can have an abnormally low body fat percentage, in spite of a normal BMI, which is in itself associated with ovulatory dysfunction and amenorrhoea.14
Subjects with HA have normal/low circulating gonadotrophins and low oestradiol levels. This reproductive hormone profile may be within the normal range for the follicular phase of the menstrual cycle and therefore needs to be interpreted by someone aware of the clinical history. The endometrial lining may be thin on transvaginal ultrasound scan, but may be normal. In a healthy premenopausal woman, the endometrial lining normally progressively thickens during the follicular phase of the menstrual cycle. It is thinnest during menstruation, with a peak thickness of up to 10 mm in the late follicular phase.36,37 In women with HA, this variability in endometrial thickness may not be observed and there is evidence to suggest that if the lining is <1.5 mm, the patient is more likely to be hypo-oestrogenic.38 HA and PCOS are the two most common causes of secondary amenorrhoea,39 other than pregnancy. Some women with HA may have a coexistent history of symptoms associated with PCOS, such as oligomenorrhoea, hirsutism and acne.40 Up to 50% of women with a nonhyperandrogenic PCOS phenotype may have FHA.41 Transvaginal ultrasound scan may therefore reveal either normal ovarian appearance or morphologically polycystic ovaries.
In patients with amenorrhoea lasting for over 6 months, or in those who have additional risk factors for low BMD, such as severe nutritional deficiency or an eating disorder, a dual-energy X-ray absorptiometry scan is advisable to indicate baseline BMD and to help guide and monitor treatment.39
A prolonged hypogonadal state during a woman’s reproductive years has potentially wide-ranging negative impacts on health. Amenorrhoea has obvious reproductive consequences by causing infertility, but the long-term effects of oestrogen deficiency extend beyond reproduction. A longitudinal study investigating the effects of amenorrhoea and amenorrhoea plus exercise on BMD of young women over 2 years found that low BMD occurs in young women with amenorrhoea and delayed menarche, regardless of exercise status, and compromises crucial bone mass accretion.42 Women with FHA experience more sexual function problems (caused by issues such as dyspareunia and low libido) and significantly higher depression and anxiety compared with women without menstrual dysfunction.43 Furthermore, women with FHA have potential increases in cardiovascular disease6 and exhibit impaired endothelial function, which may contribute to impaired vascular function.44
Treatment
Fortunately, FHA is generally reversible, and usually resolves over a period of time after a positive energy balance is restored, or the underlying stress resolves;45 however, this may take many months. A study evaluating the prognosis of FHA showed that 71% of patients recovered over a follow-up period of 7–9 years, and found that predictive factors of recovery included a higher basal BMI and lower serum cortisol values.46 There is also evidence that in women who recover from FHA serum oestradiol levels gradually increase before recovery, which is preceded by the changes in plasma cortisol concentration.47
Therefore the most successful treatment tends to focus on alleviating the underlying cause of FHA. It is important, however, that the subgroup of patients who have severe energy deficit, associated with severe bradycardia, hypotension, orthostasis and/or electrolyte imbalance are recognised and assessed for inpatient medical management.48 As FHA is often caused by a combination of factors including low weight, excessive exercise, poor nutritional intake and stress, a multidisciplinary approach is beneficial. Table 1 summarises the current treatment options for FHA.
Table 1.
Current treatment options for FHA.
| Category of treatment | Aim of treatment | Treatment options | ||
|---|---|---|---|---|
| Lifestyle advice49 | To reverse amenorrhoea | Simple advice regarding exercise/stress reduction/caloric intake | Referral to dietician | |
| Psychological | To reverse amenorrhoea | Cognitive behavioural therapy50 | Referral to multidisciplinary eating disorders unit | Pharmacotherapy for anxiety or depression for example, SSRIs |
| Hormone replacement therapy | To replace oestrogen | Combined oestrogen and progesterone – optimal preparation unclear51,52 | ||
| Assisted reproduction | To achieve pregnancy | Ovulation induction with exogenous gonadotrophins combined with timed intercourse or intrauterine insemination53 | In vitro fertilisation | |
| Experimental treatments | To reverse amenorrhoea | Recombinant leptin therapy54,55 | Kisspeptin administration56 | |
FHA, Functional hypothalamic amenorrhoea; SSRI, selective serotonin reuptake inhibitor.
