Abstract
Malignant tumors pose a major problem in the medical field. Millimeter wave (MMW) exposure have potential apoptosis-promoting effects on several types of tumors. Considering that the penetration depth of millimeter wave is usually several millimeters, we study the apoptosis-promoting effects of millimeter wave exposure on A375 human melanoma tumor cells in vitro, and this topic has not been explored in the previous literature. In this study, we use the A375 human melanoma cell line as an experimental model exposed to 35.2 GHz millimeter wave in vitro to determine any positive effect and further explore the underlying mechanisms. In this study, 2 groups namely, exposed and sham groups, were set. The exposed groups included 4 exposure time periods of 15, 30, 60, and 90 minutes. The cells in the sham group did not receive millimeter wave exposure. After millimeter wave exposure, the A375 cells in the exposed and sham groups were collected for further experimental procedures. The cell viability after exposure was determined using a cell counting kit, and the apoptosis of A375 cells was assessed by Annexin V/propidium iodide. Changes in the expression of apoptosis-related proteins, including cleaved-caspase-3, and -8, were examined by Western blot. We observed that the millimeter wave exposure could inhibit the viability and induce apoptosis in A375 cells, and the expression of cleaved caspase-3 and -8 were upregulated (P < .05). The results indicated that the millimeter wave at 35.2 GHz exerted apoptosis-promoting effects on the A375 cells via a pathway by activating of caspase-8 and -3.
Keywords: apoptosis, caspase, human melanoma cells, millimeter wave, viability
Introduction
Three main methods can be used for the treatment of the malignant tumors, but this process is still challenging. Millimeter wave (MMW) is a type of electromagnetic field with the frequency range of 30 to 300 GHz. Millimeter wave may cause various nonthermal biological effects on organisms.1-3 Several nonthermal biological effects have been applied in medical conditions. For example, MMW is widely applied as a therapeutic approach for diabetes, peptic ulcer, and wound healing.4,5 Several nonthermal biological effects of MMW are still being explored, and these studies have shown potential clinical application.6-12 For example, the tumor-suppression effects of the MMW on cell growth in vitro and tumor metastasis in vivo have been reported on certain tumoral cell systems. These findings have spurred our interest in exploring MMW as a new potential approach for the treatment of cancerous diseases.
Although related research is available, complex mechanisms underlie the nonthermal effects on tumor cells of MMW. Several studies are related to the cell environment. Some researchers have hypothesized that the changes in the physicochemical properties of water in a medium play an important role. Upon exposure to MMW, the biochemical reactions in water could affect the viability and induce a series of morphological and molecular changes in the cells.13-16 Several studies have explored the changes in the cells, such as the cell viability and apoptosis. The viability in certain tumor cell lines is inhibited by regulating apoptosis.17,18 Zhu demonstrated that wide-band MMW exposure increases the activity of caspases-3 and -9, which are the central executioners of the apoptotic pathway. The treatment also upregulated the expression of Bax and downregulated the expression of Bcl-2 in pancreatic cancer JF305 cells.19,20 They proposed that MMW exposure induces apoptosis via a mitochondria-mediated pathway.18 Similarly, Li et al 17 reported that MMW exposure inhibits the viability of human chondrosarcoma SW1353 cells. Moreover, MMW exposure on SW1353 cells decreases the membrane potential, upregulates the expression of Bax, and increases the activity of caspase-9 and -3 but does not remarkably affect the expression of Bcl-2.
The use of nonthermal biological effects of the MMW as a tumor-suppression approach is highly desirable. In this study, electromagnetic simulation and biological experiments were combined to further explore the relationship between MMW exposure and tumor cells. However, the MMW only affects the epidermis and dermis skin structures at penetration depth of up to 1 mm.21 Accordingly, the A375 cell line, which usually originates from the epidermis, dermis, subcutaneous tissue, or even skin appendages, was studied.
Materials and Methods
Electromagnetic Simulation
The qualified dosimetry distribution in the Petri dish is the precondition for the experiments in vitro. To obtain a uniformly distributed MMW exposure in the Petri dish, we carried out electromagnetic simulation to determine the practical exposure parameters with satisfactory homogeneity and intensity for subsequent experiments in vitro. The exposure mode was fixed and propagated from the base panel of the Petri dish.11 The cells were exposed in monolayer because the A375 cell line could grow and adhere to the base panel of the Petri dish. The simulation was used to explore the influence of the different distances between the base panel of the Petri dish and the antenna on the exposure dose absorbed by the A375 cells. The MMW dose absorbed by A375 cells in the Petri dish was quantified by the specific absorption rate (SAR).
