Abstract
Aims:
Interstitial lung disease (ILD) is the most common type of pulmonary involvement of extraglandular complication in patients with primary Sjögren’s syndrome (pSS), but the diagnosis of pSS-associated ILD (pSS-ILD) is still challenging. This study aimed to investigate the levels of serum tumor markers in pSS patients with or without ILD (pSS-non-ILD) and explore its diagnostic value for pSS-ILD.
Methods:
A total of 168 pSS-ILD patients and age- and sex-matched 538 pSS-non-ILD were recruited. The levels of peripheral tumor markers, including carbohydrate antigen (CA)153, CA125, CA19-9, carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), β-human chorionic gonadotropin, alpha fetoprotein, CA724, and complexed prostate specific antigen, the clinical manifestations, and general laboratory indicators were measured and collected.
Results:
Compared with pSS-non-ILD, pSS-ILD patients had higher levels of disease activity indicators, such as EULAR Sjögren’s syndrome disease activity index, ESR, and CRP, and elevated serum levels of tumor markers: NSE, CEA, CA125, and CA153. The serum levels of CA153 [odds ratio (OR) = 4.521, 95% confidence interval (CI) = [1.871, 10.928)] and CEA [OR = 2.879, 95% CI = (1.305, 6.353)] were significantly correlated with the onset of SS-ILD. CA153 was the only tumor marker with area under receiver operating characteristic curve (AUC) over 0.7 [AUC = 0.743, 95% CI = (0.70, 0.79)].
Conclusion:
Tumor markers increased in serum of pSS-ILD patients. Higher CA153 levels are significantly correlated to the increased risk of ILD in patients with pSS and may be directly involved in the pathogenesis of pSS-ILD. Serum CA153 had the best diagnostic value in those tumor markers for pSS-ILD without malignancy.
Keywords: interstitial lung disease, primary Sjögren’s syndrome, tumor markers
Introduction
Primary Sjögren’s syndrome (pSS) is a common autoimmune disease associated with multiple autoantibodies and innate and adaptive immune disorder that leads to persistent and progressive inflammation of exocrine glands, such as salivary and lacrimal glands, resulting in tissue damage and loss of function, with a prevalence of about 0.5% in the general population.1 Other organs such as liver, lungs, kidneys, and skin may also be involved. Interstitial lung disease (ILD), the most common type of pulmonary involvement of extraglandular complication, develops in approximately 25% of pSS patients.2
ILD is a highly heterogeneous lung parenchymal disease with the common pathologies (diffuse alveolar inflammation and interstitial fibrosis), clinical manifestations (cough and dyspnea), and abnormal chest radiographs (diffuse infiltration),3 which are associated with the decreased quality of life and increased mortality in Sjögren’s syndrome.4 However, early diagnosis for ILD is still a challenge.
High-resolution computed tomography (HRCT) is the most important method to detect early pulmonary pathological changes and pulmonary dysfunction at present. However, the respiratory symptoms of early pSS associated ILD (pSS-ILD) are atypical and even asymptomatic; as a consequence, most patients are not properly treated.3 Moreover, the pathogenesis of pSS-ILD is still unclear and effective treatment and accurate multiple detection methods are lacking.
Tumor markers, produced by tumoral or normal tissues, are measurable bio-chemicals in tissues, fluid, and feces, which are associated with the severity and prognosis of a malignancy.5 The commonly used clinical tumor markers for patients with cancer include carbohydrate antigen (CA) 153 (CA153), CA125, CA19-9, carcinoembryonic antigen (CEA), and neuron-specific enolase (NSE), which involves the malignant lesions of multiple systems and organs, including ovarian cancer, breast cancer, gastric cancer, pancreatic cancer, lung cancer, and some other tumors.6
However, numerous recent studies have focused on the relationship between the serum tumor markers and the incidence of ILD,7,8 and have put forward a new concept that the detection of serum tumor markers may be used as risk factors for ILD in patients with connective tissue disease (CTD), such as pSS, rheumatoid arthritis (RA), systemic sclerosis (SSc), and polymyositis/dermatomyositis with a convincing lack of clinical markers.