Lifestyle changes
Reversing the negative energy balance by restoration of body weight or fat mass and/or reduction in exercise intensity may be sufficient to restore menses and improve rates of conception in some patients with FHA.49 It is not clear what degree of weight gain is required for resolution of menstruation, but common advice is to aim for at least the same weight at which point menstruation stopped, although one study has shown that patients with low-weight eating disorders were 2 kg heavier at the time their menses resumed than when they became amenorrhoeic.57 Improving the energy deficit often requires behavioural change. In some women simply explaining the need for increased caloric intake and basic advice about how to achieve this may be adequate, whilst in others weight gain may need to be supervised, or dietary patterns discussed by a registered dietician or nutritionist. Many women with FHA have an element of disordered eating or an incipient eating disorder50,58–62 that will require psychological support in order to facilitate change in negative eating habits. In those with a formally diagnosed eating disorder, such as AN or bulimia nervosa, referral to a specialist eating disorder service is recommended to enable these patients to be appropriately treated by a multidisciplinary team, including psychiatrists.
Psychological stress is a known risk factor for the development of FHA, and whilst this may be caused by a definable stressful life event, such as bereavement, it is more commonly secondary to insidious psychological or personality characteristics, which are often associated with disordered eating. Women with FHA have been shown to have more dysfunctional attitudes (demonstrated by higher levels of control, perfectionism, rigidity of ideas and concern about judgements of others), greater difficulty in coping with daily stresses and greater interpersonal dependence than eumenorrhoeic women.15 In addition, women with FHA more commonly have a history of psychiatric disorders and primary mood disorders than eumenorrhoeic women.16,17 Behavioural and psychological interventions, such as cognitive behavioural therapy (CBT), have been shown to reverse amenorrhoea,50 associated with a reduction in nocturnal cortisol secretion and increased thyroid-stimulating hormone and leptin levels, independently of weight gain.62,63 In a study of 16 women with FHA, CBT resulted in a higher rate of resumption of ovarian function (88%) compared with observation alone (25%).50 Family-based therapy has been shown to be beneficial in the treatment of eating disorders, but has been less well described for the specific treatment of FHA. Pharmacotherapy may be considered in women to treat psychological or psychiatric morbidity, such as anxiety or depression, which can be associated with FHA, either alongside behavioural therapies, or in those where behavioural therapies have been unsuccessful. For couples wishing to conceive, the diagnosis, evaluation and treatment of infertility can be profoundly stressful, with one study finding 40% of infertility patients fulfilling the diagnosis of a psychiatric disorder, most commonly anxiety disorders and depressive disorders.64 Stress may negatively impact the success of fertility treatment,65 as well as contribute to the discontinuation of fertility treatment before pregnancy is achieved,66 and therefore it is essential that it is recognised. It has been found that psychosocial interventions for couples during infertility treatment, in particular CBT, may be effective both in reducing psychological distress and in improving clinical pregnancy rates.67
Hormonal treatment
Hormone replacement therapy may be appropriate for women with FHA without menstrual recovery despite 6–12 months of nonpharmacological therapy, or in those declining behavioural or psychological treatment. Progesterone replacement is required in addition to oestrogen in order to prevent endometrial hyperplasia. Hormone replacement therapy may be administered either transdermally or orally. Whilst hormone replacement therapy provides oestrogen replacement in patients with FHA, it does not restore gonadotrophin release or stimulate ovulation, and may offer the false reassurance of regular menses despite ongoing nutritional deficiency, energy deficit or psychological stress. Studies looking at the benefit of oestrogen replacement on BMD, specifically in FHA, are lacking, and it remains unclear what the optimal preparation and optimal dose of oestrogen replacement is in these women. In other models of hypogonadism, such as Turner’s syndrome, oral oestradiol has been effectively used as hormone replacement therapy for many years.68 However, these women are generally of normal weight and therefore it may be more helpful to compare women with FHA to those with a low-weight form of hypogonadism, such as AN. In AN it has been shown that administration of the oral contraceptive pill (OCP), providing relatively high oestrogen doses (usually ethinyloestradiol), does not improve BMD.51,69,70 In contrast, physiological transdermal oestradiol replacement may have a more positive effect in maintaining BMD.52 A potential reason for this discrepancy may be that OCPs further suppress IGF-1, an important bone trophic hormone, which is already decreased in this condition.71,72 It is plausible that OCP preparations containing the endogenous hormone oestradiol might increase BMD more effectively in women with FHA when compared with an ethinyloestradiol-containing OCP preparation. The evidence for the effectiveness of hormone replacement on BMD in exercise-associated FHA is mixed.73–77
Nonhormonal treatment
To date, there is no clear role for nonhormonal treatment in the management of FHA. Administration of naltrexone, an opioid receptor antagonist, has been shown to improve hormonal status and restore menses in HA78 and a more recent study has shown that administration of neuroactive compound acetyl-L-carnitine in combination with L-carnitine may have a positive effect on increasing LH levels in FHA.79 However, more work needs to be carried out to confirm these findings.