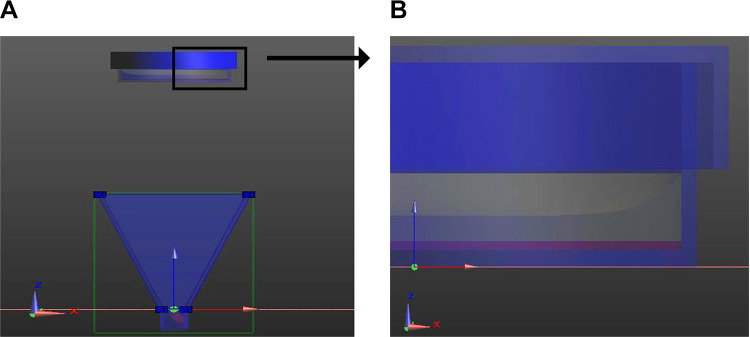
The KFA-100A MMW therapeutic instrument (Zhongcheng Kangfu Technology) was utilized. The device generated a linearly polarized and continuous sinusoidal MMW at 35.2 GHz. The device can stably generate a linearly polarized and continuous sinusoidal MMW at 35.2 GHz, which is within the frequency scope of MMW. The half-width of the 35.2-GHz signal was 110 to 115 MHz. The aperture of the antenna has a length of 46.6 mm and width of 35.2 mm. Figure 1 shows the exposure setup, including the Petri dish and the antenna (the conical blue structure) for the MMW. The cell layer was on the upper surface of the base panel of the 35-mm Petri dish with culture medium (Figure 1B shows the partial magnification of the Petri dish). We also considered the meniscus formed by the contact of the culture medium and the wall of the Petri dish to achieve an accurate model.22
Figure 1.
Electromagnetic simulation setup (unit: mm). A, Model for simulation calculation. The distance between the Petri dish and antenna was adjustable, and the direction of exposure was upward. B, Enlargement of the Petri dish showing the culture medium; the cell layer is displayed in purple.
The distance between the Petri dish and the antenna was adjusted to 0.75, 10, 30, 50, 70, and 100 mm. The finite difference time domain and uniaxial perfectly matched layers of absorbing boundary conditions were applied to calculate the SAR.23 The SAR in the cell layer was calculated as follows:24
| 1 |
where E is the electric field intensity and σ and ρ are the permeability and density of the cells, respectively. Briefly, the simulation was implemented by focusing on the culture medium and cell layer. Thus, the voxel size in the simulation model was set to 0.165 mm in the cell layer and 1.299 mm in the culture medium. The parameters set in the simulation algorithm and SAR calculation are summarized in Table 1.
Table 1.
Relative Dielectric Constant, Conductivity, and Density of Various Materials.
| Relative permittivity εr | Conductivity, S/m | Density ρ, kg/m3 | |
|---|---|---|---|
| Cell25 | 13.50 | 29.60 | 1109.00 |
| DMEM26 | 21.00 | 59.60 | 1000.00 |
| Petri dish27 | 3.11 | 0.00 | 1000.00 |
Abbreviation: DMEM, Dulbecco modified Eagles medium.
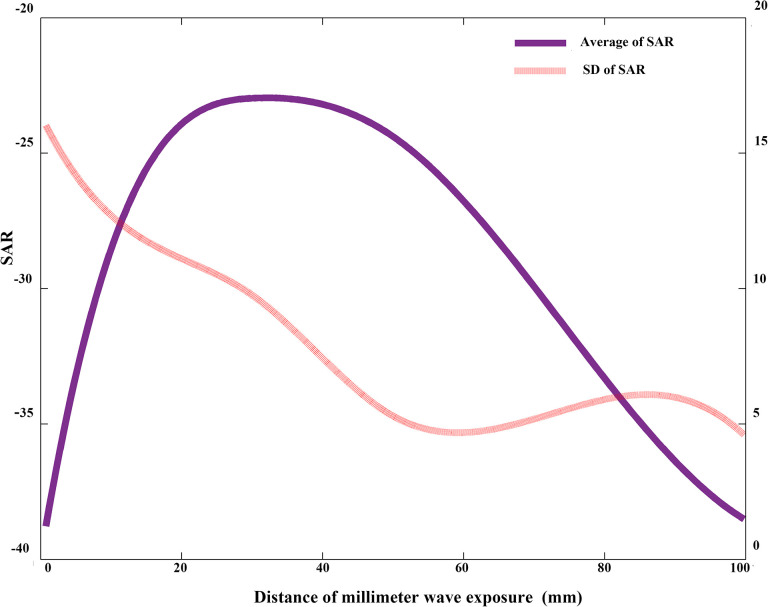
The homogeneity and intensity of the distribution of SAR in the cell layer were evaluated in terms of the standard deviation (SD) and the average value of the SAR. The normalized value of the SDs of the SARs with respect to different distances between the Petri dish and the antenna are shown in Figure 2. The simulation results indicated that the SAR homogeneity increased with the distance. The normalized value of the average SARs based on the different distances between the Petri dish and the antenna are also shown in Figure 2. The simulation results indicated that the intensity was satisfactory between 30 and 60 mm. Thus, the distance of 50 mm with satisfactory homogeneity and intensity was chosen in our subsequent practical experiment in vitro. The cellular samples were exposed under near-field conditions at the distance of 50 mm, and the boundary of the far- and near field was calculated using the Fresnel distance.11 Under this condition, the experimental power density at the base panel of the Petri dish was 0.16 mW/cm2.