This study focused on the correlation between tumor markers and ILD in patients with pSS to provide new insights for early diagnosis and treatment of pSS-ILD by evaluating the changes of serum tumor markers in pSS with and without ILD.
Materials and methods
Patients
This is a retrospective analysis of 706 patients who have a confirmed diagnosis with pSS based on the diagnosis criteria of American–European Consensus Group9 at the Second Hospital of Shanxi Medical University, between January 2015 and April 2019. All enrolled subjects underwent lung HRCT scans. Patients with the imaging ILD indicators, such as reticular abnormalities, honeycombing, and traction bronchiectasis, and clinical features of ILD were identified as pSS-ILD.10 Patients were excluded from this study if they were suffering from malignant disease, sarcoidosis, and amyloidosis, had a history of malignancy and other factors that may affect levels of tumor markers, had a recent clinically significant infection (HIV, viral hepatitis, etc.), severe liver and kidney dysfunction, or other rheumatic diseases.
Data collection
The clinical features of all enrolled individuals were carefully collected, including age, gender, pSS duration, clinical manifestations (dry mouth, dry eyes, respiratory and other systems manifestations), and EULAR Sjögren’s syndrome disease activity index (ESSDAI).
Patients also underwent biochemical assessment and collected the general biological data, including white blood count (WBC), hemoglobin (Hb), platelet (PLT), lymphocyte (LY), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), immunoglobulin (Ig) G, IgM, IgA, complement (C) 3, C4, and the levels of tumor markers, including CA153, CA125, CA19-9, CEA, NSE, β-human chorionic gonadotropin (β-HCG), alpha fetoprotein (AFP), CA724, complexed prostate specific antigen (cPSA). The normal ranges of each tumor associated antigen (TAA) are presented as follows: CA153 <35 KU/L, CA125 <35 KU/L, CA19-9 <35 KU/L, CEA <5 ng/mL, NSE <13 ng/mL, β-HCG <3 ng/mL, AFP <20 ng/mL, CA724 <6.9 U/mL, and cPSA <0.3 ng/mL.
Statistical analysis
SPSS 22.0 software was used for statistical analysis. Quantitative data was expressed as mean ± SD for normal or near-normal distribution data or the median (range) for non-normal distribution data. Categorical data was expressed as frequencies. Independent samples t-test or Mann–Whitney U test were used to analyze the quantitative data, and frequencies were analyzed by using chi-squared test. Spearman analysis was used to analyze the correlation between tumor markers and disease activity. The correlation between two variables was measured by correlation coefficient values (range from –1 to 1). Logistic regression analysis is a statistical method to predict the probability of a disease by exploring its risk factors. The strength of correlation between pSS-ILD and tumor markers and the predicted probabilities of tumor markers were calculated by constructing a logistic regression with whether or not pSS patients had ILD as the dependent variable and a single or different combinations of tumor markers with statistically significant difference between ILD and non-ILD group as the covariates. Receiver operating characteristic (ROC) curve (the test variables were the predicted probabilities of the single or combined factors and the state variable was whether or not pSS patients had ILD) was generated to analyze the discriminatory power for tumor markers. p(two-tailed) <0.05 was considered statistically significant.
Results
Characteristics of patients
The mean age of 168 pSS-ILD patients (90.5% females and 9.5% males) was 60.39 ± 7.94 years and that of 538 pSS-non-ILD cases (93.5% females and 6.5% males) was 60.46 ± 7.83 years (p > 0.05), which showed a prevalence of ILD in pSS of about 24%. A total of 123 patients (73%) with pSS-ILD presented with typical or atypical pulmonary symptoms and signs, including cough (68%), sputum (34%), progressive shortness of breath (44%), hemoptysis (1%), pleuritic pain (5%) and inspiratory crackles (41%), while others (27%) presented asymptomatic. As summarized in Table 1, both groups were comparable in terms of demographic data and other baseline variables. Patients with pSS-ILD had higher levels of ESSDAI, WBC, PLT, ESR, CRP, and IgG and lower level of LY as compared with pSS-non-ILD (p < 0.05), while other general biological indexes, such as duration, Hb, IgA, IgM, C3, and C4 were not statistically different between the two groups (p > 0.05) (Table 1).