Treatment of infertility
Hormonal treatment, with the intention to stimulate ovulation, is only clinically indicated when pregnancy is desired. It is crucial that fertility treatment is not initiated until attempts have been made to treat the underlying nutritional or psychological stress, and women have achieved, and maintained, a BMI of at least 18.5 kg/m2 in order to prevent the increased likelihood of poor obstetric and neonatal outcomes associated with low maternal weight,80 including pregnancy loss, preterm labour,81 low birth weight82 and need for caesarean section.83 Clomiphene citrate is a selective oestrogen receptor modulator and has mixed agonist and antagonist activity at oestrogen receptors at the level of the hypothalamus.84 By interfering with the oestradiol-mediated negative feedback on pituitary gonadotrophin release, clomiphene citrate increases circulating gonadotrophin levels, which stimulate ovarian folliculogenesis, leading to development of a dominant follicle and ovulation. However, clomiphene has limited efficacy in FHA since circulating levels of oestradiol are characteristically low in this form of hypogonadotrophic hypogonadism. Pulsatile GnRH therapy is an effective method of restoring menstrual cyclicity in patients with HA and has the advantage of driving the development of a single dominant follicle and therefore minimal risk of multiple pregnancy.85 However, there is currently no commercially available pump internationally. The predominant therapy used to induce ovulation in patients with HA is exogenous gonadotrophins.53 Daily FSH-containing gonadotrophin injections are administered to the patient, usually following a step-up protocol. Women with FHA are deficient in both FSH and LH and successful ovulation induction requires some exogenous LH, which is crucial for androgen steroidogenesis (substrate for oestrogen biosynthesis), alongside FSH. Preparations such as highly purified human menopausal gonadotrophin provide sufficient LH. Alternatively, if recombinant FSH is used, recombinant LH should be administered concurrently. Follicle tracking is performed using ultrasonography, and ovulation is induced using human chorionic gonadotrophin when the follicle is over 18 mm in diameter. Ovulation induction can be used in combination with timed intercourse or intrauterine insemination to achieve pregnancy, although the latter is usually reserved for those unable to have vaginal intercourse or requiring donor sperm. While gonadotrophin-based therapies are efficacious, they confer the risk of multiple pregnancy due to ovarian over-response and as a result cycle cancellation rates can be as high as 25%.86 Ovarian hyperstimulation syndrome (OHSS) occurs in a minority of cases of ovulation induction,87 but is much more prevalent with the controlled ovarian hyperstimulation protocols used for IVF. OHSS is a potentially life-threatening condition, which results from increased vascular permeability, subsequent fluid accumulation in the peritoneal and pleural spaces, renal and hepatic failure and a prothrombotic state leading to an increased risk of venous thromboembolism as a direct consequence of the pharmacological circulating gonadotrophin levels associated with therapeutic injections.