Figure 2.
Relationship between the distance and its corresponding specific absorption rate (SAR) in the cell layer. The normalized values of the standard deviation (SD) of SARs and average SARs were plotted. The distance between the antenna and the base panel of the Petri dish was considered.
Cell Culture and Cell Growth Curve
The A375 cell line was purchased from the Cell Bank of the Chinese Academy of Sciences. The cells were cultured in Dulbecco modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum, 100 µ/mL of penicillin, and 100 µ/mL of streptomycin at 37 °C in a humidified atmosphere with 5% CO2. The cells from the stock flask were suspended in trypsin, buffered with phosphate-buffered saline (PBS), and counted using a hemocytometer.
The A375 cells were grown in plates at a density of 5 × 103 cells/well and incubated for 24 hours to obtain the growth curve. The optical density (OD) of the corresponding days was detected using cell counting kit (CCK-8; Dojindo) every 24 hours through the following steps. First, 10 µL of the CCK-8 solution was added into the cell culture medium in each well. Then, the plates were incubated for 2 hours at 37 °C. Finally, the OD was determined using a microplate reader at 450 nm after incubation. The average value of 5 duplications was calculated, and the growth curve was drawn after 6 consecutive days.
Millimeter Wave Exposure
The antenna was placed at 50 mm from the Petri dish according to the simulation experiment to form an upward exposure. The cells in the exposed group were subjected to continuous wave in the near field. The A375 cells were divided into the exposed and sham groups with MMW exposure duration of 0 minutes. The exposed group was placed in a humidified and sterile incubator at 37 °C. The antenna of the MMW therapeutic instrument was placed below the Petri dishes to form the upward exposure as recommended in the electromagnetic simulation. The sham group was kept under the same experimental conditions and geometrical position, but the generator was switched off. The temperature of the samples during MMW exposure was measured using a calibrated thermocouple immersed in the medium. The rise in temperature throughout the 90-minute exposure of the cell layer did not exceed 0.1 °C. No significant increments were observed, indicating that this study mainly focused on the nonthermal effects.28
Cell Viability Assays
The effects of MMW exposure on the viability of A375 cells were investigated. The A375 melanoma cells in the exposed and sham groups were first seeded at 5 × 104 cells in the 35-mm Petri dish. The exposed group was subjected to 35.2-GHz MMW treatment for 15, 30, 60, and 90 minutes using 35.2-GHz MMW, while the sham group was kept under the same experimental conditions, except that the generator was switched off. Then, the A375 cell suspensions from both groups were removed, transferred into 96-well plates, and cultured for 4 hours at 37 °C in the incubator. The viability was assessed using CCK-8 (Dojindo). The OD value was obtained through the same procedure as that in cell growth curve. By using the obtained OD of the exposed and sham groups, the viability rates of these groups were calculated as follows:
| 2 |
where OD exposed and OD sham are the ODs of the exposed and sham group, respectively. A viability rate smaller than 100% indicated that the cell growth of A375 cells was inhibited by 35.2-GHz MMW exposure.