Table 1.
A summary of baseline demographics and disease characteristics of all enrolled pSS patients with or without ILD.
| pSS with ILD n=168 |
pSS without ILD n=538 |
t/Z/χ2 | p | |
|---|---|---|---|---|
| Gender, female/male | 152/16 | 503/35 | 1.740 | 0.187 |
| Age, years, mean ± SD | 60.39 ± 7.94 | 60.46 ± 7.83 | 0.098 | 0.922 |
| Duration, months, median (range) | 60 (2, 276) | 72 (1, 600) | 0.766 | 0.444 |
| ESSDAI, median (range) | 5 (1, 18) | 4 (1, 11) | 3.795 | <0.001 |
| WBC, ×109/L, median (range) | 5.8 (1.3, 21.9) | 5.0 (1.3, 16.7) | 3.605 | <0.001 |
| Hb, g/L, median (range) | 125 (38, 169) | 125 (46, 165) | 0.439 | 0.661 |
| PLT, ×109/L, median (range) | 214 (49, 759) | 191 (7, 1010) | 3.791 | <0.001 |
| LY, ×109/L, median (range) | 1.55 (0.26, 3.69) | 1.61 (0.09, 51.04) | 2.183 | 0.029 |
| ESR, mm/h, median (range) | 37 (2, 120) | 28 (1, 242) | 3.994 | <0.001 |
| CRP, mg/L, median (range) | 7 (1, 278) | 3 (1, 143) | 6.772 | <0.001 |
| IgG, g/L, median (range) | 17.0 (5.7, 70.3) | 13.9 (0.1, 57.5) | 5.289 | <0.001 |
| IgA, g/L, median (range) | 3.0 (0.2, 13.8) | 2.7 (0.2, 334.0) | 1.359 | 0.174 |
| IgM, g/L, median (range) | 1.3 (0.4, 22.0) | 1.1 (0.1, 9.3) | 1.566 | 0.117 |
| C3, g/L, median (range) | 0.85 (0.03, 1.92) | 0.86 (0.11, 1.65) | 0.003 | 0.998 |
| C4, g/L, median (range) | 0.20 (0.01, 25.70) | 0.19 (0.01, 93.70) | 1.640 | 0.101 |
C, complement; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ESSDAI, EULAR Sjögren’s syndrome disease activity index; Hb, hemoglobin; Ig, immunoglobulin; ILD, interstitial lung disease; LY, lymphocyte; PLT, platelet; pSS, primary Sjögren’s syndrome; WBC, white blood count.
Association between serum tumor markers and disease activity indicators
Serum levels of NSE (p < 0.001), CEA (p < 0.001), CA125 (p = 0.004), and CA153 (p < 0.001) were elevated in pSS-ILD patients compared with pSS-non-ILD patients. No significant difference was observed in the serum levels of CA199 (p = 0.139), β-HCG (p = 0.610), AFP (p = 0.495), CA724 (p = 0.535), and cPSA (p = 0.676). In addition, the study evaluated the cPSA levels in both genders and found that male patients had higher levels of PSA than female patients in the same group (Supplemental Table 1). However, there was no difference in PSA levels between patients of the same gender and different groups (p > 0.05). Further analysis showed that there was no obvious correlation between the above differential tumor markers and disease activity indicator ESSDAI in all pSS patients, and some tumor markers also had low correlation with some disease activity indexes (p < 0.05), such as CRP, IgM, and C3, which suggested that these differential tumor markers might be involved in the pathogenesis of the ILD in patients with pSS rather than pSS (Tables 2 and 3) and they may be used in the early diagnosis of pSS-ILD.