Experimental avenues of treatment
Leptin is an adipokine hormone secreted in proportion to fat mass. Circulating leptin levels positively correlate with fat mass, and levels of leptin secretion are significantly reduced following weight loss88 and acute starvation.89 Furthermore, patients with genetic leptin deficiency have hypogonadotrophic hypogonadism.90 Research has implicated recombinant leptin as a potential therapy in FHA. FHA is commonly associated with low body weight or weight loss, and subjects with HA have reduced mean circulating levels of leptin when compared with healthy female subjects. Two randomised control trials have demonstrated the potential efficacy of leptin treatment in FHA. Administration of twice daily recombinant leptin in women with FHA resulted in ovulatory menstrual cycles in 38% of women, restored LH pulsatility and significantly increased levels of oestradiol, IGF-1, thyroid hormone and bone-formation markers.54 A second study has shown that 70% of women receiving recombinant leptin therapy developed menstruation during the course of the study and significantly increased levels of oestradiol and progesterone.55 These data suggest that activity of the GnRH pulse generator is stimulated in subjects with HA following leptin administration. Interestingly, GnRH neurons do not express the leptin receptor; it is likely that kisspeptin acts as an intermediate signalling factor to mediate the stimulatory action of leptin on GnRH pulsatility.91 Subcutaneous bolus injection of kisspeptin-54 stimulates gonadotrophin secretion in healthy female subjects92,93 and acute subcutaneous administration of kisspeptin-54 potently stimulates pituitary–gonadal function in human females with HA.56 However, significantly reduced gonadotrophin responses to kisspeptin-54 administration were observed after 2 weeks of twice-daily kisspeptin-54 injections, suggesting desensitisation.94 The most promising role for kisspeptin as a therapeutic option in FHA comes from the use of a continuous infusion of kisspeptin at variable doses over 10 hours, which was associated with an increase in LH pulsatility in all women, without evidence of desensitisation.95
Oestrogens are required for pituitary responsiveness to GnRH. A recent study observed that a 10-day course of oral oestriol significantly increased LH and FSH secretion following GnRH bolus in women with FHA.96 It is possible that physiological hormone replacement therapy could provoke ovulation in women with FHA bordering on recovery. However, there is a paucity of data directly comparing ovulatory frequency in women with FHA taking hormone replacement therapy versus women with FHA not taking hormone replacement therapy.
Conclusion
HA is a common cause of amenorrhoea in women of reproductive age, which can go unrecognised. Thorough clinical evaluation is crucial in order to diagnose these women, establish the causative factor and initiate treatment appropriately. Treatment options need to be personalised and tend to focus on alleviating the underlying nutritional or psychosocial stress by lifestyle and behavioural interventions. Emerging hormonal therapies may prove useful in the future, especially in those women who do not respond to first-line behavioural treatment.
Footnotes
Author contribution(s): Rachel E. Roberts: Conceptualisation; Formal analysis; Methodology; Writing-original draft.
Linda Farahani: Investigation; Writing–review & editing.
Lisa Webber: Conceptualisation; Writing–review & editing.
Channa Jayasena: Conceptualisation; Writing–review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Rachel E. Roberts, Department of Obstetrics and Gynaecology, Queen Charlotte’s and Chelsea Hospital, London, UK
Linda Farahani, Section of Investigative Medicine, Imperial College London, London, UK, and Department of Gynaecology, St Mary’s Hospital, London, UK.
Lisa Webber, Department of Gynaecology, St Mary’s Hospital, London, UK.
Channa Jayasena, Section of Investigative Medicine, 6th Floor Commonwealth Building, Imperial College Faculty of Medicine, Hammersmith Hospital, Du Cane Road, London W120NN, UK.
References