Flow Cytometry Analysis of Cell Apoptosis
Flow cytometry analysis was conducted using the Annexin V/ propidium iodide (PI) apoptosis detection kit (BD Biosciences Pharmingen) to further confirm that the suppression of cell viability was induced by apoptosis. The A375 cells were seeded at 5 × 104 cells in 35-mm Petri dish. Then, the exposed group was subjected to MMW for 15, 30, 60, and 90 minutes in the logarithmic growth phase. By contrast, the sham group was kept under the same experimental conditions and geometrical position except that the generator was switched off. After MMW exposure, the cells were incubated at 37 °C with 5% CO2 for 24 hours. The cell suspension obtained from the digested cells was centrifuged at 1000 ×g for 5 minutes, followed by resuspension with 1× binding buffer to adjust the concentration of the A375 cells to 1 × 106/mL. Afterward, 0.1 mL of the suspension was transferred to another culture tube and stained with 5 µL of Annexin V-FITC and 5 µL of PI for 15 minutes at 25 °C in the dark. Finally, 400 µL of the 1× binding buffer was added to each tube before analyzing the samples by flow cytometry within 1 hour.
Western Blot Analysis
The expression of certain proteins in the A375 cells was changed when apoptosis occurred. After MMW exposure and 24 hours of culture, Western blot analysis was performed to identify the specific proteins in the A375 cells. The antibodies of caspase-3, caspase-8 (Cell Signaling Technology), and β-actin (Sigma-Aldrich) were used. After exposure for 0, 15, 30, 60, and 90 minutes with 35.2-GHz MMW, the A375 cells were washed twice with precooled PBS at 4 °C and lysed in radio immunoprecipitation assay buffer, followed by centrifugation at 12 000 rpm for 10 minutes at 4 °C. The bicinchoninic acid protein assay kit was used to quantify the concentration of the protein sample. Afterward, the protein was loaded onto 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to polyvinyl difluoride membranes, and then blocked with 5% nonfat dairy milk in Tris-buffered saline with 0.05% Tween-20 for 2 hours. Then, the membranes were incubated overnight with primary antibodies (caspase-3 and -8 antibodies, dilution to 1:1000 with Tris-buffered saline Tween 20 [TBST]) at 4 °C by horizontal shaking. After washing the membranes thrice with TBST, they were incubated with secondary antibodies (Horseradish Peroxidase-conjugated goat anti-rabbit IgG, dilution to 1:2000 with TBST) and shaken gently for 1 hour at room temperature. The membranes were then visualized using an enhanced chemiluminescence kit (Thermo Fisher Scientific) and scanned using the gel imaging system (Bio-Rad). The bands were analyzed using the Image J software 1.8.0.
Analysis of the Role of Caspase-3 Activation
The caspase-3 inhibitor AC-DEVD-fmk was used to determine the role of caspase-3 activation in the MMW-induced apoptosis of A375 cells. Before the MMW exposure for 90 minutes, the A375 cells were pretreated with 10 µmol AC-DEVD-fmk for 1 hour. Then, the cell viability of A375 cells was analyzed using CCK-8 as described previously.
Statistical Analysis
All data were presented as mean ± SD and analyzed by analysis of variance using the IBM SPSS Statistics version 20 software. Differences were considered as statistically significant at P < .05.
Results
Exposure to 35.2-GHz MMW Inhibited the Viability of the A375 Cells
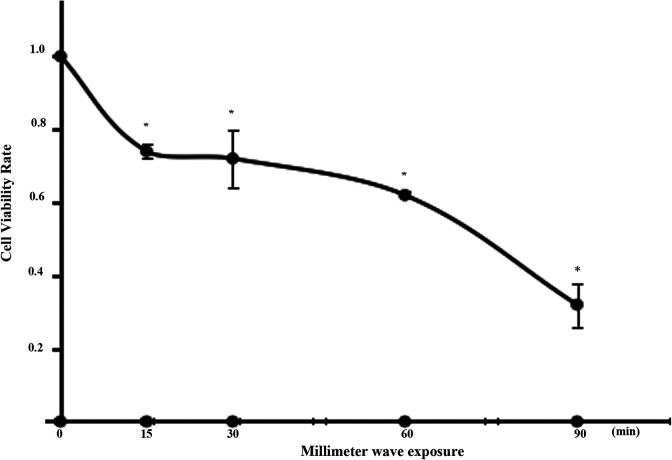
Our analysis of the growth curve of A375 cells showed that from the third day, the cell underwent logarithmic growth. Upon culturing the A375 cells for 3 days, the MMW exposure was carried out. After exposure, the cell viability was measured using the CCK-8 solution and a microplate reader. The influence of 35.2-GHz MMW exposure on the viability of A375 is shown in Figure 3. In this figure, the cell viability of A375 cells decreased with the extension of exposure time (P < .05), indicating the time cumulative effect of 35.2-GHz MMW exposure on the viability of the A375 cells.