Table 2.
Tumor markers of included patients of pSS with or without ILD [median (range)].
| pSS with ILD n=168 |
pSS without ILD n=538 |
Z | p | |
|---|---|---|---|---|
| CA199, KU/L | 8.52 (2.00, 110.06) | 8.12 (2.00, 58.26) | 1.481 | 0.139 |
| NSE, ng/mL | 3.63 (0.73, 13.00) | 2.16 (1.00, 7.99) | 6.937 | <0.001 |
| CEA, ng/mL | 1.82 (0.20, 88.91) | 1.49 (0.20, 4.30) | 4.409 | <0.001 |
| β-HCG, ng/mL | 0.32 (0.30, 2.63) | 0.30 (0.30, 117.00) | 1.776 | 0.076 |
| AFP, ng/mL | 2.16 (0.60, 79.24) | 2.37 (0.60, 20.65) | 1.212 | 0.225 |
| CA724, U/mL | 1.45 (0.60, 41.03) | 1.37 (0.30, 80.00) | 1.281 | 0.200 |
| cPSA, ng/mL | 0.31 (0.01, 2.60) | 0.30 (0.30, 9.50) | 1.264 | 0.206 |
| CA125, KU/L | 6.43 (0.62, 47.75) | 5.53 (3.00, 47.83) | 2.845 | 0.004 |
| CA153, KU/L | 8.06 (1.18, 92.91) | 4.68 (0.43, 24.97) | 9.568 | <0.001 |
AFP, alpha fetoprotein; β-HCG, beta-human chorionic gonadotropin; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; cPSA, complexed prostate specific antigen; ILD, interstitial lung disease; NSE, neuron-specific enolase; pSS, primary Sjögren’s syndrome.
Table 3.
Correlation coefficient values of tumor markers and disease characteristics of primary Sjögren’s syndrome.
| NSE | CEA | CA125 | CA153 | |
|---|---|---|---|---|
| Age, years | –0.045 | 0.303*** | –0.037 | –0.022 |
| Duration, months | –0.090 | 0.072 | –0.009 | –0.124 |
| ESSDAI | 0.105 | 0.017 | –0.031 | 0.078 |
| ESR, mm/h | –0.047 | 0.041 | 0.022 | 0.101 |
| CRP, mg/L | 0.028 | 0.015 | 0.166* | 0.043 |
| IgG, g/L | –0.088 | –0.076 | –0.101 | 0.066 |
| IgA, g/L | 0.066 | –0.113 | –0.075 | 0.070 |
| IgM, g/L | –0.015 | 0.060 | 0.231** | 0.149 |
| C3, g/L | 0.008 | –0.225* | –0.035 | –0.134 |
| C4, g/L | 0.117 | –0.140 | 0.005 | 0.071 |
p < 0.05;
p < 0.01;
p < 0.001.
C, complement; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; ESSDAI, EULAR Sjögren’s syndrome disease activity index; Ig, immunoglobulin; NSE, neuron-specific enolase.
We evaluated the strength of correlation between pSS-ILD and tumor markers by using logistic regression analysis. The serum levels of CA153 [odds ratio (OR) = 4.521, 95% confidence interval (CI) = (1.871, 10.928)] and CEA [OR = 2.879, 95% CI = (1.305, 6.353)] were significantly associated with pSS-ILD, suggesting that higher CA153 and CEA levels increased the risk of pSS-ILD (Table 4).
Table 4.
Logistic regression analysis of the association of primary Sjögren’s syndrome-associated interstitial lung disease and tumor markers.
| Variate | OR | 95% CI | p |
|---|---|---|---|
| CA153 | 4.521 | (1.871, 10.928) | <0.001 |
| NSE | 3.242 | (0.648, 16.218) | 0.130 |
| CEA | 2.879 | (1.305, 6.353) | 0.060 |
| CA125 | 1.148 | (0.407, 3.236) | 0.794 |
CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CI, confidence interval; NSE, neuron-specific enolase; OR, odds ratio.