- 1. Reindollar RH, Novak M, Tho SP, et al. Adult amenorrhea: a study of 262 patients. Am J Obstet Gynecol 1986; 155: 531–543. [DOI] [PubMed] [Google Scholar]
- 2. Caronia LM, Martin C, Welt CK, et al. A genetic basis for functional hypothalamic amenorrhea. N Engl J Med 2011; 364: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yen SS. Female hypogonadotropic hypogonadism. Hypothalamic amenorrhea syndrome. Endocrinol Metab Clin North Am 1993; 22: 29–58. [PubMed] [Google Scholar]
- 4. Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985) 1998; 84: 37–46. [DOI] [PubMed] [Google Scholar]
- 5. Reame NE, Sauder SE, Case GD, et al. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab 1985; 61: 851–858. [DOI] [PubMed] [Google Scholar]
- 6. Filicori M, Flamigni C, Vizziello G, et al. Hypothalamic control of gonadotropin secretion in the human menstrual cycle. Prog Clin Biol Res 1986; 225: 55–74. [PubMed] [Google Scholar]
- 7. Kaplan JR, Manuck SB. Ovarian dysfunction and the premenopausal origins of coronary heart disease. Menopause 2008; 15: 768–776. [DOI] [PubMed] [Google Scholar]
- 8. Kaplan JR, Chen H, Manuck SB. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am J Primatol 2009; 71: 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kennedy GC, Mitra J. Hypothalamic control of energy balance and the reproductive cycle in the rat. J Physiol 1963; 166: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab 2003; 88: 297–311. [DOI] [PubMed] [Google Scholar]
- 11. Sanborn CF, Albrecht BH, Wagner WW., Jr. Athletic amenorrhoea: lack of association with body fat. Med Sci Sports Exerc 1987; 19: 207–212. [PubMed] [Google Scholar]
- 12. Winkler LA, Frølich JS, Schulpen M, et al. Body composition and menstrual status in adults with a history of anorexia nervosa: at what fat percentage is the menstrual cycle restored. Int J Eat Disord 2017; 50: 370–377. [DOI] [PubMed] [Google Scholar]
- 13. Thralls KJ, Nichols JF, Barrack MT, et al. Body mass-related predictors of the female athlete triad among adolescent athletes. Int J Sport Nut Exerc Metab 2016; 26: 17–25. [DOI] [PubMed] [Google Scholar]
- 14. Stokić E, Srdić B, Barak O. Body mass index, body fat mass and the occurrence of amenorrhea in ballet dancers. Gynecol Endocrinol 2005; 20: 195–199. [DOI] [PubMed] [Google Scholar]
- 15. Berga SL, Girton LG. The psychoneuroendocrinology of functional hypothalamic amenorrhea. Psychiatr Clin North Am 1989; 12: 105–116. [PubMed] [Google Scholar]
- 16. Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril 1993; 60: 486–492. [PubMed] [Google Scholar]
- 17. Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril 2001; 76: 310–316. [DOI] [PubMed] [Google Scholar]
- 18. Barbarino A, De Marinis L, Folli G, et al. Corticotropin-releasing hormone inhibition of gonadotropin secretion during the menstrual cycle. Metabolism 1989; 38: 504–506. [DOI] [PubMed] [Google Scholar]
- 19. Xiao E, Luckhaus J, Niemann W, et al. Acute inhibition of gonadotropin secretion by corticotropin-releasing hormone in the primate: are the adrenal glands involved? Endocrinology 1989; 124: 1632–1637. [DOI] [PubMed] [Google Scholar]
- 20. Nikolarakis KE, Almeida OF, Herz A. Hypothalamic opioid receptors mediate the inhibitory actions of corticotropin-releasing hormone on luteinizing hormone release: further evidence from a morphine-tolerant animal model. Brain Res 1988; 450: 360–363. [DOI] [PubMed] [Google Scholar]
- 21. Kinsey-Jones JS, Li XF, Knox AM, et al. Corticotrophin-releasing factor alters the timing of puberty in the female rat. J Neuroendocrinol 2010; 22: 102–109. [DOI] [PubMed] [Google Scholar]
- 22. Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab 2007; 293: E270–E276. [DOI] [PubMed] [Google Scholar]
- 23. Lynch CD, Sundaram R, Maisog JM, et al. Preconception stress increases the risk of infertility: results from a couple-based prospective cohort study–the LIFE study. Hum Reprod 2014; 29: 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ajayi AF, Akhigbe RE, Ajayi LO. Hypothalamic-pituitary-ovarian axis in thyroid dysfunction. West Indian Med J 2013; 62: 835–838. [DOI] [PubMed] [Google Scholar]