Figure 3.
Effect of 35.2-GHz millimeter wave (MMW) exposure on A375 cell viability (relative to the sham group). The cells were seeded in 96-well plate after MMW exposure in monolayer by 35.2-GHz MMW for 0, 15, 30, 60, and 90 minutes. The cell viability was assessed using CCK-8 after MMW exposure for 4 hours. The results are expressed in terms of the ratio of the optical density in the exposed and sham groups. Each point represents the mean ± standard deviation (SD) of 6 replicates, and 3 independent experiments were performed. Values with statistically significant difference (P < .05) are marked with asterisks. CCK-8 indicates cell counting kit-8.
Exposure to 35.2-GHz MMW Induced Apoptosis in A375 Cells
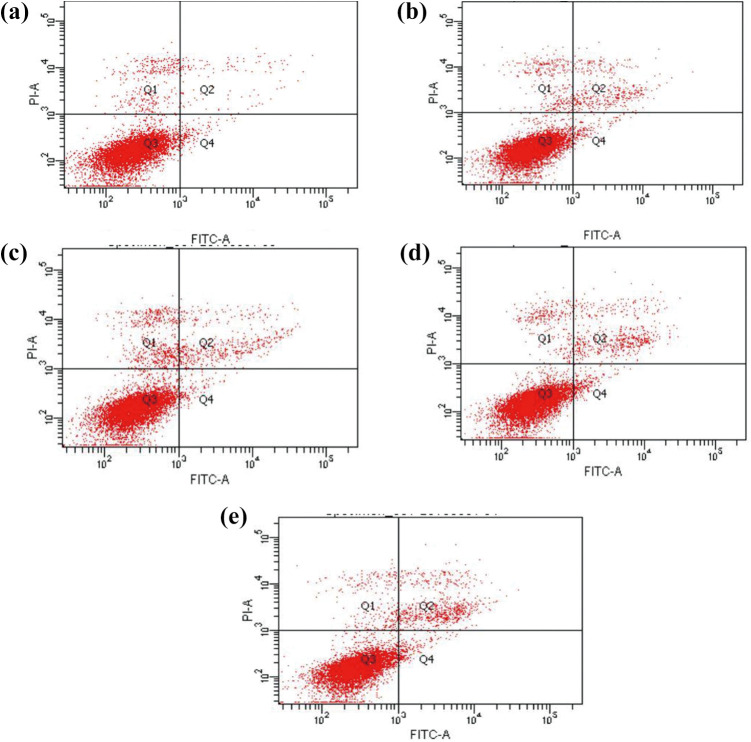
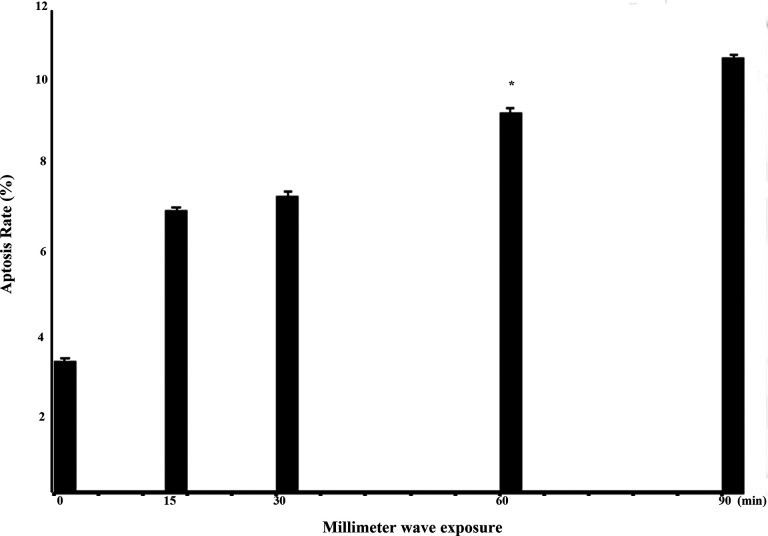
After MMW exposure, the apoptosis of A375 cells in each group was determined by AV/PI staining. Briefly, the cells were exposed with 35.2-GHz MMW in exposed groups for 15, 30, 60, and 90 minutes, and cells in sham group did not receive MMW exposure. Then, the cells were examined by cytometry via AV/PI staining. The statistical analysis of apoptosis in A375 cells induced by 35.2-GHz MMW is shown in Figures 4 and 5. The corresponding apoptosis rates were 7.23%, 7.57%, 9.73%, and 11.14% for the exposed group, and the apoptosis rate was 3.37% in the sham group. Hence, exposure to 35.2-GHz MMW induced apoptosis in A375 cells.