Clinical values of tumor markers in diagnosis of ILD in pSS patients
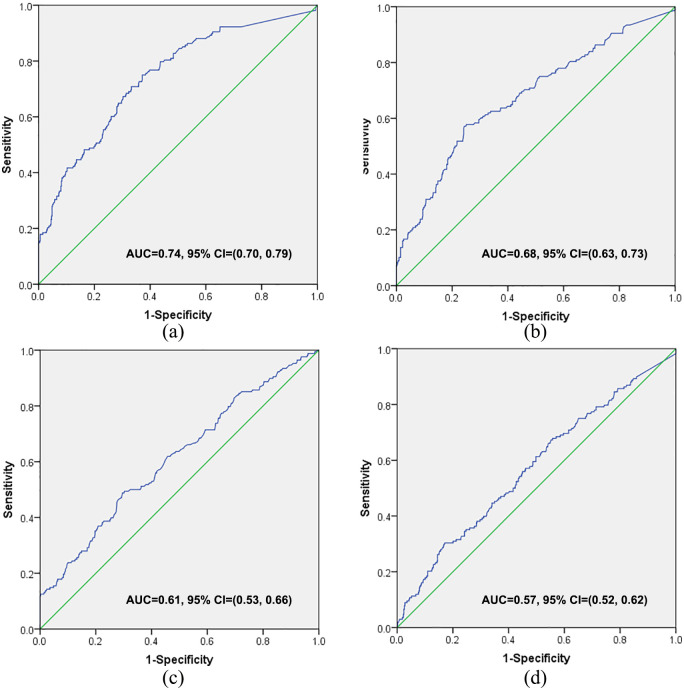
A ROC assessment was performed to evaluate the diagnostic value of studied tumor markers, including CA153, CEA, NSE, and CA125. Among them, CA153 was the only tumor marker with area under the ROC curve (AUC) over 0.7 [AUC = 0.743, 95% CI = (0.70, 0.79)], and not other tumor markers, such as CEA [AUC = 0.613, 95% CI = (0.56, 0.66)], NSE [AUC = 0.677, 95% CI = (0.63, 0.73)] (Figure 1). The diagnostic value of CA153 was significantly superior to that of other tumor markers (p < 0.05) (Supplemental Table 2).
Figure 1.
The predictive capacity of levels of tumor markers for the presence of interstitial lung disease (ILD) in patients with primary Sjögren’s syndrome (pSS). Carbohydrate antigen (CA)153 (a), neuron-specific enolase (b), carcinoembryonic antigen (c), and CA125 (d) were of certain diagnostic value for the presence of pSS-associated ILD, but CA153 was the best one.
AUC, area under the receiver operating characteristic curve; CI, confidence interval.
In order to improve the diagnostic value of tumor markers, we examined the AUC in different combinations of four differential tumor markers, including CA153, CA125, NSE, and CEA. The largest AUC was 0.777 [95% CI = (0.74, 0.82)] when diagnosed using CEA combined with NSE and CA125 or a combination of four tumor markers, which was not statistically significant compared with that of CA153 (p = 0.264) (Supplemental Table 2). These results suggested that the diagnostic value of CA153 alone for pSS-ILD without benign and malignant tumor was not weaker or even better than that of other tumor markers alone or combined.
Discussion
The pathogenesis, diagnosis, and treatment of ILD that can complicate the diagnosis and treatment of CTD have attracted attention, especially in SSc-associated ILD because ILD is the leading cause (about 35%) of disease-related deaths in SSc that is characterized by tissue injury.11 Actually, ILD was also a significant cause of death in pSS, one of the most prevalent multisystem autoimmune disease after rheumatoid arthritis;12–15 unfortunately, the research on pSS-ILD is very sparse.