- 25. Sundgot-Borgen J, Sundgot-Borgen C, Myklebust G, et al. Elite athletes get pregnant, have healthy babies and return to sport early postpartum. BMJ Open Sport Exerc Med 2019; 5: e000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mastorakos G, Pavlatou MG, Mizamtsidi M. The hypothalamic-pituitary-adrenal and the hypothalamic-pituitary-gonadal axes interplay. Pediatr Endocrinol Rev 2006; 3(Suppl. 1): 172–181. [PubMed] [Google Scholar]
- 27. Misra M, Miller KK, Bjornson J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 2003; 88: 5615–5623. [DOI] [PubMed] [Google Scholar]
- 28. Berga SL, Mortola JF, Girton L, et al. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 1989; 68: 301–308. [DOI] [PubMed] [Google Scholar]
- 29. Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril 1997; 67: 1024–1030. [DOI] [PubMed] [Google Scholar]
- 30. Misra M, Miller KK, Kuo K, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 2005; 289: E347–E356. [DOI] [PubMed] [Google Scholar]
- 31. Ackerman KE, Slusarz K, Guereca G, et al. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. Am J Physiol Endocrinol Metab 2012; 302: E800–E806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan JL, Matarese G, Shetty GK, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A 2006; 103: 8481–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCarthy MM. A piece in the puzzle of puberty. Nat Neurosci 2013; 16: 251–253. [DOI] [PubMed] [Google Scholar]
- 34. Gordon CM, Ackerman KE, Berga SL, et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2017; 102: 1413–1439. [DOI] [PubMed] [Google Scholar]
- 35. Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983; 24: 385–396. [PubMed] [Google Scholar]
- 36. Raine-Fenning NJ, Campbell BK, Clewes JS, et al. Defining endometrial growth during the menstrual cycle with three-dimensional ultrasound. BJOG 2004; 111: 944–949. [DOI] [PubMed] [Google Scholar]
- 37. Baerwald AR, Pierson RA. Endometrial development in association with ovarian follicular waves during the menstrual cycle. Ultrasound Obstet Gynecol 2004; 24: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morcos RN, Leonard MD, Smith M, et al. Vaginosonographic measurement of endometrial thickness in the evaluation of amenorrhea. Fertil Steril 1991; 55: 543–546. [DOI] [PubMed] [Google Scholar]
- 39. Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med 2010; 363: 365–371. [DOI] [PubMed] [Google Scholar]
- 40. Robin G, Gallo C, Catteau-Jonard S, et al. Polycystic ovary-like abnormalities (PCO-L) in women with functional hypothalamic amenorrhea. J Clin Endocrionol Metab 2012; 97: 4236–4243. [DOI] [PubMed] [Google Scholar]
- 41. Lauritsen MP, Pinborg A, Loft A, et al. Revised criteria for PCOS in WHO Group II anovulatory infertility – a revival of hypothalamic amenorrhoea? Clin Endocrinol (Oxf) 2015; 82: 584–591. [DOI] [PubMed] [Google Scholar]
- 42. Warren MP, Brooks-Gunn J, Fox RP, et al. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab 2002; 87: 3162–3168. [DOI] [PubMed] [Google Scholar]
- 43. Dundon CM, Rellini AH, Tonani S, et al. Mood disorders and sexual functioning in women with functional hypothalamic amenorrhea. Fertil Steril 2010; 94: 2239–2243. [DOI] [PubMed] [Google Scholar]
- 44. O’Donnell E, Goodman JM, Mak S, et al. Impaired vascular function in physically active premenopausal women with functional hypothalamic amenorrhea is associated with low shear stress and increased vascular tone. J Clin Endocrinol Metab 2014; 99: 1798–1806. [DOI] [PubMed] [Google Scholar]
- 45. Perkins RB, Hall JE, Martin KA. Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab 1999; 84: 1905–1911. [DOI] [PubMed] [Google Scholar]
- 46. Falsetti L, Gambera A, Barbetti L, et al. Long-term follow-up of functional hypothalamic amenorrhea and prognostic factors. J Clin Endocrinol Metab 2002; 87: 500–505. [DOI] [PubMed] [Google Scholar]
- 47. Kondoh Y, Uemura T, Murase M, et al. A longitudinal study of disturbances of the hypothalamic-pituitary-adrenal axis in women with progestin-negative functional hypothalamic amenorrhea. Fertil Steril 2001; 76: 748–752. [DOI] [PubMed] [Google Scholar]
- 48. Society for Adolescent Health and Medicine, Golden NH, Katzman DK, et al. Position paper of the society for adolescent health and medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health 2015; 56: 121–125. [DOI] [PubMed] [Google Scholar]