Figure 4.
Effect of 35.2-GHz millimeter wave (MMW) exposure on the apoptosis of A375 cells. After exposure to 35.2-GHz MMW for 15, 30, 60, and 90 minutes (A-E represent exposure for 0, 15, 30, 60, and 90 minutes, respectively), the A375 cells were stained by AV/PI, and then analyzed by flow cytometry.
Figure 5.
Quantification of apoptosis in A375 cells with ANOVA by using SPSS software. The data shown are averages ± standard deviation (SD; error bars). ANOVA indicates analysis of variance.
Exposure to 35.2-GHz MMW Modulated the Expression of Apoptosis-Related Proteins in the A375 Cells
To further investigate how the 35.2-GHz MMW exposure induced apoptosis in A375 cells, we used Western blot to analyze the apoptosis-related proteins in both the exposed and the sham groups. Casepase-3 and -8 play an important role in cell apoptosis. The results are shown in Figure 6. The expression of both caspase-3 and -8 in the exposed group was upregulated, indicating that exposure to 35.2-GHz MMW induced the apoptosis of A375 cells by regulating the caspase pathway.
Figure 6.
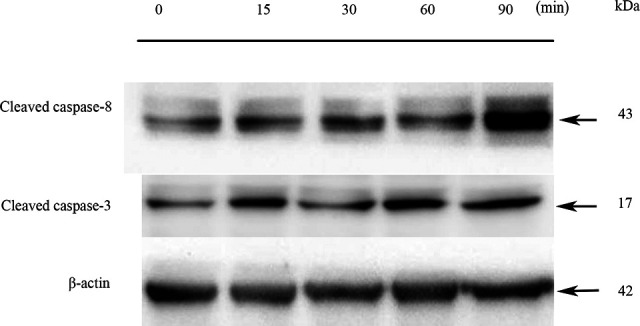
Effect of millimeter wave (MMW) exposure on the expression of caspase-3 and caspase-8 in the A375 cells. After the A375 cell monolayer was exposed to MMW for 0, 15, 30, 60, and 90 minutes and cultured 24 hours, Western blot analysis was performed to analyze the apoptosis rate in the A375 cells. β-actin was used as internal reference.
Caspase-3 Inhibitor Partially Reversed the Cell Viability Suppression Effect Induced by MMW
To verify the role of caspase-3 activation in the suppression of A375 cell viability, we used the inhibitor AC-DEVD-fmk. The result indicated that AC-DEVD-fmk pretreatment partially reversed the suppression effects induced by MMW exposure from 36.7% to 59.8%.
Discussion
Exposure to MMW can suppress the cell viability and inhibit the growth of certain tumor cell lines.6-10 The underlying mechanism was explored, and recent studies demonstrated that the suppression effect on cell viability could be induced by apoptosis in several tumor cell lines.17,18 In the present study, we determined whether 35.2-GHz MMW exposure could affect the growth and induce apoptosis in A375 melanoma cell and explored the mechanisms of the induced apoptosis. Time-dependent reduction in the viability was observed in A375 cells following exposure to 35.2-GHz MMW. The growth rate of A375 cells exposed for 90 minutes was lower than the sham group (approximately 40% of the beginning).
The development of tumor is associated with certain important events, such as cell apoptosis upon the activation of the oncogene. Therefore, the effect of apoptosis is a novel target in the search of antitumor therapy. Apoptosis is a complex process involving many biomolecules. To gain insight into the molecular mechanism involved in the A375 cells exposed with 35.2 GHz MMW, we assessed the expression of apoptosis-related proteins and their changes by Western blot in A375 cells. Caspase-3 is one of the most important executioners in the cell apoptosis procedure.29 This biomolecule can be cleaved and activated by the initiator caspases, such as caspase-8, in multiple different organisms during apoptosis.19,29 Thus, in this study, we focused on the expression changes in caspase-3 and -8 in A375 cells and found that the cleaved caspase-3 and -8 were upregulated in the A375 cells by Western blot. To further verify the dependence of caspase-3 activation in the suppression of A375 cells induced by 35.2-GHz MMW exposure, we added the caspase-3 inhibitor before exposure to 35.2-GHz MMW. The result demonstrated the caspase-3 inhibitor partially reversed the suppression effect on cell viability. These results in vitro indicated that exposure to 35.2-GHz MMW had apoptosis-promoting effects on the A375 cells. The apoptosis induced by 35.2-GHz MMW was caspase dependent because it involved the activation of caspase-8 and -3.