In the present study, the incidence of pSS-ILD patients was approximately 24%, which was similar to that of the study by Ito et al. in 2004.2 However, respiratory symptoms such as cough, wheezing, or dyspnea are not obvious in most pSS-ILD patients, which results in a lower incidence than the actual situation. We found that pSS-ILD patients had higher levels of disease activity indicators, including ESSDAI, ESR, CRP, and WBC, and lower levels of lymphocyte cells in peripheral blood, suggesting that ILD might aggravate the primary disease or, contrarily, higher disease activity leads to ILD. Therefore, it is worthwhile to identify and confirm the risk factors for systematic screening of pulmonary involvement in pSS patients, for both diagnosis and management at an early stage of ILD.16
The pathogenesis of pSS-ILD is still unclear. It has been reported that older age,8 autoantibodies such as anti-SSA antibody17and rheumatoid factor (RF),8 cigarette smoking18 and some treatment drugs for rheumatology diseases such as methotrexate19 may be involved in the incidence of ILD, but those were controversial. In recent years, tumor markers have been found to be associated with ILD in CTD.7,20,21 We found that the levels of serum tumor markers, including CA125, CA153, NSE, and CEA, were lower in pSS-ILD patients than in non-ILD pSS patients. None of these pSS-ILD patients with elevated tumor markers had detectable tumors. Obviously, the pSS patients with higher levels of serum CA153 and CEA had about four-fold and three-fold risk of pSS-ILD respectively by using logistic regression analysis, which could be used to be the risk factors for this disease. Although only few recent studies have focused on the correlation between serum tumor markers and pSS-ILD, their results were consistent with the observation that tumor markers, especially CA153, were increased in patients with ILD22,23 and some CTD-associated ILDs. Dai et al.24 reported that serum CEA and CA125 levels are often elevated in ILD patients without cancer. Sargin et al.7 also observed that patients with increase in tumor markers, especially CA153 and CA125, but not smoking rate, CRP, RF, or anti-CCP levels, should be considered for the presence of pulmonary involvement, especially ILD, rather than just a malignancy in RA patients. Another study, conducted by Lim et al.,20 demonstrated that tumor markers were not useful in malignancy screening or dermatomyositis/polymyositis (DM/PM) patients in this tertiary center, while a raised level of CA153 may be a potential indicator of the presence of ILD in those patients. Tumor markers are not only risk factors for ILD, but may also be a diagnostic indicator for early ILD with no obvious abnormality on HRCT. Serum CA153 has the superior diagnostic value in pSS-ILD patients, compared with other tumor markers.
Tumor markers are considered as biological substances synthesized and released by tumor cells or other cells in response to tumor tissue. So, in other words, tumor markers can also be produced by inflammatory cells, not just cancer cells. This may be one of the reasons why tumor markers, which may play a role in the perpetuation of inflammation, have been observed to be elevated in immune-related diseases without cancer.25 It seems to be a consensus that the main pathogenesis of ILD is the co-existence of continuous damage, over-repair and apoptosis of epithelial cells, and the formation of fibroblast foci.26 Also, CAl53, CA125 and CEA exactly reflect the proliferation and secretion of epithelial cells, which provides a novel concept to further explore the correlation between tumor markers and ILD.
In addition, the value of higher leukocyte counts in assisting in the evaluation of pSS-ILD is limited due to the large number of leukocyte classes (neutrophils, eosinophils, lymphocytes, etc.) and their susceptibility to a variety of factors, especially pathogen infection. Our previous research has confirmed that the reduced number and/or dysfunction of lymphocyte subsets, especially CD4 + CD25 + Foxp3 + Treg cells, were involved in the pathogenesis and disease activity of patients with pSS.27 Therefore, we will next analyze the absolute number of CD4+T lymphocyte subsets detected by modified flow cytometry in all patients to further assess the diagnostic and therapeutic value of lymphocytes in pSS-ILD patients.