- 49. Bates GW, Bates SR, Whitworth NS. Reproductive failure in women who practice weight control. Fertil Steril 1982; 37: 373–378. [PubMed] [Google Scholar]
- 50. Berga SL, Marcus MD, Loucks TL, et al. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril 2003; 80: 976–981. [DOI] [PubMed] [Google Scholar]
- 51. Strokosch GR, Friedman AJ, Wu SC, et al. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: a double-blind, placebo-controlled study. J Adolesc Health 2006; 39: 819–827. [DOI] [PubMed] [Google Scholar]
- 52. Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res 2011; 26: 2430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Diedrich K, Felberbaum R. New approaches to ovarian stimulation. Hum Reprod 1998; 13(Suppl. 3): 1–13. [DOI] [PubMed] [Google Scholar]
- 54. Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 2004; 351: 987–997. [DOI] [PubMed] [Google Scholar]
- 55. Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A 2011; 108: 6585–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jayasena CN, Nijher GM, Abbara A, et al. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther 2010; 88: 840–847. [DOI] [PubMed] [Google Scholar]
- 57. Golden NH, Jacobson MS, Schebendach J, et al. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 1997; 151: 16–21. [DOI] [PubMed] [Google Scholar]
- 58. Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhoea. J Clin Endocrinol Metab 1998; 83: 25–32. [DOI] [PubMed] [Google Scholar]
- 59. Warren MP, Holderness CC, Lesobre V, et al. Hypothalamic amenorrhoea and hidden nutritional insults. J Soc Gynecol Investig 1994; 1: 84–88. [DOI] [PubMed] [Google Scholar]
- 60. Pirke KM, Schweiger U, Lemmel W, et al. The influence of dieting on the menstrual cycle of healthy young women. J Clin Endocrinol Metab 1985; 60: 1174–1179. [DOI] [PubMed] [Google Scholar]
- 61. Schweiger U. Menstrual function and luteal-phase deficiency in relation to weight changes and dieting. Clin Obstet Gynecol 1991; 34: 191–197. [DOI] [PubMed] [Google Scholar]
- 62. Michopoulos V, Mancini F, Loucks TL, et al. Neuroendocrine recovery initiated by cognitive behavioral therapy in women with functional hypothalamic amenorrhea: a randomized, controlled trial. Fertil Steril 2013; 99: 2084–2091.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Drew FL. The epidemiology of secondary amenorrhea. J Chronic Dis 1961; 14: 396–407. [DOI] [PubMed] [Google Scholar]
- 64. Chen TH, Chang SP, Tsai CF, et al. Prevalence of depressive and anxiety disorders in an assisted reproductive technique clinic. Hum Reprod 2004; 19: 2313–2318. [DOI] [PubMed] [Google Scholar]
- 65. Boivin J, Schmidt L. Infertility-related stress in men and women predicts treatment outcome 1 year later. Fertil Steril 2005; 83: 1745–1752. [DOI] [PubMed] [Google Scholar]
- 66. Rajkhowa M, McConnell A, Thomas GE. Reasons for discontinuation of IVF treatment: a questionnaire study. Hum Reprod 2006; 21: 358–363. [DOI] [PubMed] [Google Scholar]
- 67. Frederiksen Y, Farver-Vestergaard I, Skovgård NG, et al. Efficacy of psychosocial interventions for psychological and pregnancy outcomes in infertile women and men: a systematic review and meta-analysis. BMJ Open 2015; 5: e006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beckett PR, Copeland KC, Flannery TK, et al. Combination growth hormone and estrogen increase bone mineralization in girls with Turner syndrome. Pediatr Res 1999; 45: 709–713. [DOI] [PubMed] [Google Scholar]
- 69. Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15: 135–143. [DOI] [PubMed] [Google Scholar]
- 70. Muñoz MT, Morandé G, García-Centenera JA, et al. The effects of estrogen administration on bone mineral density in adolescents with anorexia nervosa. Eur J Endocrinol 2002; 146: 45–50. [DOI] [PubMed] [Google Scholar]
- 71. Soyka LA, Grinspoon S, Levitsky LL, et al. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab 1999; 84: 4489–4496. [DOI] [PubMed] [Google Scholar]
- 72. Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab 1991; 72: 374–381. [DOI] [PubMed] [Google Scholar]