Before the investigation, we evaluated the SAR in the cell layer with different distances between the Petri dish and the antenna by using numerical simulation. The dose received by A375 cells can be represented by the SAR. Cells in different positions of the Petri dish may have different SARs. Subsequently, some cells were affected by MMW exposure, whereas other cells with low SAR were not affected. To ensure uniform SAR distribution at different positions, we carried out this electromagnetic simulation study. The A375 cell line grows with adherence, and the culture medium absorbs the energy and reflects the incident MMW efficiently. Thus, the upward exposure was selected. The result showed good uniformity with the increase in distance to approximately 50 mm. Hence, when examinations are performed in vitro, the distribution of the energy absorbed by cells should be considered, except for the electrical parameters such as incident power.
Conclusion
Overall, exposure to 35.2-GHz MMW induced the suppression of A375 cell viability and promoted apoptosis through a caspase-dependent pathway involving activation of caspase-8 and -3.
Abbreviations
- CCK-8
cell counting kit-8
- MMW
millimeter wave
- OD
optical density
- PBS
phosphate-buffered saline
- PI
propidium iodide
- SAR
specific absorption rate
- SD
standard deviation
- TBST
Tris-buffered saline with Tween 20.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received funding through the National Natural Science Foundation of China (grant number 61671229, 61929101), National Key Research and Development Program of China (No.2016YFC0100800, 2016YFC0100801, 2016YFC0100802), Science and Technology Program of Guangdong, China (No. 2017B020229004), Science and Technology Program of Guangzhou, China (No. 201704020091).
ORCID iD: Sherman Xuegang Xin  https://orcid.org/0000-0001-9031-2961
https://orcid.org/0000-0001-9031-2961
References
- 1. Banik S, Bandyopadhyay S, Ganguly S. Bioeffects of microwave––a brief review. Bioresour Technol. 2003;87(2):155–159. [DOI] [PubMed] [Google Scholar]
- 2. Pakhomov AG, Akyel Y, Pakhomova ON, Stuck BE, Murphy MR. Current state and implications of research on biological effects of millimeter waves: a review of the literature. Bioelectromagnetics. 1998;19(7):393–413. [DOI] [PubMed] [Google Scholar]
- 3. Sienkiewicz Z. Biological effects of electromagnetic fields and radiation. J Radiol Prot. 1998;18(3):185–193. [DOI] [PubMed] [Google Scholar]
- 4. Belyaev IY., Shcheglov VS, Alipov ED, Ushakov VL. Nonthermal effects of extremely high-frequency microwaves on chromatin conformation in cells in vivo-dependence on physical, physiological, and genetic factors. IEEE Trans Microw Theory Tech. 2000;48(11):2172–2179. [Google Scholar]
- 5. Rojavin MA, Ziskin MC. Medical application of millimetre waves. QJM. 1998;91(1):57–66. [DOI] [PubMed] [Google Scholar]
- 6. Beneduci A, Chidichimo G, de Rose R, Filippelli L, Straface SV, Venuta S. Frequency and irradiation time-dependent antiproliferative effect of low-power millimeter waves on RPMI 7932 human melanoma cell line. Anticancer Res. 2005;25(2):1023–1028. [PubMed] [Google Scholar]
- 7. Beneduci A, Chidichimo G, Tripepi S, Perrotta E. Transmission electron microscopy study of the effects produced by wide-band low-power millimeter waves on MCF-7 human breast cancer cells in culture. Anticancer Res. 2005;25(2):1009–1013. [PubMed] [Google Scholar]
- 8. Beneduci A, Chidichimo G, Tripepi S, Perrotta E, Cufone F. Antiproliferative effect of millimeter radiation on human erythromyeloid leukemia cell line K562 in culture: ultrastructural- and metabolic-induced changes. Bioelectrochemistry. 2007;70(2):214–220. [DOI] [PubMed] [Google Scholar]
- 9. Logani MK, Szabo I, Makar V, Bhanushali A, Alekseev S, Ziskin MC. Effect of millimeter wave irradiation on tumor metastasis. Bioelectromagnetics. 2006;27(4):258–264. [DOI] [PubMed] [Google Scholar]