Since this was a retrospective study, we were unable to follow up the occurrence of cancer and the influence of therapeutic drugs on the tumor marker in all enrolled patients in the following years. Longitudinal studies with larger sample size of lung biopsy are needed to explore the actual relationships between pSS-ILD and tumor markers and possible mechanisms. Although the diagnostic value of CA153 was obtained by ROC curve in this study, its specificity and sensitivity in clinical application need to be further observed due to the limitations of statistical methods and the differences in the calculation methods of the cut-off value. The lack of detailed data on smoking and health controls is an important limitation of this research, which we will supplement in future works.
Conclusion
The levels of serum CA125, CA153, CEA, and NSE were elevated in patients with pSS-ILD as compared with those in pSS without ILD. Higher CA153 and NSE levels are significantly related to the increased risk of ILD in patients with pSS, rather than the ESSDAI. Thus, those tumor markers may be directly involved in the pathogenesis of pSS-ILD. Furthermore, the diagnosis of serum CA153 was superior to any kind of tumor marker, suggesting that pSS patients with higher levels of serum CA153 may have a higher risk of ILD in the absence of other causes, such as benign and malignant tumors.
Supplemental Material
Supplemental material, supplementary_table_2 for Increases in tumor markers are associated with primary Sjögren’s syndrome-associated interstitial lung disease by Lei Shi, Xiao-Lei Han, Hong-Xia Guo, Jia Wang, Yu-Ping Tang, Chong Gao and Xiao-Feng Li in Therapeutic Advances in Chronic Disease
Footnotes
Author contribution: Study design and manuscript writing: LS, XH, and HG. Data extraction, quality assessment, analysis and interpretation of data: LS, XH, HG, and JW. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. XLi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: The Ethics Committee of the Second Hospital of Shanxi Medical University waived the need for ethics approval and the need to obtain consent for the collection, analysis and publication of the retrospectively obtained and anonymized data for this non-interventional study.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jia Wang  https://orcid.org/0000-0003-2181-0750
https://orcid.org/0000-0003-2181-0750
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lei Shi, Department of Rheumatology, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Xiao-Lei Han, Department of Mental Health, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Hong-Xia Guo, Department of Cardiology, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Jia Wang, Department of Rheumatology, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Yu-Ping Tang, Department of Rheumatology, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Chong Gao, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Xiao-Feng Li, Department of Rheumatology, The Second Hospital of Shanxi Medical University, 382 Wuyi Road, Xinghualing District, 030000, Taiyuan, Shanxi, China.
References
- 1. Nezos A, Mavragani CP. Contribution of genetic factors to Sjogren’s syndrome and Sjogren’s Syndrome Related lymphomagenesis. J Immunol Res 2015; 2015: 754825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ito I, Nagai S, Kitaichi M, et al. Pulmonary manifestations of primary Sjogren’s syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med 2005; 171: 632–638. [DOI] [PubMed] [Google Scholar]
- 3. Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther 2010; 12: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta S, Ferrada MA, Hasni SA. Pulmonary manifestations of primary Sjogren’s syndrome: underlying immunological mechanisms, clinical presentation, and management. Front Immunol 2019; 10: 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiong W, Zhao Y, Xu M, et al. The relationship between tumor markers and pulmonary embolism in lung cancer. Oncotarget 2017; 8: 41412–41421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang W, Chen XL, Zhao SY, et al. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget 2016; 7: 35423–35436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sargin G, Kose R, Senturk T. Tumor-associated antigens in rheumatoid arthritis interstitial lung disease or malignancy? Arch Rheumatol 2018; 33: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang T, Zheng XJ, Ji YL, et al. Tumour markers in rheumatoid arthritis-associated interstitial lung disease. Clin Exp Rheumatol 2016; 34: 587–591. [PubMed] [Google Scholar]