- 73. Rickenlund A, Carlström K, Ekblom B, et al. Effects of oral contraceptives on body composition and physical performance in female athletes. J Clin Endocrinol Metab 2004; 89: 4364–4370. [DOI] [PubMed] [Google Scholar]
- 74. Cobb KL, Bachrach LK, Sowers M, et al. The effect of oral contraceptives on bone mass and stress fractures in female runners. Med Sci Sports Exerc 2007; 39: 1464–1473. [DOI] [PubMed] [Google Scholar]
- 75. Castelo-Branco C, Vicente JJ, Pons F, et al. Bone mineral density in young, hypothalamic oligoamenorrheic women treated with oral contraceptives. J Reprod Med 2001; 46: 875–879. [PubMed] [Google Scholar]
- 76. Cumming DC. Exercise-associated amenorrhea, low bone density, and estrogen replacement therapy. Arch Intern Med 1996; 156: 2193–2195. [PubMed] [Google Scholar]
- 77. Gibson JH, Mitchell A, Reeve J, et al. Treatment of reduced bone mineral density in athletic amenorrhea: a pilot study. Osteoporos Int 1999; 10: 284–289. [DOI] [PubMed] [Google Scholar]
- 78. Genazzani AD, Gastaldi M, Petraglia F, et al. Naltrexone administration modulates the neuroendocrine control of luteinizing hormone secretion in hypothalamic amenorrhoea. Hum Reprod 1995; 10: 2868–2871. [DOI] [PubMed] [Google Scholar]
- 79. Genazzani AD, Despini G, Czyzyk A, et al. Modulatory effects of L-carnitine plus L-acetyl-carnitine on neuroendocrine control of hypothalamic functions in functional hypothalamic amenorrhea (FHA). Gynecol Endocrinol 2017; 33: 963–967. [DOI] [PubMed] [Google Scholar]
- 80. ESHRE Capri Workshop Group. Nutrition and reproduction in women. Hum Reprod Update 2006; 12: 193–207. [DOI] [PubMed] [Google Scholar]
- 81. Moutquin JM. Socio-economic and psychosocial factors in the management and prevention of preterm labour. BJOG 2003; 110: 56–60. [PubMed] [Google Scholar]
- 82. Koubaa S, Hällström T, Lindholm C, et al. Pregnancy and neonatal outcomes in women with eating disorders. Obstet Gynecol 2005; 105: 255–260. [DOI] [PubMed] [Google Scholar]
- 83. Hoffman ER, Zerwas SC, Bulik CM. Reproductive issues in anorexia nervosa. Expert Rev Obstet Gynecol 2011; 6: 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wolf LJ. Ovulation induction. Clin Obstet Gynecol 2000; 43: 902–915. [DOI] [PubMed] [Google Scholar]
- 85. Santoro N, Wierman ME, Filicori M, et al. Intravenous administration of pulsatile gonadotropin-releasing hormone in hypothalamic amenorrhea: effects of dosage. J Clin Endocrinol Metab 1986; 62: 109–116. [DOI] [PubMed] [Google Scholar]
- 86. White DM, Hardy K, Lovelock S, et al. Low-dose gonadotropin induction of ovulation in anovulatory women: still needed in the age of IVF. Reproduction 2018; 156: F1–F10. [DOI] [PubMed] [Google Scholar]
- 87. Elchalal U, Schenker JG. The pathophysiology of ovarian hyperstimulation syndrome – views and ideas. Hum Reprod 1997; 12: 1129–1137. [DOI] [PubMed] [Google Scholar]
- 88. Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and Ob RNA in obese and weight-reduced subjects. Nat Med 1995; 1: 1155–1161. [DOI] [PubMed] [Google Scholar]
- 89. Chan JL, Heist K, DePaoli AM, et al. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 2003; 111: 1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997; 387: 903–908. [DOI] [PubMed] [Google Scholar]
- 91. Smith JT, Acohido BV, Clifton DK, et al. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol 2006; 18: 298–303. [DOI] [PubMed] [Google Scholar]
- 92. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 2005; 90: 6609–6615. [DOI] [PubMed] [Google Scholar]
- 93. Dhillo WS, Chaudhri OB, Thompson EL, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 2007; 92: 3958–3966. [DOI] [PubMed] [Google Scholar]
- 94. Jayasena CN, Nijher GM, Chaudhri OB, et al. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 2009; 94: 4315–4323. [DOI] [PubMed] [Google Scholar]
- 95. Jayasena CN, Abbara A, Veldhuis JD, et al. Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of kisspeptin-54. J Clin Endocrinol Metab 2014; 99: E953–E961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Genazzani AD, Podfigurna-Stopa A, Czyzyk A, et al. Short-term estriol administration modulates hypothalamo-pituitary function in patients with functional hypothalamic amenorrhea (FHA). Gynaecol Endocrinol 2016; 32: 253–257. [DOI] [PubMed] [Google Scholar]