- 10. Radzievsky AA, Gordiienko OV, Szabo I, Alekseev SI, Ziskin MC. Millimeter wave-induced suppression of B16F10 melanoma growth in mice: involvement of endogenous opioids. Bioelectromagnetics. 2004;25(6):466–473. [DOI] [PubMed] [Google Scholar]
- 11. Beneduci A. Evaluation of the potential in vitro antiproliferative effects of millimeter waves at some therapeutic frequencies on RPMI 7932 human skin malignant melanoma cells. Cell Biochem Biophys. 2009;55(1):25–32. [DOI] [PubMed] [Google Scholar]
- 12. Yaekashiwa N, Otsuki S, Hayashi S, Kawase K. Investigation of the non-thermal effects of exposing cells to 70-300 GHz irradiation using a widely tunable source. J Radiat Res. 2018;59(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deghoyan A, Heqimyan A, Nikoghosyan A, Dadasyan E, Ayrapetyan S. Cell bathing medium as a target for non thermal effect of millimeter waves. Electromagn Biol Med. 2012;31(2):132–142. [DOI] [PubMed] [Google Scholar]
- 14. Ayrapetyan S. The role of cell hydration in realization of biological effects of non-ionizing radiation (NIR). Electromagn Biol Med. 2015;34(3):197–210. [DOI] [PubMed] [Google Scholar]
- 15. Nikoghosyan A, Heqimyan A, Ayrapetyan S. Non-thermal microwave radiation-induced brain tissue dehydration as a potential factor for brain functional impairment. Int J Basic Appl Sci. 2016;5(4):188–195. [Google Scholar]
- 16. Ziskin MC. Physiological mechanisms underlying millimeter wave therapy. In: Ayrapetyan SN, Markov MS, eds. Bioelectromagnetics Current Concepts. Springer; 2006; 241–251. [Google Scholar]
- 17. Li XH, Ye HZ, Cai LL, et al. Millimeter wave radiation induces apoptosis via affecting the ratio of Bax/Bcl-2 in SW1353 human chondrosarcoma cells. Oncol Rep. 2012;27(3):664–672. [DOI] [PubMed] [Google Scholar]
- 18. Zhu WH, Zhang W, Wang HY, Xu JJ, Li Y, Lv SJ. Apoptosis induced by microwave radiation in pancreatic cancer JF305 cells. Can J Physiol Pharmacol. 2014;92(4):324–329. [DOI] [PubMed] [Google Scholar]
- 19. Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. [DOI] [PubMed] [Google Scholar]
- 20. Soriano ME, Scorrano L. The interplay between Bcl-2 family proteins and mitochondrial morphology in the regulation of apoptosis. Adv Exp Med Biol. 2010;687:97–114. [DOI] [PubMed] [Google Scholar]
- 21. Alekseev SI, Radzievsky AA, Logani MK, Ziskin MC. Millimeter wave dosimetry of human skin. Bioelectromagnetics. 2008;29(1):65–70. [DOI] [PubMed] [Google Scholar]
- 22. Schonborn F, Pokovic K, Burkhardt M, Kuster N. Basis for optimization of in vitro exposure apparatus for health hazard evaluations of mobile communications. Bioelectromagnetics. 2001;22(8):547–559. [DOI] [PubMed] [Google Scholar]
- 23. Zhao JX. Numerical dosimetry for cells under millimetre-wave irradiation using Petri dish exposure set-ups. Phys Med Biol. 2005;50(14):3405–3421. [DOI] [PubMed] [Google Scholar]
- 24. Ayrapetyan G, Hayrapetyan H, Dadasyan E, et al. The non thermal effect of weak intensity millimeter waves on physicochemical properties of water and water solutions. Electromagn Biol Med. 2009;28(4):331–341. [DOI] [PubMed] [Google Scholar]
- 25. Zhao JX, Lu DQ. Effect of the cell culture dish configuration on the millimeter-wave irradiation dose. J Xidian Univ. 2004;31(1):115–118. [Google Scholar]
- 26. Li JX, Niu ZQ. Introduction to Bioelectromagnetics. XiDian University Press; 1991. [Google Scholar]
- 27. Angulo LD, Garcia SG, Pantoja MF, Sanchez CC, Martin RG. Improving the SAR distribution in petri-dish cell cultures. J Electromag Waves Appl. 2000;24(5-6):815–826. [Google Scholar]
- 28. Szabo I, Rojavin MA, Rogers TJ, Ziskin MC. Reactions of keratinocytes to in vitro millimeter wave exposure. Bioelectromagnetics. 2001;22(5):358–364. [DOI] [PubMed] [Google Scholar]
- 29. Gerald MC. Caspases: the executioners of apoptosis. Biochem J. 1997;326(pt 1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]