- 9. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69: 1809–1815. [DOI] [PubMed] [Google Scholar]
- 12. Bowman SJ, Ibrahim GH, Holmes G, et al. Estimating the prevalence among Caucasian women of primary Sjogren’s syndrome in two general practices in Birmingham, UK. Scand J Rheumatol 2004; 33: 39–43. [DOI] [PubMed] [Google Scholar]
- 13. Maciel G, Crowson CS, Matteson EL, et al. Prevalence of primary Sjogren’s syndrome in a US population-based cohort. Arthritis Care Res (Hoboken) 2017; 69: 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjogren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 1983–1989. [DOI] [PubMed] [Google Scholar]
- 15. Flament T, Bigot A, Chaigne B, et al. Pulmonary manifestations of Sjogren’s syndrome. Eur Respir Rev 2016; 25: 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roca F, Dominique S, Schmidt J, et al. Interstitial lung disease in primary Sjogren’s syndrome. Autoimmun Rev 2017; 16: 48–54. [DOI] [PubMed] [Google Scholar]
- 17. Boitiaux JF, Debray MP, Nicaise-Roland P, et al. Idiopathic interstitial lung disease with anti-SSA antibody. Rheumatology (Oxford) 2011; 50: 2245–2250. [DOI] [PubMed] [Google Scholar]
- 18. Saag KG, Kolluri S, Koehnke RK, et al. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis Rheum 1996; 39: 1711–1719. [DOI] [PubMed] [Google Scholar]
- 19. Dayton CS, Schwartz DA, Sprince NL, et al. Low-dose methotrexate may cause air trapping in patients with rheumatoid arthritis. Am J Respir Crit Care Med 1995; 151: 1189–1193. [DOI] [PubMed] [Google Scholar]
- 20. Lim CH, Tseng CW, Lin CT, et al. The clinical application of tumor markers in the screening of malignancies and interstitial lung disease of dermatomyositis/polymyositis patients: a retrospective study. SAGE Open Med 2018; 6: 2050312118781895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Celeste S, Santaniello A, Caronni M, et al. Carbohydrate antigen 15.3 as a serum biomarker of interstitial lung disease in systemic sclerosis patients. Eur J Intern Med 2013; 24: 671–676. [DOI] [PubMed] [Google Scholar]
- 22. Okada M, Suzuki K, Nakanishi T, et al. Serum levels of KL-6 are positively correlated with those of CA15-3 in patients with interstitial pneumonia associated with collagen diseases. Respirology 2006; 11: 509–510. [DOI] [PubMed] [Google Scholar]
- 23. Wong RC, Klingberg S, Wilson R. CA15-3 and cancer associated serum antigen assays are alternatives to the KL-6 assay for measuring serum MUC-1 levels in patients with interstitial lung disease associated with polymyositis/dermatomyositis. J Rheumatol 2002; 29: 2021–2022. [PubMed] [Google Scholar]
- 24. Dai H, Liu J, Liang L, et al. Increased lung cancer risk in patients with interstitial lung disease and elevated CEA and CA125 serum tumour markers. Respirology 2014; 19: 707–713. [DOI] [PubMed] [Google Scholar]
- 25. Szekanecz E, Sandor Z, Antal-Szalmas P, et al. Increased production of the soluble tumor-associated antigens CA19-9, CA125, and CA15-3 in rheumatoid arthritis: potential adhesion molecules in synovial inflammation? Ann N Y Acad Sci 2007; 1108: 359–371. [DOI] [PubMed] [Google Scholar]
- 26. Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest 2009; 136: 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miao M, Hao Z, Guo Y, et al. Short-term and low-dose IL-2 therapy restores the Th17/Treg balance in the peripheral blood of patients with primary Sjogren’s syndrome. Ann Rheum Dis 2018; 77: 1838–1840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplementary_table_2 for Increases in tumor markers are associated with primary Sjögren’s syndrome-associated interstitial lung disease by Lei Shi, Xiao-Lei Han, Hong-Xia Guo, Jia Wang, Yu-Ping Tang, Chong Gao and Xiao-Feng Li in Therapeutic Advances in Chronic Disease